Abstract
BACKGROUND
Animal studies have demonstrated better embryo development in vivo than in vitro. This pilot study tested the feasibility of using a novel in utero culture system (IUCS) to obtain normal human fertilization and embryo development.
METHODS
The IUCS device comprised a perforated silicone hollow tube. The study included 13 patients (<36 years) undergoing a first intracytoplasmic sperm injection (ICSI) treatment and 167 metaphase II oocytes in three groups. In Group 1, 1–2 h after ICSI, sibling oocytes were assigned to IUCS or conventional in vitro culture. The device was retrieved on Day 1, and all zygotes were cultured in vitro till Day 5. In Group 2, fertilized oocytes were assigned on Day 1, embryos retrieved on Day 3 and all embryos cultured till Day 5. In Group 3, after Day 0 assignment, embryos were retrieved on Day 3 for blastomere biopsy and fluorescence in situ hybridization (FISH) and cultured until Day 5. The highest quality blastocysts were transferred on Day 5.
RESULTS
Fertilization and embryo development were comparable in the in vitro and IUCS arms, with a tendency towards better embryo quality in the IUCS. FISH analysis in Group 3 revealed more normal embryos using the IUCS (P = 0.049). Three clinical pregnancies and live births were obtained: two from the IUCS arm and one from the in vitro arm.
CONCLUSIONS
Our pilot study shows that this new IUCS appears to be feasible and safe, supporting normal fertilization, embryo development and normal chromosomal segregation. Furthermore, live births are possible after the transient presence of a silicone device in the uterus.Clinicaltrials.gov: NCT00480103.
Keywords: in vivo culture, in utero culture system, ICSI, embryos, oocytes
Introduction
Between 1 and 4% of children are born through assisted reproduction techniques (ARTs) (Hansen et al., 2005). These techniques imply that fertilization occurs outside the fallopian tube, and the preimplantation embryos are cultured in vitro until transferred into the uterus. Between patients and within one patient, embryo quality may differ considerably. On the one hand, about 50% of human embryos arrested during the first week as a result of chromosomal abnormalities (Munné et al., 1995). On the other hand, suboptimal in vitro culture conditions may be associated with apoptotic events in human embryos (Hardy et al., 2001).
In the bovine model, it has been demonstrated that the post-fertilization environment affects embryo quality in terms of gene expression, cryotolerance and metabolism (Wrenzycki et al., 2005, 2007; Lonergan et al., 2006; Duranthon et al., 2008). In humans, however, there is no similar evidence from the literature.
In the current ARTs, a tendency to optimize the cost/benefit ratio exists by providing more ‘physiological’ treatment conditions, including mild ovarian stimulation and single embryo transfer. Mimicking physiological culture conditions could provide us with new insights into early embryonic development.
The present pilot study was designed to explore the safety and feasibility of a novel in utero encapsulation technology (Lysaght and Aebischer, 1999) for human embryos and aimed to compare the characteristics of the resulting embryos with their in vitro developed counterparts, in patients younger than 36 years of age undergoing a first intracytoplasmic sperm injection (ICSI) treatment. The technique involves the introduction of microinjected human oocytes into a retrievable and permeable tubing system that allows optimal exchange between the uterine maternal environment and the developing embryo.
Materials and Methods
Study design
The pilot study was carried out at the Centre for Reproductive Medicine, UZ Brussel. The in vitro fertilization laboratory is certified according to ISO15 189. All patients included in the study gave written informed consent.
Individual oocytes/embryos from patients of <36 years old undergoing a first ICSI treatment were randomly assigned to either the in vivo, in utero culture system (IUCS) or the in vitro culture system. If less than eight metaphase II (MII) oocytes were obtained on the day of oocyte retrieval, then the patient was excluded from the study. Other exclusion criteria were endometriosis, polycystic ovary syndrome and severe male-factor infertility. A total of 13 patients were included and numbered consecutively according to the order of enrolment. Overall, 167 MII oocytes were analysed. Three distinct groups were used in order to compare fertilization, embryo development and chromosomal constitution obtained in vivo and in vitro. In Group 1, half of the oocytes were inserted into the device shortly after microinjection (Day 0) and retrieved on Day 1 in order to observe fertilization. In Group 2, half of the in vitro fertilized oocytes were inserted on Day 1 and retrieved on Day 3 in order to compare embryo development. In Group 3, half of the injected oocytes were inserted into the device shortly after microinjection (Day 0) and retrieved on Day 3 in order to compare embryo development and chromosomal constitution (proof of normal fertilization). The best morphologically graded (euploid) blastocysts were transferred into the uterus on Day 5.
The in vivo culture device
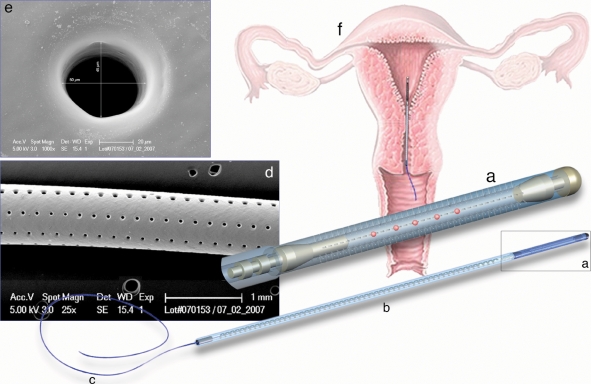
The encapsulation system (Fig. 1) is composed of a 1-cm long microperforated hollow silicone elastomer tubing with an outer diameter of 0.75 mm and an inner diameter of 0.43 mm. Eight longitudinal lines of 45 holes with a diameter of 40 µm are made with a laser-based system. The size of the holes does not allow the embryos to escape the capsule, but allows exchange of nutrients, endometrial cells and other cellular and non-cellular components. The proximal part of the tube can be opened/closed to load/retrieve oocytes/embryos using a titanium hooking device attached to an unperforated silicone tube with an outer diameter similar to the capsule. The plain silicone tubing is reinforced by a stainless steel spiral wire. A polypropylene filament is fixed to the distal end of the silicone tubing to allow the extraction of the device. This IUCS can be loaded into a standard embryo transfer catheter (Prince Medical, Ercuis, France) for introduction into the uterine cavity. A sterile tampon is placed into the vagina in order to keep the device in place. Biocompatibility and toxicity tests have been performed in an in vitro bovine model (unpublished data). Materials used for the manufacture of the IUCS device are FDA USP class VI and ISO 13 485 approved for medical applications.
Figure 1.
Diagram of the IUCS used in women undergoing ICSI: (a) microdrilled silicone segment containing the oocytes/embryos; (b) stabilization segment; (c) extraction segment; (d) scanning electron microscopy of the drilled silicone segment (magnification ×25); (e) high magnification of a laser-drilled hole (scanning electron microscopy, magnification ×1000); (f) schematic representation of the device position in the uterine cavity.
Ovarian stimulation protocol
Recombinant FSH (rFSH) (Puregon®, Organon) and GnRH antagonist Ganirelix (Orgalutran®, Organon) were used for ovarian stimulation. In summary, a low-dose, monophasic-combined oral contraceptive pill (OCP) containing 150 µg of desogestrel and 30 µg of ethinylestradiol (Marvelon®, Organon) was administered for 2 weeks starting on Day 1 of the pre-ARTs cycle. rFSH at a dose of 200 IU per day was started 5 days after discontinuation of the OCP. Ganirelix was initiated at a daily dose of 0.25 mg on Day 6 of the rFSH stimulation. Oocyte maturation was induced by the administration of 10 000 IU of hCG (Pregnyl®, NV Organon) when at least three follicles ≥17 mm diameter were present on ultrasound scan. Oocyte retrieval was carried out 36 h after Pregnyl® injection by vaginal ultrasound-guided puncture of ovarian follicles.
ICSI, fertilization and embryo culture
Pooled cumulus–oocyte complexes were denuded of cumulus cells. MII oocytes were injected by a qualified laboratory technician with a single fresh motile spermatozoon using ICSI (Van Landuyt et al., 2005). Injected oocytes were placed in individual 25 µl droplets of cleavage medium (Medicult®) under paraffin oil (Irvine Scientific®).
Group 1
Group 1 was designed to test the ability to obtain normal fertilization after 18 h in vivo incubation using the IUCS (Fig. 2). Eighty-one cumulus–oocyte complexes were retrieved from seven patients (mean of 11.6 per patient). A total of 79 MII oocytes were microinjected with ejaculated sperm from the partner. Half of the survived oocytes were subjected to in vivo culture 1 to 2 h post-injection. Randomization for allocation to in vivo or in vitro culture was performed as follows. After ICSI, oocytes were randomly allocated to individual culture droplets. In the odd-numbered patients, the first half of the culture droplets with injected oocytes was allocated to the in vitro arm and the other half was allocated to the IUCS arm. In the even-numbered patients, the opposite allocation procedure was performed, i.e. with the first half being allocated to the IUCS and the second half being further cultured in vitro. In case of an uneven oocyte number, an extra oocyte was allocated to in vitro culture. The microinjected oocytes assigned to the IUCS arm were loaded into the capsule (Fig. 1) under microscopic control at ×5 to ×10 magnification. Loading was performed in a dish filled with HEPES-buffered medium kept at 37°C. The device was inserted into a catheter and transferred to the uterus. After in vivo culture for 18 h, the IUCS was removed from the uterine cavity and immediately placed into HEPES-buffered medium at 37°C. The silicone capsule was opened at both ends, and the zygotes were recovered by aspirating the content of the capsule with a glass pipette or by flushing the contents with a blunt needle. Zygotes were individually placed into 25 µl droplets of cleavage medium under oil. Fertilization obtained in vivo or in vitro was evaluated by the appearance of two pronuclei at 18 to 20 h after injection, and all zygotes were further cultured in vitro. Embryo development was evaluated daily by the assessment of number of cells, fragmentation rate, cell size, symmetry, granulation, vacuolization and multinucleation. The different parameters were combined in an embryo score, ranging from Q1 (excellent) to Q4 (poor).
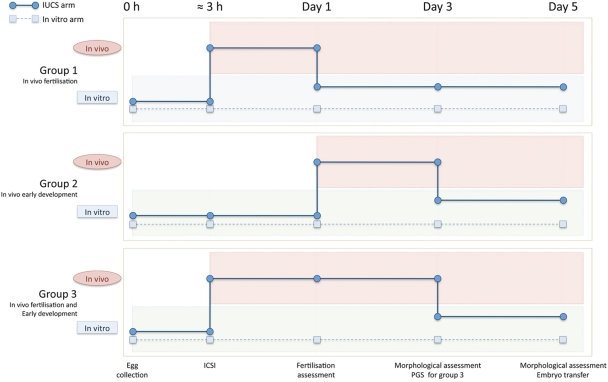
Figure 2.
Timeline for the pilot study, designed to test the utility of the IUCS in supporting normal human fertilization and embryo development. For each patient, after ICSI, half of the injected oocytes were allocated to the IUCS arm (in vivo) and the other half to the in vitro arm. The times were selected to assess the IUCS for the following aspects of early development—Group 1: Day 0 to 1, in vivo fertilization; Group 2: Day 1 to 3, in vivo early embryo development and Group 3: Day 0 to 3, in vivo fertilization and early embryo development.
In the morning of Day 3, all embryos were transferred to blastocyst medium (Medicult®). Embryos were evaluated until transfer (Day 5) or until freezing (Day 5 or 6). For blastocyst evaluation, the scoring system of Gardner and Schoolcraft (1999) was used, and a combined embryo score (Q1 to Q4) was given to the blastocysts, considering blastocyst grade, fragmentation, completeness of compaction and estimation of the number of cells in the inner cell mass and trophectoderm. The morphologically best embryos were transferred into the uterus. Only supernumerary blastocysts of Q1 and Q2 (excellent and good, respectively) were cryopreserved with glycerol as cryoprotectant.
Group 2
Group 2 was designed to compare the effect of both culture systems on early embryo development at 66 h post-injection (Fig. 2). Only normally fertilized oocytes (two pronuclei) were randomized for in vivo or in vitro culture. Forty-six cumulus–oocyte complexes were retrieved from three patients (mean of 15.3 per patient). A total of 41 MII oocytes were microinjected. On Day 1, half of the normally fertilized oocytes were cultured in vivo and the other half in vitro. Random allocation to the in vitro or the in vivo arm was similar to the procedure already described for Group 1. Embryos were retrieved from the capsule on Day 3 and further cultured in vitro up to Day 5 or 6.
Group 3
Group 3 was designed to examine the feasibility of obtaining both normal fertilization and normal early embryo development using the IUCS. From three patients, 57 cumulus–oocyte complexes were retrieved. A total of 47 MII oocytes were injected and the surviving oocytes were randomly allocated to in vivo or in vitro culture (as described earlier) 1 to 2 h after microinjection. Embryos were retrieved from the capsule on Day 3. In order to assess normal fertilization and euploidy, in vivo and in vitro embryos were biopsied and analysed by fluorescence in situ hybridization (FISH). Embryos were further cultured in vitro up to Day 5 or 6.
Embryo biopsy and FISH protocol
Embryo biopsy was performed on Day 3 embryos with ≥5 blastomeres and ≤50% fragmentation.
Before the biopsy, embryos were placed in a droplet containing Ca2+ and Mg2+ free medium (G-PGD, Vitrolife, Kungsbacka, Sweden). Laser technology was used to perforate the zona pellucida. One blastomere was withdrawn with a bevelled aspiration pipette and spread on a slide according to the HCl/Tween-20 method (Staessen et al., 2003).
In all blastomeres, five chromosomes were analysed by FISH in a one-step procedure. Centromeric probes for chromosomes X, Y and 18, and locus-specific probes for chromosomes 13 and 21 were used for hybridization (Vysis Inc., Downers Grove, IL, USA).
The percentage of normal embryos in each arm was defined as the number of euploid embryos upon the number of embryos for biopsy.
Embryo transfer and pregnancy test
In each group, the best blastocysts, regardless of culture condition (in vivo or in vitro), were transferred to the uterine cavity on Day 5. In case of similar quality, preference was given to the in vitro embryo. A rise in serum hCG on two consecutive occasions from 11 days after embryo transfer indicated pregnancy. A clinical pregnancy was defined by the presence of a gestational sac with fetal heart beat at ultrasonography after ∼7 weeks of pregnancy.
Statistical evaluation
Categorical data are presented as number of cases and percentages for each culture arm. For each group, the in vivo and in vitro culture arms were compared by using Fisher’s exact probability test, with a value of 0.05 as the limit of significance. The Statistical Package for the Social Sciences version 16.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Registration
This study was registered with number NCT00480103 in the Clinical Trial web (www.clinicaltrials.gov), and it received institutional review board approval by the Ethics Committee of the Centre for Reproductive Medicine, UZ Brussel, Brussels, Belgium.
Results
Group 1
In Group 1, 74 out of 79 MII oocytes were successfully injected and randomly assigned to in vivo or in vitro culture. Five injected oocytes degenerated shortly after ICSI, before randomization was performed. In patients with an odd number of successfully injected oocytes (six cases), the extra oocyte was allocated to in vitro culture. Overall, 40 injected oocytes were cultured in vitro, while 34 injected oocytes were loaded into the capsule and cultured within the IUCS inside the maternal uterus for 18 h (mean of 4.9 per capsule, range 3–8). From the IUCS, 32 out of 34 oocytes were successfully retrieved and 23 showed normal fertilization (2PN, 67.6%) (Table I). In vitro, 30 out of 40 oocytes showed 2PN (75%). On Day 3, 15 out of 23 embryos from the in vivo arm (65.2%) were good-quality embryos (Q1 + Q2) versus 14 of the 30 in vitro cultured siblings (46.7%). A similar trend was observed on Day 5, the day of embryo transfer for all seven patients. Three embryos were selected for transfer from the in vitro arm, and four embryos were chosen from the IUCS arm. A similar number of embryos was cryopreserved in both arms. Three of the seven patients in Group 1 showed a clinical pregnancy: two of them with a blastocyst of the IUCS arm and one with a blastocyst of the in vitro arm. Three healthy children were born: two from IUCS and one from in vitro culture.
Table I.
Results of fertilization and embryo development in vivo (using IUCS) and in vitro (Group 1)
| 7 patients |
||||
|---|---|---|---|---|
| 81 COC1 |
||||
| 79 MII2 oocytes |
||||
| 74 successfully injected MII oocytes |
||||
|
in vitro culture |
in vivo culture |
|||
| Day 0 | ||||
| Allocated injected oocytes | 40 | 34 | ||
| Day 1* | ||||
| 2PN | 30/40 | 75.0% | 23/34 | 67.6% |
| 1PN | 0/40 | 0.0% | 2/34 | 5.9% |
| ≥3PN | 7/40 | 17.5% | 4/34 | 11.8% |
| Zero PN | 3/40 | 7.5% | 3/34 | 8.8% |
| Not retrieved | 2/34 | 5.9% | ||
| Day 3** | ||||
| Q13 | 5/30 | 16.7% | 8/23 | 34.8% |
| Q24 | 9/30 | 30.0% | 7/23 | 30.4% |
| Q35 | 10/30 | 33.3% | 4/23 | 17.4% |
| Q46 | 6/30 | 20.0% | 4/23 | 17.4% |
| Day 5*** | ||||
| Q1 | 4/30 | 13.3% | 4/23 | 17.4% |
| Q2 | 6/30 | 20.0% | 6/23 | 26.1% |
| Q3 | 5/30 | 16.7% | 4/23 | 17.4% |
| Q4 | 15/30 | 50.0% | 9/23 | 39.1% |
| Embryos for ET | 3 | 4 | ||
| Embryos frozen | 9 | 8 | ||
1Cumulus–oocyte complexes, PN: pronuclei, ET: embryo transfer.
2Metaphase II.
3Q1: excellent quality.
4Q2: good quality.
5Q3: fair quality.
6Q4: poor quality.
Using Fisher’s exact probability test:
*P = 0.29, for fertilization in the in vitro versus in vivo culture arms.
**P = 0.41, for distribution of embryo quality on Day 3 in in vitro versus in vivo culture arms.
***P = 0.86, for distribution of embryo quality in in vitro versus in vivo culture arms.
Group 2
In Group 2, a total of 41 MII oocytes were microinjected, 32 survived and 29 normally fertilized zygotes were randomly assigned to in vivo or in vitro culture (Table II). Fifteen zygotes were cultured in vitro, while 14 were loaded into the capsule and cultured within the IUCS inside the uterus for 48 h (mean of 4.7 per capsule, range 3–8). On Day 3, 13 out of 14 in vivo embryos were successfully retrieved, while one was damaged. The morphological scores revealed that 8 out of 14 embryos showed good quality (Q1 + Q2) (57.1%) in the IUCS arm, while 5 out of 15 good embryos were observed (33.3%) in the in vitro arm. The morphological scores on Day 5 showed a similar trend. Two patients received one embryo for transfer and one patient exceptionally received two embryos. Three embryos were selected from the IUCS, and one embryo was chosen from the in vitro arm. One biochemical pregnancy was obtained in the patient who received one IUCS and one in vitro-derived embryo. No fetal sac was observed on ultrasound.
Table II.
Results of embryo development in vivo and in vitro (Group 2)
| 3 patients |
||||
|---|---|---|---|---|
| 46 COC |
||||
| 41 MII oocytes |
||||
| 32 successfully injected MII oocytes |
||||
| 29 2PN oocytes |
||||
|
in vitro culture |
in vivo culture |
|||
| Day 1 | ||||
| Allocated 2PN oocytes | 15 | 14 | ||
| Day 3* | ||||
| Q1 | 3/15 | 20.0% | 4/14 | 28.6% |
| Q2 | 2/15 | 13.3% | 4/14 | 28.6% |
| Q3 | 4/15 | 26.7% | 3/14 | 21.4% |
| Q4 | 6/15 | 40.0% | 2/14 | 14.3% |
| Not retrieved | 1/14 | 7.1% | ||
| Day 5** | ||||
| Q1 | 2/15 | 13.3% | 2/14 | 14.3% |
| Q2 | 1/15 | 6.7% | 3/14 | 21.4% |
| Q3 | 2/15 | 13.3% | 3/14 | 21.4% |
| Q4 | 10/15 | 66.7% | 5/14 | 35.7% |
| Not retrieved | 1/14 | 7.1% | ||
| Embryos for ET | 1 | 3 | ||
| Embryos frozen | 3 | 2 | ||
Using Fisher’s exact probability test:
*P = 0.45, comparing the distribution of embryo quality on Day 3 in in vitro and in vivo culture.
**P = 0.39, comparing the distribution of embryo quality on Day 5 in in vitro and in vivo culture.
Group 3
In Group 3, 57 cumulus–oocyte complexes were retrieved from three patients (mean of 19.0 oocytes per patient). A total of 47 MII oocytes were microinjected, 3 degenerated before randomization, resulting in 44 surviving oocytes of which 22 were cultured in vitro and 22 in the IUCS (Table III). On Day 3, 19 out of 22 embryos were successfully retrieved from IUCS. As it is not possible to evaluate the euploidy of the in vivo arm embryos at the time of morphological qualification, the proportion of embryo quality has to be calculated on allocated oocytes instead of fertilized oocytes (Table III). The morphological scores on Days 3 and 5 showed a non-significant trend in favour of the in vivo culture (63.6% versus 45.5% good quality on Day 3 and 50.0% versus 22.7% on Day 5). On Day 3, biopsy was performed in 13 in vitro embryos and 17 in vivo embryos. FISH analysis revealed euploidy in 15 out of 17 biopsied IUCS embryos and in 7 out of 13 biopsied in vitro embryos (P = 0.049, Table III). For two in vitro embryos, no FISH diagnosis was obtained. Embryo transfer was performed in two out of three patients: one patient with an embryo of the in vivo arm and one patient with an embryo of the in vitro arm. The embryos of the third patient, although genetically normal, showed insufficient morphological quality for transfer. No pregnancy was obtained.
Table III.
Results of embryo development and fluorescence in situ hybridization (chromosomes X, Y, 13, 18, 21) in vivo and in vitro (Group 3)
| 3 patients |
||||
|---|---|---|---|---|
| 57 COC |
||||
| 47 MII oocytes |
||||
| 44 successfully injected MII oocytes |
||||
|
in vitro culture |
in vivo culture |
|||
| Day 0 | ||||
| Allocated injected oocytes | 22 | 22 | ||
| Day 1 | ||||
| 2PN oocytes | 16/22 | 72.7% | ||
| Day 3* | ||||
| Q1 | 6/22 | 27.3% | 3/22 | 13.6% |
| Q2 | 4/22 | 18.2% | 11/22 | 50.0% |
| Q3 | 4/22 | 18.2% | 5/22 | 22.8% |
| Q4 | 2/22 | 9.1% | 0/22 | 0.0% |
| Not retrieved | 3/22 | 13.6% | ||
| Day 5** | ||||
| Q1 | 2/22 | 9.1% | 3/22 | 13.6% |
| Q2 | 3/22 | 13.6% | 8/22 | 36.4% |
| Q3 | 4/22 | 18.2% | 1/22 | 4.5% |
| Q4 | 7/22 | 31.8% | 7/22 | 31.8% |
| Not retrieved | 3/22 | 13.6% | ||
| Embryos for ET | 1 | 1 | ||
| Embryos frozen | 1 | 9 | ||
| FISH results*** | ||||
| Embryos biopsied | 13/22 | 59.0% | 17/22 | 77.3% |
| Normal1/embryos biopsied | 7/13 | 53.8% | 15/17 | 88.2% |
| Abnormal/embryos biopsied | 4/13 | 30.8% | 2/17 | 11.8% |
| No FISH result | 2/13 | 15.4% | 0 | 0.0% |
| Normal/allocated injected oocytes | 7/22 | 31.8% | 15/22 | 68.2% |
1For chromosomes X, Y, 13, 18, 21.
Using Fisher’s exact probability test:
*P = 0.13, comparing the distribution of embryo quality on Day 3 in in vitro and in vivo culture.
**P = 0.28, comparing the distribution of embryo quality on Day 5 (Q1 to Q4) in in vitro and in vivo culture.
***P = 0.049, comparing normal fluorescence in situ hybridization findings in in vitro and in vivo culture.
Technical issues
Initially, a total of 19 patients were included in the present pilot study. However, only 13 patients were included in the comparative embryo quality trial because of technical problems in five cases. The first technical issue (with two devices in the first four patients) was related to the introduction of the IUCS into the transfer catheter. To solve this problem, the catheter was adapted and no more difficulties occurred later on. The second problem was linked to the intrauterine stabilization of the device, as 3 out of 19 were found in the vagina on Day 3. The stabilization system was modified in order to decrease the risk of IUCS migration. In one patient, all the oocytes cultured in the IUCS were arrested in the one-cell stage at retrieval on Day 3 and the zona pellucida had disappeared. The cause of this observation could not have been elucidated.
Flushing technique and tools were improved during this pilot study and consequently the number of embryos damaged by technical procedures decreased progressively (8 embryos damaged out of 24 loaded in the first six patients, and 1 damaged out of 80 loaded in the last seven patients). No sterility problems were observed for all of the embryos cultured in utero.
Discussion
Although in animal models better embryo development has been observed in vivo than in vitro, no comparative data in the human are available from the literature so far. The present work is the first report of an IUCS allowing communication between the embryos and the endometrium and supporting the formation of normal zygotes and good-quality embryos after microinjection, as well as normal chromosomal segregation. The capsule can be inserted transiently into the uterine cavity up to 3 days without bleeding and without evidence of clinical impact on endometrial receptivity. Three clinical pregnancies leading to healthy births have been achieved, two after partial in vivo and one after in vitro culture, but all three after the transient presence of a foreign object in the uterus.
During the last three decades, many researchers have tried to optimize the in vitro environment for the developing embryo, either by mimicking the in vivo composition of genital tract secretions—the ‘back-to-nature’ strategy (Menezo et al., 1984; Gardner et al., 1996; Leese et al., 1998)—or by the ‘let the embryo choose’ strategy (Lawitts and Biggers, 1992; Biggers and McGinnis, 2001). In the 1990s, embryos were cultured in the presence of other cell types named co-culture. The most relevant systems were the use of heterologous human oviductal (Yeung et al., 1992) or endometrial cells (Plachot et al., 1994), and more recently autologous endometrium epithelial cells (Simon et al., 1999). Finally, emerging technologies, such as microfluidic systems, have tried to provide a dynamic microenvironment, in contrast to the static microdrop culture technology (Beebe et al., 2000; Glasgow et al., 2001). All these strategies are dealing with similar remaining problems inherent to the in vitro system: an absence of the complexity of cross-talk between the embryo and surrounding tissues and fluid. In addition, physicochemical conditions are far from physiological conditions, considering exposure to environmental light outside the incubators, fluctuations in temperature and pH, and elevated oxygen levels.
Our novel IUCS was designed to circumvent many of the shortcomings inherent to the in vitro systems and appears to provide a microenvironment that supports equal or superior preimplantation embryo development, as suggested by the present data. The perforated silicone membrane might allow specific and complex elements in the uterine fluid to migrate and interact with the developing embryo, and perhaps protect the embryo against harmful reactive oxygen species and heavy metal toxicity. It should, however, be recognized that culture of the oocyte and placement of the early cleavage-stage embryo into the uterus is a non-physiological condition. Nevertheless, the present data suggest that the uterine environment of the human meets the nutritional requirements for normal fertilization and embryo development, resulting in a higher proportion of euploid embryos compared with in vitro culture.
While initially some technical problems led to damage or loss of oocytes/embryos, these problems were largely solved during the course of the trial. With the numbers available, we observed a non-significant tendency to improved embryo quality in the in vivo arm of all three groups, even after short-term encapsulation for 18 h after ICSI. Although the number of oocytes/embryos in this pilot study was limited, a significant difference in the proportion of euploid embryos (chromosomes X, Y, 13, 18, 21) was observed in favour of the in vivo arm. These findings open again the discussion on in vitro culture conditions and their impact on epigenetic modifications, abnormal chromosome segregation and gene expression.
In conclusion, this new in vivo culture system, which allows communication between the embryo and the endometrium, appears to be feasible and safe, as regards fertilization and embryo development. The present pilot study further suggests superior embryo development and a higher proportion of euploid embryos after in utero culture than after conventional in vitro culture. It also demonstrates the possibility of obtaining pregnancies after transient presence of this device in the uterus. These interesting observations, however, need to be confirmed in a large RCT.
Author contribution
P.M. is the inventor of the IUCS concept (US patent No. 2004261799A1). N.B. and P.A. developed the IUC device at the Ecole Polytechnique Fédérale de Lausanne (EPFL), Switzerland in collaboration with P.M., M.V. and P.L.G. from ANECOVA S.A., Geneva, Switzerland. M.J.L.S., M.F.-S. and C.S. initiated the study of the stability and safety of the intrauterine capsule at the Instituto Valenciano de Infertilidad, Valencia, Spain. Y.H., K.H., C.W. and H.N. performed the embryo toxicity study and the loading training using bovine embryos at the FLI, Neustadt, Germany, and INRA, Jouy en Josas, France. G.V., C.B. and P.D. performed the present clinical pilot study at the Centre for Reproductive Medicine at the UZ Brussel. P.H. provided useful statistical advice.
Funding
Paul Devroey and Nicolas Bouche are shareholders of Anecova. Pascal Mock is shareholder and member of the board of Anecova. Martin Velasco is shareholder, chairman and CEO of Anecova. Philippe Le Goff is a paid consultant for Anecova. Patrick Aebischer is member of the board of Anecova. Funding is coming from Anecova.
References
- Beebe DJ, Moore JS, Bauer JM, Yu Q, Liu RH, Devadoss C, Jo BH. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature. 2000;404:588–590. doi: 10.1038/35007047. [DOI] [PubMed] [Google Scholar]
- Biggers JD, McGinnis LK. Evidence that glucose is not always an inhibitor of mouse preimplantation development in vitro. Hum Reprod. 2001;16:153–163. doi: 10.1093/humrep/16.1.153. [DOI] [PubMed] [Google Scholar]
- Duranthon V, Watson A, Lonergan P. Preimplantation embryo programming: transcription, epigenetics, and culture environment. Reproduction. 2008;135:141–150. doi: 10.1530/REP-07-0324. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–311. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Calderon I, Leeton J. Environment of the preimplantation human embryo in vivo: metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cells. Fertil Steril. 1996;65:349–353. doi: 10.1016/s0015-0282(16)58097-2. [DOI] [PubMed] [Google Scholar]
- Glasgow IK, Zeringue C, Beebe DJ, Choi SJ, Lyman JT, Chan NG, Wheeler MB. Handling individual mammalian embryos using microfluidics. IEEE Trans Biomed Eng. 2001;48:570–578. doi: 10.1109/10.918596. [DOI] [PubMed] [Google Scholar]
- Hansen M, Bower C, Mine E, de Klerk N, Kurinczuck JJ. Assisted reproductive technologies and the risk of birth defects—a systematic review. Hum Reprod. 2005;20:328–338. doi: 10.1093/humrep/deh593. [DOI] [PubMed] [Google Scholar]
- Hardy K, Spanos S, Becker D, Ianelli P, Winston RM, Stark J. From cell death to embryo arrest: mathematical models of human preimplantation embryo development. Proc Natl Acad Sci USA. 2001;98:1655–1660. doi: 10.1073/pnas.98.4.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawitts JA, Biggers JD. Joint effects of sodium chloride, glutamine, and glucose in mouse preimplantation embryo culture media. Mol Reprod Dev. 1992;31:189–194. doi: 10.1002/mrd.1080310305. [DOI] [PubMed] [Google Scholar]
- Leese HJ. Human embryo culture: back to nature. J Assist Reprod Genet. 1998;15:466–468. doi: 10.1023/A:1022526219202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan P, Fair T, Corcoran D, Evans AC. Effect of culture environment on gene expression and developmental characteristics in IVF-derived embryos. Theriogenology. 2006;65:137–152. doi: 10.1016/j.theriogenology.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Lysaght MJ, Aebischer P. Encapsulated cells as therapy. Sci Am. 1999;280:76–82. doi: 10.1038/scientificamerican0499-76. [DOI] [PubMed] [Google Scholar]
- Menezo Y, Testart J, Perrone D. Serum is not necessary in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertil Steril. 1984;42:750–755. doi: 10.1016/s0015-0282(16)48202-6. [DOI] [PubMed] [Google Scholar]
- Munné S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril. 1995;64:382–391. [PubMed] [Google Scholar]
- Plachot M, Alvarez S, Merviel P, Anoine JM, Mandelbaum J, Salat-Baroux J. Role of endometrial cells in in vitro embryo development. Assist Reprod Rev. 1994;4:85–95. [Google Scholar]
- Simon C, Mercader A, Garcia-Velasco J, Nikas G, Moreno C, Remohi J, Pellicer A. Coculture of human embryos with autologous human endometrial epithelial cells in patients with implantation failure. J Clin Endocrinol Metab. 1999;84:2638–2646. doi: 10.1210/jcem.84.8.5873. [DOI] [PubMed] [Google Scholar]
- Staessen C, Tournaye H, Van Assche E, Michiels A, Van Landuyt L, Devroey P, Liebaers I, Van Steirteghem A. PGD in 47,XXY Klinefelter’s syndrome patients. Hum Reprod. 2003;9:319–330. doi: 10.1093/humupd/dmg029. [DOI] [PubMed] [Google Scholar]
- Van Landuyt L, Devos A, Joris H, Verheyen G, Devroey P, Van Steirteghem A. Blastocyst formation in in vitro fertilization versus intracytoplasmic sperm injection cycles: influence of the fertilization process. Fertil Steril. 2005;83:1397–1403. doi: 10.1016/j.fertnstert.2004.10.054. [DOI] [PubMed] [Google Scholar]
- Wrenzycki C, Herrmann D, Lucas-Hahn A, Korsawe K, Lemme E, Niemann H. Messenger RNA expression patterns in bovine embryos derived from in vitro procedures and their implications for development. Reprod Fertil Dev. 2005;17:23–35. doi: 10.1071/rd04109. [DOI] [PubMed] [Google Scholar]
- Wrenzycki C, Herrmann D, Niemann H. Messenger RNA in oocytes and embryos in relation to embryo viability. Theriogenology. 2007;1:77–83. doi: 10.1016/j.theriogenology.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Yeung W, Ho P, Lan E, Chan S. Improved development of human embryos in vitro by a human oviductal cell co-culture system. Hum Reprod. 1992;7:1144–1149. doi: 10.1093/oxfordjournals.humrep.a137810. [DOI] [PubMed] [Google Scholar]