Abstract
The blood-brain barrier (BBB) is a multifunctional endothelial interface separating the bloodstream from the brain interior. Although the mature BBB is well characterized, the embryonic development of this complex system remains poorly understood. Embryonic neural progenitor cells (NPC) are a potential inductive cell type populating the developing brain, and their ability to influence BBB properties was therefore examined. When puromycin-purified brain microvascular endothelial cells (BMEC) were co-cultured with embryonic NPC in a two compartment Transwell system, the BMEC exhibited enhanced barrier properties in the form of increased transendothelial electrical resistance (TEER) and decreased permeability to the small molecule tracer, sodium fluorescein. These changes required the presence of NPC in the early stages of differentiation and were accompanied by alterations in the fidelity of BMEC tight junctions as indicated by occludin, claudin5 and ZO-1 redistribution at cell-cell borders. In contrast to the findings with NPC, postnatal astrocytes elicited a delayed, but longer duration response in BMEC TEER. BMEC co-culture also suppressed neuronal differentiation of NPC indicating a reciprocal signaling between the two cell populations. This study demonstrates that NPC-BMEC interactions are prevalent and for the first time demonstrates that NPC are capable of inducing BBB properties.
Keywords: Blood-brain barrier, brain microvasculature, endothelial cell, neural progenitor cell, neural stem cell
Introduction
The BBB is comprised of a specialized class of endothelium that forms a cellular barrier between the bloodstream and the interstices of the adult brain. By restricting non-specific flux of blood-borne constituents, the BBB plays an important role in maintaining parenchymal homeostasis, and strictly regulates transport of ions, small molecules, proteins, and cells into and out of the brain. The BBB accomplishes these tasks because its unique endothelium is endowed by epithelial-like tight junctions joining adjacent endothelial cells, lacks fenestrae, and possesses a rich array of molecular transport systems. Although the endothelium is the principle determinant of barrier function, perivascular non-endothelial cells in the local microenvironment have been shown to make significant contributions. Astrocytes (Stewart and Wiley 1981; Risau et al. 1986b; Janzer and Raff 1987), neurons (Tontsch and Bauer 1991) and pericytes (Balabanov and Dore-Duffy 1998; Ramsauer et al. 2002) have all been demonstrated to provide cues that result in the unique BBB endothelial phenotype.
Although the inductive properties of the aforementioned brain cell types have been confirmed through a multitude of in vivo and in vitro studies, the cell type(s) responsible for early embryonic BBB induction have not been distinguished. The developmental timecourse of embryonic BBB formation differs between species, but it is generally well accepted that the onset of BBB development begins prenatally and is followed by a gradual maturation to full BBB function (Bauer and Bauer 2000; Engelhardt 2003). For example, in rodents, vascular fenestrae disappear, pinocytosis decreases, and vessels decrease in diameter between embryonic days E11 and E17 (Bauer et al. 1993; Stewart and Hayakawa 1994; Bolz et al. 1996). The onset of tight junction formation is detectable from day E15, and tight junctions continue to increase in complexity through postnatal day P1 (Butt et al. 1990; Schulze and Firth 1992; Bauer et al. 1995; Kniesel et al. 1996; Nico et al. 1999). During this time, the transendothelial electrical resistance (TEER) of pial vessels is intermediate between peripheral vessels and the adult BBB (Butt et al. 1990; Schulze and Firth 1992; Bauer et al. 1995; Kniesel et al. 1996; Nico et al. 1999). A combination of the aforementioned attributes serves to restrict passage of protein into the embryonic brain (Risau et al. 1986a; Bauer et al. 1995; Dziegielewska et al. 2000), while a gradual decrease in BBB permeability to small tracers such as inulin and sucrose begins during embryonic development and continues postnatally (Ferguson and Woodbury 1969). Finally, transporter expression at the BBB also evolves from embryonic to postnatal stages as a result of changing nutritional needs (Johanson 1989; Gerhart et al. 1997).
The early embryonic developmental timecourse for the BBB raises the question as to what inductive factors or cell types drive the endothelial differentiation process. As mentioned above, astrocytes have long been linked with induction of BBB properties by in vitro and in vivo experiments (Stewart and Wiley 1981; Risau et al. 1986b; Janzer and Raff 1987). However, angiogenic vessels invade the immature embryonic neural environment and begin establishing BBB characteristics well in advance of the onset of gliogenesis as defined by the presence of GFAP-positive astrocytes in rodent brain (E18, (LeVine and Goldman 1988)). In addition, the developing BBB vessels have little extracellular matrix with few astrocyte contacts even just days prior to birth (Caley and Maxwell 1970). In fact, for rodents, much of the astrocyte generation takes place postnatally during which time the astrocyte sheath that surrounds mature brain capillaries is developed (Johanson 1989). Therefore, it is unlikely that astrocytes function in the early BBB induction process, but instead other cell types may be responsible for the early onset of BBB properties.
NPC are a major cell type in the developing embryonic brain, and it was recently reported that the differentiation and morphology of NPC are influenced by endothelial cells (Shen et al. 2004). In co-culture with endothelial cells, NPC show reduced neurogenesis and elevated self-renewal (Shen et al. 2004). Neural progenitors have also been observed in contact with early postnatal blood vessels, and this was implicated as an early stage in astrocyte differentiation (Zerlin and Goldman 1997). In addition, when endothelial cells and neural stem cells are grown in direct contact, it was shown that the adult neural stem cells could even produce progeny that exhibited an endothelial phenotype (Wurmser et al. 2004). Finally, adult neural stem cells are often found localized in perivascular spaces of the brain such as the subventricular zone and hippocampus, and it is thought that the vasculature is an important part of the stem cell niche (Doetsch 2003b). Given the cellular interplay in the endothelial cell to NPC direction, it would be plausible that NPC could also influence brain endothelial cell phenotype. In this study, it was demonstrated that NPC isolated from the E14 embryonic brain induced BBB properties in an in vitro model consisting of primary rat brain microvascular endothelial cells in co-culture with NPC.
Materials and Methods
Isolation of rat brain microvessel endothelial cells
The isolation of rat brain capillaries was performed as previously described (Weidenfeller et al. 2005; Calabria et al. 2006). Briefly, the meninges-free cortices from adult male Sprague Dawley rats (220–250g) were mashed with forceps, and thoroughly triturated. Capillaries were separated from surrounding tissue by sequential digestion/density centrifugation steps with type 2 collagenase (Worthington Biochemical Corporation) and Collagenase/dispase (Roche Applied Science). The capillaries were plated onto 1.12 cm2 Transwell-Clear® permeable supports (0.4 µm pore size, Corning) coated with collagen IV/fibronectin in puromycin-supplemented medium (4 µg/mL) containing DMEM, 20% bovine platelet poor plasma derived serum, 1 ng/mL human basic fibroblast growth factor (FGF-2/bFGF, R&D Systems), 1 µg/mL heparin, 2 mM L-glutamine, and an antibiotic-antimycotic solution (Penicillin-Streptomycin-Amphotericin (PSA): 100 U/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL amphotericin). Cultures were maintained in a 37°C incubator under humidified 5% CO2/95% air.
Isolation and culture of rat cortical embryonic neural progenitor cells
Rat cortical NPC were isolated as described previously (Ostenfeld et al. 2002). The cortices were dissected from E14 rat brains. The tissue was treated with accutase (Innovative Cell Technologies, San Diego, CA, USA) for 10 minutes at 37°C, washed twice in DMEM, and then dissociated into a single cell suspension. Cells were initially seeded at a density of 2×105 cells/ml in a T25 flask in defined serum-free NPC culture medium [DMEM:HAMS-F12 at 3:1 supplemented with B27 (2% v/v), epidermal growth factor (EGF, 20 ng/ml), fibroblast growth factor (FGF-2, 20 ng/ml), and heparin (5 µg/ml)]. Cells were grown as free-floating neurospheres for 3 days and then used for co-culture with BMEC on day in vitro 4 (DIV4).
Isolation of astrocytes
Cortices from neonatal rats (P6) were minced in a petridish containing ice-cold Hanks' Balanced Salt Solution (HBSS). The minced tissue was centrifuged for 2 min (500g), resuspended in HBSS containing trypsin (0.5 mg/ml) and incubated at 37°C for 25 min in a shaker bath. The trypsinized tissue was triturated and the cell suspension was filtered through a 70 µm mesh. 3×104 cells/cm2 were plated in DMEM containing 10% FBS, 10% horse serum, 2 mM L-glutamine, and PSA. Medium was changed every third day and cells were treated with 0.25 mM dibutyryl cAMP for 3 days prior to co-culture with BMEC to induce an in vivo-like phenotype (Segovia et al. 1994; Enkvist et al. 1996). The presence of GFAP-expressing astrocytes was confirmed by immunocytochemistry.
Co-culture of BMEC with NPC or astrocytes
Neurospheres were collected on DIV4, treated with accutase, and washed twice in DMEM. Live cells were counted on a hemacytometer, and 2.5 × 105 NPC/cm2 were plated in the lower compartment in either mitogen-free medium allowing NPC differentiation (DMEM:HAMS-F12 at 3:1, 2% v/v B27,1% FBS, and PSA, with poly L-lysine/laminin coating) or with mitogen-containing medium to suppress differentiation (mitogen-free medium plus 20 ng/mL EGF and 10 ng/mL FGF-2, without poly L-lysine/laminin coating) (Ostenfeld et al. 2002). The Transwell® filter containing the confluent BMEC was then added to complete the co-culture system (Fig. 1). Mitogen-mediated suppression of NPC differentiation in the presence or absence of BMEC was confirmed by anti-nestin, anti-GFAP and anti-βIII tubulin staining. In this way, it was determined that the NPC populations were GFAP and βIII tubulin negative prior to and after BMEC co-culture in mitogen-containing medium. NPC densities up to 1 × 106 NPC/cm2 were tested, but were not found to yield any increases in TEER induction above that seen with the 2.5 × 105 NPC/cm2 plating density. Embryonic mouse fibroblasts (3T3, ATCC) were used as a non-neural co-culture control.
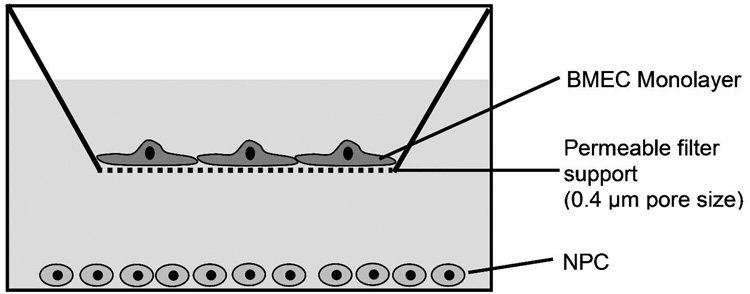
Figure 1.
In vitro model of the BBB. Primary BMEC were cultured for 2 days in the presence of puromycin to yield a nearly 100% pure BMEC monolayer on the collagen IV/fibronectin-coated filter support. On DIV 2 BMEC were switched to puromycin-free medium. After reaching confluence on DIV 3, NPC were added to the lower compartment in the presence or absence of mitogens (FGF-2, EGF). The resulting co-cultures were used to assess NPC effects on the BBB properties of the BMEC monolayer.
One day prior to co-culture, astrocytes were preconditioned in mitogen-free medium to avoid effects that serum withdrawal could have on the temporal response of astrocyte induction. After 24 hours of preconditioning, astrocytes were treated with trypsin-EDTA solution and single cells were resuspended in mitogen-free medium. A total of 6.25 × 104 astrocytes/cm2 were added to the lower compartment in mitogen-free medium, and the filter insert with the confluent BMEC monolayer was added.
Immunocytochemistry
All steps were performed at 20°C. The BMEC and NPC cultures were gently washed three times with 0.01 M PBS and fixed with paraformaldehyde (4% w/v in PBS). After blocking and permeabilization (10% goat serum containing 0.1% Triton X-100 in PBS (PBSG), 30 min), primary antibodies (anti-nestin, [BD Biosciences], rabbit anti-glial fibrillary acidic protein [GFAP, DAKO Cytomation], anti-βIII tubulin [BD Biosciences], anti-von Willebrand factor [Sigma], anti-occludin, anti-zonula occluden-1, mouse anti-claudin 5 [Invitrogen], primary antibody mix for MBP and CNPase detection [Orion Biosolutions]) were diluted in PBSG with 3% goat serum and incubated with samples for 1h. Samples were washed with PBS and incubated with secondary antibodies (Texas Red goat anti-rabbit IgG, Alexa Fluor goat anti-mouse IgG antibody) diluted in PBSG with 3% goat serum for 1 h. DAPI nuclear stain at a concentration of 300 nM in PBS was added to the wells for 5 min. For immunocytochemistry of brain tissue sections, freshly isolated E14 rat brains were embedded in tissue freezing medium, snap frozen in liquid nitrogen, sectioned, and labeled as described above.
Resistance measurements
Transendothelial electrical resistance (TEER) was measured using an EVOM voltohmmeter (World Precision Instruments). Resistance values (Ω×cm2) were corrected by subtracting the resistance of a substrate coated, empty filter. At each time point, three TEER measurements were taken per Transwell-Clear® filter to yield an average TEER value for each filter. Subsequently, TEER values for triplicate filters at each culture condition were used to compute the mean and standard deviations reported.
Permeability studies
The permeability was assessed by determining the flux of fluorescein through the BMEC monolayer. Fluorescein sodium salt in DMEM was added to the apical filter compartment to produce a uniform initial concentration of 1 µM. Subsequently, 200 µl were removed from the basolateral compartment after 0, 15, 30, 45, and 60 min. The fluorescence was measured with the FL×800 fluorescence reader (Bio-Tek Instruments) and the rates of fluorescein accumulation in the lower compartment used to determine the permeability as described previously (Perriere et al. 2005).
Quantitative analysis of cultures
Counting of NPC-derived cell types was performed by overlay of anti-nestin (undifferentiated NPC), anti-βIII tubulin (neurons), and DAPI images or by overlay of anti-nestin, anti-GFAP (astrocytes), and DAPI images. The cell distribution was assessed by determining the percentage of cells positively-labeled for a particular marker. For this determination, 5 random fields for each type of labeling (βIII tubulin/Nestin or GFAP/Nestin, ~1000 total cells each condition) were counted at a magnification of 40x. Proliferation of BMEC was evaluated by BrdU incorporation with the 5-Bromo-2′-deoxy-uridine Labeling and Detection Kit I (Roche Applied Science) according to the manufacturer’s instructions. BrdU was added to the cultures at the beginning of the 24h co-culture of BMEC with NPC. The cells were fixed after 24h, total BMEC numbers were assessed by DAPI nuclear stain, and the percentage of BMEC incorporating BrdU was determined for 6 different fields on each of 3 filters (800 cells for each condition). An analogous procedure was used to assess NPC BrdU incorporation and total cell numbers of NPC. The percentage of BMEC containing frayed junctions over a significant fraction (greater than 10%) of their total cell border was determined by randomly choosing microscope fields in phase contrast mode where junctional ultrastructure is not visible. The immunocytochemical images for occludin labeling were then acquired in fluorescence mode. Junctions between adjacent cells (100 cells per image with 5 images total for each condition) were defined as frayed if immunolabeling illuminated tight junction protrusions that are not parallel to the cell-cell border.
Results
Primary brain endothelial cell-embryonic NPC co-culture model
The influence of NPC on the barrier properties of adult BMEC was investigated using a novel in vitro model consisting of primary rat BMEC and embryonic rat cortical NPC. Since BMEC isolated from adult brains de-differentiate in vitro (Krizbai and Deli 2003), they have been widely used to study BBB induction and modulation, although they still possess some level of BBB properties. NPC and BMEC were co-cultured together using a microporous filter setup (Transwell-Clear®) with an upper compartment and a lower compartment representing the blood and brain side of the blood-brain barrier (BBB), respectively (Fig. 1). The filter setup allows the in situ measurement of the transendothelial electrical resistance (TEER) yielding information regarding the integrity of the BMEC monolayer by monitoring the paracellular flux of small electrolytes. Puromycin-purified BMEC were cultured on the upper surface of the filter membrane, and after 3 days in vitro (DIV3) had grown to confluence as determined by phase contrast microscopy. Puromycin treatment ensured a nearly 100% pure endothelial monolayer, and we and others have demonstrated that this rodent in vitro BBB model displays well defined tight junctions and forms an impermeable barrier to small molecule tracers (Perriere et al. 2005; Weidenfeller et al. 2005; Calabria et al. 2006). Importantly, the model has also proven reliable for the measurement of BMEC response to inductive factors such as cAMP, astrocytes, and glucocorticoids (Perriere et al. 2005; Weidenfeller et al. 2005; Calabria et al. 2006), and was therefore suitable for the testing of NPC inductive capacity. In order to co-culture NPC with the BMEC monolayer, freshly isolated NPC from E14 rats were expanded for 3 days in EGF and FGF-2 mitogen-containing medium (see Materials and Methods for details) and then cultured in the lower compartment such that the BMEC and NPC could interact via soluble mediators. When co-cultured in mitogen-free medium, NPC attached to the poly L-lysine/laminin coated substrate in the lower compartment, generating a mixture of NPC (nestin-positive) and differentiated progeny including astrocytes (GFAP-positive) and neurons (βIII tubulin-positive). Co-culture in the presence of mitogens supported solely proliferation as nestin-positive NPC.
NPC influence on BMEC TEER and permeability
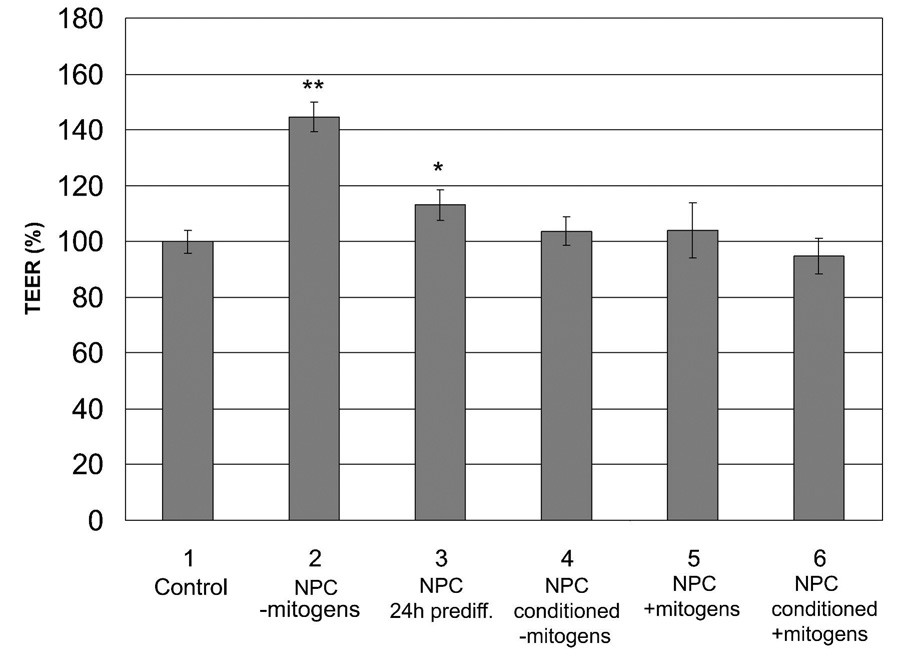
The in vitro co-culture model was used to investigate a possible involvement of NPC in the induction of BBB properties in the BMEC monolayer. In order to determine whether NPC or NPC-derived cells could affect the in vitro barrier phenotype of BMEC monolayers, the TEER was measured in the presence or absence of differentiating NPC (mitogen-free conditions). TEER measurements after 24 hours of co-culture indicated a 47% increase in monolayer TEER with NPC (110 ± 5 Ω×cm2) when compared to control BMEC cultures lacking NPC (75 ± 4 Ω×cm2), indicating an early inductive response to soluble factors released by NPC (Fig. 2, columns 1 and 2). The barrier-enhancing effect in the presence of differentiating NPC was also observed in permeability studies where NPC co-culture reduced the monolayer permeability for fluorescein sodium salt by 33% with NPC influences (Table 1). This decrease in diffusion of the BBB-impermeable fluorescein directly correlated with the increase in TEER. In addition, to support the presence of soluble factors that mediate the increases in TEER, the medium conditioned by 24 hours of co-culture was serially applied to fresh BMEC monolayers. The BMEC-NPC co-culture conditioned medium again induced BMEC TEER (150 ± 6%) compared with BMEC monolayers grown in medium conditioned by BMEC alone. Finally, even when NPC were maintained in an undifferentiated state for longer periods of 1–5 weeks in vitro prior to co-culture with BMEC, the induction properties were still observed under the mitogen-free conditions (31–43% increases in TEER).
Figure 2.
Influence of NPC co-culture and NPC-conditioned media on BMEC TEER. BMEC were cultured as follows: 1. in the absence of NPC (control), 2. in the presence of differentiating NPC (+NPC −mitogens), 3. in the presence of 24h pre-differentiated NPC (+NPC 24h prediff), 4. in NPC-conditioned medium generated by 24 hour of NPC culture in mitogen-free differentiation medium (NPC conditioned −mitogens), 5. in the presence of NPC in mitogen-containing medium (NPC +mitogens), and 6. in 24 hour conditioned mitogen-containing medium from dividing NPC (NPC conditioned +mitogens). The TEER was monitored after 24 hours for each culture condition and expressed as normalized % of control TEER values. The mitogen-containing conditions (5 and 6) were independently normalized to a mitogen-containing BMEC monoculture control. The statistical significance is given with p < 0.001 (**) and p < 0.03 (*) as determined by the unpaired Student’s t-test. Triplicate cultures were analyzed for each condition and results display mean ±SD. The results are representative of 8 independent experiments where the increase in TEER in the presence of differentiating NPC (2) ranged from 17–53%.
Table 1.
Effects of differentiating NPC on BMEC permeability, structure and proliferation.
| BMEC | Permeability (10−4 cm/min)(a) | TEER (Ω×cm2) | % Frayed (b) | Cell Density (104 cells/cm2) (c) | % BrdU+ cells |
|---|---|---|---|---|---|
| +NPC | 3.3 ± 0.5 | 105 ± 7 | 33.5 ± 3.1% | 2.56 ± 0.22 | 8.7 ± 2.4 |
| −NPC | 4.9 ± 1.1 | 65 ± 6 | 65.1 ± 2.1% | 2.49 ± 0.20 | 8.7 ± 6.3 |
Permeability: permeability to fluorescein sodium salt and the corresponding TEER values for these cultures (n=6, p<0.004 for permeability and p<0.001 for TEER).
% Frayed: the percentage of cells containing frayed edges over a significant fraction (greater than 10%) of their total cell border (p<0.001).
The number of endothelial cells per area was determined using a combination of endothelial-specific markers and DAPI nuclear staining. All data in Table 1 represent mean ±SD and statistical confidence was determined by the unpaired student’s t-test.
In contrast, conditioned medium (24h) from NPC differentiating in the absence of BMEC did not show an effect on the TEER within 24h after application to BMEC (Fig. 2, column 4). When co-cultured with NPC that were pre-differentiated for 24 hours in the absence of BMEC, TEER increases were attenuated (11 ± 6%) (Fig. 2, column 3). In order to determine whether proliferating, undifferentiated nestin-positive NPC or conditioned medium from proliferating, undifferentiated NPC can induce an increase in TEER, BMEC were co-cultured with NPC in the presence of mitogens or with NPC culture-conditioned medium from 24 hour mitogen-treated, proliferating NPC, respectively (Fig. 2, columns 5 and 6). No induction in BMEC TEER could be observed under these conditions after 24h. Finally, the effects of non-neural embryonic 3T3 fibroblasts on BMEC were investigated and like the mitogen-treated NPC, no induction was observed (data not shown). The lack of induction in the presence of undifferentiated, proliferating NPC or 3T3 fibroblasts indicated that the simple presence of another cell type was not responsible for changes in phenotype observed in the presence of differentiating NPC.
Influence of NPC co-culture on BMEC morphology
The tight junctions of BMEC cultured alone or co-cultured with NPC were investigated to determine if NPC were capable of influencing BMEC cell-cell contacts or cell morphology in a way that could account for the increased TEER and decreased fluorescein permeability. BMEC were probed with antibodies against tight junction proteins ZO-1, occludin, and claudin 5 in the presence (Fig. 3 A, C, E) or absence (Fig. 3. B, D, F) of differentiating NPC. In the presence of NPC the majority of the BMEC show well-established tight junctions and a distinct occludin, claudin 5, and ZO-1 staining. In the absence of NPC, cell-cell junctions were also evident, but tight junction staining indicated an irregular, frayed staining pattern in 65% of the BMEC while in the presence of NPC, only 34% of the BMEC exhibited such a junctional structure (Table 1, Fig. 3). The cell morphology and cell size were indistinguishable under these two conditions (Table 1). BMEC with or without NPC co-culture were probed for F-actin localization, and no difference between BMEC-NPC co-culture and BMEC monocultures was detected. In both cases, a strong peri-junctional actin localization together with intracellular actin filaments was observed (data not shown).
Figure 3.

Tight junction organization and cell morphology of BMEC in the presence (A, C, E) or absence (B, D, F) of differentiating NPC. After 24 hours of co-culture in mitogen-free medium, the BMEC were probed for ZO-1 (A, B), occludin (C, D) or claudin 5 (E, F). Note the tight junction protrusions perpendicular to the cell-cell borders that represent frayed junctions. An example denoted by the arrow is enlarged and shown in the inset of panel B. Representative fields are shown to illustrate the differences in junctional morphology. See Table 1 for the corresponding quantitative data. Scale bar represents 50 µm.
NPC effects on BMEC proliferation
Next, the filter density of BMEC was evaluated to determine whether or not the increased TEER values were the result of a tighter monolayer packing. BMEC monolayers with or without NPC co-culture were assessed using DAPI nuclear staining and 5-Bromo-2′-deoxy-uridine (BrdU) incorporation to investigate BMEC density and proliferation, respectively. As Table 1 indicates, no significant difference in the number of proliferating endothelial cells or BMEC cell density was observed between the NPC-BMEC co-cultures and BMEC mono-cultures.
Potential inductive cell types in the co-culture system
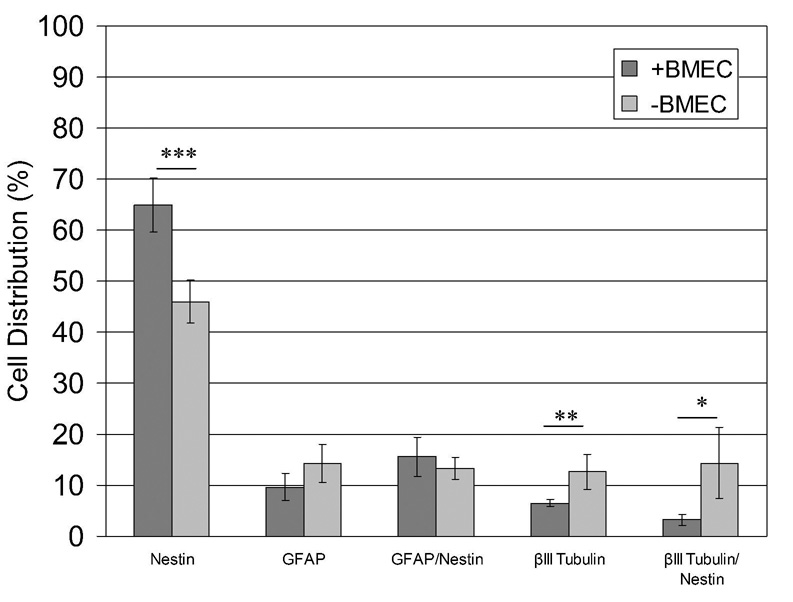
In order to determine the NPC-derived cell types that might be responsible for the induction of TEER and the observed changes in junctional structure, NPC progeny in the basolateral compartment were probed for astrocytic (GFAP), neuronal (βIII-tubulin) and progenitor cell (nestin) markers (Fig. 4). There was no significant change in the total number of NPC-derived cells in the lower compartment with or without BMEC being present, and the number of proliferating BrdU+ cells was also the same (~40%). The majority of NPC-derived cells remain nestin-positive (65 ± 5.3%) after 24 hours in co-culture with BMEC during which time the NPC induction effects are first observed. Results also indicated that NPC differentiate into neuronal and glial cells. The second largest cell population was positive for both GFAP and nestin (15.6 ± 3.8%) indicating that these cells are likely immature astrocytes. A small population (9.7 ± 2.7%) of cells was solely GFAP-positive and considered to be more mature astrocytes. Very few neurons (6.5% ± 0.7%) and immature βIII tubulin- and nestin-positive neurons (3.3 ± 1.0%) were generated in the presence of NPC in the 24-hour timeframe. In the absence of BMEC, more NPC differentiated towards a neuronal fate while the numbers of solely GFAP-positive and GFAP/nestin co-stained cells remained unaffected. The increased number of neurons was accompanied by a lower number of nestin-positive cells in the absence of BMEC (46 ± 4.2%), indicating a higher propensity for NPC to differentiate without BMEC influences. Oligodendrocytes were not detectable by immunofluorescence using antibodies against myelin basic protein or 2'3'-cyclic-nucleotide-3'phosphodiesterase (CNPase).
Figure 4.
Influence of BMEC on NPC differentiation. NPC were fixed 24h after culture in presence or in absence of BMEC. Cell types were determined by indirect immunofluorescence using cell markers (βIII tubulin, GFAP, and nestin), and cells were counted. The statistical significance is given with p < 0.001 (***), p < 0.01 (**), and p < 0.03 (*) as determined by the unpaired Student’s t-test. Results represent the means ±SD of 5 different microscope fields (~1000 cells total).
Effects of BMEC on NPC morphology
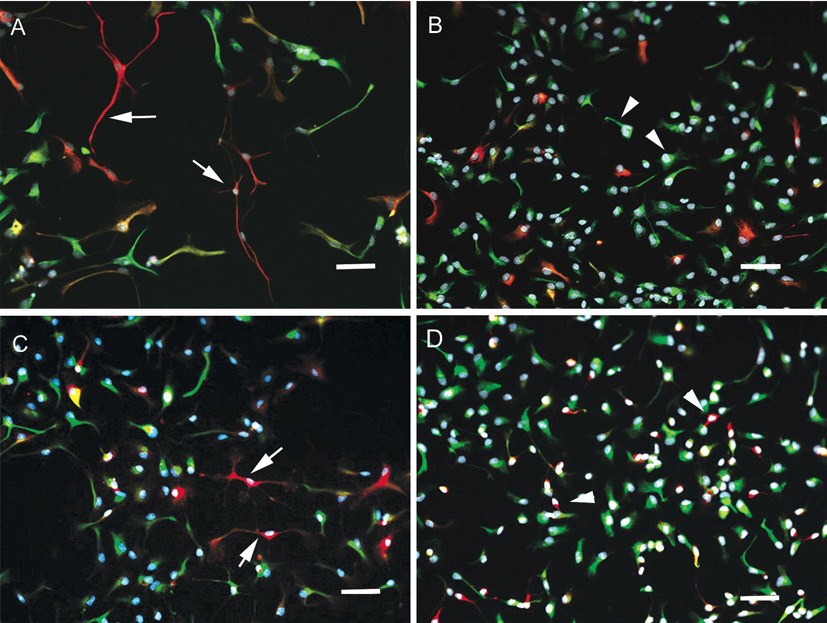
Further evidence of BMEC-NPC crosstalk was gathered by investigating the influence of BMEC on the morphology of NPC-derived cell types. Co-culturing with primary BMEC significantly influences the morphology of the neural progenitor cells. NPC that were allowed to proliferate and differentiate (mitogen-free conditions) in the absence of endothelial cells possessed small cell bodies and displayed multiple thin processes typical of maturing astrocytes and neurons (Figs. 5 A and C). In contrast, the number and length of processes is reduced in the presence of BMEC and the cells have a much more flattened precursor-like morphology (Figs. 5 B and D). This effect was detectable for each of the astrocyte, neuron, and NPC cell types.
Figure 5.
Influence of BMEC co-culture on NPC morphology. Merged images of NPC that were probed for GFAP (red), nestin (green), and DAPI (blue) (panels A and B) or βIII tubulin (red), nestin (green), and DAPI (blue) (panels B and C). Panels A and C represent differentiation in the presence of BMEC and Panels B and D in the absence of BMEC. Arrows indicate small cell bodies and elongated thin processes (panels A and C) while arrowheads point to larger cell bodies and short processes (panels B and D). Scale bar represents 20 µm.
Determination of blood vessel-NPC localization in E14 rat brain
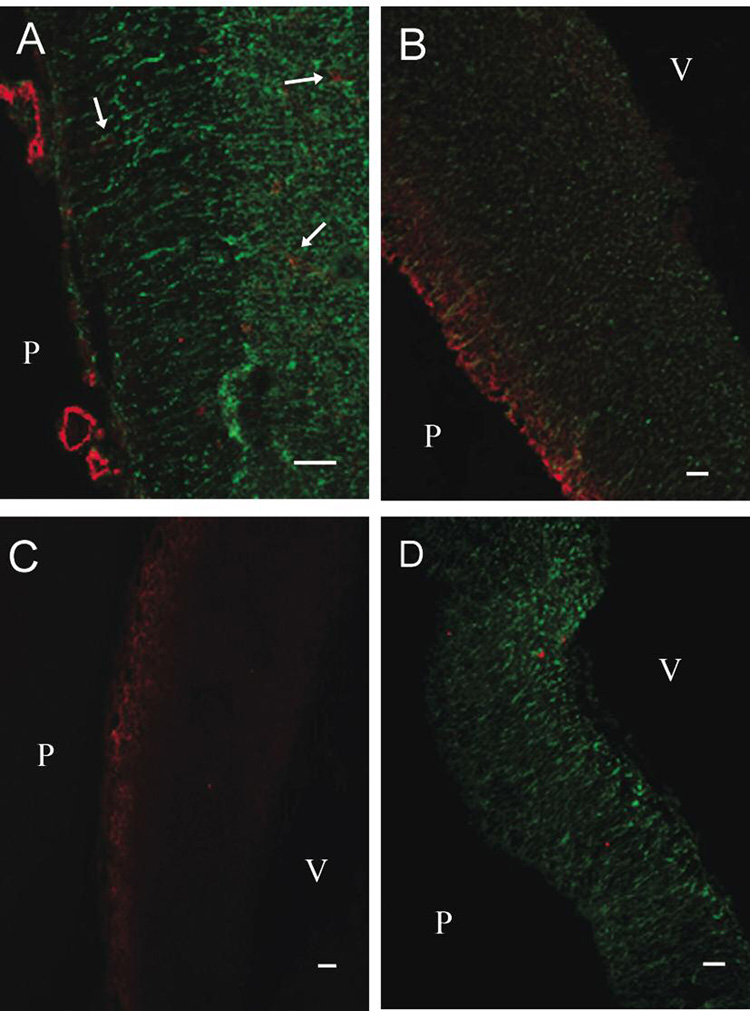
In order to correlate the in vitro results with the actual cellular distribution observed in vivo in the developing E14 rat cortex, the distributions of NPC, astrocytes, and neurons were investigated. NPC, as determined by nestin expression, could be identified throughout the whole cortex with a high density at the inner cortex close to the ventricle (Fig. 6 A, B, D). Radial glia spanned to the pial surface and were identified as elongated nestin-positive cells. In the pial-proximal area, a high density of neurons can also be observed (Fig. 6 B, C). Blood vessels could be easily identified throughout the embryonic brain by anti-Glut1, anti-PECAM1 and anti-von Willebrand factor labeling, and they were found in regions with high numbers of NPC and neurons (Fig. 6 A). In contrast, astrocytes were not detectable in the E14 rat cortex (Fig. 6 D). Finally, the tight junction protein occludin was not detectable indicating the presence of immature endothelial junctions in the E14 rat brain (Fig. 6 C).
Figure 6.
Developing rat cortex at embryonic day E14. Panel A shows von Willebrand factor-positive blood vessels (red) in the developing cortex. The vWF-positive vessels (several denoted by arrows) are localized in regions with large numbers of nestin-expressing cells (green) and are distributed from the pial surface to the ventricle. Panel B indicates a highly βIII tubulin-positive region (red) at the pial surface and homogenous distribution of neurons throughout the NPC-positive (green) ventricular zone. Panel C again indicates the distribution βIII tubulin-positive neurons in addition to the absence of the tight junction protein occludin (green). Panel D illustrates that while the E14 rat cortex is highly nestin-positive (green), the astrocytic protein GFAP (red) was not detected. P=pial surface; V=ventricle. Scale bar represents 400 µm.
Comparison of barrier induction mediated by postnatal astrocytes versus embryonic NPC
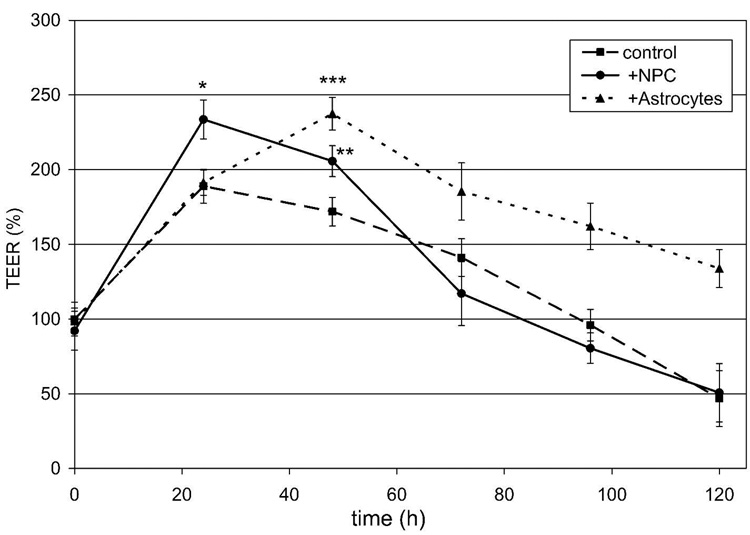
Although the majority of the NPC-derived cells in the BMEC-NPC co-cultures were nestin positive, small percentages of astrocytes (GFAP) or immature astrocytes (GFAP/nestin) were present. Thus, in an effort to distinguish between the effects commonly associated with astrocytes and those mediated by dividing and differentiating NPC, the TEER induction properties of these two situations were directly compared. In parallel to NPC-BMEC co-culture, BMEC were also co-cultured with postnatal (P6) astrocytes at a density corresponding to the number of GFAP-positive cells (25%) counted in the BMEC-NPC co-culture experiment. Based on these counts, BMEC were co-cultured either with astrocytes (6.25 × 104/cm2) or NPC (2.5 × 105/ cm2), and the TEER was monitored as a function of time (Fig. 7). The data indicate that NPC induced a 24% increase in BMEC TEER (96 ± 5 Ω×cm2) compared with the untreated control after just 24 hours (77 ± 5 Ω×cm2), while astrocytes did not induce a statistically significant increase in TEER (78 ± 4 Ω×cm2) during that time period. The maximum TEER reached by the astrocyte co-culture was similar to that achieved by NPC and was detected after 48 hours in co-culture where astrocytes (97 ± 5 Ω×cm2) result in a 38% increase in TEER. By 72 hours, the TEER in NPC co-culture decreased to the level of the monoculture control. However, in the case of astrocyte co-culture, the TEER remained elevated out to 120 hours. To investigate whether the lower overall density of astrocytes compared with NPC contributed to the delay in inductive response, co-culture of BMEC was also performed with a confluent monolayer of astrocytes. The higher density astrocyte co-culture yielded the same magnitude of TEER induction and the same temporal response as that seen for the low-density astrocyte co-culture, including the delay in TEER induction (data not shown).
Figure 7.
TEER measurements of BMEC in co-culture with NPC or astrocytes. NPC or postnatal astrocytes were co-cultured with confluent BMEC using identical mitogen-free culture medium, and the TEER timecourse was followed for 5 days. Triplicate cultures were analyzed for each condition and the resistance measurements normalized to those of the untreated control at time zero, and results display mean ±SD. Statistical significance between sample and control is indicated: p<0.006 (*), p<0.02 (**), and p<0.001 (***). The results are representative of 5 independent co-culture experiments from multiple BMEC, NPC and astrocyte isolations. Percent increases denoted in the text refer to TEER increases relative to the control at the time points of interest (24 or 48 hours). The initial increase in TEER for the control BMEC cultures observed at 24 hours was reproducible, and was a result of the reduction in serum content upon the switch to mitogen-free medium.
Discussion
In this study, the influences of NPC on the BBB properties of BMEC were investigated. An in vitro model consisting of primary rat BMEC and embryonic NPC was evaluated for its barrier properties and compared with a BMEC model lacking NPC. NPC significantly influenced BMECs by inducing TEER, reducing permeability, and affecting tight junction structure. Barrier-inducing effects were only observed in the presence of differentiating NPC, while proliferating NPC in the presence of mitogens yielded no influence on BMEC monolayer TEER. Finally, barrier-strengthening effects elicited by NPC were distinguishable from astrocytic induction in terms of both the timing and duration of TEER induction. To our knowledge, this is the first demonstration of the direct influence of NPC on BBB properties of BMEC.
The increase in BMEC TEER was detectable after 24 hours of co-culture with NPC in mitogen-free medium and correlated with a decrease in fluorescein permeability. Since fluorescein is a small molecule that does not appreciably cross the BBB in vivo (Hoffman and Olszewski 1961), these measurements serve as a barometer for the functional impermeability of BBB models. The absolute TEER values (70–120 Ω×cm2) achieved in this study were typical of TEER values reported for other rat and mouse BBB models (de Vries et al. 1996; Perriere et al. 2005; Weidenfeller et al. 2005; Calabria et al. 2006; Kis et al. 2001). Also, as a comparison, the effect of NPC on the BMEC fluorescein permeability (3.3 ± 0.5 × 10−4 cm/min, 33% permeability reduction) was of a similar magnitude to that previously observed upon co-culturing rat BMEC with astrocytes (2.7 × 10−4 cm/min, (Kis et al. 2001; Perriere et al. 2005)). It was possible that the observed effects resulted from a higher BMEC density on the filter membrane. However, NPC did not influence the endothelial cell density and did not yield higher numbers of proliferating BMEC. Therefore, it was concluded that NPC induction of BMEC properties was not simply based on a more tightly packed monolayer, nor was it a result of newly formed BMEC having optimized properties because they were generated in the presence of NPC influences. Instead, the effect correlated with tight junction fidelity as a large fraction of the BMEC possess junctions that are continuous in the presence of NPC (33.5 % frayed, 110 Ω×cm2), while in the absence of NPC cell-cell junctions are predominantly frayed (65.1 % frayed, 75 Ω×cm2). The decrease in frayed BMEC tight junctions has also been previously noted to correlate with higher TEER and lower permeability in BMEC cultures (Weidenfeller et al. 2005; Calabria et al. 2006). Similar to the case with NPC induction, treatment with BBB-inducing glucocorticoids such as corticosterone (21% frayed, 168 Ω×cm2) or hydrocortisone (12% frayed, 218 Ω×cm2) decreases the number of frayed junctions and increases the TEER while also lowering the fluorescein permeability (0.66 × 10−4 cm/min for hydrocortisone) (Weidenfeller et al. 2005; Calabria et al. 2006). Taken together, these results suggest that the improved barrier properties in the presence of NPC are likely a result of improved cell-cell junctional contacts.
Since the BMEC and NPC were separated by a microporous filter membrane and 1 mm of culture medium (Fig. 1), the barrier induction was clearly mediated by soluble factors. The induction was observed in mitogen-free medium as NPC begin differentiating into astrocytes and neurons, but it was not detectable in the presence of mitogens that keep NPC in an undifferentiated, nestin-positive state (Gage 2000; Ostenfeld et al. 2002). This finding suggests that some component of the differentiation process is likely required for BMEC barrier induction and that NPC proliferating in an undifferentiated state do not have a major influence. Although the presence of mitogens themselves might have influenced the integrity of the BMEC monolayer thereby masking the effects of factors released by proliferating, nestin-positive NPC (Sobue et al. 1999), this was not evident from TEER measurements of control BMEC in mitogen-containing medium. The lack of BMEC induction in the presence of 3T3 fibroblasts, proliferating nestin-positive NPC or postnatal astrocytes at 24 hours also indicates that the observed induction with differentiating NPC is not a generic trophic response due to the presence of another proliferating cell type, but instead shows that the properties specific to differentiating NPC are required. When BMEC were cultured in medium that was conditioned by differentiating NPC for 24h, no TEER induction was detected. Therefore, the BMEC presence during the NPC differentiation process was required for the NPC to release BBB-inducing soluble factors, and this finding implicates a bidirectional communication between BMEC and NPC. The requisite interaction between NPC and BMEC was further validated by demonstrating the capacity of co-culture conditioned medium to increase the TEER when serially applied to fresh BMEC monolayers. In order to determine if pre-differentiated NPC lacking early BMEC influences release inductive factors, NPC were pre-differentiated in the absence of mitogens and subsequently added to the BMEC monolayer. After 24h in co-culture, only a slight increase in BMEC TEER was detectable (11% pre-differentiated versus 47% co-differentiating NPC). Subsequent to 24 hours of pre-differentiation in the absence of BMEC, less than 50% of the NPC are undifferentiated (nestin-positive only), likely weakening the effect that differentiating cells can have compared with a situation where co-culture is started with a highly pure population of undifferentiated NPC. These observations support the conclusion that differentiating NPC, rather than differentiated (tubulin- or GFAP-positive) or proliferating (nestin-positive) NPC, stimulate BMEC barrier induction.
NPC proliferate as nestin-positive cells and differentiate into neurons, astrocytes, and oligodendrocytes in mitogen-free conditions (Ostenfeld et al. 2002; Ostenfeld and Svendsen 2004). Thus, NPC-derived astrocytes or neurons could be responsible for the TEER induction. Numerous studies have demonstrated that astrocytes and neurons have the potential to modulate BBB tight junctions, transporter expression, and metabolic activity in vitro and in vivo (Stewart and Wiley 1981; Risau et al. 1986b; Savettieri et al. 2000). Very few βIII tubulin-positive neurons and βIII tubulin/nestin co-positive cells (10% combined) were generated in the presence of BMEC. Also since more neurons are present when NPC are pre-differentiated prior to co-culture but the resulting inductive effect lessened, βIII tubulin-positive neurons do not appear to play a significant role in the observed induction process. Nearly 16% of the NPC-derived cells were positive for both GFAP and nestin, indicating that the second largest population of cells is committed to the astroglial fate but has not yet fully matured. Only 10% were mature astrocytes as defined by GFAP expression. Although astrocytes are strong inducers of BBB properties, the timecourse of TEER induction by NPC indicated that NPC acted earlier while astrocyte effects were more prolonged. Similarly, the pre-differentiated NPC cultures having 25% GFAP-positive cells also exhibited only weak inductive properties after 24 hours (Fig. 2, column 3). Interestingly, the NPC and astrocytes caused the BMEC to reach a similar maximum TEER value indicating a comparable absolute induction capacity of NPC and astrocytes under these experimental conditions, although the dynamics of induction clearly differed. While this study cannot entirely exclude the possibility that NPC-derived astrocytes are providing the inductive signals, these data strongly suggest that differentiating NPC and astrocytes function via distinct induction mechanisms or at the very least, different temporal programs.
In addition to the inductive signals provided by NPC, BMEC also influenced the morphology and differentiation of NPC, further implicating a bidirectional paracrine interaction. The findings of a flattened precursor-like progeny and decreased neuronal production in the presence of primary BMEC corroborate the results of a previous study that employed brain endothelial cell lines or pulmonary artery endothelial cells in embryonic neural stem cell co-cultures (Shen et al. 2004). In addition, this previous study also demonstrated that upon removal of the endothelial cells, neurogenesis was increased (Shen et al. 2004). Other investigations have also implicated endothelial involvement in NPC regulation by showing that endothelial cells assist in the recruitment of newly formed neurons (Louissaint et al. 2002) and stimulate astrocyte precursor differentiation into GFAP- and S100β-expressing mature astrocytes (Mi et al. 2001). It also has been suggested that progenitor contact with microvessels during development favors the astrocyte lineage (Zerlin and Goldman 1997). Finally, NPC are also found in various regions of the adult brain in close proximity to the vasculature in the so-called stem cell niche (Doetsch 2003b), In the adult, neurogenesis occurs in foci closely associated with blood vessels (Palmer et al. 2000). There is also evidence implying that angiogenesis and neurogenesis may be co-regulated since they are stimulated by many of the same factors, such as bFGF, VEGF, IGF-1 and TGF-β̃ In addition, endothelial cells secrete known neuronal differentiation and survival factors (bFGF, IGF-1, VEGF, PDGF, IL8 and BDNF) and a link between angiogenesis and neurogenesis is found in the adult songbird brain during testosterone-induced angiogenesis (Palmer et al. 2000; Jin et al. 2002; Louissaint et al. 2002). Thus, bidirectional BMEC-NPC communication could play important roles in both embryonic development and adult brain plasticity.
The predominant cell types in the developing brain cortex at day E14 are NPC, radial glia, neuroblasts, and neurons (Fig. 6, and references (Bass et al. 1992; Saunders et al. 2000; McCarty et al. 2002; Doetsch 2003a)). These cells are found in close proximity to and in contact with developing brain vessels in vivo. The appearance of BBB endothelial properties in vivo occurs shortly after the blood vessels invade the embryonic brain as endothelial cells begin to thin (Bauer et al. 1993; Stewart and Hayakawa 1994; Bolz et al. 1996), brain vessel permeability decreases and the TEER increases (Risau et al. 1986a; Bauer et al. 1995). These early stages of BBB development take place while astrocytes are scarce in the developing brain (Fig 6, and reference (LeVine and Goldman 1988)). Therefore, it is entirely plausible that brain cells other than astrocytes may be able to induce early BBB properties in brain endothelial cells. In the developing brain environment, the NPC differentiation process could provide the cues necessary for naïve brain EC to acquire initial BBB properties; whereas, in later stages of development, astrocytes would induce further maturation and help maintain BBB properties in differentiated EC (Fig. 7). Accordingly, the observations provided in this study indicate that differentiating NPC may be important for such early onset of BBB properties in the developing embryonic brain, although additional study will be necessary to define the exact physiological impact of the reported BMEC-NPC interactions. Despite the fact that the yields of naïve embryonic brain endothelial cells would be prohibitively low for the study described here, it is intriguing to consider using an in vitro model that employs embryonic BMEC or even stem cell-derived EC to provide additional insight into the process of BBB and NPC co-development.
Acknowledgments
This work was supported by National Institutes of Health grant AA013834. Christian Weidenfeller is a recipient of a Deutsche Forschungsgemeinschaft postdoctoral fellowship (DFG, We 4172/1-1).
Abbreviations used
- BBB
blood-brain barrier
- BMEC
brain microvascular endothelial cells
- bFGF/FGF2
basic fibroblast growth factor
- BrdU
5-Bromo-2′-deoxy-uridine
- DAPI
4',6-Diamidino-2- phenylindoldihydrochloride
- DMEM
Dulbecco's modified Eagle medium
- DIV
days in vitro
- EGF
epidermal growth factor
- FBS
fetal bovine serum
- GFAP
Glial fibrillary acidic protein
- HBSS
Hank's balanced salt solution
- NPC
neural progenitor cells
- PBS
phosphate-buffered saline
- PSA
penicillin-streptomycin-amphotericin
- TEER
transendothelial electrical resistance
- ZO-1
Zonula occluden-1.
References
- Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53:637–644. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Bass T, Singer G, Slusser J, Liuzzi FJ. Radial glial interaction with cerebral germinal matrix capillaries in the fetal baboon. Exp Neurol. 1992;118:126–132. doi: 10.1016/0014-4886(92)90029-p. [DOI] [PubMed] [Google Scholar]
- Bauer H, Sonnleitner U, Lametschwandtner A, Steiner M, Adam H, Bauer HC. Ontogenic expression of the erythroid-type glucose transporter (Glut 1) in the telencephalon of the mouse: correlation to the tightening of the blood-brain barrier. Brain Res Dev Brain Res. 1995;86:317–325. doi: 10.1016/0165-3806(95)00044-e. [DOI] [PubMed] [Google Scholar]
- Bauer HC, Bauer H. Neural induction of the blood-brain barrier: still an enigma. Cell Mol Neurobiol. 2000;20:13–28. doi: 10.1023/a:1006939825857. [DOI] [PubMed] [Google Scholar]
- Bauer HC, Bauer H, Lametschwandtner A, Amberger A, Ruiz P, Steiner M. Neovascularization and the appearance of morphological characteristics of the blood-brain barrier in the embryonic mouse central nervous system. Brain Res Dev Brain Res. 1993;75:269–278. doi: 10.1016/0165-3806(93)90031-5. [DOI] [PubMed] [Google Scholar]
- Bolz S, Farrell CL, Dietz K, Wolburg H. Subcellular distribution of glucose transporter (GLUT-1) during development of the blood-brain barrier in rats. Cell Tissue Res. 1996;284:355–365. doi: 10.1007/s004410050596. [DOI] [PubMed] [Google Scholar]
- Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabria AR, Weidenfeller C, Jones AR, de Vries HE, Shusta EV. Puromycin-purified rat brain microvascular endothelial cell cultures exhibit improved barrier properties in response to glucocorticoid induction. J Neurochem. 2006;97:922–933. doi: 10.1111/j.1471-4159.2006.03793.x. [DOI] [PubMed] [Google Scholar]
- Caley DW, Maxwell DS. Development of the blood vessels and extracellular spaces during postnatal maturation of rat cerebral cortex. J Comp Neurol. 1970;138:31–47. doi: 10.1002/cne.901380104. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003a;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003b;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Dziegielewska KM, Daikuhara Y, Ohnishi T, Waite MP, Ek J, Habgood MD, Lane MA, Potter A, Saunders NR. Fetuin in the developing neocortex of the rat: distribution and origin. J Comp Neurol. 2000;423:373–388. doi: 10.1002/1096-9861(20000731)423:3<373::aid-cne2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314:119–129. doi: 10.1007/s00441-003-0751-z. [DOI] [PubMed] [Google Scholar]
- Enkvist MO, Hamalainen H, Jansson CC, Kukkonen JP, Hautala R, Courtney MJ, Akerman KE. Coupling of astroglial alpha 2-adrenoreceptors to second messenger pathways. J Neurochem. 1996;66:2394–2401. doi: 10.1046/j.1471-4159.1996.66062394.x. [DOI] [PubMed] [Google Scholar]
- Ferguson RK, Woodbury DM. Penetration of 14C-inulin and 14C-sucrose into brain, cerebrospinal fluid, and skeletal muscle of developing rats. Exp Brain Res. 1969;7:181–194. doi: 10.1007/BF00239028. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol. 1997;273:E207–E213. doi: 10.1152/ajpendo.1997.273.1.E207. [DOI] [PubMed] [Google Scholar]
- Hoffman HJ, Olszewski J. Spread of sodium fluorescein in normal brain tissue. A study of the mechanism of the blood-brain barrier. Neurology. 1961;11:1081–1085. doi: 10.1212/wnl.11.12.1081. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE. Ontogeny and phylogeny of the blood-brain barrier. In: Neuwelt EA, editor. Implications of the blood-brain barrier and its manipulation. New York: Plenum; 1989. [Google Scholar]
- Kis B, Deli MA, Kobayashi H, Abraham CS, Yanagita T, Kaiya H, Isse T, Nishi R, Gotoh S, Kangawa K, Wada A, Greenwood J, Niwa M, Yamashita H, Ueta Y. Adrenomedullin regulates blood-brain barrier functions in vitro. Neuroreport. 2001;12:4139–4142. doi: 10.1097/00001756-200112210-00055. [DOI] [PubMed] [Google Scholar]
- Kniesel U, Risau W, Wolburg H. Development of blood-brain barrier tight junctions in the rat cortex. Brain Res Dev Brain Res. 1996;96:229–240. doi: 10.1016/0165-3806(96)00117-4. [DOI] [PubMed] [Google Scholar]
- Krizbai IA, Deli MA. Signalling pathways regulating the tight junction permeability in the blood-brain barrier. Cell Mol Biol (Noisy-le-grand) 2003;49:23–31. [PubMed] [Google Scholar]
- LeVine SM, Goldman JE. Embryonic divergence of oligodendrocyte and astrocyte lineages in developing rat cerebrum. J Neurosci. 1988;8:3992–4006. doi: 10.1523/JNEUROSCI.08-11-03992.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, Shani M, Dvorak HF, Wolburg H, Bader BL, Dvorak AM, Hynes RO. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol Cell Biol. 2002;22:7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Haeberle H, Barres BA. Induction of astrocyte differentiation by endothelial cells. J Neurosci. 2001;21:1538–1547. doi: 10.1523/JNEUROSCI.21-05-01538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nico B, Quondamatteo F, Herken R, Marzullo A, Corsi P, Bertossi M, Russo G, Ribatti D, Roncali L. Developmental expression of ZO-1 antigen in the mouse blood-brain barrier. Brain Res Dev Brain Res. 1999;114:161–169. doi: 10.1016/s0165-3806(99)00008-5. [DOI] [PubMed] [Google Scholar]
- Ostenfeld T, Svendsen CN. Requirement for neurogenesis to proceed through the division of neuronal progenitors following differentiation of epidermal growth factor and fibroblast growth factor-2-responsive human neural stem cells. Stem Cells. 2004;22:798–811. doi: 10.1634/stemcells.22-5-798. [DOI] [PubMed] [Google Scholar]
- Ostenfeld T, Joly E, Tai YT, Peters A, Caldwell M, Jauniaux E, Svendsen CN. Regional specification of rodent and human neurospheres. Brain Res Dev Brain Res. 2002;134:43–55. doi: 10.1016/s0165-3806(01)00291-7. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Perriere N, Demeuse P, Garcia E, Regina A, Debray M, Andreux JP, Couvreur P, Scherrmann JM, Temsamani J, Couraud PO, Deli MA, Roux F. Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. J Neurochem. 2005;93:279–289. doi: 10.1111/j.1471-4159.2004.03020.x. [DOI] [PubMed] [Google Scholar]
- Ramsauer M, Krause D, Dermietzel R. Angiogenesis of the blood-brain barrier in vitro and the function of cerebral pericytes. Faseb J. 2002;16:1274–1276. doi: 10.1096/fj.01-0814fje. [DOI] [PubMed] [Google Scholar]
- Risau W, Hallmann R, Albrecht U. Differentiation-dependent expression of proteins in brain endothelium during development of the blood-brain barrier. Dev Biol. 1986a;117:537–545. doi: 10.1016/0012-1606(86)90321-0. [DOI] [PubMed] [Google Scholar]
- Risau W, Hallmann R, Albrecht U, Henke-Fahle S. Brain induces the expression of an early cell surface marker for blood-brain barrier-specific endothelium. Embo J. 1986b;5:3179–3183. doi: 10.1002/j.1460-2075.1986.tb04627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders NR, Knott GW, Dziegielewska KM. Barriers in the immature brain. Cell Mol Neurobiol. 2000;20:29–40. doi: 10.1023/a:1006991809927. [DOI] [PubMed] [Google Scholar]
- Savettieri G, Di Liegro I, Catania C, Licata L, Pitarresi GL, D'Agostino S, Schiera G, De Caro V, Giandalia G, Giannola LI, Cestelli A. Neurons and ECM regulate occludin localization in brain endothelial cells. Neuroreport. 2000;11:1081–1084. doi: 10.1097/00001756-200004070-00035. [DOI] [PubMed] [Google Scholar]
- Schulze C, Firth JA. Interendothelial junctions during blood-brain barrier development in the rat: morphological changes at the level of individual tight junctional contacts. Brain Res Dev Brain Res. 1992;69:85–95. doi: 10.1016/0165-3806(92)90125-g. [DOI] [PubMed] [Google Scholar]
- Segovia J, Lawless GM, Tillakaratne NJ, Brenner M, Tobin AJ. Cyclic AMP decreases the expression of a neuronal marker (GAD67) and increases the expression of an astroglial marker (GFAP) in C6 cells. J Neurochem. 1994;63:1218–1225. doi: 10.1046/j.1471-4159.1994.63041218.x. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Sobue K, Yamamoto N, Yoneda K, Hodgson ME, Yamashiro K, Tsuruoka N, Tsuda T, Katsuya H, Miura Y, Asai K, Kato T. Induction of blood-brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci Res. 1999;35:155–164. doi: 10.1016/s0168-0102(99)00079-6. [DOI] [PubMed] [Google Scholar]
- Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Dev Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- Stewart PA, Hayakawa K. Early ultrastructural changes in blood-brain barrier vessels of the rat embryo. Brain Res Dev Brain Res. 1994;78:25–34. doi: 10.1016/0165-3806(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Tontsch U, Bauer HC. Glial cells and neurons induce blood-brain barrier related enzymes in cultured cerebral endothelial cells. Brain Res. 1991;539:247–253. doi: 10.1016/0006-8993(91)91628-e. [DOI] [PubMed] [Google Scholar]
- Weidenfeller C, Schrot S, Zozulya A, Galla HJ. Murine brain capillary endothelial cells exhibit improved barrier properties under the influence of hydrocortisone. Brain Res. 2005;1053:162–174. doi: 10.1016/j.brainres.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Nakashima K, Summers RG, Toni N, D'Amour KA, Lie DC, Gage FH. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature. 2004;430:350–356. doi: 10.1038/nature02604. [DOI] [PubMed] [Google Scholar]
- Zerlin M, Goldman JE. Interactions between glial progenitors and blood vessels during early postnatal corticogenesis: blood vessel contact represents an early stage of astrocyte differentiation. J Comp Neurol. 1997;387:537–546. doi: 10.1002/(sici)1096-9861(19971103)387:4<537::aid-cne5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]