Abstract
Context:
Many researchers have investigated the effectiveness of different types of cold application, including cold whirlpools, ice packs, and chemical packs. However, few have investigated the effectiveness of different types of ice used in ice packs, even though ice is one of the most common forms of cold application.
Objective:
To evaluate and compare the cooling effectiveness of ice packs made with cubed, crushed, and wetted ice on intramuscular and skin surface temperatures.
Design:
Repeated-measures counterbalanced design.
Setting:
Human performance research laboratory.
Patients or Other Participants:
Twelve healthy participants (6 men, 6 women) with no history of musculoskeletal disease and no known preexisting inflammatory conditions or recent orthopaedic injuries to the lower extremities.
Intervention(s):
Ice packs made with cubed, crushed, or wetted ice were applied to a standardized area on the posterior aspect of the right gastrocnemius for 20 minutes. Each participant was given separate ice pack treatments, with at least 4 days between treatment sessions.
Main Outcome Measure(s):
Cutaneous and intramuscular (2 cm plus one-half skinfold measurement) temperatures of the right gastrocnemius were measured every 30 seconds during a 20-minute baseline period, a 20-minute treatment period, and a 120-minute recovery period.
Results:
Differences were observed among all treatments. Compared with the crushed-ice treatment, the cubed-ice and wetted-ice treatments produced lower surface and intramuscular temperatures. Wetted ice produced the greatest overall temperature change during treatment and recovery, and crushed ice produced the smallest change.
Conclusions:
As administered in our protocol, wetted ice was superior to cubed or crushed ice at reducing surface temperatures, whereas both cubed ice and wetted ice were superior to crushed ice at reducing intramuscular temperatures.
Keywords: ice pack, cryotherapy, gastrocnemius muscle
Key Points.
Wetted ice was more effective than cubed ice and crushed ice in lowering surface temperature during treatment and maintaining the lower temperature during recovery.
Wetted ice and cubed ice were more effective than crushed ice in lowering intramuscular temperature during treatment and maintaining the lower temperatures during recovery.
Compared with cubed ice, crushed ice was more effective in lowering surface temperature during treatment but was less effective in maintaining the lower temperature during recovery.
A wetted-ice pack (ie, premade ice pack that has begun to melt) may be more effective than a freshly made ice pack and may be a better clinical choice than cubed-ice or crushed-ice packs for treating injuries and rapidly inducing analgesia after an injury occurs.
Ice is a therapeutic agent used in medicine as an integral part of injury treatment and rehabilitation. The use of ice packs is widespread because of their effectiveness, convenience, low cost, and ease of transportation. Ice packs can be made with any form of ice; however, 2 commonly used forms are cubed ice and crushed ice. Ice has been shown to effectively reduce pain and swelling during the inflammatory response after injury.1,2 Ice is believed to help control pain by inducing local anesthesia around the treatment area.3 Investigators have also shown that it decreases edema,4 nerve conduction velocities,5 cellular metabolism,6 and local blood flow.2
Researchers have studied the thermal effects of many methods of cold application by specifically looking at cutaneous and intramuscular tissue temperature change,7–16 the relationship between temperature change and the depth of the targeted tissue,7 and the type of cold application used.8,9,11,13,14 Researchers agree on the physiologic effects of cold therapy and concur that cold agents that pass through a phase change (from solid to liquid) while cooling are generally more effective than those that do not.9,14
Investigators8,9,11,13,14 have often compared ice packs (crushed or cubed ice) with non–ice-pack forms of cold application. Some of these application methods include ice massage,13 frozen gel packs,7–9,14 cold-water submersion,11,15 and frozen peas.8,14 In one study,14 a crushed-ice pack and mixture of chilled water and alcohol produced lower skin temperatures than those produced by frozen peas or frozen gel packs after a 20-minute treatment. In a study comparing loose ice flakes, ice flakes in a plastic bag, and a cryogenic gel pack, each wrapped in a damp terry cloth towel, surface temperatures after a 15-minute treatment were lower with loose ice flakes and ice flakes in a plastic bag than with a cryogenic gel pack.17
Although we found many studies comparing different types of cold agents, we were unable to find studies comparing the effectiveness of different types of ice when used in an ice pack. We found no published studies on the effectiveness of wetted ice (ice and water added together in an ice bag and used with a dry interface), and we found no studies that compared cubed ice with crushed ice. We were interested in wetted ice because we believed that the addition of water to an ice pack would improve the ice pack's ability to remove heat from the underlying tissue. In theory, a wetted-ice pack is comparable to a cold whirlpool or slush bucket, because the treatment interface conforms to the surface of the treatment area much better than cubed or crushed ice and enhances its ability to conduct thermal energy away from the underlying tissue, thus increasing the ice pack's ability to decrease tissue temperature. Therefore, the purpose of our study was to compare intramuscular and skin surface temperature changes among ice packs made with cubed ice, crushed ice, or wetted ice. Our specific aims were to investigate the total amount of temperature change and the length of time that the tissue remained cool after different applications of ice. We hypothesized that the wetted ice would produce a greater temperature change than both cubed ice and crushed ice and provide a longer duration of cooling effects. We also hypothesized that crushed ice would produce a greater temperature change than cubed ice.
Methods
Design
We used a repeated-measures counterbalanced design in which each participant was given separate treatments of cubed ice, crushed ice, and wetted ice, with at least 4 days between treatment sessions.
Participants
Six healthy men (age = 23.7 ± 1.0 years, height = 177.0 ± 10.3 cm, mass = 96.6 ± 22.6 kg, lateral calf skinfold = 12.8 ± 4.1 mm, calf girth = 42.4 ± 6.1 cm) and 6 healthy women (age = 22.8 ± 0.8 years, height = 165.3 ± 8.0 cm, mass = 65.4 ± 7.2 kg, lateral calf skinfold = 17.3 ± 3.4 mm, calf girth = 35.7 ± 3.0 cm) participated in the study. No participant had sustained a lower leg injury for at least 1 month before the study, had a known vascular disease in the lower leg, had a known allergy to cold or ice, had compromised circulation in the lower leg, or had sensitivity to cold. All participants were instructed about the data collection procedures and gave written informed consent. The Western Michigan University Human Subjects Internal Review Board approved the study.
Procedures
For each trial, participants were placed in a prone position on a treatment table in a laboratory. We measured each participant's calf for the location of largest girth and measured skinfold thickness using a Lange skinfold caliper (Cambridge Scientific Corp, Cambridge, MD). Each skinfold measurement was taken 3 times from the lateral portion of the posterior calf at the area with the largest girth measurement. We divided the mean skinfold measurement by 2 to determine the thickness of the subcutaneous fat layer over each participant's gastrocnemius. To determine intramuscular depth, we measured a vertical distance of 2 cm plus one-half of the mean skinfold thickness from the posterior calf and marked it on the lateral aspect of the lateral head of the gastrocnemius using a fabricated template labeled to the nearest millimeter. We shaved and sanitized the calf using 10% povidone-iodine (Betadine; Purdue Pharma, LP, Stamford, CT) followed by a 70% isopropyl alcohol swab.
We measured intramuscular temperature using a 26-gauge, 4-cm microprobe (MT-26/4; Physitemp Instruments Inc, Clifton, NJ) and cutaneous temperature using a surface thermocouple (SST-1; Physitemp Instruments Inc). The microprobe and thermocouple were sterilized with CIDEX OPA Solution (Advanced Sterilization Products, Irvine, CA), as recommended by the manufacturer. We inserted the full 4-cm length of the microprobe into the lateral aspect of the gastrocnemius, using the previously measured depth as the insertion point. Thus, the tip of the microprobe was placed at a depth of 2 cm plus one-half of the skinfold measurement below the treatment area on the posterior aspect of the gastrocnemius. The microprobe was inserted by the same investigator for all trials. This investigator wore gloves and had been instructed in safe insertion techniques by a physician. A template made of 6-mm white rayon felt with a 10 × 14-cm opening was placed over the treatment area to ensure that the participant had an equal surface area exposed to each of the 3 ice pack treatments. The surface thermocouple was applied to the center of the treatment area and fixed in position with clear plastic tape.
The microprobe and thermocouple were connected to a digital monitor (model HH23; Omega Engineering Inc, Stamford, CT), and both temperatures were recorded manually to the nearest tenth of a degree every 30 seconds for the duration of the study. Participants received each of the 3 ice pack treatments (crushed, cubed, wetted ice), with at least 4 days between treatments. Treatment order was assigned using a 3 × 12 Latin square counterbalanced design.
All ice packs were made by placing the ice into 22 × 40-cm clear, 1-mil polyethylene bags, removing excess air, and securing each bag with a knot. Cubed-ice packs were made by placing 2000 mL of cubed ice into the bag. Crushed-ice packs were made by placing 2000 mL of crushed ice into the bag. Wetted-ice packs were made by placing 2000 mL of cubed ice and 300 mL of room-temperature water into the bag. We chose 300 mL of water because it was approximately the amount of water left in the bag after a 20-minute cubed-ice treatment during pilot testing. Note that the “wet ice” described by Merrick et al9 and Belitsky et al17 was different from our wetted ice because they used a wet treatment interface, whereas we investigated a dry interface with water inside the ice pack.
Freestanding ice bags were placed on the gastrocnemius over the felt template and were not secured with any type of wrap. Temperatures were monitored and recorded every 30 seconds for a 20-minute baseline period, a 20-minute treatment period, and a 120-minute recovery period. Although other researchers have used only a 2-minute to 5-minute baseline9,11,18–20 or have required participants to remain at rest for 15 minutes before a short baseline period,12 we found during pilot testing that cutaneous and intramuscular temperatures continued to decrease at rest for several minutes before reaching a steady temperature around 15 minutes. Thus, we used a 20-minute baseline to ensure that all temperatures had reached a steady state.
At the conclusion of the recovery period, the surface thermocouple, treatment template, and microprobe were removed; the treatment area was sanitized with a 70% isopropyl alcohol swab; and the site of the microprobe insertion was covered with a self-adhesive bandage.
To calculate temperature change, we subtracted the lowest mean temperature achieved during the treatment and recovery phases from the mean baseline temperature.
Statistical Analysis
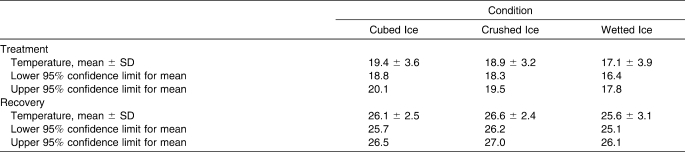
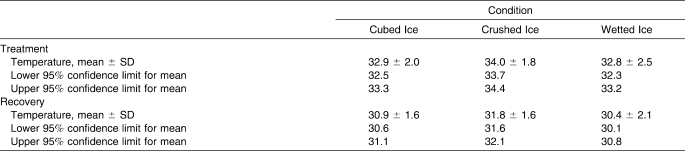
We used a 3 × 3 analysis of variance (ANOVA) with repeated measures to determine statistical differences in surface and intramuscular temperatures over time between different ice treatments. All statistical tests were 2 tailed, and the α was set a priori at .05 using SPSS (version 14.0; SPSS Inc, Chicago, IL). In the case of an interaction, post hoc testing was performed using a simple effects analysis with the Bonferroni adjustment. In the case of a main effect, specific pairwise comparisons were examined using the Bonferroni post hoc test. All data are reported as mean ± SD. Confidence intervals (CIs) for mean surface and intramuscular temperatures were also computed.
Results
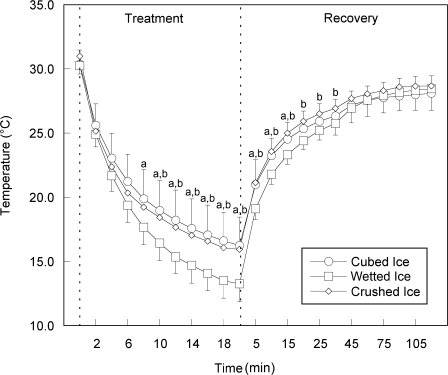
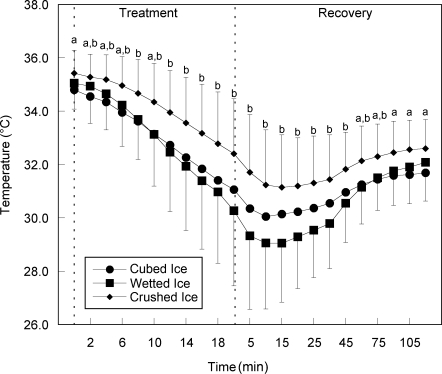
Mean changes in surface and intramuscular temperatures throughout the treatment and recovery periods are shown in Figures 1 and 2, and CIs for mean temperatures can be found in Tables 1 and 2. Crushed ice produced the smallest temperature change, decreasing 4.3°C intramuscularly and 15.0°C at the surface. Wetted ice produced the greatest temperature change, decreasing 6.0°C intramuscularly and 17.0°C at the surface. Cubed ice decreased 4.8°C intramuscularly and 14.1°C at the surface, placing its mean temperature change between that of crushed ice and wetted ice.
Figure 1. Change over time for mean surface temperature. Values are displayed in °C. a Indicates difference between wetted ice and cubed ice. b Indicates difference between wetted ice and crushed ice.
Figure 2. Change over time for mean intramuscular temperature. Values are displayed in °C. a Indicates difference between cubed ice and crushed ice. b Indicates difference between wetted ice and crushed ice.
Table 1.
Confidence Intervals and Levels for Surface Temperature (°C)
Table 2.
Confidence Intervals and Levels for Intramuscular Temperature (°C)
Time × treatment interactions were found for surface temperatures (F44,484 = 7.53, P < .001) and intramuscular temperatures (F44,484 = 5.35, P < .001). From minute 10 of treatment to minute 15 of recovery, mean surface temperatures with wetted ice were lower than temperatures for either cubed or crushed ice (P < .05). All pairwise comparisons were P < .05. The greatest difference in surface temperature occurred at minute 18 of the treatment period. Temperatures with wetted ice were 3.1°C lower than cubed ice and 2.6°C lower than crushed ice (Figure 1). Intramuscularly, wetted ice produced lower temperatures than crushed ice from minute 2 of the treatment to minute 75 of recovery (Figure 2). Although not statistically different, it did produce mean temperatures at least 0.5°C lower than those produced by cubed ice from the end of the treatment until minute 30 of the recovery.
Discussion
Previous research1–6 has shown that decreasing tissue temperature decreases pain, edema, nerve conduction, cellular metabolism, and local blood flow. Although researchers have not reached consensus regarding ideal tissue temperature after injury, Merrick et al9 suggested that, in the absence of definitive data, better treatment outcomes may result from greater and faster cooling. Based upon this idea, we also assumed that, when the tissue temperature is lowered faster and the temperature change is greater, the treatment is more effective. This idea of creating greater temperature changes also served as the major impetus for our study.
When considering temperature change while using therapeutic cold agents, remember that all temperature changes result from the transfer of thermal energy from the higher-energy tissues to the lower-energy ice packs following the laws of thermodynamics, which state that heat transfer is always unidirectional from high heat to low heat.9
For both measurement locations and all treatments, we observed temperature changes similar to those reported by other researchers during cubed-ice and crushed-ice application.8,12–15 When examining surface temperature, we found that, after the 20-minute treatment period, wetted ice had produced a lower mean temperature (13.3°C) than cubed ice (16.3°C) or crushed ice (15.9°C). From baseline to the end of the treatment period, the total change in temperature was 17.0°C for wetted ice, 14.1°C for cubed ice, and 15.0°C for crushed ice. During the recovery period, wetted ice produced a lower surface temperature than cubed ice through the 30-minute mark (25.8°C versus 26.3°C), after which time the temperature differences were negligible. Wetted ice also produced a lower surface temperature than crushed ice through the 60-minute mark (27.6°C versus 28.1°C), after which time the temperature differences were also negligible. Examination of the CIs for surface temperatures during the treatment phase revealed that wetted ice (16.4°C–17.8°C) produced the lowest temperature range when compared with both crushed ice (18.3°C–19.5°C) and cubed ice (18.8°C–20.1°C). During the recovery phase, wetted ice (25.1°C–26.1°C) continued to produce the lowest temperatures, followed by cubed ice (25.7°C–26.5°C) and crushed ice (26.2°C–27.0°C). These results suggest that wetted ice was more effective in lowering the surface treatment and recovery temperatures than the other 2 forms of ice and that, compared with cubed ice, crushed ice was more effective in lowering surface treatment temperatures but was less effective in maintaining those lower temperatures during the recovery period.
When examining intramuscular temperature, we found that after the 20-minute treatment period, wetted ice produced a lower mean temperature (30.3°C) than cubed ice (31.1°C) or crushed ice (32.4°C). From baseline to the end of the treatment period, the total change in temperature was 4.8°C for wetted ice, 3.8°C for cubed ice, and 3.0°C for crushed ice. During the recovery period, wetted ice continued to produce a lower intramuscular temperature than cubed ice through the 30-minute mark (29.8°C versus 30.6°C), after which time the differences were negligible, and also produced a lower intramuscular temperature than crushed ice through the end of the recovery period (32.1°C versus 32.6°C). Examination of the CIs for intramuscular temperatures during the treatment phase revealed that wetted ice (32.3°C–33.2°C) and cubed ice (32.5°C–33.3°C) had similar temperatures, suggesting that, during this phase, both forms of ice could lower the temperature of muscle tissue equally well when compared with crushed ice (33.7°C–34.4°C). During the recovery phase, wetted ice produced the lowest temperature range (30.1°C–30.8°C), followed by cubed (30.6°C–31.1°C) and crushed ice (31.6°C–32.1°C). These results suggest that, while no real difference existed between the two, wetted ice and cubed ice were both more effective than crushed ice in lowering intramuscular temperatures during treatment and recovery.
Basing our evaluation on the idea that colder is better, our results demonstrate that wetted ice was more effective than both cubed ice and crushed ice at decreasing skin surface temperature during and after a 20-minute treatment. This greater effectiveness may be due to increased contact between the wetted ice and the skin, because water within the pack has a greater ability to mold to the surface of the treatment area than the ice does. Water also has a much higher ability to conduct thermal energy compared with air,21 which is found between the individual ice pieces within the other 2 forms of ice pack. Cubed-ice and crushed-ice packs use air to transfer thermal energy between the individual pieces of ice, whereas a wetted-ice pack uses water to transfer the thermal energy within the pack. Thus, cubed ice and crushed ice have a decreased ability to transfer thermal energy within the ice pack when compared with wetted ice. Differences between cubed ice and crushed ice, in opposition to our hypothesis, showed that ice pack effectiveness may be less affected by moldability than by the ability to transfer thermal energy. The ice pieces in the crushed-ice pack are less dense than those in the cubed-ice pack and rely more on air to transfer thermal energy. This is important because air transfers thermal energy less effectively than ice.21
Although adding room-temperature water could increase the internal temperature of the wetted-ice pack, the pack will still be dramatically colder than the underlying tissue, and the contents will still be able to pass through the phase change from solid to liquid, which greatly affects ice pack effectiveness. One of the major clinical implications of our research is that premade ice packs that have begun to melt while sitting in coolers, such as on sidelines, are not inferior and should not be drained before use or discarded. In fact, our research indicates that these ice packs may actually be more effective than a freshly made ice pack and may be a better clinical choice for treating injuries and rapidly inducing analgesia after an injury than the more commonly used cubed-ice or crushed-ice packs. More research is needed to determine the ideal conditions for a wetted-ice pack because using different volumes and temperatures of water or using crushed ice rather than cubed ice in the pack may change the treatment outcomes.
When examining surface temperature differences, we confirmed the results of others who found that surface temperature both decreased and increased at a much faster rate and greater magnitude than intramuscular temperature.9,12 In addition, surface temperatures began to climb immediately after ice pack removal, unlike intramuscular temperatures, which continued to decline. Because of these different rates and magnitudes of temperature change, Jutte et al12 recommended that skin temperature should not be used to predict intramuscular temperature and should no longer be used as the only method for determining the efficacy of cryotherapy methods.
Intramuscular temperatures for all 3 forms of ice continued to decrease after the ice pack was removed. The lowest intramuscular temperatures were reached at 10 minutes into the recovery period for cubed ice (mean = 30.1°C) and at 15 minutes for both wetted (mean = 29.1°C) and crushed ice (mean = 31.2°C). Other researchers found that the intramuscular tissue did not reach its lowest temperature until 10 to 15 minutes after the treatment period ended.13,15 This finding is different from the findings of Merrick et al18 and Myrer et al,11 who reported the lowest intramuscular temperatures within 5 to 7 minutes of treatment conclusion using a 1-cm plus one-half skinfold measurement depth. This difference may be explained by their more superficial measurement depth. Superficial measurement locations are closer to the skin, so temperatures begin to rise faster as they absorb thermal energy from warming surface tissue.
The continued decrease in intramuscular temperature after ice pack removal was also described by Enwemeka et al.7 They suggested that this was caused, at least in part, by the deeper tissues losing their thermal energy to the cooler superficial tissues. We found that, at a depth of 2 cm plus one-half skinfold, it is possible to decrease tissue temperatures after a 20-minute treatment. Myrer et al11 also demonstrated this. However, Enwemeka et al7 found that no intramuscular changes occurred at 2-cm and 3-cm depths after a 20-minute treatment. These and other inconsistencies in the literature may be a result of different methods to obtain intramuscular temperatures, which further demonstrates the need for increased methodological consistency in future research to enable the most accurate comparisons among studies.
Researchers also have demonstrated that overlying adipose tissue can affect the amount of intramuscular temperature change during cold application.12,19 Because adipose thickness varies among participants, measuring intramuscular temperatures using depths based solely upon distance from the skin will provide different results from those obtained by measuring below the adipose tissue. Thus, measuring at a 2-cm depth may actually be measuring temperatures in different types of subcutaneous tissue. To avoid these inconsistencies, we measured at a uniform depth below the adipose tissue for all participants by adding one-half of the skinfold to our 2-cm depth. Merrick et al9 and others7,10–13,19,20 used this method for placing intramuscular thermocouples to ensure consistency among participants. We recommend that in future studies, investigators also use this method of thermocouple placement to improve consistency among studies and consider the exclusion of participants with very high or very low levels of adipose tissue, which influences the effectiveness of the cold modality being investigated. Assessing temperature changes at multiple depths below the adipose layer, such as 1 cm, 2 cm, and 3 cm, may also be prudent.
Johnson et al15 stated that, if a participant was placed in an inactive position for 4 hours after cold treatment, intramuscular temperatures could not be expected to recover to pretreatment values. This is consistent with our findings, but we only measured temperatures up to 2 hours after treatment. None of our participants' posttreatment temperatures returned to pretreatment baselines, instead remaining around 3.0°C lower than the baseline temperatures for all treatments. We recommend that future researchers examine the effects of ice packs on participants immediately after activity or on participants returning to activity during or immediately after treatment, because these circumstances would be more applicable to most clinical settings.
Conclusions
Our findings provide important insight into the effectiveness of the different forms of ice used in the clinical setting. Compared with cubed ice or crushed ice, wetted ice produced greater and more rapid temperature changes in the surface tissues of the gastrocnemius. Intramuscularly, wetted ice produced temperature changes that were similar to those of cubed ice; however, both produced temperatures that were lower than those of crushed ice, which is clinically important. This knowledge can help clinicians select which type of ice treatment is best suited for their patients and can facilitate decisions regarding ice machine purchases and the fabrication of ice packs.
Footnotes
Joseph H. Dykstra, MA, ATC; Holly M. Hill, MA, LAT, ATC; Michael G. Miller, EdD, ATC; Christopher C. Cheatham, PhD; and Timothy J. Michael, PhD, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting, critical revision, and final approval of the article. Robert J. Baker, MD, PhD, ATC, contributed to conception and design; analysis and interpretation of the data; and drafting, critical revision, and final approval of the article.
References
- 1.Bugaj R. The cooling, analgesic, and rewarming effects of ice massage on localized skin. Phys Ther. 1975;55(1):11–19. doi: 10.1093/ptj/55.1.11. [DOI] [PubMed] [Google Scholar]
- 2.Dolan M.G, Thornton R.M, Fish D.R, Mendel F.C. Effects of cold water immersion on edema formation after blunt injury to the hind limbs of rats. J Athl Train. 1997;32(3):233–237. [PMC free article] [PubMed] [Google Scholar]
- 3.Drez D, Faust D.C, Evans J.P. Cryotherapy and nerve palsy. Am J Sports Med. 1981;9(4):256–257. doi: 10.1177/036354658100900414. [DOI] [PubMed] [Google Scholar]
- 4.Hocutt J.E, Jr, Jaffe R, Rylander C.R, Beebe J.K. Cryotherapy in ankle sprains. Am J Sports Med. 1982;10(5):316–319. doi: 10.1177/036354658201000512. [DOI] [PubMed] [Google Scholar]
- 5.Halar E.M, DeLisa J.A, Brozovich F.V. Nerve conduction velocity: relationship of skin, subcutaneous and intramuscular temperatures. Arch Phys Med Rehabil. 1980;61(5):199–203. [PubMed] [Google Scholar]
- 6.Curl W.W, Smith B.P, Marr A, Rosencrance E, Holden M, Smith T.L. The effect of contusion and cryotherapy on skeletal muscle microcirculation. J Sports Med Phys Fitness. 1997;37(4):279–286. [PubMed] [Google Scholar]
- 7.Enwemeka C.S, Allen C, Avila P, Bina J, Konrade J, Munns S. Soft tissue thermodynamics before, during, and after cold pack therapy. Med Sci Sports Exerc. 2002;34(1):45–50. doi: 10.1097/00005768-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Chesterton L.S, Foster N.E, Ross L. Skin temperature response to cryotherapy. Arch Phys Med Rehabil. 2002;83(4):543–549. doi: 10.1053/apmr.2002.30926. [DOI] [PubMed] [Google Scholar]
- 9.Merrick M.A, Jutte L.S, Smith M.E. Cold modalities with different thermodynamic properties produce different surface and intramuscular temperatures. J Athl Train. 2003;38(1):28–33. [PMC free article] [PubMed] [Google Scholar]
- 10.Myrer J.W, Measom G, Durrant E, Fellingham G.W. Cold- and hot-pack contrast therapy: subcutaneous and intramuscular temperature change. J Athl Train. 1997;32(3):238–241. [PMC free article] [PubMed] [Google Scholar]
- 11.Myrer J.W, Measom G, Fellingham G.W. Temperature changes in the human leg during and after two methods of cryotherapy. J Athl Train. 1998;33(1):25–29. [PMC free article] [PubMed] [Google Scholar]
- 12.Jutte L.S, Merrick M.A, Ingersoll C.D, Edwards J.E. The relationship between intramuscular temperature, skin temperature, and adipose thickness during cryotherapy and rewarming. Arch Phys Med Rehabil. 2001;82(6):845–850. doi: 10.1053/apmr.2001.23195. [DOI] [PubMed] [Google Scholar]
- 13.Zemke J.E, Andersen J.C, Guion W.K, McMillan J, Joyner A.B. Intramuscular temperature responses in the human leg to two forms of cryotherapy: ice massage and ice bag. J Orthop Sports Phys Ther. 1998;27(4):301–307. doi: 10.2519/jospt.1998.27.4.301. [DOI] [PubMed] [Google Scholar]
- 14.Kanlayanaphotporn R, Janwantanakul P. Comparison of skin surface temperature during the application of various cryotherapy modalities. Arch Phys Med Rehabil. 2005;86(7):1411–1415. doi: 10.1016/j.apmr.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 15.Johnson D.J, Moore S, Moore J, Oliver R.A. Effect of cold submersion on intramuscular temperature of the gastrocnemius muscle. Phys Ther. 1979;59(10):1238–1242. doi: 10.1093/ptj/59.10.1238. [DOI] [PubMed] [Google Scholar]
- 16.Palmer J.E, Knight K.L. Ankle and thigh skin surface temperature changes with repeated ice pack application. J Athl Train. 1996;31(4):319–323. [PMC free article] [PubMed] [Google Scholar]
- 17.Belitsky R.B, Odam S.J, Hubley-Kozey C. Evaluation of the effectiveness of wet ice, dry ice, and cryogenic packs in reducing skin temperature. Phys Ther. 1987;67(7):1080–1084. doi: 10.1093/ptj/67.7.1080. [DOI] [PubMed] [Google Scholar]
- 18.Merrick M.A, Knight K.L, Ingersoll C.D, Potteiger J.A. The effects of ice and compression wraps on intramuscular temperatures at various depths. J Athl Train. 1993;28(3):236,238,241–245. [PMC free article] [PubMed] [Google Scholar]
- 19.Myrer J.W, Myrer K.A, Measom G.J, Fellingham G.W, Evers S.L. Muscle temperature is affected by overlying adipose when cryotherapy is administered. J Athl Train. 2001;36(1):32–36. [PMC free article] [PubMed] [Google Scholar]
- 20.Myrer J.W, Draper D.O, Durrant E. Contrast therapy and intramuscular temperature in the human leg. J Athl Train. 1994;29(4):318–322. [PMC free article] [PubMed] [Google Scholar]
- 21.Lide D.R, editor. CRC Handbook of Chemistry and Physics. 84th ed. Cleveland, OH: CRC Press; 2003. pp. 6-1–6-7. [Google Scholar]