Abstract
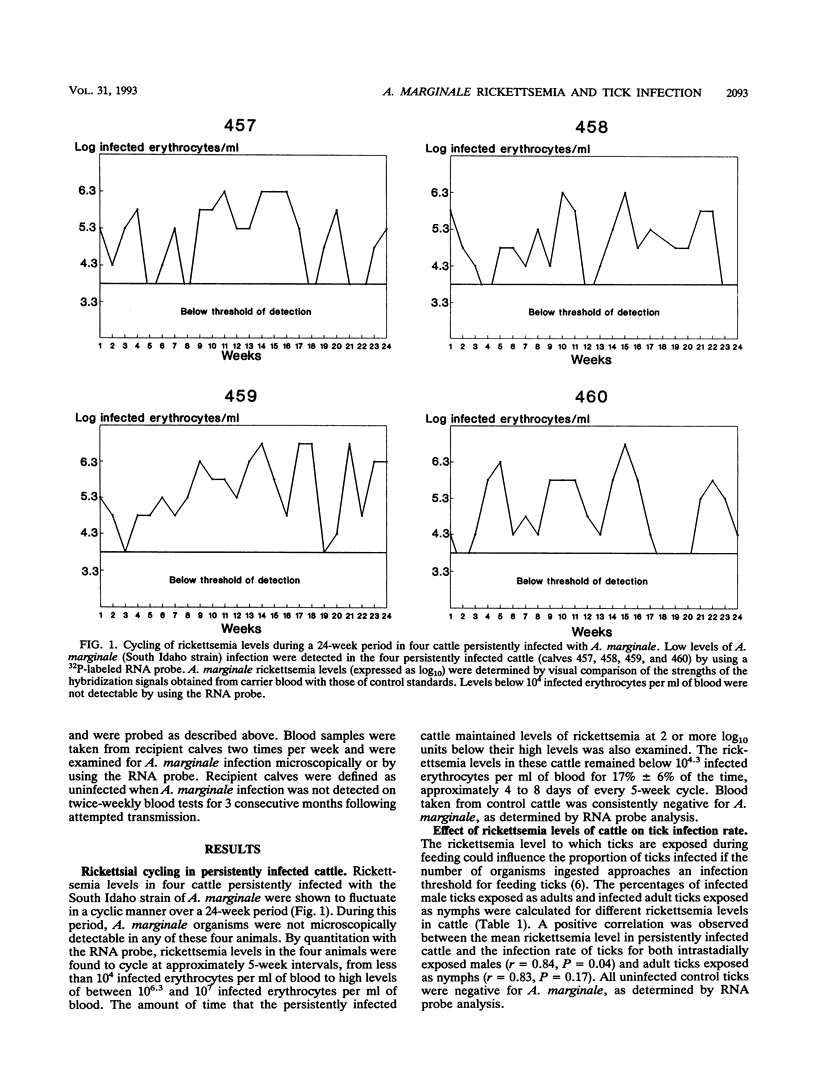
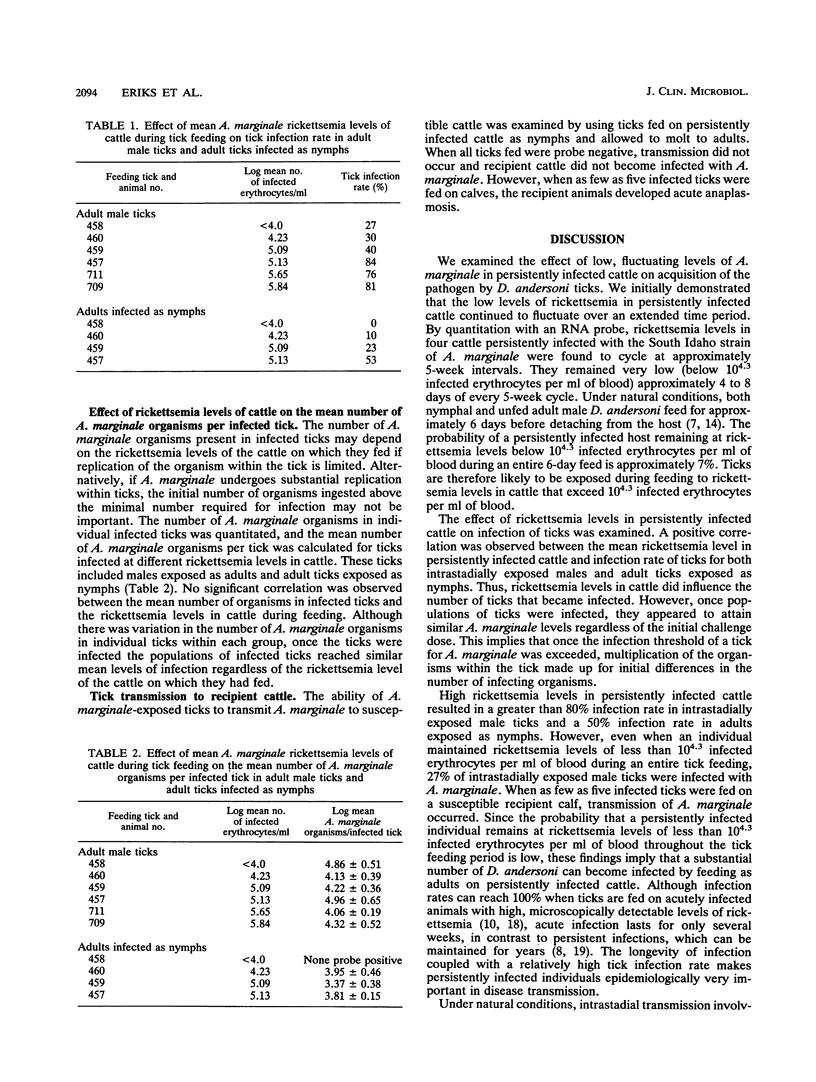
Anaplasma marginale, an intraerythrocytic rickettsia of cattle, is transmitted biologically by ticks. Because of the brevity of acute A. marginale infection, transmission may rely on the tick's ability to acquire the organism from persistently infected cattle with low rickettsemia levels. By using a nucleic acid probe to quantitate low-level infection, we found that rickettsemia levels in persistently infected cattle fluctuated at approximately 5-week intervals during a 24-week period, from < 10(4) infected erythrocytes per ml of blood to high levels of approximately 10(7) infected erythrocytes per ml of blood. Cattle maintained very low rickettsemia levels (< 10(4.3) infected erythrocytes per ml of blood) for approximately 4 to 8 days of every 5-week cycle. The effect of fluctuations in rickettsemia in persistently infected cattle on acquisition by Dermacentor andersoni nymphal and adult male ticks was examined. A positive correlation was observed between rickettsemia levels in cattle and the resulting infection rates of ticks. At high rickettsemia levels, up to 80% of ticks acquired infection, but even at extremely low rickettsemia levels, 27% of adult male ticks became infected. Moreover, once ticks acquired infection, biological replication of the organism within the ticks appeared to make up for initial differences in the infecting dose. The high infection rates in adult males, combined with their intermittent feeding behavior and the observation that only a few infected ticks were required for transmission to a susceptible host, suggest that adult male D. andersoni ticks are epidemiologically important in A. marginale transmission. Because cattle with all levels of rickettsemia were capable of efficient transmission to ticks, population control efforts must include decreasing transmission from persistently infected individuals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown K. N., Brown I. N. Immunity to malaria: antigenic variation in chronic infections of Plasmodium knowlesi. Nature. 1965 Dec 25;208(5017):1286–1288. doi: 10.1038/2081286a0. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Schwan T. G. Lyme borreliosis: a relapsing fever-like disease? Scand J Infect Dis Suppl. 1991;77:17–22. [PubMed] [Google Scholar]
- Davies C. R., Jones L. D., Nuttall P. A. A comparative study of the infection thresholds of Thogoto virus in Rhipicephalus appendiculatus and Amblyomma variegatum. Am J Trop Med Hyg. 1990 Jul;43(1):99–103. doi: 10.4269/ajtmh.1990.43.99. [DOI] [PubMed] [Google Scholar]
- Eriks I. S., Palmer G. H., McGuire T. C., Allred D. R., Barbet A. F. Detection and quantitation of Anaplasma marginale in carrier cattle by using a nucleic acid probe. J Clin Microbiol. 1989 Feb;27(2):279–284. doi: 10.1128/jcm.27.2.279-284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff W. L., Johnson W. C., Kuttler K. L. Development of an indirect fluorescent antibody test, using microfluorometry as a diagnostic test for bovine anaplasmosis. Am J Vet Res. 1985 May;46(5):1080–1084. [PubMed] [Google Scholar]
- Goff W. L., Stiller D., Roeder R. A., Johnson L. W., Falk D., Gorham J. R., McGuire T. C. Comparison of a DNA probe, complement-fixation and indirect immunofluorescence tests for diagnosing Anaplasma marginale in suspected carrier cattle. Vet Microbiol. 1990 Sep;24(3-4):381–390. doi: 10.1016/0378-1135(90)90185-x. [DOI] [PubMed] [Google Scholar]
- Goff W., Barbet A., Stiller D., Palmer G., Knowles D., Kocan K., Gorham J., McGuire T. Detection of Anaplasma-marginale-infected tick vectors by using a cloned DNA probe. Proc Natl Acad Sci U S A. 1988 Feb;85(3):919–923. doi: 10.1073/pnas.85.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser S. T., Eriks I. S., Palmer G. H. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect Immun. 1990 Apr;58(4):1117–1119. doi: 10.1128/iai.58.4.1117-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocan K. M., Barron S. J., Ewing S. A., Hair J. A. Transmission of Anaplasma marginale by adult Dermacentor andersoni during feeding on calves. Am J Vet Res. 1985 Jul;46(7):1565–1567. [PubMed] [Google Scholar]
- Kocan K. M., Barron S. J., Holbert D., Ewing S. A., Hair J. A. Influence of increased temperature on Anaplasma marginale Theiler in the gut of Dermacentor andersoni stiles. Am J Vet Res. 1982 Jan;43(1):32–35. [PubMed] [Google Scholar]
- Kocan K. M., Hair J. A., Ewing S. A., Stratton L. G. Transmission of Anaplasma marginale Theiler by Dermacentor andersoni Stiles and Dermacentor variabilis (Say). Am J Vet Res. 1981 Jan;42(1):15–18. [PubMed] [Google Scholar]
- Kocan K. M., Stiller D., Goff W. L., Claypool P. L., Edwards W., Ewing S. A., McGuire T. C., Hair J. A., Barron S. J. Development of Anaplasma marginale in male Dermacentor andersoni transferred from parasitemic to susceptible cattle. Am J Vet Res. 1992 Apr;53(4):499–507. [PubMed] [Google Scholar]
- Luedke A. J., Jones R. H., Walton T. E. Overwintering mechanism for bluetongue virus: biological recovery of latent virus from a bovine by bites of Culicoides variipennis. Am J Trop Med Hyg. 1977 Mar;26(2):313–325. doi: 10.4269/ajtmh.1977.26.313. [DOI] [PubMed] [Google Scholar]
- Mahoney D. F. Bovine babesiasis: a study of factors concerned in transmission. Ann Trop Med Parasitol. 1969 Mar;63(1):1–14. doi: 10.1080/00034983.1969.11686595. [DOI] [PubMed] [Google Scholar]
- Norval R. A., Andrew H. R., Yunker C. E. Pheromone-mediation of host-selection in bont ticks (Amblyomma hebraeum koch). Science. 1989 Jan 20;243(4889):364–365. doi: 10.1126/science.2911745. [DOI] [PubMed] [Google Scholar]
- Rurangirwa F. R., Musoke A. J., Nantulya V. M., Nkonge C., Njuguna L., Mushi E. Z., Karstad L., Grootenhuis J. Immune effector mechanisms involved in the control of parasitaemia in Trypanosoma brucei-infected wildebeest (Connochaetes taurinus). Immunology. 1986 Jun;58(2):231–237. [PMC free article] [PubMed] [Google Scholar]
- Salinovich O., Payne S. L., Montelaro R. C., Hussain K. A., Issel C. J., Schnorr K. L. Rapid emergence of novel antigenic and genetic variants of equine infectious anemia virus during persistent infection. J Virol. 1986 Jan;57(1):71–80. doi: 10.1128/jvi.57.1.71-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller D., Kocan K. M., Edwards W., Ewing S. A., Barron J. A. Detection of colonies of Anaplasma marginale in salivary glands of three Dermacentor spp infected as nymphs or adults. Am J Vet Res. 1989 Aug;50(8):1381–1385. [PubMed] [Google Scholar]
- Takahashi M., Murata M., Sasaki M., Saito T., Machida K., Hori E., Tanaka H., Kawamura A., Jr Resistance to Rickettsia tsutsugamushi and persistence of infection in different wild rodents. Jpn J Exp Med. 1990 Aug;60(4):241–245. [PubMed] [Google Scholar]
- Young A. S., Leitch B. L., Newson R. M., Cunningham M. P. Maintenance of Theileria parva parva infection in an endemic area of Kenya. Parasitology. 1986 Aug;93(Pt 1):9–16. doi: 10.1017/s0031182000049787. [DOI] [PubMed] [Google Scholar]



