Abstract
Environmental exposure to low concentration hormones can have permanent epigenetic effects in animals and humans. The consequence of long-term low-concentration glucocorticoid exposure was investigated in cell culture using glucocorticoid responsive genes organized in alternative chromatin structures. The MMTV promoter is induced by short-term glucocorticoid exposure on either an integrated (normal chromatin) or transient (unstructured chromatin) promoter. Longer hormone treatment causes a transient refractory repression of only the integrated promoter. Exposure to low-concentrations of hormone for several passages persistently represses the integrated MMTV and endogenous glucocorticoid responsive promoters. The glucocorticoid receptor cannot bind to persistently repressed promoters. Induction by androgens is also inhibited on the repressed MMTV promoter. Similarly, osmotic stress induction of the endogenous Sgk gene is repressed. Persistent repression by glucocorticoids targets glucocorticoid-responsive genes using a chromatin-dependent mechanism that disrupts binding of both GR-dependent and GR-independent transcription complexes.
Keywords: Glucocorticoid resistance, transcriptional repression, chromatin, MMTV, SGK
1. Introduction
Glucocorticoid response is essential in development and for many bodily functions. Inflammatory/immune function, cognitive processes and response to pathogens and stress are all critically dependent on response to glucocorticoids [1]. Resistance to glucocorticoids is a common feature of many diseases including autoimmune, respiratory disease, lymphoma and depression and has been linked to poor prognosis [2, 3].
Glucocorticoid resistance can involve many different mechanisms. Among these are mutations of the glucocorticoid receptor that reduce but do not eliminate glucocorticoid responsiveness [4, 5]. Glucocorticoids may be transported out of cells by the multidrug resistant p-glycoprotein [6]. High expression of the GR chaperone FKBP51 reduces GR response by reducing ligand binding [5]. Resistance to glucocorticoid-induced apoptosis in lymphoid malignancies is linked to downstream signal transduction and apoptosis [7, 8]. Glucocorticoid sensitive signal transduction and apoptosis may be restored by the mTor inhibitor rapamycin [9]. Inflammatory cytokines can modify glucocorticoid response directly by association and direct repression of GR responsive promoters. Cytokines also have indirect effects on GR isoform production and translocation [10–12]. Exposure to RU486 can modify GR translocation from cytoplasm to nucleus to induce glucocorticoid resistance [13, 14]. Long-term glucocorticoid exposure has autoregulatory effects reducing GR expression [15, 16]. Environmental endocrine disruptors and stress-induced glucocorticoid modification have long-term effects [2, 17, 18]. In particular prenatal exposure to stress or exogenous glucocorticoids can permanently alter neuroendocrine and inflammatory/immune systems and underlie common disorders [1, 2, 18, 19].
Another mechanism to permanently modify gene expression is epigenetic modifications of target genes. Exposure to environmental glucocorticoids or stress has been shown to alter DNA methylation, histone acetylation and histone methylation that are linked to changes in gene expression and diseases such as cancer, hypertension, and behavior disruption [20–22]. Glucocorticoid activation of gene expression requires chromatin remodeling of endogenous promoters and genes given prolonged exposure to glucocorticoids can become transiently refractory to glucocorticoids and associated with gene specific modification of histones [23–25].
This work investigates the effect of chronic exposure to low-level glucocorticoids in a cell culture system. Chronic exposure causes persistent repression of glucocorticoid responsive genes when the promoters are organized in a normal chromatin structure. Repression is associated with reduced binding of the hormone activated GR to chromatin promoters. Promoters repressed for glucocorticoid induction are also repressed for induction by alternative pathways. This demonstrates that long-term hormone insensitivity results from a chromatin–dependent mechanism that blocks binding of transcription factors on targeted promoters.
2. Materials and methods
2.1 Cells culture
UL3 cells are derived from the human osteosarcoma cell line U2OS by the stable addition of a rat glucocorticoid expression vector (CMV-rGR) and a full length MMTV promoter regulating a luciferase reporter [26, 27]. UL3 cells were maintained in DMEM (H21, Invitrogen Life Technologies, Carlesbad, CA), 10% fetal bovine serum, penicillin and streptomycin at 37° C and 5% CO2.
2.2 Transfection
Cells were transiently transfected with Fugene reagent (Roche Applied Science, Indianapolis, IN) with an efficiency > 80% as determined by β-galactosidase staining of cells transfected with 1μg of pSport reporter (Clonetech) [28]. Transient transfections routinely used a total of 0.5 μg of plasmid in 5 × 105 UL3 cells. The phhCAT plasmid contains 325 bp of proximal MMTV promoter driving a chloramphenicol acetyl transferase (CAT) reporter ( ). The hSgk-luc plasmid contains 3kb of Sgk promoter driving a luciferase reporter (C. Thomas, U of Iowa, Iowa City, IA). The same CMV-rGR plasmid that is integrated into UL3 cells was used in transient transfections to restore GR levels [26]. The androgen receptor was expressed from a CMV-AR expression plasmid [29].
2.3 Treatment
In these experiments cells were pretreated with hormone at different concentration and for different durations. In order to ascertain the effect (attenuation or enhancement) on hormone induction due to these treatments the cells were given an “inducing dose” of hormone (10-7M Dex for 1 hr). Hormone treatment was then discontinued and the transcription products (luciferase, CAT or RNA) were measured after allowing a suitable time for the products to accumulate. The hormone-concentration-dependent induction profile for MMTV was determined by treating cells with dexamethasone for 1 hr. Relative transcriptional activity was measured from luciferase or CAT reporters 20 hours later (Fig. 1A). The effect of the length of exposure on induction was determined by treating with either 10−7M or 10−10M hormone for various times (Fig. 1B, Fig 2). The effect of intermediate length (24–72 hrs) hormone exposure on induction was determined by treating with either 10−7M or 10−10M hormone. An inducing dose of hormone (10−7M for 1 hour) was administered in the last hour. Hormone treatment was then discontinued and reporter activity was measured 20 hours later to determine if induction was either attenuated (repressed) or enhanced (Fig 2). “Long-term-hormone” (LTH) treated cells were exposed to 10−10M dexamethasone for up to 50 passages. All measurements compared LTH with “no-long-term” (NLT) treatment controls matched for the same passage. Cells in long-term experiments were split 2 times per week resulting in passages of 3 or 4 days with replicate experiments started on alternate schedules. Induction of long term treated cells was determined after replating in the absence of hormone overnight. Induction of the transient MMTV was measured by CAT activity. Induction of the integrated MMTV was determined by luciferase activity 20 hours after hormone induction. CAT and luciferase activity was measured from the same lysates using CAT and Luciferase Assay kits (Promega, Madison, WI). Induction from endogenous genes was measured by RT-PCR of RNA levels after 3 and 6 hours.
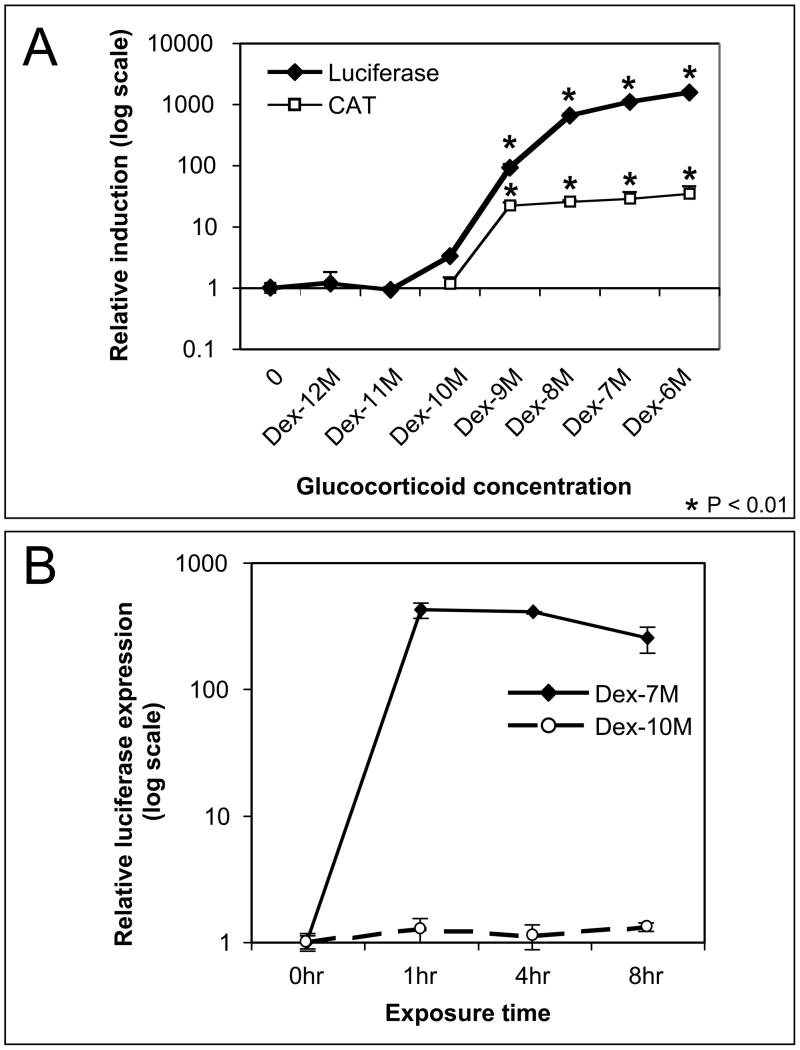
Fig 1.
Induction of MMTV by short-term exposure (hours) to hormone. (A) Cells were treated for 1 hour to different concentrations of dexamethasone. Hormone was removed after treatment and transcription was measured 20 hours later from the integrated (luciferase) and transient (CAT) reporter in the same cells. The mean of three or more trials and the standard error of the mean are shown with expression normalized to the mean uninduced control. MMTV is induced at concentrations 10−9M and higher (p<0.01) for both luciferase and CAT expression. (B) The effect of exposure between 1 and 8 hours at high (10−7M) and low (10−10M) concentration of hormone is shown on the integrated promoter. The data was analyzed as in A. No change in expression was seen for exposure to low (10−10M) concentration of hormone in this time frame.
Fig 2.
Refractory repression of the integrated MMTV after extended exposure (days) to dexamethasone. (A) Cells were exposed to a high (10−7M) or low (10−10M) dose of hormone for up to 48hrs. The integrated MMTV promoter was then tested for induction by treatment with an inducing dose of hormone (1 hr Dex 10−7M) and measured by luciferase expression. The mean of three trials and the standard error of the mean are shown normalized to the mean uninduced control. Exposure to either high or low concentration of hormone for 16 hrs or more attenuated induction (p<0.01). (B) The expression from the transient MMTV was measured by CAT expression in the same cells as in A. Exposure to either high or low concentration of hormone for 24 hrs or more enhanced induction (p<0.05). (C) Recovery of hormone induction. Cells were treated with dexamethasone for 72 hours. Hormone was then removed for 24 to 48 hours. The integrated MMTV was then induced with dexamethasone (10−7M) for 1 hour and expression was measured by luciferase activity. Recovery of induction correlates with time after exposure.
Sgk expression of the endogenous gene was measured by RNA expression. Transient Sgk expression was measured after transfecting with an Sgk-luciferase reporter. Hypertonic osmotic stress was induced by incubating cells in 0.3M sorbitol in serum free DMEM for 3 hrs. RNA expression was determined after 6 hrs. Luciferase expression was measured after 20 hrs. Androgen induction was measured in cells transfected with CMV-AR overnight then induced by 10−8M dihydrotestosterone for 1–3 hrs. RNA was measured after 6 hrs. All individual experiments were performed in triplicate and were repeated as independent biological replicates three or more times. The data was normalized by dividing each measurement by the mean of the uninduced control thus converting the relative luciferase units, CAT activity and RT-PCR units to a common scale. Data is expressed as mean and standard error of the mean. P value were determined by Student’s t test or Pearson’s correlation coefficient..
2.4 Western Blots
Cells were lysed in RIPA buffer (PBS, 1%NP40, 0.5% deoxycholate, 0.1% SDS, PMSF and Aprotinin (Sigma) buffer. Total proteins (10–25 μg) were separated on 10% NuPAGE gels (Invitrogen Life Technologies, Carlesbad, CA). Proteins were visualized with antibodies to GR (Santa Cruz Biotechnology, Santa Cruz, CA), HRP conjugated anti-IgG secondary antibody and chemiluminescence reagent (PerkinElmer Life Sciences, Boston, MA).
2.5 RNA measurement
Total RNA was purified from cells using an RNeasy mini kit (Qiagen Sciences, Valencia, CA). First strand cDNA primed with oligo dT was transcribed using a Superscript first strand kit (Invitrogen Life Technologies, Carlesbad, CA). Realtime PCR was performed with a Stratagene Mx3000P and SYBR Green detection of products to measure RNA induction (Stratgene, La Jolla, CA). Primers specific for GR, PLZF, CEBP, SGK, CSF, CD44 and GAPDH [30, 31] coding regions were used.
2.6 Chromatin immunoprecipitation (ChIP) assay
Chromatin immunoprecipitation assays were performed with a ChIP kit (Upstate, Lake Placid, NY). Immediately following treatment, cells were crosslinked with 1% formaldehyde for 10 min. Complexes were immunoprecipitated with 10 μg of antibodies to GR, H1, pH1 or rabbit IgG as a non-specific antibody (NS) (Santa Cruz Biotechnology, Santa Cruz, CA). Complexes were collected using proteinA agarose/DNA beads and washed 5 times with low salt, high salt, LiCl and TE buffers. After reversing cross-links at 65° for 4 hrs the genomic DNA was purified from eluted complexes with a QIAquick PCR purification kit (Qiagen Inc., Valencia, CA). MMTV DNA was detected by PCR amplification with primers flanking the hormone response element (GRE) (sense TTAAGTAAGTTTTTGGTTACAAACT, antisense TCTGGAAAGTGAAGGATAATGACGA) and including approximately 300 bp of the proximal promoter in the nucleosome B region of the MMTV promoter. Primers for the SGK-GRE (sense 5′CTACCCCTCCTTCGCTTGTT3′ antisense 5′GGGCGGAAATAAAAGTCGTCT3′), CMV (sense 5′ GCTCGTTTAGTGAACCGTCAGA3′ antisense 5′GGTCCCGGTGTCTTCTATGGA3′), and GAPDH (sense 5′AAAAGCGGGGAGAAAGTAGG3′, antisense 5′CTAGCCTCCCGGGTTTCTCT3′) promoters were used. Total PCR products were quantified by realtime PCR (Stratgene, La Jolla, CA) and from ethidium bromide-stained 4–12% Tris-Borate EDTA PAGE gels (Invitrogen, Carlsbad CA) using Alpha Innotech imaging software.
2.7 DNA sequencing
The MMTV and CMV promoters and GR coding region were sequenced by PCR amplification of the relevant sequence from genomic DNA using a proofreading polymerase (Pfu Ultra, Stratagene) followed by dRhodamine sequencing kit (Applied Biosystems). MMTV and CMV promoter primers are described above. GR primers corresponding to the 5′ 500bp of coding region were used.
3. Results
3.1 Induction of the MMTV promoter by glucocorticoids
Induction of transcription from glucocorticoid responsive promoters is concentration and chromatin dependent. In UL3 cells a one hour exposure to dexamethasone elicits significant induction (p<0.01) of the MMTV promoter at a concentration of 10−9M dexamethasone or higher (Fig. 1A). A concentration of 10−10M dexamethasone or less elicits no reliable increase in transcription. A similar profile of dose response is evident from the transiently transfected MMTV promoter suggesting the threshold for hormone induction is similar on the integrated and transient promoters although fold induction is 100x less on the transient than on the integrated promoter. The integrated and transient MMTV reporters are organized into two different chromatin configurations. The integrated MMTV-luciferase reporter in UL3 cells has a normal nucleosome chromatin organization. The MMTV-CAT reporter has a less structured chromatin configuration when transiently transfected into UL3 cells. Comparison of these two reporters shows significant functional differences including nucleosome association, transcription factor binding and BRG1-dependent chromatin remodeling [32]. Increasing the duration of exposure up to 8 hours does not result in any increase in the induction at either high 10−7M or low−10M hormone (Fig. 1B). Comparison of the dose response to dexamethasone on these promoters shows a similar profile suggesting neither chromatin organization nor exposure time (in this range) influences induction sensitivity.
3.2 Refractory repression of the integrated MMTV promoter
Extending the duration of hormone treatment from hours to days modifies the MMTV response. The integrated chromatin promoter becomes refractory to induction by a second dose of hormone (10−7M for 1 hour) after extended exposure to either 10−7M and 10−10M hormone (p<0.01). In contrast induction from the transient promoter is enhanced by extended exposure to either high or low dose hormone (p<0.05). A concentration of 10−10M hormone is less than required for induction at short exposures but clearly modifies the hormone response with longer exposure.
Expression from the integrated luciferase reporter begins to decline after exposure to hormone (10−7M) for more than 8 hours decreasing to less than 5% after 48 hours (Fig. 2A). Addition of fresh hormone (dexamethasone 10−7M for 1hour) did not alter the decline in response. When cells were treated with a low concentration of hormone (dexamethasone 10−10M) for 24 or 48 hours followed by treatment with an inducing concentration of hormone (10−7M for 1 hr) the low-level hormone pretreatment is also repressive. The intensity of repression is concentration dependent with low level hormone (10−10M) only repressing by 50% compared to high level hormone (10−7M) repression of 5% however repression increases with exposure at both concentrations.
In contrast to the integrated MMTV-luciferase, expression from the transient MMTV-CAT reporter increases with extended exposure to hormone. This response is the opposite of the effect of extended hormone exposure on the integrated MMTV-luciferase. Transcription from the transient CAT reporter was greater after 48 hours of hormone exposure (10−7M) than in cells exposed to hormone (10−7M) for one hour (Fig. 2B). Pretreatment with low-concentration hormone (10−10M) also enhances the MMTV-CAT expression at 48 hours. Although low concentration (10−10M) hormone cannot induce MMTV-CAT the 10−10M pretreatment enhances transcription similar to extended exposure to high concentration dexamethasone (10−7M). Long-term exposure enhances CAT induction approximately 3 fold with no appreciable concentration dependence. The chromatin structure of the promoter affects the hormone response such that the integrated chromatin promoter becomes refractory to hormone induction after prolonged treatment but the transient promoter remains responsive.
Following the termination of hormone exposure the MMTV-luciferase response recovers from refractory repression (Fig. 2C). The recovery is progressive with the less repressed cells (10−10M) regaining normal responsiveness before the more strongly repressed cells (10−7M). The ability of cells to regain normal hormone responsiveness suggests that the mechanism of refractory repression does not represent a permanent modification to either the promoter or transcription complexes.
3.3 Persistent hormone-dependent repression
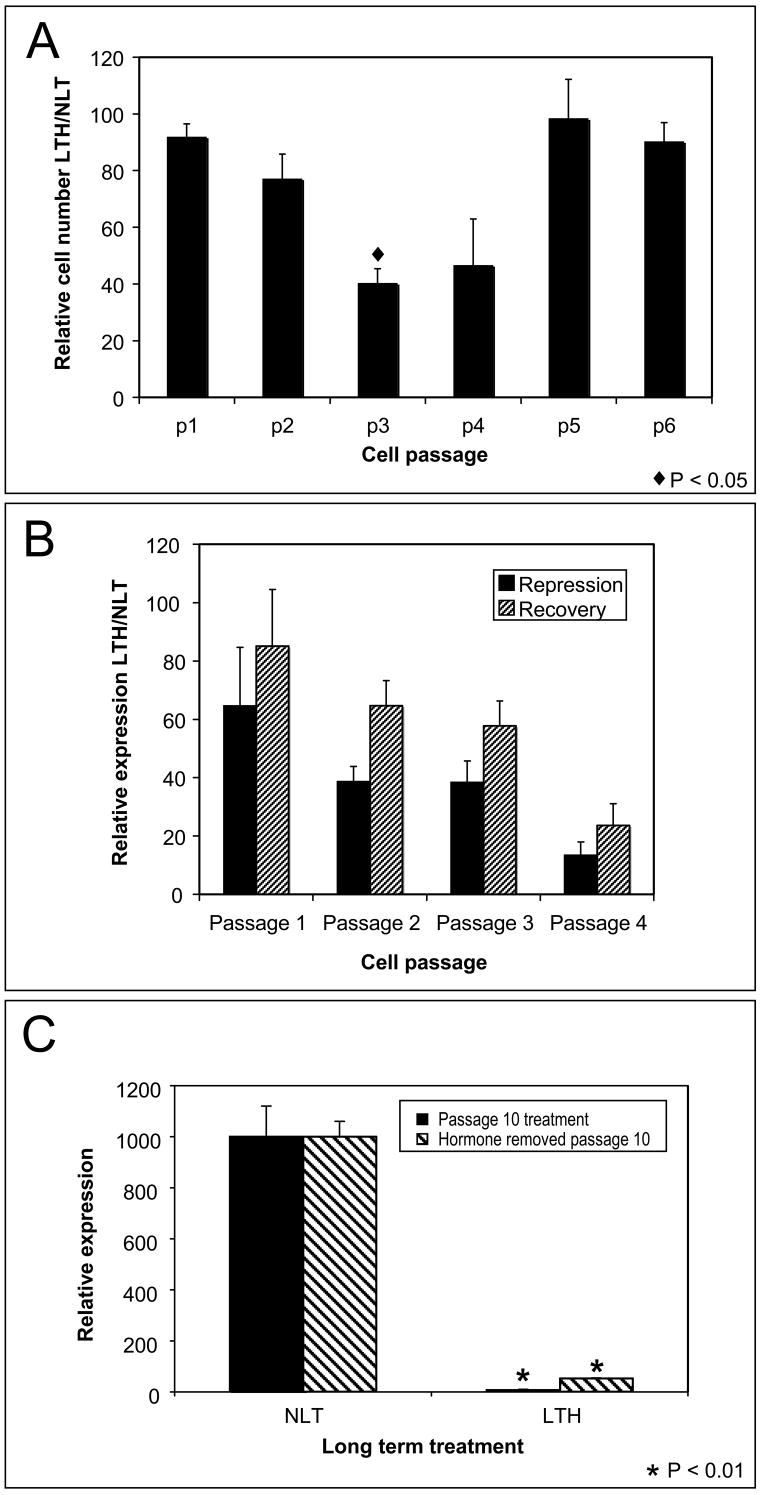
Exposure to high levels of glucocorticoids (10−7M) causes G1 growth arrest which affects the ability of UL3 cells to grow long term at these hormone levels [26]. Cells exposed to low levels of hormone (10−10M) can be grown for extended periods. Cell growth/inhibition was measured by cell number at each passage for cells maintained in low levels of hormone (LTH) and in age-matched controls grown long-term with no hormone (NLT) (Fig. 3A). LTH cells exhibit little effect on growth for 3–4 days corresponding to passage 1. A period of growth retardation is observed from passage 2 to passage 4 (p<0.05 at passage 3) with cells recovering normal growth after passage 5 (Fig. 3A). Over the same period there is a progressive decline at each passage in the induction of LTH cells relative to their NLT controls (Fig 3B). Recovery at 24 hours after the removal of hormone shows a similar progressive decline (Fig. 3B). Luciferase repression and growth retardation have independent time courses of modification. Growth retardation is limited to passages 2–4 while transcriptional repression shows a progressive decline that correlates with passage (p<0.05). The progressive decline is found beyond passage 5 as long as low level hormone treatment continues.
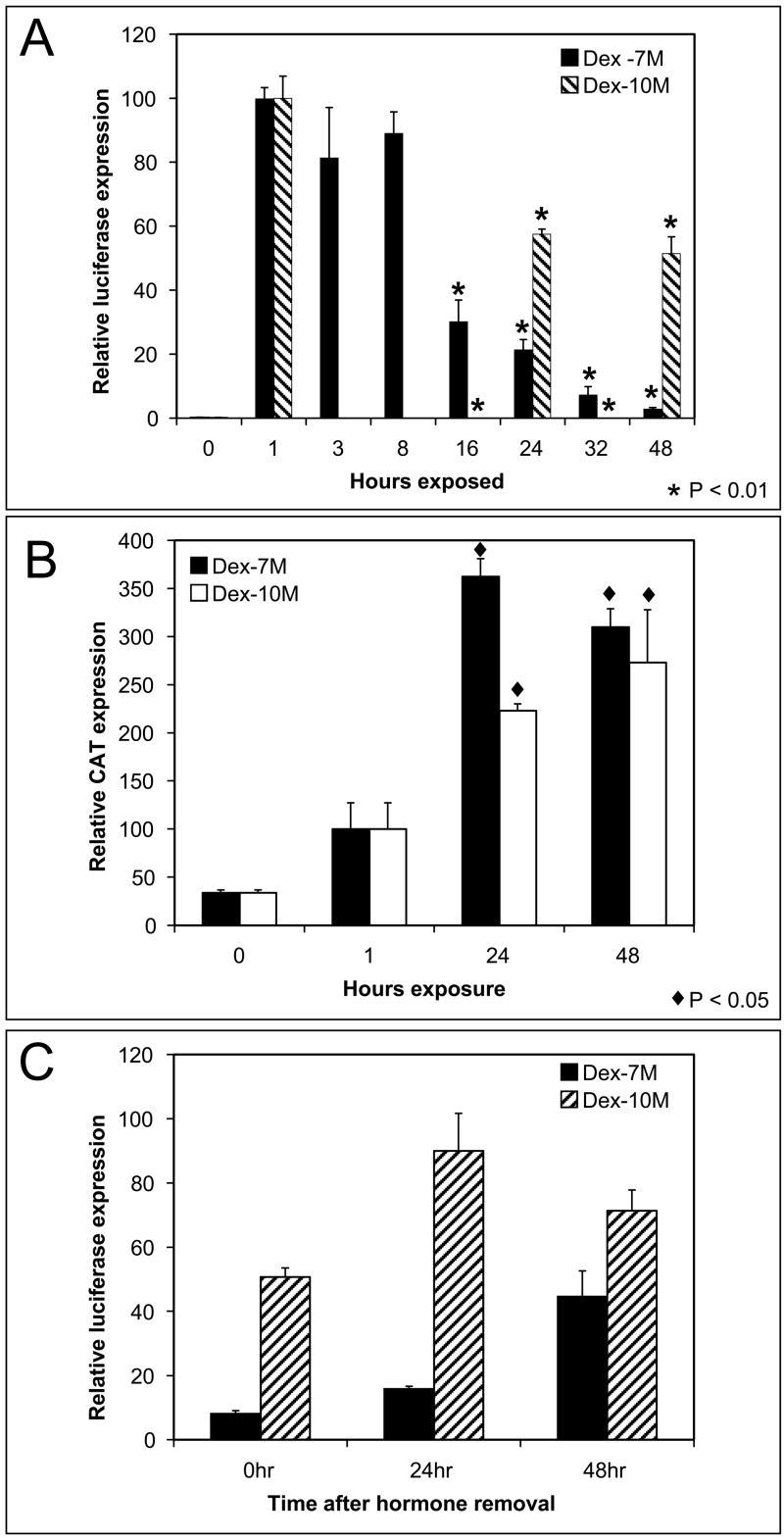
Fig 3.
Persistent repression of the MMTV promoter after long-term exposure to low level hormone. (A) Long-term-hormone (LTH) treated cells were grown for several passages in a low concentration of hormone. No-long-term (NLT) hormone treated cells were passage-matched controls. Growth inhibition was measured as LTH cell number/NLT cell number × 100 at each passage. Transient growth inhibition was observed at p3 (p<0.05) but later passages showed no inhibition. (B) A progressive attenuation of hormone induction (LTH/NLT ×100) was found at each passage for cells exposed to low hormone and after hormone removal. A significant correlation (p<0.05 determined by Pearson correlation coefficient) was found between passage and activity. (C) Attenuation of induction was measured after 10 passages in hormone and following the removal of hormone for 2 or more passages. Significant repression of MMTV-luciferase was found after the long term exposure (p<0.01) and after discontinuing hormone treatment (p<0.01).
The decline in luciferase induction was tested after 10 passages. The induction of LTH is repressed to less than 1% of NLT controls in cells continuously exposed to hormone (p<0.01) (Fig 3C). After discontinuation of low level hormone treatment from passage 10 LTH cells significant repression (p<0.01) persists. There is a partial recovery of induction after 2–3 passages but LTH induction remains less than 10% of NLT controls up to 50 passages after removal of hormone (Fig 3C). Longer exposure to hormone results in less recovery after hormone removal. This suggests that long-term hormone-dependent repression involves both a transient mechanism and a persistent mechanism for reducing hormone response.
The MMTV promoter was sequenced in the region of the glucocorticoid response element (GRE) to determine if LTH cells represent an MMTV variant with a reduced hormone response. We did not observe differences in sequence between the LTH and the NLT cells in the 200bp surrounding the GRE, suggesting a mutation of the MMTV is not responsible for the persistent repression of luciferase activity.
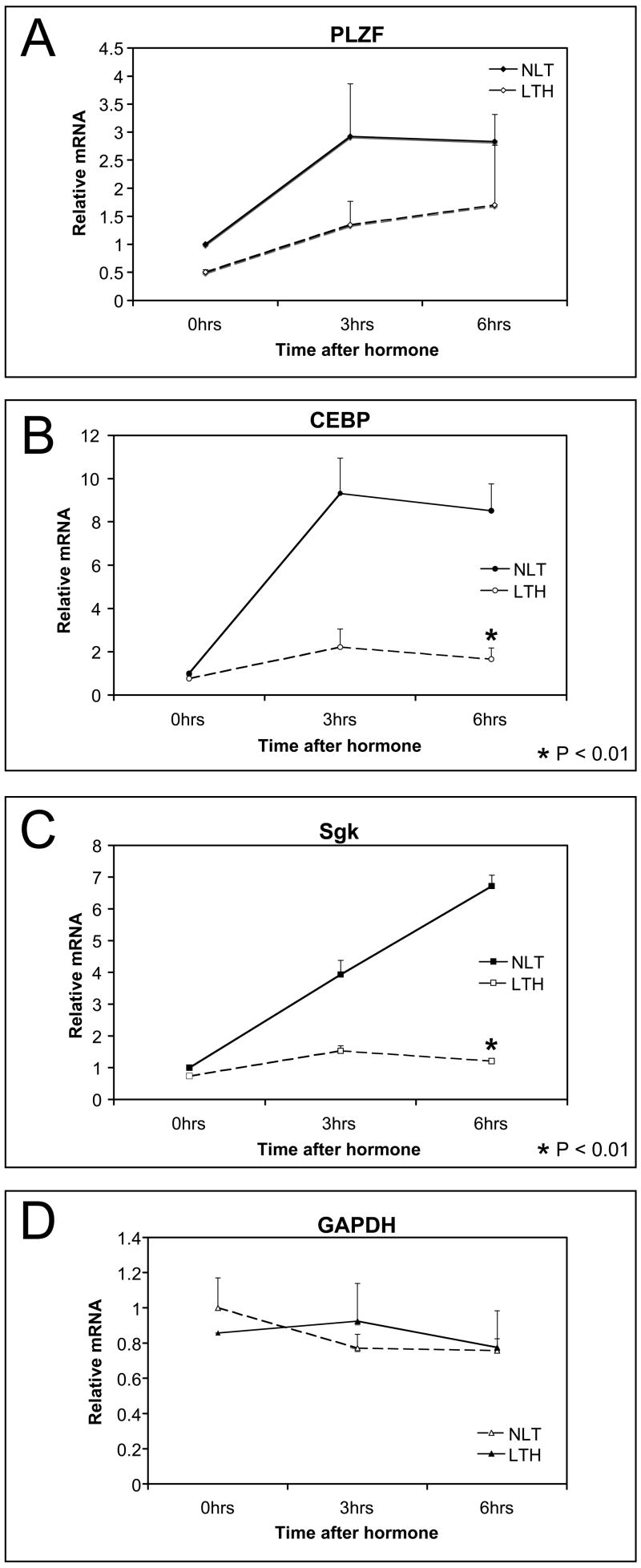
The mRNA expression of several genes was evaluated to determine if the hormone-dependent repression was MMTV specific, specific to glucocorticoid responsive genes or global. Induction of endogenous Sgk, CEBP and PLZF is hormone dependent and is repressed in LTH cells (Fig. 4). Sgk and CEBP are significantly repressed (p< 0.01) but even the weakly induced PLZF has a reduced induction in LTH cells. In contrast, GAPDH mRNA expression is unaffected in LTH cells although it normally is expressed at relatively high levels. This suggests that repression is specific to glucocorticoid responsive genes.
Fig 4.
Repression of endogenous, GR-responsive genes in LTH cells. Induction of endogenous glucocorticoid-responsive genes was measured by real time PCR of RNA in NLT and LTH cells at 3 and 6 hours after induction (1 hr Dex 10−7M). (A) PLZF. (B) CEBP. (C) SGK. (D) GAPDH was the hormone insensitive control. Significant repression (p<0.01) was found for CEBP and SGK in LTH cells.
3.4 Glucocorticoid receptor (GR) involvement
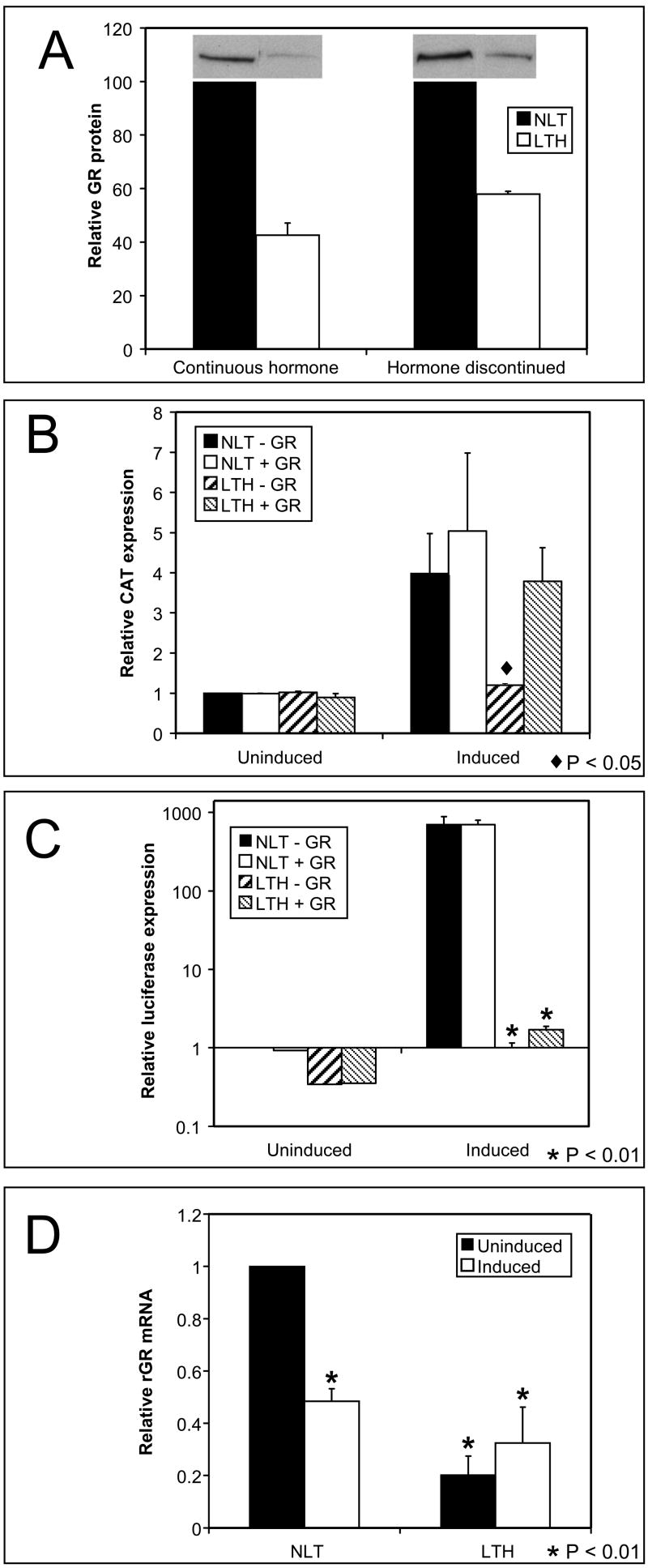
The response to glucocorticoids is mediated by the GR. Cells lacking the GR do not respond to glucocorticoids and changes in GR levels or binding at the GRE affect the degree of response. Western blots of GR in NLT and LTH cells show GR protein level is reduced in LTH cells by passage 10 compared to NLT levels and the reduction in GR is persistent as it is maintained after hormone removal (Fig. 5A). The loss of GR could account for the repression of all the glucocorticoid responsive genes. In addition it should repress the transient promoter. LTH cells transfected with the MMTV-CAT reporter are repressed for hormone induction of CAT (p<0.05) (Fig. 5B). When transfected with a CMV-GR expression plasmid along with the MMTV-CAT to restore GR levels the CAT induction in LTH cells is increased and similar to the NLT levels suggesting GR reverses repression of the transient promoter. In contrast transfection of CMV-GR does not restore normal hormone response of the integrated MMTV-luciferase in LTH cells. The MMTV luciferase is significantly repressed before (p<0.01) and after GR transfection (p<0.01) although a small increase in hormone response is evident after increasing the GR level (Fig. 5C). Increasing the GR level had little effect on either the transient or integrated MMTV in NLT cells. This suggests that the GR levels in NLT cells are sufficient to induce a maximal response and the addition of exogenous GR does not effect expression.
Fig 5.
Glucocorticoid receptor (GR) effects in repression. (A) GR protein levels were measured by western blot and quantified by phosphor-imager. GR protein is reduced in LTH cells relative to passage-matched NLT controls and persists after hormone removal. (B) Induction of the transient MMTV promoter was measured after long term hormone treatment. CAT activity was repressed (p<0.05) in LTH cells relative to the NLT control. Increasing GR levels in LTH cells by transfection of a CMV-GR expression plasmid increased CAT induction such that no repression was evident. (C) Induction of the integrated MMTV was measured by luciferase expression. MMTV is significantly repressed (p<0.01) in LTH cells and LTH cells remain significantly repressed for luciferase activity (p<0.01) following transfection with CMV-GR. CAT and luciferase activity were measured in the same cells. (D) GR RNA transcription was measured by RT-PCR. Hormone exposure reduced GR RNA after short term hormone treatment in NLT cells (p<0.01) and after long term treatment in LTH cells (p<0.01).
The sequence of the GR was compared in NLT and LTH cells to determine if the protein expressed in LTH cells was modified. No difference in the GR DNA sequence was found between the two cell types. This suggests that repression of GR protein causes repression of the transient of promoter. However, GR cannot rescue repression of the integrated promoter suggesting chromatin-dependent repression is the cause of MMTV repression and the potential cause of GR repression.
In UL3 cells the GR is expressed from an integrated CMV-GR expression plasmid. Expression of GR mRNA is regulated by glucocorticoids on the CMV promoter in both the short term and long term (Fig. 5D). Exposure of NLT cells to inducing levels of dexamethasone (10−7M) causes a decline in GR mRNA levels suggesting glucocorticoids regulate the CMV promoter. The CMV promoter has several putative binding sites for GR but it also has binding sites for NFκB and AP1 that can form repressor complexes with GR. Non-induced LTH cells have a reduced level of GR mRNA (< 20%) compared to the NLT cells. The reduced mRNA levels correlate with the reduced GR protein level. GR mRNA levels in LTH cells do not return to untreated levels after termination of long-term hormone indicating repression is persistent. Thus long-term glucocorticoid treatment represses glucocorticoid-responsive promoters regardless of whether the short-term response activates or inhibits transcription. No difference in sequence was found when the CMV promoter was compared in NLT and LTH cells suggesting a low expression variant of CMV is not the cause of reduced expression.
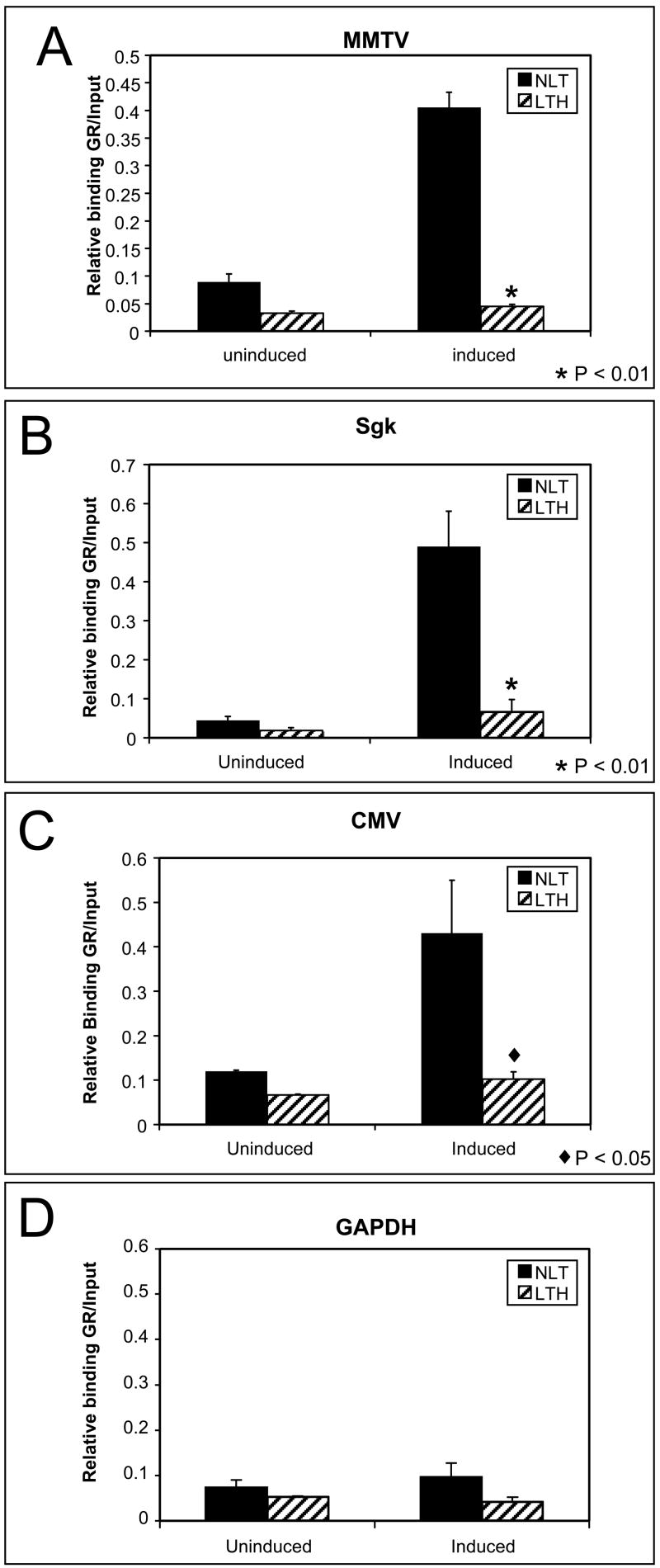
Exposure to glucocorticoids normally causes an increase in GR bound to the GRE within chromatin. The ability of the GR to bind to the integrated MMTV and endogenous GR responsive promoters was next examined in NLT and LTH cells. In the absence of hormone there is less GR bound to all the promoters in LTH compared with NLT cells. The reduction was apparent at the GAPDH promoter which does not actively bind the GR so likely reflects the lower levels of GR available in LTH cells non-specifically bound. Hormone treatment causes a four-fold increase in the amount of GR bound to the MMTV (p<0.01), Sgk (p<0.01) and CMV (p<0.05) promoters in NLT cells (Fig. 6). However there is little or no increase in GR bound to these promoters in the LTH cells. This suggests that the reduced transcriptional response is due to the failure of hormone-activated GR to bind to the repressed promoters and implicates a loss of remodeling function that facilitates binding.
Fig 6.
GR binding on repressed promoters. GR binding was measured by ChIP on hormone responsive promoters. GR binding is increased by hormone exposure in NLT cells but is significantly inhibited in LTH cells on repressed promoters. (A) MMTV (p<0.001). (B) SGK (p<0.01). (C) CMV (p<0.05). The CMV promoter has putative response elements for GR and for GR-containing repressor complexes including AP-1 and NFκB. (D) The non-hormone-responsive GAPDH promoter shows no increase in GR binding.
3.5 Previously identified chromatin-dependent mechanisms of MMTV repression
The ability of GR to bind the MMTV promoter and transcribe within chromatin requires several remodeling functions that involve both recruitment of specific transcription factors and modification of histone proteins [23, 24, 33]. The specific involvement of these mechanisms was examined in long-term repressed LTH cells.
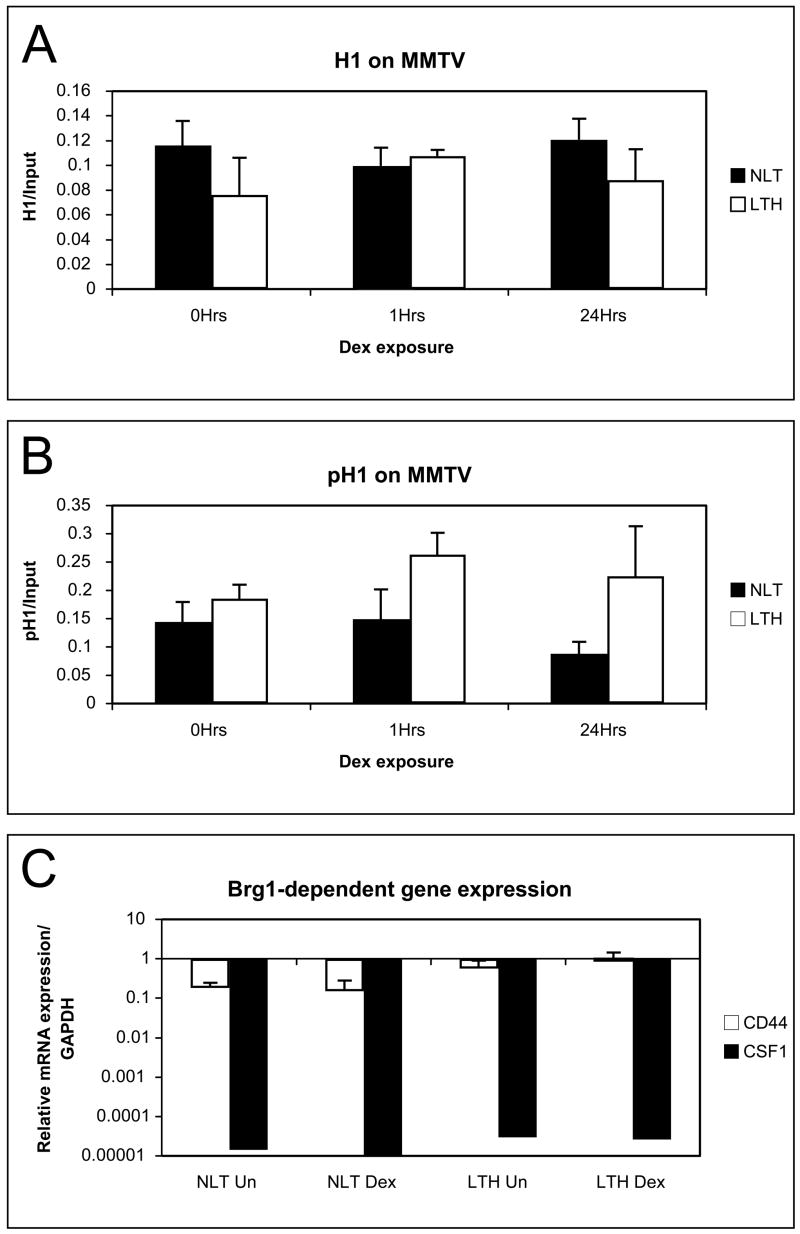
Changes to the histone H1 protein have been linked to both activation and repression of the MMTV [24, 34]. Total histone H1 and phospho-histone H1 was measured on the MMTV promoter in NLT and LTH cells untreated, after 1 hour (induction) or 24 hours (refractory repression) of 10−7M dexamethasone. The amount of histone H1 was similar on NLT and LTH cells with no change to total H1 following either one hour or longer hormone treatment (Fig. 7A). This suggests that repression is not due to an increase in total H1 at MMTV in LTH cells. The phospho-H1 levels normally decline 24 hours after hormone exposure correlating with refractory repression and disruption of GR binding. Phospho-H1 levels are similar in non-induced NLT and LTH cells so repression is not due to persistent alteration of phospho-H1 levels at repressed promoters (Fig 7B). Phospho-H1 level declines in NLT cells after 24 hours but LTH cells exhibit no decline in H1 phosphorylation. The lack of decline in phospho-H1 levels at MMTV in LTH cells suggests that the chromatin remodeling that causes refractory repression is disrupted in LTH cells. GR binding normally precedes refractory remodeling suggesting that the loss of refractory remodeling is a consequence of disruption of GR binding.
Fig 7.
Chromatin dependent repression mechanisms. (A) Total histone H1 levels in LTH and NLT cells were measured by ChIP. No significant difference was found in total histone H1 on the MMTV promoter. (B) Phospho-histone H1 levels measured by ChIP on the MMTV promoter were not significantly different in uninduced NLT and LTH cells. No hormone-dependent decline in phospho-histone H1 level was found in response to hormone induction on the repressed MMTV promoter in LTH cells. (C) Brg1-dependent RNA expression was measured by RT- PCR of CD44 and CSF1. No significant loss of expression was found in LTH compared with NLT cells.
Repression of chromatin promoters may be due to the loss of ATPase–dependent chromatin remodeling function of BRG1 that blocks induction of chromatin promoters including MMTV [23]. BRG1 also regulates RNA transcription and loss of BRG1 represses RNA expression from the endogenous genes CD44 and CSF1 [35]. RNA expression from CD44 and CSF was compared in NLT and LTH with and without dexamethasone treatment (Fig. 7C). The level of transcription for CD44 and CSF was compared between NLT and LTH cells. No decline in mRNA levels was found in LTH cells. This suggests BRG1-dependent chromatin remodeling is intact in the LTH cells and does not contribute to glucocorticoid repression.
Transcription factor NF1 bound to the chromatin MMTV is required for GR to bind the chromatin MMTV [33, 36]. Reduction of NF1 protein levels or mutation of the NF1 binding site prevents GR binding and transcription. However not all GR responsive promoters require NF1 as cofactor. The Sgk1 gene does not require NF1 for GR binding or transcription [36]. Transcription from Sgk is repressed as efficiently as MMTV in LTH cells (Fig. 4). In addition GR binding is reduced on the Sgk promoter (Fig. 6). Loss of NF1 does not affect Sgk so a remodeling function independent of histone H1, BRG1 or NF1 is implicated as the cause of long-term hormone repression.
3.6 Repression of induction by alternate pathways
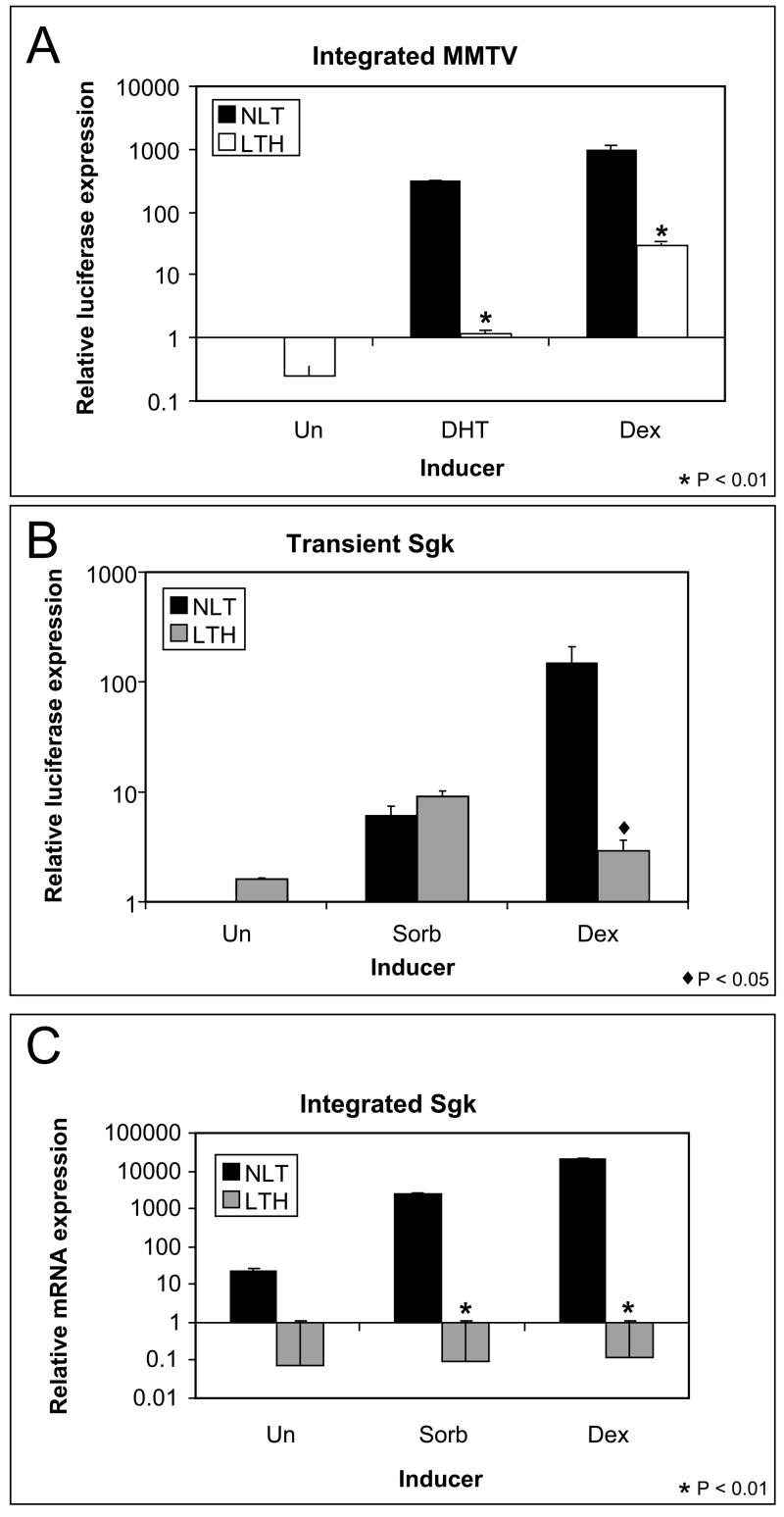
Chromatin modification that blocks transcription factor binding may affect the ability of transcription factors other than GR to activate promoters. The MMTV may be activated by several receptors including the progesterone receptor, the mineralcorticoid receptor and the androgen receptor (AR). All these receptors bind at the GRE to induce transcription. UL3 cells transfected with an androgen receptor expression plasmid were treated with dihydrotestosterone (DHT) to activate MMTV transcription. DHT-induced the expression of the integrated MMTV-luciferase from NLT cells but expression from the LTH cells was repressed (Fig 8A). The lack of DHT-dependent luciferase induction in the LTH cells suggests that the integrated MMTV promoter is modified such that induction by other steroid receptors, not just GR, is affected.
Fig 8.
Response of Dex repressed promoters to alternative inducers. (A) Induction of the integrated MMTV-luciferase was measured after induction by DHT (with a transfected androgen receptor) compared with Dex. The integrated MMTV is repressed (p<0.01) for both DHT and Dex induction in LTH cells. (B) Induction of the transient Sgk promoter was measured after osmotic stress and Dex treatment. The transient SGK-luciferase reporter is repressed for Dex induction (p<0.01) but not for osmotic stress induction. (C) Induction of the endogenous SGK gene was measured by RT-PCR after osmotic stress and hormone treatment. Sgk is repressed for both Dex (p<0.01) and osmotic stress (p<0.01) in LTH cells.
Since the AR and GR both bind the MMTV promoter at the GRE a local modification at that site could affect binding by both factors. Sgk is inducible by osmotic stress. The hyperosmotic stress-regulated element of the Sgk promoter is approximately 2 kb away from the GRE of the Sgk promoter [37, 38]. Induction of the Sgk promoter by 0.3 M sorbitol activates a transiently transfected Sgk-luciferase reporter similarly in NLT and LTH cells (Fig. 8B). In contrast, induction of endogenous Sgk mRNA by sorbitol is repressed in LTH cells (Fig. 8C). The loss of Sgk osmotic stress induction in LTH cells suggests that the promoter is cis modified and the modification must extend over at least 2kb of the Sgk promoter to disrupt regulation by the osmotic-stress response element.
4. Discussion
Exposure to glucocorticoids results in different effects on transcription from responsive genes depending on concentration and duration of exposure. In our system, induction results only after a short-term exposure to relatively high concentrations of glucocorticoids and does not require a specific chromatin structure. Glucocorticoid-dependent repression on the other hand results from a longer duration exposure at a lower dosage of hormone and requires a normal chromatin environment for expression. Moreover, glucocorticoid-dependent repression occurs in two distinct types. Refractory repression requires continuous exposure to glucocorticoids for several days and cells can recover normal hormone induction. Persistent repression requires a prolonged exposure that progressively modifies responsive genes so that they are persistently repressed in the absence of long-term hormone exposure. Persistent repression is targeted to GR-binding promoters. Cells treated long-term with glucocorticoids exhibit both forms of repression of the MMTV with refractory repression the dominant component at shorter durations of exposure (days). After an exposure of several passages, the persistent component of repression becomes dominant and eventually can become complete.
Normal glucocorticoid response requires hormone binding to the GR, translocation of the activated GR from the cytoplasm to the nucleus, binding to the GRE, and chromatin remodeling to initiate transcription. Several resistance mechanisms have been reported that disrupt different steps in this pathway. Expression of the multidrug-resistance p-glycoprotein causes resistance by reducing hormone levels in the cell [6]. Ligand binding to the GR may be reduced by the GR chaperone FKBP51 [5]. Translocation of the activated GR from cytoplasm to nucleus is blocked by exposure to RU486 in some systems [13, 14]. Association of inflammatory cytokines and transcription factors such as NFkB with the GR transcription complexes alters the function of the complex from activation to repression of glucocorticoid responsive genes and requires functional chromatin remodeling [10, 11, 39]. Mutations in the GR may reduce activity causing glucocorticoid resistance [4, 5]. This investigation has shown that none of these mechanisms can account for persistent repression.
Long-term glucocorticoid treatment reduces GR protein and mRNA expression [15, 16]. The cause of the reduction in GR RNA and protein has not previously been shown. We have shown that long-term glucocorticoids modify a chromatin-dependent mechanism to repress transcription of glucocorticoid responsive promoters including the GR. Reintroduction of the GR does not rescue persistent repression suggesting the chromatin dependent modification is the primary source of glucocorticoid resistance.
Long-term repression in UL3 cells involves both a reduction in the amount of GR available and in disruption of GR binding to integrated promoters. While both mechanisms reduce glucocorticoid response there are clear differences in the importance of each mechanism to promoters with different chromatin structures and to which mechanism is the primary cause of persistent repression. In our system transient promoters normally bind the GR and other transcription factors such as NF1 in the absence of hormone and cooperative interaction is not required for binding of GR and transcription [40]. A decrease in the level of GR represses transient promoters but restoring the level of GR restores normal induction. The recovery of normal induction indicates there is no impediment to GR binding at transient promoters. How then is the GR repressed in LTH cells? The CMV promoter regulates GR in UL3 cells and the CMV is itself regulated by glucocorticoids. The repressed CMV like other integrated and endogenous GR-dependent genes in chromatin fails to bind GR. This suggests the chromatin dependent failure to bind GR is the critical mechanism for persistent repression of CMV and reduction of GR RNA and protein is a secondary effect. Repression of mRNA from the endogenous GR has been found in long-term glucocorticoid treated cells suggesting a common mechanism [15].
In the normal chromatin environment of the integrated MMTV binding by many transcription factors is low. Hormone treatment increases the amount of GR bound to the promoter as well as other GR-associated transcription factors including BRG1, p300/CBP, p65 RelA, E1A and NF1 [23, 33, 39]. A failure of chromatin remodeling that is required for the increase in GR-associated transcription complexes on the promoter could account for the repression. The failure to remodel chromatin may be due to a defect in the remodeling machinery (a trans-acting mechanism) or a modification to the chromatin structure (a cis-acting mechanism). We examined two trans mechanisms of repression, loss of BRG1-dependent remodeling and loss of NF1-dependent GR binding. Transcription is unaffected in LTH cells for CD44 and CSF1 genes that are BRG-dependent and glucocorticoid independent suggesting BRG1 chromatin remodeling is intact. Association with NF1 is required for GR binding to MMTV. However NF1 is not required for GR activity on Sgk [36]. Since Sgk is repressed the loss of cooperative binding by GR with NF1 on chromatin is not the mechanism of repression.
The modification of histone H1 phosphorylation is a cis-acting mechanism of blocking GR binding in short term refractory repression. However the loss of pH1 is not found in persistent repression. A cis-acting mechanism is still suggested by the repression of androgen and osmotic stress transcription of MMTV and Sgk promoters. These alternative pathways involve both GR independent transcription complexes and binding sites. The chromatin promoter is the common element in repression suggesting a cis modification of the chromatin is the mechanism of repression. In addition the progressive establishment of the persistent repressed state is a feature other epigenetic modifications [41–43].
Glucocorticoids inhibit growth and selection for a variant that is not responsive to glucocorticoids is a potential mechanism to overcome growth inhibition. Many mechanisms to disrupt glucocorticoid response have been described and selection for a preexisting mutation might involve any of these mechanisms. However there is a lack of correlation between loss of growth inhibition and the MMTV repression. Alleviation of growth inhibition would relieve selective pressure in this model but cumulative repression is unaffected by the loss of growth inhibition. A requirement for specific GR sequences has been found in GR RNA repression suggesting GR directly participates in establishing repression [16]. No sequence variation was found in the MMTV, CMV or GR in this system and reintroduction of GR does not restore glucocorticoid response. Glucocorticoid resistance resulting from long-term exposure represents a syndrome involving many promoters and pathways that are modified specifically in a chromatin environment. Finally a variant selected for GR resistance is unlikely to include defects in osmotic stress and androgen induction because they do not involve either glucocorticoids or the GR.
Glucocorticoid exposure, stress or environmental chemicals have long-term consequences to adult metabolism and disease and may have intergenerational effects suggesting an epigenetic mechanism [20, 44–48]. Prenatal exposure impacts specific systems depending on the developmental time of exposure suggesting the consequence may be due to disruption of specific developmental programs in target tissues [19, 21, 45]. The development of glucocorticoid resistance in this cell culture system suggests that long-term modification of gene expression does not require a susceptible developmental stage. Persistent glucocorticoid resistance appears to be a normal response to extended exposure that epigenetically modifies genes targeted by glucocorticoid receptor binding. This is consistent with idea that steroid hormone receptor status contributes to promoter chromatin architecture and responsiveness in cells [49,50]
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, NIH; project number Z01 ES071006-09.
We would like to thank members of the Archer lab, particularly H.K. Kinyamu for helpful suggestions. R. Newbold and A. Scolcock for thoughtful review of the manuscript. G. Kissling provided statistical advice. L. Li identified transcription factor binding sites in the CMV promoter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 2.Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol. 2005;5:23–29. doi: 10.1097/00130832-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Tissing WJ, Meijerink JP, den Boer ML, Pieters R. Molecular determinants of glucocorticoid sensitivity and resistance in acute lymphoblastic leukemia. Leukemia. 2003;17:17–25. doi: 10.1038/sj.leu.2402733. [DOI] [PubMed] [Google Scholar]
- 4.Orbak Z. Glucocorticoid resistance. Biochemistry (Mosc) 2006;71:1073–1081. doi: 10.1134/s0006297906100038. [DOI] [PubMed] [Google Scholar]
- 5.Westberry JM, Sadosky PW, Hubler TR, Gross KL, Scammell JG. Glucocorticoid resistance in squirrel monkeys results from a combination of a transcriptionally incompetent glucocorticoid receptor and overexpression of the glucocorticoid receptor co-chaperone FKBP51. J Steroid Biochem Mol Biol. 2006;100:34–41. doi: 10.1016/j.jsbmb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Tsujimura S, Saito K, Tokunaga M, Nakatsuka K, Nakayamada S, Nakano K, Tanaka Y. Overcoming treatment unresponsiveness mediated by P-glycoprotein overexpression on lymphocytes in refractory active systemic lupus erythematosus. Mod Rheumatol. 2005;15:28–32. doi: 10.1007/s10165-004-0354-x. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka T, Okabe T, Gondo S, Fukuda M, Yamamoto M, Umemura T, Tani K, Nomura M, Goto K, Yanase T, Nawata H. Modification of glucocorticoid sensitivity by MAP kinase signaling pathways in glucocorticoid-induced T-cell apoptosis. Exp Hematol. 2006;34:1542–1552. doi: 10.1016/j.exphem.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Kobzdej M, Matuszyk J, Ziolo E, Strzadala L. Changes in glucocorticoid-induced apoptosis and in expression of Bcl-2 protein during long-term culture of thymic lymphoma [In Process Citation] Arch Immunol Ther Exp. 2000;48:43–46. [PubMed] [Google Scholar]
- 9.Wei G, Twomey D, Lamb J, Schlis K, Agarwal J, Stam RW, Opferman JT, Sallan SE, den Boer ML, Pieters R, Golub TR, Armstrong SA. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell. 2006;10:331–342. doi: 10.1016/j.ccr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 10.McKay LI, Cidlowski JA. Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: mechanisms of mutual antagonism. Mol Endocrinol. 1998;12:45–56. doi: 10.1210/mend.12.1.0044. [DOI] [PubMed] [Google Scholar]
- 11.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2006 doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webster JC, Oakley RH, Jewell CM, Cidlowski JA. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci U S A. 2001;98:6865–6870. doi: 10.1073/pnas.121455098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamradt MC, Mohideen N, Vaughan AT. RU486 Increases Radiosensitivity and Restores Apoptosis through Modulation of HPV E6/E7 in Dexamethasone-Treated Cervical Carcinoma Cells. Gynecol Oncol. 2000;77:177–182. doi: 10.1006/gyno.1999.5724. [DOI] [PubMed] [Google Scholar]
- 14.Kim SB, Ozawa T, Umezawa Y. A genetically encoded indicator for assaying bioactive chemicals that induce nuclear transport of glucocorticoid receptor. Anal Biochem. 2005;347:213–220. doi: 10.1016/j.ab.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Silva CM, Powell-Oliver FE, Jewell CM, Sar M, Allgood VE, Cidlowski JA. Regulation of the human glucocorticoid receptor by long-term and chronic treatment with glucocorticoid. Steroids. 1994;59:436–442. doi: 10.1016/0039-128x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 16.Burnstein KL, Jewell CM, Sar M, Cidlowski JA. Intragenic sequences of the human glucocorticoid receptor complementary DNA mediate hormone-inducible receptor messenger RNA down-regulation through multiple mechanisms. Mol Endocrinol. 1994;8:1764–1773. doi: 10.1210/mend.8.12.7708063. [DOI] [PubMed] [Google Scholar]
- 17.Odermatt A, Gumy C, Atanasov AG, Dzyakanchuk AA. Disruption of glucocorticoid action by environmental chemicals: Potential mechanisms and relevance. J Steroid Biochem Mol Biol. 2006;102:222–231. doi: 10.1016/j.jsbmb.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Koenig JI, Kirkpatrick B, Lee P. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology. 2002;27:309–318. doi: 10.1016/S0893-133X(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 19.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 20.Moritz KM, Boon WM, Wintour EM. Glucocorticoid programming of adult disease. Cell Tissue Res. 2005;322:81–88. doi: 10.1007/s00441-005-1096-6. [DOI] [PubMed] [Google Scholar]
- 21.Weaver IC, Diorio J, Seckl JR, Szyf M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann N Y Acad Sci. 2004;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- 22.Tsapis M, Lieb M, Manzo F, Shankaranarayanan P, Herbrecht R, Lutz P, Gronemeyer H. HDAC inhibitors induce apoptosis in glucocorticoid-resistant acute lymphatic leukemia cells despite a switch from the extrinsic to the intrinsic death pathway. Int J Biochem Cell Biol. 2007;39:1500–1509. doi: 10.1016/j.biocel.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Trotter KW, Archer TK. Reconstitution of glucocorticoid receptor-dependent transcription in vivo. Mol Cell Biol. 2004;24:3347–3358. doi: 10.1128/MCB.24.8.3347-3358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HL, Archer TK. Prolonged glucocorticoid exposure dephosphorylates histone H1 and inactivates the MMTV promoter. Embo J. 1998;17:1454–1466. doi: 10.1093/emboj/17.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhattacharjee RN, Banks GC, Trotter KW, Lee HL, Archer TK. Histone H1 phosphorylation by Cdk2 selectively modulates mouse mammary tumor virus transcription through chromatin remodeling. Mol Cell Biol. 2001;21:5417–5425. doi: 10.1128/MCB.21.16.5417-5425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogatsky I, Trowbridge JM, Garabedian MJ. Glucocorticoid receptor-mediated cell cycle arrest is achieved through distinct cell-specific transcriptional regulatory mechanisms. Mol Cell Biol. 1997;17:3181–3193. doi: 10.1128/mcb.17.6.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fryer CJ, Kinyamu HK, Rogatsky I, Garabedian MJ, Archer TK. Selective activation of the glucocorticoid receptor by steroid antagonists in human breast cancer and osteosarcoma cells. J Biol Chem. 2000;275:17771–17777. doi: 10.1074/jbc.M908729199. [DOI] [PubMed] [Google Scholar]
- 28.MacGregor GR, Mogg AE, Burke JF, Caskey CT. Histochemical staining of clonal mammalian cell lines expressing E.coli β-Galactosidase indicates heterologous expression of the bacterial gene. Somatic Cell Molecular Genetics. 1987;13:253–265. doi: 10.1007/BF01535207. [DOI] [PubMed] [Google Scholar]
- 29.Tam SP, Archer TK, Deeley RG. Biphasic effects of estrogen on apolipoprotein synthesis in human hepatoma cells: mechanism of antagonism by testosterone. Proc Natl Acad Sci U S A. 1986;83:3111–3115. doi: 10.1073/pnas.83.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aoyagi S, Archer TK. Dynamic histone acetylation/deacetylation with progesterone receptor-mediated transcription. Mol Endocrinol. 2007;21:843–856. doi: 10.1210/me.2006-0244. [DOI] [PubMed] [Google Scholar]
- 31.Fan HY, Trotter KW, Archer TK, Kingston RE. Swapping function of two chromatin remodeling complexes. Mol Cell. 2005;17:805–815. doi: 10.1016/j.molcel.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Kinyamu HK, Archer TK. Changes in attitude, changes in latitude: nuclear receptors remodeling chromatin to regulate transcription. Mol Endocrinol. 2006;20:1–13. doi: 10.1210/me.2005-0192. [DOI] [PubMed] [Google Scholar]
- 33.Hebbar PB, Archer TK. Nuclear factor 1 is required for both hormone-dependent chromatin remodeling and transcriptional activation of the mouse mammary tumor virus promoter. Mol Cell Biol. 2003;23:887–898. doi: 10.1128/MCB.23.3.887-898.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown DT. Histone H1 and the dynamic regulation of chromatin function. Biochem Cell Biol. 2003;81:221–227. doi: 10.1139/o03-049. [DOI] [PubMed] [Google Scholar]
- 35.Strobeck MW, DeCristofaro MF, Banine F, Weissman BE, Sherman LS, Knudsen ES. The BRG-1 subunit of the SWI/SNF complex regulates CD44 expression. J Biol Chem. 2001;276:9273–9278. doi: 10.1074/jbc.M009747200. [DOI] [PubMed] [Google Scholar]
- 36.Hebbar PB, Archer TK. Chromatin-dependent cooperativity between site-specific transcription factors in vivo. J Biol Chem. 2007;282:8284–8291. doi: 10.1074/jbc.M610554200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell LM, Leong ML, Kim B, Wang E, Park J, Hemmings BA, Firestone GL. Hyperosmotic stress stimulates promoter activity and regulates cellular utilization of the serum- and glucocorticoid-inducible protein kinase (Sgk) by a p38 MAPK-dependent pathway. J Biol Chem. 2000;275:25262–25272. doi: 10.1074/jbc.M002076200. [DOI] [PubMed] [Google Scholar]
- 38.Itani OA, Liu KZ, Cornish KL, Campbell JR, Thomas CP. Glucocorticoids stimulate human sgk1 gene expression by activation of a GRE in its 5′-flanking region. Am J Physiol Endocrinol Metab. 2002;283:E971–979. doi: 10.1152/ajpendo.00021.2002. [DOI] [PubMed] [Google Scholar]
- 39.Burkhart BA, Hebbar PB, Trotter KW, Archer TK. Chromatin-dependent E1A activity modulates NF-kappaB RelA-mediated repression of glucocorticoid receptor-dependent transcription. J Biol Chem. 2005;280:6349–6358. doi: 10.1074/jbc.M411147200. [DOI] [PubMed] [Google Scholar]
- 40.Hebbar PB, Archer TK. Chromatin remodeling by nuclear receptors. Chromosoma. 2003;111:495–504. doi: 10.1007/s00412-003-0232-x. [DOI] [PubMed] [Google Scholar]
- 41.Tryndyak VP, Kovalchuk O, Muskhelishvili L, Montgomery B, Rodriguez-Juarez R, Melnyk S, Ross SA, Beland FA, Pogribny IP. Epigenetic reprogramming of liver cells in tamoxifen-induced rat hepatocarcinogenesis. Mol Carcinog. 2007;46:187–197. doi: 10.1002/mc.20263. [DOI] [PubMed] [Google Scholar]
- 42.Pogribny IP, Tryndyak VP, Muskhelishvili L, Rusyn I, Ross SA. Methyl deficiency, alterations in global histone modifications, and carcinogenesis. J Nutr. 2007;137:216S–222S. doi: 10.1093/jn/137.1.216S. [DOI] [PubMed] [Google Scholar]
- 43.Strunnikova M, Schagdarsurengin U, Kehlen A, Garbe JC, Stampfer MR, Dammann R. Chromatin inactivation precedes de novo DNA methylation during the progressive epigenetic silencing of the RASSF1A promoter. Mol Cell Biol. 2005;25:3923–3933. doi: 10.1128/MCB.25.10.3923-3933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- 45.Seckl JR, Meaney MJ. Glucocorticoid “programming” and PTSD risk. Ann N Y Acad Sci. 2006;1071:351–378. doi: 10.1196/annals.1364.027. [DOI] [PubMed] [Google Scholar]
- 46.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Namazy JA, Schatz M. Treatment of asthma during pregnancy and perinatal outcomes. Curr Opin Allergy Clin Immunol. 2005;5:229–233. doi: 10.1097/01.all.0000168786.59335.c3. [DOI] [PubMed] [Google Scholar]
- 48.Stocker CJ, Arch JR, Cawthorne MA. Fetal origins of insulin resistance and obesity. Proc Nutr Soc. 2005;64:143–151. doi: 10.1079/pns2005417. [DOI] [PubMed] [Google Scholar]
- 49.Archer TK, Fryer CJ, Lee H-L, Zaniewski E, Liang T, Mymryk JS. Steroid hormone receptor status defines the MMTV promoter chromatin structure in vivo. J Steroid Biochem Mol Biol. 1995;53:421–429. doi: 10.1016/0960-0760(95)00088-h. [DOI] [PubMed] [Google Scholar]
- 50.Kinyamu HK, Archer TK. Modifying chromatin to permit steroid hormone receptor-dependent transcription. BBA Gene Structure and Expression. 2004;1677:30–45. doi: 10.1016/j.bbaexp.2003.09.015. [DOI] [PubMed] [Google Scholar]