Abstract
Introduction
In clinical tissue-engineering-based approaches to articular cartilage repair, various types of flaps are frequently used to retain an implanted construct within the defect, and they are usually affixed by suturing. We hypothesize that the suturing of articular cartilage is associated with a loss of chondrocytes from and osteoarthritis-like changes within the peri-sutural area.
Materials and Methods
We established a large, partial-thickness defect model in the femoral groove of adult goats. The defects were filled with bovine fibrinogen to support a devitalized flap of autologous synovial tissue, which was sutured to the surrounding articular cartilage with single, interrupted stitches. The perisutural and control regions were analyzed histologically, histochemically and histomorphometrically shortly after surgery and 3 weeks later.
Results
Compared to control regions, chondrocytes were lost from the perisutural area even during the first few hours of surgery. During the ensuing 3 weeks, the numerical density of cells in the perisutural area decreased significantly. The cell losses were associated with a loss of proteoglycans from the extracellular matrix. Shortly after surgery, fissures were observed within the walls of the suture channels. By the third week, their surface density had increased significantly and they were filled with avascular mesenchymal tissue.
Conclusions
The suturing of articular cartilage induces severe local damage, which is progressive and reminiscent of that associated with the early stages of osteoarthritis. This damage could be most readily circumvented by adopting an alternative mode of flap affixation, such as glueing with a biological adhesive.
Keywords: surgical suturing, articular cartilage, osteoarthritis fissures, proteoglycan loss
Introduction
Structural lesioning of the articular cartilage layer is a common consequence of traumatic or pathological events in large human joints. Such lesions are frequently associated with pain, diminished joint functionality and reduced life-quality 1, 2. The symptoms tend to be most severe when the lesions arise pathologically during the course of osteoarthritis 3, 4, 5, 6. It is generally believed that these debilitating symptoms can be best relieved by inducing the lesions to heal. With this aim in view, diverse surgical strategies have been elaborated to induce a spontaneous tissue repair response. These include surgical stimulation of the subchondral bone marrow by, for example, Pridie drilling, microfracturing or abrasive chondroplasty (for review, see Hunziker 2002) 7. These interventions are reasonably successful in the short- and mid-terms (months to a few years) 8, 9, but not in the long-run 10. Against this background, great efforts have been made to improve the repair of articular cartilage lesions by novel means, such as tissue engineering. One such approach that has been avidly adopted in clinical practice is the autologous chondrocyte implantation technique 11, 12. This procedure involves the suturing of an autologous periosteal flap to the border of the articular cartilage lesion, into which a suspension of autologous chondrocytes is then injected 12. The introduction of this methodology into clinical practice has popularized the surgical suturing of diverse types of flap to articular cartilage lesions that have been variously treated to induce their repair. After more than a decade’s experience with the autologous chondrocyte implantation technique, it is now evident that it confers no advantage over conventional bone-marrow-stimulating approaches, such as microfracturing 10. To improve the results yielded by tissue-engineering approaches, efforts must now be made not only to improve the therapeutic principles upon which they are based, but also to minimize iatrogenic damage and to eliminate surgical manoeuvres that compromise the repair response of the articular cartilage tissue.
The surgical suturing of articular cartilage is known to be a delicate task, owing to the tissue’s avascular, aneural and alymphatic nature and to its poor healing capacity. Suturing involves the insertion of a surgical needle into the tissue, thereby creating a channel which, in effect, is a partial-thickness lesion. We hypothesize that these channel-like defects are associated with damage to the adjacent tissue, and that the lesions not only fail to heal but enlarge with time and joint usage.
To test this hypothesis, we established a large, partial-thickness defect model in the trochlear femoral groove of adult goats. The defects were filled with bovine fibrinogen to support a thin flap of devitalized synovial tissue, which was sutured to the surrounding articular cartilage. The animals were killed either 2–3 hours after surgery or 3 weeks later. The perisutural area and control regions were analyzed histologically, histochemically (metachromatic staining of the extracellular matrix with Toluidine Blue to assess the proteoglycan status) and histomorphometrically (numerical and volume densities of chondrocytes, and their mean volume; surface and volume densities of fissures within the walls of the suture channels).
Materials and Methods
Experimental Outline
The purpose of this study was to investigate the effects of surgical suturing on normal hyaline articular cartilage tissue within the synovial knee-joints of adult goats, both at the time of surgery and 3 weeks later. These effects were analyzed histologically, histochemically and histomorphometrically.
Surgical Procedure
Sixteen adult Saanen goats were used for this investigation, eight of which were sacrificed 2–3 hours after surgery and the other eight 3 weeks later. The study was approved by the local ethical committee for animal experimentation and conducted in accordance with its regulations. General anaesthesia was induced by an intravenous injection of butorphenol and diazepam and maintained by ketamine. In one leg per animal, the trochlear femoral groove was exposed layer by layer. One purely chondral defect, 5 mm in width and 10 mm in length (in the saggital plane), was created within the lateral facet of the patellar groove using a custom-made cutting tool (see Hunziker and Rosenberg, 1996) 13. Bovine fibrinogen was deposited within the defect to support the flap of synovial tissue that was sutured to its roof. This material was excised from the lateral wall of the synovial joint. Its surface area slightly exceeded that of the defect. Prior to placement, it was devitalized by three freezing-thawing cycles, and then trimmed to precisely match the defect area. It was sutured to the lesion borders with single stitches of Vicryl 7-0 thread (Fig. 1). The wound was closed layer by layer.
Fig. 1.
Macroscopic view of a synovial tissue flap sutured to the borders of a chondral defect.
Eight of the goats were killed within 2–3 hours of surgery, and the other eight 3 weeks later. In the latter category, a soft cast was applied to the treated leg [to hinder the loss of flaps (Driesang and Hunziker, 2000)] 14. This cast restricted movement of the knee to 3–4 degrees, but permitted full loading of the joint. The animals were able to stand up and lie down of their own accord (see Hunziker, 2001) 15.
After the 3-week period had elapsed, the goats were anaesthetized (as described above) and infused with an overdose of potassium chloride to induce cardiac arrest.
The treated joints were excised and transferred to a moist chamber for the removal of soft tissues and the preparation of tissue blocks that comprised the entire defect area.
Tissue Processing
The tissue blocks were prefixed in 4% buffered formaldehyde solution (Merck, Darmstadt, Germany) for 1–2 hours at ambient temperature. They were then cut into smaller units using an Exact saw. Tissue fixation was continued for 5 days at ambient temperature. The blocks were then rinsed in tap water, dehydrated in ethanol (Alco Suisse, Bern, Switzerland), and embedded in methylmethacrylate (Merck, Darmstadt, Germany).
Tissue-Sampling Protocol
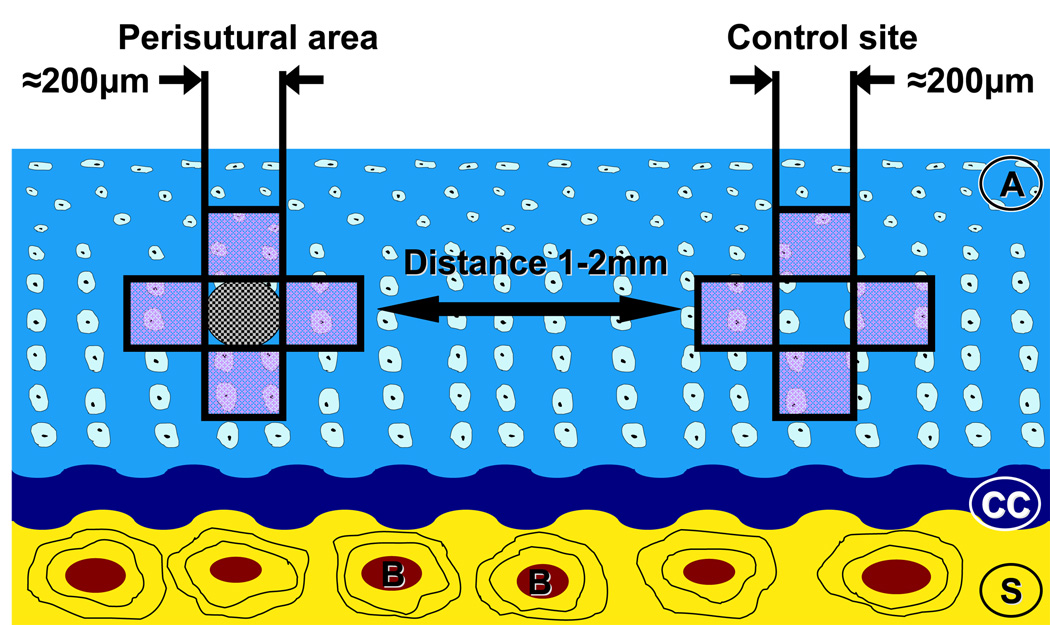
The tissue blocks were sawed perpendicular to the long axis of the defect area. With a random start at the left-hand margin of the tissue block (systematic random-sampling principle) 16, the specimens were cut into 600-µm-thick slices. Each specimen yielded 8–10 saw-cuts per defect volume. After glueing to section-holders, the saw-cuts were milled to a final thickness of 100–150 µm using a Polycut-E microtome (Leica, Vienna, Austria). They were then surface polished with fine-grained sand-paper prior to surface staining with McNeal’s Tetrachrome, Toluidine Blue O and basic fuchsine, according to the procedure described by Schenk et al., 1984 17. Every second tissue slice was used for the morphometric analysis. The specimens were examined in an Olympus Vanox AH2 microscope, which was equipped with a digital camera. Digital images were printed in colour. The region of interest, namely, the perisutural area, was defined as the 200-µm-diameter space around the suture channel (Fig. 2). Normal (unsutured) areas of tissue, 1–2 mm from the suture channels, served as control regions. Perisutural and control areas were photographed according to a systematic random-sampling protocol (see Fig. 2) using the x20-, x60- and x100-objectives (the latter for determining only the mean cell volume). The digital images were printed at final magnifications of x340, x1100 and x1700 for the morphometric analysis.
Fig. 2.
Scheme depicting the perisutural area and a control site. The regions analyzed are boxed in black. A: articular cartilage; CC: calcified cartilage, S: subchondral bone.
Morphometric Analysis
(i) Volume density of chondrocytes, VV
The volume density (or volume fraction) of chondrocytes describes the proportion of chondrocytes in the cartilage tissue as a percentage value. This parameter was estimated for the perisutural and the control regions using the point-counting technique18.
(ii) Mean cell volume, v̄(c)
The mean volume of chondrocytes was estimated using the nucleator methodology described by Gundersen et al., 1988 18. The measurements were made on photographic prints at a final magnification of x1700. All chondrocytes that were sectioned through the nucleus were counted 18, 19. The diameters of the nucleated cell profiles were measured using a system of concentric circles. The mean cell radius (r) was then used to calculate the mean cell volume [v̄(c)] according to the expression:
(Gundersen et al., 1988) 18
(iii) Numerical density of chondrocytes, Nv
The numerical cell density was calculated from the cell volume density (Vv) and the mean cell volume [v̄(c)] using the following equation:
(Howard and Reed, 2005) 20
(iv) Surface area density of fissures, Sv
The walls of the suture channels were frequently cracked. The total surface area implicated in these fissures was estimated using a cycloid test system 21. The surface area density of the fissures was then determined according to the following equation:
where Y is the object of interest, Ii is the number of points of intersection, px is the test-grid constant, and Pi is the total number of points in the reference space 20, 21.
(iv) Volume density of fissures, Vv
The volume density of the fissures was estimated using the point-counting technique18.
Statistical analysis
SPSS and GraphPad Prism software were used for the statistical analysis. Numerical data are presented as mean values together with the standard error of the mean (SEM). Differences between sets of data were statistically analyzed using Student’s unpaired t-test, the level of significance being set at p < 0.05.
Results
Descriptive Histology
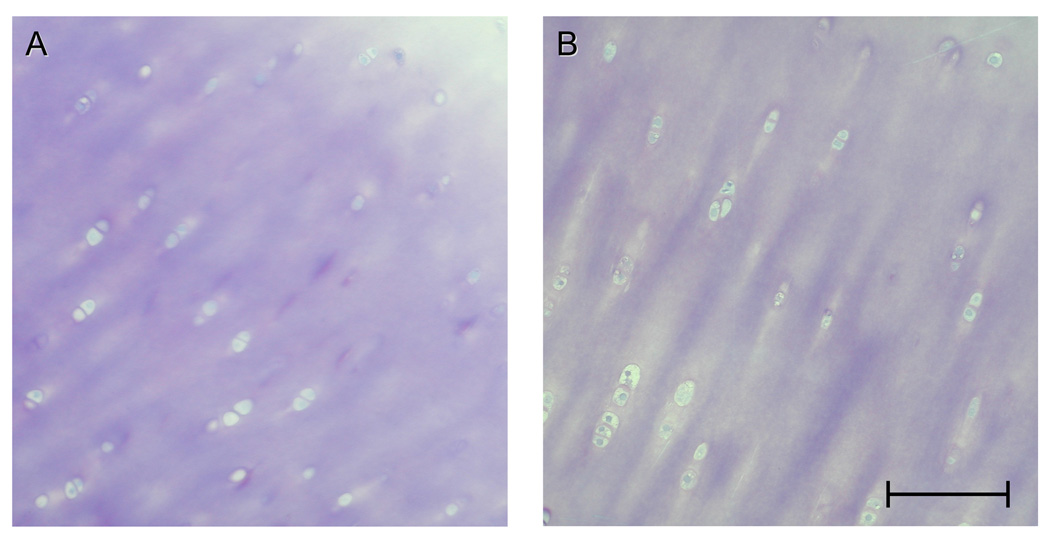
Light microscopy of the control (unsutured) areas of articular cartilage tissue revealed a typical distribution of the chondrocytes 22, 23, both 2–3 hours after surgery and 3 weeks later. The pattern of staining of the extracellular matrix was regular in each zone (Figs. 3, A & B), with a slight and gradual increase in intensity from the superficial zone to the lower radial zone. This gradient reflects an increase in the concentration of proteoglycans 24, 25.
Fig. 3.
A, B: Light micrographs of normal articular cartilage tissue derived from control sites 2–3 hours after surgery, revealing a regular arrangement of the chondrocytes and a regular pattern of staining of the extracellular matrix. Bar = 100 µm.
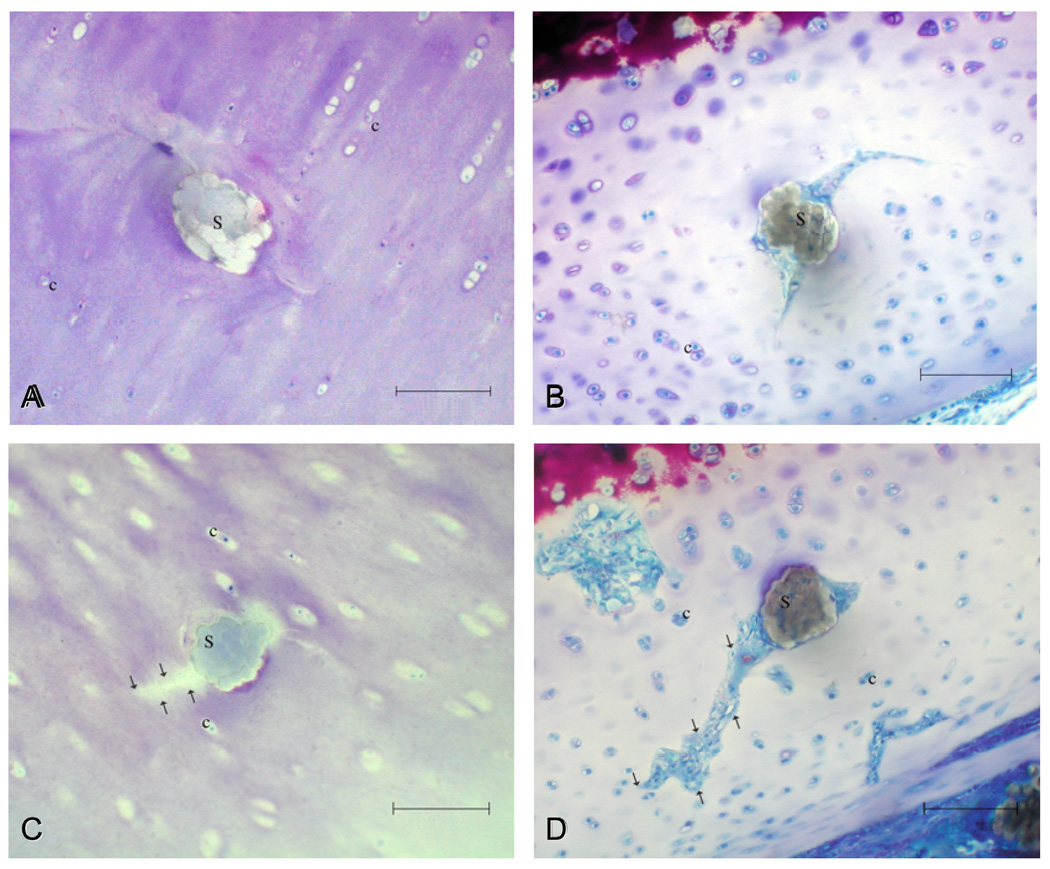
Shortly after surgery, the perisutural area was characterized by a reduction in the numerical density of cells and by a decrease in the staining intensity of the extracellular matrix (Fig. 4, A & C). Both of these effects were more marked by the third postoperative week (Fig. 4, B & D and Fig. 5). At the 3-week juncture, a few chondrocyte clusters (indicative of limited mitotic division) were encountered in the perisutural area. Shortly after surgery, fissures were apparent within the walls of the suture channels. At this stage, the channels themselves were filled with suture material (Fig. 4, A & C), whereas the fissures could be distinguished as “empty” spaces (Fig. 4C). By the third postoperative week, the fissures had elongated (Fig. 4D). At this stage, both the suture channels and the fissures were partially filled with a loose, avascular type of mesenchymal tissue (Fig. 4, B & D and Fig. 5). There was no evidence of its differentiation into a cartilaginous type of repair tissue.
Fig. 4.
Light micrographs of cross-sectioned sutures (S) 2–3 hours after surgery (A, C) and 3 weeks later (B, D). Fissures that developed in the walls of the suture channels [see arrows in (C) and (D)] propagated with time, and at the 3-week juncture, they were filled with a primitive type of avascular scar tissue (D). c = chondrocytes. Bar = 100 µm.
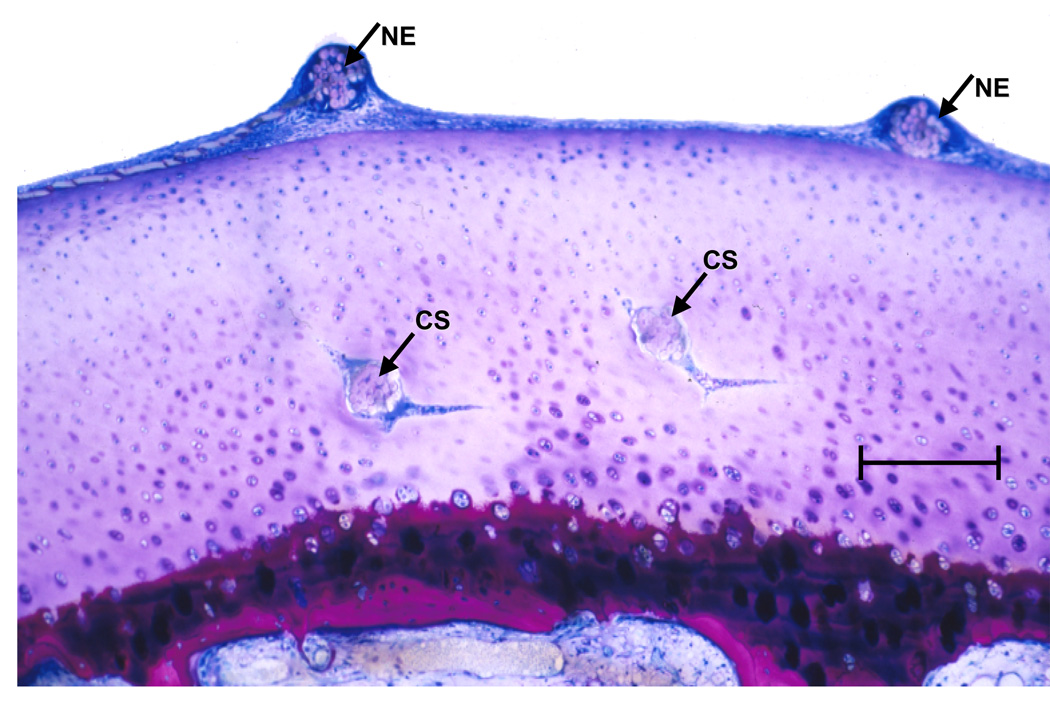
Fig. 5.
Low-magnification overview of a vertical section through the sutured articular cartilge layer, 3 weeks after surgery, illustrating the entry points of two sutures on the surface (NE) and their traversal of the flat part of the “U”-turn taken by the needle [cross-sectioned (CS)] before it returns to the surface. Bar = 300 µm.
Histomorphometry
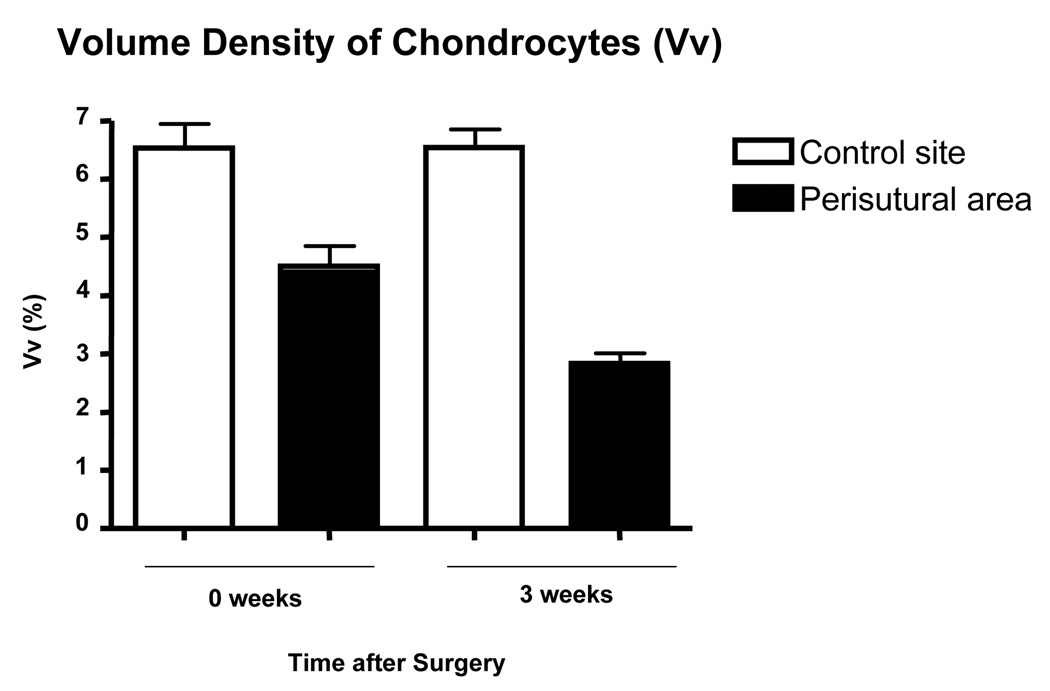
The descriptive histological findings were confirmed by the morphometric analysis. Shortly after surgery, the volume density of chondrocytes in the perisutural area (4.5%) was significantly lower (p=0.0004) than in the control region (6.5%) [Fig. 6]. By the third postoperative week, the value in the perisutural area had undergone a further significant decrease (p<0.0001) to 2.8% (Fig. 6).
Fig. 6.
Volume density of chondrocytes in the control site and in the perisutural area 2–3 hours after surgery (0 weeks) and 3 weeks later. Mean values (+ SEM) are represented (n = 8 for each site and for each juncture). The values at both junctures differ significantly from each other (p = 0.0004 at “0 weeks”; p = 0.0001 at “3 weeks”).
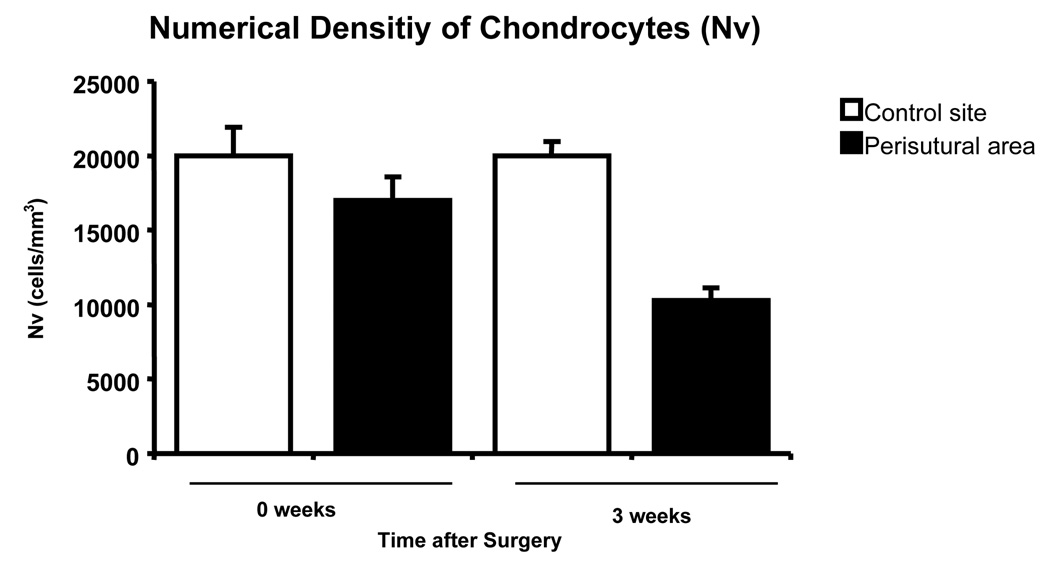
A few hours after surgery, the numerical density of chondrocytes in the perisutural area [16980 (± 1610) cells per mm3] did not differ significanty (p=0.23) from that in the control region [19990 (± 1910) cells per mm3] (Fig. 7). However, by the third postoperative week, the cellularity in the perisutural area had decreased significantly (p<0.0001) to 10290 (± 860) chondrocytes per mm3 of tissue (Fig. 7).
Fig. 7.
Numerical density of chondrocytes in the control site and in the perisutural area 2–3 hours after surgery (0 weeks) and 3 weeks later. Mean values (+ SEM) are represented (n = 8 for each site and for each juncture). The “3-week” values differ significantly from each other (p = 0.0001); the “0-week” ones do not.
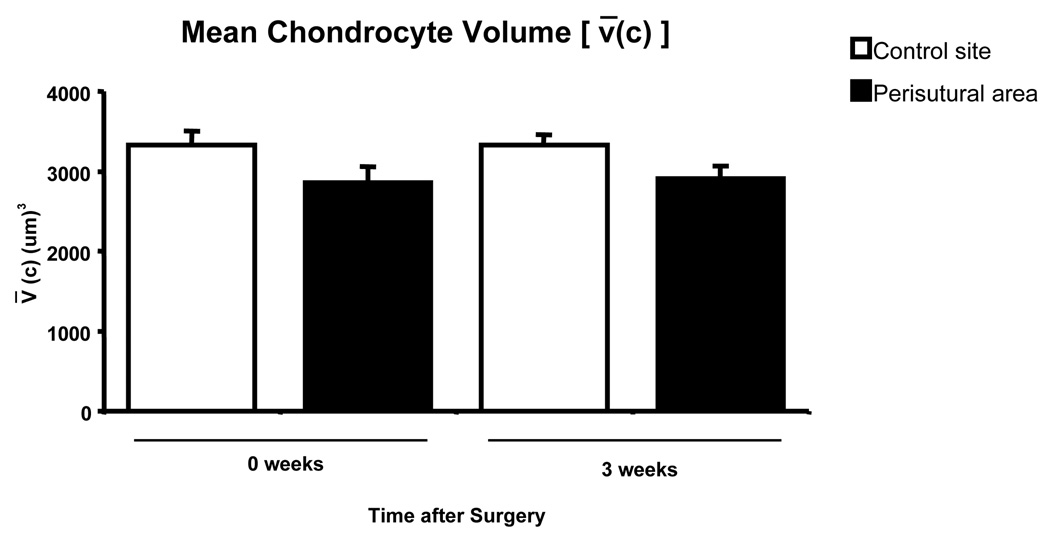
Shortly after surgery, the mean chondrocyte volume in the perisutural area [2860 (± 200)] was slightly, but not significantly smaller (p = 0.09) than that in the control region [3330 (± 180)] (Fig. 8). Three weeks after surgery, the mean chondrocyte volume in the perisutural area [2910 (± 150)] was slightly smaller (p = 0.04) than that in the control region [3330 (± 130)] (Fig. 8).
Fig. 8.
Mean volume of chondrocytes (+ SEM) in the control site and in the perisutural area 2–3 hours after surgery (0 weeks) and 3 weeks later (n = 8 for each site and for each juncture). The values differ significantly from each other at “3 weeks” (p = 0.0478), but not at “0 weeks” (p = 0.09).
In the perisutural area, the estimated decrease in the volume density of chondrocytes (Fig. 6) was proportional to the decrease in their numerical density (Fig. 7) at both junctures, namely, 15% a few hours after surgery and 50% after the third postoperative week.
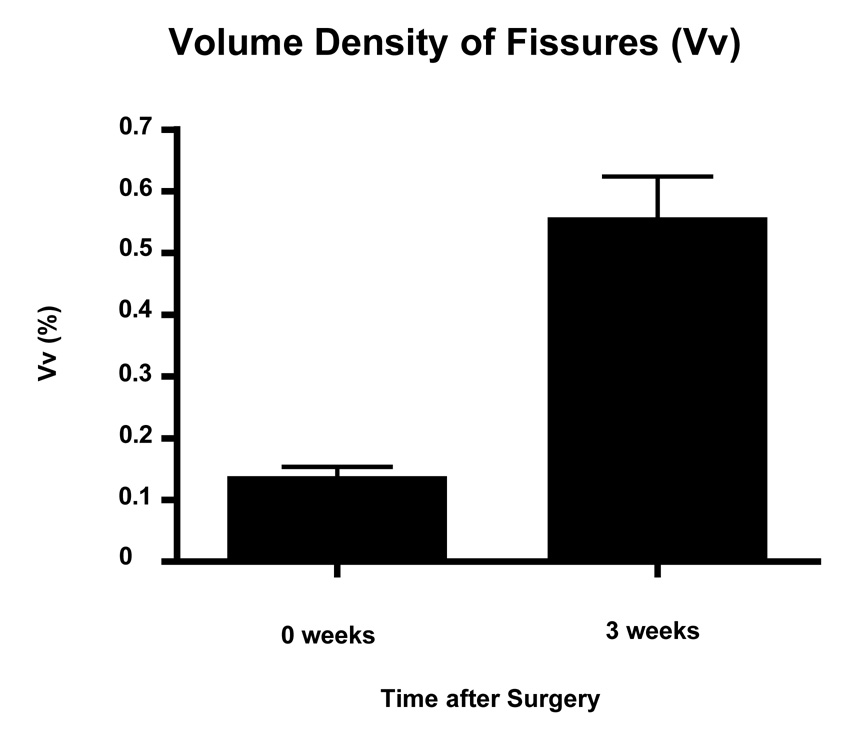
The surface area density of the fissures (Fig. 9) increased from 0.002 mm2 per mm3 of tissue shortly after surgery to 0.0047 mm2 per mm3 of tissue at the 3-week juncture (Fig. 9). The same trend was observed for the volume density of the fissures (Fig. 10).
Fig. 9.
Mean surface density of fissures (+ SEM) that were observed to develop in the suture channels, 2–3 hours after surgery (0 weeks) and 3 weeks later (n = 8 for each juncture). The values differ significantly from each other (p = 0.0001).
Fig. 10.
Mean volume density of fissures (+ SEM) that were observed to develop in the suture channels, 2–3 hours after surgery (0 weeks) and 3 weeks later (n = 8 for each juncture). The values differ significantly from each other
Discussion
In clinical tissue-engineering-based approaches to articular cartilage repair, flaps of autologous or xenogeneic periosteal or perichondrial tissue, or of synthetic material, are frequently used to retain an implanted construct within the defect. In most instances, these flaps are affixed to the surrounding tissue by surgical suturing. In the case of articular cartilage, the suturing process itself is not unproblematic. The highly anisotropic organization of this tissue, which is essential for its mechanical competence and functionality, is readily disturbed by structural discontinuities (lesions). Moreover, given the poor repair potential of articular cartilage tissue, these discontinuities are unlikely to heal 13, 26, 27.
Surgically-induced wounding of articular cartilage tissue is known to induce a loss of chondrocytes, probably due to apoptosis, and to elicit metabolic changes in the surviving population of cells close to the wound margin 28, 29. We hypothesized that the suturing of articular cartilage might be associated with a loss of chondrocytes from the perisutural area, and that the suture channels themselves might not only fail to heal but enlarge with time and joint usage. To test this hypothesis, we established a large, partial-thickness defect model in the femoral groove of adult goats. The defects were filled with bovine fibrinogen to support a devitalized synovial flap, which was sutured to the surrounding articular cartilage. The perisutural area (200 µm in diameter) and control regions were analyzed histologically, histochemically and histomorphometrically 2–3 hours after surgery and 3 weeks later.
Our data revealed a loss of both chondrocytes and proteoglycans from the peri-sutural area even during the first few hours of suturing, and these losses were exacerbated with time (3 weeks). Fissures were observed within the walls of the suture channels shortly after surgery, and they elongated with time. These findings are disconcerting. The histological changes are reminiscent of those that occur during the early stages of osteoarthritis 30, 31, 32, which are likewise progressive.
The loss of chondrocytes from the perisutural area may be a consequence of necrosis, which could be induced by the high mechanical forces that are generated during the insertion of the suturing needle. The tissue may be thereby compressed to an injuriously high degree. Such high forces can also induce the apoptosis of chondrocytes 28, 29. However, the loss of cells was not confined to the immediate margin of the suture channel. It embraced a 200-µm-diameter perisutural area. This finding indicates that the metabolic activity of chondrocytes spanning a wide reach is impaired and tipped in a catabolic direction. That the synthetic activity of these perisutural chondrocytes was indeed suppressed is evidenced by their inability to maintain the proteoglycan levels within the extracellular matrix. The slight but significant decrease in the mean volume of these perisutural cells 3 weeks after surgery could reflect a decrease in the volume of their organellar synthetic equipment. Not surprisingly, the surviving population of chondrocytes evinced but a poor capacity to proliferate, only a few small cell clusters being observed in the vicinity of the suture channels.
The early development of fissures within the walls of the suture channels is probably directly correlated with the suturing process per se. As aforementioned, the action of pushing the suturing needle through the articular cartilage layer generates forces of an order of magnitude that will deform the tissue locally, thereby subjecting it to stresses and strains that may cause its rupture 33. The “empty” fissures observed shortly after surgery can be considered as tiny partial-thickness defects created by the action of suturing. Three weeks after surgery, many of these fissures were partially or completely filled with a loose, avascular type of mesenchymal tissue. Such a primitive type of scar tissue is known to be deposited spontaneously within partial-thickness defects that have been artificially created 13. But this tissue does not spontaneously differentiate into cartilage; a chondrogenic agent must be introduced to trigger the differentiation process 13, 15. Conceivably, the suturing thread could be impregnated with a chondrogenic agent, and in such a form as to ensure its gradual release over a period of several weeks. Such growth fator would induce the primitive type of scar tissue to differentiate into repair cartilage 13, 15. Moreover, an anabolic agent such as BMP-7 would also hinder the osteoarthritis-like changes 34.
The elongation of the fissures with time could partially reflect the “osteoarthritis-like” metabolic conditions that are operating within the perisutural area. However, the mechanical forces that are acting on the joint during its all but normal usage (knee movement restricted to 3–5 degrees, but normal loading permitted) are probably a more important cause of this phenomenon. Indeed, the articular cartilage layer is prone to cracking when high deformation forces are brought to bear during even physiological activities, such as walking or jumping 33, 35, 36. And the cracking phenomenon will be accentuated if the adjacent phases have different mechanical properties, as is the case when articular cartilage tissue abuts on an empty suture channel 33. Unphysiologically high stress phenomena acting along the interface between the channel wall and the tissue border will favour the propagation of existing cracks 36. To avoid this effect, the suturing thread would need to have the stiffness properties of articular cartilage. But the thread is bioresorbable and dissolves slowly with time. Hence, its material properties are continually changing. Moreover, the primitive scar tissue that is deposited within the fissures is probably much less stiff than articular cartilage. Hence, also when the fissures are tissue-filled, the interfacial stress phenomena will still be sufficiently high to promote their propagation, since, as aforementioned, this primitive type of scar tissue does not spontaneously differentiate into hyaline cartilage 13. The propagation of these fissures could be arrested by inducing the mesenchymal tissue to differentiate into cartilage, in the manner indicated above 15. Unless such a measure is taken, it is unlikely that the “osteoarthritis-like” changes induced by surgical suturing will be checked.
In summary, our study demonstrates that the suturing of tissue flaps to articular cartilage defects leads to a significant and progressive loss of cells from the peri-sutural area. Furthermore, the walls of the channels created by suturing are subject to cracking, which is likewise a progressive phenomenon. And these fissures do not heal. Hence, a treatment modality that aims to induce the repair of damaged articular cartilage, namely, the suturing of a tissue flap to retain an engineered construct within a defect, actually promotes further injury, which is progressive. This problem could be tackled either by inducing the repair of the suture channels and the fissures by impregnating the suturing thread with appropriate chondrogenic 15 and anaboloic 34 agents at the time of surgery, or by seeking an alternative means of flap of fixation. The use of a biological adhesive, such as tissue transglutaminase 37, might prove to be a viable option.
Acknowledgements
This study was supported by the University of Bern, Switzerland and by a grant from the NIH, USA (NIAMS, grant number 1 R01 AR 52766-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paradowski PT, Bergman S, Sunden-Lundius A, Lohmander LS, Roos EM. Knee complaints vary with age and gender in the adult population. Populationbased reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS) BMC Musculoskelet Disord. 2006;7:38. doi: 10.1186/1471-2474-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarzi-Puttini P, Cimmino MA, Scarpa R, Caporali R, Parazzini F, Zaninelli A, et al. Osteoarthritis: an overview of the disease and its treatment strategies. Semin Arthritis Rheum. 2005;35:1–10. doi: 10.1016/j.semarthrit.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Buckwalter JA, Martin JA. Osteoarthritis. Adv Drug Deliv Rev. 2006;58:150–167. doi: 10.1016/j.addr.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Wluka AE, Forbes A, Wang Y, Hanna F, Jones G, Cicuttini FM. Knee cartilage loss in symptomatic knee osteoarthritis over 4.5 years. Arthritis Res Ther. 2006;8:R90. doi: 10.1186/ar1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2006;14:1033–1040. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current concepts review. Am J Sports Med. 2006;34:1181–1191. doi: 10.1177/0363546506290127. [DOI] [PubMed] [Google Scholar]
- 7.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 8.Henderson I, Gui J, Lavigne P. Autologous chondrocyte implantation: natural history of postimplantation periosteal hypertrophy and effects of repair-site debridement on outcome. Arthroscopy. 2006;22:1318–1324. doi: 10.1016/j.arthro.2006.07.057. e1. [DOI] [PubMed] [Google Scholar]
- 9.Ruano-Ravina A, Jato Diaz M. Autologous chondrocyte implantation: a systematic review. Osteoarthritis Cartilage. 2006;14:47–51. doi: 10.1016/j.joca.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A:455–464. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Grande DA, Pitman MI, Peterson L, Menche D, Klein M. The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J Orthop Res. 1989;7:208–218. doi: 10.1002/jor.1100070208. [DOI] [PubMed] [Google Scholar]
- 12.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 13.Hunziker EB, Rosenberg LC. Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg Am. 1996;78:721–733. doi: 10.2106/00004623-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Driesang IM, Hunziker EB. Delamination rates of tissue flaps used in articular cartilage repair. J Orthop Res. 2000;18:909–911. doi: 10.1002/jor.1100180609. [DOI] [PubMed] [Google Scholar]
- 15.Hunziker EB. Growth-factor-induced healing of partial-thickness defects in adult articular cartilage. Osteoarthritis Cartilage. 2001;9:22–32. doi: 10.1053/joca.2000.0346. [DOI] [PubMed] [Google Scholar]
- 16.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 17.Schenk RK, Olah AJ, Herrmann W. Preparation of calcified tissues for light microscopy. In: Dickson GR, editor. Methods of Calcified Tissue Preparation. Vol. 1. Amsterdam: Elsevier Science Publishers B.V.; 1984. pp. 1–56. [Google Scholar]
- 18.Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 19.Cruz-Orive LM, Gual-Arnau X. Precision of circular systematic sampling. J Microsc. 2002;207:225–242. doi: 10.1046/j.1365-2818.2002.01057.x. [DOI] [PubMed] [Google Scholar]
- 20.Howard CV, Reed MG. Unbiased Stereology. Three-Dimensional Measurement in Microscopy. Second Edition. Oxford: Bios Scientific Publishers; 2005. [Google Scholar]
- 21.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 22.Hunziker EB, Quinn TM, Hauselmann HJ. Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis Cartilage. 2002;10:564–572. doi: 10.1053/joca.2002.0814. [DOI] [PubMed] [Google Scholar]
- 23.Eggli PS, Hunziker EB, Schenk RK. Quantitation of structural features characterizing weight- and less-weight-bearing regions in articular cartilage: a stereological analysis of medial femoral condyles in young adult rabbits. Anat Rec. 1988;222:217–227. doi: 10.1002/ar.1092220302. [DOI] [PubMed] [Google Scholar]
- 24.Carney SL, Muir H. The structure and function of cartilage proteoglycans. Physiol Rev. 1988;68:858–910. doi: 10.1152/physrev.1988.68.3.858. [DOI] [PubMed] [Google Scholar]
- 25.Wong M, Wuethrich P, Eggli P, Hunziker E. Zone-specific cell biosynthetic activity in mature bovine articular cartilage: a new method using confocal microscopic stereology and quantitative autoradiography. J Orthop Res. 1996;14:424–432. doi: 10.1002/jor.1100140313. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Markel MD, Swain C, Kaplan LD. Development of partial thickness articular cartilage injury in an ovine model. J Orthop Res. 2006;24:1974–1982. doi: 10.1002/jor.20249. [DOI] [PubMed] [Google Scholar]
- 27.Mankin HJ. The reaction of articular cartilage to injury and osteoarthritis (first of two parts) N Engl J Med. 1974;291:1285–1292. doi: 10.1056/NEJM197412122912406. [DOI] [PubMed] [Google Scholar]
- 28.Walker EA, Verner A, Flannery CR, Archer CW. Cellular responses of embryonic hyaline cartilage to experimental wounding in vitro. J Orthop Res. 2000;18:25–34. doi: 10.1002/jor.1100180105. [DOI] [PubMed] [Google Scholar]
- 29.Hunziker EB, Quinn TM. Surgical removal of articular cartilage leads to loss of chondrocytes from cartilage bordering the wound edge. J Bone Joint Surg Am. 2003;85-A Suppl 2:85–92. doi: 10.2106/00004623-200300002-00011. [DOI] [PubMed] [Google Scholar]
- 30.Paulsen FP, Tillmann BN. Osteoarthritis in cricoarytenoid joint. Osteoarthritis Cartilage. 1999;7:505–514. doi: 10.1053/joca.1999.0253. [DOI] [PubMed] [Google Scholar]
- 31.Martin JA, Buckwalter JA. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology. 2002;3:257–264. doi: 10.1023/a:1020185404126. [DOI] [PubMed] [Google Scholar]
- 32.Hough AJ. Pathology of osteoarthritis. In: Moskowitz RW, Howell DS, Altman RD, Buckwalter JA, Goldberg VM, editors. Osteoarthritis. 3rd Edition. Philadelphia: Diagnosis and Medical/Surgical Management, W.B. Saunders Company; 1984. pp. 69–99. [Google Scholar]
- 33.Li X, Haut RC, Altiero NJ. An analytical model to study blunt impact response of the rabbit P-F joint. J Biomech Eng. 1995;117:485–491. doi: 10.1115/1.2794212. [DOI] [PubMed] [Google Scholar]
- 34.Fan Z, Chubinskaya S, Rueger DC, Bau B, Haag J, Aigner T. Regulation of anabolic and catabolic gene expression in normal and osteoarthritic adult human articular chondrocytes by osteogenic protein-1. Clin Exp Rheumatol. 2004;22:103–106. [PubMed] [Google Scholar]
- 35.Morel V, Berutto C, Quinn TM. Effects of damage in the articular surface on the cartilage response to injurious compression in vitro. J Biomech. 2006;39:924–930. doi: 10.1016/j.jbiomech.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 36.Quinn TM, Grodzinsky AJ, Hunziker EB, Sandy JD. Effects of injurious compression on matrix turnover around individual cells in calf articular cartilage explants. J Orthop Res. 1998;16:490–499. doi: 10.1002/jor.1100160415. [DOI] [PubMed] [Google Scholar]
- 37.Jurgensen K, Aeschlimann D, Cavin V, Genge M, Hunziker EB. A new biological glue for cartilage-cartilage interfaces: tissue transglutaminase. J Bone Joint Surg Am. 1997;79:185–193. doi: 10.2106/00004623-199702000-00004. [DOI] [PubMed] [Google Scholar]