Abstract
Objective To test the depression-distortion hypothesis in pediatric type 1 diabetes. Methods In a sample of 187 youth with type 1 diabetes, caregivers completed the Center for Epidemiologic Studies Depression (CES-D) scale and the Children's Depression Inventory (CDI): parent proxy report. Youth completed the CDI. To test whether caregiver depressive symptoms (CES-D) moderated the proxy report of youth depressive symptoms (CDI:P), the CDI, CES-D, and their interactions were entered as predictors in to a regression analysis. Results The regression was significant, F (8,178) = 9.26, p <.0001, R2 =.29, and all three variables were significant predictors. Post-hoc probing of the interaction showed that caregivers with high CES-D scores reported high levels of youth depressive symptoms at both high and low levels of youth-reported depressive symptoms. In contrast, caregivers with low CES-D scores reported similar levels as the youth. Conclusions These results support the depression-distortion hypothesis in a pediatric chronic disease sample.
Keywords: caregivers, depression, depression-distortion, proxy, type 1 diabetes
There is a well-documented association between the psychological functioning of caregivers and the development of the child. For example, the presence of depression in a caregiver can increase the risk of academic, social, and emotional difficulties in the child (Cicchetti, Rogosch, & Toth, 1998; Cytryn et al., 1984; Hay, Pawlby, Angold, Harold, & Sharp, 2003; Lyons-Ruth, Connell, Grunebaum, & Botein, 1990; Orvaschel, Weissman, & Kidd, 1980; Radke-Yarrow, Zahn-Waxler, Richardson, Susman, & Martinez, 1994; Silverstein, Augustyn, Cabral, & Zuckerman, 2006; Walker et al., 2007). Further, caregiver depression has been linked with negative health outcomes in children with and without chronic physical conditions. In a sample of 1,528 inner-city youth with asthma, there was a 2-fold increase in child hospitalization in the presence of caregiver depression (Weil et al., 1999). On a larger scale, multiple social risks including “low maternal mental health”, predicted poorer child health in the 2003 National Survey of Children's Health (Larson, Russ, Crall, & Halfon, 2008). These examples collectively show that the psychological health of caregivers is a key factor in child development and health outcomes.
Across various settings (e.g., medical and psychological care and clinical research), multiple informants are frequently asked to report on the psychological functioning of the child or adolescent. These assessments rely heavily on informants, typically the primary caregiver, as a method of ascertaining information about the child or adolescent to inform treatment decisions (Hawley & Weisz, 2003; Holmbeck, Li, Schurman, Friedman, & Coakley, 2002; Loeber, Green, Lahey, & Stouthamer-Loeber, 1991; Tates & Meeuwesen, 2001). Clinicians and researchers therefore require accurate, and as much as possible, uniform reports across informants in order to make appropriate decisions about care. There are, however, a host of factors that can promote discrepancies across informants. De Los Reyes & Kazdin (2005) provided a comprehensive review of individual, family, and system characteristics that contribute to discrepancies and highlighted that informant depression can lead to distorted reports of child behavior or emotions. More specifically, caregiver depression has fairly consistently led to reports of child behavior and emotions that are inflated compared to other reports including those of the child. This “depression-distortion hypothesis” has garnered empiric support across a number of child and adolescent psychological and psychiatric conditions (Breslau, Davis, & Prabucki, 1988; Chi & Hinshaw, 2002; Chilcoat & Breslau, 1997; Griest, Forehand, Wells, & McMahon, 1980; Jensen, Traylor, Xenakis, & Davis, 1988; Webster-Stratton, 1988; Webster-Stratton & Hammond, 1988; Youngstrom, Loeber, & Stouthamer-Loeber, 2000). Distorted reports may be the result of different attributions about the cause of the behaviors or emotions, differing perceptions about the need for treatment, as well as potential biases in memory recall (i.e., present mood influences recall) (De Los Reyes & Kazdin, 2005). Nevertheless, across a host of studies, relatively consistent findings point to the presence of depression in the caregiver distorting their report of youth depressive symptoms.
The depression-distortion hypothesis did not, however, come without opposing views and alternative interpretations of the findings (Richters, 1992; Richters & Pellegrini, 1989). Richters argued that necessary and sufficient criteria for “distortion” were not met for a large number of studies, particularly concerning child behavior problems. For example, the absence of a “gold standard” to compare reports of various informants against is problematic when attempting to establish which informant, the caregiver or the child, is actually providing the distorted report. Further, decisions made by researchers with regard to measurement of depressive symptoms (e.g., continuous score versus use of a clinical cutoff) and statistical approaches to measure associations may yield different and potentially misleading findings. Considering these caveats raised by Richters, several studies conducted after his review employed more sophisticated methodology and analysis (Chilcoat & Breslau, 1997; Najman et al., 2000) to provide stronger support for the depression-distortion hypothesis. While there are still critics of the depression-distortion hypothesis, empiric evidence tends to support the occurrence of distorted reports by depressed caregivers.
One important shortcoming of the research on the depression-distortion hypothesis, however, is the relative lack of studies in samples in which a child has a chronic disease. While several studies have investigated this topic in general pediatric clinics (Braaten et al., 2001; Wildman, Stancin, Golden, & Yerkey, 2004), a review of the literature identified no studies that specifically addressed this hypothesis in pediatric chronic disease. This is both surprising and concerning for a number of reasons. First, the number of daily treatment decisions made by families seen in general pediatric settings likely pales in comparison to the daily treatment decisions that have to be made in pediatric chronic disease. Given that a number of these management activities are performed as part of a “team” with the youth (Anderson, Ho, Brackett, Finkelstein, & Laffel, 1997; Drotar & Ievers, 1994; Rapoff, 1999), a caregiver's distorted perception of more depressive symptoms in the youth may impact this teamwork. The caregiver could expect less out of the “depressed” youth and assume more of the daily management activities, subsequently leading to greater burden and more depressive symptoms for the caregiver. Second, the misidentification of clinically significant depressive symptoms in a youth with a chronic disease due to caregiver depression may lead to a treatment plan that is not the appropriate match for the youth's problems. For example, individual treatment may be prescribed, but the problems are rooted in family variables (e.g., family conflict) and disease-specific management demands. Without a family-based treatment that targets disease-specific contextual variables, there may be little positive change in psychological functioning and disease management. Finally, given that multiple pediatric chronic diseases are associated with increased prevalence of elevated depressive symptoms in caregivers (Jaser, Whittemore, Ambrosino, Lindemann, & Grey, 2008; Quittner, DiGirolamo, Michel, & Eigen, 1992; Schulz & Quittner, 1998), there is a higher likelihood for the presence of caregiver depression-distortion in the pediatric chronic disease population. For these reasons, a test of the depression-distortion hypothesis in a pediatric chronic disease sample is timely and has both individual and public health significance.
In this study, the depression-distortion hypothesis was examined in a large sample of youth with type 1 diabetes and their caregivers. Type 1 diabetes is an appropriate pediatric chronic disease to test this hypothesis for several reasons. First, type 1 diabetes is an autoimmune disease characterized by the body's own destruction of the insulin-producing β cells (Atkinson & Eisenbarth, 2001). When the body no longer (or insufficiently) produces insulin, type 1 diabetes must be managed via a regimen of insulin administration and close monitoring of blood glucose levels, dietary intake, and physical activity. So, the management of type 1 diabetes is a complex and intensive process that requires youth and caregiver teamwork as well as collaboration with a multidisciplinary diabetes team (Silverstein et al., 2005). Second, type 1 diabetes is one of the most common pediatric chronic diseases, presently affecting 1 in 500 youth (Liese et al., 2006; NIDDK, 2005) with nearly 15,000 new diagnoses expected this year in the United States (Dabelea et al., 2007). Further, type 1 diabetes is linked to increased risk of suboptimal psychological functioning as evidenced by elevated rates of depression found in both youth and their caregivers (Grey, Whittemore, & Tamborlane, 2002; Hood et al., 2006; Jaser et al., 2008; Kovacs, Goldston, Obrosky, & Bonar, 1997). Thus, in this prevalent pediatric chronic disease, a higher proportion of youth and caregivers will experience depression providing sufficient numbers to test this hypothesis. Third, there appears to be a higher degree of caregiver–youth agreement about the presence of youth depressive symptoms in pediatric type 1 diabetes than in the general population. Specifically, a prior report in a separate sample of youth with type 1 diabetes (Hood et al., 2006) found a substantially higher correlation (r =.60 versus r =.41) between caregiver proxy and youth reports of depressive symptoms on the Children's Depression Inventory (CDI) compared to the rate published in the CDI manual (Kovacs, 2003). This increased agreement may be due to the demands of type 1 diabetes management within the family and this added intensity and supervision contributes to similar perceptions of youth functioning across caregivers and youth. However, that particular study (Hood and colleagues) did not consider the rate of caregiver depression and its potential influence on agreement.
Considering the need to address the depression-distortion hypothesis in pediatric chronic disease and the appropriateness of using a type 1 diabetes sample, the overarching goal of this study was to systematically test whether caregivers with elevated levels of depressive symptoms provide distorted reports. It was hypothesized that a higher degree of depressive symptoms in caregivers would inflate their proxy reports of youth depressive symptoms in comparison to the youth's report of symptoms, thus providing support for the depression-distortion hypothesis.
Method
Participants and Procedures
Study participants included 187 children and adolescents with type 1 diabetes and their caregivers receiving care at a tertiary pediatric diabetes center from a multidisciplinary team. All participants had type 1 diabetes diagnosed according to the American Diabetes Association (ADA) practice guidelines (Silverstein et al., 2005) and were between 10 and 18 years of age. Exclusion criteria included: major psychiatric or neurocognitive disorder that would limit the youth's ability to complete surveys (e.g., mental retardation); significant medical disease other than type 1 diabetes or treated thyroid disorders or celiac disease; present participation in a psychosocial intervention; or inability to read or understand English. During the study recruitment period, we approached 270 families and 196 (73%) agreed to participate. Of the families that declined participation, most did so because of lack of time or interest in study participation. We subsequently excluded 9 families from data analysis due to substantial missing or incomplete data for a final study sample of 187 youth and their caregivers. These 9 youth were not different from the final sample of 187 on family and sociodemographic characteristics. Prior to implementing the study procedures, the institutional Committee on Human Studies approved the protocol. A research assistant obtained written informed consent from participating caregivers and assent from the youth, then administered the questionnaires in the pediatric and adolescent clinic.
Measures
Youth depressive symptoms were assessed with the CDI (Kovacs, 2003), a self-report questionnaire consisting of 27 items rated from 0 (no symptom) to 2 (distinct symptom). CDI scores can range from 0–54 with a clinical cutoff score of 13 or higher indicative of elevated depressive symptoms and suggestive of further evaluation (Grey et al., 2002; Kovacs, 2003). A high degree of internal consistency in participant responses was found in this sample (coefficient α =.83). Caregivers provided a proxy report of the child's depressive symptoms on the 17-item parent version (CDI:P). A score of 17 and higher is indicative of significant child depressive symptomatology. In this sample of caregivers, there was a high degree of internal consistency (α =.84). The CDI manual reports good agreement between child and parent proxy versions of the CDI (r =.41), but a prior study has shown a higher correlation (r =.60) in a sample of youth with type 1 diabetes (Hood et al., 2006).
The 20-item Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977) was used to assess depressive symptoms in caregivers. This measure is widely used and large sample normative data and clinical cutoff scores (≥16) are available for the CES-D. Caregivers respond to each item by endorsing 0 (not experiencing that symptom) to 3 (experiencing that symptom all the time) over the past week. Internal consistency was high on this measure as well (α =.91).
Family demographic data were obtained during the clinic visit via self-report questionnaire completed by the child's primary caregiver. The frequency of blood glucose monitoring was obtained via meter download. The daily average was calculated from available data up to 2 weeks prior to the clinic visit. Each child provided blood for hemoglobin A1c, measured by high-performance liquid chromatography (reference range 4.0–6.0%, Tosoh 2.2; Foster City, CA, USA). Physical data (e.g., measurement of weight, height, and blood pressure) and diabetes regimen information—type 1 diabetes duration, frequency of insulin injections or use of Continuous Subcutaneous Insulin Infusion (CSII)—were documented by the child's medical provider and later ascertained through chart review.
Statistical Analysis
First, descriptive statistics, frequencies, and univariate analyses (i.e., t-tests and chi-square analyses) were calculated to examine differences between caregivers who met the CES-D cutoff for elevated depression and those who did not. Next, the depression-distortion hypothesis was tested in a series of regression analyses modeled after work on interactions and moderating effects (Aiken & West, 1991; Holmbeck, 1997) and post-hoc probing of interactions (Holmbeck, 2002). The caregiver proxy report of youth depressive symptoms (CDI:P) was conceptualized as the outcome of interest (i.e., dependent variable), youth report of depressive symptoms (CDI) as the true estimate of youth depressive symptoms (i.e., predictor variable), and caregiver depressive symptoms (CES-D) as the potentially influential moderating variable. For the regression analyses, predictor and moderator variables were first centered by subtracting the sample means on each variable from each individual score. Then, the predictor variables (CDI and CES-D separately) were entered along with the interaction between the two (CDI × CES-D) in to a regression model predicting caregiver proxy report (CDI:P). Covariates (child age, gender, race/ethnicity, type 1 diabetes duration, and type of caregiver) were entered in the model as well. If the interaction term was significant, a conditional moderator variable was created based on individual CES-D scores in relation to the SD of the sample and used in follow-up regression equations. The first regression equation was generated for caregivers with high CES-D scores (i.e., +1 SD above the mean) and the second equation for those with low CES-D scores (Holmbeck, 2002). Equations were then plotted to depict the interaction term and graphically display the presence of distortion. Finally, a chi-square analysis was conducted to supplement the moderation analyses. In this analysis, rates of caregiver depressive symptoms were compared across cases of caregiver–youth agreement and disagreement about youth depressive symptoms on the CDI and CDI:P. All analyses were performed with SAS 9.1 (SAS Institute, Cary, NC, USA).
Results
Descriptive Statistics and Univariate Comparisons
Table I provides details on the characteristics of the study sample. In brief, the 187 children and adolescents in this study had a mean age of 14.4 ± 2.4 years, were predominantly white (87%) and the majority (80%) resided in two-caregiver families. These youth had a mean duration of type 1 diabetes of 6.5 ± 3.9 years and a mean A1c of 9.0 ± 1.5%. Nearly 78% of youth monitored blood glucose levels four or more times daily and 47% received insulin via CSII. Caregivers included 153 mothers (82%), 27 fathers (14%), and 7 who identified themselves as “other caregiver” (4%). There were no differences between mothers, fathers, and other caregivers on the CES-D or CDI:P. There were 42 caregivers (22%) who met or exceeded the clinical cutoff on the CES-D. There were 30 youth (16%) who scored at or above 13 on the CDI and 46 youth (25%) who met the CDI:P cutoff according to their caregivers. Table I shows the similarities for caregiver proxy report between those who met or exceeded the clinical cutoff on the CES-D and those with little to no depressive symptoms. Caregiver groups were not significantly different on any variables and in fact, were nearly identical across age, gender, type 1 diabetes duration, and A1c.
Table I.
Participant Characteristics
| Characteristic | Total sample (n = 187) | CES-D <16 (n = 145) | CES-D ≥16 (n = 42) |
|---|---|---|---|
| Age (in years) | 14.4 ± 2.4 | 14.4 ± 2.4 | 14.6 ± 2.3 |
| Gender (% female) | 45% | 46% | 43% |
| Ethnicity | |||
| White, not of Hispanic origin | 162 (87%) | 128 (88%) | 34 (81%) |
| Black/African American | 11 (6%) | 8 (6%) | 3 (7%) |
| Hispanic/Latino | 9 (5%) | 5 (3%) | 4 (10%) |
| Asian/Pacific Islander | 5 (<3%) | 4 (3%) | 1 (2%) |
| Education level of primary caregiver (% with at least college degree) | 58% | 60% | 52% |
| Insurance Status | |||
| Private insurance | 85% | 87% | 83% |
| Public insurance | 15% | 13% | 17% |
| Family status (% with two caregivers in home) | 80% | 81% | 79% |
| Type 1 diabetes duration (in years) | 6.5 ± 3.9 | 6.6 ± 3.8 | 6.4 ± 4.1 |
| Hemoglobin A1c | 9.0 ± 1.5% | 9.0 ± 1.5% | 9.0 ± 1.5% |
| Blood glucose monitoring frequency (checks per day) | 4.8 ± 1.9 | 4.9 ± 1.9 | 4.4 ± 1.9 |
| Method of insulin delivery | |||
| Multiple daily injections | 53% | 52% | 60% |
| CSII | 47% | 48% | 40% |
| CDI score | 6.3 ± 5.3 | 5.9 ± 5.3 | 7.4 ± 5.2 |
Note: Scores shown as mean ± SD; CSII, continuous subcutaneous insulin infusion.
Tests of Moderation
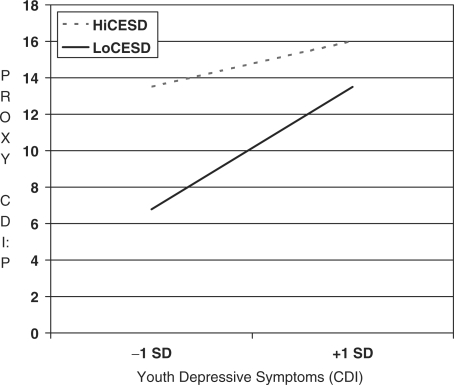
To test the hypothesis that caregivers with elevated depressive symptoms provide distorted reports of youth depressive symptoms, a regression model was run that contained the CDI, CES-D, their interaction, and covariates as predictors of the CDI:P. The overall model was significant, F (8,178) = 9.26, p <.0001, R2 =.29, and three variables were significant predictors: CDI (p <.0001), CES-D (p <.0001), and the interaction term (p <.02). No covariates (child age, gender, race/ethnicity, type 1 diabetes duration, or type of caregiver) were significant predictors of the CDI:P. Post-hoc probing of caregivers with high CES-D scores (i.e., +1 SD above the mean) showed the following regression equation, CDI:P = 14.77 + 0.24 (CDI), when the conditional moderator variable was substituted with zero. The simple slope of this equation was significant (p <.03). Likewise, caregivers with low CES-D scores (i.e., −1 SD below the mean) had the following regression equation, CDI:P = 10.13 + 0.64 (CDI). This simple slope was also significant (p <.0001). These findings are depicted in Fig. 1 and support the depression-distortion hypothesis; caregivers with high levels of depressive symptoms reported levels of youth depressive symptoms that are elevated at both high and low levels of youth-reported depressive symptoms. In contrast, caregivers with little to no depressive symptoms provide reports of youth depressive symptoms that are similar to the level reported by the youth (i.e., as the youth reports increasing symptoms, so do the caregivers).
Figure 1.
Influence of caregiver depression on proxy report.
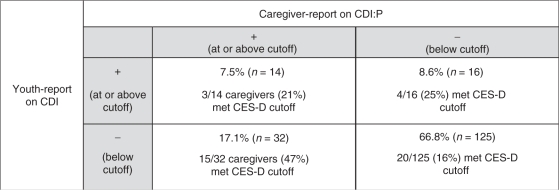
In this sample, there were 48 caregiver–youth dyads that disagreed on the presence of youth depressive symptoms and 139 dyads that agreed (Table II). Chi-square comparisons revealed that the disagreement group in which caregivers reported elevated youth depressive symptoms but the youth did not contained the highest proportion of caregivers that met the CES-D cutoff (χ2 = 14.0, p <.003). The rate in this group (47%) was nearly twice that of any other group. This finding provides further support of the influence of caregiver depressive symptoms on their proxy reports of youth depressive symptoms.
Table II.
Percentage of Caregivers with Elevated CES-D Scores by Agreement Group
 |
Discussion
This study is the first to test the depression-distortion hypothesis in caregivers of children and adolescents with a chronic disease. Results of this study confirmed the presence of distorted reports of youth depressive symptoms due to elevations in caregiver depressive symptoms. In contrast, caregivers with little to no depressive symptoms provided reports of youth depressive symptoms that were similar to what was reported by the youth. These findings are more compelling when one considers that caregivers above and below the clinical cutoff on the CES-D were nearly identical on relevant family and diabetes-specific variables and there were no covariates that significantly predicted the CDI:P score. Further, the use of regression techniques and post-hoc probing of interactions addresses several of the methodologic critiques raised in prior studies about the depression-distortion hypothesis (Richters, 1992). Overall, the findings support the collection of studies in nonchronic disease samples that show distortion and extend those findings to pediatric chronic disease.
Given the evidence of depression-distortion in this sample of caregivers of youth with type 1 diabetes, the possible reasons for distortion should be considered. The first set of possible reasons focus on the study sample. As De Los Reyes and Kazdin (2005) note, possible reasons for distortion can include attributions about the causes of symptoms or what the report of those symptoms may mean for treatment. Given that this was a non-clinical sample (i.e., not referred for depression or other psychological concerns) drawn from a large pediatric diabetes clinic, it is unlikely that caregivers and youth were focused on the causes of youth depressive symptoms or what the report of symptoms would mean in terms of treatment. Thus, it is unlikely that distortion was the result of different attributions of the causes of depression or need for treatment. An alternative explanation is that the presence of elevated depressive symptoms in caregivers negatively biased their recall of youth depressive symptoms to be more consistent with their own mood. Indeed, there is fairly strong empiric support for this general principle of mood-congruent memory (Johnson, Hashtroudi, & Lindsay, 1993; Tversky & Marsh, 2000), yet there is no way to determine this with any certainty in this sample. Further, this explanation also rests on the assumption that the youth's report of depressive symptoms is a true estimate and that the youth who did not report depressive symptoms provided accurate reports. While mindful of the inability to empirically document the mechanism for depression-induced distortion in this sample, this explanation is potentially the most plausible of the possible reasons raised by De Los Reyes and Kazdin with regard to individual variables.
The reasons that caregivers in this study showed distorted reports of youth depressive symptoms should also be considered from a methodologic perspective. In this study, a multisource approach to collection of data on youth depressive symptoms was adopted. This approach offered the advantage of determining the unique influence on proxy reports of both youth-reported depressive symptoms and caregiver depressive symptoms (Holmbeck et al., 2002). The results of the regression analyses support the influence of each of the separate variables along with their interaction. However, there was no evaluation of other sources of data, possibly observations from other caregivers or healthcare providers, and only one method was employed (i.e., self-report questionnaires). In the absence of multimethod data (e.g., a diagnostic interview for assessing youth depression), there is no “gold standard” for assessing depression across informants (Richters, 1992). While this study did employ statistical techniques recommended when there are discrepancies between multiple sources (Holmbeck et al., 2002), there would be additional benefit to combining questionnaire, interview, and clinician-observed data in order to provide the most accurate assessment of this construct in future studies.
Although the explanation for this depression-induced distortion can be considered from multiple angles, the core finding of distortion still remains. Considering that assessments (informal or formal) that occur in clinical and research settings rely on caregivers to provide reports of the youth's psychological functioning and that treatment decisions or entry in to intervention studies may be closely tied to these reports, the findings of the current study have both clinical and research implications. For example, multidisciplinary specialty care in pediatric type 1 diabetes (Silverstein et al., 2005) and specific recommendations from working groups in the American Diabetes Association about psychological care for youth with type 1 diabetes (Delamater et al., 2001) highlight the importance of routine assessment of the child's psychological functioning. Given the rate of elevated depressive symptoms found in this study as well as a host of prior studies (Grey et al., 2002; Kovacs, Obrosky, Goldston, & Drash, 1997), routine assessment appears warranted. However, no specific recommendations for assessing caregiver psychological functioning have been made. Caregivers are charged with continuing to address the developmental needs of the child or adolescent while simultaneously ensuring effective management of the disease. This additional burden of management may create fertile ground for the development of depression. More specifically in the case of type 1 diabetes, caregivers are typically mindful of the direct association between disease management and maintenance of near-normal levels of blood glucose and the prevention of long-term complications associated with the disease. Thus, the daily burden of management and the awareness of the importance of management to long-term health may serve to promote depressive symptoms via feelings of “diabetes burnout” (Polonsky, 2000) and potential feelings of helplessness in preventing long-term problems. Taken together, it seems important to identify a brief method to assess caregiver psychological functioning, either through directed questions from the child's healthcare provider or through a brief measure like the CES-D, that could be implemented in clinics as either a “mail-out” prior to the child's appointment or during periods of waiting in clinics. While logistical barriers inherent in a multidisciplinary pediatric diabetes clinic are there, the need to assess caregiver psychological functioning remains.
This point is further emphasized when the context of clinical research is considered. Many family-based interventions are effective in addressing the diabetes-specific needs of the family (Anderson, Brackett, Ho, & Laffel, 1999; Laffel et al., 2003; Wysocki et al., 2006); however, general distress in caregivers is potentially missed and untreated. Thus, the outcomes experienced by the child as well as the caregiver's perception of child or family outcomes may be jeopardized if the caregiver has significant elevations in depressive symptoms. It may be necessary to directly target psychological distress in the caregiver either before or simultaneously during family-based interventions. While there is still a high rate of caregiver–youth agreement about depressive symptoms (Hood et al., 2006), caregiver depression clearly influences this agreement as shown in the current study. Considering our understanding of the nature of depression in caregivers of youth with and without a chronic disease, it seems that intervention strategies could be delineated and implemented. It may be necessary to address aspects of caregiver psychological functioning (e.g., strategies for obtaining social support) at the same time attention is paid to diabetes-specific burden (e.g., strategies for more effective sharing of treatment responsibilities).
The results of this study should be considered in the context of several limitations. Beyond the issues raised about the lack of multimethod data in this study, the reliance on the CES-D and the CDI to document “depression” has limitations. The CES-D is a widely used measure, but is more so an indication of psychological distress than diagnosable depression (Fechner-Bates, Coyne, & Schwenk, 1994). Considering a diabetes-specific context, a large study of adults with diabetes that examined the differences between distress and depression (Fisher et al., 2007) found that adults with elevated CES-D scores only met diagnostic criteria for depression one-third of the time. While the caregivers in the present study did not have diabetes, they all lived in a family where diabetes was present and had significant management responsibilities. Interestingly, 22% of the present study's sample and 22% of the Fisher and colleagues’ sample scored above the clinical cutoff on the CES-D. Considering all of these points, the finding of depression-distortion may be somewhat limited due to the use of this measure over one that can be used to make a diagnosis of depression, especially in the context of diabetes. Similar limitations apply to the use of the CDI even though this measure demonstrates better predictive ability toward diagnosable depression (Timbremont, Braet, & Dreessen, 2004) relative to the CES-D.
Another limitation involves assumptions made about the direction of relationships. In this study, the conceptualization of the relationships between the CES-D, CDI, and CDI:P was made to test the depression-distortion hypothesis. There is, however, the likelihood for bi-directional relationships between informants’ levels of depressive symptoms. In addition, an unmeasured factor that could have potentially influenced these relationships is active treatment for depression, either in the form of antidepressant medication or psychotherapy. These variables were not assessed in youth or their caregivers. The use of a single caregiver variable may also represent a limitation of this study. While there were no differences on the CES-D or CDI:P across different types of caregivers and the type of caregiver was not a significant predictor of proxy reports in regression analyses, the implications of the study findings are likely more appropriate when considering maternal depressive symptoms given that 82% of the caregivers were mothers. There were only a small proportion of fathers and other caregivers in this sample. One of the reasons that there were no differences may be because this breakdown of caregivers represents a “primary” versus “secondary” distinction among potential caregivers and the study sample was made up of “primary” caregivers. During recruitment, the study team did encourage participation from the caregiver with the most treatment responsibilities. Nevertheless, a larger sample of fathers and other caregivers would further inform any differences between types of caregivers. A final limitation of this study also concerns the representativeness of the sample. While large, this sample of children and adolescents with type 1 diabetes was predominantly white, made up of two-caregiver families, and contained a large percentage of caregivers who reported higher levels of education than seen in the general population. Even though this sample may fit the general backdrop of pediatric type 1 diabetes (i.e., over-representation of whites), these findings may not generalize to samples that are different from the study sample.
In sum, this study demonstrates that caregiver reports of youth depressive symptoms can be influenced by the caregiver's own depressive symptoms, and importantly, this occurs in the context of a pediatric chronic disease. Future work to further understand the causes of this depression-induced distortion as well as its impact on disease management behaviors (i.e., adherence) and health outcomes appears warranted. Likewise, research and clinical practice protocols to provide or make referrals for psychological treatment for depressed caregivers need to be developed and implemented more readily. The demands of being a caregiver to a child or adolescent with a chronic disease and the need for optimal psychological functioning in these caregivers should be at the forefront of our pediatric research and clinical practice agendas.
Funding
National Institutes of Health (K23 DK-073340).
Conflicts of interest: None declared.
Acknowledgments
The author thanks Dr Lori Laffel for her mentorship during the conduct of the research presented in this article. The author also thanks Dr Grayson Holmbeck and members of the Adherence Center for comments on earlier drafts of this article and the families who participated in this study for generously giving of their time.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Anderson B, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. Journal of Pediatrics. 1997;130(2):257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Brackett J, Ho J, Laffel LM. An office-based intervention to maintain parent-adolescent teamwork in diabetes management. Impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care. 1999;22(5):713–721. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: New perspectives on disease pathogenesis and treatment. Lancet. 2001;358(9277):221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- Braaten EB, Biederman J, DiMauro A, Mick E, Monuteaux MC, Muehl K, et al. Methodological complexities in the diagnosis of major depression in youth: An analysis of mother and youth self-reports. Journal of Child and Adolescent Psychopharmacology. 2001;11(4):395–407. doi: 10.1089/104454601317261573. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Prabucki K. Depressed mothers as informants in family history research—are they accurate? Psychiatry Research. 1988;24(3):345–359. doi: 10.1016/0165-1781(88)90115-1. [DOI] [PubMed] [Google Scholar]
- Chi TC, Hinshaw SP. Mother-child relationships of children with ADHD: The role of maternal depressive symptoms and depression-related distortions. Journal of Abnormal Child Psychology. 2002;30(4):387–400. doi: 10.1023/a:1015770025043. [DOI] [PubMed] [Google Scholar]
- Chilcoat HD, Breslau N. Does psychiatric history bias mothers' reports? An application of a new analytic approach. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):971–979. doi: 10.1097/00004583-199707000-00020. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Toth SL. Maternal depressive disorder and contextual risk: Contributions to the development of attachment insecurity and behavior problems in toddlerhood. Developmental Psychopathology. 1998;10(2):283–300. doi: 10.1017/s0954579498001618. [DOI] [PubMed] [Google Scholar]
- Cytryn L, McKnew DH, Zahn-Waxler C, Radke-Yarrow M, Gaensbauer TJ, Harmon RJ, et al. A developmental view of affective disturbances in the children of affectively ill parents. American Journal of Psychiatry. 1984;141(2):219–222. doi: 10.1176/ajp.141.2.219. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Bell RA, D’Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, et al. Incidence of diabetes in youth in the United States. Journal of the American Medical Association. 2007;297(24):2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- De Los Reyes A, Kazdin AE. Informant discrepancies in the assessment of childhood psychopathology: A critical review, theoretical framework, and recommendations for further study. Psychological Bulletin. 2005;131(4):483–509. doi: 10.1037/0033-2909.131.4.483. [DOI] [PubMed] [Google Scholar]
- Delamater AM, Jacobson AM, Anderson B, Cox D, Fisher L, Lustman P, et al. Psychosocial therapies in diabetes: Report of the psychosocial therapies working group. Diabetes Care. 2001;24(7):1286–1292. doi: 10.2337/diacare.24.7.1286. [DOI] [PubMed] [Google Scholar]
- Drotar D, Ievers C. Age differences in parent and child responsibilities for management of cystic fibrosis and insulin-dependent diabetes mellitus. Journal of Developmental and Behavioral Pediatrics. 1994;15(4):265–272. [PubMed] [Google Scholar]
- Fechner-Bates S, Coyne JC, Schwenk TL. The relationship of self-reported distress to depressive disorders and other psychopathology. Journal of Consulting and Clinical Psychology. 1994;62(3):550–559. doi: 10.1037//0022-006x.62.3.550. [DOI] [PubMed] [Google Scholar]
- Fisher L, Skaff MM, Mullan JT, Arean P, Mohr D, Masharani U, et al. Clinical depression versus distress among patients with type 2 diabetes: Not just a question of semantics. Diabetes Care. 2007;30(3):542–548. doi: 10.2337/dc06-1614. [DOI] [PubMed] [Google Scholar]
- Grey M, Whittemore R, Tamborlane W. Depression in type 1 diabetes in children: Natural history and correlates. Journal of Psychosomatic Research. 2002;53(4):907–911. doi: 10.1016/s0022-3999(02)00312-4. [DOI] [PubMed] [Google Scholar]
- Griest DL, Forehand R, Wells KC, McMahon RJ. An examination of differences between nonclinic and behavior-problem clinic-referred children and their mothers. Journal of Abnormal Psychology. 1980;89(3):497–500. doi: 10.1037//0021-843x.89.3.497. [DOI] [PubMed] [Google Scholar]
- Hawley KM, Weisz JR. Child, parent, and therapist (dis)agreement on target problems in outpatient therapy: The therapist's dilemma and its implications. Journal of Consulting and Clinical Psychology. 2003;71(1):62–70. doi: 10.1037//0022-006x.71.1.62. [DOI] [PubMed] [Google Scholar]
- Hay DF, Pawlby S, Angold A, Harold GT, Sharp D. Pathways to violence in the children of mothers who were depressed postpartum. Developmental Psychology. 2003;39(6):1083–1094. doi: 10.1037/0012-1649.39.6.1083. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN. Toward terminological, conceptual, and statistical clarity in the study of mediators and moderators: Examples from the child-clinical and pediatric psychology literatures. Journal of Consulting and Clinical Psychology. 1997;65(4):599–610. doi: 10.1037//0022-006x.65.4.599. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27(1):87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN, Li ST, Schurman JV, Friedman D, Coakley RM. Collecting and managing multisource and multimethod data in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27(1):5–18. doi: 10.1093/jpepsy/27.1.5. [DOI] [PubMed] [Google Scholar]
- Hood KK, Huestis S, Maher A, Butler D, Volkening L, Laffel LM. Depressive symptoms in children and adolescents with type 1 diabetes: Association with diabetes-specific characteristics. Diabetes Care. 2006;29(6):1389–1391. doi: 10.2337/dc06-0087. [DOI] [PubMed] [Google Scholar]
- Jaser SS, Whittemore R, Ambrosino JM, Lindemann E, Grey M. Mediators of depressive symptoms in children with type 1 diabetes and their mothers. Journal of Pediatric Psychology. 2008;33(5):509–519. doi: 10.1093/jpepsy/jsm104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PS, Traylor J, Xenakis SN, Davis H. Child psychopathology rating scales and interrater agreement: I. Parents’ gender and psychiatric symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 1988;27(4):442–450. doi: 10.1097/00004583-198807000-00012. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychological Bulletin. 1993;114(1):3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Kovacs M. North Tonawanda. NY: Multi-Health Systems; 2003. The Children's Depression Inventory (CDI): Technical manual. [Google Scholar]
- Kovacs M, Goldston D, Obrosky DS, Bonar LK. Psychiatric disorders in youths with IDDM: Rates and risk factors. Diabetes Care. 1997;20(1):36–44. doi: 10.2337/diacare.20.1.36. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Obrosky DS, Goldston D, Drash A. Major depressive disorder in youths with IDDM. A controlled prospective study of course and outcome. Diabetes Care. 1997;20(1):45–51. doi: 10.2337/diacare.20.1.45. [DOI] [PubMed] [Google Scholar]
- Laffel LM, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. Journal of Pediatrics. 2003;142(4):409–416. doi: 10.1067/mpd.2003.138. [DOI] [PubMed] [Google Scholar]
- Larson K, Russ SA, Crall JJ, Halfon N. Influence of multiple social risks on children's health. Pediatrics. 2008;121(2):337–344. doi: 10.1542/peds.2007-0447. [DOI] [PubMed] [Google Scholar]
- Liese AD, D’Agostino RB, Jr, Hamman RF, Kilgo PD, Lawrence JM, Liu LL, et al. The burden of diabetes mellitus among US youth: Prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118(4):1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- Loeber R, Green SM, Lahey BB, Stouthamer-Loeber M. Differences and similarities between children, mothers, and teachers as informants on disruptive child behavior. Journal of Abnormal Child Psychology. 1991;19(1):75–95. doi: 10.1007/BF00910566. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Connell DB, Grunebaum HU, Botein S. Infants at social risk: Maternal depression and family support services as mediators of infant development and security of attachment. Child Development. 1990;61(1):85–98. doi: 10.1111/j.1467-8624.1990.tb02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najman JM, Williams GM, Nikles J, Spence S, Bor W, O’Callaghan M, et al. Mothers’ mental illness and child behavior problems: Cause-effect association or observation bias? Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(5):592–602. doi: 10.1097/00004583-200005000-00013. [DOI] [PubMed] [Google Scholar]
- NIDDK. National diabetes statistics: Prevalence of diagnosed diabetes in people aged 20 years or younger. United States. Bethesda, MD: U.S. Department of Health and Human Services; 2005. [Google Scholar]
- Orvaschel H, Weissman MM, Kidd KK. Children and depression—the children of depressed parents; the childhood of depressed patients; depression in children. Journal of Affective Disorders. 1980;2(1):1–16. doi: 10.1016/0165-0327(80)90017-8. [DOI] [PubMed] [Google Scholar]
- Polonsky WH. Diabetes burnout: What to do when you can't take it anymore. New York: American Diabetes Association; 2000. [Google Scholar]
- Quittner AL, DiGirolamo AM, Michel M, Eigen H. Parental response to cystic fibrosis: A contextual analysis of the diagnosis phase. Journal of Pediatric Psychology. 1992;17(6):683–704. doi: 10.1093/jpepsy/17.6.683. [DOI] [PubMed] [Google Scholar]
- Radke-Yarrow M, Zahn-Waxler C, Richardson DT, Susman A, Martinez P. Caring behavior in children of clinically depressed and well mothers. Child Development. 1994;65(5):1405–1414. doi: 10.1111/j.1467-8624.1994.tb00825.x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rapoff MA. Adherence to pediatric medical regimens. New York, NY: Kluwer Academic/Plenum Publishers; 1999. [Google Scholar]
- Richters J. Depressed mothers as informants about their children: A critical review of the evidence for distortion. Psychological Bulletin. 1992;112(3):485–499. doi: 10.1037/0033-2909.112.3.485. [DOI] [PubMed] [Google Scholar]
- Richters J, Pellegrini D. Depressed mothers’ judgments about their children: An examination of the depression-distortion hypothesis. Child Development. 1989;60(5):1068–1075. doi: 10.1111/j.1467-8624.1989.tb03537.x. [DOI] [PubMed] [Google Scholar]
- Schulz R, Quittner AL. Caregiving for children and adults with chronic conditions: Introduction to the special issue. Health Psychology. 1998;17(2):107–111. [PubMed] [Google Scholar]
- Silverstein JH, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, et al. Care of children and adolescents with type 1 diabetes: A statement of the American Diabetes Association. Diabetes Care. 2005;28(1):186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- Silverstein M, Augustyn M, Cabral H, Zuckerman B. Maternal depression and violence exposure: Double jeopardy for child school functioning. Pediatrics. 2006;118(3):e792–e800. doi: 10.1542/peds.2005-1841. [DOI] [PubMed] [Google Scholar]
- Tates K, Meeuwesen L. Doctor-parent-child communication. A (re)view of the literature. Social Science & Medicine. 2001;52(6):839–851. doi: 10.1016/s0277-9536(00)00193-3. [DOI] [PubMed] [Google Scholar]
- Timbremont B, Braet C, Dreessen L. Assessing depression in youth: Relation between the Children's Depression Inventory and a structured interview. Journal of Clinical Child and Adolescent Psychology. 2004;33(1):149–157. doi: 10.1207/S15374424JCCP3301_14. [DOI] [PubMed] [Google Scholar]
- Tversky B, Marsh EJ. Biased retellings of events yield biased memories. Cognitive Psychology. 2000;40(1):1–38. doi: 10.1006/cogp.1999.0720. [DOI] [PubMed] [Google Scholar]
- Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: Risk factors for adverse outcomes in developing countries. Lancet. 2007;369(9556):145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- Webster-Stratton C. Mothers’ and fathers’ perceptions of child deviance: Roles of parent and child behaviors and parent adjustment. Journal of Consulting and Clinical Psychology. 1988;56(6):909–915. doi: 10.1037//0022-006x.56.6.909. [DOI] [PubMed] [Google Scholar]
- Webster-Stratton C, Hammond M. Maternal depression and its relationship to life stress, perceptions of child behavior problems, parenting behaviors, and child conduct problems. Journal of Abnormal Child Psychology. 1988;16(3):299–315. doi: 10.1007/BF00913802. [DOI] [PubMed] [Google Scholar]
- Weil CM, Wade SL, Bauman LJ, Lynn H, Mitchell H, Lavigne J. The relationship between psychosocial factors and asthma morbidity in inner-city children with asthma. Pediatrics. 1999;104(6):1274–1280. doi: 10.1542/peds.104.6.1274. [DOI] [PubMed] [Google Scholar]
- Wildman BG, Stancin T, Golden C, Yerkey T. Maternal distress, child behaviour, and disclosure of psychosocial concerns to a paediatrician. Child: Care, Health and Development. 2004;30(4):385–394. doi: 10.1111/j.1365-2214.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Taylor A, et al. Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. Journal of Pediatric Psychology. 2006;31(9):928–938. doi: 10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- Youngstrom E, Loeber R, Stouthamer-Loeber M. Patterns and correlates of agreement between parent, teacher, and male adolescent ratings of externalizing and internalizing problems. Journal of Consulting and Clinical Psychology. 2000;68(6):1038–1050. doi: 10.1037//0022-006x.68.6.1038. [DOI] [PubMed] [Google Scholar]