Abstract
Ebola virus-like particles (VLPs) were produced in insect cells using a recombinant baculovirus expression system and their efficacy for protection against Ebola virus infection was investigated. Two immunizations with 50 ug Ebola VLPs (high dose) induced a high level of antibodies against Ebola GP that exhibited strong neutralizing activity against GP-mediated virus infection and conferred complete protection of vaccinated mice against lethal challenge by a high dose of mouse-adapted Ebola virus. In contrast, two immunizations with 10 ug Ebola VLPs (low dose) induced 5-fold lower levels of antibodies against GP and these mice were not protected against lethal Ebola virus challenge, similar to control mice that were immunized with 50 ug SIV Gag VLPs. However, the antibody response against GP were boosted significantly after a third immunization with 10 ug Ebola VLPs to similar levels as those induced by two immunizations with 50 ug Ebola VLPs, and vaccinated mice were also effectively protected against lethal Ebola virus challenge. Furthermore, serum viremia levels in protected mice were either below the level of detection or significantly lower compared to the viremia levels in control mice. These results show that effective protection can be achieved by immunization with Ebola VLPs produced in insect cells, which give high production yields, and lend further support to their development as an effective vaccine strategy against Ebola virus.
INTRODUCTION
Ebola virus, along with Marburg virus, belongs to the Filoviridae family and causes severe viral hemorrhagic fevers with a high fatality rate up to 90%, for which there is no effective treatment or licensed vaccine at present. Since its first identification in the 1977 outbreak in Africa, Ebola virus outbreaks have caused over 1800 human infections with over 1300 deaths and such outbreaks have become increasingly frequent in recent years (Groseth et al., 2007). Recent studies indicate that African fruit bats may be natural reservoirs for both Ebola and Marburg viruses (Leroy et al., 2005; Towner et al., 2007), suggesting that these viruses will remain endemic in these regions and outbreaks will continue to occur through zoonotic transmission. Outbreaks in humans are likely to stem from contact with infected animals followed by spread among humans through close person-to-person contacts. Ebola viruses cause acute infection in humans with an incubation period usually between 4–10 days, that typically starts with headache, chill, myalgia, as well as other signs of infection, followed by more severe symptoms including weight loss, delirium, shock, massive hemorrhaging, and multi-organ dysfunction that eventually lead to death in about two to three weeks (Bwaka et al., 1999; Peters and DeLuc, 1999). Although outbreaks of Ebola virus have largely been confined to endemic regions, their high fatality rate, ability to transmit person-to-person, and low lethal infectious dose make Ebola virus a dangerous threat to public health and pose a great risk for researchers working with these viruses as well as health care personnel treating patients during outbreaks. Furthermore, their potential to be developed into aerosolized biological weapons also causes grave concern for their use as a bioterrorism agent (Brey, 2003). These features together with the lack of effective treatment underscore the need to develop an efficacious vaccine strategy against Ebola virus infection.
While there is no licensed vaccine, significant progress has been made and results from recent studies demonstrate that viral hemorrhagic fevers caused by Ebola virus infection can be successfully controlled in animals, including non-human primates, by effective vaccinations (Hart, 2003). Highly promising results have been obtained with viral-vector based vaccine approaches. The first vaccine strategy that was shown to successfully protect non-human primates against Ebola virus infection employed an immunization regimen of DNA vaccine priming followed by recombinant adenovirus vaccine boosting (Sullivan et al., 2000). Subsequent studies showed that a single immunization with recombinant adenoviruses expressing the Ebola GP and NP proteins or the GP alone was sufficient to confer complete protection against EBOV infection in non-human primates (Sullivan et al., 2003; 2006). More recently, recombinant VSV and recombinant human PIV3 virus based vaccines that express Ebola virus GP were also developed and shown to confer complete protection of non-human primates against Ebola virus infection (Jones et al., 2005; Bukreyev et al., 2007). However, the pre-existing immune response against the adenovirus viral vector may potentially reduce the efficacy of recombinant adenovirus replicon-based vaccines in human applications. Also, the recombinant VSV and PIV3 viral-vector based vaccines are replication competent and may raise safety concerns for use as a preventive human vaccine.
These limitations of viral vector-based vaccines underscore the need for developing alternative vaccine strategies that can meet both safety and efficacy criteria for prevention of Ebola virus infection. In particular, expression of viral structural proteins leads to assembly and release of particles from the cells, which are designated as virus-like particles (VLPs) due to their resemblance to virions in size and morphology (Johnson and Chiu, 2000). Like many viruses, expression of filovirus structural proteins VP40 and GP leads to assembly and release of VLPs that are similar to infectious virions in morphology (Aman et al., 2003, Hartlieb and Weissenhorn, 2006). Expression of VP40 alone leads to efficient assembly and budding of VLPs from mammalian 293T cells and co-expression of VP40 and GP proteins results in release of VLPs containing both proteins (Bavari et al., 2002, Noda et al., 2002). Further, co-expression of GP was found to significantly enhance the release of VP40 proteins in the form of VLPs, indicating that it may interact with VP40 and facilitate VLP assembly and release from cells (Bavari et al., 2002, Licata et al., 2004). Of particular interest, recent studies on Ebola virus-like particles (VLPs) demonstrated their potential as an effective vaccine strategy against Ebola infection. It has been shown that three immunizations with Ebola VLPs produced in mammalian 293T cells successfully protected mice against lethal challenge by mouse-adapted Ebola virus (Warfield et al., 2003). Furthermore, when given in combination with an adjuvant, these VLPs were able to protect against lethal Ebola virus challenge by a two-dose immunization regimen in mice (Warfield et al., 2005) and by a single-dose immunization regimen in guinea pigs (Swenson et al., 2005). Moreover, it was recently reported that mammalian cell-produced Ebola VLPs also effectively protected non-human primates against lethal Ebola virus challenge when given in formulation with an adjuvant (Warfield et al., 2007a). These results show that Ebola VLPs represent a promising alternative to viral vector-based vaccines for protection against Ebola virus infection.
We have previously reported that Ebola VLPs produced in insect cells exhibit dendritic cell stimulating activities similar to Ebola VLPs produced in mammalian cells and can induce neutralizing antibodies against Ebola GP mediated virus infection (Ye et al., 2006). However, it was also observed that Ebola virus GP proteins exhibit a smaller molecular weight compared to those produced in mammalian cells, possibly due to differences in glycosylation between mammalian and insect cells (Mellquist-Reimenschneider et al., 2003). This raises the question as to whether EBOV VLPs produced in insect cells are capable to induce protective immune responses against Ebola virus infection, as observed for those produced in mammalian cells. In this study, we investigated the effect of dose and number of immunizations on immune responses induced by immunization with Ebola VLPs produced in insect cells, and evaluated their efficacy to protect vaccinated mice against Ebola virus infection.
RESULTS
EBOV VLPs induce strong antibody responses
VLPs were produced by co-infection of Sf9 insect cells with recombinant baculoviruses expressing Ebola VP40 and GP proteins as described previously (Ye et al., 2006). Because the EBOV GP is the primary antigen for eliciting protective immune responses, we further determined the levels of GP incorporation in EBOV VLP preparations by a quantitative ELISA, using purified EBOV GP1-histag proteins as a standard. As shown in Figure 1A, the amount of GP proteins in EBOV VLPs was similar between two separate preparations, at 23 and 19 ng/ug respectively. Analysis by Western blot also detected similar levels of EBOV GP and VP40 proteins in the two separate VLP preparations (Figure 1B), indicating consistency for this production method. Further characterization by Coomassie blue staining (Figure 1C) revealed the major band as VP40 with no visible GP in both VLP preparations, similar as shown in our previous studies (Ye at al., 2006). VLPs were also examined by negative staining under an electron microscope. As shown in Figure 1D, the VLP preparations were found to contain filamentous particles that are typical of Ebola virus as well as irregular vesicles that may be broken particles or vesicles containing GP as reported previously (Ye et al., 2006). After characterization, VLPs preparation 1, which contains relatively higher level of GP, were used in immunization studies as outlined in Figure 2 to evaluate their immunogenicity and protective efficacy against EBOV infection.
Figure 1.
A. Determining the amount of GP protein in Ebola VLPs by a quantitative ELISA. Ebola VLPs were coated onto a microtiter plate and the level of GP proteins in VLPs was detected by ELISA using a monoclonal antibody against GP. A standard curve was constructed by coating the plate with serial dilutions of purified Ebola GP1-histag protein with known concentrations, and the amount of GP proteins in Ebola VLP preparations was calculated and expressed as nanograms of GP protein per microgram of total proteins in VLPs (ng/ug). B. Western blot analysis of Ebola VLP preparations. Ebola VLPs were produced by co-infection of Sf9 insect cells with recombinant baculoviruses expressing GP and VP40 at the MOI of 5 and 2 respectively, and purified as described in the Materials and Methods. The VLP preparations were resuspended in PBS at the concentration of 1 ug/ul and 5 ug of VLP preparations were mixed with reducing protein sample buffer, heated at 95 °C for 5 min, and then subjected to SDS-PAGE followed by Western blot analysis using monoclonal antibodies against Ebola VP40 or GP proteins as indicated. Lanes 1, control SIV Gag VLPs; lanes 2, Ebola VLPs (preparation 1); lanes 3, Ebola VLPs (preparation 2). C. Coomassie blue staining of VLP preparations. Lanes 1, control SIV Gag VLPs; lanes 2, Ebola VLPs (preparation 1); lanes 3, Ebola VLPs (preparation 2). D. Negative staining of Ebola VLPs. The VLP preparations were stained with 1% uranyl acetate followed by examination under a Hitachi-H7500 transmission electron microscope.
Figure 2. Schematic diagram of immunization and challenge study design.
Four groups of mice (groups of 6) were immunized with different doses of Ebola VLPs or control SIV Gag VLPs as indicated. Mice of Groups 1, 2, and 3 were challenged by ip injection of 1000 pfu of mouse adapted Ebola virus at 4 weeks after the second immunization and mice of Group 4 were challenged at 10 weeks after the third immunization. Mice that survived the first lethal challenge (group 3) were challenged again 10 weeks later in parallel with mice in Group 4.
We first compared immune responses induced by immunization with two different doses of Ebola VLPs. Mice (groups of 6) were immunized twice at 4-week intervals with 10 ug or 50 ug Ebola VLPs produced in Sf9 insect cells. In parallel, a control group was immunized with 50 ug of SIV Gag VLPs that were similarly produced in SF9 insect cells. Blood samples were collected at 2 weeks after the second immunization for analysis of antibody responses against the Ebola GP antigen. As shown in Figure 3A, analysis of antibody responses by ELISA showed that two immunizations with 50 ug Ebola VLPs (EboVLP-50) induced strong antibody responses against GP, which were more than 5-fold higher than the responses induced by two immunizations with 10 ug Ebola VLPs (EboVLP-10). No GP-specific antibody response was detected in samples from the control group mice that were immunized with SIV Gag VLPs. Also, Ebola VLP-induced antibodies against GP were mainly of the IgG2a subtype along with significant levels of IgG2b antibodies but only minimal levels of IgG1 antibodies. This was observed for both high and low dose immunizations, indicating that the dose of VLPs does not significantly affect the profile of induced antibody responses.
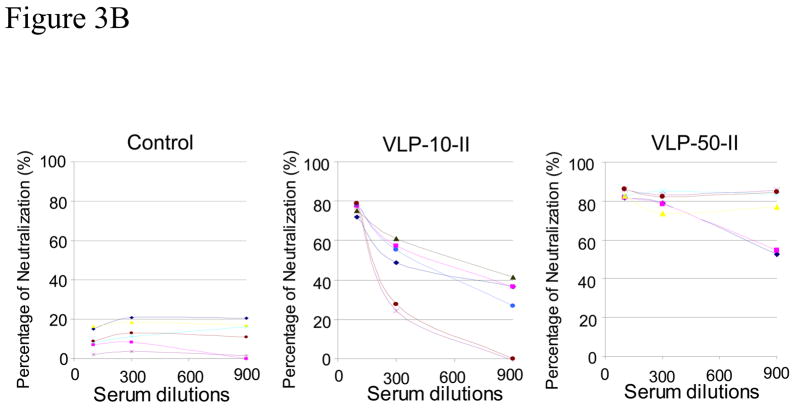
Figure 3. Two immunizations with Ebola VLPs induce strong antibody responses against GP dose dependently.
A. Analysis of antibody responses by ELISA. Mice (groups of 6) were immunized intramuscularly with different doses (10 or 50 ug) of Ebola VLPs or 50 ug SIV Gag VLPs twice at 4-week intervals. Serum samples were collected at two weeks after the second immunization and assayed for Ebola GP specific antibodies by ELISA using purified His-tagged GP1 as coating antigen. A standard curve was generated using purified mouse antibodies for each mouse antibody subtype and used for calculation of the equivalent amount of GP-binding antibodies in serum samples. Control, mice immunized with control SIV Gag VLPs; EboVLP-10, mice immunized with 10 ug Ebola VLPs; EboVLP-50, mice immunized with 50 ug Ebola VLPs. B. Neutralization of Ebola GP pseudotyped HIV. Serum samples collected after the second immunization were mixed with 500 pfu of Ebola GP-HIV pseudovirions at final dilutions of 1:100, 1:300, and 1:900 as indicated. After incubation at 37 °C for 1 hr, the mixtures were added to JC53 cells seeded in a 96-well plate and incubated at 37 °C for 2 days. The level of β-galactosidase activity in each well was determined by hydrolysis of the CPRG substrate by measuring the O.D. value at 590 nm as described in Materials and Methods. A standard curve was constructed by infecting JC53BL cells with serial dilutions of Ebola GP-HIV pseudovirions and the neutralizing activity of serum samples is expressed as the percentage reduction of virus titers in sample wells compared to the titers in control wells without mouse sera [(virus titer in control well-virus titer in sample well)/virus titer in control well x 100%].
We further compared the neutralizing activity of antibody responses against Ebola GP-mediated virus entry induced by immunization with different doses of VLPs using a pseudovirus-neutralization assay. As shown in Figure 3B, sera from high-dose Ebola VLP immunized mice (EboVLP-50) exhibited a neutralizing activity of about 90% on average at 1:100 dilution, which was maintained above 80% at 1:300 dilution. On the other hand, sera from the low-dose Ebola VLP immunized mice (EboVLP-10) exhibit a neutralizing activity of about 80% on average at 1:100 dilution, which dropped to below 60% at 1:300 dilution (significantly lower compared to the EboVLP-50 group, p<0.05). Moreover, sera from mice immunized with high-dose Ebola VLPs retained neutralizing activities above 50% at 1:900 dilution with sera from 4 of 6 mice exhibiting neutralizing activities above 80%, whereas sera from mice immunized with low-dose Ebola VLPs showed neutralizing activity below 50% at this dilution. As summarized in Table 1, the 50% endpoint neutralizing titers (50%NT) in the high-dose group are higher than those in the low-dose group, correlating with the level of antibody responses. These results show that strong neutralizing antibody responses against the Ebola GP were induced by two immunizations with the higher dose of Ebola VLPs produced in insect cells, correlating with the levels of GP-specific antibody responses as determined be ELISA.
Table 1.
Viremia level, weight loss, and survival rate after lethal Ebola virus challenge (I).
| Mouse | Antibody level | 50% NT* | viremia | Weight loss | Survival/death | |
|---|---|---|---|---|---|---|
| Control | 1 | ND | ND | 6.03 × 1010 | >25% | died |
| 2 | ND | ND | 6.74 × 1010 | > 25% | died | |
| 3 | ND | ND | 8.29 × 1010 | > 25% | died | |
| 4 | ND | ND | 4.03 × 1010 | > 25% | died | |
| 5 | ND | ND | 3.74 × 1010 | > 25% | died | |
| 6 | ND | ND | ND | 20% | survived | |
| VLP-50-II | 1 | 10822 ng/ml | 1:900 | ND | NC | survived |
| 2 | 13070 ng/ml | 1:2700 | ND | NC | survived | |
| 3 | 22033 ng/ml | 1:8100 | ND | NC | survived | |
| 4 | 16668 ng/ml | 1:2700 | ND | NC | survived | |
| 5 | 16743 ng/ml | 1:2700 | ND | NC | survived | |
| 6 | 9959 ng/ml | 1:900 | 1.78 × 109 | 10% | survived | |
| VLP-10-II | 1 | 2832 ng/ml | 1:300 | 4.21 × 1010 | > 25% | died |
| 2 | 1972 ng/ml | 1:100 | 9.17 × 1010 | > 25% | died | |
| 3 | 2052 ng/ml | 1:100 | 5.33 × 1010 | > 25% | died | |
| 4 | 2027 ng/ml | 1:300 | 3.31 × 1010 | > 25% | died | |
| 5 | 2432 ng/ml | 1:300 | 1.64 × 1010 | > 25% | died | |
| 6 | 2797 ng/ml | 1:300 | 5.09 × 1010 | > 25% | died |
Note. BALB/c mice were vaccinated with Ebola VLPs or control SIV Gag VLPs and challenged at 4 weeks after the last immunization. Blood samples were collected on day 4 post challenge for determination of viremia by qRT-PCR. Mice were monitored for 21 days after challenge and the largest percentage of weight loss was presented.
ND: Not detected; NC: No change (<5%).
: The endpoint neutralizing titer is provided as the highest sera dilution that gives at least 50% neutralization of pseudovirions (50% NT).
Two immunizations with the high-dose Ebola VLPs protect mice against lethal Ebola virus challenge
Encouraged by the high-level antibody responses induced by Ebola VLPs, we challenged the immunized mice after two immunizations. At 4 weeks after the second immunization, mice were challenged with 1000 pfu of mouse-adapted Ebola virus (approximately 30,000 LD50), which was originally obtained by Bray et al. through passage in suckling balb/c mouse and has been shown to be highly lethal for mice by intra-peritoneal injection with an estimated 30 LD50 per pfu as tittered in Vero E6 cells (Bray et al., 1998). Mice were monitored daily for signs of disease and weight changes after challenge and blood samples were collected on day 4 after challenge for comparison of viremia levels. Shown in Figure 4 are the weight changes of mice after challenge. Five of the six mice that were immunized with control SIV Gag VLPs showed a rapid weight loss on day 4 post-challenge, with one of these mice died on day 4 and the other four were sacrificed on day 5 in accordance with IACUC guidelines. Similarly, all six mice of the EboVLP-10 group that were immunized with 10 ug Ebola VLPs experienced rapid weight loss on day 4 post-challenge and succumbed to the challenge on day 5. In contrast, all six mice of the EboVLP-50 group that were immunized with 50 ug Ebola VLPs survived the challenge. Among these, only one mouse exhibited weight loss of about 10% but showed no other signs of disease such as hunched back or reduced activity. Continued monitoring showed that this mouse started to gain weight on day 8 and regained its weight by day 11 after challenge. Blood samples were collected from all mice on day 4 after challenge and the levels of serum viremia were determined by a quantitative RT-PCR assay (qRT-PCR) using in vitro transcribed GP RNA segment as standards. As shown in Table 1, high levels of viremia ranging from 1.64 to 9.17 × 1010 copies of Ebola viral genome equivalent per ml of blood were detected in all animals in the EboVLP-10 group as well as in five of six mice in the control group. In contrast, viremia was only detected in one mouse in the EboVLP-50 group, and the level (1.78 × 109) was 10-fold lower than those detected in the other groups. Of note, this mouse was the same one that exhibited weight loss after challenge as shown in Figure 4 and has the lowest antibody titer and neutralizing titer comparing to other animals in this group as shown in Table 1. In addition, blood samples were also collected from all survived mice on day 28 after challenge and no viremia was detected (data not shown).
Figure 4. Mice immunized with two high doses of Ebola VLPs are protected against lethal Ebola virus challenge.
Mice were immunized by different VLP preparations as indicated in Figure 2. Four weeks after the second immunization, mice were challenged by i.p. injection of 1000 pfu mouse-adapted Ebola virus. Mice were monitored daily and weighed on indicated days post challenge for recording of weight changes after challenge. Mice in the Ebo-10 group (6 mice) and the control group (5 of 6 mice) were sacrificed in accordance with IACUC guidelines.
A third low-dose EBOV VLP immunization effectively boosts antibody responses against GP and protects mice against Ebola virus challenge
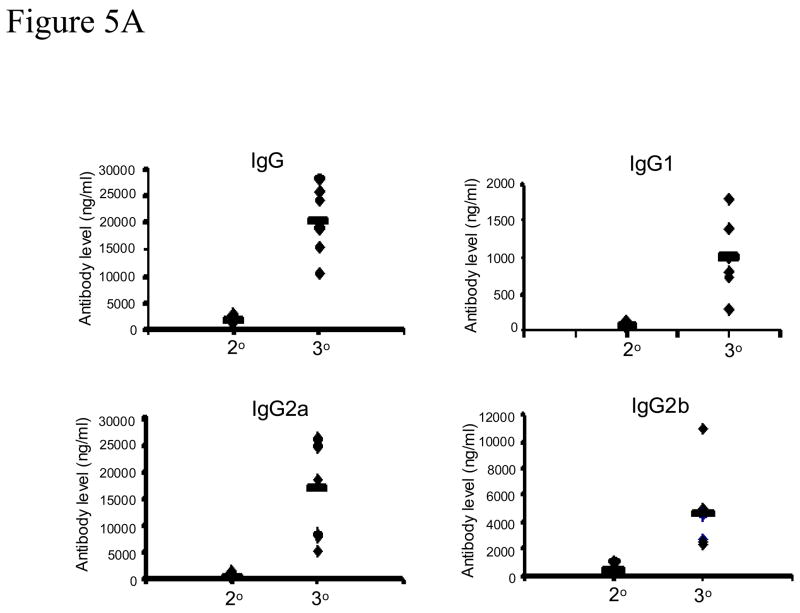
As outlined in Figure 2, the Group 4 mice (VLP-10-III) were immunized three times with 10 ug EBOV VLPs at 4-week intervals, a similar regimen as previously reported for mammalian cell-produced Ebola VLPs (Warfield et al., 2003). Blood samples from this group were collected at 2 weeks after the second and third immunizations for comparison of antibody responses. As shown in Figure 5A, low levels of antibody response against GP were detected in sera after the second immunization, similar in range to the levels detected in Group 3 mice (VLP-10-II) that received two immunizations with 10 ug Ebola VLPs as shown in Figure 3. However, the antibodies against GP were significantly boosted after the third immunization, resulting in levels that were comparable to those in Group 2 mice (VLP-50-II) that received two immunizations with 50 ug Ebola VLPs. Moreover, sera collected from low-dose VLP-vaccinated mice after the third immunization exhibited similar levels of neutralizing activity against Ebola GP-mediated virus infection as those from high-dose VLP-vaccinated mice (Figure 5B). At 12 weeks after the third immunization, mice in Group 4 were challenged with 1000 pfu of mouse-adapted Ebola virus. As shown in Figure 6, three mice in this group showed weight loss and one of them died on day 6 after challenge. As presented in Table 2, viremia was detected in the three mice that lost weight and the levels were over 100-fold lower compared to those detected in control group mice (Group 1). These results show that a third immunization with lose-dose Ebola VLPs significantly boosted immune responses against GP that were able to confer an effective protection against lethal Ebola virus challenge at 12 weeks after the final immunization. In addition, mice in Group 2 (VLP-50-II) that survived the first challenge were re-challenged in parallel. After re-challenge, two mice showed about 10% weight loss and viremia was also detected in these mice at levels that were 1000-fold lower compared to those detected in control mice (Group 1). Of note, one additional mouse also showed about 10% weight loss and died on day 4 after re-challenge. Incidentally, this is the same animal in which viremia was detected after the first challenge (#6 in the VLP-50-II Group). Surprisingly, no viremia was detected in serum samples collected on day 4 after re-challenge from this mouse. Thus it is also possible that the death may result from other factors and not due to virus infection. However, the exact reason could not be determined in this single incidence.
Figure 5. A third low dose Ebola VLP immunization significantly boosts the level of antibody responses against GP.
A. Analysis of antibody responses by ELISA. Mice (groups of 6) were immunized intramuscularly with 10 ug Ebola VLPs or 50 ug SIV Gag VLPs three times at 4-week intervals. Serum samples were collected at two weeks after the second and third immunizations, and assayed for Ebola GP specific antibodies by ELISA using purified His-tagged GP1 as coating antigen. A standard curve was generated using purified mouse antibodies for each mouse antibody subtype and used for calculation of the equivalent amount of GP-binding antibodies in serum samples. 2°, antibody responses after two immunizations with 10 ug Ebola VLPs; 3°, antibody responses after three immunizations with 10 ug Ebola VLPs. B. Neutralization of Ebola GP pseudotyped HIV. Serum samples collected after two immunizations with 50 ug Ebola VLPs (VLP-50-II) or three immunizations with 10 ug Ebola VLPs (VLP-10-III) were mixed with 500 pfu of Ebola GP-HIV pseudovirions at final dilutions of 1:100, 1:300, and 1:900 as indicated. After incubation at 37 °C for 1 hr, the mixtures were added to JC53 cells seeded in a 96-well plate and incubated at 37 °C for 2 days. The level of β-galactosidase activity in each well was determined by hydrolysis of the CPRG substrate by measuring the O.D. value at 590 nm as described in Materials and Methods. A standard curve was constructed by infecting JC53BL cells with serial dilutions of Ebola GP-HIV pseudovirions and the neutralizing activity of serum samples is expressed as the percentage reduction of virus titers in sample wells compared to the titers in control wells without mouse sera [(virus titer in control well-virus titer in sample well)/virus titer in control well x 100%].
Figure 6. Mice immunized with three low doses of Ebola VLPs are protected against lethal Ebola virus challenge.
Mice were immunized three times at 4-week intervals by 10 ug Ebola VLPs. Twelve weeks after the third immunization, mice were challenged by i.p. injection of 1000 pfu mouse-adapted Ebola virus. In addition, the 6 mice of group EboVLP-50 that survived the first challenge were re-challenged at the same time. Mice were monitored daily and weighed on indicated days post challenge for recording of weight changes after challenge. Mice that exhibit substantial weight loss and signs of severe disease were sacrificed in accordance with IACUC guidelines.
DISCUSSION
VLPs are highly attractive for vaccine development in several aspects: 1) They lack viral genomic material and thus are non-infectious and safe for broad application; 2) They can be administered repeatedly to vaccinated individuals for boosting immune responses; 3) They present viral glycoproteins in their native conformation for eliciting neutralizing antibodies. Currently, VLP-based vaccines are under investigation for a number of viruses including filoviruses (Bertolotti-Ciarlet et al., 2003; Crum et al., 2003; Li et al., 1997; Park et al., 2003; Pushko et al., 2005; Swenson et al., 2005; Warfield et al., 2003; Yao et al., 2003). Of specific interest, Ebola VLPs produced in mammalian 293T cells by DNA transfection have been shown to potently activate dendritic cells (DCs) and to confer effective protections against lethal Ebola virus challenge in animal models including non-human primates (Swenson et al., 2005; Warfield et al., 2003; 2005; 2007a). However, production of Ebola VLPs in mammalian 293T cells by DNA transfection gives low yield and the process will be difficult to scale-up for manufacturing under Good Manufacturing Practice (GMP) conditions. To address this issue, we and others have investigated production of Ebola VLPs using the recombinant baculovirus expression system and shown that co-expression of Ebola VP40 and GP proteins in insect cells using the baculovirus expression system led to release of filamentous VLPs which stimulate DCs similarly to VLPs produced in mammalian 293T cells (Warfield et al., 2007b; Ye et al., 2006). Warfield et al. also showed that Ebola VLPs produced in High 5 insect cells are able to protect mice against lethal Ebola virus challenge when given in formulation with an adjuvant (Warfield et al., 2007b). Here we show that immunization with unadjuvanted Ebola VLPs produced in Sf9 insect cells also induced strong protective immune responses in mice against lethal challenge by a high dose of mouse-adapted Ebola virus. Our results agree with and extend the findings reported by Warfield et al. in their recent study, further demonstrating that Ebola VLPs produced in insect cells are highly efficacious for protection against Ebola virus infection.
While the immune correlates for protection against Ebola virus infection have not been defined, current data from animal studies indicates that the Ebola glycoprotein GP is the primary target for inducing protective immune responses (Bukreyev et al., 2007; Jones et al., 2005; Sullivan et al., 2006; Swenson et al., 2005). In this study, we observed that two immunizations with a higher dose induced strong antibody responses against the GP and protected mice against lethal Ebola virus challenge whereas two immunizations with a lower dose induced significantly lower levels of antibody responses against GP and no protection against lethal Ebola virus challenge was observed. Further, mice immunized with a high dose of SIV Gag VLPs that were similarly produced in insect cells did not induce detectable antibody response against the GP and these mice all succumbed to challenge by Ebola virus. These results indicate that effective protection requires induction of specific immune responses against GP and correlate with the level of antibody responses to GP. This is in agreement with results reported by Sullivan et al. in their recent study, which showed that monkeys immunized by a lower dose of recombinant adenovirus expressing Ebola GP showed significantly lower titers of antibody response against GP and these animals were not protected against lethal Ebola virus challenge (Sullivan et al., 2006). However, it is possible that the antibody titers may just an indicator of the overall level of immune responses, and that both antibody and T cell responses contribute to the protection. Future studies are needed to determine whether passive transfer of sera from VLP-vaccinated mice alone will be able to confer protection against Ebola challenge. On the other hand, while protection against Ebola virus infection is likely to be mediated by both humoral and cellular immune responses (Warfield et al., 2005), the level of GP-specific antibody responses may serve as an useful indicator to determine whether a boosting immunization is necessary for achieving effective protection against Ebola virus infection. However, the criteria will need to be determined for different vaccine formulations and immunization regimens in evaluation of their efficacy.
Because of the unpredictability of Ebola virus outbreaks and their large endemic region, it will be cost prohibitive and logistically difficult to vaccinate the whole population at risk. However, there are two possible applications for a potential Ebola vaccine: 1) to control the spread of an emerging outbreak; and 2) to protect people who are at higher risk of infection. Under the first scenario, it will be highly desirable for a candidate filovirus vaccine strategy to elicit protective immune responses rapidly for mounting a quick response to a suspected outbreak to protect people who are at immediate risk. In this respect, several viral-vector-based Ebola vaccines hold a potential advantage as they have been shown to induce protective immune responses by a single immunization despite their perceived limitations on overcoming pre-existing immunity against viral vectors or safety concerns (Bukreyev et al., 2007; Jones et al., 2005; Sullivan et al., 2006). In contrast, while it has been shown that adjuvanted Ebola VLPs were able to protect guinea pigs against lethal Ebola challenge by a single immunization (Swenson et al., 2005), protection of non-human primates against lethal Ebola virus challenge was only shown by three immunizations with adjuvanted Ebola VLPs (Warfield et al., 2007a). Nonetheless, it is encouraging to note that the antibody responses induced after two immunizations reached similar levels as those detected after three immunizations in non-human primates (Warfield et al., 2007a). Our results show that immunization with a higher dose of unadjuvanted Ebola VLPs can induce strong immune responses against Ebola GP and confer complete protection against lethal Ebola virus challenge by two immunizations. Further investigations are needed to optimize the VLP vaccine dose, its formulation with an adjuvant, as well as the immunization regimen for achieving rapid protection against Ebola virus infection in non-human primates. Under the second scenario, it will require an Ebola vaccine strategy that can provide long-term protection for people who may be at constant risk of infection, such as researchers working with these highly dangerous viruses, health care providers working in endemic areas, as well as personnel to be deployed in such regions. We observed in this study that the sub-optimal immune responses induced by two immunizations with a lower dose of Ebola VLPs do not seem to benefit vaccinated animals against lethal Ebola virus challenge at all, with no reduction in serum viremia levels and no delay in time to death after challenge. Therefore, it will likely be necessary to give boosting immunizations for maintaining the levels of protective immune responses against Ebola virus infection. On the other hand, our results also demonstrate that the sub-optimal immune responses could be effectively boosted by an additional vaccination with Ebola VLPs which resulted in effective protection against lethal challenge by a high dose of Ebola virus. Thus, in the second scenario, Ebola VLP vaccines, which can be administered repeatedly to boost the levels of immune responses, will likely to be more advantageous compared to viral vector-based vaccines, which also induce immune responses against the vector that may limit their efficacy in boosting immunizations. It is also noted that the protective efficacy in the second challenge study, in which challenge was given at 12 weeks after the immunizations, seems to be less effective (as shown in Table 2). Similar results were also obtained with the re-challenged group. It is possible that immune responses at this later time point may become lower over time and need to be re-activated to control virus replication. This observation further underscores the need to give boosting immunizations for maintaining the levels of protective immune responses against Ebola virus infection.
Table 2.
Viremia level, weight loss, and survival rate after lethal Ebola virus challenge (II).
| Mouse | Antibody level | 50% NT* | viremia | Weight loss | Survival/death | |
|---|---|---|---|---|---|---|
| VLP-10-III | 1 | 25735 ng/ml | 1:8100 | ND | NC | survived |
| 2 | 11929 ng/ml | 1:2700 | ND | NC | survived | |
| 3 | 4535ng/ml | 1:900 | 3.33 × 107 | 17% | survived | |
| 4 | 3223 ng/ml | 1:900 | 2.51 × 107 | 15% | died | |
| 5 | 9370 ng/ml | 1:2700 | ND | NC | survived | |
| 6 | 7823 ng/ml | 1:2700 | 5.09 × 107 | 10% | survived | |
| VLP-50-II (re-challenge) | 1 | 10822 ng/ml | 1:900 | ND | NC | survived |
| 2 | 13070 ng/ml | 1:2700 | 3.7 × 106 | 10% | survived | |
| 3 | 22033 ng/ml | 1:8100 | ND | NC | survived | |
| 4 | 16668 ng/ml | 1:2700 | 5.2 × 106 | 12% | survived | |
| 5 | 16743 ng/ml | 1:2700 | ND | NC | survived | |
| 6 | 9959 ng/ml | 1:900 | ND | 10% | died |
Note. BALB/c mice were vaccinated Ebola VLPs and challenged at 12 weeks after the third immunization (VLP-10-III) or re-challenged at 12 weeks after the first challenge (VLP-50-II). Blood samples were collected on day 4 post challenge for determination of viremia by qRT-PCR. Mice were monitored for 21 days after challenge and the largest percentage of weight loss was presented.
ND: Not detected; NC: No change (<5%).
: The endpoint neutralizing titer is provided as the highest sera dilution that gives at least 50% neutralization of pseudovirions (50% NT).
In summary, Ebola VLPs produced in insect cells are able to induce strong antibody responses against Ebola GP that effectively neutralize GP-mediated virus infection. The immune responses induced by immunization with insect cell-derived Ebola VLPs are highly efficacious for protection against infection by Ebola virus. Further, when given in formulation with an adjuvant, Ebola VLP vaccine dose can be significantly reduced and still confer effective protection against lethal Ebola virus challenge (Warfield et al., 2007b). Given the high yield for production of VLPs in insect cells using the recombinant baculovirus expression system and the ease to scale-up the process for manufacturing under GMP conditions, the insect cell-produced Ebola VLPs represent an attractive and cost-effective approach for the development of an effective vaccine strategy against Ebola virus infection. Of note, the Ebola VLPs in current form are far from optimal. In characterization of Ebola VLPs, we found that the GP constitutes roughly 2% of the total protein in VLP preparations. While it is possible that the antibodies used in the quantitative ELISA analysis may be more reactive to purified GP that was prepared in mammalian cells and thus resulting in a lower estimation of the amount of GP in VLPs that were produced in insect cells, these results also indicate that the process for Ebola VLP production may be further optimized. Thus, additional studies on Ebola VLP assembly and release from insect cells are needed to improve the level of GP incorporation in VLPs that may enhance their immunogenicity. Moreover, VLPs are also highly versatile for manipulation during production to incorporate immune-stimulatory molecules (Guo et al., 2003, Sailaja et al., 2007; Skountzou et al., 2007), and similar strategies may also be applied to the design of Ebola VLP vaccines to augment induction of protective immune responses against Ebola virus infection. Future studies to optimize Ebola VLP design and formulation will lead to development of more potent VLP-based vaccines that may rapidly induce protective immune responses as shown by recombinant viral vector-based vaccines.
MATERIALS AND METHODS
Cells and antibodies
Spodoptera frugiperda Sf9 insect cells were cultured in SF-900 II serum-free medium with penicillin/streptomycin in suspension. Monoclonal antibodies against Ebola VP40 and GP proteins were kindly provided by Dr. Y. Kawaoka (Univ. Wisconsin, Madison.). Polyclonal antiserum against Ebola virus was kindly provided by Dr P. Rollin (CDC).
Virus and Biosafety
The mouse adapted Ebola virus Zaire strain was obtained from USAMRIID, which was originally obtained by Bray et al. through passage in suckling balb/c mouse (Bray et al., 1998). Mouse adapted Ebola Zaire stock was propagated in Vero E6 cells. The flasks were infected at an MOI of 0.01. On day seven post-infection, the virus was harvested and the infectious titer was determined by a plaque assay. All experiments involving infectious Ebola virus were performed at the maximum containment facility at the Southwest Foundation for Biomedical Research, San Antonio, Texas.
Production and characterization of Ebola VLPs
Generation of recombinant baculoviruses expressing Ebola VP40 (rBV-VP40) or GP (rBV-GP) proteins has been described previously (Ye et al., 2006). For VLP production, Sf9 cells (2 × 106/ml) were co-infected with rBV-VP40 and rBV-GP at MOIs (multiplicity of infection) of 2 and 5 respectively, and VLPs released into the medium were collected at 60 hr post infection. After clarification of cell debris, VLPs were concentrated by ultra-centrifugation and further purified through a discontinuous sucrose gradient (10–50%). Purified VLPs were then concentrated by ultra-centrifugation and re-suspended in PBS. Protein concentrations of VLPs as well as purified Ebola GP were determined using a Bradford assay kit as well as a BCA assay kit, which gave similar results in this study. The VLP preparations were adjusted by PBS, giving a final protein concentration of 1 ug/ul.
Purified VLPs were characterized by Coomassie blue as well as Western blot analysis for the presence of EBOV VP40 and GP proteins, and examined by electron microscopy under a Hitachi-H7500 transmission electron microscope by negative staining with 1% uranyl acetate, following established protocols described previously (Ye et al., 2006). The amount of Ebola GP incorporated in Ebola VLPs was determined by a quantitative Elisa. Briefly, 96-well plates were coated with an anti-GP monoclonal antibody overnight at 4 °C followed by wash and blocking with PBS-T (PBS plus 0.5% Tween-20) plus 2% BSA, and then addition of serial dilutions of VLPs in triplicates for 2 hr at room temperature. The plates were washed again and then incubated with rabbit antibodies against Ebola virus for 2 hr at room temperature, followed by addition of HPR-conjugated goat-anti-rabbit secondary antibodies and then development of color with TMB (Sigma). A standard curve was obtained by using known amounts of purified Ebola GP1-histag proteins, which were produced in HeLa cells using the recombinant vaccinia virus expression system as described previously (Ye et al., 2006), to determine the amount of Ebola GP in Ebola VLPs. After characterization, VLPs were stored in aliquots at −80 °C until use.
Immunization of mice, sample collection, and challenge
Female BALB/c mice (6–8 weeks old) were purchased from Charles River Laboratory and housed at the Emory University Animal Facility or the SFBR Animal Facility. All animal studies were carried out following approved IACUC protocols. Immunization of mice was carried out by intramuscular injection of VLP vaccines at both sides quadriceps at 4-week intervals using doses as indicated for each group. Blood samples were collected from the retro-orbital sinus under anesthesia at 2 weeks after each immunization, and stored at −80 °C until further analysis.
Lethal EBOV challenge studies were carried out in the BSL-4 animal facility at SFBR. After the final immunization, mice were challenged by intra peritoneal injection with 1000 pfu of mouse-adapted EBOV diluted in PBS. After challenge, mice were monitored daily for weight changes and signs of disease. Blood was collected on day 4 post-challenge for determination of viremia.
Isolation of total RNA and qRT-PCR
Total RNA from mouse blood was isolated using the Mouse RiboPure-Blood RNA isolation kit (Ambion) according to the manufacturer’s recommendations and used in a qRT-PCR assay. The qRT-PCR was performed using RNA UltraSense one-step qRT-PCR system (Invitrogen) using Ebola Zaire specific primers and Probe (forward primer: 5′-ACTGATCACAGGCGGGAGAA-3′; Probe: FAM-5′-AATTGTCAATGCTCAACCCAAA TGC- 3′-TAMRA; reverse primer: 5′-GCTGGCCCGAAATATGGTATC- 3′). Reactions (20 μl) contained reaction buffer, E-mix, BSA, 0.25 μM of each primer, 0.25 μM TaqMan probe, and total RNA from mouse samples. Amplifications were performed using a LightCycler 1.5 instrument (Roche) set at the default thermal profile (RT reaction at 45 °C for 30 min, Platinum Taq polymerase activation at 95 °C for 2 min, 45 cycles of denaturation at 95 °C for 15 sec and annealing/extension at 60 °C for 30 sec). Ebola Zaire GP RNA copy numbers (expressed as copies per ml of blood) in blood samples were quantified by extrapolating from the standard curve plot of Ct values generated similarly using reactions containing T7 in vitro transcribed Ebola Zaire GP RNA transcript prepared as 10-fold RNA dilution series.
ELISA
Ebola GP-specific antibodies were measured in individual mouse serum samples by an enzyme-linked immunoabsorbent assay (ELISA) using purified His-tagged GP1 proteins as coating antigens. Briefly, the assays were performed in 96-well polystyrene microtiter plates (Nunc) coated overnight at 4 °C with purified His-tagged GP1 and VP40 respectively at a concentration of 2ug/ml. Serial dilutions of serum samples were incubated at RT for 2 hrs on coated and blocked ELISA plates, and the bound immunoglobulins were detected with HRP-conjugated goat against mouse IgG, IgG1, IgG2a, or IgG 2b secondary antibodies (Southern Biotechnology Associates). The wells were developed with TMB (Sigma). The color reaction was stopped with hydrochloric acid (0.2N) and the absorbance at 450 nm was read in an EL312 Bio-Kinetics microplate reader (Bio-Tek Instruments Inc., Winooski, VT). A standard curve was constructed by coating each ELISA plate with serial 2-fold dilutions of purified mouse IgG, IgG1, IgG2a, or IgG2b with known concentrations respectively, and the concentrations of Ebola GP-specific antibodies in serum samples were calculated using obtained standard curves and expressed as the amount of antigen-specific antibody per ml of serum sample (ng/ml).
Detection of neutralizing activity against EBOV GP-pseudovirus
Neutralizing antibodies against Ebola GP were analyzed using a single-round infectivity assay as used in our previous studies (Ye et al., 2006) with slight modifications. Briefly, Ebola GP pseudotyped HIV was prepared by cotransfection of 293T cells with DNA vectors for the HIV backbone and the Ebola GP and the titer of released pseudovirions was determined in the JC53BL cells by staining for β-galactosidase expressing cells. For analysis of neutralizing antibodies, serial 3-fold dilutions of heat-inactivated serum samples were incubated with 500 pfu of Ebola GP-HIV pseudovirions for 1 h at 37 °C, and then added to JC53BL cells seeded in a 96-well plate in the presence of DEAE-dextran. The JC53BL cells in control wells were infected with 500 pfu of Ebola GP-HIV pseudovirions in the absence of mouse sera. After incubation at 37 °C for 2 days, 5 ul of 20% Nonidet P-40 (V/V) was added to each well to lyse the cells, and the level of β-galactosidase activity in each well was determined as described previously (Yang and Compans, 1997). Briefly, 50 ul of cell lysate from each well with 50 ul of CPRG (chlorophenol red-3-D-galactopyranoside) substrate solution (16 mM CPRG, 0.12 M Na2HP04, 0.08 M NaH2PO4, 0.02M KCI, 0.002 M MgSO4, 0.01 M β-mercaptoethanol) for 30 min at room temperature followed by measuring the O.D. value at 590 nm with an ELISA reader. A standard curve was constructed by infecting JC53BL cells with serial dilutions of Ebola GP-HIV pseudovirions for calculation of virus titers in each sample well. Neutralizing activity is expressed as the percentage reduction of virus titers in sample wells compared to the titers in control wells without mouse sera [(virus titer in control well-virus titer in sample well)/virus titer in control well × 100%]. The endpoint neutralizing titer is provided as the highest sera dilution that gives at least 50% neutralization of pseudovirions (50% NT).
Acknowledgments
This work was supported by Public Service Grants from the National Institute of Health (AI053514, AI057266, AI048638, AI056499, DK057665, AI056957, AI057157), the National Institutes of Health (NIH) for Regional Centers of Excellence for Biodefense and EmergingInfectious Diseases (grants U54 AI057156 and U54 AI57168), and by an NIH laboratory construction grant (1C06RR12087). We thank Robert Geiger, Michelle Reynolds, and April Villar for technical support and helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aman MJ, Bosio CM, Panchal RG, Burnett JC, Schmaljohn A, Bavari S. Molecular mechanisms of filovirus cellular trafficking. Microbes Infect. 2003;5:639–49. doi: 10.1016/s1286-4579(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Bavari S, Bosio CM, Wiegand E, Ruthel G, Will AB, Geisbert TW, Hevey M, Schmaljohn C, Schmaljohn A, Aman MJ. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J Exp Med. 2002;195:593–602. doi: 10.1084/jem.20011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti-Ciarlet A, Ciarlet M, Crawford SE, Conner ME, Estes MK. Immunogenicity and protective efficacy of rotavirus 2/6-virus-like particles produced by a dual baculovirus expression vector and administered intramuscularly, intranasally, or orally to mice. Vaccine. 2003;21:3885–900. doi: 10.1016/s0264-410x(03)00308-6. [DOI] [PubMed] [Google Scholar]
- Bray M, Davis K, Geisbert T, Schmaljohn C, Huggins J. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J Infect Dis. 1998;178:651–61. doi: 10.1086/515386. [DOI] [PubMed] [Google Scholar]
- Bray M. Defense against filoviruses used as biological weapons. Antiviral Res. 2003;57:53–60. doi: 10.1016/s0166-3542(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Bukreyev A, Rollin PE, Tate MK, Yang L, Zaki SR, Shieh WJ, Murphy BR, Collins PL, Sanchez A. Successful topical respiratory tract immunization of primates against Ebola virus. J Virol. 2007;81:6379–88. doi: 10.1128/JVI.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bwaka MA, Bonnet MJ, Calain P, Colebunders R, De Roo A, Guimard Y, Katwiki KR, Kibadi K, Kipasa MA, Kuvula KJ, Mapanda BB, Massamba M, Mupapa KD, Muyembe-Tamfum JJ, Ndaberey E, Peters CJ, Rollin PE, Van den Enden E, Van den Enden E. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis. 2007;179 Suppl 1:S1–S7. doi: 10.1086/514308. [DOI] [PubMed] [Google Scholar]
- Crum CP, Rivera MN. Vaccines for cervical cancer. Cancer J. 2003;9:368–76. doi: 10.1097/00130404-200309000-00006. [DOI] [PubMed] [Google Scholar]
- Groseth A, Feldmann H, Strong JE. The ecology of Ebola virus. Trends Microbiol. 2007;5:408–16. doi: 10.1016/j.tim.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Guo L, Lu X, Kang SM, Chen C, Compans RW, Yao Q. Enhancement of mucosal immune responses by chimeric influenza HA/SHIV virus-like particles. Virology. 2003;313:502–13. doi: 10.1016/s0042-6822(03)00372-6. [DOI] [PubMed] [Google Scholar]
- Hart MK. Vaccine research efforts for filoviruses. Int J Parasitol. 2003;33:583–95. doi: 10.1016/s0020-7519(03)00064-x. [DOI] [PubMed] [Google Scholar]
- Hartlieb B, Weissenhorn W. Filovirus assembly and budding. Virology. 2006;34:64–70. doi: 10.1016/j.virol.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Chiu W. Structures of virus and virus-like particles. Curr Opin Struct Biol. 2000;10:229–35. doi: 10.1016/s0959-440x(00)00073-7. [DOI] [PubMed] [Google Scholar]
- Jones SM, Feldmann H, Ströher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, Daddario KM, Hensley LE, Jahrling PB, Geisbert TW. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11:786–90. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, Délicat A, Paweska JT, Gonzalez JP, Swanepoel R. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–6. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- Li TC, Yamakawa Y, Suzuki K, Tatsumi M, Razak MA, Uchida T, Takeda N, Miyamura T. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J Virol. 1997;71:7207–13. doi: 10.1128/jvi.71.10.7207-7213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata JM, Johnson RF, Han Z, Harty RN. Contribution of ebola virus glycoprotein, nucleoprotein, and VP24 to budding of VP40 virus-like particles. J Virol. 2004;78:7344–51. doi: 10.1128/JVI.78.14.7344-7351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellquist-Riemenschneider JL, Garrison AR, Geisbert JB, Saikh KU, Heidebrink KD, Jahrling PB, Ulrich RG, Schmaljohn CS. Comparison of the protective efficacy of DNA and baculovirus-derived protein vaccines for EBOLA virus in guinea pigs. Virus Res. 2003;92:187–93. doi: 10.1016/s0168-1702(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Noda T, Sagara H, Suzuki E, Takada A, Kida H, Kawaoka Y. Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP. J Virol. 2002;76:4855–65. doi: 10.1128/JVI.76.10.4855-4865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Oh YK, Kang MJ, Kim CK. Enhanced mucosal and systemic immune responses following intravaginal immunization with human papillomavirus 16 L1 virus-like particle vaccine in thermosensitive mucoadhesive delivery systems. J Med Virol. 2003;70:633–41. doi: 10.1002/jmv.10442. [DOI] [PubMed] [Google Scholar]
- Peters CJ, LeDuc JW. An introduction to Ebola: the virus and the disease. J Infect Dis. 1999;179 Suppl 1:ix–xvi. doi: 10.1086/514322. [DOI] [PubMed] [Google Scholar]
- Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005;23:5751–9. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- Sailaja G, Skountzou I, Quan FS, Compans RW, Kang SM. Human immunodeficiency virus-like particles activate multiple types of immune cells. Virology. 2007;362:331–41. doi: 10.1016/j.virol.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skountzou I, Quan FS, Gangadhara S, Ye L, Vzorov A, Selvaraj P, Jacob J, Compans RW, Kang SM. Incorporation of glycosylphosphatidylinositol-anchored granulocyte- macrophage colony-stimulating factor or CD40 ligand enhances immunogenicity of chimeric simian immunodeficiency virus-like particles. J Virol. 2007;81:1083–94. doi: 10.1128/JVI.01692-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–9. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZY, Roederer M, Koup RA, Jahrling PB, Nabel GJ. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424:681–4. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan NJ, Geisbert TW, Geisbert JB, Shedlock DJ, Xu L, Lamoreaux L, Custers JH, Popernack PM, Yang ZY, Pau MG, Roederer M, Koup RA, Goudsmit J, Jahrling PB, Nabel GJ. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med. 2006;3:e177. doi: 10.1371/journal.pmed.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson DL, Warfield KL, Negley DL, Schmaljohn A, Aman MJ, Bavari S. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine. 2005;23:3033–42. doi: 10.1016/j.vaccine.2004.11.070. [DOI] [PubMed] [Google Scholar]
- Towner JS, Pourrut X, Albariño CG, Nkogue CN, Bird BH, Grard G, Ksiazek TG, Gonzalez JP, Nichol ST, Leroy EM. Marburg virus infection detected in a common African bat. PLoS ONE. 2007;2:e764. doi: 10.1371/journal.pone.0000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield KL, Bosio CM, Welcher BC, Deal EM, Mohamadzadeh M, Schmaljohn A, Aman MJ, Bavari S. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci U S A. 2003;100:15889–94. doi: 10.1073/pnas.2237038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield KL, Olinger G, Deal EM, Swenson DL, Bailey M, Negley DL, Hart MK, Bavari S. Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J Immunol. 2005;175:1184–91. doi: 10.4049/jimmunol.175.2.1184. [DOI] [PubMed] [Google Scholar]
- Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis. 2007a;196 Suppl 2:S430–7. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- Warfield KL, Posten NA, Swenson DL, Olinger GG, Esposito D, Gillette WK, Hopkins RF, Costantino J, Panchal RG, Hartley JL, Aman MJ, Bavari S. Filovirus-like particles produced in insect cells: immunogenicity and protection in rodents. J Infect Dis. 2007b;196 Suppl 2:S421–9. doi: 10.1086/520612. [DOI] [PubMed] [Google Scholar]
- Yang C, Compans RW. Analysis of the murine leukemia virus R peptide: delineation of the molecular determinants which are important for its fusion inhibition activity. J Virol. 1997;71:8490–6. doi: 10.1128/jvi.71.11.8490-8496.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Bu Z, Vzorov A, Yang C, Compans RW. Virus-like particle and DNA-based candidate AIDS vaccines. Vaccine. 2003;21:638–43. doi: 10.1016/s0264-410x(02)00572-8. [DOI] [PubMed] [Google Scholar]
- Ye L, Lin J, Sun Y, Bennouna S, Lo M, Wu Q, Bu Z, Pulendran B, Compans RW, Yang C. Ebola virus-like particles produced in insect cells exhibit dendritic cell stimulating activity and induce neutralizing antibodies. Virology. 2006;352:74–85. doi: 10.1016/j.virol.2006.03.021. [DOI] [PubMed] [Google Scholar]