Abstract
Schaffer collateral synapses in hippocampus show target-cell specific short-term plasticity. Using GFP-expressing Inhibitory Neuron (GIN) transgenic mice that express enhanced green fluorescent protein (EGFP) in a subset of somatostatin-containing interneurons (SOM interneurons), we previously showed that Schaffer collateral synapses onto SOM interneurons in stratum (S.) radiatum have unusually large (up to 6-fold) paired-pulse facilitation. This results from a low initial release probability and the enhancement of facilitation by synaptic activation of presynaptic kainate receptors. Here we further investigate the properties of these kainate receptors and examine their effects on short-term facilitation during physiologically derived stimulation patterns, using excitatory postsynaptic currents recorded in S. radiatum interneurons during Schaffer collateral stimulation in acute slices from juvenile GIN mice. We find that GluR5 and GluR6 antagonists decrease short-term facilitation at Schaffer collateral synapses onto SOM interneurons with no additive effects, suggesting that the presynaptic kainate receptors are heteromers containing both GluR5 and GluR6 subunits. The calcium-permeable receptor antagonist 1-napthyl acetyl spermine (NASPM) both mimics and occludes the effect of the kainate receptor antagonists, indicating that the presynaptic kainate receptors are calcium permeable. Furthermore, Schaffer collateral synapses onto SOM interneurons show up to 11-fold short-term facilitation during physiologically derived stimulus patterns, in contrast to other interneurons that have less than 1.5-fold facilitation. Blocking the kainate receptors reduces facilitation in SOM interneurons by ∼50% during the physiologically derived patterns and reduces the dynamic range. Activation of calcium-permeable kainate receptors containing GluR5/GluR6 causes a dramatic increase in short-term facilitation during physiologically derived stimulus patterns, a mechanism likely to be important in regulating the strength of Schaffer collateral synapses onto SOM interneurons in vivo.
INTRODUCTION
A delicate balance between excitatory and inhibitory synaptic transmission is crucial for the correct functioning of neuronal circuits. It is important to understand mechanisms regulating both excitatory and inhibitory neurons, including the excitatory synapses that drive these neurons to fire action potentials. Because the firing of a single interneuron can have a powerful influence on the overall properties of the hippocampal network (Cobb et al. 1995), the strength and dynamics of excitatory synapses onto inhibitory interneurons can have a profound influence on the magnitude and spatial properties of inhibition.
The strength of excitatory synapses is not static, but it changes during different patterns of activity as a result of short-term plasticity. Short-term plasticity is often target-cell specific (reviewed in Pelkey and McBain 2007; Thomson 2003; Toth and McBain 2000), where the same type of axon has different dynamic properties onto excitatory versus inhibitory neurons (e.g., Koester and Johnston 2005; Markram et al. 1998; Scanziani et al. 1998; Thomson 1997) and different subtypes of inhibitory neurons (e.g., Ali et al. 1998; Losonczy et al. 2002; Reyes et al. 1998; Thomson et al. 2002). In hippocampus, Schaffer collateral axons from CA3 pyramidal cells provide excitatory input to both CA1 pyramidal cells and feed-forward interneurons in stratum (S.) radiatum (Freund and Buzsaki 1996). In general, Schaffer collateral synapses onto s. radiatum interneurons have less short-term facilitation than synapses onto CA1 pyramidal cells (Sun et al. 2005). However, interneurons are heterogeneous in nature (Freund and Buzsaki 1996; Parra et al. 1998; Somogyi and Klausberger 2005), and s. radiatum interneurons exhibit heterogeneity in the short-term plasticity of their Schaffer collateral inputs (Sun et al. 2005).
While most s. radiatum interneurons have little or no short-term facilitation, a subset of s. radiatum interneurons containing the neuropeptide somatostatin is unusual in having extremely large short-term facilitation, even larger than at Schaffer collateral synapses onto CA1 pyramidal cells (Sun and Dobrunz 2006). These interneurons, which we refer to as SOM interneurons, can be targeted for study using the GFP-expressing Inhibitory Neuron (GIN) line of transgenic mice (Oliva et al. 2000). The large short-term facilitation at Schaffer collateral synapses onto SOM interneurons is caused by a low initial release probability and by synaptic activation of presynaptic kainate receptors that increases release probability on subsequent pulses (Sun and Dobrunz 2006). It is similar to the large short-term facilitation observed at mossy fiber synapses onto CA3 pyramidal cells, which also contain presynaptic kainate autoreceptors (Contractor et al. 2001; Lauri et al. 2001b; Schmitz et al. 2001). At Schaffer collateral synapses, however, presynaptic kainate receptors that increase glutamate release and enhance short-term facilitation are specific to synapses onto SOM interneurons and are not found at Schaffer collateral synapses onto CA1 pyramidal cells or other s. radiatum interneurons (Sun and Dobrunz 2006).
The subunit composition of kainate receptors affects their physiological properties and enables specialized roles in regulating synaptic transmission (reviewed in Lerma 2006; Pinheiro and Mulle 2006). In the hippocampus, GluR5- and GluR6-containing kainate receptors have been shown to play an active role in the regulation of synaptic transmission (Bortolotto et al. 1999; Bureau et al. 1999; Clarke and Collingridge 2002; Contractor et al. 2001; Lauri et al. 2001b, 2003; Partovi and Frerking 2006; Sun and Dobrunz 2006; Vignes et al. 1998). We have previously shown that the GluR6 subunit is involved in the large facilitation of Schaffer collateral synapses onto SOM interneurons (Sun and Dobrunz 2006) as has been reported for mossy fiber synapses in CA3 (Contractor et al. 2001). Studies have also indicated that presynaptic kainate receptors containing the GluR5 subunit can increase glutamate release and regulate short-term plasticity at some synapses (Campbell et al. 2007; Lauri et al. 2001b). In addition, GluR5 and GluR6 can form functional heteromeric kainate receptors, which have different properties than homomeric receptors (Cui and Mayer 1999; Mulle et al. 2000; Paternain et al. 2000). However, it is not known whether kainate receptors containing the GluR5 subunit contribute to the large facilitation of Schaffer collateral synapses onto SOM interneurons.
The underlying mechanism by which presynaptic kainate receptors enhance glutamate release is unclear. Kainate receptors containing unedited GluR5 and/or GluR6 subunits are calcium permeable (reviewed in Lerma 2003). An influx of calcium through presynaptic calcium-permeable kainate receptors would cause an increase in presynaptic calcium, resulting in an increase in the release probability per synaptic vesicle. This would increase glutamate release and enhance short-term facilitation (Dobrunz 2002); this mechanism was predicted by our previous mathematical modeling (Sun and Dobrunz 2006). We therefore tested whether the kainate receptors involved in the large short-term facilitation at Schaffer collateral synapses onto SOM interneurons are calcium permeable.
While presynaptic kainate receptors increase short-term facilitation during simple stimulus paradigms (Sun and Dobrunz 2006), it is not known to what extent they modulate facilitation during temporally complex stimulus patterns such as Schaffer collateral synapses receive in vivo. The strength of Schaffer collateral synapses onto CA1 pyramidal cells varies over a wide dynamic range during physiologically derived stimulus patterns taken from in vivo recordings of hippocampal firing patterns (Dekay et al. 2006; Dobrunz and Stevens 1999; Klyachko and Stevens 2006; Speed and Dobrunz 2008). These patterns, which contain high-frequency bursts separated by long intervals with little or no activity (e.g., Fenton and Muller 1998), are referred to as natural stimulus patterns (NSPs) to reflect their physiological origin (Dobrunz and Stevens 1999). It is not known how Schaffer collateral synapses onto SOM interneurons respond during stimulation with NSPs or how much of an effect the activation of presynaptic kainate receptors has on this form of short-term plasticity or on the dynamic range over which these synapses operate.
Here we investigate the subunit composition and calcium permeability of the presynaptic kainate receptors involved in short-term facilitation at Schaffer collateral synapses onto SOM interneurons and explore their role in short-term facilitation in response to physiologically derived NSPs. We find that heteromeric kainate receptors containing both GluR5 and GluR6 are involved in the large short-term facilitation at these synapses and show that these receptors are calcium permeable. Furthermore, we show that Schaffer collateral synapses onto SOM interneurons have extremely large (≤11-fold) facilitation in response to temporally complex natural pattern stimulation and demonstrate that activation of presynaptic calcium-permeable kainate receptors is one important mechanism underlying this facilitation.
METHODS
Slice preparation
GIN transgenic mice (11–16 days old) that express EGFP in a subset of interneurons containing somatostatin (Oliva et al. 2000) were anesthetized with isoflurane and decapitated, and their brains were removed rapidly. Coronal slices of the brain, 400 μm thick, were cut using an oscillating tissue slicer (EMS-4000; Electron Microscopy Sciences, Fort Washington, PA) or a vibrating microtome (VT1000S; Leica, Bannockburn, IL). Slicing and dissection of the hippocampi were done in ice-cold (1–3°C) dissecting solution containing the following (in mM): 120 NaCl, 3.5 KCl, 0.7 CaCl2, 4.0 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose, bubbled with 95% O2-5% CO2, pH 7.35–7.45. Slices were stored at room temperature in a holding chamber containing the dissecting solution and bubbled with 95% O2-5% CO2 for ≥1 h before recording.
Electrophysiology
During the experiment, slices were held in a submersion recording chamber perfused with external recording solution composed of the following (in mM): 120 NaCl, 3.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose. The solution was bubbled with 95% O2-5% CO2, pH 7.35–7.45. Picrotoxin (100 μM) was added to the external solution to block inhibitory synaptic responses mediated by GABAA receptors; the CA3 region of hippocampus was removed to prevent recurrent excitation. The solution also contained 100 μM d-2-amino-5-phosphonopentanoic acid (APV) to block N-methyl-d-aspartate (NMDA) receptor-mediated currents and prevent postsynaptic short-term plasticity as well as to prevent long-term potentiation (LTP) and long-term depression (LTD). All experiments were performed at room temperature (∼24°C). APV, 6,7,8,9-tetrahydro-5-nitro-1H-benz(g)indole-2,3-dione 3-oxime (NS-102), 1-napthyl acetyl spermine (NASPM), and philanthotoxin-433 are from Sigma-Aldrich (St. Louis, MO); (S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxybenzyl)pyrimidine-2,4-dione (UBP302) and 4,6-bis(benzoylamino)-1,3-benzenedicarboxylic acid (NS3763) are from Tocris Bioscience (Ellisville, MO); 3S,4aR,6S,8aR)-6-(4-carboxyphenyl) methyl-1,2,3,4,4a,5, 6,7,8,8a-decahydroisoquinoline-3-carboxylic acid (LY382884) is from Eli Lilly (Indianapolis, IN).
Interneurons expressing EGFP (which are a subset of SOM interneurons) and non-EGFP interneurons were identified visually in CA1 using infrared differential inference contrast optics and epifluorescent optics on a Nikon (New York) E600FN upright microscope. Interneurons recorded had somata located in s. radiatum. Targeted neurons were patched in the voltage-clamp configuration and recorded at a holding potential of −60 mV using an Axopatch 200B amplifier (Molecular Devices, Union City, CA). Patch electrodes (3–4.5MΩ) were filled with internal solution composed of the following (in mM): 100 Cs-gluconate, 0.6 EGTA, 5.0 MgCl2, and 40 HEPES, pH was adjusted to 7.2 with CsOH. The internal solution also contained 10 mM BAPTA to block interneuron LTP and LTD (Laezza et al. 1999) and to inhibit postsynaptic calcium-mediated effects of G-protein-coupled receptors and prevent postsynaptic short-term plasticity; N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium chloride (QX-314; 5 mM) to improve space clamp and reduce nonlinear effects caused by voltage-gated channels in dendrites while recording from the soma; 10 mM ATP was added to partially chelate intracellular spermine and spermidine and prevent possible postsynaptic short-term plasticity at postsynaptic calcium-permeable AMPA receptors (Bahring et al. 1997; McBain 1998; Rozov and Burnashev 1999; Toth et al. 2000). The access resistance and holding current (<200 pA) were monitored continuously. Recordings were rejected if either access resistance or holding current increased ∼20% during the experiment.
Excitatory postsynaptic currents (EPSCs) were recorded in response to extracellular stimulation of Schaffer collateral axons by a bipolar tungsten microelectrode (FHC, Bowdoinham, ME) placed in s. radiatum. Stimulation was generated from a Master-8-cp stimulator (AMPI, Jerusalem, Israel) and applied with a BSI-2 biphasic stimulus isolator (BAK Electronics, Mount Airy, MD).
During the recording, the stimulating electrode was positioned and the stimulus strength adjusted (30–50 μA for a 100 μs pulse) to produce a single-peak EPSC with fixed latency. There were two stimulation patterns used in the experiments: 1) paired-pulse stimulation with different intervals (in ms: 20, 40, 80, 200, and 500), applied in a random sequence and repeated 10 times at 0.1 Hz. The averaged paired-pulse ratio (EPSC2/EPSC1) was calculated after recording; and 2) NSP. As described in Dobrunz and Stevens (1999), the stimulus patterns were taken from the timing of action potentials recorded in vivo from hippocampal place cells of awake, freely moving rats. These spike patterns were generously provided by Dr. Robert Muller (see Fenton and Muller 1998 for details of the recording methods). We refer to the stimulation patterns used here as NSPs because they more closely reflect the temporal patterns of input that hippocampal synapses actually receive. Details of the methods for the NSP experiments are described in Dekay et al. (2006) and Dobrunz and Stevens (1999). In each experiment, the same pattern of 46 points was presented three to seven times, separated by control sections of 14 points at a slow constant frequency (0.1 Hz). The data for each cell are normalized by the average value of the last 8 points of the control period. The stimulus amplitude and duration were held constant during each experiment.
Statistical analysis
Data are presented as means ± SE. Except where noted, statistical comparisons were made using paired t-test with P < 0.05 considered significant. Where noted, statistical comparisons were made using the Student's t-test or one-way ANOVA, with P < 0.05 considered significant. In figures, * indicates a statistically significant difference.
RESULTS
GluR5 involvement in the facilitation at Schaffer collateral synapses onto EGFP-expressing SOM interneurons
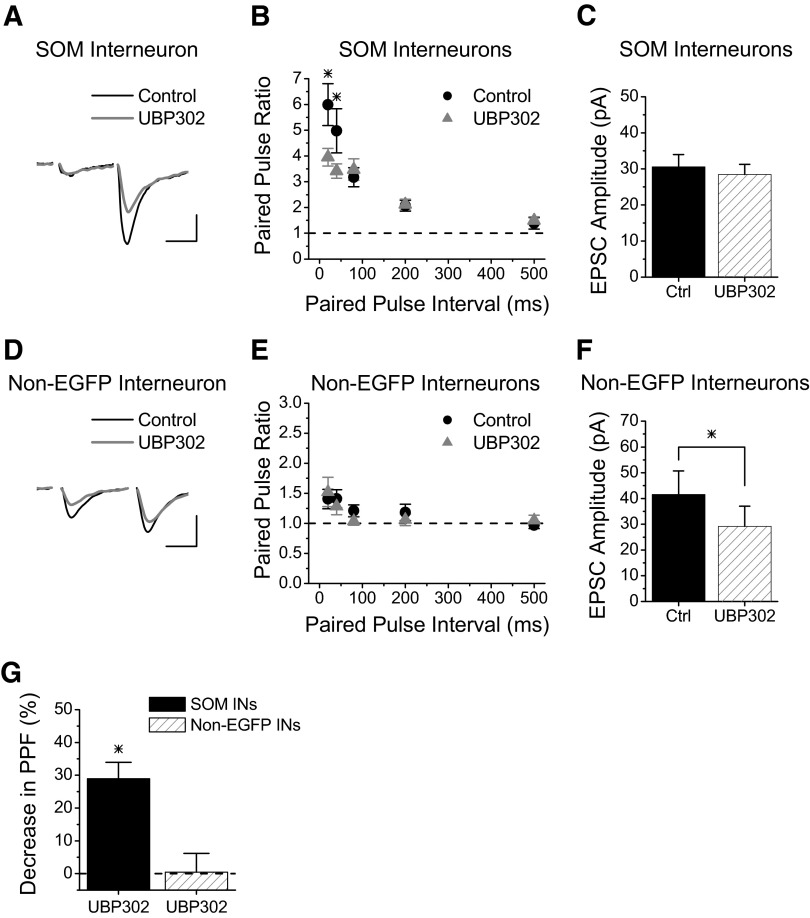
We first tested whether GluR5 containing kainate receptors also contribute to the large short-term facilitation seen at Schaffer collateral synapses onto SOM interneurons, as has been shown to occur with GluR6 containing kainate receptors (Sun and Dobrunz 2006). Figure 1 A shows examples of paired-pulse EPSCs (40-ms interval) recorded in an EGFP-expressing SOM interneuron before (black line) and during (gray line) the application of UBP302 (15 μM), a GluR5 selective kainate receptor antagonist (More et al. 2004; Partovi and Frerking 2006). UBP302 reduced the amount of paired-pulse facilitation (PPF), although the remaining facilitation was still large. Group results (Fig. 1B) show that the reduction in paired-pulse facilitation was significant at short paired-pulse intervals (20–40 ms, P < 0.05, n = 13). However, there was no effect on the size of the first EPSC (Fig. 1C, P > 0.5), indicating that the effect of UBP302 was presynaptic. The effect was completely reversed on drug washout (data not shown). The involvement of GluR5 containing kainate receptors was further confirmed with another specific GluR5 antagonist, LY382884 (10 μM) (Bortolotto et al. 1999; Lauri et al. 2001b), which also caused a reduction in paired-pulse facilitation at the 20- and 40-ms intervals (P < 0.05, n = 4, data not shown). The magnitude of the decrease in facilitation (combining the 20- and 40-ms intervals) was not different between the two GluR5-specific antagonists (29.0 ± 4.9% UPB302 vs. 31.7 ± 8.8% LY382884, Student's t-test, P > 0.7). These data indicate that paired-pulse facilitation is enhanced by synaptically released glutamate activating kainate autoreceptors that contain GluR5.
FIG. 1.
GluR5 is involved in Schaffer collateral synapse facilitation onto SOM interneurons. A: Example of EPSCs recorded from an s. radiatum EGFP-expressing SOM interneuron before and during application of the GluR5-specific kainate receptor antagonist UBP302 (15 μM). Scale bars: 20 ms, 60 pA. B: Group results for paired-pulse ratios versus interval from EGFP-expressing SOM interneurons before (black circles) and during application of UBP302 (gray triangles) (n = 13). UBP302 caused a significant decrease in paired-pulse facilitation for intervals from 20 to 40 ms. C: Average amplitude of first EPSC for experiments in B was not different in UBP302. D: Example of EPSCs recorded from an s. radiatum non-EGFP interneuron before and during application of 15 μM UBP302. Scale bars: 20 ms, 35 pA. E: Group results for paired-pulse ratios versus interval from non-EGFP interneurons before (black circles) and during application of UBP302 (gray triangles) (n = 7) show no change in paired-pulse facilitation. F: Average amplitude of first EPSC for experiments in E. G: Percent decrease in paired-pulse facilitation with UBP302 at 20 ms and 40 ms paired-pulse intervals (combined) for each cell type. * = significant, P < 0.05.
The effect is specific for Schaffer collateral synapses onto SOM interneurons, with no effect of UBP302 on paired-pulse facilitation at Schaffer collateral synapses onto non-EGFP interneurons (Fig. 1, D and E, P > 0.7, n = 7). Interestingly, UBP302 caused a reduction in the amplitude of the first EPSC in non-EGFP interneurons (Fig. 1F, P < 0.05, n = 7). This most likely reflects the block of postsynaptic kainate receptors because postsynaptic kainate receptors containing GluR5 have been found on some CA1 interneurons (Clarke et al. 1997; Cossart et al. 1998; Mulle et al. 2000). Figure 1G shows the average percentage decrease in PPF (combining the 20- and 40-ms intervals) in response to UBP302, which shows a ∼30% decrease for SOM interneurons and no change for non-EGFP interneurons. These data are consistent with our previous results that showed that the kainate receptors that caused a reduction in paired-pulse facilitation are only present at Schaffer collateral inputs onto SOM interneurons (Sun and Dobrunz 2006). Therefore the GluR5- and GluR6-containing kainate receptors that enhance short-term facilitation are target-cell specific.
Kainate receptors that increase short-term facilitation at synapses onto EGFP-expressing SOM interneurons are heteromeric
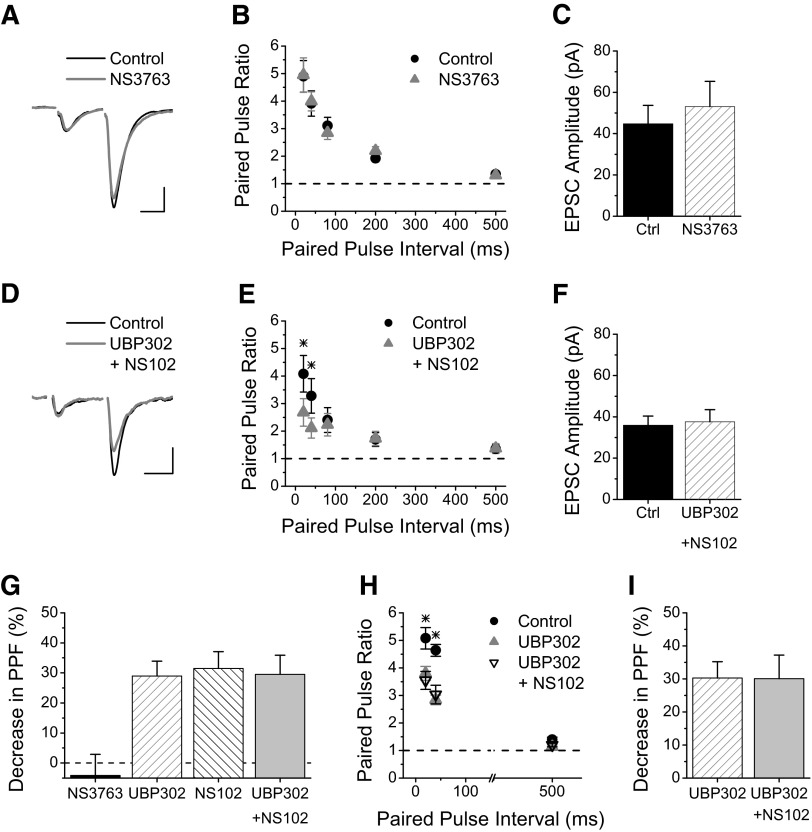
Kainate receptors containing the GluR5 subunit can either be homomeric or heteromeric. We tested if GluR5 homomeric receptors contribute to the facilitation at this synapse through application of NS3763 (10 μM), which is a specific antagonist for homomeric GluR5 kainate receptors (Christensen et al. 2004a,b; Partovi and Frerking 2006). NS3763 had no effect on the paired-pulse facilitation of Schaffer collateral synapses onto EGFP-expressing SOM interneurons (Fig. 2, A and B), indicating that the GluR5-containing kainate receptors that enhance facilitation at these synapses are not homomeric and therefore must be heteromeric. Because our previous results showed an involvement of GluR6-containing kainate receptors (Sun and Dobrunz 2006), and the results from Fig. 1 show GluR5 involvement, this suggests that Schaffer collateral synapses targeting SOM interneurons either contain two populations of kainate receptors (some with GluR5 and others with GluR6) or contain heteromeric GluR5/6 kainate receptors.
FIG. 2.
Kainate receptors at Schaffer collateral synapses onto SOM interneurons are heteromeric and contain GluR5/GluR6. A: Example of EPSCs recorded from an s. radiatum EGFP-expressing SOM interneuron before and during application of the GluR5-specific homomeric kainate receptor antagonist NS3763 (10 μM). Scale bars: 20 ms, 50 pA. B: Group results for paired-pulse ratios versus interval from EGFP-expressing SOM interneurons before (black circles) and during application of NS3763 (gray triangles) (n = 7). Blockade of homomeric GluR5 receptors had no effect on paired-pulse facilitation at Schaffer collateral synapses onto SOM interneurons. C: Average amplitude of first EPSC for experiments in B was not different in NS3763. D: Example traces of EPSCs recorded from an s. radiatum EGFP-expressing SOM interneuron before and during application of UBP302 (15 μM) and NS102 (20 μM), to block all kainate receptors containing GluR5 and GluR6. Scale bars: 20 ms, 20 pA. E: Group results for paired-pulse ratios showing that blockade of GluR5 and GluR6 containing kainate receptors with co-application of UBP302 and NS102 reduces paired-pulse facilitation in SOM interneurons (n = 12). The combination of UBP302 and NS102 causes a significant decrease in the paired-pulse facilitation for short intervals. F: Average amplitude of first EPSC for experiments in E. G: Percent decrease in paired-pulse facilitation in SOM interneurons (combining the 20 ms and 40 ms intervals) for NS3763 (n = 7), UPB302 (n = 13), NS102 (n = 6), and UBP302 plus NS102 (n = 12). No additive effect is seen for UBP302+NS102. H, Group results for paired-pulse facilitation with UBP302 versus UBP302+NS102 (n = 9). Order of application: Control (no drug, black circles), UBP302 alone (gray triangles), co-application of UBP302 and NS102 (black open triangles). While both UBP302 and UBP302+NS102 have less paired-pulse facilitation compared with Control at 20 and 40 ms, they are not different from each other. I, Percent decrease in paired-pulse facilitation in SOM interneurons (combining 20 ms and 40 ms intervals) for UBP302 versus UBP302+NS102 (from experiments in H) are not different. * = significant, P < 0.05.
If the GluR5 and GluR6 subunits are different populations of kainate receptors, then blocking both subunits should cause a greater reduction of short-term facilitation compared with blockade of only one of the subunit containing kainate receptors. To test this possibility, we applied UBP302 (15 μM) together with NS102 (20 μM), an antagonist for GluR6 containing kainate receptors (Chittajallu et al. 1999). The combined drugs caused a significant reduction in the paired-pulse facilitation at the 20- and 40-ms intervals (Fig. 2, D and E, P < 0.05, n = 12), with no change in the amplitude of the first response (Fig. 2F, P > 0.35). However, the effect of UBP302 plus NS102 (Fig. 2E) was not greater than the effect seen with either UBP302 alone (Fig. 1B) or our previous data with NS102 alone (Sun and Dobrunz 2006). Figure 2G shows that the average magnitude of the reduction in the paired-pulse facilitation (combining the 20- and 40-ms intervals) is the same for UPB302 (from Fig. 2B), NS102 (from Sun et al. 2006), and UBP302 plus NS102 (from Fig. 2E, one-way ANOVA, P > 0.9). Because there was no additive effect of the GluR5 and GluR6 antagonists, this suggests that the large facilitation seen at Schaffer collateral synapses onto EGFP-expressing SOM interneurons is due to heteromeric GluR5/6 kainate receptors rather than separate populations of GluR5 kainate receptors and GluR6 kainate receptors. To confirm this, we tested whether UBP302 occluded a further effect of NS102, focusing on the shorter intervals (20 and 40 ms) where kainate receptors are involved (Fig. 2H). Although PPF was significantly reduced (compared with control) in both UBP302 and UBP302+NS102 (one-way ANOVA, P < 0.05), there was no further reduction in PPF caused by the addition of NS102 (Fig. 2I, one-way ANOVA, P > 0.8). Together, our results show that the presynaptic kainate receptors that contribute to the large short-term facilitation at Schaffer collateral synapses onto SOM interneurons are GluR5/GluR6 heteromers.
Kainate receptors that increase short-term facilitation at Schaffer collateral synapses onto SOM interneurons are calcium permeable
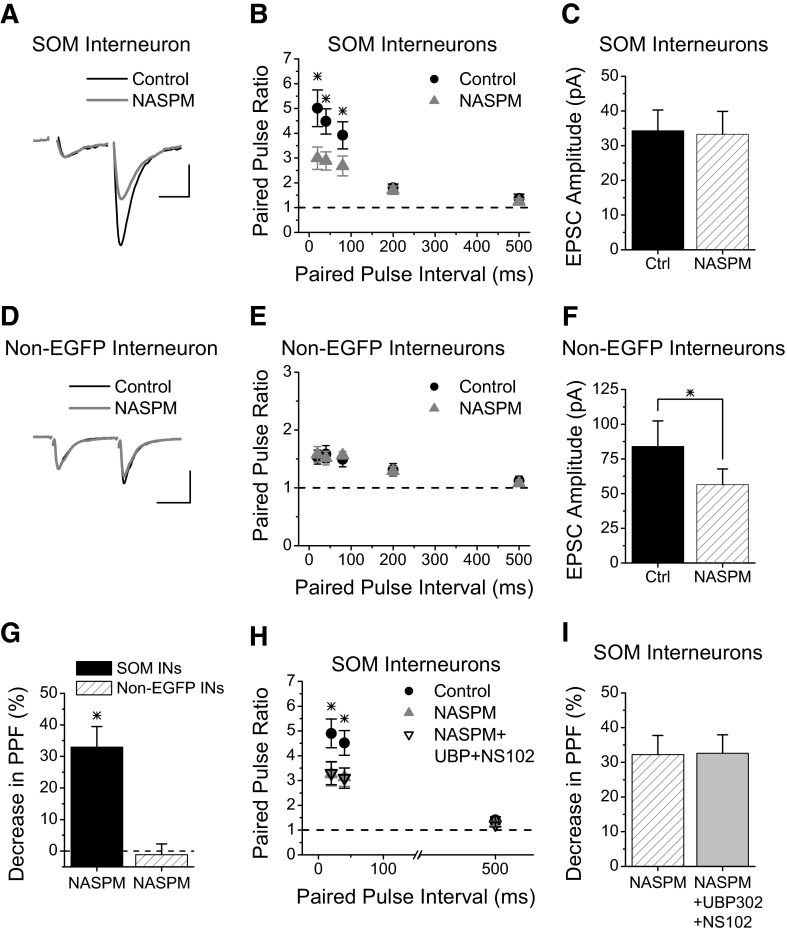
We next tested whether the kainate receptors that increase short-term facilitation at Schaffer collateral synapses onto SOM interneurons are calcium permeable. Application of NASPM (200 μM), an antagonist of calcium-permeable AMPA and kainate receptors (Koike et al. 1997), caused a decrease in paired-pulse facilitation at short intervals (Fig. 3, A and B). The reduction in paired-pulse facilitation with NASPM is not due to an effect at postsynaptic calcium-permeable AMPA receptors, since there is no change in the size of the first EPSC (Fig. 3C, P > 0.4). We also saw a reduction in paired-pulse facilitation at synapses onto SOM interneurons using Philanthotoxin-433 (5 μM) (Supplemental Fig. S1),1 another antagonist of calcium-permeable kainate and AMPA receptors (Bahring and Mayer 1998; Washburn and Dingledine 1996). As with the NASPM, the Philanthotoxin-433 had no effect on the size of the first EPSC (Supplemental Fig. S1C), indicating that these cells do not have postsynaptic calcium-permeable AMPA receptors. Together, these data show that Schaffer collateral synapses onto SOM interneurons have calcium-permeable kainate receptors that enhance their paired-pulse facilitation.
FIG. 3.
Presynaptic kainate receptors are calcium permeable at Schaffer collateral synapses onto SOM interneurons. A: Example of EPSCs recorded from an s. radiatum EGFP-expressing SOM interneuron before and during application of NASPM (200 μM), an antagonist of calcium-permeable AMPA and kainate receptors. Scale bars: 20 ms, 30 pA. B: Group results for paired-pulse ratios showing that blockade of calcium-permeable kainate receptors with application of NASPM reduces paired-pulse facilitation in SOM interneurons at short intervals (n = 12). C: Average amplitude of first EPSC for experiments in B. D: Example of EPSCs recorded from an s. radiatum non-EGFP-expressing interneuron before and during application of NASPM (200 μM). Scale bars: 20 ms, 50 pA. E: Group results for paired-pulse ratios versus interval from non-EGFP-expressing interneurons before and during application of NASPM (n = 10). NASPM caused no change in the paired-pulse facilitation. F: Average amplitude of first EPSC for experiments in E. G: Percent decrease in paired-pulse facilitation with NASPM at 20 ms and 40 ms paired-pulse intervals (combined) for both cell types. H, Group results for paired-pulse ratios versus interval from SOM interneurons for NASPM versus NASPM+UBP302+NS102. Order of application: Control (no drug, black circles), NASPM alone (gray triangles), co-application of NASPM with UBP302 and NS102 (black open triangles). While both NASPM and NASPM+UBP302+NS102 have less paired pulse facilitation compared with Control at 20 and 40 ms, they are not different from each other. I, Percent decrease in paired-pulse facilitation in SOM interneurons (combining 20 ms and 40 ms intervals) for NASPM versus NASPM+UBP302+NS102 (from experiments in H) are not different. * = significant, P < 0.05.
We previously showed that the effect of presynaptic kainate receptors to increase release probability and enhance short-term facilitation is specific to Schaffer collateral synapses onto SOM interneurons with no effect at Schaffer collateral synapses onto non-EGFP interneurons or pyramidal cells (Sun and Dobrunz 2006). Here we confirm that non-EGFP-expressing interneurons do not use this novel mechanism of calcium influx through presynaptic calcium-permeable kainate receptors to increase their facilitation. Figure 3, D and E, show that NASPM does not alter paired-pulse facilitation at Schaffer collateral synapses onto non-EGFP interneurons. NASPM does cause a significant decrease in the average size of the first EPSC in non-EGFP interneurons, as shown in Fig. 3F (n = 10, P < 0.05), indicating that these cells do have postsynaptic calcium-permeable AMPA receptors. The effect of NASPM on the first EPSC amplitude was variable; NASPM caused a large reduction in the first EPSC size in a subgroup of cells and no effect in the others (Supplemental Fig. S2, A and C), consistent with the fact that calcium-permeable AMPA receptors are found only in a subset of interneurons (Buldakova et al. 2007; Washburn and Dingledine 1996). However, NASPM did not cause a reduction in paired-pulse facilitation in either group (Supplemental Fig. S2, B and D). NASPM also had no effect on paired-pulse facilitation at Schaffer collateral synapses onto pyramidal cells (Supplemental Fig. S3); the percent reduction in the paired-pulse ratio at 20 and 40 ms (combined) was −3.2 ± 7.4% (n = 4, P > 0.5). Together, these data show that the effect of NASPM to reduce short-term facilitation is specific to Schaffer collateral synapses onto SOM interneurons and not found at other Schaffer collateral synapses.
Both GluR5 and GluR6 are capable of forming calcium-permeable kainate receptors, and the size of the reduction in PPF at Schaffer collateral synapses onto SOM interneurons is the same for NASPM as was seen with the combination of kainate receptor antagonists (32.9 ± 6.6% NASPM; 29.6 ± 6.4% UBP302 + NS102, Student's t-test, P > 0.8). This suggests that the effect of the NASPM on PPF occurs through the block of calcium-permeable kainate receptors and that the combination of kainate receptor antagonists is blocking all of the calcium-permeable kainate receptors. However, it is possible that the kainate receptor antagonists and NASPM maybe reducing the PPF through two different mechanisms. To determine whether NASPM and the kainate receptor antagonists were blocking the same or different receptors, we tested whether NASPM occludes a further effect of UBP302+NS102. As shown in Fig. 3H, addition of UPB302+NS102 causes no further change in paired-pulse facilitation in cells when NASPM is present (one-way ANOVA, P > 0.8). Figure 3I shows the percent decrease in paired-pulse facilitation (20 and 40 ms combined) is not different for NASPM compared with NASPM+UBP302+NS102 (n = 6, P > 0.5), although both are significantly reduced compared with control values (P < 0.05). This shows that NASPM is acting on presynaptic kainate receptors and confirms that the GluR5/GluR6 kainate receptors are responsible for enhancing the short-term facilitation at Schaffer collateral synapses onto SOM interneurons are calcium permeable.
Schaffer collateral synapses onto SOM interneurons show very large short-term facilitation in response to natural stimulus patterns
While simple stimulus paradigms such as paired pulse are very useful in studying the properties of synapses and mechanisms of short-term plasticity, they are not representative of the types of input patterns hippocampal synapses receive in vivo. Because short-term plasticity can be nonlinear, it is difficult to predict the responses to complex patterns from the results with simple stimuli. The NSP, which is derived from in vivo recordings of action potential timing in hippocampal neurons, provides a temporally complex stimulus for investigation of the interactions between the different forms of short-term plasticity in a behaviorally relevant context (Dobrunz and Stevens 1999). We next investigated the responses of Schaffer collateral synapses onto SOM interneurons and non-EGFP interneurons in response to the NSP.
Figure 4 A shows the timing of the stimulus pattern that was applied that contains several high-frequency bursts of stimuli separated by long intervals with little or no activity. Figure 4A, bottom, shows one of the bursts on an expanded time scale, illustrating that the timing within the burst is also highly variable. Figures 4B and C show example traces in response to that burst from a SOM interneuron and a non-EGFP interneuron, respectively. Figure 4D shows the summary data, which is normalized to the control response during 0.1-Hz constant frequency stimulation (last 14 points) and plotted against stimulus number, not time. The pattern of responses to the NSP is dramatically different between Schaffer collateral synapses onto the two types of interneurons. Schaffer collateral synapses onto SOM interneurons show mainly facilitation in response to the physiologically derived NSP. The amount of facilitation is very large (up to eightfold) and varies greatly throughout the pattern (Fig. 4, B and D). In contrast, non-EGFP interneurons have hardly any facilitation in response to the NSP (maximum is 1.4-fold facilitation; Fig. 4, C and D). The range of responses (maximum-minimum) is significantly larger for Schaffer collateral synapses onto the SOM interneurons (Fig. 4E, P < 0.05), indicating that Schaffer collateral synapses onto SOM interneurons operate over a much wider dynamic range of synaptic strength than synapses onto non-EGFP interneurons. There is also a large difference in the overall average amplitude of the responses during the NSP (Fig. 4F, P < 0.05). Interestingly, the difference between EGFP-expressing SOM and non-EGFP interneurons occurs mainly at shorter interstimulus intervals of the NSP (Fig. 4G). One possible mechanism that might be involved in the enhanced facilitation at Schaffer collateral synapses onto SOM interneurons during the shorter interstimulus intervals of the NSP is the activation of presynaptic kainate receptors, as we have seen during paired-pulse stimulation (Figs. 1–3).
FIG. 4.
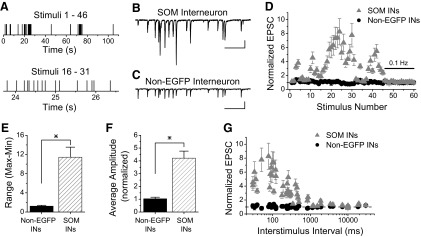
Natural pattern stimulation induces large short-term facilitation in SOM interneurons. A: Timing of NSP is highly variable. Top panel: timing of whole NSP (46 stimuli). Bottom panel: timing of one cluster of stimuli shown on an expanded time scale. B: Example trace of EPSCs recorded in response to a region (stimuli 16–31) of the NSP applied to Schaffer collateral synapses onto an s. radiatum EGFP-expressing SOM interneuron. Scale bars: 500 ms, 35 pA. C: Example trace of EPSCs recorded in response to stimuli 16 through 31 of the NSP applied to Schaffer collateral synapses onto a non-EGFP-expressing interneuron. Scale bars: 500 ms, 25 pA. D: Group results show very large short-term facilitation at Schaffer collateral synapses onto EGFP-expressing SOM interneurons (gray triangles, n = 8) in contrast to non-EGFP interneurons (black circles, n = 7) which show little facilitation. Stimuli 47–60 are control period at 0.1 Hz for normalization. E: The dynamic range over which the Schaffer collateral synapses operate (maximum-minimum, from experiments in D) is larger for EGFP-expressing SOM interneurons compared with other s. radiatum interneurons. F: The average amplitude of the entire NSP (the first 46 points, from experiments in D) is significantly different between the two different interneuronal groups. G: Responses to natural pattern stimulation plotted versus interstimulus interval shows that large facilitation occurs at shorter intervals in EGFP-expressing SOM interneurons. * = significant, P < 0.05.
Large facilitation of Schaffer collateral synapses onto SOM interneurons during NSPs is mediated in part by calcium-permeable kainate receptors
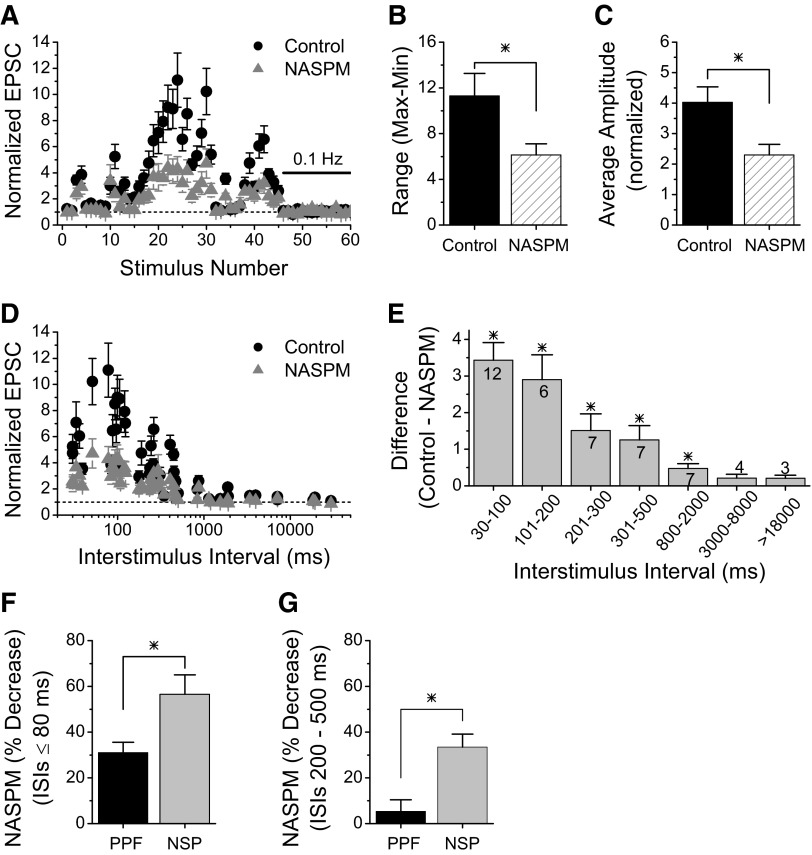
We tested whether calcium-permeable kainate receptors contributed to the large short-term facilitation seen during the NSP by comparing the responses before and during application of NASPM. Figure 5 A shows that blocking the kainate receptors (gray triangles) causes a large reduction in the amount of facilitation at many points during the pattern. This causes a reduction in the dynamic range (maximum –minimum, Fig. 5B, n = 6, P < 0.05), indicating that the synaptic activation of presynaptic kainate receptors normally acts to increase range of synaptic strength over which these synapses operate. While not all points are affected by the NASPM, there was a significant reduction in the overall average amplitude of the responses (averaged for the 46 points of the NSP) as shown in Fig. 5C (n = 6, P < 0.05). A reduction in short-term facilitation during the NSP was also seen when kainate receptors were blocked by the application of NS102 plus UBP302 (Supplemental Fig. S4). The magnitude of the reduction in the overall average amplitude was similar to that with NASPM (NS102+UBP302 34.6 ± 9.9%, n = 8; NASPM 38.4 ± 14.2%, n = 6, student's t-test, P > 0.5), indicating that the reduction of short-term facilitation by NASPM was occurring through the block of kainate receptors.
FIG. 5.
Large short-term facilitation during natural pattern stimulation is mediated in part through calcium-permeable kainate receptors. A: Group results showing that blocking calcium-permeable kainate receptors with 200 μM NASPM (gray triangles) reduces short-term facilitation at Schaffer collateral synapses onto SOM interneurons in response to NSP (n = 6). B: Average range of responses (maximum –minimum, from experiments in A) is reduced by NASPM. C: Average amplitude of the responses to the NSP is reduced by NASPM. D: Responses to NSP plotted versus interstimulus interval show that the effect of NASPM is greatest at shorter intervals. E: Difference between control responses and responses in NASPM for different ranges of interstimulus intervals. Numbers on the bars indicate the number of stimuli (out of 46) that were in each range. F: Percent reduction of short-term facilitation with NASPM is greater during natural stimulus pattern (NSP, from experiments in A) than during paired-pulse stimulation (PPF, from experiments in 3B) for interstimulus intervals (ISIs) of ≤80 ms. G: Percent reduction with NASPM is greater during NSP than PPF for intervals of 200 –500 ms. * = significant, P < 0.05.
The effect of NASPM on short-term facilitation occurs at points with short interstimulus intervals as can be more clearly seen in Fig. 5D in which the data are plotted versus the interstimulus interval. We quantified this by calculating the magnitude of the difference in the normalized EPSC for each point (control –NASPM) and averaging them for different ranges of interstimulus interval. As shown in Fig. 5E, there is a statistically significant increase in short-term facilitation caused by calcium-permeable kainate receptors at all interval ranges except the longest two (interstimulus intervals >3 s). Interestingly, the enhancement of short-term facilitation by kainate receptor activation occurs at longer intervals during natural pattern stimulation (Fig. 5, D and E) than during paired-pulse stimulation, where the effect was limited to ≤80 ms (Fig. 3D). In addition, the magnitude of the reduction in facilitation with NASPM is greater during the NSP than during paired-pulse stimulation. Figure 5F shows that for intervals of ≤80 ms, the average reduction in facilitation with NASPM is 31% for paired pulse stimulation but over 50% for the NSP (P < 0.05). For intervals of 200–500 ms (Fig. 5G), there is no significant effect of NASPM during paired-pulse stimulation (P > 0.3), while the decrease in facilitation during the NSP is >30% (P < 0.05). These results show that the pattern of stimulation is important for the activation of presynaptic kainate receptors that enhance facilitation, and not just the stimulus frequency. Together, these results indicate that activation of presynaptic calcium-permeable kainate receptors by synaptically released glutamate is likely to be a physiologically important mechanism to increase short-term facilitation at these synapses and increase their dynamic range during burst stimulation such as these synapses would receive in vivo.
DISCUSSION
Here we show that the presynaptic kainate receptors that increase facilitation at Schaffer collateral synapses onto s. radiatum SOM interneurons are heteromeric receptors that contain the GluR5 subunit as well as the GluR6 subunit. They are calcium permeable, suggesting that an increase in presynaptic calcium may be the mechanism by which activation of these kainate receptors increases release probability and enhances short-term facilitation. Furthermore, we show that short-term facilitation at Schaffer collateral synapses onto SOM interneurons is extremely large (≤11-fold) during physiologically derived stimulus patterns, in contrast to Schaffer collateral synapses onto other interneurons that have small facilitation (<1.5-fold). This is due in large part to the activation of the presynaptic kainate receptors at synapses onto the SOM interneurons.
The enhancement of short-term facilitation by synaptic activation of presynaptic kainate receptors is a unique function for Schaffer collateral axons that occurs at only a subset of synapses based on their postsynaptic targets, the SOM interneurons. Schaffer collateral synapses onto CA1 pyramidal cells, in contrast, contain presynaptic kainate receptors whose activation can depress glutamate release (Clarke and Collingridge 2002; Frerking et al. 2001; Kamiya and Ozawa 1998; Lauri et al. 2006; Partovi and Frerking 2006; Vignes et al. 1998). Because the GluR5 subunit has been implicated in both types of kainate receptors (Clarke and Collingridge 2002; Lauri et al. 2006; Partovi and Frerking 2006), it is possible that other kainate receptor subunits determine whether the receptor activation facilitates or depresses glutamate release. Schaffer collateral synapses onto other s. radiatum interneurons, which have a higher initial release probability and lower short-term facilitation than Schaffer collateral synapses onto SOM interneurons (Sun and Dobrunz 2006), appear to lack high-affinity presynaptic kainate autoreceptors (Sun and Dobrunz 2006; present study). It is not yet known if they have low-affinity kainate receptors that depress release.
Presynaptic kainate receptors containing GluR5 that enhance glutamate release are also found at excitatory synapses onto CA3 interneurons during the first postnatal week (Lauri et al. 2005). Heteromeric kainate receptors containing both GluR5 and GluR6 have been shown to be located presynaptically at perforant path synapses onto CA3 pyramidal cells (Contractor et al. 2000), and postsynaptically in CA1 s. radiatum interneurons (Mulle et al. 2000). Presynaptic kainate receptors at excitatory synapses onto layer II/III pyramidal cells in cortex, in contrast, contain GluR5 but not GluR6 (Campbell et al. 2007). The subunit composition of presynaptic kainate receptors at mossy fiber synapses onto CA3 pyramidal cells is still controversial but has been reported to contain GluR5 (Bortolotto et al. 2003; Lauri et al. 2001a; but see Contractor et al. 2001), GluR6 (Contractor et al. 2001; Pinheiro et al. 2007), GluR7 (Pinheiro et al. 2007), and KA2 subunits (Contractor et al. 2003). While our data support the idea that presynaptic kainate receptors at Schaffer collateral synapses onto SOM interneurons contain GluR5/6 heteromers, our results do not rule out a possible role for other kainate receptor subunits, such as KA1 or KA2. Studies have shown that GluR5 and GluR6 kainate receptor subunits can form heteromeric receptors with either KA1 or KA2 subunits (Alt et al. 2004; Kayadjanian et al. 2007; Lerma 2003, 2006; Pinheiro and Mulle 2006; Ruiz et al. 2005), which can modify the receptor properties. For example, the affinity of the presynaptic kainate receptors at mossy fiber synapses in CA3 is decreased when KA2 subunits are genetically ablated (Contractor et al. 2003). Future experiments will be needed to investigate whether other subunits in addition to GluR5 and GluR6 are important at Schaffer collateral synapses onto SOM interneurons.
In our previous study (Sun and Dobrunz 2006), we showed that kainate receptor activation increases release probability at Schaffer collateral synapses onto SOM interneurons but did not determine the underlying mechanism for this effect. However, our mathematical modeling results predicted that the kainate receptor mechanism would be calcium dependent because our model was able to fit the data for the large facilitation of the Schaffer collateral inputs onto SOM interneurons by incorporating a calcium-dependent increase in the release probability per vesicle (Sun and Dobrunz 2006). Here we show that the presynaptic kainate receptors at Schaffer collateral synapses onto SOM interneurons are calcium permeable as has been shown for the presynaptic kainate receptor autoreceptors that enhance short-term facilitation at mossy fiber synapses onto CA3 pyramidal cells (Pinheiro et al. 2007). This means that calcium influx through these receptors could be the mechanism through which they increase release probability and enhance short-term plasticity. However, our results do not directly demonstrate that it is the calcium influx through the receptors that enhances facilitation. At mossy fiber synapses in CA3, the activation of presynaptic kainate receptors may also enhance glutamate release through a direct depolarizing effect on the presynaptic membrane, leading to enhanced calcium influx through voltage gated calcium channels (Kamiya et al. 2002). It is not known whether this mechanism is also involved in enhancing facilitation at Schaffer collateral synapses onto SOM interneurons. However, our results demonstrate that the presynaptic kainate receptors at Schaffer collateral synapses onto SOM interneurons are calcium permeable like presynaptic kainate receptors at mossy fiber synapses in CA3.
Because our experiments use pharmacological tools to investigate the subunit composition and calcium permeability of the presynaptic kainate receptors, the interpretation of some of our results depends on the selectivity of the antagonists used. Given the numerous possible combinations of the various subunits, the potential heterogeneity of kainate receptors also complicates the assessment of pharmacological properties (Pinheiro and Mulle 2006). Because most kainate receptor antagonists are characterized in heterologous expression systems, it is possible that effectiveness of these compounds on heteromeric kainate receptors in acute slices may differ. Whenever possible, we used several different antagonists to confirm the results. A reduction on paired-pulse facilitation occurred with both UBP302 and LY382884, both of which are reported to be GluR5 specific. Similarly, two different blockers of calcium-permeable kainate/AMPA receptors, NASPM and philanthotoxin-433, each caused a reduction on paired-pulse facilitation. It is also possible that UPB302 plus NS102 may not be completely blocking all of the presynaptic autoreceptors at the Schaffer collateral synapses onto SOM interneurons, although this is unlikely because there was no additive effect when UBP302 and NS102 were applied together. If so, this would cause an underestimate of the effect of presynaptic kainate receptors on short-term facilitation. However, the close agreement between the reduction in paired-pulse facilitation with UBP302 plus NS102 as compared with that seen with NASPM suggests that the combination of kainate receptor antagonists is blocking all of the calcium-permeable kainate receptors. Together, our results therefore support the idea that the presynaptic kainate receptors at Schaffer collateral synapses onto SOM interneurons are calcium-permeable heteromers containing GluR5 and GluR6.
While using the NSP provides stimulation that is more physiologically relevant in its temporal complexity and mixture of frequencies, there are several respects in which our experiments differ from in vivo conditions. Because our experiments are focused on presynaptic short-term plasticity, we block NMDA receptors to prevent postsynaptic short-term plasticity that might occur through activity-dependent relief of the magnesium block of these receptors. In addition, this prevents possible NMDA-receptor-dependent long-term plasticity, which can be induced by the NSP when NMDA receptors are active (Dobrunz and Stevens 1999). We also block GABAA receptors to isolate the responses from excitatory Schaffer collateral synapses; with inhibition intact, the results would be more difficult to interpret due to short-term plasticity of inhibitory responses. Future experiments will be needed to examine the interactions between these different forms of plasticity, which will clearly be important to the overall functioning of the hippocampal circuit in vivo. However, blocking NMDA receptors and GABAA receptors is unlikely to significantly alter the effects of short-term plasticity to modulate glutamate release by Schaffer collateral synapses during temporally complex stimulation.
These experiments also differ from the in vivo conditions by being performed at room temperature rather than physiological temperature. Recording at room temperature enables the SOM interneurons to stay healthy longer, which is important due to the long duration of the NSP experiments. We have previously shown that recording at near physiological temperature compared with room temperature causes a slight increase in the amount of paired-pulse and five-pulse facilitation at SC synapses onto SOM interneurons (Sun and Dobrunz 2006). In addition, recording at higher temperature causes a small increase in paired-pulse facilitation and facilitation during the NSP at SC synapses onto CA1 pyramidal cells (Speed and Dobrunz 2008). Based on this, we predict that recording at warmer temperature would also cause a small increase in the amount of short-term facilitation at SC synapses onto SOM interneurons during the NSP. As a result, the extremely large short-term facilitation we record here may actually be an underestimate of what would occur in vivo.
The EGFP-expressing interneurons in the GIN mice, which we refer to here as SOM interneurons for simplicity, are a subset (∼20% in hippocampus) of the interneurons that contain somatostatin (Oliva et al. 2000). It is not yet known whether other somatostatin interneurons that do not express EGFP also have presynaptic kainate receptors that contribute to large facilitation of their SC synapses or whether the EGFP-expressing subset is somehow unique. However, we have never detected a non-EGFP interneuron that had such large short-term facilitation or that showed any evidence of presynaptic kainate receptors that increased facilitation.
The fact that Schaffer collateral synapses onto SOM interneurons also have extremely large short-term facilitation in response to physiologically derived NSPs suggests that they are likely to have very large presynaptic short-term facilitation in vivo. In addition, these synapses respond over a very wide dynamic range during the temporally complex pattern. This indicates the strength of these synapses can rapidly change from weak to very strong during high-frequency bursts and therefore that the frequency and timing of Schaffer collateral firing has a very large ability to modulate the postsynaptic response of the SOM interneuron. In contrast, we show that Schaffer collateral synapses onto the non-EGFP interneurons have very little short-term facilitation during the NSP and operate over a much narrower dynamic range. Schaffer collateral synapses onto non-SOM interneurons are therefore less sensitive to stimulus frequency and would provide a more consistent level of excitatory drive onto their postsynaptic target interneurons. Previous results using field potential recordings (Dekay et al. 2006; Speed and Dobrunz 2008, 2009) and whole cell recordings (Dobrunz and Stevens 1999) have shown that Schaffer collateral synapses onto CA1 pyramidal cells have robust short-term facilitation in response to NSPs and that they operate over a fairly wide dynamic range (Dobrunz and Stevens 1999). However, the amount of short-term facilitation is over twice as large and the dynamic range is more than five times larger at Schaffer collateral synapses onto SOM interneurons observed here as compared with the results from CA1 pyramidal cells (Dobrunz and Stevens 1999).
The very large short-term facilitation of Schaffer collateral synapses onto SOM interneurons during the NSP is due in large part to the synaptic activation of the kainate autoreceptors because short-term facilitation at Schaffer collateral synapses onto SOM interneurons is reduced by > 40% by the antagonist NASPM. A similar result was obtained using the combination of UBP302 and NS102, indicating that the effect was due to block of calcium-permeable kainate receptors. This shows that synaptically released glutamate activates calcium-permeable kainate receptors during physiologically based stimulus patterns, leading to an increase in release probability and enhanced short-term facilitation on subsequent pulses in the train. While blocking the kainate receptors causes a reduction in the overall average amplitude and the dynamic range of responses during the NSP, both are still larger than what is observed in CA1 pyramidal cells (Dobrunz and Stevens 1999). This is consistent with the fact that Schaffer collateral synapses onto SOM interneurons have a lower initial release probability than Schaffer collateral synapses onto pyramidal cells (Sun and Dobrunz 2006), which also contributes to their large short-term facilitation. The activation of presynaptic kainate autoreceptors at Schaffer collateral synapses onto SOM interneurons is therefore a key mechanism that enables these synapses to have unusually large short-term facilitation and operate over an extremely large dynamic range.
Interestingly, the effect of presynaptic kainate receptors lasts considerably longer during the NSP than during a pair of pulses because a reduction in facilitation with NASPM is seen at intervals up to 2 s, as compared with only 80 ms during paired-pulse stimulation. In addition, the reduction in facilitation with NASPM is almost twice as large during the NSP than during paired-pulse stimulation at interstimulus intervals of ≤80 ms. This could indicate that during longer stimulus trains more kainate receptors are activated, perhaps by spillover of glutamate from neighboring synapses. Alternatively, another signaling mechanism could be involved that is triggered by activation of the presynaptic kainate receptors.
The modulation of transmitter release by presynaptic kainate receptors at some synapses has been reported to involve a metabotropic action (Frerking et al. 2001; Rodriguez-Moreno and Lerma 1998), which can be mediated by KA1 and KA2 subunits (Pinheiro and Mulle 2006; Ruiz et al. 2005). The rapid activation of presynaptic kainate receptors at Schaffer collateral synapses onto SOM interneurons is fast enough (<20 ms) to make it unlikely that a metabotropic function is required during paired-pulse facilitation. However, this does not preclude the possible involvement of a G-protein-coupled process in the presynaptic terminal during the natural stimulation pattern because the effect of blocking the kainate receptors is seen at interstimulus intervals up to 2 s. Future experiments will be needed to determine whether KA1 and/or KA2 subunits are also found at these kainate autoreceptors and to investigate whether metabotropic kainate receptor functions are activated during longer stimulation patterns and contribute to the enhanced short-term facilitation at longer intervals.
Another mechanism by which kainate receptor activation could potentially contribute to the enhanced short-term facilitation at longer intervals of the NSP is through triggering the release of calcium from intracellular stores. There is evidence that calcium stores are involved in synaptic short-term plasticity at a variety of synapses in the CNS (Berridge 1998; Emptage et al. 2001; Lauri et al. 2003). For example, calcium stores have been shown to contribute to calcium transients during brief high-frequency activation (Liang et al. 2002). In contrast, depletion of calcium stores has been reported to have no effect on low-frequency transmission (Carter et al. 2002; Lauri et al. 2003). Release of calcium from intracellular stores can be triggered by several different mechanisms (Irving et al. 1992; Lauri et al. 2003; Rae et al. 2000), including activation of calcium-permeable kainate receptors (Lauri et al. 2003). For example, calcium influx through calcium-permeable presynaptic kainate receptors causes release of calcium from intracellular stores that contributes to the large short-term facilitation and to the induction of long-term potentiation at mossy fiber synapses in CA3 (Lauri et al. 2003). Future experiments will be needed to determine whether calcium release from intracellular stores plays any role in the kainate-receptor-mediated enhancement of short-term facilitation at Schaffer collateral synapses onto SOM interneurons. However, our experiments showing synaptic activation of presynaptic kainate autoreceptors during the NSP demonstrate that these receptors should be activated at Schaffer collateral synapses onto SOM interneurons by bursts of action potentials in vivo and that they are likely to play an important role in regulating the function of these synapses.
Our results also show that there are considerable differences in the excitatory input to different subgroups of interneurons in response to high-frequency activity (Sun et al. 2005; present study). Short-term facilitation will increase the excitatory drive onto specific interneurons and promote action potential firing in these neurons, whereas short-term depression will cause a decrease firing of other interneurons. Because Schaffer collateral synapses onto SOM interneurons have much greater high-frequency facilitation than synapses onto other interneurons, they are likely to fire more often during high-frequency bursts than other types of interneurons. Most interneurons in s. radiatum provide inhibitory input to the soma and proximal dendrites of CA1 pyramidal cells (Freund and Buzsaki 1996). In contrast, many SOM interneurons in s. radiatum have extensive axonal arborization in s. lacunosum-moleculare (Oliva et al. 2000) where they provide inhibitory input onto the most distal dendrites of CA1 pyramidal cells. It remains to be determined whether there are also differences in short-term plasticity at the inhibitory synapses made onto CA1 pyramidal cells by SOM interneurons compared with other interneurons during temporally complex firing patterns. However, differences in the dynamic properties of Schaffer collateral synapses onto SOM interneurons compared with other interneurons may result in frequency-dependent changes in the spatial distribution of inhibition on CA1 pyramidal cells.
In addition to the Schaffer collateral input onto their proximal dendrites, CA1 pyramidal cells also receive excitatory input from the direct perforant pathway from entorhinal (ER) cortex that makes excitatory synapses onto the distal dendrites in s. lacunosum-moleculare (Hjorth-Simonsen and Jeune 1972). This pathway, which is also referred to as the temporoammonic pathway (Maccaferri and McBain 1995), has been shown to be important for memory consolidation (Remondes and Schuman 2004). CA1 pyramidal cells are the main output neurons of the hippocampus (Amaral 1993), and their firing properties are determined by the dynamics of both Schaffer collateral synapses and temporoammonic synapses (Jarsky et al. 2005; Kocsis et al. 1999). Because they receive excitatory input from Schaffer collateral synapses and provide inhibition to CA1 pyramidal cells at the site of temporoammonic synapses (Oliva et al. 2000), SOM interneurons in s. radiatum are uniquely poised to regulate the input from ER cortex in response to Schaffer collateral input and therefore act as “input biasing” neurons (Oliva et al. 2000).
Our results show that Schaffer collateral synapses that drive SOM interneurons have unique properties that enable them to operate over an unusually large dynamic range, which we propose is important for enabling them to regulate the temporoammonic input in a frequency-dependent manner. During low-frequency stimulation, Schaffer collateral synapses onto SOM interneurons have a low probability of release (Sun and Dobrunz 2006), and therefore SOM interneurons are unlikely to fire action potentials. In contrast, high-frequency stimulation from the Schaffer collateral pathway will activate the presynaptic calcium-permeable kainate receptors and increase the release probability. This enhancement in synaptic strength will increase the firing of the SOM interneurons and therefore should increase the inhibition they provide onto CA1 pyramidal cells in s. lacunosum-moleculare, thereby reducing the effect of the temporammonic input from ER cortex. Our results therefore suggest that the very large short-term facilitation at Schaffer collateral synapses onto SOM interneurons, caused in large part by the synaptic activation of presynaptic kainate receptors, could function to dynamically alter the inhibitory gating of the temporoammonic pathway during temporally complex stimuli such as these synapses receive in vivo.
GRANTS
This work has been supported by National Institutes oF Heatlh Grant R01 MH-65328 to L. E. Dobrunz, a Civitan International Research Center Emerging Scholars Award to H. Y. Sun, NIH Grant HD-38985, and NIH Neuroscience Blueprint Core Grant NS-57098 to the University of Alabama at Birmingham.
Supplementary Material
Acknowledgments
We thank Dr. Smadar Lapidot for assistance in editing and Dr. Lori McMahon for helpful comments on the manuscript. We also thank Drs. Robert Muller and Andre Fenton for generously providing the in vivo spike timing patterns used in these experiments and John Hablitz for providing us with LY382884.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Ali et al. 1998.Ali AB, Deuchars J, Pawelzik H, Thomson AM. CA1 pyramidal to basket and bistratified cell EPSPs: dual intracellular recordings in rat hippocampal slices. J Physiol 507: 201–217, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt et al. 2004.Alt A, Weiss B, Ogden AM, Knauss JL, Oler J, Ho K, Large TH, Bleakman D. Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharmacology 46: 793–806, 2004. [DOI] [PubMed] [Google Scholar]
- Amaral 1993.Amaral DG Emerging principles of intrinsic hippocampal organization. Curr Opin Neurobiol 3: 225–229, 1993. [DOI] [PubMed] [Google Scholar]
- Bahring et al. 1997.Bahring R, Bowie D, Benveniste M, Mayer ML. Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. J Physiol) 502: 575–589, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahring and Mayer 1998.Bahring R, Mayer ML. An analysis of philanthotoxin block for recombinant rat GluR6(Q) glutamate receptor channels. J Physiol 509: 635–650, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge 1998.Berridge MJ Neuronal calcium signaling. Neuron 21: 13–26, 1998. [DOI] [PubMed] [Google Scholar]
- Bortolotto et al. 1999.Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature 402: 297–301, 1999. [DOI] [PubMed] [Google Scholar]
- Bortolotto et al. 2003.Bortolotto ZA, Lauri S, Isaac JT, Collingridge GL. Kainate receptors and the induction of mossy fibre long-term potentiation. Philos Trans R Soc Lond B Biol Sci 358: 657–666, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buldakova et al. 2007.Buldakova SL, Kim KK, Tikhonov DB, Magazanik LG. Selective blockade of Ca2+ permeable AMPA receptors in CA1 area of rat hippocampus. Neuroscience 144: 88–99, 2007. [DOI] [PubMed] [Google Scholar]
- Bureau et al. 1999.Bureau I, Bischoff S, Heinemann SF, Mulle C. Kainate receptor-mediated responses in the CA1 field of wild-type and GluR6-deficient mice. J Neurosci 19: 653–663, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell et al. 2007.Campbell SL, Mathew SS, Hablitz JJ. Pre- and postsynaptic effects of kainate on layer II/III pyramidal cells in rat neocortex. Neuropharmacology 53: 37–47, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter et al. 2002.Carter AG, Vogt KE, Foster KA, Regehr WG. Assessing the role of calcium-induced calcium release in short-term presynaptic plasticity at excitatory central synapses. J Neurosci 22: 21–28, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu et al. 1999.Chittajallu R, Braithwaite SP, Clarke VR, Henley JM. Kainate receptors: subunits, synaptic localization and function. Trends Pharmacol Sci 20: 26–35, 1999. [DOI] [PubMed] [Google Scholar]
- Christensen et al. 2004a.Christensen JK, Paternain AV, Selak S, Ahring PK, Lerma J. A mosaic of functional kainate receptors in hippocampal interneurons. J Neurosci 24: 8986–8993, 2004a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen et al. 2004b.Christensen JK, Varming T, Ahring PK, Jorgensen TD, Nielsen EO. In vitro characterization of 5-carboxyl-2,4-di-benzamidobenzoic acid (NS3763), a noncompetitive antagonist of GLUK5 receptors. J Pharmacol Exp Ther 309: 1003–1010, 2004b. [DOI] [PubMed] [Google Scholar]
- Clarke et al. 1997.Clarke VR, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakman D. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature 389: 599–603, 1997. [DOI] [PubMed] [Google Scholar]
- Clarke and Collingridge 2002.Clarke VR, Collingridge GL. Characterisation of the effects of ATPA, a GLU(K5) receptor selective agonist, on excitatory synaptic transmission in area CA1 of rat hippocampal slices. Neuropharmacology 42: 889–902, 2002. [DOI] [PubMed] [Google Scholar]
- Cobb et al. 1995.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378: 75–78, 1995. [DOI] [PubMed] [Google Scholar]
- Contractor et al. 2003.Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2-/- mice. J Neurosci 23: 422–429, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor et al. 2001.Contractor A, Swanson G, Heinemann SF. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron 29: 209–216, 2001. [DOI] [PubMed] [Google Scholar]
- Contractor et al. 2000.Contractor A, Swanson GT, Sailer A, O'Gorman S, Heinemann SF. Identification of the kainate receptor subunits underlying modulation of excitatory synaptic transmission in the CA3 region of the hippocampus. J Neurosci 20: 8269–8278, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart et al. 1998.Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci 1: 470–478, 1998. [DOI] [PubMed] [Google Scholar]
- Cui and Mayer 1999.Cui C, Mayer ML. Heteromeric kainate receptors formed by the coassembly of GluR5, GluR6, and GluR7. J Neurosci 19: 8281–8291, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekay et al. 2006.Dekay JG, Chang TC, Mills N, Speed HE, Dobrunz LE. Responses of excitatory hippocampal synapses to natural stimulus patterns reveal a decrease in short-term facilitation and increase in short-term depression during postnatal development. Hippocampus 16: 66–79, 2006. [DOI] [PubMed] [Google Scholar]
- Dobrunz 2002.Dobrunz LE Release probability is regulated by the size of the readily releasable vesicle pool at excitatory synapses in hippocampus. Intl J Dev Neurosci 20: 225–236, 2002. [DOI] [PubMed] [Google Scholar]
- Dobrunz 1999.Dobrunz LE, Stevens CF. Response of hippocampal synapses to natural stimulation patterns. Neuron 22: 157–166, 1999. [DOI] [PubMed] [Google Scholar]
- Emptage et al. 2001.Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca(2+) entry, and spontaneous transmitter release. Neuron 29: 197–208, 2001. [DOI] [PubMed] [Google Scholar]
- Fenton and Muller 1998.Fenton AA, Muller RU. Place cell discharge is extremely variable during individual passes of the rat through the firing field. Proc Natl Acad Sci USA 95: 3182–3187, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking et al. 2001.Frerking M, Schmitz D, Zhou Q, Johansen J, Nicoll RA. Kainate receptors depress excitatory synaptic transmission at CA3–>CA1 synapses in the hippocampus via a direct presynaptic action. J Neurosci 21: 2958–2966, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund and Buzsaki 1996.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus 6: 347–470, 1996. [DOI] [PubMed] [Google Scholar]
- Hjorth-Simonsen and Jeune 1972.Hjorth-Simonsen A, Jeune B. Origin and termination of the hippocampal perforant path in the rat studied by silver impregnation. J Comp Neurol 144: 215–232, 1972. [DOI] [PubMed] [Google Scholar]
- Irving et al. 1992.Irving AJ, Collingridge GL, Schofield JG. Interactions between Ca2+ mobilizing mechanisms in cultured rat cerebellar granule cells. J Physiol 456: 667–680, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky et al. 2005.Jarsky T, Roxin A, Kath WL, Spruston N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat Neurosci 8: 1667–1676, 2005. [DOI] [PubMed] [Google Scholar]
- Kamiya and Ozawa 1998.Kamiya H, Ozawa S. Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus. J Physiol 509: 833–845, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya et al. 2002.Kamiya H, Ozawa S, Manabe T. Kainate receptor-dependent short-term plasticity of presynaptic Ca2+ influx at the hippocampal mossy fiber synapses. J Neurosci 22: 9237–9243, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayadjanian et al. 2007.Kayadjanian N, Lee HS, Pina-Crespo J, Heinemann SF. Localization of glutamate receptors to distal dendrites depends on subunit composition and the kinesin motor protein KIF17. Mol Cell Neurosci 34: 219–230, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyachko and Stevens 2006.Klyachko VA, Stevens CF. Excitatory and feed-forward inhibitory hippocampal synapses work synergistically as an adaptive filter of natural spike trains. PLoS Biol 4: e207, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis et al. 1999.Kocsis B, Bragin A, Buzsaki G. Interdependence of multiple theta generators in the hippocampus: a partial coherence analysis. J Neurosci 19: 6200–6212, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester and Johnston 2005.Koester HJ, Johnston D. Target cell-dependent normalization of transmitter release at neocortical synapses. Science 308: 863–866, 2005. [DOI] [PubMed] [Google Scholar]
- Koike et al. 1997.Koike M, Iino M, Ozawa S. Blocking effect of 1-naphthyl acetyl spermine on Ca(2+)-permeable AMPA receptors in cultured rat hippocampal neurons. Neurosci Res 29: 27–36, 1997. [DOI] [PubMed] [Google Scholar]
- Laezza et al. 1999.Laezza F, Doherty JJ, Dingledine R. Long-term depression in hippocampal interneurons: joint requirement for pre- and postsynaptic events. Science 285: 1411–1414, 1999. [DOI] [PubMed] [Google Scholar]
- Lauri et al. 2001a.Lauri SE, Bortolotto ZA, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. A critical role of a facilitatory presynaptic kainate receptor in mossy fiber LTP. Neuron 32: 697–709, 2001a. [DOI] [PubMed] [Google Scholar]
- Lauri et al. 2003.Lauri SE, Bortolotto ZA, Nistico R, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron 39: 327–341, 2003. [DOI] [PubMed] [Google Scholar]
- Lauri et al. 2001b.Lauri SE, Delany C, VR JC, Bortolotto ZA, Ornstein PL, J TRI, Collingridge GL. Synaptic activation of a presynaptic kainate receptor facilitates AMPA receptor-mediated synaptic transmission at hippocampal mossy fiber synapses. Neuropharmacology 41: 907–915, 2001b. [DOI] [PubMed] [Google Scholar]
- Lauri et al. 2005.Lauri SE, Segerstrale M, Vesikansa A, Maingret F, Mulle C, Collingridge GL, Isaac JT, Taira T. Endogenous activation of kainate receptors regulates glutamate release and network activity in the developing hippocampus. J Neurosci 25: 4473–4484, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri et al. 2006.Lauri SE, Vesikansa A, Segerstrale M, Collingridge GL, Isaac JT, Taira T. Functional maturation of CA1 synapses involves activity-dependent loss of tonic kainate receptor-mediated inhibition of glutamate release. Neuron 50: 415–429, 2006. [DOI] [PubMed] [Google Scholar]
- Lerma 2003.Lerma J Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci 4: 481–495, 2003. [DOI] [PubMed] [Google Scholar]
- Lerma 2006.Lerma J Kainate receptor physiology. Curr Opin Pharmacol 6: 89–97, 2006. [DOI] [PubMed] [Google Scholar]
- Liang et al. 2002.Liang Y, Yuan LL, Johnston D, Gray R. Calcium signaling at single mossy fiber presynaptic terminals in the rat hippocampus. J Neurophysiol 87: 1132–1137, 2002. [DOI] [PubMed] [Google Scholar]
- Losonczy et al. 2002.Losonczy A, Zhang L, Shigemoto R, Somogyi P, Nusser Z. Cell type dependence and variability in the short-term plasticity of EPSCs in identified mouse hippocampal interneurones. J Physiol 542: 193–210, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri and McBain 1995.Maccaferri G, McBain CJ. Passive propagation of LTD to stratum oriens-alveus inhibitory neurons modulates the temporoammonic input to the hippocampal CA1 region. Neuron 15: 137–145, 1995. [DOI] [PubMed] [Google Scholar]
- Markram et al. 1998.Markram H, Wang Y, Tsodyks M. Differential signaling via the same axon of neocortical pyramidal neurons. Proc Natl Acad Sci USA 95: 5323–5328, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain 1998.McBain CJ A short-term mechanism of plasticity for interneurones? J Physiol 511: 331, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More et al. 2004.More JC, Nistico R, Dolman NP, Clarke VR, Alt AJ, Ogden AM, Buelens FP, Troop HM, Kelland EE, Pilato F, Bleakman D, Bortolotto ZA, Collingridge GL, Jane DE. Characterisation of UBP296: a novel, potent and selective kainate receptor antagonist. Neuropharmacology 47: 46–64, 2004. [DOI] [PubMed] [Google Scholar]
- Mulle et al. 2000.Mulle C, Sailer A, Swanson GT, Brana C, O'Gorman S, Bettler B, Heinemann SF. Subunit composition of kainate receptors in hippocampal interneurons. Neuron 28: 475–484, 2000. [DOI] [PubMed] [Google Scholar]
- Oliva et al. 2000.Oliva AA, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci 20: 3354–3368, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra et al. 1998.Parra P, Gulyas AI, Miles R. How many subtypes of inhibitory cells in the hippocampus? Neuron 20: 983–993, 1998. [DOI] [PubMed] [Google Scholar]
- Partovi and Frerking 2006.Partovi D, Frerking M. Presynaptic inhibition by kainate receptors converges mechanistically with presynaptic inhibition by adenosine and GABAB receptors. Neuropharmacology 51: 1030–1037, 2006. [DOI] [PubMed] [Google Scholar]
- Paternain et al. 2000.Paternain AV, Herrera MT, Nieto MA, Lerma J. GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassemble to form functional receptors. J Neurosci 20: 196–205, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey and McBain 2007.Pelkey KA, McBain CJ. Differential regulation at functionally divergent release sites along a common axon. Curr Opin Neurobiol 17: 366–373, 2007. [DOI] [PubMed] [Google Scholar]
- Pinheiro and Mulle 2006.Pinheiro P, Mulle C. Kainate receptors. Cell Tissue Res 326: 457–482, 2006. [DOI] [PubMed] [Google Scholar]
- Pinheiro et al. 2007.Pinheiro PS, Perrais D, Coussen F, Barhanin J, Bettler B, Mann JR, Malva JO, Heinemann SF, Mulle C. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci USA 104: 12181–12186, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae et al. 2000.Rae MG, Martin DJ, Collingridge GL, Irving AJ. Role of Ca2+ stores in metabotropic L-glutamate receptor-mediated supralinear Ca2+ signaling in rat hippocampal neurons. J Neurosci 20: 8628–8636, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remondes and Schuman 2004.Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature 431: 699–703, 2004. [DOI] [PubMed] [Google Scholar]
- Reyes et al. 1998.Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci 1: 279–285, 1998. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno and Lerma 1998.Rodriguez-Moreno A, Lerma J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron 20: 1211–1218, 1998. [DOI] [PubMed] [Google Scholar]
- Rozov and Burnashev 1999.Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature 401: 594–598, 1999. [DOI] [PubMed] [Google Scholar]
- Ruiz et al. 2005.Ruiz A, Sachidhanandam S, Utvik JK, Coussen F, Mulle C. Distinct subunits in heteromeric kainate receptors mediate ionotropic and metabotropic function at hippocampal mossy fiber synapses. J Neurosci 25: 11710–11718, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani et al. 1998.Scanziani M, Gahwiler BH, Charpak S. Target cell-specific modulation of transmitter release at terminals from a single axon. Proc Natl Acad Sci USA 95: 12004–12009, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz et al. 2001.Schmitz D, Mellor J, Nicoll RA. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science 291: 1972–1976, 2001. [DOI] [PubMed] [Google Scholar]
- Somogyi and Klausberger 2005.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol 562: 9–26, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed and Dobrunz 2008b.Speed HE, Dobrunz LE. Developmental changes in short-term facilitation are opposite at temporoammonic synapses compared to Schaffer collateral synapses onto CA1 pyramidal cells. Hippocampus 19: 187–204, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed and Dobrunz 2008.Speed HE, Dobrunz LE. Developmental decrease in Short-term facilitation at schaffer collateral synapses in hippocampus is mGluR1 sensitive. J Neurophysiol 99: 799–813, 2008. [DOI] [PubMed] [Google Scholar]
- Sun and Dobrunz 2006.Sun HY, Dobrunz LE. Presynaptic kainate receptor activation is a novel mechanism for target cell-specific short-term facilitation at Schaffer collateral synapses. J Neurosci 26: 10796–10807, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al. 2005.Sun HY, Lyons SA, Dobrunz LE. Mechanisms of target-cell specific short-term plasticity at Schaffer collateral synapses onto interneurones versus pyramidal cells in juvenile rats. J Physiol 568: 815–840, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson 1997.Thomson AM Activity-dependent properties of synaptic transmission at two classes of connections made by rat neocortical pyramidal axons in vitro. J Physiol 502: 131–147, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson 2003.Thomson AM Presynaptic frequency- and pattern-dependent filtering. J Comput Neurosci 15: 159–202, 2003. [DOI] [PubMed] [Google Scholar]
- Thomson et al. 2002.Thomson AM, Bannister AP, Mercer A, Morris OT. Target and temporal pattern selection at neocortical synapses. Philos Trans R Soc Lond B Biol Sci 357: 1781–1791, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth and McBain 2000.Toth K, McBain CJ. Target-specific expression of pre- and postsynaptic mechanisms. J Physiol 525: 41–51, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth et al. 2000.Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci 20: 8279–8289, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignes et al. 1998.Vignes M, Clarke VR, Parry MJ, Bleakman D, Lodge D, Ornstein PL, Collingridge GL. The GluR5 subtype of kainate receptor regulates excitatory synaptic transmission in areas CA1 and CA3 of the rat hippocampus. Neuropharmacology 37: 1269–1277, 1998. [DOI] [PubMed] [Google Scholar]
- Washburn and Dingledine 1996.Washburn MS, Dingledine R. Block of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. J Pharmacol Exp Ther 278: 669–678, 1996. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.