Abstract
Context:
The body of knowledge concerning shoulder kinematics in patients with rotator cuff tears is increasing. However, the level of understanding regarding how pain and tear size affect these kinematic patterns is minimal.
Objective:
To identify relationships between pain associated with a full-thickness rotator cuff tear, tear size, and scapulohumeral rhythm (SHR) and to determine whether pain and tear size serve as predictors of SHR.
Design:
A test-retest design was used to quantify pain and SHR before and after a subacromial lidocaine injection. Correlation and multivariate analyses were used to identify relationships among pain, tear size, and SHR.
Setting:
Orthopaedic biomechanics research laboratory.
Patients or Other Participants:
Fifteen patients (age range, 40–75 years) with diagnosed full-thickness rotator cuff tears participated. They were experiencing pain at the time of testing.
Intervention(s):
Shoulder kinematic data were collected with an electromagnetic tracking system before and after the patient received a lidocaine injection.
Main Outcome Measure(s):
Pain was rated using a visual analog scale. Three-dimensional scapular kinematics and glenohumeral elevation were assessed. Scapular kinematics included anterior-posterior tilt, medial-lateral tilt, and upward-downward rotation. A regression model was used to calculate SHR (scapular kinematics to glenohumeral elevation) for phases of humeral elevation and lowering.
Results:
Linear relationships were identified between initial pain scores and SHR and between tear size and SHR, representing an increased reliance on scapular motion with increasing pain and tear size. Pain was identified as an independent predictor of SHR, whereas significant findings for the effect of tear size on SHR and the interaction between pain and tear size were limited.
Conclusions:
We noted an increased reliance on scapular contributions to overall humeral elevation with increasing levels of pain and rotator cuff tear size. Pain associated with a rotator cuff tear serves as a primary contributor to the kinematic patterns exhibited in patients with rotator cuff tears.
Keywords: shoulder kinematics, scapula, biomechanics
Key Points.
Pain levels associated with a full-thickness rotator cuff tear, independent of cuff tear size, contributed to the observed movement patterns of the shoulder.
Increased levels of pain were directly related to increased values of scapulohumeral rhythm (SHR) in patients with full-thickness rotator cuff tears.
Scapulohumeral rhythm increased as tear size increased.
Pain was an independent predictor of SHR; however, tear size and the interaction between pain and tear size contributed to but were not predictors of SHR.
Altered shoulder kinematic patterns have been well documented in patients with shoulder pathologies.1–9 An increased reliance on scapular contribution to overall shoulder motion has been identified in patients with rotator cuff tears (RCTs).6,7,9 It has been speculated that the elevated scapular contribution could serve to improve the length-tension relationships of the deltoid and unaffected cuff muscles in order to generate the forces necessary for shoulder motion; to enhance clearance of the acromion, limiting compression of the cuff and the resultant pain; or to compensate for weakness and pain associated with the tear.6,7,9
The level of awareness of shoulder kinematics in patients with full-thickness RCTs is increasing, but the understanding of how pain and tear size affect these kinematic patterns is minimal.1,7,9,10 There is some evidence that would suggest that there is a difference in the shoulder kinematic patterns exhibited by shoulder impingement patients.6 However, it is unclear if the amount of pain or even the degree of change in pain levels affects scapulothoracic and glenohumeral (GH) joint function. Similarly, clinicians have a limited understanding of the impact that the RCT, and specifically the size of the tear itself, has on shoulder kinematic function.
The overall goal of our study was to develop a better understanding of the coordinated movement of the scapula and humerus during humeral elevation and lowering activities in patients with full-thickness RCTs. The data regarding changes in scapulohumeral rhythm (SHR) after a reduction in pain have been presented in a previous article,10 but the purpose of this segment of our study was to address 4 different hypotheses. First, we hypothesized that a positive relationship exists between pain and SHR and results in an increase in scapular contributions to humeral elevation. Second, we hypothesized that a direct relationship also exists between the changes in pain (from preinjection to postinjection of medication) and the change in SHR. Third, we hypothesized that a relationship exists between tear size and SHR. Fourth, we hypothesized that pain and tear size serve both independently and collectively as predictors of SHR. We believed that the extent of the cuff tear and pain associated with it would affect movement of the scapula and humerus and that altering those pain levels would result in a decreased reliance on scapulothoracic contributions to overall humeral elevation.
Methods
A sample of 15 patients (mean age, 60.2 ± 8.9 years [range, 40–75 years]; mean height, 1.72 ± 0.10 m; mean mass, 85.43 ± 18.32 kg; 9 men and 6 women) who were diagnosed with a chronic (>3 months) full-thickness RCT, measuring at least 1 cm2, confirmed by sonographic or magnetic resonance imaging, was studied. Patients were identified through a search of the patient records. The sample included 8 individuals with moderate tears (1 to <3 cm), 3 with large tears (≥3 to 5 cm), and 4 with massive tears (>5 cm).11 Of the patients, 9 had supraspinatus tears only, 1 had a subscapularis tear only, 4 had supraspinatus and infraspinatus tears, and 1 had supraspinatus, infraspinatus, and subscapularis tears. The sample size was determined through a power analysis conducted on data from a previous study.6 The study was powered to 80% with an effect size of 50%. Inclusion criteria were full-thickness RCT patients having typical signs and symptoms associated with shoulder impingement and rotator cuff tendinopathy for 3 months or more. Patients were excluded if they exhibited any neurologic condition resulting in muscle weakness or decreased range of motion, had any additional shoulder pathology thought to alter their shoulder kinematics, had a history of prior shoulder surgery, were diagnosed with rheumatoid arthritis, were currently pregnant, or exhibited any contraindications associated with the use of lidocaine.
All subjects completed the informed consent document approved by the University of Michigan Institutional Review Board for Human Subject Research (Ann Arbor, MI). They also completed the patient portion of the American Shoulder and Elbow Surgeons shoulder assessment questionnaire.12 Patients rated their current shoulder pain on a visual analog scale (range from 0 to 10) and their symptoms and level of function by use of the American Shoulder and Elbow Surgeons questionnaire. They rated their pain before the testing protocol and again after the subacromial lidocaine injection.
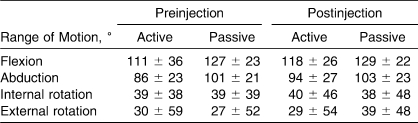
Before data collection, we also measured range of motion in the shoulder and strength. Range of motion was assessed both actively and passively for shoulder flexion, abduction, internal rotation, and external rotation (Table 1). Manual muscle testing was also performed for all strength measures. The patients demonstrated weakness (5/5) in each of the ranges. Sixty percent exhibited weakness with flexion; 73%, with abduction; 86%, with external rotation; and 73%, with internal rotation.
Table 1.
Descriptive Range-of-Motion Measures for the Involved Shoulder Before and After a Lidocaine Injection (Mean ± SD)
To acquire shoulder kinematic data, sensors from the MotionStar electromagnetic tracking system (Ascension Technology, Burlington, VT) were attached to the sternum, scapula, humerus, and wrist on the involved side. The sternum and wrist sensors were attached with double-sided tape. A humeral cuff was used to measure the movements of the humerus,13 whereas a scapula tracker followed the motion of the scapula.14 The system transmitter was rigidly fixed to a wooden chair (devoid of all metal), and a digitizing probe was used to digitize reference landmarks.15 Raw kinematic data were collected at 100 Hz by use of the MotionStar electromagnetic tracking system (a multiple-sensor implementation of the Flock of Birds technology [Ascension Technology]) and the MotionMonitor software package (Innovative Sports Technology, Chicago, IL). A short-range transmitter was used to minimize measurement error, and the sensors were kept within 75 cm of the transmitter. Metal mapping was performed before each data collection session to compensate for any potential interference caused by existing metal within the testing area. Corrections from the mapping session were accounted for during the data collection sessions by the MotionMonitor software. To aid in the data collection and postprocessing, triggers were attached to the targets, one on the bottom target and one on the top target. The triggers used were pressure-sensitive electric switches that, when depressed through an analog signal, interrupted data collection via the MotionMonitor data system. When the subject's hand was removed from the trigger, the MotionMonitor system would immediately resume humeral elevation and lowering data collection. With the inclusion of the triggers on the targets, it was possible to determine when the subject initiated the motion, reached the desired point of humeral elevation, initiated the return, and returned to the starting position. By using the triggers, we were certain that the desired data had been captured in its entirety through the complete arc of motion that we were assessing.
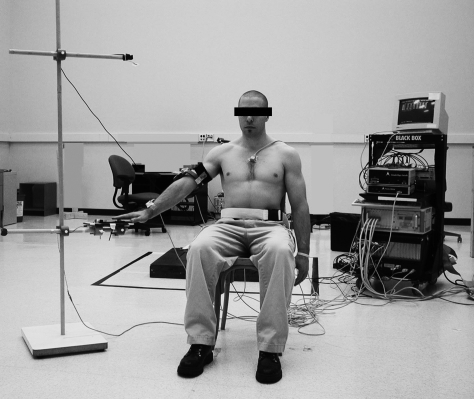
By use of the digitizing probe, 15 anatomic landmarks were digitized to construct the local 3-dimensional anatomic coordinate system necessary to compute the Euler angles of the individual bony segments. With the use of these landmarks and the local anatomic coordinate systems of each bony segment, the position, orientation, and displacement of the individual coordinate systems, with respect to one another, can be calculated. The digitization process, the specific landmarks, and the rotation matrices used to calculate these angles were based on the recommendation of the International Society of Biomechanics and the specifications of the humeral cuff.13,16 To complete the patient setup, targets were set at arm's length and positioned so that subjects, during the humeral elevation tasks, would be performing sagittal-, scapular-, and frontal-plane elevation. The scapular plane was defined as the plane 30° anterior to the frontal plane. The height of the targets was set relative to subject anthropometry, with the top target set at the height of the subject's chin, while standing, and the bottom target set to the height of the superior pole of the patella, while the subject was seated (Figure 1). A lap belt was also used to limit the movement of the hips and upper torso during setup and testing.
Figure 1. Patient testing setup. Each patient performed the humeral elevation and lowering tasks between the anthropometrically assigned hand triggers. We attached electromagnetic tracking sensors to the sternum, upper arm, forearm, and scapula (not shown in photograph) of each patient while he or she sat in a wooden chair. Adapted from the Journal of Shoulder and Elbow Surgery, 17(1), Scibek JS, Mell AG, Downie BK, Carpenter JE, Hughes RE, Shoulder kinematics in patients with full-thickness rotator cuff tears following a subacromial injection, 172–181, 2008, with permission from Elsevier.10.
Shoulder kinematic data were collected before and after a subacromial lidocaine injection. For the preinjection kinematic data acquisition, the initial plane of motion was systematically randomized for each subject. The pressure-sensitive triggers on each target were designed to accommodate the palmar and dorsal surfaces of the hand when they came into contact. The use of the triggers in the study setup did not require the subjects to stray from the desired plane or arc of elevation and lowering. They began each humeral elevation task with their fingers depressing the trigger on the bottom target and, when instructed, proceeded to move at a self-selected, comfortable pace to the top target. On reaching the top target, they activated the trigger on the target and then returned their hand to the starting position. The triggers were designed to turn off and on as the hand left and came into contact with the trigger, respectively. Both target triggers were electrically interfaced with the data collection system to determine when the hand left or reached the target. Using the triggers, we were certain that the desired data had been captured in their entirety through the complete arc of motion being assessed. Each subject practiced the elevation task for each plane 3 times, allowing them to gain comfort and familiarity with the various motions. After the practice trials, they performed shoulder elevation and lowering in each of the corresponding planes 3 times, with 10 seconds' rest between repetitions and 3 minutes' rest between planes.
The subjects received a subacromial injection in the involved shoulder after the initial kinematic data acquisition. To prevent potential adverse reactions to the injection, they were carefully questioned about medication allergies and reactions to lidocaine and other commonly used local anesthetics before study enrollment and again before the time of injection. A clinician performed the injections using a standard sterile technique. No additional modalities were used to ensure needle placement. However, a posterior approach was used to assist in the identification of appropriate placement of the injection within the subacromial space. The subject's subacromial space was identified by palpating the spine of the scapula and following it laterally until the posterior angle of the acromion and lateral border of the acromion were palpable. After identifying the posterior angle of the acromion, the clinician palpated the sulcus just inferior to the posterior angle of the acromion (approximately 1 to 1.5 cm directly caudal to the acromion) and prepared the skin 3 times with povidone-iodine solution. The clinician applied gentle caudal traction to the humerus at the elbow with the shoulder in neutral rotation. A 10-mL syringe with a 1.5-in (3.81-cm) 22-gauge needle was used to deliver 10 mL of 1% lidocaine into the subacromial space. The lidocaine was injected toward the position of the coracoid on the anterior aspect of the shoulder. Patients undergoing this procedure are expected to experience a decrease in pain within 5 minutes of having received the injection, with the effects lasting from 1 to 3 hours.17
After the injection, the subjects were provided with a 10-minute rest period to adapt to the decrease in pain associated with the injection. During this rest period, they moved the involved arm, gradually increasing the amplitude to circulate the medication and to acclimate to the decreased sensation of pain. After the rest period, they were allotted 5 minutes to rerate their pain level using the aforementioned visual analog scale. At the conclusion of the standardized rest period and pain level rating, the shoulder elevation protocol was repeated.
Data Processing
The independent variables for the study included pain (preinjection [initial pain level] and postinjection [final pain level]), which was measured with the visual analog scale, and tear size. The change in pain was calculated as the difference between preinjection and postinjection. The dependent measure was SHR. Included in the SHR measures were SHR for anterior-posterior tilt (SHRAP Tilt), medial-lateral tilt (SHRML Tilt), and scapular upward-downward rotation (SHRRotation). Scapulohumeral rhythm for humeral elevation was defined by the ratio of the specified scapular motion observed during humeral elevation (posterior tilt, medial tilt, or upward rotation) to GH elevation, and SHR for humeral lowering was defined by the ratio of scapular motion observed for humeral lowering (anterior tilt, lateral tilt, or downward rotation) to GH lowering.
To calculate scapular and humeral kinematics, Euler angles were computed from the MotionStar sensor data, after capture via the MotionMonitor system and reduction through custom MATLAB processing code (The Math- Works, Natick, MA). Data were smoothed by use of a 2-way, low-pass, fourth-order Butterworth filter with an 8-Hz cutoff frequency.6,18 The digitized landmarks, segmental anatomic coordinate systems, and Euler angles used were those proposed and adopted by the International Society of Biomechanics.19 For the purposes of our study, humeral elevation and scapular motion were assessed with respect to the sternum. Scapular anterior-posterior tilt was defined as scapular motion with respect to the sternum about an axis directed horizontally relative to the scapular plane, whereas scapular medial tilt and scapular lateral tilt occurred about an axis directed vertically relative to the plane of the scapula. Scapular upward rotation and downward rotation were defined as scapular motion with respect to the sternum about an axis directed anteriorly relative to the plane of the scapula. GH joint elevation was defined by motion of the humerus with respect to the scapula.
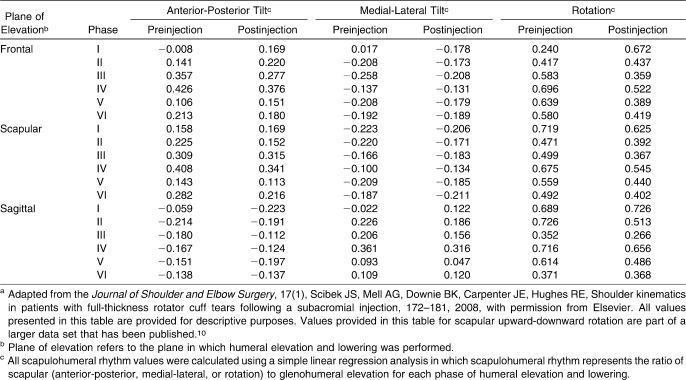
Because of the anthropometric variability, although the subjects performed similar arcs of humeral elevation and lowering, the data were divided into 3 equal phases for both elevation and lowering to compare the data between subjects (Figure 2). Ultimately, 6 phases were defined. The humeral elevation arc was divided into 3 equal phases, and 3 equal phases were defined for the humeral lowering arc. For each phase of elevation and lowering, scapular rotation and GH elevation were plotted against each other, and SHR was calculated for each trial (Figure 3). Delineation of the phases of motion within each arc of humeral motion was performed to allow for comparisons while minimizing anthropometric variability and has been cited as a method by which to assess SHR more accurately.20 Furthermore, this data processing technique was used in a previous study to compare shoulder kinematics associated with humeral elevation between groups of both healthy and impaired subjects.6 Simple linear regression analyses were used to compute SHR relative to triplanar scapular tilt with the slope corresponding to SHR. The regression analyses were performed for each of the 3 individual phases associated with both elevation and lowering.
Figure 2. Arc of motion divided into phases for humeral elevation and lowering. Humeral motion was plotted against time, and the maximum point of elevation was determined for each trial. Total arc of motion achieved during the humeral elevation was calculated by subtracting the point at which humeral elevation was initiated (baseline) from the maximum point of elevation. The arc of motion was divided into 3 equal segments and labeled as phases I, II, and III for humeral elevation and phases IV, V, and VI for humeral lowering. Adapted from the Journal of Shoulder and Elbow Surgery, 17(1), Scibek JS, Mell AG, Downie BK, Carpenter JE, Hughes RE, Shoulder kinematics in patients with full-thickness rotator cuff tears following a subacromial injection, 172–181, 2008, with permission from Elsevier.10.
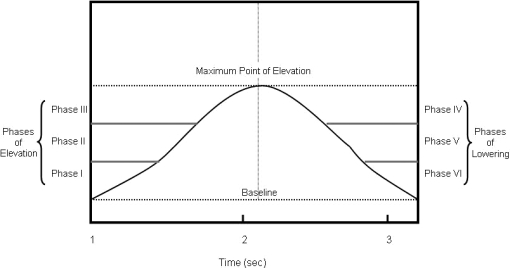
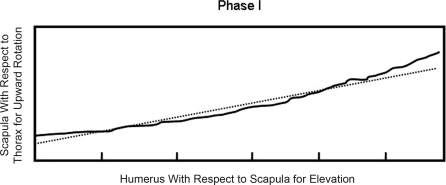
Figure 3. Diagram for scapulohumeral rhythm calculation with respect to the slope of the regression. The slope obtained during the simple linear regression analyses corresponded to scapulohumeral rhythm. These calculations represent scapular upward-downward rotation phase I. The solid line represents real motion for the humerus (elevation) and scapula (scapular rotation); the dashed line represents the line based on the slope for the linear regression. The same calculations also were performed for scapulohumeral rhythm for anterior-posterior tilt and scapulohumeral rhythm for medial-lateral tilt for each phase of elevation and lowering.
Data Analysis
Means and SDs were calculated for preinjection pain, change in pain, SHRRotation, SHRAP Tilt, and SHRML Tilt for each plane of elevation. Three sets of Spearman correlation analyses were conducted to determine if any relationship existed between (1) preinjection pain and preinjection SHR, (2) change in pain and change in SHR (preinjection to postinjection), and (3) tear size and SHR. Spearman correlation analyses were used because both pain scores and tear sizes were tabbed as ordinal data. The criteria used for evaluating the strength of correlation coefficients were as follows: 0.00 to 0.25 equaled no or little relationship; 0.25 to 0.50, fair relationship; 0.50 to 0.75, moderate to good relationship; and >0.75, good to excellent relationship.21
To perform the multivariate analysis of pain and tear size as predictors of SHR, the following model was used:
where Yi is SHR for the ith patient, Xi1 is the pain score for the ith patient, Xi2 is the tear size for the ith patient, β0 is the y intercept, β1 is the regression coefficient for pain, β2 is the regression coefficient for tear size, β3 is the interaction term for pain and tear size, and εi is the error for the ith patient.
For SHRRotation, SHRAP Tilt, and SHRML Tilt, we conducted a multivariate analysis for each plane of elevation across each phase of humeral elevation and lowering. Through this analysis, we were able to observe whether pain and tear size contributed to the model independently and/or collectively. Additionally, a Spearman correlation analysis was used to identify any relationship between pain and tear size.
For all of the statistical analyses, the α level was set a priori at .05. All statistical analyses were performed using SPSS (version 13.0; SPSS, Inc, Chicago, IL).
Results
Pain Versus SHR
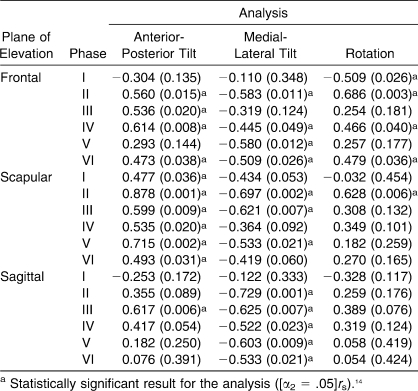
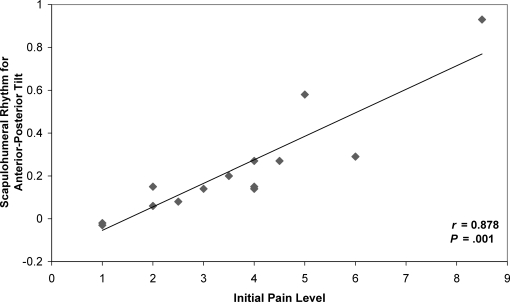
As Scibek et al10 reported, changes were observed in SHR during humeral elevation and lowering after a decline in pain (Table 2). Positive relationships were identified between initial pain and SHR for each scapular motion across both phases and planes of elevation (Table 3). These direct linear relationships indicated that patients experiencing higher levels of pain also exhibited higher values for SHR, corresponding to an increased reliance on scapular contributions to overall humeral motion. The data presented in Figure 4 indicate the positive linear relationships observed between preinjection pain and SHR for the phase that was identified as statistically significant with respect to scapular motion and plane of elevation.
Table 2.
Mean Values of Scapulohumeral Rhythm for Planes of Humeral Elevation by Scapular Motiona
Table 3.
Spearman Correlation Analysis Between Preinjection Pain and Scapulohumeral Rhythm Measures
Figure 4. Positive linear relationship between preinjection pain and scapulohumeral rhythm for anterior-posterior tilt for phase II of scapular plane humeral motion.
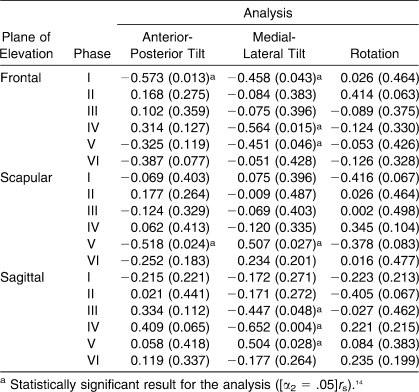
Linear relationships also were identified between the change in pain levels and changes in SHR (Table 4). The correlation analyses revealed moderate-to-good positive linear relationships for SHRML Tilt in phase IV of frontal-plane elevation and in phases IV of sagittal-plane elevation. Unlike the relationships observed between preinjection pain and SHR, no positive correlations were identified between change in pain and changes in SHRRotation and SHRAP Tilt for sagittal-, scapular-, or frontal-plane humeral elevation and lowering.
Table 4.
Spearman Correlation Analysis Between the Change in Pain and the Change in Scapulohumeral Rhythm Measures
Tear Size Versus SHR
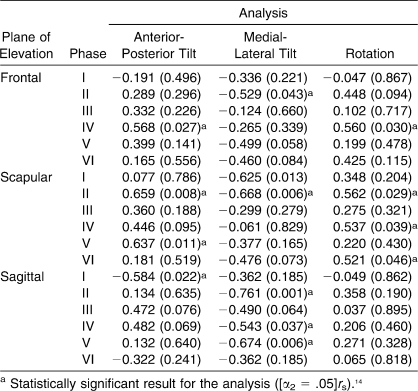
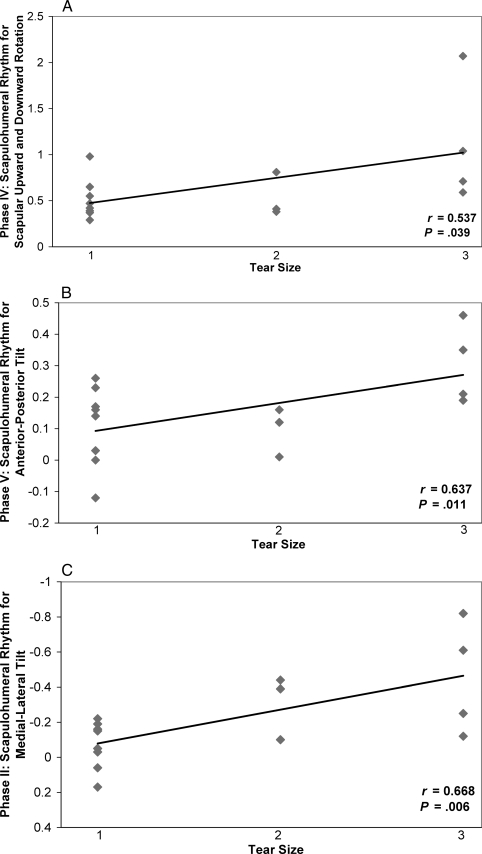
Upon review and analysis of the SHR data with respect to the tear size, it was evident that SHR tended to be higher in patients with larger RCTs (Table 5). These positive correlations indicated that, as tear size increased, SHR increased. These results further indicated that, because of the increase in full-thickness RCTs, the reliance on scapular contributions to overall shoulder motion increased to compensate for the structural deficit. The data presented in Figure 5 represent what we observed for the positive relationships that were identified for the respective phases in each plane of elevation.
Table 5.
Spearman Correlation Analysis Between Tear Size and Scapulohumeral Rhythm Measures
Figure 5. Positive linear relationships between tear size and scapulohumeral rhythm for scapular-plane humeral elevation. A, Tear size versus scapular upward-downward rotation for phase IV. B, Tear size versus scapulohumeral rhythm for anterior-posterior tilt for phase V. C, Tear size versus scapulohumeral rhythm for medial-lateral tilt for phase II. 1 indicates small or moderate tears; 2, large tears; and 3, massive tears.
Pain and Tear Size Versus SHR
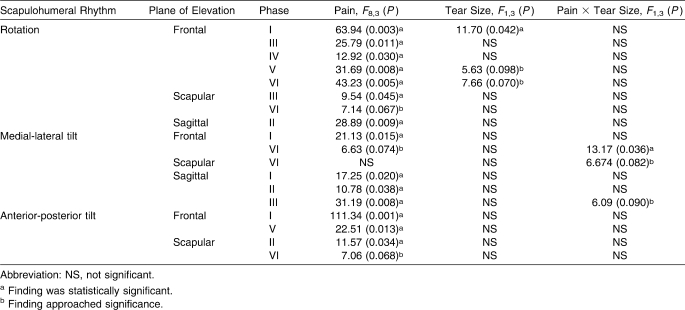
Multivariate analysis showed that pain was an independent predictor of SHRRotation, SHRAP Tilt, and SHRML Tilt for each plane of shoulder elevation (Table 6). Tear size was identified as a contributor for SHRRotation only for frontal-plane elevation. Additionally, remarkable interactions were identified between pain and tear size for frontal-, scapular-, and sagittal-plane elevation with respect to SHRML Tilt. Unlike the results observed for pain, the results observed for tear size alone and the interaction between pain and tear size exhibited limited instances of statistical significance, with other results only approaching significance (Table 6). Further examination of the multivariate analysis and the adjusted R2 values revealed that, when pain, tear size, or the interaction of pain and tear size were significant or approaching statistical significance, the proposed model accounted for 80% to 98% of the variance. Additionally, results of the correlation analysis for pain and tear size revealed a positive relationship approaching statistical significance ([α2 = .05]rs)14 = 0.507, P = .054).
Table 6.
Results of Multivariate Analysis for Pain, for Tear Size, and for the Interaction of Pain and Tear Size
Interparticipant Variability
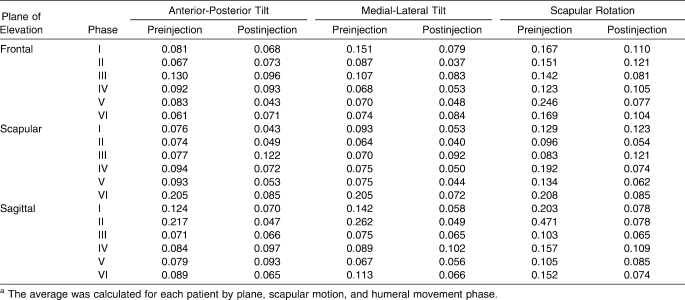
Average SDs for preinjection and postinjection SHR were calculated by plane, scapular motion, and phase (Table 7). A wide distribution of interparticipant variability was identified, with average SDs ranging from 0.040 to 0.471.
Table 7.
Average of SDs for Scapulohumeral Rhythm Measuresa
Discussion
The purpose of this investigation was to identify the relationships between pain associated with RCTs, the size of the RCT, and SHR. We also attempted to identify whether pain and tear size, both independently and collectively, serve as predictors of SHR. We identified numerous positive relationships between initial levels of pain and SHR and between tear size and SHR. These results confirmed our belief that the extent of the RCT and pain associated with it affect movement of the scapula and humerus. However, our results did not overwhelmingly support a relationship between changes in pain level and changes in scapulothoracic contributions to overall shoulder motion. Our findings also indicated that pain levels associated with an RCT, independent of RCT size, contribute to the observed movement patterns of the shoulder.
Our data were consistent with our hypotheses that increased levels of pain are directly related to increased values of SHR in patients with RCTs and that pain is an independent predictor of SHR. We not only found an overwhelming amount of evidence when looking at the relationship between pain and SHRAP Tilt and SHRML Tilt, but we also identified positive linear relationships for all 3 SHR ratios over each plane of humeral elevation. The relationships ranged from fair to excellent, with most falling within the moderate-to-good and good-to-excellent categories. However, we did not observe these positive relationships to the same extent when looking at the relationship between pain and SHRRotation. Based on the results of the multivariate analysis, we noted that pain served as a factor in the prediction of SHR in patients with RCTs for each plane of humeral elevation. Although the role of SHRRotation in shoulder kinematics continues to be emphasized, an ever-increasing body of knowledge supports the important contributions of both the anterior-posterior and medial-lateral tilting of the scapula to overall shoulder function and supports their possible implications with respect to shoulder injury and disability.2,3,22,23 The reason behind our limited number of significant findings with respect to SHRRotation is unclear; however, these results do coincide with what investigators have observed when looking at changes in SHR after a reduction in pain.10
Authors of some studies have indicated that patients experiencing shoulder impingement exhibit reduced scapular motion, which results in limited acromion clearance and added compression of subacromial structures.1,2 However, little evidence is available to support whether these changes in shoulder kinematics are causing the pain and dysfunction associated with the condition or whether these changes are the cumulative effect of the impingement and pain. Although investigators have observed altered kinematic patterns in patients with RCTs, the association between pain and altered kinematic patterns has not been established.6,7,9 The positive relationships that we identified show that, as pain levels associated with an RCT increase, SHR also increases, indicating that changes in SHR may be, in part, a compensatory reaction to overall pain associated with an RCT.
We also identified positive linear relationships between changes in pain and changes in SHR. However, these relationships were not observed to the anticipated extent, based on the relationships identified between initial pain levels and SHR. Significant relationships were categorized as moderate-to-good positive linear relationships. However, these results were sporadic, were not present for all 3 planes of humeral elevation, and did not extend throughout the measures of triplanar SHR. These relationships were only identified for SHRML Tilt for phase IV of frontal-plane elevation and phase IV of sagittal-plane elevation. As a result, clinicians must remain cautious in supporting our hypothesis regarding the relationship between changes in pain and SHR after a lidocaine injection.
Drawing further conclusions on the selective relationships observed between changes in RCT pain and changes in SHR is difficult. The chronic nature of the injury and the long-term pain associated with it could affect the relationship. Chronic pain likely led to certain adaptations in the movement pattern and muscle activity.24
Although the literature2,3,4,6,7,9,25 supports the idea that patients with shoulder disorders rely on compensatory mechanisms to cope with pain and damage associated with RCTs and shoulder impingement, investigators26–31 have shown that modifications in stimuli and exposure to practice and teaching elements foster reorganization in movement patterns and adaptations associated with brain functioning, both immediately and over time. Although some of the SHR changes correlated with the immediate changes in pain, it is unrealistic to believe that all of these formerly acquired kinematic patterns could be eliminated upon the immediate reduction in pain, especially because many of these patients had been experiencing pain for a long time.
The positive linear relationships identified for tear size indicate that, as RCT size increases, SHR increases, which translates to a greater reliance on scapular contributions to overall shoulder motion in patients with larger RCTs. Our findings again were consistent with our hypothesis and provided additional support to the idea that compensatory scapular kinematic patterns are adopted to compensate for alterations in the ability of the rotator cuff to function. Participants in the moderate, large, and massive RCT groups could generate the necessary muscle activity and force to perform humeral elevation and to move between the anthropometrically aligned triggers used in the study. However, compared with their counterparts, patients with large and massive RCTs relied to a greater extent on scapular motion to achieve the same degree of overall humeral motion, translating into a relative shift in contributions of the scapula and humerus to compensate for the disruption in the rotator cuff. The results obtained regarding tear size and SHR corroborate similar findings by Mell et al,5 who assessed differences in SHR among healthy controls, patients with small or moderate RCTs, and patients with large or massive tears. We both noted that, as the size of the RCT increased, the reliance on scapular contributions to humeral elevation also increased. Although we both identified similar variations in SHR as the extent of the injury changes, the exact reason behind these adaptations is unclear. The lack of significant findings throughout each phase for scapular motion, however, also remains puzzling. The absence of research findings in this area makes it difficult to further explain our findings. Additional investigation may be necessary to determine whether an interaction exists between tear size location and both plane and phase of humeral elevation.
Unlike our findings for the linear relationship between tear size and SHR, our results indicated that tear size is a contributor to, but not an independent predictor of, SHR. One instance of statistical significance was identified for frontal-plane SHRRotation, with only 2 other instances approaching significance. Again, clinicians must cautiously consider our hypothesis concerning tear size as an independent factor in the prediction of SHR, and the mechanisms behind these significant relationships between tear size and SHR need to be further studied. Although a relationship does appear to exist between tear size and SHR, the true involvement of the RCT and the compensatory mechanisms around the shoulder for rotator cuff insufficiency are not as well understood.
Although pain and tear size collectively could contribute to the scapulohumeral kinematic patterns observed in our patients with RCTs, our results provide little evidence to support this hypothesis. We identified only 1 statistically significant interaction between pain and tear size, with 2 other interactions approaching the level of significance. When statistical significance was achieved or approached by 1 or more factors, the model accounted for a large percentage of the variability. However, much of that could be attributed to the independent involvement of pain, followed by tear size and then by the limited interaction of both factors. Although a positive relationship may exist between tear size and pain, the extent to which they interact and, thus, collectively affect SHR, is still unknown. In kinematic studies of patients with symptomatic RCTs, researchers6,7,9 have observed increased scapulothoracic contributions during humeral elevation and have speculated on the involvement of pain and tear size. Furthermore, they have tabbed the resultant increase in scapulothoracic contributions as an attempt to limit pain associated with the tear and to facilitate the adjustment of length-tension relationships of the existing rotator cuff muscles.6,7,9 Although our results are not overwhelmingly favorable regarding the interaction between pain and tear size and their effect on SHR, we are the first to have specifically examined the involvement of pain and tear size collectively on shoulder kinematics in patients with symptomatic full-thickness RCTs.
Limitations and Future Research Considerations
With the design of our study, we were unable to determine by what mechanism pain is causing these shoulder kinematic patterns. However, because of the observed influence of pain on SHR, further investigation is required. Similar to previous studies involving lifting mechanics32 and gait patterns,33 our results provide evidence to support the effect of pain on body mechanics. The mechanisms by which pain alters kinematics may include changes in muscle activity,34 muscle inhibition,35,36 decreases in proprioception and kinesthetic awareness,37,38 and alterations in neuromuscular control.39 Although the use of a lidocaine injection may have affected any one of these mechanisms, the lidocaine may have decreased pain and muscle inhibition, resulting in the relationships that we observed between pain and SHR. Modifications in study design would be necessary to truly ascertain this information. Furthermore, researchers investigating the involvement of chronic pain and muscle inhibition specifically should attempt to address both the documented physical24 and psychosocial factors35,36 involved.
Continued investigation into the mechanical modifications due to rotator cuff deficiency also is required. Relationships between tear size and SHR have been identified in this and another study5; yet the biomechanical explanation for this phenomenon is still only speculative. Our results and the ability of each patient to perform the required tasks indicate that compensatory shifts in SHR enhance the length-tension relationships of the rotator cuff in an effort to combat limitations caused by structural deficiency. Additionally, little is known about the effect of tear location and configuration on shoulder kinematics and function. Future investigation into these areas could provide additional insight into the effects of rotator cuff injury on SHR.
When considering the relationships observed between tear size and SHR, note the large intersubjects variability with respect to SHR and the potential outliers associated with the correlation analyses. Based upon the current body of knowledge regarding shoulder kinematics, the reason that some of our patients experienced a negative SHR that was equal in magnitude to others in the same tear size group is unclear. Consideration must be given to the movement environment and the potential need for an increased level of control to reduce potential sources of between-subjects variability. However, we also must consider the findings of other investigators2,6,10,40 who identified shoulder kinematic patterns in injured shoulders that stray from what is normal in magnitude, direction, and presentation and must consider that the patterns that we observed, in fact, may be the clinical presentation of SHR in a subset of patients with RCTs. Additionally, some readers may question the limited number of participants and the inclusion of data points on the scatter plot that could present as outliers in other data sets. Future researchers should include both a larger sample size and even distribution of those patients over the respective tear-size groups. However, because of the limited amount of research addressing RCTs and shoulder kinematics, clearly identifying whether some of the data points from our data set are indeed outliers or are consistent for this patient population is difficult. The current literature might suggest that the inconsistencies being observed in the data are, in fact, consistent for this patient population and require further investigation.
When quantifying shoulder kinematics in patients with full-thickness RCTs, future investigators may need to consider additional clinical elements, including capsular patterns,40–42 muscle length,43 muscle fatigue and strength,23,44–46 and rehabilitative efforts4,47 because they may have had an effect on our findings. Patients included in our study had no other shoulder disorders; however, evidence presented on patients with adhesive capsulitis,41,42 multidirectional instability,40 and shortened pectoralis minor length43 suggests that careful evaluation of capsular patterns and muscle length is warranted because of observed differences in, and their abilities to contribute to, shoulder kinematic patterns. The role of rotator cuff and scapula-stabilizing muscle strength in shoulder kinematics also has been examined.20,23,45,47 Muscle fatigue and movement of the shoulder against external resistance or loading in some cases has been shown to affect SHR.20,23,45 A small amount of evidence suggests that shoulder strengthening and stretching exercises influence shoulder kinematic patterns.47 However, limited quantitative data are available to compare the strength of specific muscles with selected scapulohumeral movement patterns.44 We manually measured shoulder ranges of motion and strength before testing, but employing an isokinetic or handheld dynamometer both before and after the injection may be useful in confirming the effect that pain reduction has on muscle strength and the potential role that specific muscle strength may have on scapular kinematic patterns. Additionally, although some patients were engaged in a rehabilitation program at the time of testing and others had been involved before testing, the specifics of those rehabilitation efforts (ie, rotator cuff and scapula-stabilizing muscle strengthening) were unknown. Certainly, the areas of emphasis within each program could have implications for the shoulder kinematic patterns observed in patients with shoulder disorders; however, we are uncertain to what degree the programs themselves and the variability among them would have on observed shoulder kinematic patterns.4,47
Clinical Relevance
Based on our findings, the ability of the clinician to properly manage pain associated with an RCT has major implications for shoulder motion. Before repair of an RCT, restoration of “normal” shoulder function will remain a challenge because of the chronic nature of this injury, the pain associated with it, and the amount of structural damage involved. However, the compensatory kinematic adaptations both to pain and to the RCT will enable the patient to maintain shoulder function, so our attention to pain control and scapulothoracic function, which is a cornerstone in shoulder rehabilitation,48,49 is even more critical. Although our results do not provide insight into long-term pain control, it is plausible that, with added pain relief and rehabilitation, an additional effect on these kinematic patterns may be observed. The acquisition of movement patterns developing over some period as the body adapts to new and repeated stimuli is well documented.26–28,30,31 As we sporadically observed, certain aspects are assimilated rapidly; however, others require additional time to materialize. Typically, the goal of both the patient and the clinician is to make progress during individual rehabilitation sessions and over time. Multiple factors (ie, pain, tissue healing, and improvements in motion and strength) could influence those developments during the recovery process, with each factor contributing to the restoration of optimal function. Scapulohumeral rhythm may continue to be challenged if the structural deficiency is not corrected, but, to facilitate their outcomes-based rehabilitative efforts, clinicians must continue to monitor and quantify adaptations in kinematic patterns as pain levels change and progression in the rehabilitation process occurs.
Acknowledgments
This study was funded by the Great Lakes Athletic Trainers' Association Research Assistance Funds via the Bob Behkne Research Assistance Award. We thank Dr Riann Palmieri-Smith, Dr Kirsten Namesnik, Dr Susan Brown, and Dr Barry McDonough for their assistance with this study; Dr James Carpenter, Dr Bruce Miller, and Dr Waldomar Roeser for providing access to their patients; the University of Michigan Orthopaedic Research Laboratories and MedSport for their contributions to this project; and the Great Lakes Athletic Trainers' Association for providing assistance and financial support of this investigation.
Footnotes
Jason S. Scibek, PhD, ATC; James E. Carpenter, MD; and Richard E. Hughes, PhD, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting, critical revision, and final approval of the article.
Portions of the Introduction and Methods sections are reprinted from the Journal Of Shoulder And Elbow Surgery, 17(1), Scibek JS, Mell AG, Downie BK, Carpenter JE, Hughes RE, Shoulder kinematics in patients with full-thickness rotator cuff tears following a subacromial injection, 172–181, 2008, with permission from Elsevier.
References
- 1.Borstad J.D, Ludewig P.M. Comparison of scapular kinematics between elevation and lowering of the arm in the scapular plane. Clin Biomech (Bristol, Avon) 2002;17(9–10):650–659. doi: 10.1016/s0268-0033(02)00136-5. [DOI] [PubMed] [Google Scholar]
- 2.Ludewig P.M, Cook T.M. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80(3):276–291. [PubMed] [Google Scholar]
- 3.Lukasiewicz A.C, McClure P, Michener L, Pratt N, Sennett B. Comparison of 3-dimensional scapular position and orientation between subjects with and without shoulder impingement. J Orthop Sports Phys Ther. 1999;29(10):574–586. doi: 10.2519/jospt.1999.29.10.574. [DOI] [PubMed] [Google Scholar]
- 4.McClure P.W, Bialker J, Neff N, Williams G, Karduna A. Shoulder function and 3-dimensional kinematics in people with shoulder impingement syndrome before and after a 6-week exercise program. Phys Ther. 2004;84(9):832–848. [PubMed] [Google Scholar]
- 5.Mell A.G, Hughes R.E, Carpenter J.E. Effect of rotator cuff tear size on shoulder kinematics. In: Transactions of the 51st Annual Meeting of the Orthopaedic Research Society; February 20–23, 2005; Washington, DC. Poster 0623.
- 6.Mell A.G, LaScalza S, Guffey P, et al. Effect of rotator cuff pathology on shoulder rhythm. J Shoulder Elbow Surg. 2005;14(1 suppl S):58S–64S. doi: 10.1016/j.jse.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Paletta G.A, Jr, Warner J.J, Warren R.F, Deutsch A, Altchek D.W. Shoulder kinematics with two-plane x-ray evaluation in patients with anterior instability or rotator cuff tearing. J Shoulder Elbow Surg. 1997;6(6):516–527. doi: 10.1016/s1058-2746(97)90084-7. [DOI] [PubMed] [Google Scholar]
- 8.Warner J.J, Micheli L.J, Arslanian L.E, Kennedy J, Kennedy R. Scapulothoracic motion in normal shoulders and shoulders with glenohumeral instability and impingement syndrome: a study using Moiré topographic analysis. Clin Orthop Relat Res. 1992;285:191–199. [PubMed] [Google Scholar]
- 9.Yamaguchi K, Sher J.S, Anderson W.K, et al. Glenohumeral motion in patients with rotator cuff tears: a comparison of asymptomatic and symptomatic shoulders. J Shoulder Elbow Surg. 2000;9(1):6–11. doi: 10.1016/s1058-2746(00)90002-8. [DOI] [PubMed] [Google Scholar]
- 10.Scibek J.S, Mell A.G, Downie B.K, Carpenter J.E, Hughes R.E. Shoulder kinematics in patients with full-thickness rotator cuff tears following a subacromial injection. J Shoulder Elbow Surg. 2008;17(1):172–181. doi: 10.1016/j.jse.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins R.J, Morin W.D, Bonutti P.M. Surgical treatment of full-thickness rotator cuff tears in patients 40 years of age or younger. J Shoulder Elbow Surg. 1999;8(3):259–265. doi: 10.1016/s1058-2746(99)90139-8. [DOI] [PubMed] [Google Scholar]
- 12.Richards R.R, An K.N, Bigliani L.U, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347–352. doi: 10.1016/S1058-2746(09)80019-0. [DOI] [PubMed] [Google Scholar]
- 13.LaScalza S, Gallo L.N, Carpenter J.E, Hughes R.E. A method for measuring Euler rotation angles and helical axis of upper arm motion. J Appl Biomech. 2002;18(4):374–383. [Google Scholar]
- 14.Karduna A.R, McClure P.W, Michener L.A, Sennett B.J. Dynamic measurements of three-dimensional scapular kinematics: a validation study. J Biomech Eng. 2001;123(2):184–190. doi: 10.1115/1.1351892. [DOI] [PubMed] [Google Scholar]
- 15.Meskers C.G.M, Fraterman H, Van der Helm F.C.T, Vermeulen H.M, Rozing P.M. Calibration of the “Flock of Birds” electromagnetic tracking device and its application in shoulder motion studies. J Biomech. 1999;32(6):629–633. doi: 10.1016/s0021-9290(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 16.van der Helm F.C.T. A standardized protocol for motion recordings of the shoulder. In: Veeger H.E.J, van der Helm F.C.T, Rozing P.M, editors. Proceedings of the First Conference of the International Shoulder Group, Delft University of Technology. Aachen, Germany: Shaker Publishing BV; 1997. pp. 7–14. [Google Scholar]
- 17.Bach B.R, Jr, Bush-Joseph C.A. Subacromial space injections: a tool for evaluating shoulder pain. Physician Sportsmed. 1992;20(2):93–97. doi: 10.1080/00913847.1992.11947410. [DOI] [PubMed] [Google Scholar]
- 18.Yu B, Gabriel D, Noble L, An K.N. Estimate of the optimum cutoff frequency for the Butterworth low-pass digital filter. J Appl Biomech. 1999;15(3):318–329. [Google Scholar]
- 19.Wu G, van der Helm F.C, Veeger H.E, Makhsous M, Van Roy P, Anglin C, Nagels J, Karduna A.R, McQuade K, Wang X, Werner F.W, Buchholz B. International Society of Biomechanics. ISB recommendation on definition of joint coordinate systems of various joints for the reporting of human joint motion–Part II: shoulder, elbow, wrist and hand. J Biomech Eng. 2005;38:981–992. doi: 10.1016/j.jbiomech.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 20.McQuade K.J, Smidt G.L. Dynamic scapulohumeral rhythm: the effects of external resistance during elevation of the arm in the scapular plane. J Orthop Sports Phys Ther. 1998;27(2):125–133. doi: 10.2519/jospt.1998.27.2.125. [DOI] [PubMed] [Google Scholar]
- 21.Portney L.G, Watkins M.P. Foundations of Clinical Research: Applications to Practice. Upper Saddle River, NJ: Prentice Hall Health; 2000. pp. 494–495. [Google Scholar]
- 22.McClure P.W, Michener L.A, Sennett B.J, Karduna A.R. Direct 3-dimensional measurement of scapular kinematics during dynamic movements in vivo. J Shoulder Elbow Surg. 2001;10(3):269–277. doi: 10.1067/mse.2001.112954. [DOI] [PubMed] [Google Scholar]
- 23.McQuade K.J, Hwa Wei S, Smidt G.L. Effects of local muscle fatigue on three-dimensional scapulohumeral rhythm. Clin Biomech (Bristol, Avon) 1995;10(3):144–148. doi: 10.1016/0268-0033(95)93704-w. [DOI] [PubMed] [Google Scholar]
- 24.Lund J.P, Donga R, Widmer C.G, Stohler C.S. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69(5):683–694. doi: 10.1139/y91-102. [DOI] [PubMed] [Google Scholar]
- 25.Poppen N.K, Walker P.S. Normal and abnormal motion of the shoulder. J Bone Joint Surg Am. 1976;58(2):195–201. [PubMed] [Google Scholar]
- 26.Classen J, Liepert J, Wise S.P, Hallett M, Cohen L.G. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- 27.Kleim J.A, Barbay S, Nudo R.J. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80(6):3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 28.Dorris M.C, Pare M, Munoz D.P. Immediate neural plasticity shapes motor performance. J Neurosci. 2000;20(1):1–5. doi: 10.1523/JNEUROSCI.20-01-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernardi M, Solomonow M, Nguyen G, Smith A, Baratta R. Motor unit recruitment strategy changes with skill acquisition. Eur J Appl Physiol Occup Physiol. 1996;74(1–2):52–59. doi: 10.1007/BF00376494. [DOI] [PubMed] [Google Scholar]
- 30.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21(14):5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karni A, Meyer G, Rey-Hipolito C, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95(3):861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Commissaris D.A, Nilsson-Wikmar L, van Dieen J.H, Hirschfeld H. Joint coordination during whole-body lifting in women with low back pain after pregnancy. Arch Phys Med Rehabil. 2002;83(9):1279–1289. doi: 10.1053/apmr.2002.33641. [DOI] [PubMed] [Google Scholar]
- 33.Benedetti M.G, Bonato P, Cantani F, et al. Myoelectric activation pattern during gait in total knee replacement: relationship with kinematics, kinetics, and clinical outcome. IEEE Trans Rehabil Eng. 1999;7(2):140–149. doi: 10.1109/86.769404. [DOI] [PubMed] [Google Scholar]
- 34.Ruwe P.A, Pink M, Jobe F.W, Perry J, Scovazzo M.L. The normal and the painful shoulders during the breaststroke: electromyographic and cinematographic analysis of twelve muscles. Am J Sports Med. 1994;22(6):789–796. doi: 10.1177/036354659402200610. [DOI] [PubMed] [Google Scholar]
- 35.Verbunt J.A, Seelen H.A, Vlaeyen J.W, et al. Pain-related factors contributing to muscle inhibition in patients with chronic low back pain: an experimental investigation based on superimposed electrical stimulation. Clin J Pain. 2005;21(3):232–240. doi: 10.1097/00002508-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Al-Obaidi S.M, Nelson R.M, Al-Awadhi S, Al-Shuwaie N. The role of anticipation and fear of pain in the persistence of avoidance behavior in patients with chronic low back pain. Spine. 2000;25(9):1126–1131. doi: 10.1097/00007632-200005010-00014. [DOI] [PubMed] [Google Scholar]
- 37.Newcomer K.L, Jacobson T.D, Gabriel D.A, Larson D.R, Brey R.H, An K.N. Muscle activation patterns in subjects with and without low back pain. Arch Phys Med Rehabil. 2002;83(6):816–821. doi: 10.1053/apmr.2002.32826. [DOI] [PubMed] [Google Scholar]
- 38.Newcomer K.L, Laskowski E.R, Yu B, Johnson J.C, An K.N. Differences in repositioning error among patients with low back pain compared with control subjects. Spine. 2000;25(19):2488–2493. doi: 10.1097/00007632-200010010-00011. [DOI] [PubMed] [Google Scholar]
- 39.Svensson P, Wang K, Sessle B.J, Arendt-Nielsen L. Associations between pain and neuromuscular activity in the human jaw and neck muscles. Pain. 2004;109(3):225–232. doi: 10.1016/j.pain.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 40.Ogston J.B, Ludewig P.M. Differences in 3-dimensional shoulder kinematics between persons with multidirectional instability and asymptomatic controls. Am J Sports Med. 2007;35(8):1361–1370. doi: 10.1177/0363546507300820. [DOI] [PubMed] [Google Scholar]
- 41.Vermeulen H.M, Stokdijk M, Eilers P.H, Meskers C.G, Rozing P.M, Vliet Vlieland T.P. Measurement of three dimensional shoulder movement patterns with an electromagnetic tracking device in patients with a frozen shoulder. Ann Rheum Dis. 2002;61(2):115–120. doi: 10.1136/ard.61.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rundquist P.J. Alterations in scapular kinematics in subjects with idiopathic loss of shoulder range of motion. J Orthop Sports Phys Ther. 2007;37(1):19–25. doi: 10.2519/jospt.2007.2121. [DOI] [PubMed] [Google Scholar]
- 43.Borstad J.D, Ludewig P.M. The effect of long versus short pectoralis minor resting length on scapular kinematics in healthy individuals. J Orthop Sports Phys Ther. 2005;35(4):227–238. doi: 10.2519/jospt.2005.35.4.227. [DOI] [PubMed] [Google Scholar]
- 44.Ewy R, Hudson S, Miller C, Rhoads A. Scapular strength in presence of scapular winging and tipping in female athletes who participate in overhead sports. http://hdl.handle.net/10057/715. Accessed July 10, 2008.
- 45.McQuade K.J, Dawson J, Smidt G.L. Scapulothoracic muscle fatigue associated with alterations in scapulohumeral rhythm kinematics during maximum resistive shoulder elevation. J Orthop Sports Phys Ther. 1998;28(2):74–80. doi: 10.2519/jospt.1998.28.2.74. [DOI] [PubMed] [Google Scholar]
- 46.Tsai N.T, McClure P.W, Karduna A.R. Effects of muscle fatigue on 3-dimensional scapular kinematics. Arch Phys Med Rehabil. 2003;84(7):1000–1005. doi: 10.1016/s0003-9993(03)00127-8. [DOI] [PubMed] [Google Scholar]
- 47.Wang C.H, McClure P.W, Pratt N.E, Nobilini R. Stretching and strengthening exercises: their effect on three-dimensional scapular kinematics. Arch Phys Med Rehabil. 1999;80(8):923–929. doi: 10.1016/s0003-9993(99)90084-9. [DOI] [PubMed] [Google Scholar]
- 48.Kibler W.B. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325–337. doi: 10.1177/03635465980260022801. [DOI] [PubMed] [Google Scholar]
- 49.Wilk K.E, Arrigo C.A. Current concepts in the rehabilitation of the athletic shoulder. J Orthop Sports Phys Ther. 1993;18(1):365–378. doi: 10.2519/jospt.1993.18.1.365. [DOI] [PubMed] [Google Scholar]