Abstract
Previous seizure models have demonstrated genetic differences in generalized seizure threshold (GST) in inbred mice, but the genetic control of epileptogenesis is relatively unexplored. The present study examined, through analysis of inbred strains of mice, whether the seizure characteristics observed in the flurothyl kindling model are under genetic control. Eight consecutive, daily generalized seizures were induced by flurothyl in mice from five inbred strains. Following a 28-day rest period, mice were retested with flurothyl. The five strains of mice demonstrated inter-strain differences in GST, decreases in GST across seizure trials, and differences in the behavioral seizure phenotypes expressed. Since many of the seizure characteristics that we examined in the flurothyl kindling model were dissociable between C57BL/6J and DBA/2J mice, we analyzed these strains in detail. Unlike C57BL/6J mice, DBA/2J mice had a lower GST on trial 1, did not demonstrate a decrease in GST across trials, nor did they show an alteration in seizure phenotype upon flurothyl retest. Surprisingly, [C57BL/6JxDBA/2J] F1-hybrids had initial GST on trial 1 and GST decreases across trials similar to what was found for C57BL/6J, but they did not undergo the alteration in behavioral seizure phenotype that had been observed for C57BL/6J mice. Our data establish the significance of the genetic background in flurothyl-induced epileptogenesis. The [C57BL/6JxDBA/2J] F1-hybrid data demonstrate that initial GST, the decrease in GST across trials, and the change in seizure phenotype differ from the characteristics of the parental strains, suggesting that these phenotypes are controlled by independent genetic loci.
Keywords: epileptogenesis, flurothyl, inbred mice, generalized seizure threshold, kindling, clonic, tonic, brainstem, forebrain
Introduction
Epilepsy is one of the most common neurological diseases, affecting approximately 1 – 4% of the total population by the age of 80 (Engel and Pedley, 1998). While many of the current treatments for epilepsy block the symptoms of the epilepsy through seizure suppression, there are no specific therapies that target the changes in the brain that render it hyperexcitable in the epileptic state. The alteration in the excitability of cellular activity in the brain is known as epileptogenesis (Schwartzkroin, et al., 2004) and has been demonstrated both in vitro and in vivo (Dichter, 2006; Schwartzkroin, 1986; Schwartzkroin and Franck, 1986). Often these changes in brain excitability result in alterations in seizure threshold and in seizure phenotype (Leite, et al., 2002; McNamara, 1986; Mhyre and Applegate, 2003; Onat, et al., 2007; Wlaz, et al., 1998). The presence of spontaneous/unprovoked seizures can occur in some experimental models (i.e., kainate- or pilocarpine-induced epileptogenesis), but not in others (i.e., electrical kindling)(reviewed in (Leite, et al., 2002; Sharma, et al., 2007)). Analyses in animal models of epilepsy have led to the identification of loci controlling seizure susceptibility, but the genetic factors controlling epileptogenesis remain uncharacterized. Therefore, elucidation of the molecular mechanisms responsible for epileptogenesis will be important, if we are to gain a better understanding of epilepsy, and if we are to identify new therapies that target epileptogenesis.
Epileptic disorders are complex, quantitative traits controlled by genetic and environmental factors. Genetic heterogeneity is evident in inbred strains of mice that demonstrate different generalized seizure thresholds (GST) induced by seizure-inducing stimuli such as convulsant drugs (i.e., kainic acid, pentylenetetrazol, or bicuculline) or non-pharmacological manipulations (i.e., maximal electroshock or audiogenic seizures)(Engstrom and Woodbury, 1988; Ferraro, et al., 2002; Ferraro, et al., 2001; Ferraro, et al., 2004; Ferraro, et al., 1997; Ferraro, et al., 1999; Ferraro, et al., 1998; Frankel, et al., 2001; Frankel, et al., 1995; Hain, et al., 2000; Kosobud and Crabbe, 1990; Schauwecker, et al., 2004). However, no systematic mouse studies have been performed to reveal the genetic determinants of the processes that contribute to epileptogenesis.
One of the most popular models of epileptogenesis is electrical kindling. Electrical kindling is defined as a process whereby repeated and focal administrations of an initially subconvulsive electrical current, through brain electrodes, produce clonic seizures that become progressively more severe with each trial (epileptogenesis) eventually resulting in the consistent and permanent expression of generalized clonic-forebrain seizures (Goddard, et al., 1969; McNamara, et al., 1993; Sutula, et al., 1986). Thus, in kindling, a reorganization of the brain occurs, such that fully-kindled animals, when retested at a later date, show a seizure response identical to the one that was displayed when they were last stimulated (Goddard, et al., 1969; McNamara, et al., 1993). This indicates the presence of a sustained hyperexcitability of neuronal activity in the kindled brain. In addition, electrically-kindled animals do not develop spontaneous/unprovoked seizures in traditional electrical kindling paradigms; only after large numbers (>100) of kindled seizures can spontaneous/unprovoked seizures occasionally be observed (Lothman, et al., 1992; Michalakis, et al., 1998; Pinel and Rovner, 1978; Pinel and Rovner, 1978; Sharma, et al., 2007). However, electrical kindling is not amenable to high-throughput genetic studies of epileptogenesis, since it requires surgical implantation of electrodes. As an alternative, the flurothyl kindling model is a non-invasive protocol that allows the examination of baseline GST and epileptogenic processes.
The flurothyl kindling model was previously characterized in C57BL/6J mice that received daily exposures to a 10% flurothyl solution over eight days. These exposures resulted in the expression of generalized clonic-forebrain seizures (Ferland and Applegate, 1998; Ferland and Applegate, 1998; Samoriski and Applegate, 1997; Samoriski, et al., 1998). Over the course of the 8-day induction period, the GST of C57BL/6J mice decreased and then plateaued. When the mice were retested after a 28-day rest period, flurothyl exposure produced a GST equal to the plateaued level. Upon this flurothyl re-exposure, C57BL/6J mice displayed a change in the behavioral seizure phenotype expressed, from a generalized clonic-forebrain seizure (as observed in the induction-phase) to a generalized clonic-forebrain seizure that rapidly progressed into a generalized brainstem seizure (another epileptogenic process, hereafter referred to as a forebrain→brainstem seizure)(Applegate, et al., 1997; Ferland and Applegate, 1998; Ferland and Applegate, 1998; Samoriski and Applegate, 1997; Samoriski, et al., 1998). These experiments demonstrated the occurrence of epileptogenic processes in the flurothyl kindling model: decreases in GST over trials (kindling) and a change in seizure phenotype.
One major epileptogenic process, observed in the flurothyl kindling model, is a kindling of GST due to repeated seizure stimulation (Applegate, et al., 1997; Ferland and Applegate, 1998; Ferland and Applegate, 1998; Samoriski and Applegate, 1997; Samoriski, et al., 1998). This permanent decrease in GST is one of the hallmarks of classical kindling (Barnes and Pinel, 2001). Although the stimulation in the flurothyl kindling model is not subconvulsive, Ferland and Applegate (1999) demonstrated bidirectional transfer of kindling between the flurothyl kindling model and classical electrical kindling in C57BL/6J mice. That is, after 8 flurothyl-induced seizures, the rate of electrical kindling was increased. Similarly, when animals were first electrically kindled, increases in flurothyl-induced seizure susceptibility were seen. Importantly, mice that were electrically kindled, given a 28-day rest period, and exposed to one (first) trial with flurothyl, demonstrated the same change in seizure phenotype to forebrain→brainstem seizures (Ferland and Applegate, 1999). Overall, these results illustrated that the decreases in GST and change in seizure phenotype were independent of the method of seizure induction, and further supports the idea that animals in the flurothyl kindling model undergo epileptogenic processes (Ferland and Applegate, 1999).
In the current study, we examine the seizure characteristics of C57BL/6J, DBA/2J, 129S1/SvImJ, BALB/cJ, and C3H/HeJ strains of mice in the flurothyl kindling model. Differences in behavioral seizure characteristics in the flurothyl kindling model were most pronounced between C57BL/6J and DBA/2J mice; therefore, we focused on those two strains for detailed comparisons. C57BL/6J mice had a high initial GST, demonstrated a decrease in GST across trials, and showed an alteration in seizure phenotype following flurothyl retest. Conversely, DBA/2J mice had a lower initial GST, did not demonstrate a decrease in GST across trials, and did not show an alteration in seizure phenotype upon flurothyl retest. [C57BL/6J x DBA/2J] F1 hybrid mice were similar to C57BL/6J mice, with respect to initial GST and decreases of GST across 8 trials (kindling), but they did not undergo the alteration in behavioral seizure phenotype that was observed in C57BL/6J mice. Our results suggest genetic dissociation of epileptogenic characteristics observed in the flurothyl kindling model.
Materials and methods
Animals
Adult male DBA/2J (n = 28), C57BL/6J (n = 28), [C57BL/6J x DBA/2J] F1 hybrid (n = 28), 129S1/SvImJ (n = 12), BALB/cJ (n = 10), and C3H/HeJ (n = 10) mice (7 weeks of age) were used to examine genetic differences in the flurothyl kindling model using the standard 10%-flurothyl concentration (Applegate, et al., 1997; Ferland and Applegate, 1999; Samoriski and Applegate, 1997). Additional adult male DBA/2J (n = 10), C57BL/6J (n = 10), and 129S1/SvImJ (n = 10) mice (7 weeks of age) were used to test flurothyl at a lower concentration (5% flurothyl) in the flurothyl kindling model. Lastly, DBA/2J (n = 10) and C57BL/6J (n = 10) mice (12 weeks of age) were used to test whether age had an effect on the change in seizure phenotype that is observed in the flurothyl kindling model. All mice were obtained from the Jackson Laboratories (Bar Harbor, ME, USA).
All mice were acclimated to the housing facilities for at least 1 week prior to seizure testing. Mice were maintained on a normal 12 hour light-dark cycle with lights on at 6:00 AM, with unlimited access to food and water. All testing occurred between 7:00 AM and 12:00 PM. All testing was performed under approval of the Institutional Animal Care and Use Committees of both Rensselaer Polytechnic Institute and the Wadsworth Center (NY State Department of Health), in accordance with The National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental Design
Mice (7 weeks of age) were allowed to acclimate to the testing room for at least 30 minutes before testing commenced. All seizure testing was performed in the same room, under the same chemical rated fume hood, throughout the experiment. Mice were each placed into a 2.4 liter closed Plexiglas chamber. A 10% (or 5%) flurothyl solution (Bis(2,2,2-trifluoroethyl) ether; Sigma Aldrich, St. Louis, MO, USA) made in 95% ethanol was infused into the chamber at a rate of 100 μl/min, using a 20-ml B-D Multifit syringe attached to a syringe pump. Flurothyl was dripped onto a gauze pad that was suspended inside the upper part of the chamber; it quickly evaporated inside the enclosed chamber, exposing the animal to the flurothyl vapors. One mouse at a time was placed in the chamber containing a dry, unused gauze pad. Once the top of the chamber was secured, flurothyl exposure began. The generalized seizure threshold was determined as the time at which the animal fell to one side, losing its posture. All strains tested demonstrated a loss of posture that was occasionally and immediately preceded by rearing and bilateral forelimb clonus. Most animals, upon regaining postural control, reared and had bilateral forelimb clonus. If brainstem seizures were observed, they were always following a clonic-forebrain seizure. This is denoted by the fact that such seizures are referred to as forebrain→brainstem seizures. At the start of the generalized forebrain seizure (which again was usually loss of postural control), the top of the chamber was removed, exposing the mouse to room air. Once an animal had recovered, it was placed in a separate cage from the untested animals. All animals were returned to their home cages and placed back in the colony at the completion of each day of seizure testing. The latency to the loss of postural control, the time to regain posture, the duration of the seizure (calculated as the time from the start of the generalized seizure to the time when the animal regained its posture and stopped showing clonus of the limbs), and the type of seizure were recorded.
The behavioral seizures were graded according to a number classification scheme (Applegate, et al., 1997; Samoriski and Applegate, 1997): Grade 1 (clonic/forebrain) - a loss of posture, clonus of hindlimbs and/or forelimbs, and facial clonus including chewing; Grade 2 (clonic/forebrain) - grade 1 and low intensity bouncing; Grade 3 (brainstem) - grade 2 and wild running and hopping; Grade 4 (brainstem) - grade 3 and hindlimb and/or forelimb treading; Grade 5 (tonic/brainstem) -grade 4 and bilateral tonic extension of the forelimbs; Grade 6 (tonic/brainstem) - grade 5 and bilateral extension of the hindlimbs; and Grade 7 (tonic/brainstem) - grade 6 followed by death. Grades 1 and 2 seizures were categorized as clonic-forebrain seizures whereas Grades 3–7 seizures were categorized as forebrain→brainstem seizures (Samoriski, et al., 1997; Samoriski, et al., 1998).
The exposure procedure for induction of seizures was repeated once daily over 8 consecutive days (induction-phase). Mice were then given a 28-day rest period (incubation-phase), in which they remained in their home cages with no further seizure induction. Following the incubation-phase, mice were re-exposed to flurothyl (retest) for 1 trial, and the seizure parameters were again recorded.
To examine whether the change in behavioral seizure phenotype was due to developmental (age) factors, we exposed 12-week old DBA/2J and C57BL/6J mice (n = 10 per strain) to a single flurothyl trial (using a 10% flurothyl solution). Twelve weeks corresponds to the age at which the animals in the full flurothyl kindling model were retested with flurothyl.
Statistical analysis
One-way analyses of variance (ANOVA), followed by Tukey Honest Significant Difference (HSD) post hoc comparisons, were used to assess differences in generalized seizure thresholds on trial 1 of the induction-phase of the flurothyl kindling model. Repeated measures ANOVA, followed by Tukey HSD post hoc comparisons, were used to assess changes in generalized seizure thresholds (kindling) and seizure durations observed during the flurothyl induction period (across the 8 trials). Student’s t-tests were employed to examine differences in seizure characteristics between trial 8 of the induction-phase and the retest that followed the 28-day rest. Evaluation of differences (%) in the numbers of mice exhibiting clonic-forebrain seizures and the numbers exhibiting forebrain→brainstem seizures, after flurothyl exposure, employed Fisher Exact analyses. All statistical analyses were conducted with the Statistica software package (StatSoft, Tulsa, OK).
Results
To examine the genetic effects on various phenotypic characteristics in the flurothyl paradigm, we evaluated several inbred strains of mice (C57BL/6J, 129S1/SvImJ, C3H/HeJ, BALB/cJ, and DBA/2J). Since many of the seizure characteristics examined in the flurothyl paradigm were found to be dissociable between C57BL/6J and DBA/2J mice, we tested [C57BL/6J x DBA/2J] F1 hybrid mice in the flurothyl kindling model.
Inbred Strain Variability in Initial (Trial 1) Generalized Seizure Threshold with 10% Flurothyl
A generalized seizure was defined to start at the moment when a mouse fell on its side, losing its posture, which occasionally was immediately preceded by rearing and forelimb clonus (Applegate, et al., 1997; Samoriski and Applegate, 1997). We recorded the latency to generalized seizures on trial 1 of the flurothyl induction-phase to determine differences in the susceptibility to flurothyl-induced seizures among inbred strains of mice (Table 1). In the flurothyl kindling model, the latency to a generalized seizure is considered the generalized seizure threshold (GST)(Samoriski and Applegate, 1997). There were significant differences in the generalized seizure latency among C3H/HeJ, BALB/cJ, C57BL/6J, 129S1/SvImJ, and DBA/2J mice on the first exposure to flurothyl (Table 1; F4,77 = 20.41, P < 0.000001). There were no significant differences in initial GST between C3H/HeJ, BALB/cJ, and C57BL/6J mice. Of the five strains, DBA/2J mice were most sensitive to seizures induced by flurothyl, with the lowest GST (shortest latency to the expression of a generalized seizure)(Table 1; P < 0.02; Tukey HSD).
Table 1.
Latency to loss of postural control (a measure of generalized seizure threshold) in inbred mouse strains upon initial exposure to 10% flurothyl.
| Mouse Strain | Latency to Generalized Seizure (sec) |
|---|---|
| C3H/HeJ | 474.6 +/− 41.5 * |
| BALB/cJ | 433.8 +/− 40.1 * |
| C57BL/6J | 422.2 +/− 14.1 * |
| 129S1/SvImJ | 345.8 +/− 15.2 *$ |
| DBA/2J | 252.0 +/− 12.3 |
| [C57BL/6J x DBA/2J] F1 hybrid mice | 366.9 +/− 11.2 @^ |
Values represent the mean +/− SEM.
Comparisons of all inbred strains (except [C57BL/6J x DBA/2J] F1 hybrid mice: F4,77 = 20.41, P < 0.000001 followed by Tukey HSD post hoc comparisons
denotes significance of P < 0.02 as compared to DBA/2J
denotes significance of P < 0.006 as compared to C3H/HeJ
Comparisons of C57BL/6J, DBA/2J, and [C57BL/6J x DBA/2J] F1 hybrid mice: F2,73 = 47.2, P < 0.0001 followed by Tukey HSD post hoc comparisons
denotes significance of P < 0.0001 as compared to DBA/2J
denotes significance of P < 0.008 as compared to C57BL/6J
Inbred Strain Variability on Decreases in Generalized Seizure Threshold (Kindling) with 10% Flurothyl
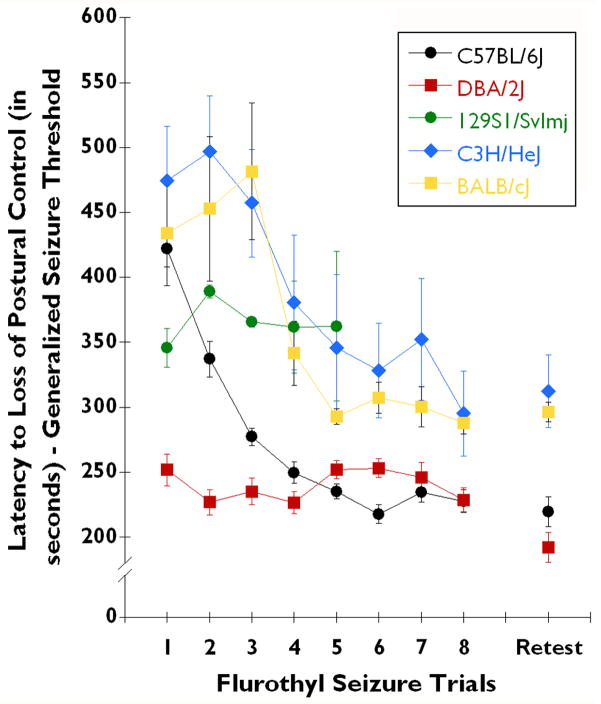
C57BL/6J, C3H/HeJ, and BALB/cJ mice had a different GST profile across flurothyl seizure trials than did the DBA/2J and 129S1/SvImJ strains (Fig. 1). Both C57BL/6J and BALB/cJ strains demonstrated significant decreases in generalized clonic-forebrain seizure threshold across seizure trials (C57BL/6J: F7,168 = 66.61, P < 0.0001; BALB/cJ: F7,56 = 5.82, P < 0.0001), an effect currently referred to as kindling (Fig. 1). However, kindling developed after multiple trials in BALB/cJ mice, whereas C57BL/6J mice kindled following trial 1 (Fig. 1). C3H/HeJ mice demonstrated a trend towards kindling, but it did not attain statistical significance (F7,28 = 1.88, P = 0.11). Since more than half of the C3H/HeJ mice had lethal forebrain→brainstem seizures during the induction-phase (Table 2), their ability to kindle could not be accurately determined. Also, the presence or absence of kindling could not be confirmed in 129S1/SvImJ mice, since mice in this strain had lethal forebrain→brainstem seizures between trials 1 to 5 of the induction-phase (Table 2). Few animals remained on trials 4 and 5 of the induction-phase (Fig. 1; Table 2). Interestingly, the DBA/2J strain of mice did not kindle (Fig. 1: F7,168 = 1.52, not significant), suggesting an impairment in this hallmark of epileptogenesis in this strain in the flurothyl kindling model.
Fig. 1. Inbred strain differences in 10% flurothyl-induced generalized seizure thresholds over the 8-trials of the induction phase and following incubation and retest.
The latency to loss of postural control (generalized seizure threshold (GST)) in C57BL/6J (n=28), DBA/2J (n=28), BALB/cJ (n=10), C3H/HeJ (n=10), and 129S1/SvImJ (n=12) strains of mice in the flurothyl (10%) kindling model. The GST of C57BL/6J mice (black circles) decreased over the 8 induction trials (F7,168 = 66.61, P < 0.0001), and remained low upon flurothyl retest. Significant decreases in GST also were observed in BALB/cJ mice (yellow squares) (F7,56 = 5.82, P < 0.0001). The GST of C3H/HeJ mice (blue diamonds) did not significantly decrease over the eight induction trials (F7,28 = 1.88, P = 0.11). 129S1/SvImJ mice (green circles) displayed a consistent high GST, however, these mice did not survive the 8 trials since they expressed lethal forebrain→brainstem seizures (100% lethal by trial 5). DBA/2J mice (red squares) exhibited a low GST that remained constant over the 8 induction-phase trials (F7,168 = 1.52, not significant). Upon retest, C57BL/6J, C3H/HeJ, and BALB/cJ mice were found to have maintained the GST from trial 8 of the induction-phase. However, DBA/2J mice tended to have a lower GST upon retest, compared to the GST for the last trial in the induction-phase (t39 = 2.5, P < 0.02). One BALB/cJ, 5 C3H/HeJ, and all 129S1/SvlmJ mice had lethal forebrain→brainstem seizures during the induction-phase, whereas no DBA/2J or C57BL/6J mice had this lethality (or expressed forebrain-->brainstem seizures).
Table 2.
Percentage of mice expressing a forebrain→brainstem seizure during the flurothyl induction-phase/total number of animals tested on each trial
| Flurothyl Trial | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Strain | ||||||||
| C57BL/6J | 0%/25 | 0%/25 | 0%/25 | 0%/25 | 0%/25 | 0%/25 | 0%/25 | 0%/25 |
| DBA/2J | 0%/25 | 0%/25 | 0%/25 | 0%/25 | 0%/25 | 0%/25 | 0%/25 | 0%/25 |
| BALB/cJ | 30%/10 | 40%/10 | 50%/10 | 22%/9 | 11%/9 | 22%/9 | 22%/9 | 0%/9 |
| C3H/HeJ | 60%/10 | 86%/7 | 100%/5 | 40%/5 | 20%/5 | 20%/5 | 20%/5 | 20%/5 |
| 129S1/SvImJ | 50%/12 | 50%/10 | 43%/7 | 50%/4 | 50%/2 | 100%/1 | NA | NA |
| F1 Hybrid | 0%/26 | 0%/26 | 0%/26 | 0%/26 | 0%/26 | 0%/26 | 0%/26 | 0%/26 |
Variability on Decreases in Generalized Seizure Threshold (Kindling) with 5% Flurothyl
The lack of kindling in DBA/2J mice could be due to a floor effect if the 10% flurothyl concentration used was too high to permit decreased latencies to be discerned over the course of the induction period. Additionally, the lethality in 129S1/SvImJ mice during the induction-phase could have resulted from the 10% flurothyl concentration rapidly overcoming their brainstem seizure thresholds. To test these hypotheses, we exposed C57BL/6J, 129S1/SvImJ and DBA/2J mice to the same flurothyl paradigm, but with a concentration of flurothyl only half as high (5%) (Fig. 2). With the 5% concentration of flurothyl, the GSTs of DBA/2J, C57BL/6J, and 129S1/SvmJ mice were higher than the GSTs of the same strains of mice tested at the 10% flurothyl concentration. However, DBA/2J mice still failed to kindle across trials in the flurothyl paradigm (Fig. 2: F7,56 = 1.12, not significant). The 129S1/SvImJ mice were able to kindle (F7,35 = 10.41, P < 0.0001), and at the lower concentration over half of the 129S1/SvImJ mice were able to survive throughout the induction-phase. C57BL/6J mice were able to kindle even at the lower concentration of flurothyl (Fig. 2: F7,35 = 25.58, P < 0.0001).
Fig. 2. Inbred strain differences in 5% flurothyl-induced generalized seizure thresholds over the 8-trials of the induction phase and following incubation and retest.
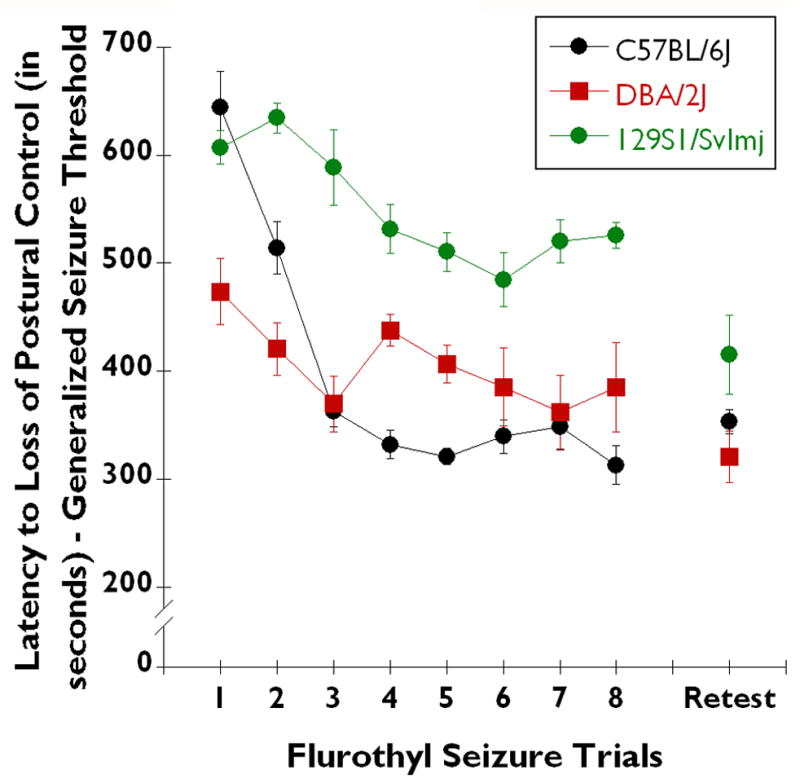
The latency to loss of postural control (generalized seizure threshold (GST)) in C57BL/6J (n=28), DBA/2J (n=28), and 129S1/SvImJ (n=12) mice when a lower, 5% concentration of flurothyl was used in the testing. The GST of C57BL/6J mice (black circles) decreased over the 8 induction trials (F7,35 = 25.58, P < 0.0001), and remained low upon flurothyl retest. 129S1/SvImJ mice (green circles) displayed an initially high GST, which significantly decreased over the 8 induction trials (F7,35 = 10.41, P < 0.0001). There was a significant decrease between the trial 8 GST and the 28-day retest GST in 129S1/SvImJ mice (t10 = 2.87, P < 0.02). However, DBA/2J mice (red squares) failed to demonstrate a significant overall decrease in GST across the 8 induction-phase trials (F7,56 = 1.12, not significant). In DBA/2J mice, the flurothyl retest GST remained at levels similar to those of trial 8 of the induction-phase. When tested with 5% flurothyl, four 129S1/SvlmJ mice had lethal forebrain→brainstem seizures during the induction-phase; no DBA/2J or C57BL/6J mice had lethal forebrain→brainstem seizures.
Inbred Strain Variability on Induction-phase Seizure Expression with 5% or 10% Flurothyl
During the 8 trial flurothyl induction-phase, both C57BL/6J and DBA/2J mice always expressed generalized clonic-forebrain seizures upon exposure to flurothyl, at either a 5% or 10% flurothyl concentration. However, BALB/cJ, C3H/HeJ, and 129S1/SvImJ mice often had forebrain→brainstem seizures on some trials, intermixed with clonic-forebrain seizures on other trials, during the 10% flurothyl induction-phase (Table 2). Specifically, one BALB/cJ mouse (10%) and five C3H/HeJ mice (50%) had lethal forebrain→brainstem seizures during the induction-phase (Table 2). In addition, all 129S1/SvImJ mice had lethal forebrain→brainstem seizures by day 5 of the flurothyl (10%) induction-phase (Table 2). With 5% flurothyl, four 129S1/SvImJ mice died during the induction-phase from lethal forebrain→brainstem seizures (data not shown).
In both BALB/cJ and C3H/HeJ mice, animals tended to have fewer forebrain→brainstem seizures as the number of flurothyl exposures increased, suggesting alterations in the balance between the forebrain and brainstem seizure thresholds with repeated seizures and time (Table 2). This pattern was also observed in 129S1/SvImJ mice exposed to the flurothyl kindling model using 5% flurothyl (data not shown). Overall, the seizure expression phenotypic data from BALB/cJ, C3H/HeJ, and 129S1/SvImJ mice suggest strain-specific sensitivities to flurothyl-induced forebrain→brainstem seizure expression during the induction-phase.
Inbred Strain Variability on the Alteration in Behavioral Seizure Phenotype in Response to Flurothyl (10%) Retest
Following the eighth induction trial and the 28-day incubation-phase, a significant percentage of C57BL/6J mice (75%; P < 0.0001; Fisher Exact) displayed an alteration in behavioral seizure phenotype upon flurothyl retest: from a generalized clonic-forebrain seizure to a forebrain→brainstem seizure. This alteration in seizure phenotype upon retest occurred, while this strain maintained a GST consistent with the trial 8 GST (Fig. 1). None of the other strains experienced this change in seizure phenotype on retest, even though some of these strains tended to have forebrain→brainstem seizures during the early trials of the induction-phase (Table 2). C3H/HeJ and BALB/cJ mice maintained the GST, upon flurothyl retest, that was measured following the last flurothyl seizure of the induction-phase (trial 8) (Fig. 1). However, DBA/2J mice tended to have a decreased GST following flurothyl retest, as compared to the last trial in the induction-phase (t39 = 2.5, P < 0.02) (Fig. 1). Given that none of the DBA/2J, C3H/HeJ, and BALB/cJ mice had a forebrain→brainstem seizure upon flurothyl retest, these strains clearly did not undergo an alteration in behavioral seizure phenotype.
Variability on the Alteration in Behavioral Seizure Phenotype in Response to Flurothyl (5%) Retest
Exposure of the C57BL/6J, 129S1/SvImJ and DBA/2J strains to the flurothyl paradigm at the lower concentration of flurothyl (5%), gave results similar to those found for the 10% concentration. C57BL/6J mice showed an alteration in seizure phenotype from 0% expressing forebrain→brainstem seizures, during the induction-phase, to 60% expressing forebrain→brainstem seizures upon flurothyl retest. No DBA/2J mice or any of the remaining 129S1/SvImJ mice (6 of the original 10) displayed this change in seizure phenotype (data not shown). Moreover, for 5% flurothyl, C57BL/6J and DBA/2J mice maintained the trial 8 GST upon retest (Fig. 2), but the surviving 129S1/SvImJ mice showed a drop in their GST on retest (t10 = 2.9, P < 0.02) (Fig. 2).
The Change in Behavioral Seizure Phenotype in Response to Flurothyl Retest is not due to Aging
To rule out any age related effects on this change in seizure phenotype, we administered a single flurothyl trial to C57BL/6J and DBA/2J mice at 12 weeks of age (the age of animals that have completed the flurothyl induction-phase, 28-day incubation, and flurothyl retest, when started at 7 weeks of age). Twelve week-old mice and 7 week-old mice of a given strain had indistinguishable GSTs, following one exposure to flurothyl. Again, in the 1 trial experiment, C57BL/6J mice had a high GST, and DBA/2J mice exemplified a low GST (data not shown). Both strains expressed clonic-forebrain seizures in the 1 trial experiment. Therefore, unlike the C57BL/6J mice in the full flurothyl kindling model (8 trials and retest), which typically displayed forebrain→brainstem seizures in the retest, the single flurothyl-trial C57BL/6J mice at 12 weeks of age expressed clonic-forebrain seizures.
Initial (Trial 1) Generalized Seizure Threshold in [C57BL/6J x DBA/2J] F1 Hybrid Mice
In comparing the five inbred strains of mice in the flurothyl kindling model, we determined that three seizure traits were dissociable between C57BL/6J and DBA/2J mice. DBA/2J mice had a lower GST on trial 1 than did C57BL/6J mice; they did not demonstrate the kindling effect, nor did they have an alteration in seizure phenotype upon flurothyl retest.
Previous work in other epilepsy models has demonstrated complex genetic control over seizure susceptibility between C57BL/6J and DBA/2J mice, with [C57BL/6J x DBA/2J] F1 hybrid mice showing intermediate seizure susceptibilities (Ferraro, et al., 1997; Ferraro, et al., 1999; Ferraro, et al., 1998). We examined the seizure characteristics of [C57BL/6J x DBA/2J] F1 hybrid mice to determine whether the hybrid is similarly intermediate in the flurothyl kindling model.
A significant difference in the initial GST (trial 1) (F2,73 = 47.2, P < 0.0001) was observed among C57BL/6J, DBA/2J, and [C57BL/6J x DBA/2J] F1 hybrid mice (Table 1). However, the [C57BL/6J x DBA/2J] F1 hybrid mice demonstrated an intermediate resistance to a generalized clonic-forebrain seizure on trial 1 of the induction-phase that was closer to the value for C57BL/6J mice (P < 0.008; Tukey HSD) than that for DBA/2J mice (P < 0.0001; Tukey HSD).
[C57BL/6J x DBA/2J] F1 Hybrid Mice Show Decreases in the Generalized Seizure Threshold across Flurothyl Trials (Kindling)
Direct and detailed comparison of C57BL/6J and DBA/2J mice demonstrated that the difference in flurothyl kindling was significant between these two groups (F1,48 = 31.6, P < 0.0001) and across seizure trials (F7,336 = 31.0, P < 0.0001); there was also a significant group by trial interaction (F7,336 = 29.9, P < 0.0001). Specifically, flurothyl trials 1–3 were significantly different between the C57BL/6J and DBA/2J strains (trial 1: P < 0.0002; trial 2: P < 0.0002; trial 3: P < 0.002; Tukey HSD). The remaining trials were not significantly different, with the GSTs of C57BL/6J mice decreasing to levels comparable to those of DBA/2J mice, except for trial 6, in which the C57BL/6J mice had a lower latency to a generalized seizure than did the DBA/2J mice (P < 0.002; Tukey HSD)(Fig. 3; for ease of comparison, we have re-plotted the original parental strains on this graph).
Fig. 3. Comparison of [C57BL/6JxDBA/2J] F1 hybrid mice with parental strains in 10% flurothyl-induced generalized seizure thresholds over the 8-trials of the induction phase and following incubation and retest.
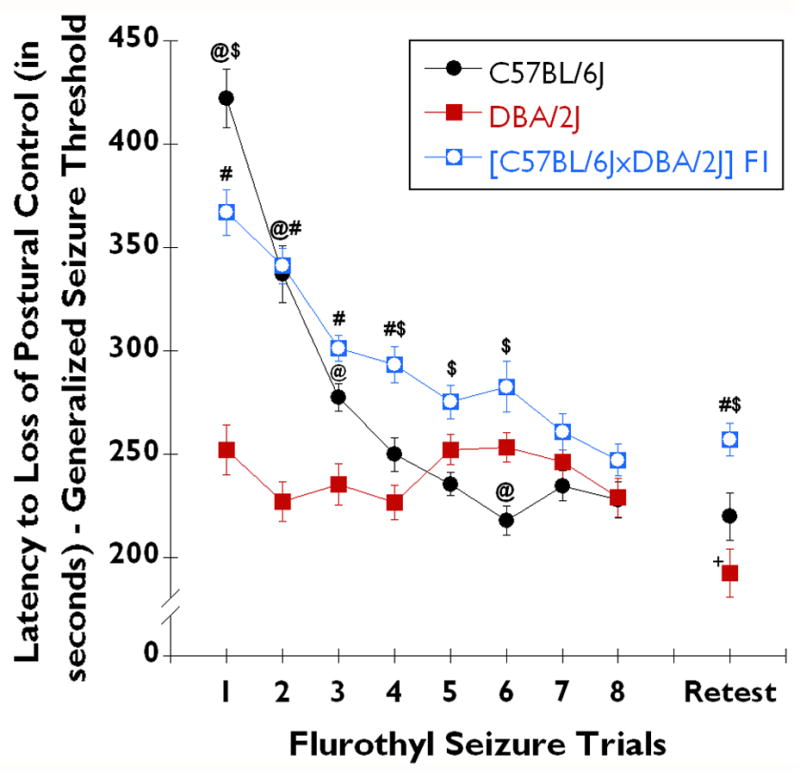
The latency to loss of postural control (generalized seizure threshold (GST)) throughout the full regimen of the flurothyl kindling model in C57BL/6J (n=28), DBA/2J (n=28), and [C57BL/6J x DBA/2J] F1 hybrid (n=28) mice (data from C57BL/6J and DBA/2J mice used in Figure 1 are re-plotted for comparison). The first exposure to flurothyl (10%) on trial 1 serves as an indicator of the baseline GST for each strain. C57BL/6J mice (black circles) had the highest GST (most resistant), while the DBA/2J mice (red squares) had the lowest GST (most susceptible). The [C57BL/6J x DBA/2J] F1 hybrid (blue squares with open white circles) mice had an intermediate GST. Successive daily exposures to flurothyl for 8-days resulted in a decreased latency to loss of postural control in both C57BL/6J and in [C57BL/6J x DBA/2J] F1 hybrid mice, which was not seen in DBA/2J mice. Throughout the entire 8 trial flurothyl kindling procedure, C57BL/6J, DBA/2J, and [C57BL/6J x DBA/2J] F1 hybrid mice all expressed clonic-forebrain seizures. Following a 28-day rest period, mice were again exposed to flurothyl. On this retest, C57BL/6J (filled circles) and [C57BL/6J x DBA/2J] F1 hybrid mice had the same latency to loss of postural control as they had had on trial 8 of the induction-phase. However, the GST of DBA/2J mice significantly decreased upon retest (P < 0.02). No DBA/2J, C57BL/6J, or [C57BL/6J x DBA/2J] F1 hybrid mice had lethal forebrain→brainstem seizures during the induction-phase. @ denotes significance of difference from DBA/2J (P < 0.03); $ denotes significance of difference from [C57BL/6J x DBA/2J] F1 hybrid (P < 0.03); # denotes significance of difference from DBA/2J (P < 0.0002); + denotes significance of difference from DBA/2J - trial 8 of the induction-phase (P < 0.02).
Similar to C57BL/6J mice, the [C57BL/6J x DBA/2J] F1 hybrid mice demonstrated significant decreases in GST (kindling) in response to repeated 10% flurothyl exposures (F7,175 = 19.5, P < 0.0001)(Fig. 3), whereas DBA/2J mice failed to kindle when exposed to either 5% or 10% flurothyl (Figs. 1 & 2). Direct pair-wise comparisons of C57BL/6J and DBA/2J mice with [C57BL/6J x DBA/2J] F1 hybrid mice demonstrated that the differences in flurothyl kindling was significant among these groups (F2,73 = 47.9, P < 0.0001) and between trials (F7,511 = 48.6, P < 0.0001); there was also a significant group by trial interaction (F14,511 = 15.8, P < 0.0001). Specifically, flurothyl trials 1–4 were significantly different between DBA/2J mice and [C57BL/6J x DBA/2J] F1 hybrid mice (P < 0.0002; Tukey HSD); trials 1 and 4–6 were significantly different between C57BL/6J mice and [C57BL/6J x DBA/2J] F1 hybrid mice (P < 0.008; Tukey HSD). Overall, these results suggest that flurothyl kindling in [C57BL/6J x DBA/2J] F1 hybrid mice is most similar to flurothyl kindling in the C57BL/6J parental line.
The Change in Seizure Phenotype upon Flurothyl Retest does not occur in [C57BL/6J x DBA/2J] F1 Hybrid Mice
Throughout the flurothyl induction-phase [C57BL/6J x DBA/2J] F1 hybrids showed clonic-forebrain seizure expression, similar to the parental strains. Following the 28-day rest period and flurothyl retest, none of the [C57BL/6J x DBA/2J] F1 hybrid mice had a forebrain→brainstem seizure. Thus, their behavioral seizure phenotype differed from that of C57BL/6J mice, and was most similar to the DBA/2J parental strain. The [C57BL/6J x DBA/2J] F1 hybrid mice, upon flurothyl retest, had a GST that was similar to this hybrid strain’s GST from the last trial of the flurothyl induction-phase (trial 8: t47 = 0.9, not significant)(Fig. 3). As a whole, these data suggest that the hybrid strain’s change in behavioral seizure phenotype observed following flurothyl retest is dissociable from the kindling phenotype, and that this alteration in seizure phenotype is most similar to the behavioral seizure phenotype of the DBA/2J parental strain.
The Effects of Inbred Strain Variability on the Duration of the Generalized Seizure Threshold
Since variations in seizure duration could potentially have accounted for the differences between mouse strains, we analyzed whether the total duration of the seizures changed across the flurothyl regimen (8 trials and retest) in C57BL/6J, DBA/2J, and [C57BL/6J x DBA/2J] F1 hybrid mice (Fig. 4). Seizure durations for all three groups of mice were not statistically different across the eight flurothyl induction-phase trials (strains: [F2,71 = 1.9, not significant]; trials [F7,497 = 0.6, not significant]; strain by trial interaction [F14,497 = 1.6, not significant]). Significant differences in seizure duration were only observed following flurothyl retest: C57BL/6J mice had the longest seizure duration, and [C57BL/6J x DBA/2J] F1 hybrid mice had the shortest seizure duration (F2,56 = 22.2, P < 0.0001). DBA/2J mice had intermediate seizure durations. Moreover, comparisons of the retest seizure duration with the trial-8 seizure duration indicated that C57BL/6J (t43 = 9.2, P < 0.0001), DBA/2J (t39 = 6.5, P < 0.0001), and [C57BL/6J x DBA/2J] F1 hybrid (t47 = 2.7, P < 0.01) mice had significantly longer seizures on retest. However, the major difference in seizure duration was related to the type of seizure expressed: C57BL/6J mice, which had forebrain→brainstem seizures upon retest had seizures that were longer than those of mice having clonic-forebrain seizures (i.e., DBA/2J and [C57BL/6J x DBA/2J] F1 hybrid mice).
Fig. 4. Comparison of [C57BL/6JxDBA/2J] F1 hybrid mice with parental strains in 10% flurothyl-induced generalized seizure durations over the 8-trials of the induction phase and following incubation and retest.
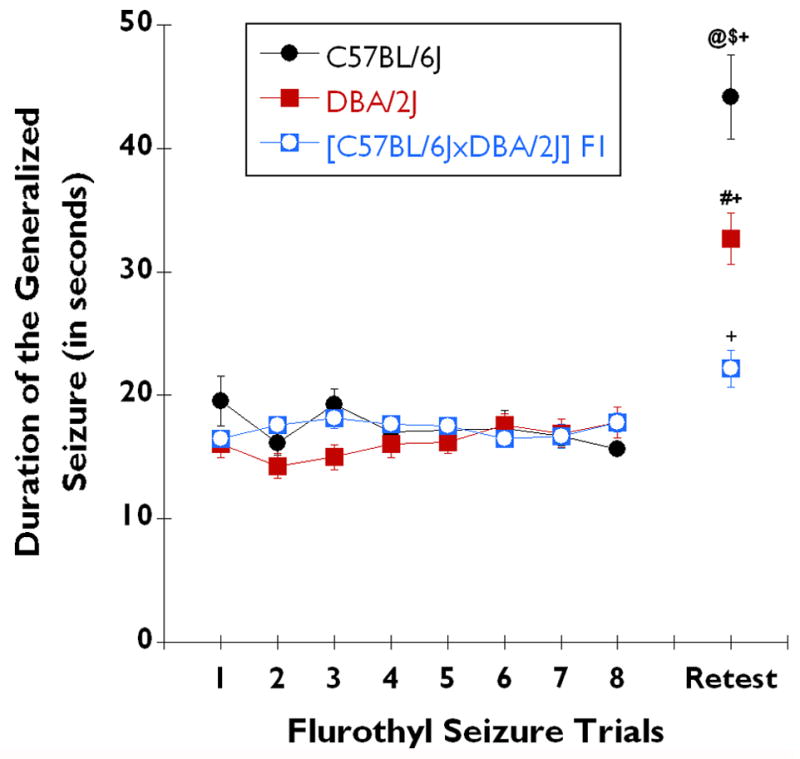
The total seizure duration on each flurothyl trial during the full regimen of the flurothyl kindling model for C57BL/6J (n=28), DBA/2J (n=28), and [C57BL/6J x DBA/2J] F1 hybrid (n=28) mice. C57BL/6J (black circles), DBA/2J (red squares), and [C57BL/6J x DBA/2J] F1 hybrid (blue squares with open white circles) mice had similar seizure durations throughout the flurothyl induction trials (1–8). Successive daily exposures to flurothyl over 8-days resulted in no significant change in the total seizure duration, in any of the three strains of mice. However, after incubation followed by flurothyl retest, C57BL/6J mice showed an increase in the total seizure duration that was significantly higher than the mean increase for either DBA/2J (P < 0.008), or [C57BL/6J x DBA/2J] F1 hybrid (P < 0.0002) mice. Moreover, C57BL/6J (P < 0.0001) DBA/2J (P < 0.0001), and [C57BL/6J x DBA/2J] F1 hybrid (P < 0.01) mice had significant increases in the total seizure duration upon flurothyl retest as compared to the duration in trial 8 of the induction-phase. @ denotes significantly different from DBA/2J (P < 0.008); $ denotes significantly different from [C57BL/6J x DBA/2J] F1 hybrid (P < 0.0002); # denotes significantly different from [C57BL/6J x DBA/2J] F1 hybrid (P < 0.02); + denotes significantly different from trial 8 of the induction-phase for each strain (P < 0.01).
Discussion
Differences in seizure threshold among inbred strains of mice have been established using a variety of seizure-inducing stimuli (Engstrom and Woodbury, 1988; Ferraro, et al., 2002; Ferraro, et al., 2001; Ferraro, et al., 2004; Ferraro, et al., 1997; Ferraro, et al., 1999; Ferraro, et al., 1998; Frankel, et al., 2001; Frankel, et al., 1995; Hain, et al., 2000; Kosobud and Crabbe, 1990; Schauwecker, et al., 2004). Using the flurothyl kindling model, we have found differences in seizure susceptibility and epileptogenic characteristics among five inbred mouse strains. We examined susceptibility to seizures, decreases in generalized seizure threshold (GST) over repeated trials (an assessment of epileptogenesis), and alterations (if any) in behavioral seizure phenotype (an evaluation of epileptogenesis/plasticity). Overall, we found significant differences in these seizure characteristics among the five inbred strains studied; however, we focused on the C57BL/6J and DBA/2J strains, and on [C57BL/6J x DBA/2J] F1 hybrids, since the seizure characteristics of the two parental strains had been found to be the most interesting with respect to phenotype dissociation. For the [C57BL/6J x DBA/2J] F1 hybrid mice, the initial GST was between that of C57BL/6J and DBA/2J mice; however, it was closer to the C57BL/6J strain. The overall decrease in GST across flurothyl trials (kindling) was seen in both C57BL/6J and [C57BL/6J x DBA/2J] F1 hybrid mice, suggesting that the genetic contributions from C57BL/6J are dominant for seizure susceptibility and kindling. In contrast, [C57BL/6J x DBA/2J] F1 hybrids did not have a change in seizure phenotype upon flurothyl retest at 28-days, which is characteristic of the DBA/2J strain. The two discrepant patterns suggest that these traits are under distinct genetic control from dominant alleles from the DBA/2J background and from the C57BL/6J background; however, further genetic experiments must be conducted to prove or disprove this hypothesis. Overall, the results of the current study suggest dissociation between epileptogenic characteristics observed in the flurothyl kindling model.
Given that our model requires the animal to inhale the convulsant, it is possible that the respiration or expiration of flurothyl could impact the strain effect in our system. However, strain differences in lung function have been previously determined in BALB/cJ, C3H/HeJ, C57BL/6J, 129S1/SvImJ and DBA/2J mice (Anh, et al., 2006). Importantly, there is no correlation between initial GST measurements and respiratory rate, tidal volume, minute volume, or expiratory duration. Furthermore, no correlations with our GST data and cardiovascular function were observed in the mouse phenome database (http://phenome.jax.org).
On trial 1 of the flurothyl kindling model, C3H/HeJ, BALB/cJ, and C57BL/6J mice had the highest GSTs, while the DBA/2J strain had the lowest GST. The initial GST of 129S1/SvImJ mice was intermediate. These results illustrated the range in baseline seizure susceptibility among inbred strains of mice, as has been noted in previous studies that used different seizure-inducing stimuli (Engstrom and Woodbury, 1988; Ferraro, et al., 1997; Ferraro, et al., 1998; Frankel, et al., 2001).
BALB/cJ and C57BL/6J mice exhibited a kindling effect on trials 1–8 of the induction-phase as evidenced by an overall significant decrease in GST across flurothyl trials. C3H/HeJ mice did not display a significant decrease in GST; however, they did show a trend of decreasing GST over trials 1–8 of the induction-phase. DBA/2J mice were the only strain examined that did not exhibit a decreasing GST. The lack of perceptible kindling in DBA/2J mice was due either to an inability to kindle in response to flurothyl, or to a possible floor effect for GST in mice. The seizure assay conducted at a lower (5%) concentration of flurothyl produced higher baseline latencies to generalized seizure expression in DBA/2J mice, comparable to the GST of C57BL/6J mice at 10% flurothyl exposure. Even with increased latencies to generalized seizure expression, however, DBA/2J mice were unable to kindle; thus, we eliminated the possibility of a floor effect. An inability to kindle during the induction-phase suggests that perhaps the brains of DBA/2J mice have less plasticity than do the brains of the other strains examined (Bampton, et al., 1999; Grice, et al., 2007; Jones, et al., 2001; McIntyre, et al., 2002; Middei, et al., 2007; Morimoto, et al., 2004; Restivo, et al., 2006; Schubert, et al., 2005; Yeh, et al., 1989). As a whole, our data suggest that a genetic heterogeneity, which mediates flurothyl kindling, exists among inbred strains. It would be of interest to determine inbred strain variability with corneal kindling to assess the presence or absence of kindling in this model (Potschka and Loscher, 1999; Wlaz, et al., 1998).
C57BL/6J and DBA/2J mice had clonic-forebrain seizures throughout the flurothyl induction-phase, whereas BALB/cJ and C3H/HeJ mice had a mixture of clonic-forebrain and forebrain→brainstem seizures on the early trials of the induction-phase, and strictly clonic-forebrain seizures on the later induction trials. The occurrence of forebrain→brainstem seizures early in the induction phase could be due to a differential imbalance in the forebrain seizure system threshold and the brainstem seizure system threshold in BALB/cJ and C3H/HeJ mice as compared to C57BL/6J and DBA/2J mice. C57BL/6J and DBA/2J mice have solely clonic-forebrain seizures throughout the induction phase suggesting that in these strains, the forebrain seizure system threshold is lower than the brainstem seizure system threshold. However, it appears early in the induction phase, BALB/cJ and C3H/HeJ mice tend to have a forebrain seizure system threshold that is similar to the brainstem seizure system threshold, thereby accounting for the expression of forebrain→brainstem seizures in these strains. Interestingly, as the BALB/cJ and C3H/HeJ animals continue to receive flurothyl-induced seizures, these strains eventually express solely clonic-forebrain seizures on later induction-phase trials. This result suggests that these additional flurothyl trials could either be increasing the brainstem seizure system threshold or more likely decreasing the forebrain seizure system threshold (Coffey, et al., 1996; Hirsch, et al., 1997), which would explain the occurrence of only clonic-forebrain seizures on later trials of the flurothyl kindling model in BALB/cJ and C3H/HeJ mice. As compared to the BALB/cJ and C3H/HeJ mice, 129S1/SvlmJ mice, using 5% flurothyl, also had intermixed clonic-forebrain and forebrain→brainstem seizures, possibly due to similar forebrain/brainstem seizure system threshold changes (Coffey, et al., 1996; Frankel, et al., 2001; Fuller, 1985; Hirsch, et al., 1997; Kriscenski-Perry, et al., 2002). While the genetic mechanisms underlying brainstem seizure thresholds in inbred strains are relatively unexplored, our results suggest genetic control over the brainstem seizure system threshold. The 129S1/SvImJ, BALB/cJ, and C3H/HeJ strains could be useful in the mapping of quantitative trait loci (QTL) responsible for production of these lower brainstem seizure thresholds.
Flurothyl retest, after a 28-day rest period, resulted in a change in behavioral seizure phenotype from a generalized clonic-forebrain seizure to a forebrain→brainstem seizure in C57BL/6J mice. Although the change in seizure phenotype was dramatic, the retest GST was maintained at a value matching the GST of trial 8 of the induction-phase. Surprisingly, none of the other strains showed an alteration in seizure phenotype upon flurothyl retest, including the strains that had forebrain→brainstem seizures early in the flurothyl induction-phase. These results indicate the occurrence of a change in the brain, during the course of the flurothyl regimen in C57BL/6J mice, resulting in an increased susceptibility specifically to forebrain→brainstem seizures upon flurothyl retest (Goddard, et al., 1969; Mason and Cooper, 1972). Ferland and Applegate (1998) showed that C57BL/6J mice, examined in the flurothyl kindling model, had a decrease in brainstem seizure threshold in the post-induction phase, as assessed by minimal corneal electroconvulsive shock that correlated with the increased occurrence of forebrain→brainstem seizures. The decrease in brainstem seizure threshold in C57BL/6J mice could be contributing to the behavioral seizure phenotype change observed upon retest (Cain, 1980; Chiba and Wada, 1995; Ferland and Applegate, 1998; Omori, et al., 2001). It would be of interest to measure the brainstem seizure thresholds of DBA/2J, BALB/cJ, and C3H/HeJ mice that do not have a change in seizure phenotype upon flurothyl retest, to determine whether or not a change in brainstem seizure threshold correlates with the presence or absence of the change in seizure phenotype. If the change in seizure phenotype after flurothyl kindling and incubation is mediated solely by a change in the brainstem seizure threshold, then one might predict that DBA/2J, BALB/cJ, and C3H/HeJ mice would not have lowered brainstem seizure thresholds. However, if DBA/2J, BALB/cJ, and C3H/HeJ mice did have lower brainstem thresholds following flurothyl kindling and incubation, then this would suggest an alternative mechanism for the change in seizure phenotype observed in the flurothyl kindling model with C57BL/6J mice.
Previous research has suggested that the ventromedial nucleus of the hypothalamus (VMH) has a fundamental role in the forebrain→brainstem seizure change. Immunohistochemistry for c-Fos, a neuronal activation marker, demonstrated that bilateral VMH activation only occurred in animals having the change in seizure phenotype (Samoriski, et al., 1997). Also, bilateral lesions of the VMH prevented the change in seizure phenotype in C57BL/6J mice (Ferland and Applegate, 1998), indicating a critical role of the VMH in the change in seizure phenotype observed in the flurothyl kindling model.
Our results from [C57BL/6J x DBA/2J] F1 hybrid mice indicate genetic dissociations of certain seizure characteristics in the flurothyl kindling model. The initial GST in [C57BL/6J x DBA/2J] F1 hybrids appeared closest to the GST in C57BL/6J mice. Similarly, the overall kindling of GST appeared to be influenced by C57BL/6J genes; like the C57BL/6J parent, [C57BL/6J x DBA/2J] F1 hybrids did exhibit kindling. It is interesting to note that on later induction trials, the GST was higher in [C57BL/6J x DBA/2J] F1 hybrid mice than both parental strains. Since the seizure characteristics observed in the flurothyl kindling model are likely complex quantitative traits, the differences noted in flurothyl kindling in the [C57BL/6J x DBA/2J] F1 hybrid mice could be due to multiple seizure resistance genes acquired from both parental strains. Thus, the initial GST observed with 10% flurothyl in C57BL/6J, DBA/2J, and [C57BL/6J x DBA/2J] F1 hybrid mice is similar to the GST seen in other seizure susceptibility models; models that have been proposed to be due to multiple genetic effects (Engstrom and Woodbury, 1988; Ferraro, et al., 2002; Ferraro, et al., 2001; Ferraro, et al., 2004; Ferraro, et al., 1997; Ferraro, et al., 1999; Ferraro, et al., 1998; Frankel, et al., 2001; Frankel, et al., 1995; Hain, et al., 2000; Kosobud and Crabbe, 1990; Schauwecker, et al., 2004).
Surprisingly, C57BL/6J’s alteration in seizure phenotype on retest, from a clonic-forebrain seizure phenotype to a forebrain→brainstem seizure phenotype, was not observed in the [C57BL/6J x DBA/2J] F1 hybrid mice. In this respect, then, the hybrid resembled DBA/2J mice instead. Seizure durations were similar between C57BL/6J, DBA/2J, and [C57BL/6J x DBA/2J] F1 hybrid mice, with the exception of C57BL/6J mice having significantly longer seizure durations upon retest. This was expected, since C57BL/6J mice had forebrain→brainstem seizures, and therefore had a longer recovery to regain posture. Although DBA/2J and [C57BL/6J x DBA/2J] F1 hybrid mice differed in seizure duration upon retest, their seizure phenotype was the same, having clonic-forebrain seizures. Overall, these results indicate that the trait of resistance to seizure propagation from the forebrain circuitry to the brainstem circuitry is dissociable from the traits of GST and kindling in [C57BL/6J x DBA/2J] F1 hybrid mice. Moreover, the fact that the [C57BL/6J x DBA/2J] F1 hybrid does not show an altered behavioral seizure phenotype upon retest suggests that genetic loci present in the DBA/2J genome or the lack of such loci from the C57BL/6J genome affect neuronal plasticity, such that the propagation of seizure discharge from the forebrain seizure system to the brainstem seizure system is altered.
To rule out age related effects on the change in seizure phenotype, we gave one flurothyl trial to 12 week old C57BL/6J and DBA/2J mice. These mice responded to flurothyl similarly to 7 week-old mice on trial 1 of the full flurothyl regimen; C57BL/6J mice displayed a high GST, and DBA/2J mice had a low GST. Additionally, when both strains of mice were given one flurothyl trial at 12 weeks of age, both strains expressed clonic-forebrain seizures, indicating that the change in seizure phenotype in flurothyl-kindled C57BL/6J mice is not age-related. Overall, these results suggest that the alteration in the brain, which accounts for the change in seizure phenotype, does not occur in DBA/2J mice, and is not the result of developmental changes associated with age in C57BL/6J mice. Although brain changes are likely, alterations in flurothyl metabolism could also contribute to this phenomenon and require further investigation.
Overall, the above described data highlight some of the phenotypic differences between the C57BL/6J and DBA/2J strains. They establish baseline phenotypic data that can be used with either recombinant inbred lines (Johnson, et al., 1992; Justice, et al., 1992; Plomin, et al., 1991) or chromosome-substitution strains (consomics)(Davis, et al., 2005; Hill, et al., 2006; Singer, et al., 2004; Williams, 1999) for the mapping of QTLs that control seizure threshold (i.e., seizure susceptibility or resistance), kindling, and behavioral seizure phenotypic changes. The use of these genetic tools to elucidate the genetic differences seen among inbred strains could provide insight into the process of epileptogenesis.
Acknowledgments
This work was partly supported by NIH grants 1K01MH71801 (to RJF) and R01MH065400 (to BJH). We wish to thank members of the Ferland laboratory and Dr. Fern P. Finger (RPI) for critical review of the manuscript. The authors wish to thank Dr. Adriana Verschoor (Wadsworth Center) for critical reading and input on our manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anh DB, Faisca P, Desmecht DJ. Differential resistance/susceptibility patterns to pneumovirus infection among inbred mouse strains. Am J Physiol Lung Cell Mol Physiol. 2006;291:L426–435. doi: 10.1152/ajplung.00483.2005. [DOI] [PubMed] [Google Scholar]
- 2.Applegate CD, Samoriski GM, Ozduman K. Effects of valproate, phenytoin, and MK-801 in a novel model of epileptogenesis. Epilepsia. 1997;38:631–636. doi: 10.1111/j.1528-1157.1997.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 3.Bampton ET, Gray RA, Large CH. Electrophysiological characterisation of the dentate gyrus in five inbred strains of mouse. Brain Res. 1999;841:123–134. doi: 10.1016/s0006-8993(99)01811-9. [DOI] [PubMed] [Google Scholar]
- 4.Barnes SJ, Pinel JP. Conditioned effects of kindling. Neurosci Biobehav Rev. 2001;25:745–751. doi: 10.1016/s0149-7634(01)00054-9. [DOI] [PubMed] [Google Scholar]
- 5.Cain DP. Effects of kindling or brain stimulation on pentylenetetrazol-induced convulsion susceptibility. Epilepsia. 1980;21:243–249. doi: 10.1111/j.1528-1157.1980.tb04069.x. [DOI] [PubMed] [Google Scholar]
- 6.Chiba S, Wada JA. Kindling of the interpeduncular nucleus and its influence on subsequent amygdala kindling in rats. Epilepsia. 1995;36:410–415. doi: 10.1111/j.1528-1157.1995.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 7.Coffey LL, Reith ME, Chen NH, Mishra PK, Jobe PC. Amygdala kindling of forebrain seizures and the occurrence of brainstem seizures in genetically epilepsy-prone rats. Epilepsia. 1996;37:188–197. doi: 10.1111/j.1528-1157.1996.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 8.Davis RC, Schadt EE, Smith DJ, Hsieh EW, Cervino AC, van Nas A, Rosales M, Doss S, Meng H, Allayee H, Lusis AJ. A genome-wide set of congenic mouse strains derived from DBA/2J on a C57BL/6J background. Genomics. 2005;86:259–270. doi: 10.1016/j.ygeno.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Dichter MA. Models of epileptogenesis in adult animals available for antiepileptogenesis drug screening. Epilepsy Res. 2006;68:31–35. doi: 10.1016/j.eplepsyres.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Engel J, Pedley TA. Introduction: what is epilepsy? In: Engel J, Pedley TA, editors. Epilepsy: a Comprehensive Textbook. Lippincott-Raven; Philadelphia: 1998. pp. 1–7. [Google Scholar]
- 11.Engstrom FL, Woodbury DM. Seizure susceptibility in DBA and C57 mice: the effects of various convulsants. Epilepsia. 1988;29:389–395. doi: 10.1111/j.1528-1157.1988.tb03736.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferland RJ, Applegate CD. Decreased brainstem seizure thresholds and facilitated seizure propagation in mice exposed to repeated flurothyl-induced generalized forebrain seizures. Epilepsy Res. 1998;30:49–62. doi: 10.1016/s0920-1211(97)00093-4. [DOI] [PubMed] [Google Scholar]
- 13.Ferland RJ, Applegate CD. The role of the ventromedial nucleus of the hypothalamus in epileptogenesis. Neuroreport. 1998;9:3623–3629. doi: 10.1097/00001756-199811160-00013. [DOI] [PubMed] [Google Scholar]
- 14.Ferland RJ, Applegate CD. Bidirectional transfer between electrical and flurothyl kindling in mice: evidence for common processes in epileptogenesis. Epilepsia. 1999;40:144–152. doi: 10.1111/j.1528-1157.1999.tb02067.x. [DOI] [PubMed] [Google Scholar]
- 15.Ferraro TN, Golden GT, Smith GG, DeMuth D, Buono RJ, Berrettini WH. Mouse strain variation in maximal electroshock seizure threshold. Brain Res. 2002;936:82–86. doi: 10.1016/s0006-8993(02)02565-9. [DOI] [PubMed] [Google Scholar]
- 16.Ferraro TN, Golden GT, Smith GG, Longman RL, Snyder RL, DeMuth D, Szpilzak I, Mulholland N, Eng E, Lohoff FW, Buono RJ, Berrettini WH. Quantitative genetic study of maximal electroshock seizure threshold in mice: evidence for a major seizure susceptibility locus on distal chromosome 1. Genomics. 2001;75:35–42. doi: 10.1006/geno.2001.6577. [DOI] [PubMed] [Google Scholar]
- 17.Ferraro TN, Golden GT, Smith GG, Martin JF, Lohoff FW, Gieringer TA, Zamboni D, Schwebel CL, Press DM, Kratzer SO, Zhao H, Berrettini WH, Buono RJ. Fine mapping of a seizure susceptibility locus on mouse Chromosome 1: nomination of Kcnj10 as a causative gene. Mamm Genome. 2004;15:239–251. doi: 10.1007/s00335-003-2270-3. [DOI] [PubMed] [Google Scholar]
- 18.Ferraro TN, Golden GT, Smith GG, Schork NJ, St Jean P, Ballas C, Choi H, Berrettini WH. Mapping murine loci for seizure response to kainic acid. Mamm Genome. 1997;8:200–208. doi: 10.1007/s003359900389. [DOI] [PubMed] [Google Scholar]
- 19.Ferraro TN, Golden GT, Smith GG, St Jean P, Schork NJ, Mulholland N, Ballas C, Schill J, Buono RJ, Berrettini WH. Mapping loci for pentylenetetrazol-induced seizure susceptibility in mice. J Neurosci. 1999;19:6733–6739. doi: 10.1523/JNEUROSCI.19-16-06733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferraro TN, Golden GT, Snyder R, Laibinis M, Smith GG, Buono RJ, Berrettini WH. Genetic influences on electrical seizure threshold. Brain Res. 1998;813:207–210. doi: 10.1016/s0006-8993(98)01013-0. [DOI] [PubMed] [Google Scholar]
- 21.Frankel WN, Taylor L, Beyer B, Tempel BL, White HS. Electroconvulsive thresholds of inbred mouse strains. Genomics. 2001;74:306–312. doi: 10.1006/geno.2001.6564. [DOI] [PubMed] [Google Scholar]
- 22.Frankel WN, Valenzuela A, Lutz CM, Johnson EW, Dietrich WF, Coffin JM. New seizure frequency QTL and the complex genetics of epilepsy in EL mice. Mamm Genome. 1995;6:830–838. doi: 10.1007/BF00292431. [DOI] [PubMed] [Google Scholar]
- 23.Fuller JL. Effects of maturation and priming on audiogenic seizure thresholds in mice. Dev Psychobiol. 1985;18:141–149. doi: 10.1002/dev.420180206. [DOI] [PubMed] [Google Scholar]
- 24.Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- 25.Grice DE, Reenila I, Mannisto PT, Brooks AI, Smith GG, Golden GT, Buxbaum JD, Berrettini WH. Transcriptional profiling of C57 and DBA strains of mice in the absence and presence of morphine. BMC Genomics. 2007;8:76. doi: 10.1186/1471-2164-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hain HS, Crabbe JC, Bergeson SE, Belknap JK. Cocaine-induced seizure thresholds: quantitative trait loci detection and mapping in two populations derived from the C57BL/6 and DBA/2 mouse strains. J Pharmacol Exp Ther. 2000;293:180–187. [PubMed] [Google Scholar]
- 27.Hill AE, Lander ES, Nadeau JH. Chromosome substitution strains: a new way to study genetically complex traits. Methods Mol Med. 2006;128:153–172. doi: 10.1385/1-59745-159-2:153. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch E, Danober L, Simler S, Pereira de Vasconcelos A, Maton B, Nehlig A, Marescaux C, Vergnes M. The amygdala is critical for seizure propagation from brainstem to forebrain. Neuroscience. 1997;77:975–984. doi: 10.1016/s0306-4522(96)00503-9. [DOI] [PubMed] [Google Scholar]
- 29.Johnson TE, DeFries JC, Markel PD. Mapping quantitative trait loci for behavioral traits in the mouse. Behav Genet. 1992;22:635–653. doi: 10.1007/BF01066635. [DOI] [PubMed] [Google Scholar]
- 30.Jones MW, Peckham HM, Errington ML, Bliss TV, Routtenberg A. Synaptic plasticity in the hippocampus of awake C57BL/6 and DBA/2 mice: interstrain differences and parallels with behavior. Hippocampus. 2001;11:391–396. doi: 10.1002/hipo.1053. [DOI] [PubMed] [Google Scholar]
- 31.Justice MJ, Jenkins NA, Copeland NG. Recombinant inbred mouse strains: models for disease study. Trends Biotechnol. 1992;10:120–126. doi: 10.1016/0167-7799(92)90193-y. [DOI] [PubMed] [Google Scholar]
- 32.Kosobud AE, Crabbe JC. Genetic correlations among inbred strain sensitivities to convulsions induced by 9 convulsant drugs. Brain Res. 1990;526:8–16. doi: 10.1016/0006-8993(90)90243-5. [DOI] [PubMed] [Google Scholar]
- 33.Kriscenski-Perry E, Applegate CD, Serour A, Mhyre TR, Leonardo CC, Pearce DA. Altered flurothyl seizure induction latency, phenotype, and subsequent mortality in a mouse model of juvenile neuronal ceroid lipofuscinosis/batten disease. Epilepsia. 2002;43:1137–1140. doi: 10.1046/j.1528-1157.2002.16002.x. [DOI] [PubMed] [Google Scholar]
- 34.Leite JP, Garcia-Cairasco N, Cavalheiro EA. New insights from the use of pilocarpine and kainate models. Epilepsy Res. 2002;50:93–103. doi: 10.1016/s0920-1211(02)00072-4. [DOI] [PubMed] [Google Scholar]
- 35.Lothman EW, Stringer JL, Bertram EH. The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res Suppl. 1992;7:301–313. [PubMed] [Google Scholar]
- 36.Mason CR, Cooper RM. A permanent change in convulsive threshold in normal and brain-damaged rats with repeated small doses of pentylenetetrazol. Epilepsia. 1972;13:663–674. doi: 10.1111/j.1528-1157.1972.tb04401.x. [DOI] [PubMed] [Google Scholar]
- 37.McIntyre DC, Poulter MO, Gilby K. Kindling: some old and some new. Epilepsy Res. 2002;50:79–92. doi: 10.1016/s0920-1211(02)00071-2. [DOI] [PubMed] [Google Scholar]
- 38.McNamara JO. Kindling model of epilepsy. Adv Neurol. 1986;44:303–318. [PubMed] [Google Scholar]
- 39.McNamara JO, Bonhaus DW, Shin C. The kindling model of epilepsy. In: Schwartzkroin PA, editor. Epilepsy: Models, mechanisms, and concepts. Cambridge University Press; Cambridge: 1993. pp. 20–47. [Google Scholar]
- 40.Mhyre TR, Applegate CD. Persistent regional increases in brain-derived neurotrophic factor in the flurothyl model of epileptogenesis are dependent upon the kindling status of the animal. Neuroscience. 2003;121:1031–1045. doi: 10.1016/s0306-4522(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 41.Michalakis M, Holsinger D, Ikeda-Douglas C, Cammisuli S, Ferbinteanu J, DeSouza C, DeSouza S, Fecteau J, Racine RJ, Milgram NW. Development of spontaneous seizures over extended electrical kindling. I. Electrographic, behavioral, and transfer kindling correlates. Brain Res. 1998;793:197–211. doi: 10.1016/s0006-8993(98)00155-3. [DOI] [PubMed] [Google Scholar]
- 42.Middei S, Vetere G, Sgobio C, Ammassari-Teule M. Landmark-based but not vestibular-based orientation elicits mossy fiber synaptogenesis in the mouse hippocampus. Neurobiol Learn Mem. 2007;87:174–180. doi: 10.1016/j.nlm.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Omori N, Ishimoto T, Mutoh F, Chiba S. Kindling of the midbrain periaqueductal gray in rats. Brain Res. 2001;903:162–167. doi: 10.1016/s0006-8993(01)02436-2. [DOI] [PubMed] [Google Scholar]
- 45.Onat FY, Aker RG, Gurbanova AA, Ates N, van Luijtelaar G. The effect of generalized absence seizures on the progression of kindling in the rat. Epilepsia. 2007;48(Suppl 5):150–156. doi: 10.1111/j.1528-1167.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 46.Pinel JP, Rovner LI. Electrode placement and kindling-induced experimental epilepsy. Exp Neurol. 1978;58:335–346. doi: 10.1016/0014-4886(78)90145-0. [DOI] [PubMed] [Google Scholar]
- 47.Pinel JP, Rovner LI. Experimental epileptogenesis: kindling-induced epilepsy in rats. Exp Neurol. 1978;58:190–202. doi: 10.1016/0014-4886(78)90133-4. [DOI] [PubMed] [Google Scholar]
- 48.Plomin R, McClearn GE, Gora-Maslak G, Neiderhiser JM. Use of recombinant inbred strains to detect quantitative trait loci associated with behavior. Behav Genet. 1991;21:99–116. doi: 10.1007/BF01066330. [DOI] [PubMed] [Google Scholar]
- 49.Potschka H, Loscher W. Corneal kindling in mice: behavioral and pharmacological differences to conventional kindling. Epilepsy Res. 1999;37:109–120. doi: 10.1016/s0920-1211(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 50.Restivo L, Roman FS, Ammassari-Teule M, Marchetti E. Simultaneous olfactory discrimination elicits a strain-specific increase in dendritic spines in the hippocampus of inbred mice. Hippocampus. 2006;16:472–479. doi: 10.1002/hipo.20174. [DOI] [PubMed] [Google Scholar]
- 51.Samoriski GM, Applegate CD. Repeated generalized seizures induce time-dependent changes in the behavioral seizure response independent of continued seizure induction. J Neurosci. 1997;17:5581–5590. doi: 10.1523/JNEUROSCI.17-14-05581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samoriski GM, Piekut DT, Applegate CD. Differential spatial patterns of Fos induction following generalized clonic and generalized tonic seizures. Exp Neurol. 1997;143:255–268. doi: 10.1006/exnr.1996.6368. [DOI] [PubMed] [Google Scholar]
- 53.Samoriski GM, Piekut DT, Applegate CD. Regional analysis of the spatial patterns of Fos induction in brain following flurothyl kindling. Neuroscience. 1998;84:1209–1222. doi: 10.1016/s0306-4522(97)00571-x. [DOI] [PubMed] [Google Scholar]
- 54.Schauwecker PE, Williams RW, Santos JB. Genetic control of sensitivity to hippocampal cell death induced by kainic acid: a quantitative trait loci analysis. J Comp Neurol. 2004;477:96–107. doi: 10.1002/cne.20245. [DOI] [PubMed] [Google Scholar]
- 55.Schubert M, Siegmund H, Pape HC, Albrecht D. Kindling-induced changes in plasticity of the rat amygdala and hippocampus. Learn Mem. 2005;12:520–526. doi: 10.1101/lm.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartzkroin PA. Hippocampal slices in experimental and human epilepsy. Adv Neurol. 1986;44:991–1010. [PubMed] [Google Scholar]
- 57.Schwartzkroin PA, Franck JE. Electrophysiology of epileptic tissue: what pathologies are epileptogenic? Adv Exp Med Biol. 1986;203:157–172. doi: 10.1007/978-1-4684-7971-3_12. [DOI] [PubMed] [Google Scholar]
- 58.Schwartzkroin PA, Roper SN, Wenzel HJ. Cortical dysplasia and epilepsy: animal models. Adv Exp Med Biol. 2004;548:145–174. doi: 10.1007/978-1-4757-6376-8_12. [DOI] [PubMed] [Google Scholar]
- 59.Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW. Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol Pathol. 2007;35:984–999. doi: 10.1080/01926230701748305. [DOI] [PubMed] [Google Scholar]
- 60.Singer JB, Hill AE, Burrage LC, Olszens KR, Song J, Justice M, O’Brien WE, Conti DV, Witte JS, Lander ES, Nadeau JH. Genetic dissection of complex traits with chromosome substitution strains of mice. Science. 2004;304:445–448. doi: 10.1126/science.1093139. [DOI] [PubMed] [Google Scholar]
- 61.Sutula T, Harrison C, Steward O. Chronic epileptogenesis induced by kindling of the entorhinal cortex: the role of the dentate gyrus. Brain Res. 1986;385:291–299. doi: 10.1016/0006-8993(86)91075-9. [DOI] [PubMed] [Google Scholar]
- 62.Williams RW. A targeted screen to detect recessive mutations that have quantitative effects. Mamm Genome. 1999;10:734–738. doi: 10.1007/s003359901081. [DOI] [PubMed] [Google Scholar]
- 63.Wlaz P, Potschka H, Loscher W. Frontal versus transcorneal stimulation to induce maximal electroshock seizures or kindling in mice and rats. Epilepsy Res. 1998;30:219–229. doi: 10.1016/s0920-1211(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 64.Yeh GC, Bonhaus DW, Nadler JV, McNamara JO. N-methyl-D-aspartate receptor plasticity in kindling: quantitative and qualitative alterations in the N-methyl-D-aspartate receptor-channel complex. Proc Natl Acad Sci U S A. 1989;86:8157–8160. doi: 10.1073/pnas.86.20.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]