Abstract
We recorded from rostral vestibular (VN) and rostral fastigial nuclei (FN) neurons that did not respond to eye movements during three-dimensional (3D) vestibular and optokinetic stimulation (OKS). The majority of neurons in both areas (76 and 69% in VN and FN, respectively) responded during both rotational and translational motion. Preferred directions scattered throughout 3D space for translation but showed some preference for pitch/roll over yaw for rotation. VN/FN neurons were also tested during OKS while monkeys suppressed their optokinetic nystagmus by fixating a head-fixed target. Only a handful of cells (VN: 17%, FN: 6%) modulated during 0.5-Hz OKS suppression, but the number of responsive cells increased (VN: 40%, FN: 48%) during 0.02-Hz OKS. Preferred directions for rotation and OKS were not matched on individual neurons, and OKS gains were smaller than the respective gains during rotation. These results were generally similar for VN and FN neurons. We conclude that optokinetic-vestibular convergence might not be as prevalent as earlier studies have suggested.
INTRODUCTION
Many studies have qualified the responses of primate vestibular nuclei (VN) and rostral fastigial nuclei (FN) neurons with no eye movement–related activity during rotation and translation (VN: Angelaki and Dickman 2000; Chen-Huang and Peterson 2006; Dickman and Angelaki 2002; Musallam and Tomlinson 2002; FN: Büttner et al. 1991; Gardner and Fuchs 1975; Shaikh 2004; Shaikh et al. 2005a; Siebold et al. 1997, 1999; Zhou et al. 2001). With the exception of Chen-Huang and Peterson (2006), characterization of responses to translation was limited to the horizontal plane. Responsiveness to rotation and translation in these earlier studies was taken as the signature of otolith/canal convergence. More recently, however, it has become increasingly clear that the two types of convergence are not necessarily identical. In particular, there is little argument that responses to both yaw rotation and translation suggest otolith/canal convergence. However, modulation to translational but not rotational stimuli does not necessarily exclude semicircular canal inputs to the cell. This occurs because otolith afferents respond during both translation and rotation (e.g., roll and pitch from an upright orientation). Thus the only way for central neurons to respond exclusively to translation is by canal signals canceling out otolith activation during tilt movements relative to gravity (Angelaki et al. 2004; Shaikh et al. 2005b; Yakusheva et al. 2007, 2008).
In contrast to vestibular stimulation, studies testing the responses of central vestibular neurons during visual or optokinetic stimulation (OKS) have been limited. Waespe and Henn (1977a,b, 1978, 1979) were the first to show modulation of macaque VN neurons during constant velocity yaw optokinetic nystagmus (OKN) and optokinetic after-nystagmus (OKAN). These results were generally in agreement to those in the goldfish (Allum et al. 1976; Dichgans et al. 1973), rabbit (Dichgans and Brandt 1972), rat (Precht 1981), cat (Precht 1981), and guinea pig (Azzena et al. 1974). Using sinusoidal stimulation, Boyle et al. (1985) showed that yaw OKS modulation of VN neurons was limited to low frequencies. Although not quantified in detail, rostral FN neurons also modulate during OKS (Büttner et al. 1991). Most OKS studies were limited to a single axis of stimulation and characterized neural responses while the animals elicited an optokinetic nystagmus. As a result, the retinal stimulus was continuously varying, thus preventing quantification of the true visual responsiveness of the neurons.
Here we have evaluated the responsiveness of macaque VN and FN neurons to three-dimensional (3D) optokinetic stimulation at two frequencies: 0.5 and 0.02 Hz (one above and the other within the frequency range of the velocity storage mechanism; Cohen et al. 1977). In contrast to previous studies, we characterized neural responses during OKS cancellation (i.e., while monkeys fixated a head-fixed target), thus providing a controlled retinal stimulus. In addition, we tested VN and FN responses during OKS and vestibular stimulation in 3D, allowing for the first time a comparison between preferred vestibular and OKS responsiveness.
METHODS
Experiments were performed on three juvenile macaque monkeys (Macaca mulatta), weighing 5–7 kg. Our general experimental procedures were similar to previous studies (Angelaki and Dickman 2000; Angelaki et al. 2001; Gu et al. 2006; Meng et al. 2005). Briefly, animals were chronically implanted with a circular, delrin ring and scleral search coils for measuring horizontal and vertical eye movements using a three-field magnetic search coil system (Robinson 1963). All surgical and experimental procedures were performed under sterile conditions, in accordance with the Institutional Animal Care and Use Committee at Washington University and National Institutes of Health guidelines.
Sinusoidal 3D motion was delivered using a six degrees of freedom motion platform (6DOF2000E, MOOG, East Aurora, NY). In all experiments, the head was positioned such that the horizontal stereotaxic plane was earth-horizontal, with the axis of rotation always centered at the center of the head. Because we did not have access to a 3D optokinetic sphere, the visual stimuli used here were generated by a three-chip DLP projector (Mirage 2000, Christie Digital Systems, Cypress, CA) that was mounted on top of the motion platform and rear-projected images onto a 60 × 60-cm tangent screen that was viewed by the monkey from a distance of 30 cm (thus subtending 90 × 90° of visual angle; see Gu et al. 2006 for details). The tangent screen was mounted on the front of the field coil frame. The sides, top, and back of the coil frame were covered with black enclosures such that the monkey's field of view was restricted to visual stimuli presented on the screen.
The optokinetic stimuli simulated yaw, pitch, and roll rotation inside a sphere (70 cm radius) whose inner surface consisted of spots of varying sizes (density of 0.01 dots/cm2 and dot sizes randomly chosen from a Gaussian distribution centered at 7° with an SD of 1.5°). This was achieved by generating stereoscopic images of the simulated sphere (using an OpenGL accelerator board; nVidia Quadro FX 3000G), which were projected onto the flat screen and were viewed binocularly through Kodak written filters (29 and 61, Kodak, Rochester, NY) mounted on custom-made goggles. Two OpenGL cameras were required for accurate rendering of sphere rotation, binocular disparity, and texture cues (for details, see Gu et al. 2006). This provided an effective optokinetic stimulus that generated a robust nystagmus during both low and high frequencies.
Behavioral control and data acquisition were controlled by Spike2 scripts and the CED system (Cambridge Electronic Design, Cambridge, UK). During all neural recordings, animals suppressed their OKN/VOR by fixating on a central target that was front-projected onto the screen using a head-fixed laser. The behavioral performance of the animal was continuously monitored using electronic windows, which ensured that right and/or left eye positions were maintained within 2.5° of ideal target fixation. This “eye-in-window” signal was monitored by the CED for on-line juice reward delivery and was saved for off-line analyses. Juice rewards were typically given at a frequency of once every ∼3 s, as long as eye position was within the specified behavioral windows. To ensure that these stimuli were adequate to generate optokinetic nystagmus, horizontal and vertical eye movements were also collected in the absence of any behavioral control.
Extracellular recordings were obtained using epoxy-coated, etched tungsten microelectrodes using standard electrophysiological techniques. We recorded from vestibular only (VO) cells in the rostral FN and rostral VN (mostly rostral medial and caudal superior nuclei), as identified by the absence of eye movement–related activity during 0.5-Hz, ±10° horizontal and vertical pursuit eye movements. Once a VO cell was identified, we tested its responsiveness during the following protocols: 1) 0.5-Hz, ±7° yaw, pitch, and roll rotation; 2) 0.5-Hz, ±10 cm lateral, fore-aft, and vertical translation; and 3) yaw, pitch, and roll OKS at two frequencies: 0.5 (±7°) and 0.02 Hz (±175°). For both frequencies, peak velocity was kept constant at 22°/s, a stimulus sufficient to elicit robust OKN and OKAN (Cohen et al. 1977; Waespe and Henn 1977a). Note that, because we wanted to characterize neural responses during OKN cancellation (i.e., in the absence of reflexive eye movements, for a consistent retinal stimulus), constant velocity OKN and OKAN were not used in these experiments.
The data were analyzed off-line using custom-written MATLAB scripts (MathWorks, Natick, MA). Eye position was calibrated and slow phase eye velocity computed by filtered differentiation after removal of fast phases of nystagmus through a semiautomated computer algorithm (see Angelaki 1998; Angelaki and Hess 1994 for details). Neural activity was expressed as instantaneous firing rate (IFR), calculated as 1/interspike interval, and assigned to the middle of the interval. IFRs from multiple stimulus cycles were folded in time into a single cycle instantaneous frequency response for each stimulus condition. This procedure provides no averaging, because all spike occurrences are represented in time (Fig. 1; see Meng et al. 2005 for details). For OKN/VOR cancellation tasks, only portions of data in which the eye position was within 2.5° of the target were included in the folding and further analyses.
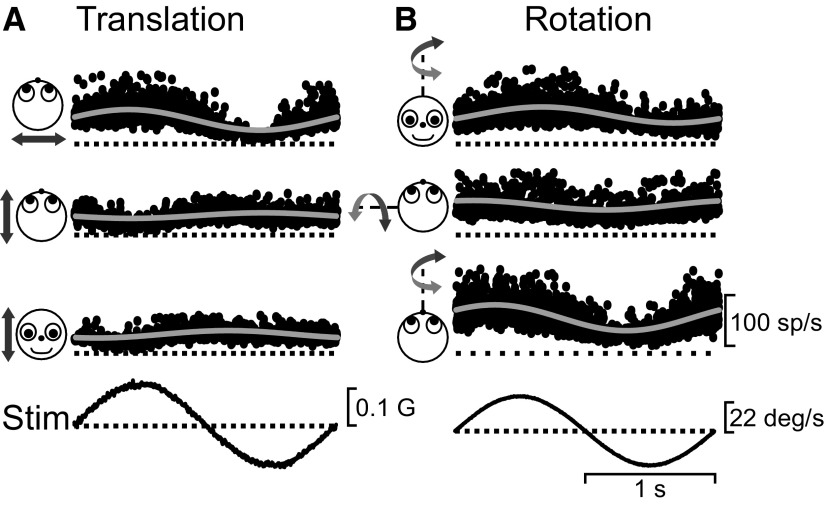
FIG. 1.
Responses from a rostral vestibular nuclei (VN) neuron during 3-dimensional (3D) vestibular stimulation. Small dots show instantaneous firing rate during (A) translation and (B) rotation at 0.5 Hz (12–16 cycles superimposed). Motion directions are indicated by the cartoon drawings. The solid gray lines show the sinusoidal fits. The stimulus traces (Stim) show linear acceleration (A) or angular velocity (B). Response gains were as follows—A: 209 (P ≪ 0.001, lateral), 55 (P = 0.05, fore-aft), and 74 sp/s/G (P = 0.01, vertical); B: 0.75 (P ≪ 0.001, yaw), 0.43 (P ≪ 0.001, pitch), and 1.19 sp/s/deg/s (P ≪ 0.001, roll).
Gain and phase were computed by fitting a sine function (1st and 2nd harmonics, and a DC offset) using a nonlinear least-squares algorithm (see Dickman and Angelaki 2002; Meng et al. 2005; Shaikh et al. 2005a for details). To assign a statistical significance in the response modulation for each neuron, we also computed average binned histograms (40 bins per cycle) and a Fourier ratio (FR), defined as the ratio of the fundamental over the maximum of the first 20 harmonics. The statistical significance of FR was based on a permutation analysis. Briefly, the 40 response bins were shuffled randomly, thus destroying the systematic modulation in the data but maintaining the inherent variability of the responses. The FR was computed from those randomly permuted histograms, and the randomization process was repeated 1,000 times. If the FR for the original data exceeded that for 99% of the permuted data sets, we considered the temporal modulation to be statistically significant (P < 0.01).
Preferred response directions are described in spherical coordinates as combinations of azimuth and elevation. A spatio-temporal convergence (STC) function was used to calculate each cell's preferred direction (Angelaki 1991, 1992; Schor and Angelaki 1992). This was done by first computing the preferred direction in the horizontal plane. The polar angle of this vector defined the azimuth of the 3D-preferred response. We computed its elevation by applying the spatio-temporal model in a vertical plane defined by the preferred direction in the horizontal plane and the vertical axis. This procedure allows computation of the azimuth and elevation of the 3D-preferred direction in spherical coordinates, the gain and phase of the 3D-preferred direction, and the tuning ratio (i.e., ratio of the minimum over maximum response) in each of the horizontal and vertical planes.
RESULTS
Neural responses to 3D rotation and translation
We recorded from 53 neurons in the rostral VN (rostral medial and caudal superior nuclei) and 89 neurons in the rostral FN during pitch/roll/yaw rotation and/or lateral/fore-aft/vertical translation at 0.5 Hz. Data were collected exclusively from neurons without any eye movement sensitivity. An example response from a VN neuron that showed some modulation during motion along all six axes (lateral/fore-aft/vertical translation and yaw/pitch/roll rotation) is shown in Fig. 1, A and B. Based on these responses, the cell was classified as a convergent neuron. Note that the present classification of VN/FN neurons as convergent or nonconvergent applies to their responsiveness during 3D translation and 3D rotation and not to their presumed canal and otolith inputs. As shown previously, many central neurons that selectively modulate during translation, but not during rotation, receive signals from both otolith and semicircular canals (Angelaki et al. 2004; Shaikh et al. 2005a,b; Yakusheva et al. 2007).
The majority of VN and FN neurons (32/42, 76% and 59/85, 69%, respectively) were significantly modulated for at least one of the three cardinal directions during both translation and rotation; thus they were considered convergent cells. In contrast, 7 cells (17%) in the VN and 20 cells (24%) in the FN were only significantly modulated during translation. The remaining three (7%) VN and six (7%) FN neurons were only modulated during rotation.
Using responses from all three axes, we computed the 3D-preferred direction of 47 VN and 82 FN neurons significantly tuned to translation, as well as 38 VN and 66 FN neurons significantly tuned to rotation (Fig. 2, A and B, respectively; definitions of azimuth and elevation are shown in Fig. 2C). The corresponding gain distributions along each cell's preferred direction are shown in Fig. 2, E and F. Preferred directions, plotted in the form of azimuth and elevation scatter plots, were distributed throughout the 3D space. Translation response preferences were uniformly distributed in both horizontal and vertical planes (uniformity test, P > 0.05). Tuning ratios in each of the horizontal and vertical planes were broadly distributed, with 45% of the VN and 22% of the FN neurons having both tuning ratios <0.2. Approximately 19% (VN) and 28% (FN) had tuning ratios >0.2 in both horizontal and vertical planes. For rotational response preferences, there was a weak departure from uniformity for both azimuth and elevation in FN cells (Fig. 2B, red; P = 0.03) and for elevation in VN cells (Fig. 2B, blue; P = 0.01). When comparing the azimuth of the 3D-preferred directions for rotation and translation (Fig. 2D), data points were scattered throughout the range, suggesting the absence of tilt-aligned rotation/translation preferences, as previously described in rats (Angelaki et al. 1993; Bush et al. 1993). That is, cells that prefer forward (lateral) motion do not necessarily prefer pitch (roll) rotations and vice versa.
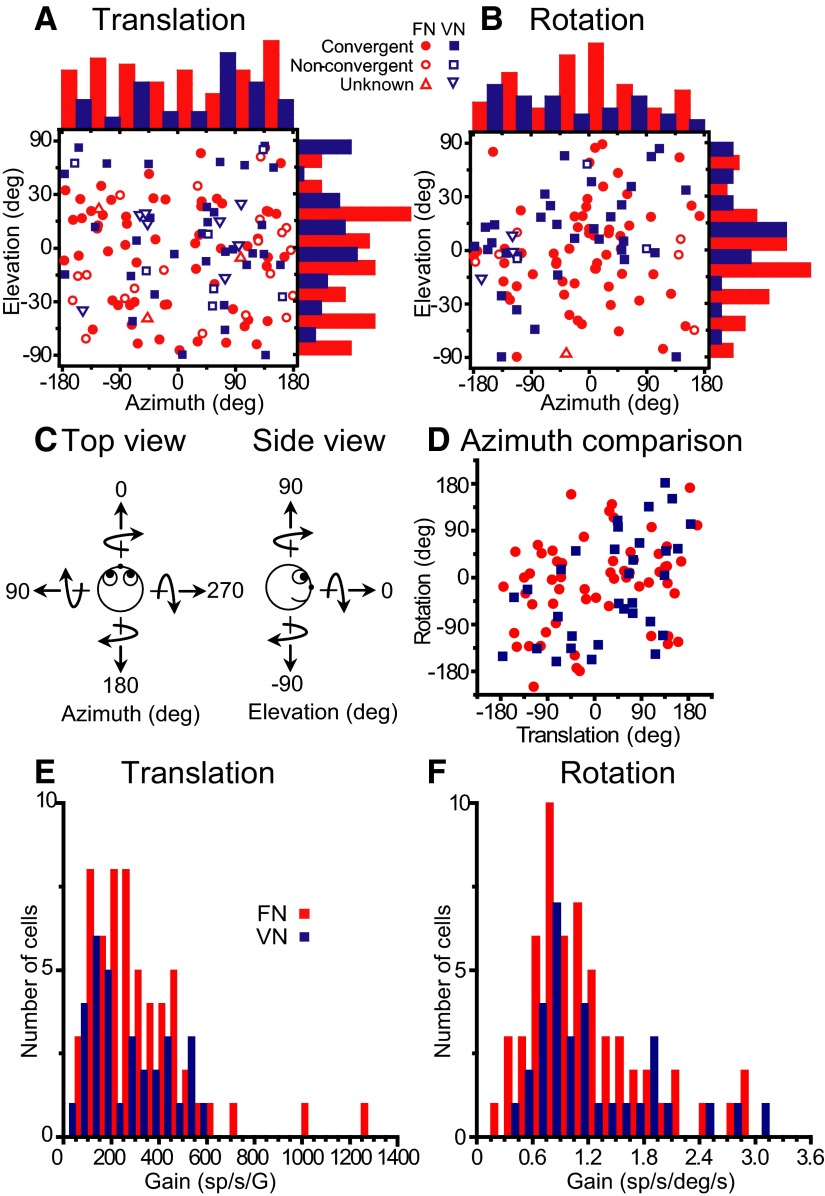
FIG. 2.
Summary of rotation/translation responses in 3D. A and B: distributions of preferred directions (shown in spherical coordinates as azimuth and elevation) during translation and rotation, respectively. Each data point in the scatter plots corresponds to the preferred azimuth (abscissa) and elevation (ordinate) of a single neuron with significant responses along at least 1 motion direction. The data are plotted on Cartesian axes that represent the Lambert cylindrical equal-area projection of the spherical stimulus space (see Gu et al. 2006 for details). Histograms along the top and right sides of each scatter plot show the marginal distributions. Filled symbols indicate cells with significant modulation along at least 1 direction during both rotation and translation (convergent). Open symbols show nonconvergent cells that only modulate during either translation (A) or rotation (B). Cells that were not tested during both rotation and translation are shown with open triangles. C: definition of azimuth and elevation: Top and side view. Straight arrows depict the direction of translation, whereas curved arrows show the direction of rotation around each of the movement axes (based on the right-hand rule). D: scatter plot comparing preferred azimuths for rotation and translation (shown only for convergent cells). For tilt-aligned rotation/translation preferences, data points should fall along the ±90° diagonals (e.g., forward translation is defined with an azimuth of 0°, but a pitch rotation-tilt has an azimuth of 90/270°). E and F: distribution of neural response gains along the 3D preferred direction for translation and rotation, respectively. Fastigial nuclei (FN) neurons, red symbols/bars; VN neurons, blue symbols/bars.
Neural responses to 3D optokinetic stimulation
Forty-one of the vestibular-sensitive cells in the VN and FN were also tested with optokinetic stimuli. In the absence of the head-fixed fixation target during yaw and pitch OKS, animals generated a robust horizontal and vertical optokinetic nystagmus (Fig. 3). Figure 3, A and B, shows representative examples of the nystagmus evoked during 0.5- and 0.02-Hz OKS, respectively. Figure 3, C and D, summarizes the distributions of peak horizontal and vertical eye velocities evoked during yaw and pitch OKS, respectively [mean amplitude: 17.7°/s for yaw, 23.3°/s for pitch OKN (0.5 Hz) and 18.9°/s for yaw, 24.0°/s for pitch OKN (0.02 Hz)]. To have a greater control over the retinal stimulus, VN/FN neurons were tested while the animal suppressed its optokinetic nystagmus by fixating a head-fixed target. Only a small percentage of VN/FN neurons were significantly modulated along at least one stimulus direction; VN: 2/12 (17%) at 0.5 Hz and 4/10 (40%) at 0.02 Hz; FN: 2/31 (6%) at 0.5 Hz and 15/31 (48%) at 0.02 Hz.
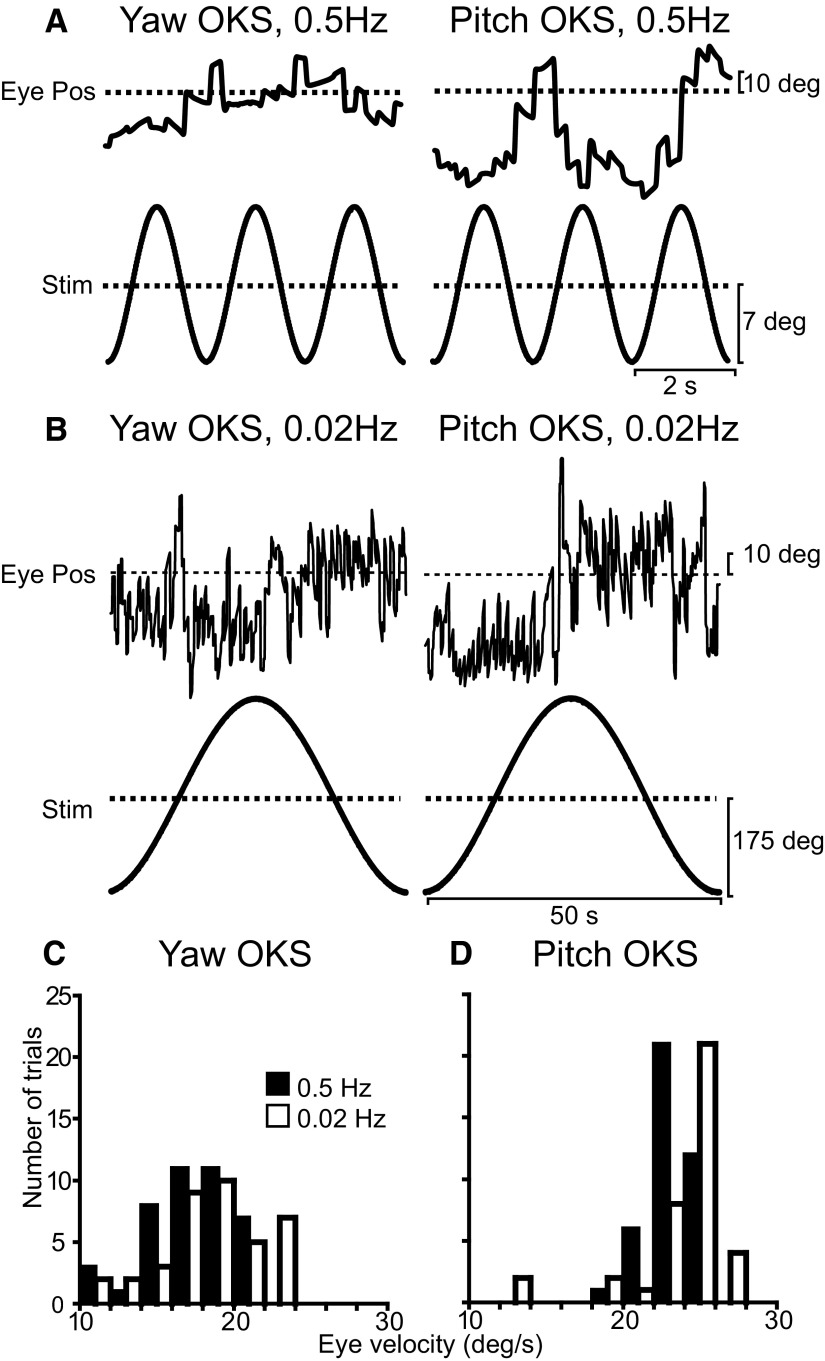
FIG. 3.
Nystagmus elicited during yaw and pitch optokinetic stimulation (OKS). A and B: examples of horizontal (left) and vertical (right) eye position during yaw and pitch OKS at 0.5 and 0.02 Hz, respectively. C and D: histograms of peak horizontal and peak vertical slow phase eye velocity elicited during yaw and pitch OKS, respectively. Data are shown separately for 0.5 (filled bars) and 0.02 Hz (open bars).
Even for responsive cells, modulation amplitude was small, as shown with the example in Fig. 4A that shows one of the most responsive FN neurons to yaw, pitch, and roll OKS at 0.5 (top) and 0.02 Hz (bottom). Like the vestibular responses, we computed the preferred direction and gain of the OKS responses of VN/FN neurons. Figure 4B compares the respective gains for all cells that were significantly modulated to at least one direction during rotation and optokinetic stimulation (VN: 5 cells; FN: 16 cells). Data at both 0.5 Hz (filled symbols) and 0.02 Hz (open symbols) fall above the unity line, indicating poorer response modulation to OKS than vestibular stimulation. Preferred directions were not matched, as shown in Fig. 4C, which plots the difference in 3D-preferred directions between 0.5-Hz rotation and 0.02-Hz OKS responses (because of the small number of OKS-responsive cells at 0.5 Hz, this comparison is not included). Distributions were not significantly different from uniform (uniformity test, P > 0.05).
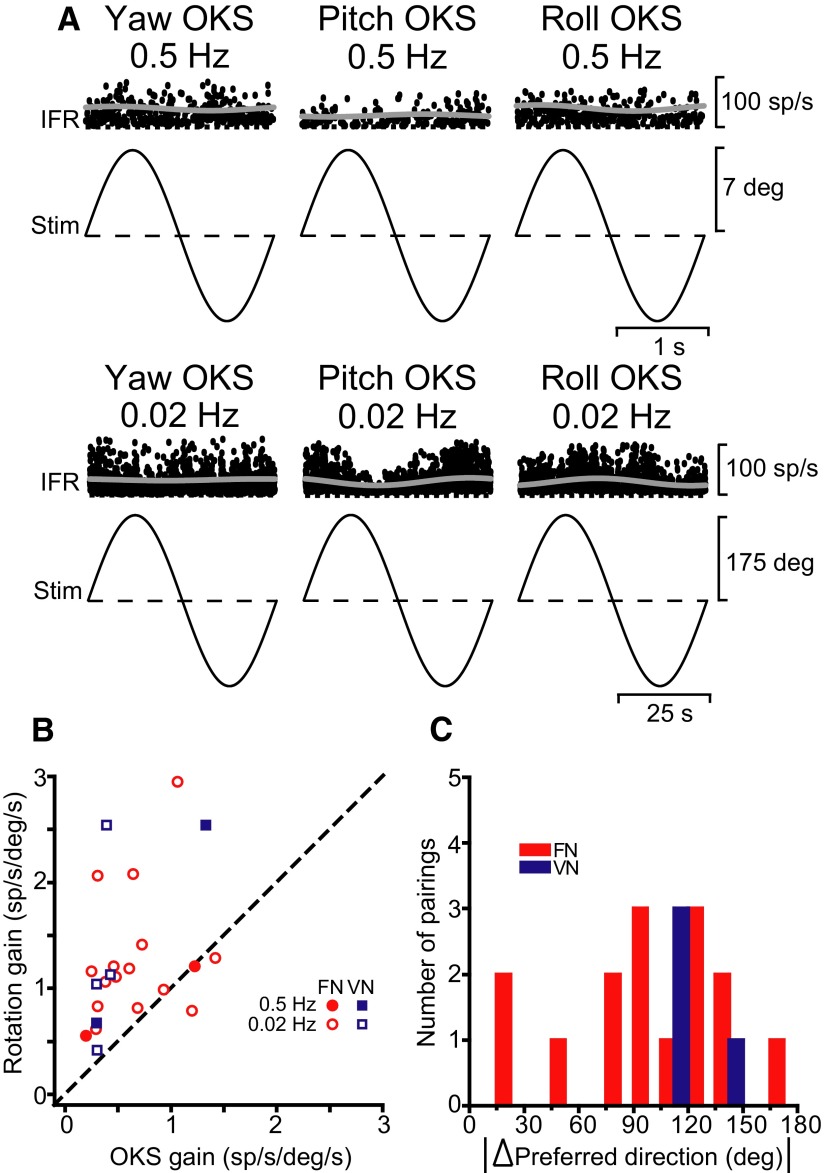
FIG. 4.
A: examples of neural responses from a FN cell during yaw, pitch, and roll OKS at 0.5 (top, ∼15 cycles superimposed) and 0.02 Hz (bottom, ∼3 cycles superimposed). Response gains were as follows—0.5 Hz: 0.20 (P = 0.6, yaw OKS), 0.12 (P = 0.5, pitch OKS), and 0.28 sp/s/deg/s (P = 0.3, roll OKS); 0.02 Hz: 0.07 (P = 0.09, yaw OKS), 0.34 (P ≪ 0.001, pitch OKS), and 0.33 sp/s/deg/s (P = 0.03, roll OKS). B: scatter plot comparing 3D neural response gains during rotation and OKS, shown separately for FN (red symbols) and VN (blue symbols) at 0.5 (filled symbols) and 0.02 Hz (open symbols). Data are only shown for cells with significant modulation during both rotation and OKS. C: distribution of the difference in 3D preferred directions for responses to 0.5-Hz rotation and 0.02-Hz OKS stimulation. A difference of 180° signifies alignment of preferred directions; 0° represents anti-alignment.
DISCUSSION
We characterized vestibular and OKS responsiveness during 3D stimulation in the rostral VN and rostral FN. Unlike previous reports that have implied that all units that were activated by vestibular stimulation could also be activated by moving optokinetic patterns (Buettner and Büttner 1979; Henn et al. 1974; Waespe and Henn 1977a,b), we found that, at most, one half of the vestibular-responsive neurons modulated during sinusoidal OKS at 0.02 Hz. The fact that this number decreased drastically down to only less than a handful of responsive neurons at 0.5 Hz is in agreement with the findings of Boyle et al. (1985), who showed that most VN neurons only modulate during low-frequency yaw OKS. In particular, only a few neurons responded at 0.2 Hz and none >1 Hz (Boyle et al. 1985). The larger proportion of OKS-responding neurons at 0.02 Hz, a frequency that is within the range of velocity storage mechanism (Raphan et al. 1979), suggests that non-eye movement-sensitive VN/FN neurons might mediate the indirect, slow component, but not the direct, fast component of OKN (Cohen et al. 1977).
Despite these general similarities with earlier studies, even responsive cells typically exhibited small modulation during OKS. This observation differs from the robust responses reported previously by Waespe and Henn (1977a,b, 1978, 1979). There are multiple differences between these experiments and those of previous studies. First, the stimuli are different: we used 0.5- and 0.02-Hz sinusoidal stimulation, whereas the studies of Waespe and Henn used constant velocity OKN and OKAN. Second, unlike Waespe and Henn (1977a,b, 1978, 1979), our animals suppressed reflexive eye movements. Indeed, Buettner and Büttner (1979) also reported reduced neuronal responses in the majority of VN neurons during OKS suppression. Third, previous studies used actual drum rotation, whereas our optokinetic stimuli were simulated using OpenGL. Although we believe that this is an adequate stimulus (because it elicits robust optokinetic nystagmus; Fig. 3), we cannot exclude the possibility that neural responses were less robust because sphere rotation was not real but simulated. Finally, we want to emphasize that our aim for these experiments was to characterize neural responses during OKN cancellation (i.e., in the absence of reflexive eye movements) as part of a greater effort to study the visual motion responses of subcortical neurons. This could not have been possible by recording (like previous studies did) neural activities during OKAN. Perhaps responses are different during sinusoidal stimulation in the absence of reflexive eye movements (this study) and OKAN (Waespe and Henn 1977a,b, 1978, 1979). However, the latter does not test for visual (sensory) responsiveness. Neuronal modulation during optokinetic nystagmus and afternystagmus cannot be interpreted as either sensory- or motor-driven. Previous studies have instead shown that VN neurons modulate during activation of the velocity storage mechanism. In contrast, these experiments, where we controlled the retinal motion stimulus by suppressing reflexive eye movements, aimed to test for visual (sensory) responsiveness. Results point out that, unlike a widely accepted belief in the field, true visual/vestibular interactions might be rather limited in the vestibular and rostral fastigial nuclei.
To our knowledge, this is the first study to compare the preferred OKS and rotational preference for VN/FN neurons. We found no relationship between the two, although the number of cells used for this comparison (requiring neurons responsive to both) was small. This result is surprising, given the strong behavioral relationship between the rotational vestibulo-ocular and optokinetic reflexes (Cohen et al. 1977; Raphan et al. 1979). Additional data, with this comparison available for a larger number of neurons, might be necessary to reliably compare these relationships.
Precht (1981) showed that lesions of the nucleus of the optic tract (NOT) or nucleus reticularis tegmental pontis (NRTP) in the cat and rat abolished OKS responses in the VN. In primates, it is generally thought that descending projections from the nucleus of the optic tract (NOT) and possibly the dorsal terminal nucleus (DTN) project through the central tegmental tract to the ipsilateral vestibular complex, where diffuse terminals are seen in the medial vestibular nucleus (Büttner-Ennever et al. 1996). The lateral terminal nucleus (LTN) is also known to project to the superior and medial VN (Blanks et al. 2000). A similar but smaller projection is also found from the medial terminal nucleus (MTN) to the superior and lateral VN.
Finally, optokinetic signals to the VN/FN can also be carried by Purkinje cell projections from the ipsilateral nodulus and uvula (Carleton and Carpenter 1983; Voogd et al. 1996; Wylie et al. 1994). Although the OKS responses of nodulus/uvula Purkinje cells have yet to be quantified in alert primates, there is evidence that simple spike responses in the anesthetized rabbit modulate during OKS (Kano et al. 1991a,b). Further experiments are necessary to quantify the properties and origin of optokinetic modulation in vestibular-responsive brain stem and cerebellar areas, as well as potential differences in responses between low-frequency sinusoidal versus constant velocity OKN and OKAN.
GRANTS
This work was supported by National Eye Institute rant R01 EY-12814.
Acknowledgments
The authors thank H. Meng and T. Yakusheva for assistance with the neural recordings.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Allum et al. 1976.Allum JH, Graf W, Dichgans J, Schmidt CL. Visual-vestibular interactions in the vestibular nuclei of the goldfish. Exp Brain Res 26: 463–485, 1976. [DOI] [PubMed] [Google Scholar]
- Angelaki 1991.Angelaki DE Dynamic polarization vector of spatially tuned neurons. IEEE Trans Biomed Eng 38: 1053–1060, 1991. [DOI] [PubMed] [Google Scholar]
- Angelaki 1992.Angelaki DE Spatio-temporal convergence (STC) in otolith neurons. Biol Cybern 67: 83–96, 1992. [DOI] [PubMed] [Google Scholar]
- Angelaki 1998.Angelaki DE Three-dimensional organization of otolith-ocular reflexes in rhesus monkeys. III. Responses to translation. J Neurophysiol 80: 680–695, 1998. [DOI] [PubMed] [Google Scholar]
- Angelaki et al. 1993.Angelaki DE, Bush GA, Perachio AA. Two-dimensional coding of linear acceleration in vestibular nuclei neurons. J Neurosci 13: 1403–1417, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelaki and Dickman 2000.Angelaki DE, Dickman JD. Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses. J Neurophysiol 84: 2113–2132, 2000. [DOI] [PubMed] [Google Scholar]
- Angelaki et al. 2001.Angelaki DE, Green AM, Dickman JD. Differential sensorimotor processing of vestibulo-ocular signals during rotation and translation. J Neurosci 21: 3968–3985, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelaki and Hess 1994.Angelaki DE, Hess BJ. Inertial representation of angular motion in the vestibular system of rhesus monkeys. I. Vestibuloocular reflex. J Neurophysiol 71: 1222–1249, 1994. [DOI] [PubMed] [Google Scholar]
- Angelaki et al. 2004.Angelaki DE, Shaikh AG, Green AM, Dickman JD. Neurons compute internal models of the physical laws of motion. Nature 430: 560–564, 2004. [DOI] [PubMed] [Google Scholar]
- Azzena et al. 1974.Azzena GB, Azzena MT, Marini R. Optokinetic nystagmus and the vestibular nuclei. Exp Neurol 42: 158–168, 1974. [DOI] [PubMed] [Google Scholar]
- Blanks et al. 2000.Blanks RHI, Giolli RA, van der Want JJL. Neural circuitry and neurotransmitters in the pretectal and accessory optic systems. In: Neurochemistry of the Vestibular System, edited by Beitz AJ, Anderson JH. Boca Raton, FL: CRC, 2000.
- Boyle et al. 1985.Boyle R, Büttner U, Markert G. Vestibular nuclei activity and eye movements in the alert monkey during sinusoidal optokinetic stimulation. Exp Brain Res 57: 362–369, 1985. [DOI] [PubMed] [Google Scholar]
- Buettner et al. 1991.Büttner U, Fuchs AF, Markert-Schwab G, Buckmaster P. Fastigial nucleus activity in the alert monkey during slow eye and head movements. J Neurophysiol 65: 1360–1371, 1991. [DOI] [PubMed] [Google Scholar]
- Buettner and Büttner 1979.Buettner UW, Büttner U. Vestibular nuclei activity in the alert monkey during suppression of vestibular and optokinetic nystagmus. Exp Brain Res 37: 581–593, 1979. [DOI] [PubMed] [Google Scholar]
- Buettner-Ennever et al. 1996.Büttner-Ennever JA, Cohen B, Horn AK, Reisine H. Pretectal projections to the oculomotor complex of the monkey and their role in eye movements. J Comp Neurol 366: 348–359, 1996. [DOI] [PubMed] [Google Scholar]
- Bush et al. 1993.Bush GA, Perachio AA, Angelaki DE. Encoding of head acceleration in vestibular neurons. I. Spatiotemporal response properties to linear acceleration. J Neurophysiol 69: 2039–2055, 1993. [DOI] [PubMed] [Google Scholar]
- Carleton and Carpenter 1983.Carleton SC, Carpenter MB. Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. Brain Res 278: 29–51, 1983. [DOI] [PubMed] [Google Scholar]
- Chen-Huang and Peterson 2006.Chen-Huang C, Peterson BW. Three dimensional spatial-temporal convergence of otolith related signals in vestibular only neurons in squirrel monkeys. Exp Brain Res 168: 410–426, 2006. [DOI] [PubMed] [Google Scholar]
- Cohen et al. 1977.Cohen B, Matsuo V, Raphan T. Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol 270: 321–344, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichgans and Brandt 1972.Dichgans J, Brandt T. Visual-vestibular interaction and motion perception. Bibl Ophthalmol 82: 327–338, 1972. [PubMed] [Google Scholar]
- Dichgans et al. 1973.Dichgans J, Schmidt CL, Graf W. Visual input improves the speedometer function of the vestibular nuclei in the goldfish. Exp Brain Res 18: 319–322, 1973. [DOI] [PubMed] [Google Scholar]
- Dickman and Angelaki 2002.Dickman JD, Angelaki DE. Vestibular convergence patterns in vestibular nuclei neurons of alert primates. J Neurophysiol 88: 3518–3533, 2002. [DOI] [PubMed] [Google Scholar]
- Gardner and Fuchs 1975.Gardner EP, Fuchs AF. Single-unit responses to natural vestibular stimuli and eye movements in deep cerebellar nuclei of the alert rhesus monkey. J Neurophysiol 38: 627–649, 1975. [DOI] [PubMed] [Google Scholar]
- Gu et al. 2006.Gu Y, Watkins PV, Angelaki DE, DeAngelis GC. Visual and nonvisual contributions to three-dimensional heading selectivity in the medial superior temporal area. J Neurosci 26: 73–85, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn et al. 1974.Henn V, Young LR, Finley C. Vestibular nucleus in alert monkeys are also influenced by moving visual fields. Brain Res 71: 144–149, 1974. [DOI] [PubMed] [Google Scholar]
- Kano et al. 1991a.Kano M, Kano MS, Maekawa K. Optokinetic response of simple spikes of Purkinje cells in the cerebellar flocculus and nodulus of the pigmented rabbit. Exp Brain Res 87: 484–496, 1991a. [DOI] [PubMed] [Google Scholar]
- Kano et al. 1991b.Kano M, Kano MS, Maekawa K. Simple spike modulation of Purkinje cells in the cerebellar nodulus of the pigmented rabbit to optokinetic stimulation. Neurosci Lett 128: 101–104, 1991b. [DOI] [PubMed] [Google Scholar]
- Meng et al. 2005.Meng H, Green AM, Dickman JD, Angelaki DE. Pursuit-vestibular interactions in brain stem neurons during rotation and translation. J Neurophysiol 93: 3418–3433, 2005. [DOI] [PubMed] [Google Scholar]
- Musallam and Tomlinson 2002.Musallam S, Tomlinson RD. Asymmetric integration recorded from vestibular-only cells in response to position transients. J Neurophysiol 88: 2104–2113, 2002. [DOI] [PubMed] [Google Scholar]
- Precht 1981.Precht W Visual-vestibular interaction in vestibular neurons: functional pathway organization. Ann NY Acad Sci 374: 230–248, 1981. [DOI] [PubMed] [Google Scholar]
- Raphan et al. 1979.Raphan T, Matsuo V, Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res 35: 229–248, 1979. [DOI] [PubMed] [Google Scholar]
- Robinson 1963.Robinson DA A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10: 137–145, 1963. [DOI] [PubMed] [Google Scholar]
- Schor and Angelaki 1992.Schor RH, Angelaki DE. The algebra of neural response vectors. Ann NY Acad Sci 656: 190–204, 1992. [DOI] [PubMed] [Google Scholar]
- Shaikh 2004.Shaikh AG Multiple reference frames for motion in the primate cerebellum. J Neurosci 24: 4491–4497, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh et al. 2005a.Shaikh AG, Ghasia FF, Dickman JD, Angelaki DE. Properties of cerebellar fastigial neurons during translation, rotation and eye movements. J Neurophysiol 93: 853–863, 2005a. [DOI] [PubMed] [Google Scholar]
- Shaikh et al. 2005b.Shaikh AG, Green AM, Ghasia FF, Newlands SD, Dickman JD, Angelaki DE. Sensory convergence solves a motion ambiguity problem. Curr Biol 15: 1657–1662, 2005b. [DOI] [PubMed] [Google Scholar]
- Siebold et al. 1997.Siebold C, Glonti L, Glasauer S, Büttner U. Rostral fastigial nucleus activity in the alert monkey during three-dimensional passive head movements. J Neurophysiol 77: 1432–1446, 1997. [DOI] [PubMed] [Google Scholar]
- Siebold et al. 1999.Siebold C, Kleine JF, Glonti L, Tchelidze T, Büttner U. Fastigial nucleus activity during different frequencies and orientations of vertical vestibular stimulation in the monkey. J Neurophysiol 82: 34–41, 1999. [DOI] [PubMed] [Google Scholar]
- Voogd et al. 1996.Voogd J, Gerrits NM, Ruigrok TJ. Organization of the vestibulocerebellum. Ann NY Acad Sci 781: 553–579, 1996. [DOI] [PubMed] [Google Scholar]
- Waespe and Henn 1977a.Waespe W, Henn V. Neuronal activity in the vestibular nuclei of the alert money during vestibular and optokinetic stimulation. Exp Brain Res 27: 523–538, 1977a. [DOI] [PubMed] [Google Scholar]
- Waespe and Henn 1977b.Waespe W, Henn V. Vestibular nuclei activity during optokinetic after-nystagmus (OKAN) in the alert monkey. Exp Brain Res 30: 323–330, 1977b. [DOI] [PubMed] [Google Scholar]
- Waespe and Henn 1978.Waespe W, Henn V. Conflicting visual-vestibular stimulation and vestibular nucleus activity in alert monkeys. Exp Brain Res 33: 203–211, 1978. [DOI] [PubMed] [Google Scholar]
- Waespe and Henn 1979.Waespe W, Henn V. The velocity response of vestibular nucleus neurons during vestibular, visual and combined angular acceleration. Exp Brain Res 37: 337–347, 1979. [DOI] [PubMed] [Google Scholar]
- Wylie et al. 1994.Wylie DR, De Zeeuw CI, DiGiorgi PL, Simpson JI. Projections of individual Purkinje cells of identified zones in the ventral nodulus to the vestibular and cerebellar nuclei in the rabbit. J Comp Neurol 349: 448–463, 1994. [DOI] [PubMed] [Google Scholar]
- Yakusheva et al. 2008.Yakusheva TA, Blazquez PM, Angelaki DE. Frequency-selective coding of translation and tilt in macaque cerebellar nodulus and uvula. J Neurosci 28: 9997–10009, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakusheva et al. 2007.Yakusheva TA, Shaikh AG, Green AM, Blazquez PM, Dickman JD, Angelaki DE. Purkinje cells in posterior cerebellar vermis encode motion in an inertial reference frame. Neuron 54: 973–985, 2007. [DOI] [PubMed] [Google Scholar]
- Zhou et al. 2001.Zhou W, Tang BF, King WM. Responses of rostral fastigial neurons to linear acceleration in an alert monkey. Exp Brain Res 137: 111–115, 2001. [DOI] [PubMed] [Google Scholar]