Abstract
Background
Digoxin reduces hospitalizations due to heart failure (HF) and may also reduce mortality at low serum digoxin concentrations (SDC). Most HF patients are ≥ 65 years, yet the effects of digoxin on outcomes in these patients have not been well studied.
Methods
Of the 7788 ambulatory chronic HF patients in normal sinus rhythm in the Digitalis Investigation Group trial (1991–1995), 5548 (2890 were ≥ 65 years) were alive at 1 month and were either receiving placebo or had data on SDC. Of these patients, 982 had low (0.5–0.9 ng/mL) and 705 had high (≥ 1 ng/mL) SDC.
Results
Among patients ≥ 65 years, compared with 38% placebo patients, 34% low SDC patients died during 39 months of median follow-up (adjusted hazard ratio [AHR] = 0.81; 95% confidence interval [CI] = 0.68–0.96; p = .017). All-cause hospitalizations occurred in 70% of placebo and 68% of low-SDC patients (AHR = 0.86; 95% CI = 0.76–0.98; p = .019). Reduction in hospitalizations for HF occurred in both low and high SDC groups. High SDC was not independently associated with all-cause hospitalization or all-cause mortality. Age, impaired renal function, and pulmonary congestion reduced the odds of low SDC. Low-dose digoxin (≥ 0.125 mg/d) was the strongest independent predictor of low SDC (adjusted odd ratio = 2.37; 95% CI = 1.65–3.39); p < 0.0001).
Conclusions
Digoxin at low SDC was associated with a reduction in mortality and hospitalization in chronic geriatric HF, and low-dose digoxin was the strongest predictor of low SDC.
MOST heart failure (HF) patients are ≥ 65 years, and HF is the number one hospital discharge diagnosis in this age group (1). Data from the randomized Digitalis Investigation Group (DIG) trial suggest that digoxin reduce HF hospitalization in patients with HF (2,3). A recent comprehensive post hoc analysis of the DIG trial suggests that digoxin reduces HF hospitalizations regardless of serum digoxin concentrations (SDC) (4,5). However, in patients with low SDC (0.5–0.9 ng/mL), digoxin also reduces all-cause mortality and all-cause hospitalizations (4).
Digoxin is approved by the U.S. Food and Drug Administration (FDA) for use in HF, and national HF guidelines recommend use of digoxin to reduce HF symptoms and HF hospitalizations (6,7). However, evidence from recent large national HF registries suggest that use of digoxin in HF is on the decline (8,9). The rate of nonuse of digoxin may be even higher in geriatric HF due to previous reports about ineffectiveness or inappropriateness of digoxin in this population (10–12). Elderly patients are also more likely to develop high SDC and digoxin toxicity than are younger patients (4,13). The decline in the appropriate use of digoxin in HF patients may have negative consequences for both HF patients and the health care system. The bulk of the cost of HF care is spent for inpatient care, and HF is the number one reason for hospitalization among older adults (14,15). However, little is known about the effect of digoxin at low and high SDC on outcomes in geriatric HF. The objective of this study was to determine the effect of digoxin at low and high SDC on mortality and hospitalizations, and to determine independent predictors of low SDC in older and younger adults with chronic systolic and diastolic HF.
Methods
DIG Trial
The randomized placebo-controlled DIG trial was conducted during 1991–1995 in the United States (186 centers) and Canada (116 centers). The goal of the DIG trial was to determine the effects of digoxin in ambulatory adults with systolic and diastolic HF and normal sinus rhythm receiving background therapy with diuretics and angiotensin-converting enzyme (ACE) inhibitors. Patients received digoxin or placebo at the daily doses of 0.125 mg, 0.25 mg, 0.375 mg, or 0.50 mg (2). The design and the results of the DIG trial have been previously described in detail (2).
Patients
Of the 7788 patients enrolled in the DIG trial, 6800 had systolic HF or left ventricular ejection fraction ≤ 45%, and 988 had diastolic HF or ejection fraction > 45%. For the current study, we analyzed data from 5548 patients who were alive at 1 month, and were either receiving placebo or had data on SDC based on specimens collected at least 6 hours after the last dose of digoxin.
Of the 5548 patients, 2658 were < 65 years and 2890 were ≥ 65 years, and 982 had low (0.5–0.9 ng/mL) and 705 had high (≥ 1 ng/mL) SDC. SDC has been shown to be an important determinant of the effect of digoxin on HF (4,16–18). Patients were randomly chosen for SDC measurement, except in life-threatening emergencies, when it was dictated by clinical indications (2). SDC was categorized into low (0.5–0.9 ng/mL) or high (≥ 1.0 ng/mL) based on cut points used in the literature (4,17).
Outcomes
Primary outcomes for this analysis were all-cause mortality and hospitalizations due to HF and all causes at 39.9 months of median follow-up (41.3 months for < 65 years and 39.1 months for ≥ 65 years). Data on outcomes were 98.9% complete by December 31, 1995 (19).
Statistical Analysis
Patients were categorized as either < 65 or ≥ 65 years of age, and separate analyses were conducted for each age group. We compared baseline characteristics of placebo, low, and high SDC patients. Kaplan–Meier survival analysis and log-rank tests were used to estimate the effects of low and high SDC on outcomes relative to placebo. Chronic kidney disease (CKD) was defined as estimated baseline glomerular filtration rate (GFR) < 60 mL/1.73 square meters of body surface area (20).
Chi-square and bivariate Cox proportional hazards regression models were used to determine the unadjusted associations of low and high SDC with all-cause mortality, HF hospitalization, and all-cause hospitalization. To control for confounding variables, we used multivariable Cox regression models for each of the outcomes. In the multivariable models, low and high SDC were used as dummy variables (using placebo as the reference category). The covariates used in the multivariable models were age; sex; race; body mass index; duration of HF; etiology of HF; prior myocardial infarction, current angina, hypertension, or diabetes; pretrial use of digoxin; use of ACE inhibitors, diuretics, and combination of hydralazine and nitrates; current dyspnea at rest and dyspnea on exertion; heart rate; systolic and diastolic blood pressure; current jugular venous distension; third heart sound; pulmonary râles; lower extremity edema; New York Heart Association (NYHA) functional class; pulmonary congestion by chest x-ray; cardiothoracic ratio > 0.5; estimated GFR; and ejection fraction.
Finally, we examined the association between low-dose (≤ 0.125 mg/d) digoxin and low SDC (0.5–0.9 ng/mL) among patients receiving digoxin (n = 1687), separately for patients < 65 (n = 881) and ≥ 65 years (n = 806). Covariates previously shown to be associated with SDC (4), were stepwise entered into the multivariable logistic regression model: (i) age (> median age = 1, else = 0), sex (female = 1), and race (nonwhite = 1); (ii) CKD, diuretic use, and pulmonary congestion by chest x-ray. Median age for patients < 65 years and ≥ 65 years were, respectively, 58 and 71 years. Both models were fit to data during all steps of the regression analyses. Hosmer and Lemeshow goodness of fit test Chi-square at the final steps for < 65 and ≥ 65 years were, respectively, 7.2 (p = .512) and 7.8 (p = .455). All statistical tests were evaluated using a two-tailed 95% confidence level. All data analyses were performed using SPSS for Windows, version 14 (21).
Results
Patient Characteristics
Patients < 65 years (n = 2658; low SDC = 510, high SDC = 296, and placebo = 1852) had a median age of 57 years; 22% were women, 18% were nonwhite, and 9% had an ejection fraction > 45% (Table 1). Patients ≥ 65 years (n = 2890; low SDC = 472, high SDC = 409, and placebo = 2009), in contrast, had a median age of 71 years; 26% were women, 9% were nonwhite, and 13% had and ejection fraction > 45% (Table 1). Among the 881 patients ≥ 65 years receiving digoxin, the mean (±standard deviation [SD]) daily dose of digoxin was 0.23 (±0.06) mg/d. The mean (±SD) daily dose for those < 65 years was 0.29 (±0.07) mg/d.
Table 1.
Baseline Characteristics of Heart Failure (HF) Patients by Age Groups and Serum Digoxin Concentration (SDC)
| < 65 Years (n = 2658) | ≥ 65 Years (n = 2890) | |||||||
|---|---|---|---|---|---|---|---|---|
| N (%) or Mean (±SD) | Placebo (N = 1852) | SDC 0.5–0.9 (N = 510) | SDC ≥1.0 (N = 296) | Overall p | Placebo (N = 2009) | SDC 0.5–0.9 (N = 472) | SDC ≥ 1.0 (N = 409) | Overall p |
| Age, y* | 55 (±8) | 55 (±8) | 56 (±8) | .289 | 72 (±5) | 71 (±5) | 72 (±5) | .005 |
| Women* | 399 (22%) | 103 (20%) | 78 (26%) | .109 | 556 (28%) | 114 (24%) | 116 (28%) | .256 |
| Nonwhites* | 336 (18%) | 77 (15%) | 53 (18%) | .325 | 227 (11%) | 49 (10%) | 33 (8%) | .152 |
| BMI, kg/m2* | 28.4 (±5.7) | 28.3 (±5.3) | 27.9 (±5.8) | <.0001 | 26.5 (±4.5) | 26.0 (±4.4) | 26.0 (±4.9) | .039 |
| Ejection fraction, %* | 31 (±12) | 31 (±11) | 30 (±12) | .637 | 33 (±13) | 33 (±13) | 327 (±13) | .093 |
| NYHA class III–IV* | 526 (28%) | 132 (28%) | 87 (29%) | .457 | 668 (33%) | 141 (30%) | 144 (35%) | .218 |
| HF duration, mo | 30 (±35) | 32 (±36) | 37 (±39) | .003 | 30 (±37) | 32 (±41) | 33 (±38) | .159 |
| Ischemic etiology* | 1201 (65%) | 337 (66%) | 194 (66%) | .866 | 1453 (73%) | 335 (71%) | 313 (77%) | .144 |
| Hypertension* | 827 (45%) | 214 (42%) | 145 (49%) | .154 | 999 (50%) | 219 (46%) | 189 (46%) | .239 |
| Diabetes | 513 (28%) | 139 (27%) | 96 (32%) | .215 | 596 (30%) | 126 (27%) | 119 (29%) | .441 |
| Chronic kidney disease* | 573 (31%) | 117 (23%) | 129 (44%) | <.0001 | 1222 (61%) | 251 (53%) | 286 (70%) | <.0001 |
| Prior digoxin use† | 836 (45%) | 251 (49%) | 148 (50%) | .114 | 838 (42%) | 206 (44%) | 192 (47%) | .137 |
| ACE inhibitor use† | 1765 (95%) | 480 (94%) | 277 (94%) | .314 | 1852 (92%) | 451 (96%) | 387 (95%) | .014 |
| Diuretic use* | 1382 (75%) | 357 (70%) | 247 (83%) | <.0001 | 1635 (81%) | 358 (76%) | 339 (83%) | .011 |
| Dose of study medication* | 0.27 (±0.1) | 0.27 (±0.1) | 0.27 (±0.1) | .120 | 0.22 (±0.1) | 0.22 (±0.1) | 0.23 (±0.1) | .043 |
| Pulmonary congestion | 243 (13%) | 57 (11%) | 51 (17%) | .049 | 292 (15%) | 57 (12%) | 83 (20%) | .002 |
| Cardiothoracic ratio > 0.5* | 1067 (57%) | 289 (57%) | 174 (59%) | .839 | 1257 (63%) | 269 (57%) | 267 (65%) | .028 |
| Serum creatinine, mg/dL* | 1.2 (±0.3) | 1.1 (±0.3) | 1.3 (±0.4) | <.0001 | 1.4 (±0.4) | 1.3 (±0.4) | 1.4 (±0.4) | <.0001 |
| eGFR, ml/min/1.73 m2* | 70 (±23) | 73 (±19) | 64 (±19) | <.0001 | 57 (±24) | 60 (±17) | 53 (±18) | <.0001 |
| Serum potassium, mEq/L | 4.3 (±0.4) | 4.3 (±0.5) | 4.3 (±0.4) | .863 | 4.4 (±0.4) | 4.4 (±0.4) | 4.4 (±0.4) | .817 |
Note: SD = standard deviation; ACE = angiotensin-converting enzyme; BMI = body mass index; eGFR = estimate glomerular filtration rate; NYHA = New York Heart Association.
p < .0001 and
p <. 05 for comparisons between patient < 65 and ≥ 65 years
Among patients ≥ 65 years, compared to those receiving placebo, low SDC was associated with younger age, lower mean serum creatinine, fewer pulmonary congestion, and less diuretic use (Table 1). High SDC, in contrast, was associated with a higher dose of digoxin and higher serum creatinine. An asterisk next to a variable in Table 1 identifies a significant differences in patient characteristic between the two age groups.
Digoxin and Mortality in Younger Patients
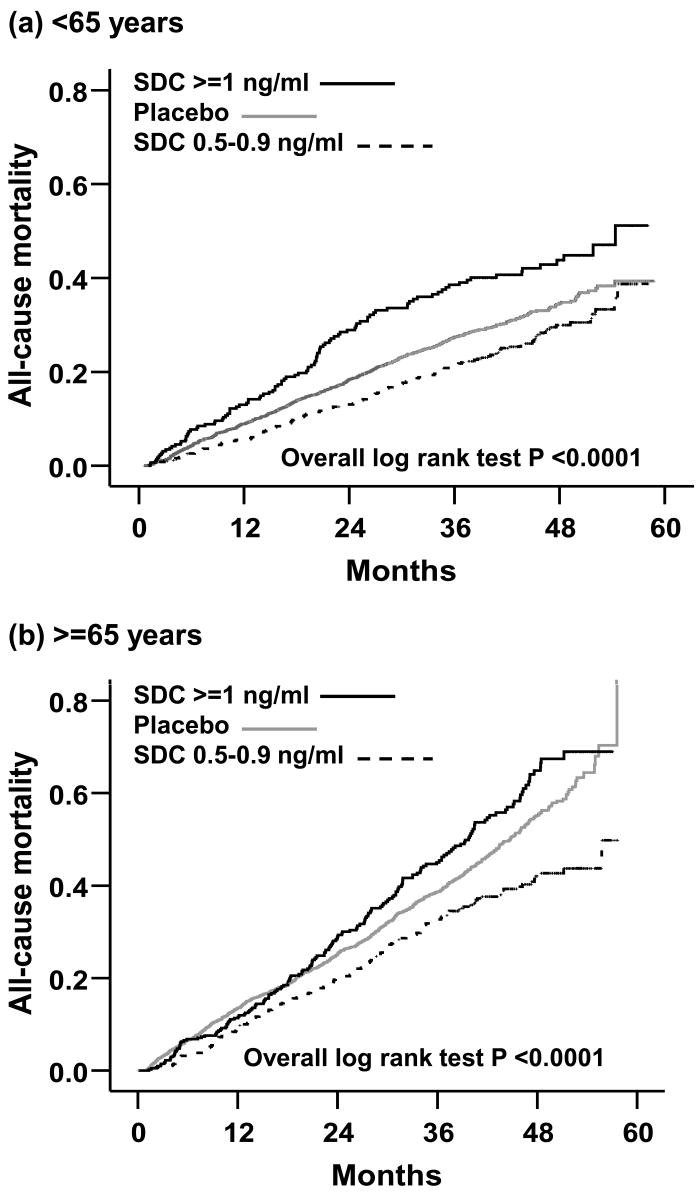
During 41 months of median follow-up, 735 (28%) patients died from all causes, including 597 (23%) from cardiovascular causes and 233 (9%) from worsening HF. Compared with 27% of patients receiving placebo, 26% with low SDC (hazard ratio [HR] = 0.61; 95% confidence interval [CI] = 0.42–0.89; p = .010), and 36% with high SDC (HR = 1.18; 95% CI = 0.81–1.72; p = .391) died (Table 2). Multivariable adjustment for baseline covariates did not significantly alter associations of low and high SDC with mortality (Table 2). The Kaplan–Meier plots for mortality are displayed in Figure 1a.
Table 2.
Effects of Digoxin on All-Cause Mortality
| Patient Subgroups | Absolute Risk (% Mortality/Total) | Crude HR (95% CI) | p Value | Adjusted* HR (95% CI) | p Value |
|---|---|---|---|---|---|
| < 65 Years (n = 2658) | |||||
| Placebo (n = 1852) | 27% | 1 | Reference | 1 | Reference |
| SDC 0.5–0.9 ng/mL (n = 510) | 26% | 0.61 (0.42–0.89) | .010 | 0.62 (0.42–0.90) | .012 |
| SDC ≥ 1 ng/mL (n = 296) | 36% | 1.18 (0.81–1.72) | .391 | 0.97 (0.66–1.43) | .885 |
| ≥ 65 Years n = 2890) | |||||
| Placebo (n = 2009) | 38% | 1 | Reference | 1 | Reference |
| SDC 0.5–0.9 ng/mL (n = 472) | 34% | 0.76 (0.64–0.90) | .001 | 0.81 (0.68–0.96) | .017 |
| SDC ≥ 1 ng/mL (n = 409) | 46% | 1.13 (0.96–1.32) | .136 | 1.03 (0.88–1.21) | .727 |
Notes: HR = hazard ratio; CI = confidence interval; SDC = serum digoxin concentration.
Adjusted for age, sex, race, body mass index, duration of heart failure, etiology of heart failure, prior myocardial infarction, current angina, hypertension, diabetes, pretrial use of digoxin, use of angiotensin-converting enzyme inhibitors, diuretics, and combination of hydralazine and nitrates, current dyspnea at rest and dyspnea on exertion, heart rate, systolic and diastolic blood pressure, current jugular venous distension, third heart sound, pulmonary râles, lower extremity edema, New York Heart Association functional class, pulmonary congestion by chest x-ray, cardiothoracic ratio > 0.5, estimated glomerular filtration rate, and ejection fraction.
Figure 1.
Kaplan Meier plots for all-cause mortality in heart failure patients (a) < 65 years and (b) ≥ 65 years
Digoxin and Mortality in Older Patients
During 39 months of median follow-up, 1119 (39%) patients ≥ 65 years died from all causes, including 859 (30%) from cardiovascular causes and 417 (14%) from worsening HF. Compared with 38% of patients receiving placebo, 34% with low SDC (HR = 0.76; 95% CI = 0.64–0.90; p = .001) and 46% with high SDC (HR = 1.13; 95% CI = 0.96–1.32; p = .136) died (Table 2). Multivariable adjustment for baseline covariates did not significantly alter these associations of low and high SDC with mortality (Table 2). The Kaplan–Meier plots for mortality among HF patients ≥ 65 years receiving placebo and among those with low and high SDC are displayed in Figure 1b.
Digoxin and Hospitalizations in Younger Patients
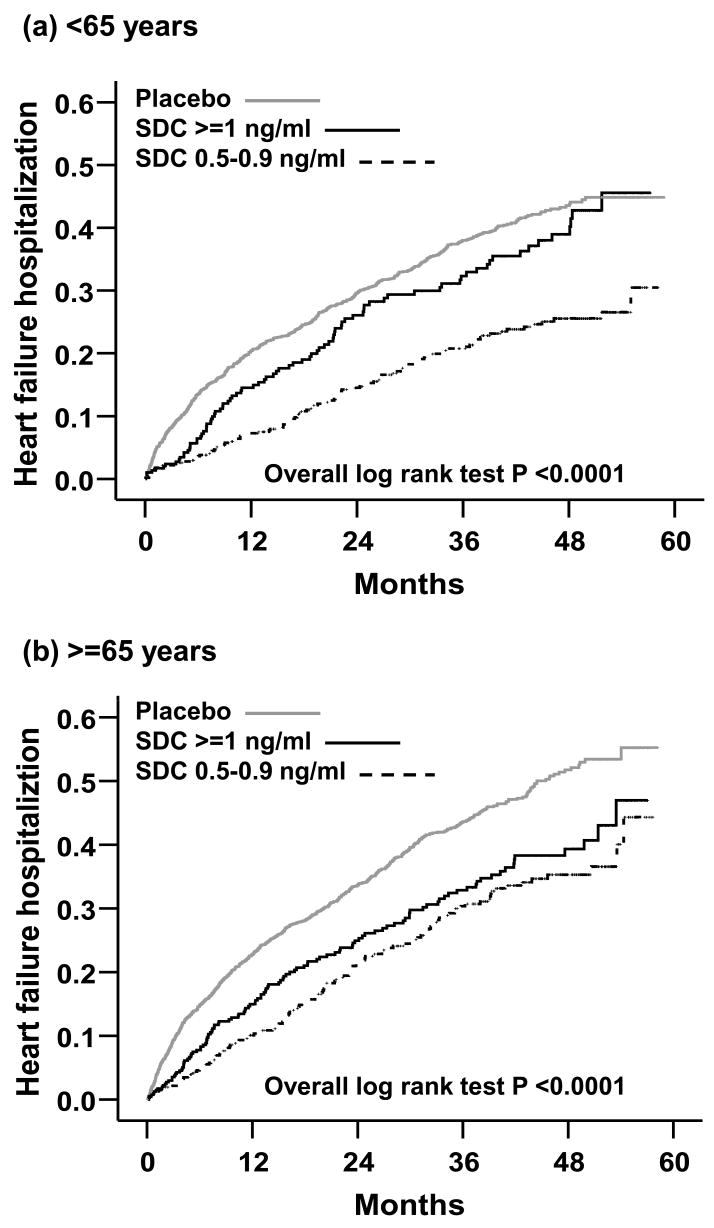
Overall, 1693 (64%) patients < 65 years were hospitalized due to all causes, 1379 (52%) were hospitalized due to cardiovascular causes, and 773 (29%) due to worsening HF. Compared with 32% of patients receiving placebo, 20% with low SDC (HR = 0.56; 95% CI = 0.45–0.69; p < .0001) and 29% with high SDC (HR = 0.88; 95% CI = 0.70–1.11; p = .275) were hospitalized due to worsening HF (Table 3). Multivariable adjustment for baseline covariates did not significantly alter the association of low SDC and HF hospitalization (Table 3). However, the association between high SDC and HF hospitalization became stronger and significant after multivariable adjustment for baseline covariates (Table 3). The Kaplan–Meier plots for first HF hospitalization are displayed in Figure 2a.
Table 3.
Effects of Digoxin on Heart Failure Hospitalization
| Patient Subgroups | Absolute Risk % (Events/Total) | Crude HR (97.5% CI) | p Value | Adjusted* HR (97.5% CI) | p Value |
|---|---|---|---|---|---|
| < 65 Years (n = 2658) | |||||
| Placebo (n = 1852) | 32% | 1 | Reference | 1 | Reference |
| SDC 0.5–0.9 ng/mL (n = 510) | 20% | 0.56 (0.45–0.69) | <.0001 | 0.54 (0.43–0.66) | <.0001 |
| SDC ≥ 1 ng/mL (n = 296) | 29% | 0.88 (0.70–1.11) | .275 | 0.70 (0.56–0.88) | .002 |
| ≥ 65 Years (n = 2890) | |||||
| Placebo (n = 2009) | 35% | 1 | Reference | 1 | Reference |
| SDC 0.5–0.9 ng/mL (n = 472) | 27% | 0.66 (0.54–0.79) | <.0001 | 0.71 (0.58–0.86) | <.0001 |
| SDC ≥ 1 ng/mL (n = 409) | 33% | 0.75 (0.62–0.91) | .004 | 0.66 (0.54–0.81) | <.0001 |
Note: HR = hazard ratio; CI = confidence interval; SDC = serum digoxin concentration.
Adjusted for the same covariates as in Table 2.
Figure 2.
Kaplan Meier plots for hospitalization due to worsening heart failure in patients (a) <65 years and (b) ≥ 65 years
Compared with 64% of patients receiving placebo, 60% with low SDC (HR = 0.82; 95% CI = 0.72–0.93; p = .002) and 69% of high SDC (HR = 1.22; 95% CI = 0.97–1.30; p = .131) patients were hospitalized due to all causes (Table 4). Multivariable adjustment for baseline covariates did not significantly alter these associations of low and high SDC with all-cause hospitalization (Table 4). Compared with 53% of patients receiving placebo, 46% with low SDC (HR = 0.76; 95% CI = 0.67–0.88; p < .0001) and 55% with high SDC (HR = 1.06; 95% CI = 0.90–1.25; p = .522) were hospitalized due to cardiovascular causes. Multivariable adjustment for baseline covariates did not significantly alter these associations.
Table 4.
Effects of Digoxin on All-Cause Hospitalization
| Patient Subgroups | Absolute Risk % (Events/Total) | Crude HR (97.5% CI) | p Value | Adjusted* HR (97.5% CI) | p Value |
|---|---|---|---|---|---|
| < 65 Years (n = 2658) | |||||
| Placebo (n = 1852) | 64% | 1 | Reference | 1 | Reference |
| SDC 0.5–0.9 ng/mL (n = 510) | 60% | 0.82 (0.72–0.93) | .002 | 0.80 (0.71–0.91) | .001 |
| SDC ≥ 1 ng/mL (n = 296) | 69% | 1.22 (0.97–1.30) | .131 | 0.99 (0.85–1.15) | .844 |
| ≥ 65 Years (n = 2890) | |||||
| Placebo (n = 2009) | 70% | 1 | Reference | 1 | Reference |
| SDC 0.5–0.9 ng/mL (n = 472) | 68% | 0.83 (0.73–0.93) | .002 | 0.86 (0.76–0.98) | .019 |
| SDC ≥ 1 ng/mL (n = 409) | 72% | 0.98 (0.86–1.11) | .712 | 0.92 (0.81–1.05) | .216 |
Note: HR = hazard ratio; CI = confidence interval; SDC = serum digoxin concentration.
Adjusted for the same covariates as in Table 2.
Digoxin and Hospitalizations in Older Patients
Overall, 2024 (70%) patients ≥ 65 years were hospitalized due to all causes, 1541 (53%) were hospitalized due to cardiovascular causes, and 939 (23%) due to worsening HF. Compared with 35% of patients receiving placebo, 27% with low SDC (HR = 0.66; 95% CI = 0.54–0.79; p < .0001) and 33% with high SDC (HR = 0.75; 95% CI = 0.62–0.91; p = .004) were hospitalized due to worsening HF (Table 3). Multivariable adjustment for baseline covariates did not significantly alter these associations of low and high SDC with HF hospitalization (Table 3). The Kaplan–Meier plots for first HF hospitalization among HF patients ≥ 65 years receiving placebo and among those with low and high SDC are displayed in Figure 2b.
Compared with 70% of patients receiving placebo, 68% with low SDC (HR = 0.83; 95% CI = 0.73–0.93; p = .002) and 72% of high SDC (HR = 0.98; 95% CI = 0.86–1.11; p = .712) patients were hospitalized due to all causes (Table 4). Multivariable adjustment for covariates did not significantly alter these associations (Table 4). Compared with 54% of patients receiving placebo, 50% with low SDC (HR = 0.80; 95% CI = 0.69–0.92; p = .001) and 55% with high SDC (HR = 0.97; 95% CI = 0.84–1.12; p = .643) were hospitalized due to cardiovascular causes. These associations remained essentially unchanged after multivariable covariate adjustment.
Hospitalizations Due to Digoxin Toxicity
During the entire follow-up, 26 (1.0%) patients < 65 years and 35 (1.2%) patients ≥ 65 years were hospitalized due to suspected digoxin toxicity (Chi-square p = .441). Among younger patients, compared with 0.8% receiving placebo, 1.2% with low SDC (HR = 1.46; 95% CI = 0.56–3.80; p = .439) and 2.0% with high SDC (HR = 2.77; 95% CI = 1.06–7.20; p = .037) were hospitalized for suspected digoxin toxicity. Among older patients, compared with 1.0% receiving placebo, 1.3% with low SDC (HR = 1.15; 95% CI = 0.46–2.87; p = .766) and 2.2% with high SDC (HR = 2.12; 95% CI = 0.97–4.66; p = .061) were hospitalized for suspected digoxin toxicity.
Low-Dose Digoxin and Low SDC
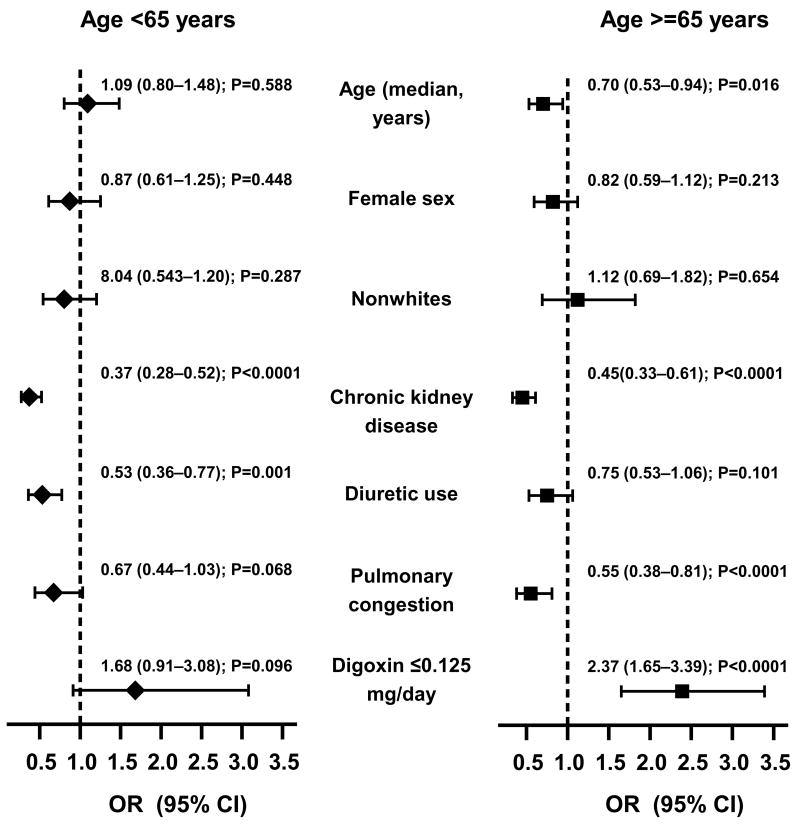
Among patients receiving digoxin (n = 1687), 260 (15%) were receiving digoxin at low doses (≥ 0.125 mg/d) and 982 (58%) had low SDC (0.5–0.9 ng/mL). Compared with 63% of younger patients, 54% of older patients developed low SDC (Chi-square p < .0001). Among patients < 65 years, impaired renal function and diuretics were independent negative predictors of low SDC (Figure 3). In contrast, among patients ≥ 65 years, age, impaired renal function, and pulmonary congestion were independent negative predictors of low SDC. Low-dose digoxin was the strongest independent predictor of low SDC (Figure 3). In these patients, low-dose digoxin was associated with lower odds of high SDC (adjusted OR = 0.42; 95% CI = 0.30–0.61; p < .0001).
Figure 3.
Independent predictors of low (0.5 – 0.9 ng/ml) serum digoxin concentrations among patients < 65 years (left panel: n=806; median age, 58 years) and ≥ 65 years (right panel: n=881; median age, 72 years) [Chronic kidney disease=estimated glomerular filtration rate <60 ml/m/1.73 square meters; OR=odds ratio, CI=confidence interval
Discussion
The results of the current study demonstrate that, as in younger HF patients, in ambulatory older adults with HF use of digoxin was associated with significant reduction in HF hospitalizations regardless of SDC. In addition, at low SDC, use of digoxin was also associated with reduction in all-cause mortality and all-cause hospitalization in both age groups. Among older HF patients, low-dose digoxin (≥ 0.125 mg/d) was the strongest independent predictor of low SDC.
These findings are important because the vast majority of HF patients are older adults, HF is the number one reason for hospitalization among older adults, and with the aging of the U.S. population, the number of older adults with HF is likely to double in the coming decades. The benefits of low SDC are observed in patients who were receiving ACE inhibitors and diuretics, which is also an important consideration, because many elderly HF patients cannot tolerate or afford beta-blockers, and nearly half of all HF patients do not receive beta-blockers (8,22).
Possible Mechanistic Explanations
The beneficial effects of digoxin in HF are believed to be mediated via its favorable neurohormonal properties (6,23). Recent evidence suggests that digoxin inhibits sympathetic nervous system activities in HF by inhibiting the sodium–potassium (Na+–K+) adenosine triphosphatase (ATPase) enzyme in vagal afferent nerve fibers (24,25). Digoxin also inhibits the rennin-angiotensin-aldosterone system by inhibiting the Na+–K+ ATPase in the renal tubules (23,26). The effects of digoxin have long been known to be dependent on doses and SDC (27,28). The favorable effects of digoxin at low SDC on broader natural history endpoints such as all-cause hospitalizations and all-cause mortality are probably mediated via the neurohormonal modulating properties of digoxin. It has been suggested that digoxin exerts its neurohormonal properties best at low SDC (4,17,18,23,25). However, the effect of digoxin on HF hospitalization was evident regardless of SDC. It is possible that this was in part mediated via hemodynamic properties of digoxin (32,33). The inotropic properties of digoxin are believed to be less SDC-dependent than are its neurohormonal properties.
Comparison With Previous Studies
To the best of our knowledge, this is the first comprehensive post hoc analysis of DIG data examining the effect of SDC in elderly men and women with systolic and diastolic HF. The results of this analysis are consistent with those reported by Rich and colleagues, who did not find any significant interaction between age and digoxin (3). However, SDC was not accounted for in that analysis.
Clinical Implications
Digoxin is the oldest HF drug and is also inexpensive. It is FDA approved for HF use and is recommended by HF guidelines (6,7). However, recent evidence suggests that the use of digoxin in HF has declined (8,22). Although the reasons for this decline are complex and have not been well studied, it is suspected that the age of the DIG trial in the pre-beta-blocker era and lack of mortality benefit of digoxin tempered enthusiasm for digoxin use in today’s HF patients. However, there is no evidence that beta-blockers recommended for systolic HF are beneficial in diastolic HF, (6,34,35), and about half of all systolic HF patients do not receive beta-blockers (8,22). There is also evidence that digoxin and beta-blockers are beneficial in the presence of each other (36). Finally, many elderly HF patients remain symptomatic despite therapy with ACE inhibitors and beta-blockers. Therefore, digoxin can play an important role in reducing HF hospitalization in older adults with symptomatic HF (4,37) and thus improve quality of life and ease the burden on the health care system. However, digoxin is used in low doses and care is taken to achieve low SDC, digoxin may also reduce all-cause mortality and all-cause hospitalizations.
Importance of Low – Dose Digoxin in Older Adults
Digoxin should be used in low doses in older adults with symptomatic HF with or without atrial fibrillation. Specifically, digoxin should be considered for treatment of HF symptoms before high-dose non-potassium-sparing diuretics. Diuretics may activate neurohormones and increase mortality (38). Digoxin should also be considered before referring geriatric HF patients for invasive and expensive device-based therapies (39).
The results of the current analysis also highlight the relative importance of low-dose digoxin (≤ 0.125 mg/d) in older adults. Low-dose digoxin was an independent predictor of low SDC in older adults, but not in younger adults. It is thus prudent to treat older adults with HF with digoxin ≤ 0.125 mg/d. Lower doses (0.125 mg every other day or 0.625 mg/d) should be used for patients who are ≥ 72 years or those who have CKD or pulmonary congestion. It may also be prudent and cost-effective to check SDC in these patients to guide therapy.
Strengths and Limitations
This is the first comprehensive analysis of the DIG trial that demonstrates that digoxin at low SDC is as effective in older adults as in younger adults suggesting that the pharmacodynamic (what drug does to the body) properties of digoxin are similar, regardless of age. However, pharmacokinetics (what body does to the drug) of digoxin may be more variable at different ages. We noted that kidney function, a major determinant of excretion of digoxin, had similar effect of SDC, regardless of age. However, unlike in younger adults, age and digoxin dose were independent predictors of SDC in older adults, suggesting important age-related changes in digoxin pharmacokinetics with aging. We also noted that there was no age-related difference in the incidence of hospitalizations due to suspected digoxin toxicity.
Participants in the DIG trial were predominantly white, male, and relatively younger, with mild to moderate HF and normal sinus rhythm, thus limiting generalizability. However, the mean age of patients ≥ 65 years in our subgroup analysis was 72 years (vs 55 years for those < 65 years). The results of this study are based on post hoc analysis and should be interpreted with caution. However, in the absence of randomized clinical trial evidence, these provide the best interim evidence of the effect of digoxin on long-term broader natural history end points in geriatric patients with HF.
Conclusions
Digoxin reduced HF hospitalizations in older adults with HF regardless of SDC. However, at low SDC (0.5–0.9 ng/mL), digoxin also reduced all-cause mortality and all-cause hospitalization and should be considered in symptomatic geriatric HF patients. Low-dose digoxin (≤ 0.125 mg/d) is likely to achieve low SDC. However, SDC should be checked in frail elderly patients with impaired renal function to guide therapy.
Acknowledgments
Dr. Ahmed is supported by the National Institutes of Health through grants from the National Institute on Aging (1-K23-AG19211-04) and the National Heart, Lung, and Blood Institute (1-R01-HL085561-01 and P50-HL077100).
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
References
- 1.American Heart Association. Heart Disease and Stroke Statistics: 2005 Update. Dallas, TX: American Heart Association; 2005. [Google Scholar]
- 2.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 3.Rich MW, McSherry F, Williford WO, Yusuf S. Effect of age on mortality, hospitalizations and response to digoxin in patients with heart failure: the DIG study. J Am Coll Cardiol. 2001;38:806–813. doi: 10.1016/s0735-1097(01)01442-5. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brophy JM. Rehabilitating digoxin. Eur Heart J. 2006;27:127–129. doi: 10.1093/eurheartj/ehi686. [DOI] [PubMed] [Google Scholar]
- 6.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 7.Adams K, Lindenfeld J, Arnold J, et al. Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006;12:10–38. doi: 10.1016/j.cardfail.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Gheorghiade M, Zannad F, Sopko G, et al. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112:3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 10.Aronow WS, Starling L, Etienne F. Lack of efficacy of digoxin in treatment of compensated congestive heart failure with third heart sound and sinus rhythm in elderly patients receiving diuretic therapy. Am J Cardiol. 1986;58:168–169. doi: 10.1016/0002-9149(86)90264-x. [DOI] [PubMed] [Google Scholar]
- 11.Aronow WS. Prevalence of appropriate and inappropriate indications for use of digoxin in older patients at the time of admission to a nursing home. J Am Geriatr Soc. 1996;44:588–590. doi: 10.1111/j.1532-5415.1996.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 12.Casiglia E, Tikhonoff V, Pizziol A, et al. Should digoxin be proscribed in elderly subjects in sinus rhythm free from heart failure? A population-based study. Jpn Heart J. 1998;39:639–651. doi: 10.1536/ihj.39.639. [DOI] [PubMed] [Google Scholar]
- 13.Aronson JK, Grahame-Smith DG, Wigley FM. Monitoring digoxin therapy. The use of plasma digoxin concentration measurements in the diagnosis of digoxin toxicity. Q J Med. 1978;47:111–122. [PubMed] [Google Scholar]
- 14.Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am Heart J. 1999;137:352–360. doi: 10.1053/hj.1999.v137.95495. [DOI] [PubMed] [Google Scholar]
- 15.O’Connell JB, Bristow MR. Economic impact of heart failure in the United States: time for a different approach. J Heart Lung Transplant. 1994;13:S107–S112. [PubMed] [Google Scholar]
- 16.Smith TW, Butler VP, Jr, Haber E. Determination of therapeutic and toxic serum digoxin concentrations by radioimmunoassay. N Engl J Med. 1969;281:1212–1216. doi: 10.1056/NEJM196911272812203. [DOI] [PubMed] [Google Scholar]
- 17.Adams KF, Jr, Patterson JH, Gattis WA, et al. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: a retrospective analysis. J Am Coll Cardiol. 2005;46:497–504. doi: 10.1016/j.jacc.2005.02.091. [DOI] [PubMed] [Google Scholar]
- 18.Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–878. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 19.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–730. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 20.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 21.SPSS. SPSS for Windows, Rel. 14. Chicago, IL: SPSS Inc; 2006. [Google Scholar]
- 22.Fonarow GC, Abraham WT, Albert NM, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148:43–51. doi: 10.1016/j.ahj.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Gheorghiade M, Ferguson D. Digoxin. A neurohormonal modulator in heart failure? Circulation. 1991;84:2181–2186. doi: 10.1161/01.cir.84.5.2181. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson DW, Berg WJ, Sanders JS, Roach PJ, Kempf JS, Kienzle MG. Sympathoinhibitory responses to digitalis glycosides in heart failure patients. Direct evidence from sympathetic neural recordings. Circulation. 1989;80:65–77. doi: 10.1161/01.cir.80.1.65. [DOI] [PubMed] [Google Scholar]
- 25.van Veldhuisen DJ, Man in ‘t Veld AJ, Dunselman PH, et al. Double-blind placebo-controlled study of ibopamine and digoxin in patients with mild to moderate heart failure: results of the Dutch Ibopamine Multicenter Trial (DIMT) J Am Coll Cardiol. 1993;22:1564–1573. doi: 10.1016/0735-1097(93)90579-p. [DOI] [PubMed] [Google Scholar]
- 26.Covit AB, Schaer GL, Sealey JE, Laragh JH, Cody RJ. Suppression of the renin-angiotensin system by intravenous digoxin in chronic congestive heart failure. Am J Med. 1983;75:445–447. doi: 10.1016/0002-9343(83)90346-7. [DOI] [PubMed] [Google Scholar]
- 27.Smith TW. Measurement of serum digitalis glycosides: clinical implications. Circulation. 1971;43:179–182. doi: 10.1161/01.cir.43.2.179. [DOI] [PubMed] [Google Scholar]
- 28.Slatton ML, Irani WN, Hall SA, et al. Does digoxin provide additional hemodynamic and autonomic benefit at higher doses in patients with mild to moderate heart failure and normal sinus rhythm? J Am Coll Cardiol. 1997;29:1206–1213. doi: 10.1016/s0735-1097(97)00057-0. [DOI] [PubMed] [Google Scholar]
- 29.Bertler A, Redfors A. Plasma-digoxin concentration. Lancet. 1971;2:50. doi: 10.1016/s0140-6736(71)90044-4. [DOI] [PubMed] [Google Scholar]
- 30.van Veldhuisen DJ. Low-dose digoxin in patients with heart failure. Less toxic and at least as effective? J Am Coll Cardiol. 2002;39:954–956. doi: 10.1016/s0735-1097(02)01710-2. [DOI] [PubMed] [Google Scholar]
- 31.Gheorghiade M, Adams KF, Jr, Colucci WS. Digoxin in the management of cardiovascular disorders. Circulation. 2004;109:2959–2964. doi: 10.1161/01.CIR.0000132482.95686.87. [DOI] [PubMed] [Google Scholar]
- 32.Remme WJ. Inotropic agents for heart failure: what if digoxin increases mortality? Br Heart J. 1994;72:S92–S99. doi: 10.1136/hrt.72.3_suppl.s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy S, Benatar D, Gheorghiade M. Update on digoxin and other oral positive inotropic agents for chronic heart failure. Curr Opin Cardiol. 1997;12:233–241. doi: 10.1097/00001573-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 35.Aronow WS, Ahn C, Kronzon I. Effect of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction > or = 40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol. 1997;80:207–209. doi: 10.1016/s0002-9149(97)00320-2. [DOI] [PubMed] [Google Scholar]
- 36.Eichhorn EJ, Lukas MA, Wu B, Shusterman N. Effect of concomitant digoxin and carvedilol therapy on mortality and morbidity in patients with chronic heart failure. Am J Cardiol. 2000;86:1032–5. A10–1. doi: 10.1016/s0002-9149(00)01146-2. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary Digitalis Investigation Group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young JB, Abraham WT, Smith AL, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]