Abstract
Context:
Sodium replacement during prolonged exercise in the heat may be critically important to maintaining fluid and electrolyte balance and muscle contractility.
Objective:
To examine the effectiveness of sodium-containing sports drinks in preventing hyponatremia and muscle cramping during prolonged exercise in the heat.
Design:
Randomized crossover study.
Patients or Other Participants:
Thirteen active men.
Intervention(s):
Participants completed 4 trials of an exercise protocol in the heat (30°C) consisting of 3 hours of exercise (alternating 30 minutes of walking and cycling at a heart rate of 130 and 140 beats per minute, respectively); a set of standing calf raises (8 sets of 30 repetitions); and 45 minutes of steep, brisk walking (5.5 km⋅h−1 on a 12% grade). During exercise, participants consumed fluids to match body mass loss. A different drink was consumed for each trial: carbohydrate-electrolyte drink containing 36.2 mmol/L sodium (HNa), carbohydrate-electrolyte drink containing 19.9 mmol/L sodium (LNa), mineral water (W), and colored and flavored distilled water (PL).
Main Outcome Measure(s):
Serum sodium, plasma osmolality, plasma volume changes, and muscle cramping frequency.
Results:
During both HNa and LNa trials, serum sodium remained relatively constant (serum sodium concentration at the end of the protocol was 137.3 mmol/L and 136.7 mmol/L, respectively). However, a clear decrease was observed in W (134.5 ± 0.8 mmol/L) and PL (134.4 ± 0.8 mmol/L) trials compared with HNa and LNa trials (P < .05). The same trends were observed for plasma osmolality (P < .05). Albeit not significant, plasma volume was preserved during the HNa and LNa trials, but a reduction of 2.5% was observed in the W and PL trials. None of the volunteers experienced cramping.
Conclusions:
The data suggest that sodium intake during prolonged exercise in the heat plays a significant role in preventing sodium losses that may lead to hyponatremia when fluid intake matches sweat losses.
Keywords: endurance, fluid replacement, hydration, hyponatremia, plasma volume, sports drinks
Key Points.
Under environmental conditions in which fluid intake matched body mass loss, relatively moderate amounts of sodium added to the hydration solution attenuated the decline in sodium concentration of plasma and preserved plasma volume at pre-exercise levels.
Sodium supplementation did not increase serum sodium even when participants consumed a carbohydrate-electrolyte drink containing 36.2 mmol/L sodium.
A relationship between sodium supplementation and the prevention of exercise-associated muscle cramps cannot be established from the environmental and exercise factors of this study.
Further research using appropriate exercise protocols is needed to clarify the effect of sodium supplementation on the propensity to cramp during exercise.
Exercising in the heat often leads to dehydration. Dehydration may increase cardiovascular strain by disproportionately elevating heart rate with a concomitant reduction in cardiac output,1 may decrease the body's ability to dissipate heat,2 and may ultimately hamper performance during endurance exercise.3,4 Researchers have found that the sodium content of rehydration solutions provided after exercise-induced dehydration is a significant factor in the restoration of body water loss, especially plasma volume.5,6 It has also been demonstrated that administration of high-sodium solutions results in a selective restoration of plasma volume at even higher levels than predehydration values.5,6 When investigators included small quantities of sodium in fluid-replacement drinks during exercise, they did not find consistent improvements in retention of ingested fluid in the vascular compartment, reporting either a better maintenance of plasma volume7,8 or no effect.9–11 Possibly, the sodium test drinks in these studies failed to produce a clear difference in plasma-sodium concentration compared with control drinks because of the small quantities of the sodium provided7 or because of the exercise protocol's short duration.9 Maintenance of plasma sodium during prolonged exercise would theoretically benefit the athlete because it would promote the stabilization of plasma volume and would limit the cardiovascular strain of exercise.
Given the increasing popularity of long-lasting endurance events, researchers have focused on the effects of drinking patterns (both quantity and composition of sports drinks) on exertional hyponatremia. Although severe hyponatremia is commonly associated with ultra-endurance sport events, a high incidence of mild hyponatremia also has been documented in sports of shorter duration, such as the marathon.12 Proposed mechanisms for exercise-induced hyponatremia include excessive sodium loss from sweating; ingestion of low-sodium fluids and solid foods during exercise13; overload of the extracellular fluids by excessive fluid intake13–15; impairment of renal function13; failure to suppress antidiuretic hormone secretion14,15; and the use of medications, such as nonsteroidal anti-inflammatory drugs.13
The efficacy of sodium supplementation in preventing hyponatremia during exercise has not been systematically examined. Twerenbold et al16 showed that consumption of sodium-containing drinks in excess of fluid needs during a 4-hour run decreased the incidence of hyponatremia compared with overconsumption of sodium-free fluids. Vrijens and Rehrer17 found that sodium-free fluid intake during 3 hours of low-intensity exercise at a rate to match fluid loss decreased plasma sodium compared with ingestion of a carbohydrate-electrolyte drink. However, Speedy et al18 and Hew-Butler et al19 reported that large amounts of sodium supplemented in the form of tablets (700 mg/h and 40 tablets that each contained 620 mg of table salt) during the Ironman triathlon did not confer any advantage in the preservation of serum sodium. It is important to note that in these studies, participants had a weight loss of about 4% at the end of the race.
Another incident that could limit endurance performance is muscle cramps. Although experimental data are lacking, proposed theories for the cause of exercise-associated cramping include abnormalities in substrate metabolism, fluid balance, and electrolyte concentrations, especially under extreme environmental conditions of heat or cold.20 Consumption of a carbohydrate-electrolyte solution has been shown to delay the onset of exercise-associated muscle cramping during calf-fatiguing exercise compared with no fluid ingestion.21 In a study of athletes participating in an ultra-endurance run, Schwellnus et al22 found that plasma sodium concentration at the end of the race was lower in those runners who experienced muscle cramping than in those who did not, although at nonhyponatremic values. In addition, Stofan et al23 showed that football players with a history of cramping had larger exercise-induced sodium losses compared with players who had no history of cramps. However, such experimental protocols do not allow conclusions to be drawn about the effect of electrolyte or water balance on the development of muscle cramps.
The purpose of our study was to examine the effectiveness of drinks with different sodium concentrations in maintaining plasma volume and preventing hyponatremia during long-duration exercise in the heat when fluid supplementation matches fluid lost to sweating. We hypothesized that a high-sodium drink would maintain plasma sodium concentration, resulting in preservation of plasma volume. Additionally, we hypothesized that a higher plasma sodium concentration would minimize cramping and related symptoms, such as pain and muscle stiffness.
Methods
Participants
Thirteen healthy, physically active, untrained men (age = 24.5 ± 2.1 years, height = 178 ± 8 cm, mass = 77.9 ± 10.4 kg, body fat = 17.4 ± 5.4%) were recruited to participate in the study. Each participant gave written informed consent, and the protocol was approved by the institutional review board. Eligibility criteria for participation in the study included a normal physical examination; absence of any metabolic, cardiovascular, or renal disease; and normal body weight and fat for height (body mass index <25 kg/m2). Body composition was assessed by dual-energy x-ray absorptiometry (model DPX-MD; GE Lunar Corp, Madison, WI).
Experimental Protocol
We used a randomized crossover design with 4 different test drinks during exercise. The 4 test drinks consisted of the following: (1) a low-sodium carbohydrate drink (LNa, 19.9 mmol/L sodium) (Gatorade Endurance; PepsiCo Inc, Purchase, NY); (2) a high-sodium carbohydrate drink (HNa, 36.2 mmol/L sodium) (European Gatorade Thirst Quencher; PepsiCo Inc); (3) artificially sweetened, flavored, and colored distilled water (PL) that was calorie free and sodium free (Crystal Light; Kraft Foods Inc, Northfield, IL); and (4) mineral water (W) with trace amounts of sodium (<0.3 mmol/L of sodium). The LNa drink and HNa drink contained 6% (6 g per 100 mL) carbohydrates and 3.2 mmol/L potassium and 9.6 mmol/L potassium, respectively.
All trials were performed between 8 and 10 am (within a designated hour for each participant) after an overnight fast of at least 10 hours and at an ambient temperature of approximately 30°C. Trials were separated by at least 1 week. Participants were instructed to refrain from vigorous physical activity the day before each trial, to drink liberally, and to consume a high-carbohydrate diet. At the end of the first trial, participants noted the evening meal consumed on the previous day and were instructed to consume the same evening meal at the same time on the day before each subsequent trial.
On their arrival at the laboratory, participants were instructed to void their bladders, and a venous catheter was placed into a forearm vein and kept patent by periodic flushing with heparinized saline solution (20 U/mL). Special attention was given to preventing a substantial amount of heparin from entering the circulation. After 20 minutes of rest, blood samples were collected, and participants began to exercise.
The first phase of the exercise protocol included 30-minute intervals alternating between walking and cycling for 3 hours to induce sweating sodium loss. Each 30-minute period consisted of 25 minutes of exercise and 5 minutes of rest. The intensity of exercise was adjusted to elicit a heart rate of 130 and 140 beats per minute during cycling and walking, respectively. Body mass was recorded at the beginning of the protocol and immediately after every 25-minute period of exercise. Body mass loss was replenished by an equal amount of the test drink.
The second phase of the exercise protocol included 8 sets of standing calf raises (30 repetitions per minute) in 1∶1 minutes-of-exercise-to-rest intervals. This protocol has been shown to induce glycogen depletion of the gastrocnemius muscles by 75%.24 After completion of this phase, body mass loss was also recorded, and an equal amount of fluid was provided.
The third phase of the protocol included steep walking at 5.5 km/h on a 12% grade on a treadmill for 45 minutes. During this phase, drinks were provided at a rate of 150 mL every 15 minutes. This rate was chosen because it is within the range of guidelines for fluid replenishment of the American College of Sports Medicine25 and because preliminary testing showed that this fluid supply is sufficient to prevent sweat-induced body mass reduction at the given exercise and environmental conditions.
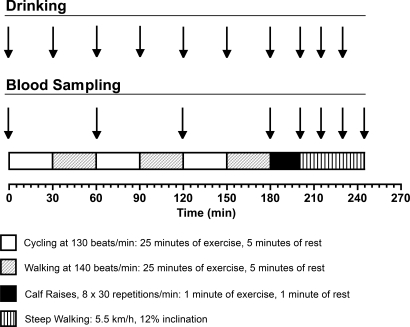
Blood samples were collected every 60 minutes during phase 1 of exercise, at the end of phase 2, and every 15 minutes during phase 3. The experimental design of the study is presented schematically in Figure 1. Participants were asked to report any muscle cramping or precramping symptoms, such as deep pain and stiffness, during the last 2 phases of the exercise protocol.
Figure 1. Schematic presentation of the study's experimental design.
Analytical Measurements
Blood samples (approximately 15 mL in each draw) were analyzed immediately in triplicate for hematocrit (microhematocrit method) and hemoglobin (cyanmethemoglobin method, Drabkin reagent; Sigma-Aldrich, St Louis, MO), and the Dill and Costill26 equation was used to calculate changes in plasma volume. Lactate was also measured in whole blood in duplicate (Accutrend Lactate system; Roche Diagnostics, Mannheim, Germany). In the remaining blood, serum and plasma were separated from the blood cells by centrifugation (10 000 rpm for 10 minutes at 4°C). Aliquots of blood plasma were used fresh for the determination of plasma osmolality by freezing-point depression (3D3 Osmometer; Advanced Instruments Inc, Norwood, MA). The remaining blood plasma and serum were stored frozen (−80°C) for subsequent analysis of plasma glucose and free fatty acids, serum sodium, potassium, and aldosterone. Glucose and free fatty acids were measured by enzymatic analysis in an automated biochemical analyzer (ACE; Schiapparelli Biosystems Inc, Fairfield, NJ), and electrolytes were measured by selective electrode conductivity in an automated analyzer (Ektachem DT60 II system; Eastman Kodak Co, Rochester, NY). Aldosterone was measured by a radioimmunoassay kit (Coat-A-Count Aldosterone; Siemens, Los Angeles, CA).
Statistical Analysis
Data were analyzed using analysis of variance with repeated measures with 2 within-subjects factors (drink, time). When a main effect was detected, we used the Tukey honest significant difference test to perform post hoc comparisons. The α level was set at .05. Values are presented as mean ± 1 SEM.
Results
The amount of fluid provided was the same in all trials and resulted in body mass stability throughout the exercise protocol, as required by the study design. Mean cumulative fluid intake was 2108 ± 209 mL for HNa, 2008 ± 245 mL for LNa, 2015 ± 323 mL for PL, and 1931 ± 213 mL for W (F3 = 0.585, P = .629).
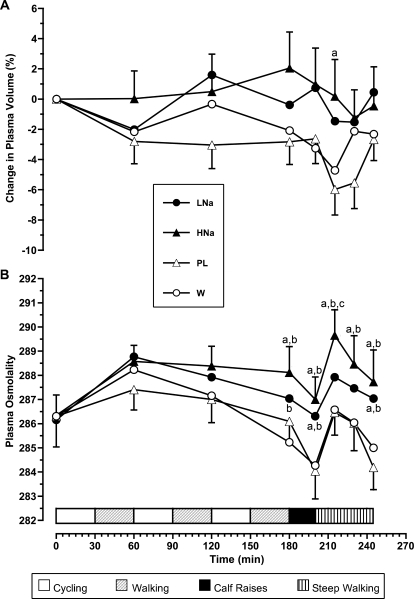
Figure 2 shows changes in plasma volume and osmolality during the experiment. Sodium supplementation during HNa and LNa trials resulted in stable plasma volume (changes at the end of the protocol were −0.5 and 0.5%). In the PL and W trials, plasma volume tended to decrease over time, reaching −2.7% and −2.3% at the end of the protocol (P > .05). After the first 15 minutes of steep walking, plasma volume was greater for the HNa trial than for the PL trial (P = .003). Sodium-containing drinks also caused a small increase in plasma osmolality, whereas in PL and W trials, we noted a tendency for decrease at the end of calf raises and at the end of the whole protocol. Plasma osmolality values were different for HNa compared with W and PL at the end of phase 1 and thereafter (P < .001 for both comparisons). Similar trends were shown for LNa, but differences were significant only at the end of phases 2 and 3 for both comparisons (P < .001). The HNa drink resulted in slightly higher plasma osmolality values compared with the LNa drink, with the difference being significant only at 15 minutes into phase 3 (P = .012).
Figure 2. Changes in A, plasma volume and B, plasma osmolality during the experiment. Values are means ± SEs. Abbreviations: LNa, carbohydrate-electrolyte drink containing 19.9 mmol/L sodium; HNa, carbohydrate-electrolyte drink containing 36.2 mmol/L sodium; PL, colored and flavored distilled water; W, mineral water. a Denotes difference compared with colored and flavored distilled water trial (P < .05). b Denotes difference compared with mineral water trial (P < .05). c Denotes difference compared with carbohydrate-electrolyte drink containing 19.9 mmol/L sodium (P < .05).
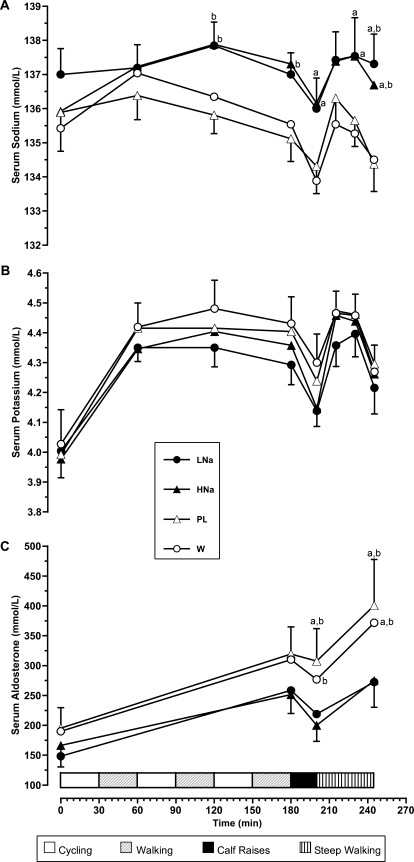
Serum sodium concentration showed a similar pattern to that seen with plasma osmolality, but no differences were observed for LNa and HNa trials (Figure 3A). During PL and W trials, participants experienced reductions in serum sodium concentrations, reaching values less than 135 mmol/L at the end of the exercise protocol (PL versus LNa, P = .001; PL versus HNa, P = .064; W versus LNa, P < .001; W versus HNa, P = .018). No differences were observed for serum potassium among trials at any time (Figure 3B). Serum aldosterone increased over time, with a more profound increase during the PL and W trials (Figure 3C). Aldosterone responses between sodium supplementation trials (LNa and HNa) were not different during the whole experiment.
Figure 3. A, Serum sodium; B, serum potassium; and C, serum aldosterone concentrations during the experiment. Values are means ± SEs. Abbreviations: LNa, carbohydrate-electrolyte drink containing 19.9 mmol/L sodium; HNa, carbohydrate-electrolyte drink containing 36.2 mmol/L sodium; PL, colored and flavored distilled water; W, mineral water. a Denotes difference compared with colored and distilled flavored water trial (P < .05). b Denotes difference compared with mineral water trial (P < .05).
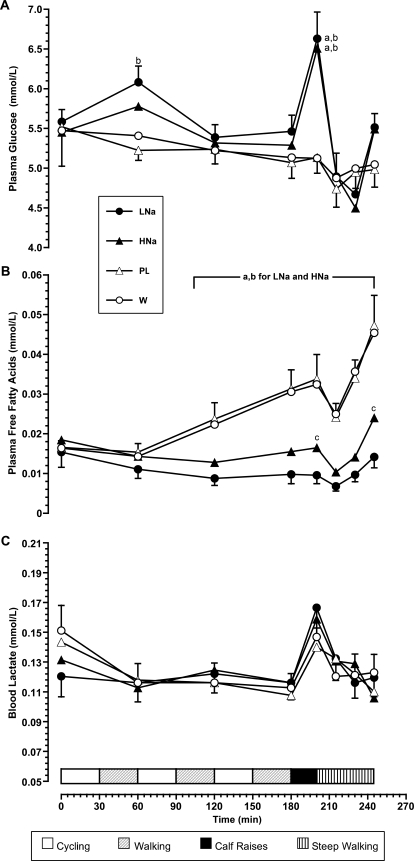
Figure 4 depicts plasma glucose, plasma free fatty acids, and blood lactate values. The LNa and HNa drinks, which contained carbohydrates, produced higher plasma glucose values at the end of phase 2 (P < .05) than W or PL. At the end of the whole protocol, glucose values for PL and W trials were slightly lower than initial values and LNa and HNa trials, but differences were not significant (P > .05). Marked fatty acid mobilization was observed for PL and W trials at 120 minutes of exercise and increased toward the end of the protocol (P < .001 for PL or W compared with HNa or LNa for all points after 120 minutes). We also observed a slight increase in free fatty acid concentration during the HNa trial that was different from the corresponding value during the LNa trial at the end of the protocol (P < .001). Because work output was the same during all trials, lactate values did not differ among the 4 trials, as expected.
Figure 4. Changes in A, plasma volume and B, plasma osmolality during the experiment. Values are means ± SEs. Abbreviations: LNa, carbohydrate-electrolyte drink containing 19.9 mmol/L sodium; HNa, carbohydrate-electrolyte drink containing 36.2 mmol/L sodium; PL, colored and flavored distilled water; W, mineral water. a Denotes difference compared with colored and flavored distilled water trial (P < .05). b Denotes difference compared with mineral water trial (P < .05). c Denotes difference compared with carbohydrate-electrolyte drink containing 19.9 mmol/L sodium trial (P < .05).
Four of the 13 participants experienced precramping signs with pain and stiffness in the gastrocnemius muscle. Three of these 4 participants developed signs during either the PL or W trial, whereas the fourth participant developed the same signs during the LNa trial. No participant reported muscle cramping throughout the 4 trials.
Discussion
We examined the effectiveness of sport drinks with different sodium content to prevent hyponatremia, a decrease in plasma volume, and muscle cramping during prolonged exercise of moderate intensity in the heat. Our major finding was that under environmental conditions in which fluid intake matched body mass loss, even a moderate amount of sodium added to the hydration solution was adequate to attenuate the decline in sodium concentration of plasma and to preserve plasma volume at pre-exercise levels.
Our results regarding sodium concentrations are in agreement with those of Vrijens and Rehrer,17 who showed that a low-sodium drink prevented the decline in plasma sodium concentration observed when a sodium-free fluid is ingested. In both their study and ours, fluids were ingested at a rate similar to the rate of water loss. However, plasma volume responses during exercise were completely different between the studies. Vrijens and Rehrer17 found a plasma volume decline at the end of the 3-hour exercise period of approximately 10%, whereas we found a conservation of plasma volume. We have no apparent explanation for this discrepancy, but it may reflect differences in the exercise protocol between the studies. Nevertheless, the conservation effect of sodium supplementation in plasma volume has also been confirmed in another study by Sanders et al27 in which fluid consumption equaled fluid loss during prolonged exercise of moderate intensity. Specifically, they showed that, although a drink of 5 mmol/L sodium decreased extracellular fluid, a drink containing 50 mmol/L sodium stabilized extracellular fluids. In addition, they found that a drink of 100 mmol/L sodium resulted in a small increase in the extracellular fluid at the end of exercise.
In our study, sodium supplementation was unable to produce an increase in serum sodium concentration even during the HNa trial. We propose 2 possible mechanisms for this observation. First, excessive sodium intake during exercise may have resulted in an increased excretion of sodium in the urine or the sweat. Second, part of the additional sodium provided with sodium-containing drinks was diluted in the plasma volume that was retained compared with the no-sodium supplementation trials. Both mechanisms could play a role. Note that both sodium supplementation trials suppressed the increase in plasma aldosterone during the experiment, indicating that the overall sodium load during these trials was adequate to produce physiologic responses regarding electrolyte balance.
Given the virtually identical values in serum sodium and plasma volume at the end of exercise in sodium supplementation trials, some degree of excessive excretion of sodium should have existed in the HNa trial compared with the LNa trial. However, because sodium losses in the urine and sweat were not measured, definite conclusions cannot be drawn. Sanders et al27 showed that although a solution of 50 mmol/L sodium did not suppress urine volume during prolonged exercise, it did increase overall sodium loss in the urine. They also found that sodium excretion in sweat was insensitive to sodium supplementation even at relatively high sodium intakes.
The possibility that the sodium supplemented during exercise is diluted in the preserved plasma volume is better supported in both laboratory and field studies in which the researchers did not observe a decline in blood sodium when participants drank water alone.7,18,27 In all these studies, drinking sodium-free solutions caused a marked decrease in plasma volume that was possibly more profound than sodium losses, resulting in normal blood sodium during exercise. When sodium was also provided, the conservation of plasma volume prevented an increase in blood sodium. These observations may be much more critically important when fluid intake is higher than fluid losses during exercise.
Exercise-associated hyponatremia may result in confusion, disorientation, nausea, vomiting, pulmonary edema, cardiorespiratory arrest, coma, and even death.13 Most cases of exertional hyponatremia have been attributed to overload of fluids due to excessive water intake.15 To prevent the development of hyponatremia during exercise, Hew-Butler et al14 recommended that athletes participating in long-lasting endurance events should avoid excessive drinking and that drinking only according to thirst could be an effective strategy to avoid overdrinking. However, it is well-established that thirst perception is an insufficient means to match fluid losses during exercise.28 In addition, a body mass reduction of 3% or even less during 16 km of walking or running under the extreme environmental condition of heat stress29 or during a marathon30 has been associated with a decrease in plasma volume. These studies suggest that restoration of fluids lost via sweating should be promoted during prolonged, moderate-intensity exercise in the heat to ensure cardiovascular and thermoregulatory stability. However, according to our results, replacement of sodium should also be promoted to prevent the drop in serum sodium concentration.
Another aim of our study was to examine the effect of sodium supplementation during exercise on the prevention of muscle cramps. We applied a mixed-exercise protocol of both endurance and dynamic exercise that was predicted to simultaneously challenge electrolyte balance and fluid dynamics, glycogen stores, and muscle contractility fatigue. However, none of our volunteers experienced muscle cramping symptoms in any of the 4 trials. Based on our results, a relationship between sodium supplementation and the prevention of exercise-associated muscle cramps cannot be established at the given environmental and exercise conditions. The exercise protocol that we applied may not have sufficiently targeted a specific muscle in a way that could alter its neuromuscular function and subsequently induce cramping. Jung et al21 examined the effect of a much more targeted and more intense exercise protocol on muscle cramping. Cramping was observed in most of their participants. In their study, participants were tested only during euhydration with a carbohydrate-electrolyte drink or during dehydration with no fluids provided. Further research that applies appropriate exercise protocols is needed to clarify the effect of sodium supplementation on the propensity to cramping during exercise.
Conclusions
Relatively small amounts of sodium (19.9 mmol/L) in a sport drink consumed at a rate equal to body mass change effectively prevented the decrease in plasma sodium concentration typically observed when sodium-free fluids are ingested during prolonged exercise. Additional sodium intake did not confer further advantage under these specific conditions. Possible limitations of our study include inadequate control of dietary sodium intake before each trial and the lack of measurements of sodium losses in the urine and sweat.
As recently suggested by the American College of Sports Medicine,4 endurance athletes should avoid excessive dehydration during exercise; however, when sweating is excessive and the goal is to restore fluid loss during exercise, special attention should be paid to the replenishment of sodium. This may be accomplished either by consuming a sodium-containing sport drink or by consuming solid foods containing sodium along with adequate fluids.
Acknowledgments
The study was supported by a research grant from The Gatorade Sports Science Institute (Barrington, IL) and Pepsico Inc (Purchase, NY).
Footnotes
Costas A. Anastasiou, PhD, contributed to conception and design, analysis and interpretation of the data, and drafting and final approval of the article. Stavros A. Kavouras, PhD, contributed to conception and design, analysis and interpretation of the data, and critical revision and final approval of the article. Giannis Arnaoutis, MS; Aristea Gioxari, MS; Maria Kollia, MS; and Efthimia Botoula, BS, contributed to acquisition and analysis and interpretation of the data and critical revision and final approval of the article. Labros S. Sidossis, PhD, contributed to conception and design, analysis and interpretation of the data, and critical revision and final approval of the article.
References
- 1.Montain S.J, Coyle E.F. Influence of graded dehydration on hyperthermia and cardiovascular drift during exercise. J Appl Physiol. 1992;73(4):1340–1350. doi: 10.1152/jappl.1992.73.4.1340. [DOI] [PubMed] [Google Scholar]
- 2.Fortney S.M, Wenger C.B, Bove J.R, Nadel E.R. Effect of hyperosmolality on control of blood flow and sweating. J Appl Physiol. 1984;57(6):1688–1695. doi: 10.1152/jappl.1984.57.6.1688. [DOI] [PubMed] [Google Scholar]
- 3.Barr S.I, Costill D.L, Fink W.J. Fluid replacement during prolonged exercise: effects of water, saline, or no fluid. Med Sci Sports Exerc. 1991;23(7):811–817. [PubMed] [Google Scholar]
- 4.Sawka M.N, Burke L.M, et al. American College of Sports Medicine. American College of Sports Medicine position stand: exercise and fluid replacement. Med Sci Sports Exerc. 2007;39(2):377–390. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 5.Nose H, Mack G.W, Shi X.R, Nadel E.R. Involvement of sodium retention hormones during rehydration in humans. J Appl Physiol. 1988;65(1):332–336. doi: 10.1152/jappl.1988.65.1.332. [DOI] [PubMed] [Google Scholar]
- 6.Wemple R.D, Morocco T.S, Mack G.W. Influence of sodium replacement on fluid ingestion following exercise-induced dehydration. Int J Sport Nutr. 1997;7(2):104–116. doi: 10.1123/ijsn.7.2.104. [DOI] [PubMed] [Google Scholar]
- 7.Criswell D, Renshler K, Powers S.K, Tulley R, Cicale M, Wheeler K. Fluid replacement beverages and maintenance of plasma volume during exercise: role of aldosterone and vasopressin. Eur J Appl Physiol Occup Physiol. 1992;65(5):445–451. doi: 10.1007/BF00243512. [DOI] [PubMed] [Google Scholar]
- 8.Maughan R.J, Fenn C.E, Gleeson M, Leiper J.B. Metabolic and circulatory responses to the ingestion of glucose polymer and glucose/electrolyte solutions during exercise in man. Eur J Appl Physiol Occup Physiol. 1987;56(3):356–362. doi: 10.1007/BF00690905. [DOI] [PubMed] [Google Scholar]
- 9.Gisolfi C.V, Lambert G.P, Summers R.W. Intestinal fluid absorption during exercise: role of sport drink osmolality and [Na+] Med Sci Sports Exerc. 2001;33(6):907–915. doi: 10.1097/00005768-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Anastasiou C.A, Kavouras S.A, Koutsari C, et al. Effect of maltose-containing sports drinks on exercise performance. Int J Sport Nutr Exerc Metab. 2004;14(6):609–625. doi: 10.1123/ijsnem.14.6.609. [DOI] [PubMed] [Google Scholar]
- 11.Maughan R.J, Fenn C.E, Leiper J.B. Effects of fluid, electrolyte and substrate ingestion on endurance capacity. Eur J Appl Physiol Occup Physiol. 1989;58(5):481–486. doi: 10.1007/BF02330701. [DOI] [PubMed] [Google Scholar]
- 12.Almond C.S, Shin A.Y, Fortescue E.B, et al. Hyponatremia among runners in the Boston Marathon. N Engl J Med. 2005;352(15):1550–1556. doi: 10.1056/NEJMoa043901. [DOI] [PubMed] [Google Scholar]
- 13.Montain S.J, Sawka M.N, Wenger C.B. Hyponatremia associated with exercise: risk factors and pathogenesis. Exerc Sport Sci Rev. 2001;29(3):113–117. doi: 10.1097/00003677-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Hew-Butler T, Almond C, Ayus J.C, et al. Consensus statement of the 1st International Exercise-Associated Hyponatremia Consensus Development Conference, Cape Town, South Africa 2005. Clin J Sport Med. 2005;15(4):208–213. doi: 10.1097/01.jsm.0000174702.23983.41. [DOI] [PubMed] [Google Scholar]
- 15.Noakes T.D, Sharwood K, Speedy D, et al. Three independent biological mechanisms cause exercise-associated hyponatremia: evidence from 2,135 weighed competitive athletic performances. Proc Natl Acad Sci U S A. 2005;102(51):18550–18555. doi: 10.1073/pnas.0509096102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Twerenbold R, Knechtle B, Kakebeeke T.H, et al. Effects of different sodium concentrations in replacement fluids during prolonged exercise in women. Br J Sports Med. 2003;37(4):300–303. doi: 10.1136/bjsm.37.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vrijens D.M, Rehrer N.J. Sodium-free fluid ingestion decreases plasma sodium during exercise in the heat. J Appl Physiol. 1999;86(6):1847–1851. doi: 10.1152/jappl.1999.86.6.1847. [DOI] [PubMed] [Google Scholar]
- 18.Speedy D.B, Thompson J.M, Rodgers I, Collins M, Sharwood K, Noakes T.D. Oral salt supplementation during ultradistance exercise. Clin J Sport Med. 2002;12(5):279–284. doi: 10.1097/00042752-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Hew-Butler T.D, Sharwood K, Collins M, Speedy D, Noakes T. Sodium supplementation is not required to maintain serum sodium concentrations during an Ironman triathlon. Br J Sports Med. 2006;40(3):255–259. doi: 10.1136/bjsm.2005.022418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwellnus M.P, Derman E.W, Noakes T.D. Aetiology of skeletal muscle “cramps” during exercise: a novel hypothesis. J Sports Sci. 1997;15(3):277–285. doi: 10.1080/026404197367281. [DOI] [PubMed] [Google Scholar]
- 21.Jung A.P, Bishop P.A, Al-Nawwas A, Dale R.B. Influence of hydration and electrolyte supplementation on incidence and time to onset of exercise-associated muscle cramps. J Athl Train. 2005;40(2):71–75. [PMC free article] [PubMed] [Google Scholar]
- 22.Schwellnus M.P, Nicol J, Laubscher R, Noakes T.D. Serum electrolyte concentrations and hydration status are not associated with exercise associated muscle cramping (EAMC) in distance runners. Br J Sports Med. 2004;38(4):488–492. doi: 10.1136/bjsm.2003.007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stofan J.R, Zachwieja J.J, Horswill C.A, Murray R, Anderson S.A, Eichner E.R. Sweat and sodium losses in NCAA football players: a precursor to heat cramps. Int J Sport Nutr Exerc Metab. 2005;15(6):641–652. doi: 10.1123/ijsnem.15.6.641. [DOI] [PubMed] [Google Scholar]
- 24.Price T.B, Rothman D.L, Taylor R, Avison M.J, Shulman G.I, Shulman R.G. Human muscle glycogen resynthesis after exercise: insulin-dependent and -independent phases. J Appl Physiol. 1994;76(1):104–111. doi: 10.1152/jappl.1994.76.1.104. [DOI] [PubMed] [Google Scholar]
- 25.Convertino V.A, Armstrong L.E, Coyle E.F, et al. American College of Sports Medicine position stand: exercise and fluid replacement. Med Sci Sports Exerc. 1996;28(1):i–vii. doi: 10.1097/00005768-199610000-00045. [DOI] [PubMed] [Google Scholar]
- 26.Dill D.B, Costill D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37(2):247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 27.Sanders B, Noakes T.D, Dennis S.C. Sodium replacement and fluid shifts during prolonged exercise in humans. Eur J Appl Physiol. 2001;84(5):419–425. doi: 10.1007/s004210000371. [DOI] [PubMed] [Google Scholar]
- 28.Greenleaf J.E, Sargent F., 2nd Voluntary dehydration in man. J Appl Physiol. 1965;20(4):719–724. doi: 10.1152/jappl.1965.20.4.719. [DOI] [PubMed] [Google Scholar]
- 29.Mudambo K.S, Leese G.P, Rennie M.J. Dehydration in soldiers during walking/running exercise in the heat and the effects of fluid ingestion during and after exercise. Eur J Appl Physiol Occup Physiol. 1997;76(6):517–524. doi: 10.1007/s004210050284. [DOI] [PubMed] [Google Scholar]
- 30.Maughan R.J, Whiting P.H, Davidson R.J. Estimation of plasma volume changes during marathon running. Br J Sports Med. 1985;19(3):138–141. doi: 10.1136/bjsm.19.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]