Abstract
An abnormal naevus phenotype is associated with an increased risk of melanoma. We report a pooled analysis conducted using individual naevus data from 15 case-control studies (5,421 melanoma cases and 6,966 controls). The aims were to quantify better the risk, and to determine whether relative risk varied by latitude. Bayesian unconditional logistic random coefficients models were employed to study the risk associated with naevus characteristics. Participants with whole body naevus counts in the highest of four population-based categories had a greatly increased risk of melanoma compared with those in the lowest category (pooled odds ratio (pOR) 6.9 (95% confidence interval (CI): 4.4, 11.2) for those aged <50 years and pOR 5.1 (95% CI: 3.6, 7.5) for those aged ≥50). The pOR for presence compared with absence of any clinically atypical naevi was 4.0 (95% CI: 2.8, 5.8). The pORs for 1–2 and ≥3 large naevi on the body compared with none were 2.9 (95% CI: 1.9, 4.3) and 7.1 (95% CI: 4.7, 11.6), respectively. The relative heterogeneities among studies were small for most measures of naevus phenotype, except for the analysis of naevus counts on the arms, which may have been due to methodological differences among studies. The pooled analysis also suggested that an abnormal naevus phenotype is associated most with melanomas on intermittently sun-exposed sites. The presence of increased numbers of naevi, large naevi and clinically atypical naevi on the body are robust risk factors for melanoma showing little variation in relative risk among studies performed at different latitudes.
Keywords: melanoma, naevus, latitude, pooled analysis, Bayesian
Introduction
Although a number of phenotypic risk factors for melanoma have been identified, the level of risk associated with those factors in different populations living at very different latitudes (and therefore different exposures to the major aetiological environmental exposure: sunlight) is not well established. We set out to quantify better that risk and to compare risk across different studies to determine whether there is variation by latitude. Our ultimate aim is to construct a web-based risk estimation tool and therefore a secondary aim was to identify the most robust measures for use in such a tool.
The number of common and atypical naevi have been shown to be important risk factors for cutaneous malignant melanoma in multiple studies.1 The methods used to count naevi however have varied considerably among studies, and risk estimates have been obtained using different statistical methods and with adjustment for different confounding factors. These differences have hampered the comparisons among studies and meta-analyses. Gandini et al1 conducted a systematic meta-analysis, and reported a pooled relative risk of 6.9 (based on published results from 26 studies) for the presence of 101–120 common naevi compared with <15 naevi on the whole body, and a relative risk of 4.8 (based on published results from 17 studies) for 11–15 naevi on the arms compared with no naevi on the arms, and a relative risk of 6.4 (based on 13 published studies) for 5 atypical naevi compared with none using linear dose-response models. Significant heterogeneity among studies was observed in Gandini et al,1 however, the differences between risks could not be explained by population differences, counting methods (self or physician assessment) or adjustment for confounding factors.
We report a pooled data analysis that allows differences to be assessed in the distribution of naevus phenotype among studies. Pooled analysis of the original data allows us to quantify more precisely the risk associated with naevus phenotype using unified statistical models. Other phenotypic risk factors such as hair colour, skin type and freckling were investigated as possible confounders.
Materials and Methods
Identification and recruitment of case-control study data sets
It is noted in Gandini et al1 that small studies tended to report more positive results than larger studies, which is likely due to publication bias. For this reason, and since small sized studies would require similar analytic effort but contribute little to the overall analysis, inclusion in the pooled analysis was restricted to studies with at least 100 cases and 100 controls. We further restricted the sources of controls to be primarily population-based or non hospital-based as our final aim is to construct a risk prediction tool for the general public. Multi-country studies were excluded if each country involved had less than 100 cases and 100 controls or had included hospital-based controls.
Participant groups of the Melanoma Genetics Consortium, GenoMEL (www.genomel.org) were invited to take part in the pooled analyses, as were other investigators who had conducted melanoma case-control studies. Forty-six studies included in Gandini et al1, 2 were screened to determine those that satisfied the criteria for inclusion in the pooling project. Those studies included in Gandini et al1, 2 were published prior to September 2002, and a new search was conducted to identify case-control studies published from January 2002 until 2007. The MEDLINE search was conducted using key words: melanoma, case control* and case-control* tagged on title and abstract, and restricted to human research only. One hundred and twenty nine articles were found, and 10 review articles and 15 clinical trials were excluded. All relevant articles were evaluated using the title and abstract, and seven new case-control studies other than those in Gandini et al1, 2 were identified. Screening was restricted to studies of cutaneous melanoma in adults; uveal melanoma studies were not considered.
Of the 53 studies identified by Gandini et al1, 2 and as a result of the MEDLINE search performed for studies reported after January 2002, 18 studies were excluded due to smaller study size (8 cohort studies, 4 case-control studies using hospital controls, 6 case-control studies using non-hospital controls). Eleven studies using hospital-based controls and one study with mixed races were further excluded. There was a total of 26 studies which passed the inclusion criteria, and the principal investigators were invited to participate. We were not able to make contact with seven of the study investigators, one expressed interest but could not locate data in electronic format, and two declined to take part in the pooled analyses. Fifteen studies were ultimately included for the naevus phenotype analyses. A diagram of the selection process for the studies included in the pooled analyses is given in Supplementary Figure S1.
Investigators who expressed an interest in collaboration were initially asked to send in sample data based on 10 people including some or all of the following information: disease status, hair and eye colour, freckling, naevus phenotype, sun damage including solar keratoses, sun exposure, latitude of residence, family history of melanoma, sex, age, previous sun bed use, reported sun sensitivity, and MC1R and CDKN2A genotype if available.
Investigators were asked to provide a data set including all variables that related to the above risk factors. Each study was analyzed separately before pooling using its original design and categorization. If there were discrepancies in results from the original article, the data and results were checked with the original study investigators. The corrected datasets were then recoded for each risk factor uniformly across studies wherever possible.
Categorization of risk factors across studies
Most of the studies recruited only newly diagnosed melanoma cases with no selection on the basis of family history, although two studies3, 4 included a mixture of prevalent and incident cases. Eleven studies recorded melanoma history in first-degree relatives. Two hundreds and sixty five out of 5326 controls (5%) and 349 out of 4342 cases (8%) reported at least one first-degree relative with a diagnosis of melanoma. The median time interval between melanoma diagnosis and interview or naevus phenotype assessment was less than one year, and more than 90% of cases were interviewed within 4 years of diagnosis. Age at interview (and hence at skin examination) was adopted for age in the analysis for both cases and controls; age at diagnosis was substituted for cases if age at interview was missing. Four studies had a median age of cases between 40 and 49 years, and 11 studies had a median age of cases between 50 and 59 years. All analyses for common naevi were conducted by first stratifying the data into participants aged <50 and ≥ 50 years since naevi are thought to develop in the earlier years of life and then involute.5 Fifty years was chosen as the cut-off, and this age gave reasonable numbers of cases and controls in each age subgroup.
Hair colour was categorized wherever possible as red (including red, reddish brown, auburn, strawberry blond), blond (light blond, medium blond, dark blond, ash blond), brown (light brown, pale brown, dark brown) or black. If hair colour in childhood, adolescence or early adulthood was recorded, then it was used instead of hair colour at the time of interview.
Reported skin type was evaluated using the 6 Fitzpatrick skin types6 (I: always burns, never tans to IV: never burns, tans very easily) or based on skin reaction after a few days repeated sun exposure in summer, such as ability to tan. If information regarding ability to tan was not recorded in a study, then skin reaction to first summer sun exposure or any other assessment of propensity to burn, if available, was substituted in the analyses.
There was considerable variation in recording of freckling and naevus phenotype among data sets. For example, freckling was measured as either some-or-none, percentage of coverage (0–100%), or using a freckling chart (degree 1–6) in different studies. Some studies only measured freckling on the face, and others included the arms and back. Freckling on the face was favoured in our analyses, if available, and otherwise freckling on the arms or back was used. Freckling was characterized as absence or presence in the pooled analysis.
Common naevi were counted differently among studies. Most studies included only naevi at least 2 mm in size. Six studies included common naevus counts on the entire body; some counted naevi on the arms. In addition to naevi at least 2mm in size, seven studies also recorded the number of palpable naevi on arms. Several studies also recorded the number of large naevi (variously defined as ≥5, ≥7, or ≥8mm), and clinically atypical (or dysplastic) naevi.
For studies that recorded common naevi ≥2mm or ≥3mm on the entire body, there were low correlations between age and naevus count (ranging from −0.2 to 0.0) in the controls within the two age groups. Raw naevus counts on the whole body or arms of the control participants were divided into four study-specific quarters for each age group (aged <50 and ≥ 50 years) separately. Data from men and women were pooled to ensure a reasonable number of controls in each age group. If more than 25% of the controls had no naevi on the whole body or arms, then all controls with a zero naevus count were included in the lowest naevus count group, and the remaining controls were equally divided into three groups representing thirds of controls with non-zero naevus counts. The quartile (tertile) cut-points obtained from the controls were then applied to melanoma cases in the same study and age group. The resulting four study-specific naevus-count groups represent low, intermediate-low, intermediate-high and high naevus count categories. If a study used pre-defined naevus count categories (such as low, medium, and high), the originally defined groups were used.
In addition to common naevi of size larger than 2 or 3 mm, seven studies recorded the number of palpable naevi on the arms, and the actual counts were grouped into three categories (0, 1–2 and ≥ 3). The number of large naevi (≥5, ≥7, or ≥8mm) was categorized as 0, 1–2, ≥ 3 on the entire body (five studies) or 0, 1, ≥ 2 on the arms (four studies). Four studies recorded the presence of atypical naevi, and atypical naevi were classified by presence or absence in the pooled analysis.
Statistical methods
Bayesian unconditional logistic random coefficients models were employed to study the overall effects of naevus phenotype and to account for the heterogeneity among studies in the risk of developing melanoma. Unlike the two-stage approach in most meta-analyses, a random coefficients model enabled us to estimate the pooled odds ratios (pORs) and the variations among study-specific log ORs simultaneously. The among-study variance quantifies the degree of heterogeneity in the effects of the corresponding risk factor among studies. The coefficient of variation, i.e. the ratio of the estimated among-study standard deviation and log OR, was used to assess the ‘relative heterogeneity’ and robustness among different naevus characteristics when applicable. Multivariable logistic random coefficients models were fitted to all records adjusting for age, sex, hair colour, ability of skin to tan (or propensity to burn) and freckling (if available). Dummy variables were created for naevus count categories where the lowest category was assigned as the reference.
To examine the potential influence of latitude on the risk associated with naevi, we also allowed the pORs to vary between the two pre-defined geographical regions (Northern region: latitude ≥ 50N, and Mid/Southern region: latitude < 50N). However, the among-study variance was assumed to be the same across the two regions.
The Bayesian Markov chain Monte Carlo (MCMC) method was employe d to estimate the risk associated with naevus characteristics, and WinBUGS software7 was adopted for this purpose. Flat priors with low precision were assigned for all parameters. Details of the statistical models, prior specifications and their implementations are given in Supplementary Statistical Methods.
Results
Fifteen case-control studies in which naevus phenotype information was collected were included, and Table 1 summarizes the studies included in the naevus phenotype pooled analysis.3, 4, 8–20 The pooled dataset consists of studies from Europe, North America, Hawaii and Australia. Eight studies conducted in latitude ≥ 50N was grouped into Northern region, and 7 studies located in attitude < 50N was classified into Mid/Southern region. A total of 5,421 melanoma cases and 6,966 controls were included in the analysis.
Table 1.
Description of studies and of their naevus counting methods in the pooled analysis (ordered by latitude).
| Study | Diagnosis year | Geographic location | Latitudee | Cases/Controls | Matching | Naevus counts on body | Naevus counts on arms |
|---|---|---|---|---|---|---|---|
| Westerdahl, 19948 | 1988–1990 | South Sweden | 56N | 400/640 | Stratified by age, sex, parish | Self reported: left arm ≥3mm total + ≥3mm raised Exact count |
|
| Westerdahl, 20009 | 1995–1997 | South Sweden | 56N | 571/913 | Stratified by age, sex, parish | Self reported: left arm ≥3mm total + ≥3mm raised Exact count |
|
| Osterlind, 198810a | 1982–1985 | East Denmark | 55N | 293/536 | Stratified by age, sex | Interviewer (blind): both arms below axilla <5mm palpable + ≥5mm palpable Exact count |
|
| Swerdlow, 198611 | 1979–1984 | Scotland, UK | 55N | 180/197 | Stratified by age, sex, treatment city | Dermatologist: whole body ≥2mm total + ≥7mm total Exact count |
Dermatologist: both arms ≥2mm total + ≥7mm total Exact count |
| Elwood, 199012 | 1984–1986 | E. Midlands, UK | 53N | 195/195 | Pair matched by age, sex | Interviewer (blind): both arms ≥3mm total + ≥6mm total + all raised Exact count |
|
| Bataille, 199613 | 1989–1993 | N.E. Thames, UK | 52N | 418/417 | Stratified by age, sex | Dermatologist: whole body 2–5mm + 5mm–1cm + > 1cm + all atypical Exact count |
Interviewer: both arms 2–5mm + 5mm–1cm + > 1cm Exact count |
| Kennedy, 200314b | 1991–1998 | Leiden, the Netherlands | 52N | 123/385 | Stratified by age, sex | Dermatologist: whole body ≥2mm total + all atypical Exact count |
|
| Mössner, 20073c | Munich, Göttingen, Germany | 50N | 319/345 | Stratified by sex | Interviewer: both arms ≥2mm total Exact count |
||
| Titus-Ernstoff, 200515 | 1995–1998 | New Hampshire, USA | 43N | 323/424 | Stratified by age, sex | Physician: whole body ≥3mm total + all atypical Exact count |
Physician: both arms ≥3mm total Exact count |
| Berwick, 199616 | 1987–1989 | Connecticut, USA | 41N | 650/549 | Stratified by age, sex | Interviewer: back ≥2mm flat + ≥5mm total + all raised Exact count |
Interviewer: both arms ≥2mm flat + ≥5mm total + all raised Exact count |
| Kanetsky, 200117d | 1997–1999 | Pennsylvania, USA | 39N | 363/150 | Friend controls | Research nurse: whole body excluding scalp and genitalia ≥2mm total (truncated at 100) + ≥8mm total + all atypical Exact count |
|
| Holly, 199518 | 1981–1986 | San Francisco, California, USA | 37N | 452/935 | Stratified by age, county, women only | Self reported: whole body, including removed plus [check for atypical] ≥5mm total Exact count |
|
| Holman, 198420 | 1980–1981 | Western AU | 31S | 511/511 | Pair matched by age, sex | Interviewer: both arms below axilla Palpably raised Exact count |
|
| Green, 198519 | 1979–1992 | Queensland, AU | 27S | 232/232 | Pair matched by age, sex | Interviewer: left arm ≥2mm raised or macular Grouped count: 0, 1–4, 5–10, >10 |
|
| Le Marchand, 20064 | 1986–1987 (prevalent) 1988–1992 (incident) |
Hawaii, USA | 21N | 278/278 | Pair matched by age, sex | Self reported (instructed to follow structured protocol with a mirror): whole body All size + ≥5mm Exact count |
Interviewer: both arms ≥5mm flat + ≥2mm palpable + ≥5mm palpable Exact count |
Women only.
Included unpublished data in the pooled analysis.
Three cases and 2 controls with missing age were deleted from the pooled analysis.
Patients coming to the Pigmented Lesion Clinic for presence of pigmented lesions but without diagnoses of melanoma in the original study were excluded from pooled analysis.
Two geographical regions were defined as: Northern region (latitude ≥ 50N), and Mid/Southern region (latitude < 50N)
Six studies collected data on whole body naevus counts (≥2 or ≥3mm, Supplementary Figure S2). Whole body naevus examinations were performed by dermatologists or physicians in these six studies except for the Pennsylvania17 and the Hawaiian study.4 One research nurse completed all the skin examinations in the Pennsylvania study, and participants in the Hawaiian study were instructed in self-reported whole body naevus counts.
Data on arm naevus counts were given by 10 studies (≥2 or ≥3mm, Supplementary Figure S3), usually both arms. Green et al19 (Queensland, Australia) used pre-defined arm naevus count categories (0, 1–4, 5–10, >10) in the original study, and we therefore substituted the mid-point of the corresponding category and 15 for the highest category. We report here on the pooled whole body naevus counts data and data on arms counts where available.
Skin type, freckling and hair colour
The estimated pORs for fair skin type I/II, presence of freckles, red hair colour and blond hair colour overall were 1.7 (95% CI: 1.2, 2.4), 1.7 (95% CI: 0.7, 5.6), 1.9 (95% CI: 1.1, 3.3) and 2.0 (95% CI: 1.3, 3.0), respectively, in the younger age group. Over the age of 50 years the corresponding estimated pORs were 1.8 (95% CI: 1.2, 2.6), 1.8 (95% CI: 1.3, 2.7), 1.6 (95% CI: 1.0, 2.7) and 1.4 (95% CI: 1.0, 2.0).
Whole body naevus count
Cases had a higher total numbers of naevi than did controls in all applicable studies both under and over the age of 50 years (Supplementary Figure S2). There were some differences among studies however. Most notably, the three European studies that recorded whole body naevi ≥ 2mm11, 13, 14 showed a higher count of naevi in younger controls than did those in the United States. The New Hampshire study15 recorded only naevi ≥ 3mm, so that differences in counting methods among studies probably contributed to this difference (Table 1). Number of whole body naevi in the controls differed less in the older people than in younger people.
The distributions of the whole body naevus count categories and their estimated pORs are given in Table 2. The estimated among-study variance of the effect of body naevus categories was 0.07 in the younger than 50 age group. The magnitude of among-study variance gives the degree of heterogeneity of the effect among studies, assumed equal across naevus count categories. The relative heterogeneity (coefficient of variation) was 14% for the highest body naevus count category. For the three European studies (in the Northern latitude region) combined11, 13, 14 that recorded whole body naevus counts, the estimated pORs for body naevus count categories intermediate-low, intermediate-high and high in the < 50 years age group were 1.6 (95% CI: 0.7, 3.6), 2.7 (95% CI: 1.2, 5.7) and 7.9 (95% CI: 3.8, 17.4), respectively. The corresponding estimated pORs for the three US studies (in the Mid/Southern latitude region)4, 15, 17 were 1.0 (95% CI: 0.5, 2.2), 2.5 (95% CI: 1.2, 5.5) and 6.2 (95% CI: 3.0, 13.5), respectively, which were similar to the risk estimates in the Northern region. Figure 1(a) shows the forest plots of study-specific ORs for the highest category of naevus counts compared with the lowest reference category, together with the pORs across latitudes.
Table 2.
Total number of melanoma patients and controls (column percentage in parentheses), the pooled odds ratios (pORs) and 95% Bayesian confidence intervals (CI) and relative heterogeneity (RH) for the whole body naevus count categories and the arm naevus count categories.
| Site and category of naevus counts | Age < 50 | Age ≥ 50 | ||||||
|---|---|---|---|---|---|---|---|---|
| Whole body naevus count1 | Controls | Cases | pORs (95% CI) | RH | Controls | Cases | pORs (95% CI) | RH |
| Low | 197 (26.5%) |
77 (10.3%) |
1 | 352 (34.3%) |
147 (16.7%) |
1 | ||
| Intermediate-low | 184 (24.7%) |
84 (11.2%) |
1.3 (0.8, 2.1) |
182 (17.7%) |
109 (12.3%) |
1.1 (0.7, 1.7) |
||
| Intermediate-high | 179 (24.1%) |
170 (22.7%) |
2.7 (1.7, 4.2) |
248 (24.1%) |
195 (22.1%) |
1.9 (1.3, 2.8) |
||
| High | 184 (24.7%) |
417 (55.8%) |
6.9 (4.4,11.2) |
14% | 245 (23.9%) |
432 (48.9%) |
5.1 (3.6, 7.5) |
11% |
| Total | 744 | 748 | 1,027 | 883 | ||||
| Arms naevus count2 | Controls | Cases | pORs (95% CI) | RH | Controls | Cases | pORs (95% CI) | RH |
| Low | 772 (44.3%) |
312 (23.6%) |
1 | 1,425 (64.3%) |
839 (40.8%) |
1 | ||
| Intermediate-low | 336 (19.3%) |
204 (15.4%) |
1.6 (1.0, 2.5) |
326 (14.7%) |
355 (17.3%) |
1.6 (1.0, 2.5) |
||
| Intermediate-high | 316 (18.1%) |
266 (20.1%) |
2.8 (1.8, 4.4) |
233 (10.5%) |
276 (13.4%) |
1.8 (1.1, 3.0) |
||
| High | 318 (18.3%) |
540 (40.1%) |
5.8 (3.8, 9.1) |
32% | 233 (10.5%) |
586 (28.5%) |
4.0 (2.5, 6.4) |
44% |
| Total | 1,742 | 1,322 | 2,217 | 2,056 | ||||
Figure 1.
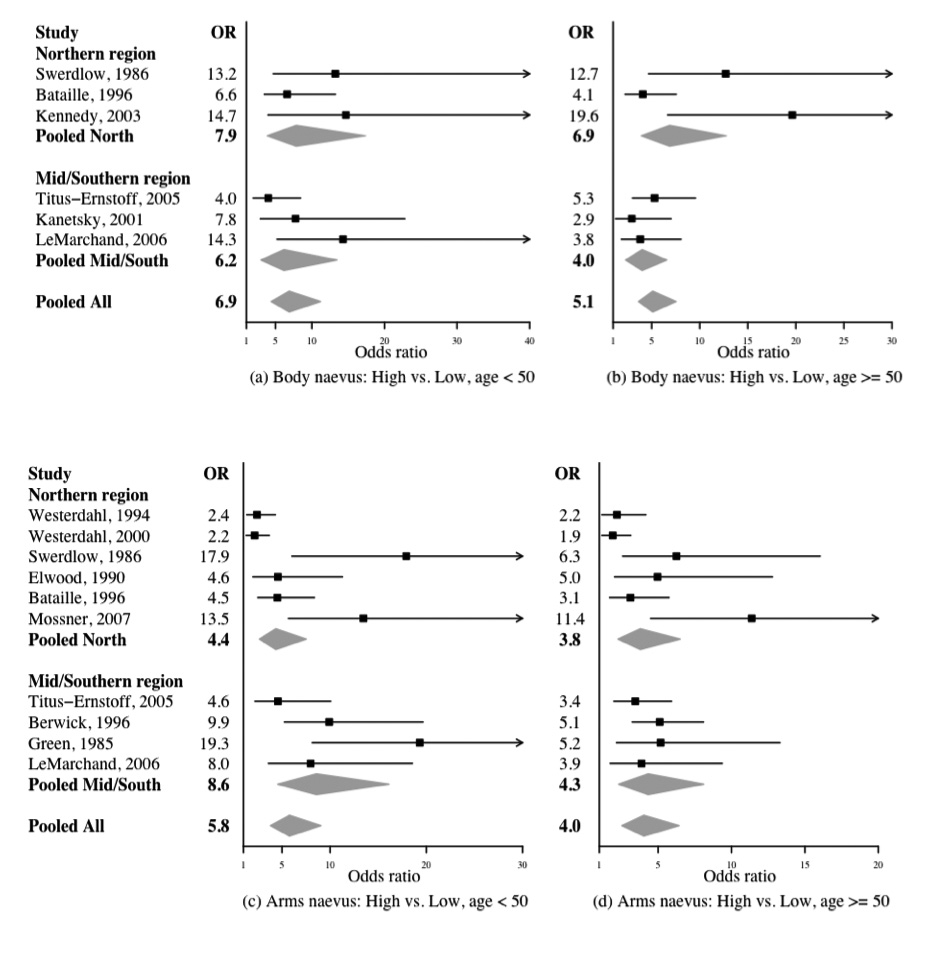
Forest plot of the association between the highest body naevus category and melanoma risks in (a) age < 50 subgroup, and (b) age ≥ 50 subgroup, and the highest arm naevus counts category and melanoma risks in (c) age < 50 subgroup, and (d) age ≥ 50 subgroup. Each line represents results from an individual study with the width of the horizontal line indicating the 95% Bayesian confidence intervals, and the squared box indicating the study-specific OR for the highest naevus count category (‘High’). Pooled ORs and 95% Bayesian confidence intervals are represented by grey diamonds.
In the age ≥ 50 years subgroup, the estimated among-study variance for the effect of body naevus categories was smaller at 0.03. The relative heterogeneity was 11%, slightly less than that in the < 50 age group for the highest body naevus count category. The estimated pORs for these naevus count categories were slightly lower than in the younger age group and comparable between the two latitude regions. Study-specific associations among whole body naevus count categories and melanoma risks at age ≥ 50 are given in Figure 1(b).
For those with extreme body naevus counts (greater than the 9th decile of naevus counts in controls), the estimated pORs in the Northern and Mid/Southern regions were 9.9 (95% CI: 4.8, 22.3) and 10.0 (95% CI: 5.0, 21.7), respectively, for the younger age group. The overall pOR for the two regions combined was 9.8 (95% CI: 6.2, 16.4). For the ≥50 age group the estimated pORs in the Northern and Mid/Southern regions were 10.5 (95% CI: 5.6, 21.5) and 5.1 (95% CI: 2.7, 9.6), respectively. Although in the ≥ 50 age group the estimated pOR is higher in the Northern region than the Mid/Southern region, the 95% CI is wide. The pOR for the two regions combined was 6.9 (95% CI: 4.6, 10.7).
Because of the differences in counting method among studies, no pooled analyses of whole body naevus counts were conducted using dose-response models. In contrast to the naevus category analyses, the forest plots that were based on dose-response models indicated great variation among studies, where the 95% CI of study-specific ORs did not overlap among many studies (Supplementary Figure S4).
Arm naevus count
Cases had a higher numbers of naevi on arms than did controls in all applicable studies both under and over the age of 50 years (Supplementary Figure S4). The distributions of the arm naevus count categories and the estimated pORs are given in Table 2. Forest plots of study-specific associations of the highest arm naevus count categories compared with the lowest reference set are displayed in Figures 2(c) and 2(d) for the two age groups. Here there was considerably more variation among studies than for whole body naevus count. The estimated among-study variances were 0.33 in the <50 age group and 0.39 in the older group. The relative heterogeneities were 32% and 44% for the highest arm naevus category in the two age groups. These coefficients were much larger than those for the highest naevus category on the whole body.
Figure 2.
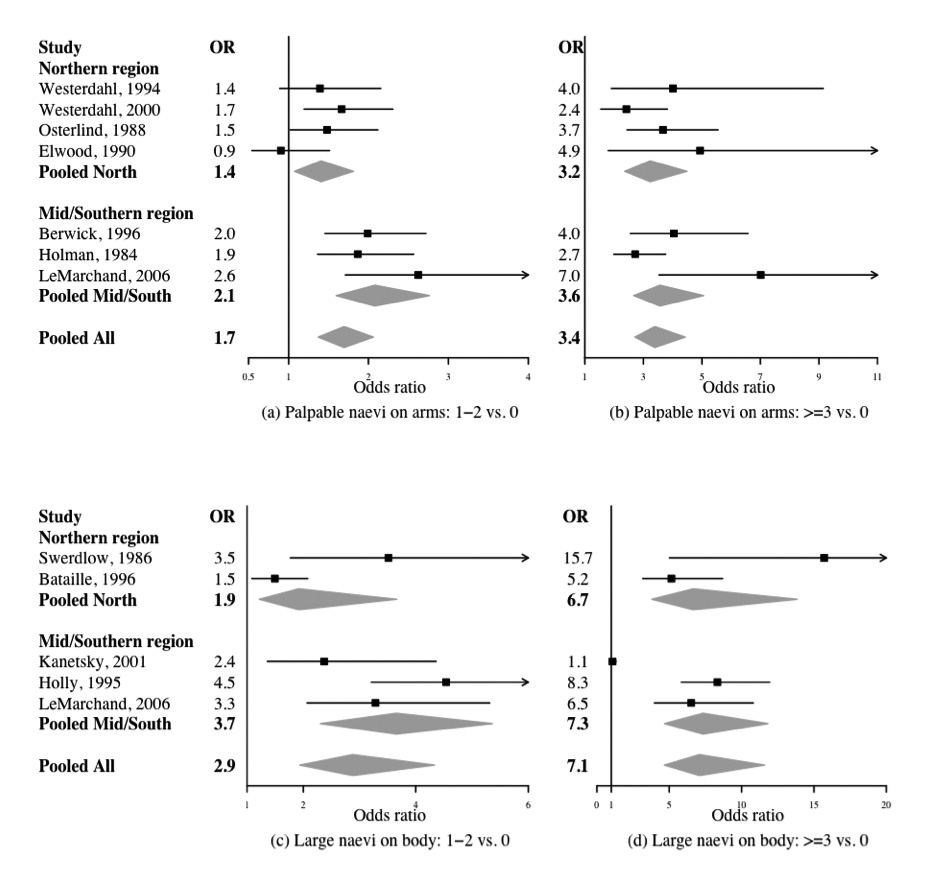
Forest plot of the association between the number of palpable naevi on arms (a) 1–2 versus none, (b) ≥3 versus none and melanoma risks, and the number of large naevi on the whole body (c) 1–2 versus none, (d) ≥3 versus none and melanoma risks. Each line represents results from an individual study with the width of the horizontal line indicating the 95% Bayesian confidence intervals, and the squared box indicating the study-specific OR. Pooled ORs and 95% Bayesian confidence intervals are represented by grey diamond
For the six studies in the Northern region, the estimated pORs for arm naevus count categories intermediate-low, intermediate-high and high in the < 50 years age group were 1.0 (95% CI: 0.6, 1.7), 2.1 (95% CI: 1.3, 3.6) and 4.4 (95% CI: 2.7, 7.5), respectively. The corresponding estimated pORs for the Mid/Southern latitude region were 3.1 (95% CI: 1.6, 5.9), 4.0 (95% CI: 2.0, 8.2) and 8.6 (95% CI: 4.6, 16.1), respectively. These pORs were higher than the risk estimates in the Northern region but had wider confidence intervals. The estimated pORs were slightly lower in the older age group and more comparable between the two latitude regions.
Palpable naevi
The among-study variance for palpable naevi on the arms was small in magnitude with a median of 0.02 and a relative heterogeneity of 12%. Figures 2(a)–(b) show the forest plots of study-specific ORs for palpable naevi on the arms together with the corresponding pORs of 1.7 (95% CI: 1.4, 2.1) and 3.4 (95% CI: 2.7, 4.4) for 1–2 or ≥3 palpable naevi, respectively, compared with none. The pORs for having 1–2 or ≥3 palpable naevi present on the arms compared with none in the Northern region were 1.4 (95% CI: 1.1, 1.8) and 3.2 (95% CI: 2.4, 4.5), respectively. The corresponding estimated pORs for the Mid/Southern latitude region were 2.1 (95% CI: 1.6, 2.8) and 3.6 (95% CI: 2.7, 5.1), respectively. The estimated pORs were comparable between the Northern and Mid/Southern regions, and showed little variation among studies performed at different latitudes.
Atypical naevi and large naevi
The pooled analyses by total number of atypical naevi, large naevi on the body and large naevi on the arms are listed in Table 3. On average the melanoma patients had more atypical naevi and large naevi compared with controls. The percentage of melanoma cases with ≥3 large naevi on the body was more than twice that in controls. In four studies 99 (8.4% of) of cases had ≥2 large naevi on the arms compared with only 39 (2.9% of) of controls.
Table 3.
Distributions of palpable naevi, atypical naevi and large naevi across studies (row percentage in parentheses), the corresponding pooled odds ratios (pORs) and 95% Bayesian confidence intervals (CI) and relative heterogeneity (RH).
| Type and site of naevi | No. of naevi | |||
|---|---|---|---|---|
| Palpable naevi on arms1 | None | 1 – 2 | ≥ 3 | Total |
| Controls | 2,609 (76.1%) | 578 (16.9%) | 240 (7.0%) | 3,427 |
| Cases | 1,661 (60.1%) | 614 (22.2%) | 489 (17.7%) | 2,764 |
| pORs (95% CI) | 1 | 1.7 (1.4, 2.1) | 3.4 (2.7, 4.4) | |
| RH | 12% | |||
| Atypical naevi on body2 | None | ≥ 1 | Total | |
| Controls | 1,075 (90.8%) | 109 (9.2%) | 1,184 | |
| Cases | 818 (72.1%) | 317 (27.9%) | 1,135 | |
| pORs (95% CI) | 1 | 4.0 (2.8, 5.8) | ||
| RH | 7% | |||
| Large naevi on body3 | None | 1 – 2 | ≥ 3 | Total |
| Controls | 1,117 (59.4%) | 512 (27.2%) | 252 (13.4%) | 1,881 |
| Cases | 671 (41.3%) | 503 (31.0%) | 451 (27.7%) | 1,625 |
| pORs (95% CI) | 1 | 2.9 (1.9, 4.3) | 7.1 (4.7, 11.6) | |
| RH | 17% | |||
| Large naevi on arms4 | None | 1 | ≥ 2 | Total |
| Controls | 1,219 (89.0%) | 111 (8.1%) | 39 (2.9%) | 1,369 |
| Cases | 941 (79.4%) | 144 (12.2%) | 99 (8.4%) | 1,184 |
| pORs (95% CI) | 1 | 1.6 (1.0, 2.4) | 3.2 (1.9, 5.3) | |
| RH | 16% | |||
For the four studies recording atypical naevi, the estimated among-study variance was 0.01 with a relative heterogeneity of 7%. The estimated pOR of having at least one atypical naevus compared with having no atypical naevi was 4.0 (95% CI: 2.8, 5.8).
Five studies recorded the number of large naevi on the body. The estimated among-study variance for large naevi on the body was 0.11, and the relative heterogeneity was 17% for the class with ≥3 large naevi on the body. The forest plots of study-specific and pORs for large naevi on the entire body a re given in Figures 2(c)–(d). The pORs for having 1–2 and ≥3 large naevi present on the body compared with none in the Northern region were 1.9 (95% CI: 1.2, 3.7) and 6.7 (95% CI: 3.8, 13.8), respectively. The corresponding estimated pORs for the Mid/Southern latitude region were 3.7 (95% CI: 2.3, 5.4) and 7.3 (95% CI: 4.7, 11.8), respectively. Although the estimated pORs are higher in the Mid/Southern region compared to the Northern region, their 95% CIs overlap.
Four studies recorded the number of large naevi on the arms. The estimated among-study variance for large naevi on the arms was 0.04, and the relative heterogeneity was 16%. The pORs for having one and ≥2 large naevi present on the arms compared with none in the Northern region were 1.2 (95% CI: 0.6, 2.3) and 2.8 (95% CI: 1.3, 6.4), respectively. The corresponding estimated pORs for the Mid/Southern latitude region were 2.0 (95% CI: 1.1, 4.1) and 3.5 (95% CI: 1.6, 7.9), respectively, which were similar to those in the Northern region.
Correlations among different measures of naevus phenotype
A few studies recorded more than one measure of naevus phenotype. The Spearman correlation between arm naevus count category and large naevi on the arms ranged between 0.1 and 0.2 for the two age groups respectively (based on three studies). Based on five studies, correlations between arm naevus count categories and palpable naevi on the arms were 0.5 for both age groups. Spearman correlations between arm naevus count categories and whole body naevus count categories were 0.8 and 0.7 for the <50 and ≥ 50 years age groups (based on four studies). Spearman correlations between whole body naevus count categories and large naevi on the body were 0.4 and 0.4 for the two age groups (based on four studies). Presence and number of large naevi on the body increased substantially with increasing whole body naevus count in both controls and cases (P < 0.0001, Supplementary Table S1). Patients with atypical naevi on the body also tended to have higher whole body naevus counts (P < 0.0001).
Naevus count and site of melanoma
In cases, the probability that the melanoma would be on the trunk generally increased with increasing whole body naevus count categories; the opposite trend was seen for melanoma on the head and neck and, to a lesser extent, limbs (P < 0.0001, Table 4). Probability that the melanoma would be on the trunk did not change with increasing naevus counts on the arms. Probability that it would be on the limbs increased a little while probability that it would be on the head and neck fell with increasing naevus counts on the arms as it did with increasing total counts (P < 0.0001).
Table 4.
Distributions of the total body naevus count categories, arm naevus count categories and large naevi on the body and melanoma tumour sites (row percentages in parentheses).
| Type and site of naevi | Melanoma tumour site | ||
|---|---|---|---|
| Total body naevus count category1 | Trunk | Limbs | Head & neck |
| Low | 33 (20.4%) | 90 (55.6%) | 39 (24.1%) |
| Intermediate-low | 36 (24.2%) | 90 (60.4%) | 23 (15.4%) |
| Intermediate-high | 118 (40.4%) | 135 (46.2%) | 39 (13.4%) |
| High | 263 (39.5%) | 328 (49.3%) | 74 (11.1%) |
| Total | 450 | 643 | 175 |
| P-value4 | - | < 0.0001 | < 0.0001 |
| Arms naevus count category2 | Trunk | Limbs | Head & neck |
| Low | 378 (36.8%) | 438 (42.6%) | 211 (20.5%) |
| Intermediate-low | 177 (36.2%) | 232 (47.4%) | 80 (16.4%) |
| Intermediate-high | 198 (39.1%) | 241 (47.5%) | 68 (13.4%) |
| High | 398 (38.9%) | 515 (50.3%) | 110 (10.8%) |
| Total | 1,151 | 1,426 | 469 |
| P-value4 | - | 0.254 | <0.0001 |
| Number of large naevi on body3 | Trunk | Limbs | Head & neck |
| 0 | 195 (29.1%) | 373 (55.7%) | 102 (15.2%) |
| 1–2 | 165 (33.1%) | 276 (55.4%) | 57 (11.4%) |
| 3 | 160 (36.4%) | 233 (53.1%) | 46 (10.5%) |
| Total | 520 | 882 | 205 |
| P-value4 | - | 0.044 | 0.004 |
arm naevus count and melanoma tumour site data available from 9 studies3, 4, 8, 9, 11–13, 15, 16, 19
number of large naevi on the entire body and melanoma tumour site data available from 5 studies4, 11, 13, 17, 18
Chi-square test on whether naevus distributions for different tumour sites’ patients differ from that of trunk’s
Discussion
Using original data from 15 case-control studies from different geographic regions, we examined the relationship between naevus phenotype and risk of melanoma. The selection criteria for inclusion of studies in the pooled analysis were the use of primarily population-based controls and moderate to large sample size, and are therefore unlikely to have led to biased estimates. Indeed the exclusion of small studies, although partly based on practicality, offers some protection against the publication bias observed in small studies.
Overall, although there were some differences among studies, the variation in the estimated relative risk associated with higher total body naevus counts was moderate under the age of 50 and smaller over the age of 50 years. The increased risk associated with naevus number was remarkably consistent across studies despite variable counting techniques and the likely presence of inter-observer variation. When total naevus number was assessed for Northern latitudes compared with Mid/Southern latitudes there was consistency in elevated relative risk among individuals with more and less naevi. Our pooled naevus category ORs indicated steeply rising risks associated with increasing numbers of naevi on the body. Naevus number on the arms was also a marker of risk for melanoma, but with a lower gradient of risk increase and with greater variation among studies than for the entire body naevus numbers. In addition, a strongly increased risk of melanoma associated with the presence of large naevi and atypical naevi was supported by our pooled analyses.
Classifications based on actual counts of palpable naevi, large naevi and atypical naevi have been used in our pooled analyses. However, we have used quantile methods for the analysis of common naevi on the body and arms (≥2 or ≥3mm). This classification rather than absolute naevus counts was used because of the differences between counting methods and differences among populations. The relative heterogeneity of study-specific log ORs was larger for categories of common naevi on the arms than for body naevus count categories. This may have been due to the variable definitions and counting methods for common naevi on the arms. Variations among study-specific log ORs were less when we examined actual counts of large naevi and palpable naevi on the arms. Various questionnaire designs and counting methods complicated the pooled analyses, but this also provided the opportunity to assess the robustness of different measures of the naevus phenotype.
Gandini et al1 reported pooled estimates of 1.019 and 1.129 for an increase of one naevus on the whole body and the arms respectively, using the regression of average risk on average exposure model.21 In view of the great diversity in the counting methods among studies as well as potential differences among populations across studies it seemed inappropriate here to perform pooled analysis using a dose-response model.
Few studies have compared the risk of melanoma with whole body naevus count at different latitudes. A joint case-control study in Australia and the UK found that common naevi were significant risk factors with similar ORs in the two countries,22 despite a greater median number of common naevi in Australian controls than UK for the younger age group (< 50 years old).
Several studies that passed our inclusion criteria but were not included in the pooled analysis have investigated the risks for a very high number of body naevi in their populations. The estimated ORs were 11.9 (95% CI: 4.4, 31.9) in the Italian study23 (at least 30 naevi > 2mm) and 16.1 (95% CI: 4.3, 60.7) in the French study24 (at least 120 naevi > 1mm). In Canada, the risk of having moderate or many naevi on the body was 10.7 (95% CI: 6.6, 17.4) compared with none using whole body naevus density diagrams.25 These published results were similar to our estimated pORs for those with extreme body naevus count.
The smaller variance associated with palpable naevi compared with common naevi on the arms in our pooled analysis indicated that this may be a more uniformly defined trait across studies, and that examiners might be less likely to confuse palpable naevi with other pigmented lesions such as freckles. However, palpable naevi are mature naevi exhibiting melanocyte senescence and are biologically less associated with the increased melanocytic proliferation that typifies the atypical mole syndrome and melanoma risk.26 In this pooled analysis they appeared to be associated with a more modest increase in risk of melanoma than were total number of arm naevi or whole body naevus number.
A strongly increased risk of melanoma with presence of large naevi on the body and arms was supported by our pooled analyses. In patients with the atypical mole syndrome there is usually an increased number of both common (above 2mm) and large or clinically atypical naevi (equal or above 5mm). Therefore there is an increased tendency to develop new naevi in adulthood in subjects with atypical mole syndrome and for many of these naevi to reach bigger size in time. In a twin study in the UK, Bataille et al27 reported a significant association between the number of common naevi and large naevi. Similarly, our estimated pORs for larger naevi on the body and arms corresponded well with the estimated pORs for the highest categories of common naevi on the body and on the arms.
Our pOR for having at least one atypical naevus compared with participants without any atypical naevi was smaller than that found by Gandini et al1 who reported a 10-fold risk. In the subgroup meta-analyses of case-control studies, they obtained a considerable reduction in the estimated relative risk using a dose-response model with relative risks ranging from 1.5 (95% CI: 1.3, 1.6) for the presence of a single atypical naevus to 6.4 (95% CI: 3.8, 10.3) for five atypical naevi.1 The drawback of regression of average risks on average exposure described in Greenland28 might partially explain the difference in our results from those of Gandini et al.1
Twin studies have provided strong evidence that naevus number is predominantly genetically determined,29–31 although there is also evidence for a smaller effect of sun exposure.32 For melanoma overall, intermittent sun exposure, as is typically experienced by truncal skin, is most associated with increased risk and therefore we examined the association between naevus phenotype and melanoma site. In this pooled analysis, the higher the total body naevus counts the more likely the melanoma was to be on the trunk, principally at the ‘expense’ of melanoma on the head and neck. For naevi on the arms, probability that the melanoma would be on the limbs increased with increasing counts, entirely at the ‘expense’ of melanoma on the head and neck. These observations support the view that an abnormal naevus phenotype is associated mostly with melanomas on intermittently sun-exposed sites, as has been suggested by others.33–36
In summary, in a detailed pooled analysis we have shown that naevus phenotype is predictive of melanoma risk and that the size of the relative risk is consistent across studies and across latitudes. The presence of increased numbers of naevi, large naevi and clinically atypical naevi on the body a re robust risk factors for melanoma.
Supplementary Material
Acknowledgments
This GenoMEL research has been supported by the European Commission under the 6th Framework Programme, contract no: LSHC-CT-2006-018702. The Section of Epidemiology and Biostatistics, Leeds Institute of Molecular Medicine receives funding from Cancer Research UK programme grant C588/A994. The fundings from NCI RO1-CA52345 (E. A. Holly, PI) and P0-1 CA42101 (M. Berwick, PI) are acknowledged. Bruce Armstrong’s research is supported by the University of Sydney Medical Foundation Program Grant. The authors thank the funders of the contributing studies, who are acknowledged in the original study publications listed in the references to this paper, and other investigators for those studies, who are authors of the original study publication. Dr JN Bouwes Bavinck is thanked for putting the melanoma database together for Leiden University Medical Center, the Netherlands. Lund Melanoma Study Group is thanked for compiling the Swedish data. Mr John Taylor is thanked for recoding the New Hampshire study for the pooled analysis. We thank also Dr Margaret Karagas who provided original data from the East Denmark, Scotland, East Midlands, San Francisco, Queensland and Western Australian studies, which she had compiled for pooled analysis of other variables.
References
- 1.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Abeni D, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer. 2005;41:28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41:45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Mössner R, Anders N, König IR, Krüger U, Schmidt D, Berking C, Ziegler A, Brockmöller J, Kaiser R, Volkenandt M, Westphal GA, Reich K. Variations of the melanocortin-1 receptor and the glutathione-S transferase T1 and M1 genes in cutaneous malignant melanoma. Arch Dermatol Res. 2007;298:371–379. doi: 10.1007/s00403-006-0708-7. [DOI] [PubMed] [Google Scholar]
- 4.Le Marchand L, Saltzman BS, Hankin JH, Wilkens LR, Franke AA, Morris SJ, Kolonel LN. Sun exposure, diet, and melanoma in Hawaii Caucasians. Am J Epidemiol. 2006;164:232–245. doi: 10.1093/aje/kwj115. [DOI] [PubMed] [Google Scholar]
- 5.MacKie RM, English J, Aitchison TC, Fitzsimons CP, Wilson P. The number and distribution of benign pigmented moles (melanocytic naevi) in a healthy British population. Br J Dermatol. 1985;113:167–174. doi: 10.1111/j.1365-2133.1985.tb02060.x. [DOI] [PubMed] [Google Scholar]
- 6.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 7.Lunn DJ, Thomas A, Best N, D S. WinBUGS - a Bayesian modeling framework: concept, structure, and extensibility. Statistics and Computing. 2000;10:325–337. [Google Scholar]
- 8.Westerdahl J, Olsson H, Måsbäck A, Ingvar C, Jonsson N, Brandt L, Jönsson PE, Möller T. Use of sunbeds or sunlamps and malignant melanoma in southern Sweden. Am J Epidemiol. 1994;140:691–699. doi: 10.1093/oxfordjournals.aje.a117317. [DOI] [PubMed] [Google Scholar]
- 9.Westerdahl J, Ingvar C, Måsbäck A, Jönsson N, Olsson H. Risk of cutaneous malignant melanoma in relation to use of sunbeds: further evidence for UV-A carcinogenicity. Br J Cancer. 2000;82:1593–1599. doi: 10.1054/bjoc.1999.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osterlind A, Tucker MA, Hou-Jensen K, Stone BJ, Engholm G, Jensen OM. The Danish case-control study of cutaneous malignant melanoma. I. Importance of host factors. Int J Cancer. 1988;42:200–206. doi: 10.1002/ijc.2910420210. [DOI] [PubMed] [Google Scholar]
- 11.Swerdlow AJ, English J, MacKie RM, O'Doherty CJ, Hunter JA, Clark J, Hole DJ. Benign melanocytic naevi as a risk factor for malignant melanoma. Br Med J (Clin Res Ed) 1986;292:1555–1559. doi: 10.1136/bmj.292.6535.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elwood JM, Whitehead SM, Davison J, Stewart M, Galt M. Malignant melanoma in England: risks associated with naevi, freckles, social class, hair colour, and sunburn. Int J Epidemiol. 1990;19:801–810. doi: 10.1093/ije/19.4.801. [DOI] [PubMed] [Google Scholar]
- 13.Bataille V, Bishop JA, Sasieni P, Swerdlow AJ, Pinney E, Griffiths K, Cuzick J. Risk of cutaneous melanoma in relation to the numbers, types and sites of naevi: a case-control study. Br J Cancer. 1996;73:1605–1611. doi: 10.1038/bjc.1996.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy C, Bajdik CD, Willemze R, De Gruijl FR, Bouwes Bavinck JN. The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol. 2003;120:1087–1093. doi: 10.1046/j.1523-1747.2003.12246.x. [DOI] [PubMed] [Google Scholar]
- 15.Titus-Ernstoff L, Perry AE, Spencer SK, Gibson JJ, Cole BF, Ernstoff MS. Pigmentary characteristics and moles in relation to melanoma risk. Int J Cancer. 2005;116:144–149. doi: 10.1002/ijc.21001. [DOI] [PubMed] [Google Scholar]
- 16.Berwick M, Begg CB, Fine JA, Roush GC, Barnhill RL. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17–23. doi: 10.1093/jnci/88.1.17. [DOI] [PubMed] [Google Scholar]
- 17.Kanetsky PA, Holmes R, Walker A, Najarian D, Swoyer J, Guerry D, Halpern A, Rebbeck TR. Interaction of glutathione S-transferase M1 and T1 genotypes and malignant melanoma. Cancer Epidemiol Biomarkers Prev. 2001;10:509–513. [PubMed] [Google Scholar]
- 18.Holly EA, Aston DA, Cress RD, Ahn DK, Kristiansen JJ. Cutaneous melanoma in women. II. Phenotypic characteristics and other host-related factors. Am J Epidemiol. 1995;141:934–942. doi: 10.1093/oxfordjournals.aje.a117360. [DOI] [PubMed] [Google Scholar]
- 19.Green A, MacLennan R, Siskind V. Common acquired naevi and the risk of malignant melanoma. Int J Cancer. 1985;35:297–300. doi: 10.1002/ijc.2910350303. [DOI] [PubMed] [Google Scholar]
- 20.Holman CD, Armstrong BK. Pigmentary traits, ethnic origin, benign nevi, and family history as risk factors for cutaneous malignant melanoma. J Natl Cancer Inst. 1984;72:257–266. [PubMed] [Google Scholar]
- 21.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 22.Bataille V, Grulich A, Sasieni P, Swerdlow A, Newton Bishop J, McCarthy W, Hersey P, Cuzick J. The association between naevi and melanoma in populations with different levels of sun exposure: a joint case-control study of melanoma in the UK and Australia. Br J Cancer. 1998;77:505–510. doi: 10.1038/bjc.1998.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carli P, Massi D, Santucci M, Biggeri A, Giannotti B. Cutaneous melanoma histologically associated with a nevus and melanoma de novo have a different profile of risk: results from a case-control study. J Am Acad Dermatol. 1999;40:549–557. doi: 10.1016/s0190-9622(99)70436-6. [DOI] [PubMed] [Google Scholar]
- 24.Grob JJ, Gouvernet J, Aymar D, Mostaque A, Romano MH, Collet AM, Noe MC, Diconstanzo MP, Bonerandi JJ. Count of benign melanocytic nevi as a major indicator of risk for nonfamilial nodular and superficial spreading melanoma. Cancer. 1990;66:387–395. doi: 10.1002/1097-0142(19900715)66:2<387::aid-cncr2820660232>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 25.Marrett LD, King WD, Walter SD, From L. Use of host factors to identify people at high risk for cutaneous malignant melanoma. Cmaj. 1992;147:445–453. [PMC free article] [PubMed] [Google Scholar]
- 26.MacKie RM. Multiple melanoma and atypical melanocytic naevi--evidence of an activated and expanded melanocytic system. Br J Dermatol. 1982;107:621–629. doi: 10.1111/j.1365-2133.1982.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 27.Bataille V, Snieder H, MacGregor AJ, Sasieni P, Spector TD. Genetics of risk factors for melanoma: an adult twin study of nevi and freckles. J Natl Cancer Inst. 2000;92:457–463. doi: 10.1093/jnci/92.6.457. [DOI] [PubMed] [Google Scholar]
- 28.Greenland S. Problems in the average-risk interpretation of categorical dose-response analyses. Epidemiology. 1995;6:563–565. doi: 10.1097/00001648-199509000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Easton DF, Cox GM, Macdonald AM, Ponder BA. Genetic susceptibility to naevi--a twin study. Br J Cancer. 1991;64:1164–1167. doi: 10.1038/bjc.1991.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu G, Montgomery GW, James MR, Trent JM, Hayward NK, Martin NG, Duffy DL. A genome-wide scan for naevus count: linkage to CDKN2A and to other chromosome regions. Eur J Hum Genet. 2007;15:94–102. doi: 10.1038/sj.ejhg.5201729. [DOI] [PubMed] [Google Scholar]
- 31.Wachsmuth RC, Gaut RM, Barrett JH, Saunders CL, Randerson-Moor JA, Eldridge A, Martin NG, Bishop TD, Newton Bishop JA. Heritability and gene-environment interactions for melanocytic nevus density examined in a U.K. adolescent twin study. J Invest Dermatol. 2001;117:348–352. doi: 10.1046/j.0022-202x.2001.01415.x. [DOI] [PubMed] [Google Scholar]
- 32.Wachsmuth RC, Turner F, Barrett JH, Gaut R, Randerson-Moor JA, Bishop DT, Bishop JA. The effect of sun exposure in determining nevus density in UK adolescent twins. J Invest Dermatol. 2005;124:56–62. doi: 10.1111/j.0022-202X.2004.23548.x. [DOI] [PubMed] [Google Scholar]
- 33.Cress RD, Holly EA, Ahn DK, LeBoit PE, Sagebiel RW. Cutaneous melanoma in women: anatomic distribution in relation to sun exposure and phenotype. Cancer Epidemiol Biomarkers Prev. 1995;4:831–836. [PubMed] [Google Scholar]
- 34.Bataille V, Sasieni P, Grulich A, Swerdlow A, McCarthy W, Hersey P, Newton Bishop JA, Cuzick J. Solar keratoses: a risk factor for melanoma but negative association with melanocytic naevi. Int J Cancer. 1998;78:8–12. doi: 10.1002/(sici)1097-0215(19980925)78:1<8::aid-ijc2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 35.Whiteman DC, Watt P, Purdie DM, Hughes MC, Hayward NK, Green AC. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806–812. doi: 10.1093/jnci/95.11.806. [DOI] [PubMed] [Google Scholar]
- 36.Cho E, Rosner BA, Colditz GA. Risk factors for melanoma by body site. Cancer Epidemiol Biomarkers Prev. 2005;14:1241–1244. doi: 10.1158/1055-9965.EPI-04-0632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


