Abstract
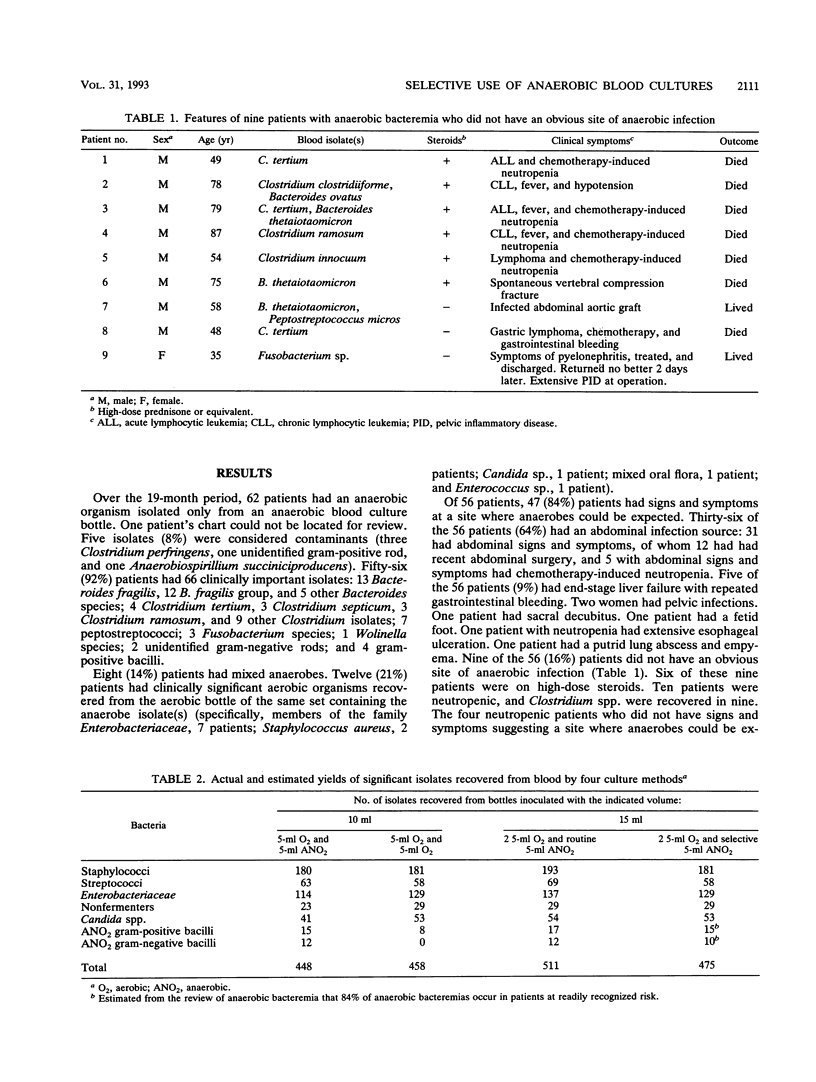
Because of the declining frequency of anaerobic bacteremia, routinely using half the collected blood volume for anaerobic culture has been challenged. There is no data indicating whether more clinically relevant isolates would be recovered if all or most of the given blood sample were cultured aerobically. In this two-part study, we reviewed cases of anaerobic bacteremia to determine what proportion occurred in situations when anaerobes would be expected and then estimated the yield of different culture approaches by reanalyzing the data from a large prospective clinical blood culture study. The records of 61 patients who had an anaerobic isolate (excluding Propionibacterium species) recovered only from an anaerobic bottle were examined to define clinical settings in which such isolates occur. Fifty-six (92%) patients had clinically important isolates, and the source of infection was obvious at the time of culture in 47 of the 56 (84%). Of 56 patients, 36 (64%) had abdominal signs and symptoms, including 12 with recent abdominal surgery. Of nine patients without an obvious source of infection, six were on high-dose steroids. Relative yields were compared for (i) one aerobic bottle and one anaerobic bottle (5 ml to each) for all blood cultures, (ii) two aerobic bottles (5 ml to each), or (iii) two aerobic bottles plus an extra anaerobic bottle (only for clinically suspected anaerobic sepsis) (5 ml to each). The third approach had the highest yield (475 isolates), because the routine use of two aerobic bottles recovered more Candida spp., members of the family Enterobacteriaceae, and nonfermenters than did the first approach (448 isolates) (P < 0.02), and clinically directed culturing for anaerobes would recover anaerobes missed with the second approach (458 isolates). Our data suggest that the use of two aerobic bottles with selective culturing for anaerobes could increase the number of clinically relevant isolates by at least 6% compared with the current practice of inoculating an aerobic bottle and an anaerobic bottle with equal volumes of blood.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G., Condon R. E., Gorbach S. L., Clarke J. S., Nichols R. L., Ochi S. Veterans Administration Cooperative Study on Bowel Preparation for Elective Colorectal Operations: impact of oral antibiotic regimen on colonic flora, wound irrigation cultures and bacteriology of septic complications. Ann Surg. 1978 Aug;188(2):249–254. doi: 10.1097/00000658-197808000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodner S. J., Koenig M. G., Goodman J. S. Bacteremic Bacteroides infections. Ann Intern Med. 1970 Oct;73(4):537–544. doi: 10.7326/0003-4819-73-4-537. [DOI] [PubMed] [Google Scholar]
- Brook I. Anaerobic bacterial bacteremia: 12-year experience in two military hospitals. J Infect Dis. 1989 Dec;160(6):1071–1075. doi: 10.1093/infdis/160.6.1071. [DOI] [PubMed] [Google Scholar]
- Case C. P., MacGowan A. P., Brown N. M., Reeves D. S., Whitehead P., Felmingham D. Prophylactic oral fluconazole and candida fungaemia. Lancet. 1991 Mar 30;337(8744):790–790. doi: 10.1016/0140-6736(91)91406-k. [DOI] [PubMed] [Google Scholar]
- Chow A. W., Guze L. B. Bacteroidaceae bacteremia: clinical experience with 112 patients. Medicine (Baltimore) 1974 Mar;53(2):93–126. [PubMed] [Google Scholar]
- Cuchural G. J., Jr, Tally F. P., Jacobus N. V., Aldridge K., Cleary T., Finegold S. M., Hill G., Iannini P., O'Keefe J. P., Pierson C. Susceptibility of the Bacteroides fragilis group in the United States: analysis by site of isolation. Antimicrob Agents Chemother. 1988 May;32(5):717–722. doi: 10.1128/aac.32.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchural G. J., Jr, Tally F. P., Jacobus N. V., Cleary T., Finegold S. M., Hill G., Iannini P., O'Keefe J. P., Pierson C. Comparative activities of newer beta-lactam agents against members of the Bacteroides fragilis group. Antimicrob Agents Chemother. 1990 Mar;34(3):479–480. doi: 10.1128/aac.34.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida A. E., De Uzeda M. Susceptibility to five antimicrobial agents of strains of the Bacteroides fragilis group isolated in Brazil. Antimicrob Agents Chemother. 1987 Apr;31(4):617–618. doi: 10.1128/aac.31.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsher C. W., Rosenblatt J. E., Wilson W. R., Ilstrup D. M. Anaerobic bacteremia: decreasing rate over a 15-year period. Rev Infect Dis. 1991 Jul-Aug;13(4):633–636. doi: 10.1093/clinids/13.4.633. [DOI] [PubMed] [Google Scholar]
- Downes J., Andrew J. H. Susceptibility of 114 isolates of the Bacteroides fragilis group to imipenem and eight other antimicrobial agents. Pathology. 1988 Jul;20(3):260–263. doi: 10.3109/00313028809059504. [DOI] [PubMed] [Google Scholar]
- Edson R. S., Rosenblatt J. E., Lee D. T., McVey E. A., 3rd Recent experience with antimicrobial susceptibility of anaerobic bacteria: increasing resistance to penicillin. Mayo Clin Proc. 1982 Dec;57(12):737–741. [PubMed] [Google Scholar]
- Elhag K. M., Mustafa A. K., Pazhoor A. A. The changing pattern of antibiotic susceptibilities of Bacteroides fragilis in Kuwait. J Antimicrob Chemother. 1989 Nov;24(5):699–707. doi: 10.1093/jac/24.5.699. [DOI] [PubMed] [Google Scholar]
- Felner J. M., Dowell V. R., Jr "Bacteroides" bacteremia. Am J Med. 1971 Jun;50(6):787–796. doi: 10.1016/0002-9343(71)90187-2. [DOI] [PubMed] [Google Scholar]
- Finegold S. M. Anaerobes: problems and controversies in bacteriology, infections, and susceptibility testing. Rev Infect Dis. 1990 Jan-Feb;12 (Suppl 2):S223–S230. doi: 10.1093/clinids/12.supplement_2.s223. [DOI] [PubMed] [Google Scholar]
- Hadfield T. L., Smith M. B., Winn R. E., Rinaldi M. G., Guerra C. Mycoses caused by Candida lusitaniae. Rev Infect Dis. 1987 Sep-Oct;9(5):1006–1012. doi: 10.1093/clinids/9.5.1006. [DOI] [PubMed] [Google Scholar]
- Henderson G., Garner J., Morris A. Antimicrobial susceptibility of anaerobic bacteria in Auckland 1987-90. N Z Med J. 1992 Jan 22;105(926):11–12. [PubMed] [Google Scholar]
- Ingram C. W., Cooper J. N. Clostridial bloodstream infections. South Med J. 1989 Jan;82(1):29–31. doi: 10.1097/00007611-198901000-00009. [DOI] [PubMed] [Google Scholar]
- Katlic M. R., Derkac W. M., Coleman W. S. Clostridium septicum infection and malignancy. Ann Surg. 1981 Mar;193(3):361–364. doi: 10.1097/00000658-198103000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi D. P., Engleberg N. C. Anaerobic bacteremia: incidence, patient characteristics, and clinical significance. Am J Med. 1992 Jan;92(1):53–60. doi: 10.1016/0002-9343(92)90015-4. [DOI] [PubMed] [Google Scholar]
- Merz W. G. Candida lusitaniae: frequency of recovery, colonization, infection, and amphotericin B resistance. J Clin Microbiol. 1984 Dec;20(6):1194–1195. doi: 10.1128/jcm.20.6.1194-1195.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morace G., Manzara S., Dettori G. In vitro susceptibility of 119 yeast isolates to fluconazole, 5-fluorocytosine, amphotericin B and ketoconazole. Chemotherapy. 1991;37(1):23–31. doi: 10.1159/000238828. [DOI] [PubMed] [Google Scholar]
- Murray P. R., Traynor P., Hopson D. Critical assessment of blood culture techniques: analysis of recovery of obligate and facultative anaerobes, strict aerobic bacteria, and fungi in aerobic and anaerobic blood culture bottles. J Clin Microbiol. 1992 Jun;30(6):1462–1468. doi: 10.1128/jcm.30.6.1462-1468.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musial C. E., Rosenblatt J. E. Antimicrobial susceptibilities of anaerobic bacteria isolated at the Mayo Clinic during 1982 through 1987: comparison with results from 1977 through 1981. Mayo Clin Proc. 1989 Apr;64(4):392–399. doi: 10.1016/s0025-6196(12)65727-9. [DOI] [PubMed] [Google Scholar]
- Peláez M. T., Cercenado E., Rodríguez-Créixems M., Bouza E. Resistance of anaerobic bacteria to antimicrobial agents. Rev Infect Dis. 1991 Jan-Feb;13(1):183–183. doi: 10.1093/clinids/12.5.183. [DOI] [PubMed] [Google Scholar]
- Persons D. A., Laughlin M., Tanner D., Perfect J., Gockerman J. P., Hathorn J. W. Fluconazole and Candida krusei fungemia. N Engl J Med. 1991 Oct 31;325(18):1315–1315. [PubMed] [Google Scholar]
- Speirs G., Warren R. E., Rampling A. Clostridium tertium septicemia in patients with neutropenia. J Infect Dis. 1988 Dec;158(6):1336–1340. doi: 10.1093/infdis/158.6.1336. [DOI] [PubMed] [Google Scholar]
- Thaler M., Gill V., Pizzo P. A. Emergence of Clostridium tertium as a pathogen in neutropenic patients. Am J Med. 1986 Oct;81(4):596–600. doi: 10.1016/0002-9343(86)90543-7. [DOI] [PubMed] [Google Scholar]
- Valtonen M., Sivonen A., Elonen E. A cluster of seven cases of Clostridium tertium septicemia in neutropenic patients. Eur J Clin Microbiol Infect Dis. 1990 Jan;9(1):40–42. doi: 10.1007/BF01969532. [DOI] [PubMed] [Google Scholar]
- Warnock D. W., Burke J., Cope N. J., Johnson E. M., von Fraunhofer N. A., Williams E. W. Fluconazole resistance in Candida glabrata. Lancet. 1988 Dec 3;2(8623):1310–1310. doi: 10.1016/s0140-6736(88)92919-4. [DOI] [PubMed] [Google Scholar]
- Wexler H. M., Harris B., Carter W. T., Finegold S. M. Six-year retrospective survey of the resistance of Bacteroides fragilis group species to clindamycin and cefoxitin. Diagn Microbiol Infect Dis. 1986 Mar;4(3):247–253. doi: 10.1016/0732-8893(86)90104-5. [DOI] [PubMed] [Google Scholar]
- Wilson M. L., Weinstein M. P., Reimer L. G., Mirrett S., Reller L. B. Controlled comparison of the BacT/Alert and BACTEC 660/730 nonradiometric blood culture systems. J Clin Microbiol. 1992 Feb;30(2):323–329. doi: 10.1128/jcm.30.2.323-329.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W. R., Martin W. J., Wilkowske C. J., Washington J. A., 2nd Anaerobic bacteremia. Mayo Clin Proc. 1972 Sep;47(9):639–646. [PubMed] [Google Scholar]
- Wingard J. R., Merz W. G., Rinaldi M. G., Johnson T. R., Karp J. E., Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991 Oct 31;325(18):1274–1277. doi: 10.1056/NEJM199110313251803. [DOI] [PubMed] [Google Scholar]



