Abstract
Animals actively regulate the position and movement of their sensory systems to boost the quality and quantity of the sensory information they obtain. The rat vibrissal system is recognized to be an important model system in which to investigate such “active sensing” capabilities. The current study used high-speed video analysis to investigate whisker movements in untrained, freely moving rats encountering unexpected, vertical surfaces. A prominent feature of rat vibrissal movement is the repeated posterior–anterior sweep of the whiskers in which the macrovibrissae are seen to move largely in synchrony. Here we show that a second significant component of whisking behavior is the size of the arc, or “spread,” between the whiskers. Observed spread is shown to vary over the whisk cycle and to substantially decrease during exploration of an unexpected surface. We further show that the timing of whisker movements is affected by surface contact such that 1) the whiskers rapidly cease forward protraction following an initial, unexpected contact, and may do so even more rapidly following contact with the same surface in the subsequent whisk cycle, and 2) retraction velocity is reduced following this latter contact, leading to longer second-contact durations. This evidence is taken to support two hypotheses: 1) that the relative velocities of different whiskers may be actively controlled by the rat and 2) that control of whisker velocity and timing may serve to increase the number and duration of whisker–surface contacts while ensuring that such contacts are made with a light touch.
INTRODUCTION
Adult rats sweep their large facial whiskers (macrovibrissae) back and forth in a rhythmic behavior known as whisking, observed under most conditions of natural locomotion and exploration (Carvell and Simons 1990; Gustafson and Felbain-Keramidas 1977; Hartmann 2001; Vincent 1912). Recent research has demonstrated that exploratory whisking, in freely moving animals, often diverges from the regular, largely bilaterally symmetric and synchronous motor pattern that has been recorded when immobilized rats are trained to whisk in air (e.g., Bermejo et al. 2002; Gao et al. 2001). Specifically, asymmetries, asynchronies, and changes in whisk amplitude and frequency have been documented (Berg and Kleinfeld 2003; Carvell and Simons 1990; Mitchinson et al. 2007; Sachdev et al. 2003; Sellien et al. 2005; Towal and Hartmann 2006, 2008) that may be the consequence of “active sensing” control strategies. Such strategies use sensor information, or task knowledge, to control the position and movement of the sensory apparatus in a manner likely to boost the amount of useful sensory information obtained (Aloimonos et al. 1988; Ballard 1991; Gibson 1962; Lungarella et al. 2005). In the current study we set out to discover whether other aspects of whisker control might also be subject to this kind of active regulation and to identify some of the likely sensory consequences of these forms of anticipatory control.
Our specific focus concerns how the rat modifies the movements of its whiskers, and of its head and body, over the course of a sequence of whisk cycles during which contact is made with an unexpected vertical surface. We have previously demonstrated immediate, contact-induced changes in the timing of whisker control and longer-term, anticipatory modulations of whisk amplitude (Mitchinson et al. 2007). Here we will present evidence of changes in a further whisking parameter: the relative spacing, or “spread,” between adjacent whiskers. We will provide data suggesting that this measure not only captures a significant, and previously unquantified, fraction of variance in whisking behavior, but that it also appears to be modified by active control mechanisms following contact and in anticipation of further contacts.
During exploratory whisking, the initial contact with a vertical surface triggers a rapid cessation of whisker protraction (and commencement of retraction) such that the whiskers palpate the surface with a relatively light touch (Mitchinson et al. 2007). We have termed this active control strategy “minimal impingement,” since whisker movements appear to be modulated to reduce whisker bending (impingement) against the contacted surface. The investigation of such whisking control strategies is important to understanding the nature of the tactile stimuli that are processed in upstream neural centers, such as the somatosensory cortex, during natural behavior. For instance, evidence for “minimal impingement” strategies suggests that the whisker stimuli that are transmitted to these structures may be less intense than might otherwise be expected, whereas “maximal contact” strategies that involve regulating whisk amplitude or spread, could lead us to expect greater numbers of whisker–surface contacts than if control is not subject to such modulation. A further important parameter for understanding upstream sensory processing is the duration of whisker–surface contacts: while the whiskers are in contact with the surface the animal may be able to extract useful information about surface shape or texture. Our investigation will therefore also consider how differential control of whisker movements during interactions with surfaces could influence these important determinants of the tactile signals processed by the rat brain.
METHODS
Experimental procedures
ANIMALS.
Ten male Royal College of Surgeons (RCS) dystrophic rats, aged 6–18 mo and weighing 250–350 g, were used. All animals had genetic retinal degeneration (dystrophy) and thus minimal vision; they were therefore strongly reliant on tactile information from their whiskers during locomotion and exploration behavior. Hetherington et al. (2000) previously showed that the capacity to orient to whisker stimulation is unimpaired in dystrophic RCS animals compared with other rat strains, although they did note a deficit in orienting to a tactile stimulus on the flank. Observations in our laboratory of dystrophic and nondystrophic RCS animals and of sighted Hooded Lister rats suggest that whisking control in dystrophic animals does not deviate in any marked way from that of normally sighted rats. All animals were kept in a 12-h dark/light cycle at 22°C, with unrestricted access to water and food; tested during the dark (active) part of their daily cycle; and handled prior to being placed in the experimental arena. All procedures were approved by the local Ethics Committee and UK Home Office, under the terms of the UK Animals (Scientific Procedures) Act of 1986.
APPARATUS.
Digital video recordings were made using a Photron Fastcam PCI camera, recording at 500 frames/s, shutter speed of 0.5 ms, and resolution of 1,024 × 1,024. The camera was suspended from the ceiling above a custom-built rectangular (40 × 40 cm) viewing arena with a glass floor, ceiling, and end-wall (see Supplemental Fig. S1).1 The arena contained a front-silvered mirror, positioned behind the end-wall, to afford two viewpoints to the camera. The camera was also positioned so that it looked directly down the end and side walls in the overhead view; the front-silvered mirror was angled at about 45° so that the camera looked along the surface of the floor in the reflected, end-on view. The arena was illuminated from below by a custom-built, high-power light box, and from the far end of the arena facing the end-wall by a second, suitably angled mirror. The design and geometry of the arena thus provided uniform backlighting in two dimensions, with the field of view of the camera covering close to 20 × 20 cm in the overhead view and 8 × 20 cm in the end-on view. To obtain an accurate measure of camera/arena geometry a recording was also made of a three-dimensional (3D) calibration tool, with known shape and location, following initial positioning of the camera.
RECORDING.
Opportunistic recordings, each 3–4 s in length, were taken of awake, unrestrained animals engaged in active, exploratory behavior. Each animal was filmed on between 2 and 11 occasions with ≤12 clips recorded, per rat, in each filming session. Each recording was initiated by an experimenter viewing the camera scene on a monitor window and pressing a trigger when the animal entered the field of view. In all, 334 recordings were obtained in this way with between 15 and 82 per animal.
Data selection
Video clips were selected for whisker tracking and detailed analysis as follows. To qualify for analysis each video was required to contain three consecutive whisks during which the rat approached and contacted an identified arena wall, with whiskers on the side of the snout ipsilateral to the initial contact clearly visible in the overhead view throughout. Furthermore, these three whisks were required to be of the following types:
) An initial precontact whisk, in which the whiskers did not contact the identified wall
) A first-contact whisk, in which at least two whiskers made contact with this vertical surface
) A second-contact whisk, in which the same two whiskers, and possibly others, made a further contact with the same surface
The constraint that there should be contacts on at least two whiskers in each cycle was enforced to reduce the possibility of including clips in which surface contacts were so slight that they might be below the rat's detection thresholds (Stuttgen et al. 2006). The requirement that the same two whiskers made the initial contact with the wall, in both the first- and second-contact whisks, was enforced to exclude some of the more atypical interactions with surfaces. In all, 60 clips (between 1 and 15 per animal) were identified that satisfied these criteria.
We note that the clips selected for analysis do not, in general, record the animal's first experience of the experimental arena. However, in sightless animals, anticipation of a surface prior to whisker contact would require the synthesis of path integration information with acquired knowledge of the arena configuration. Due to the accumulation of error in neuronal path integration mechanisms and evidence that such mechanisms may be specialized toward encoding position with respect to a home base (Etienne et al. 1996), it is unlikely that the RCS rat will have access to an accurate estimate of the relative position of a wall following a period of roaming, during which there was no tactile contact with that surface. In the clips we analyzed, then, the initial contact with the wall, in the first-contact whisk, was considered to be “unexpected” in the sense that the animal is assumed to lack accurate prior knowledge of its own position relative to that surface prior to contact. More generally, our results do not require that the rat has no prior knowledge of nearby surfaces, but rather that the initial whisker contact provides information that greatly improves the animal's capacity to localize a nearby surface and is therefore useful for directing subsequent exploratory behavior.
Whisker tracking and head tracking
For the 60 selected clips, and for each of the three selected whisks, five whiskers, ipsilateral to the initial contact, were tracked on an LCD flat-screen monitor by a human observer using uncompressed video and a purpose-built tracking tool. Whisker tracking, in this case, used only the overhead view and involved tracking two points, one near the base of the whisker, the other two thirds of the way out along the whisker shaft (see Supplemental Fig. S1). This is referred to as the overhead tracking set in the following text. The tracked whiskers were assumed to correspond to whisker columns 0–4, although our results do not depend on this assumption. To estimate head movements, the tip of the snout and the midpoint of the head were also tracked in the overhead view and the height of the snout above the floor in the end-on view. Note that, although technologies have recently been developed for automated tracking of whisker movement (Knutsen et al. 2005, 2008; Voigts et al. 2008), the difficult problem of tracking multiple whiskers in a complete, intact whisker field has yet to be automated. No smoothing was performed on the tracked whisker angular position or head-movement data; for whisker velocity a moving average filter that computes a running average of three adjacent points was used to reduce any effects of tracking inaccuracies.
The overhead tracking data for each clip were analyzed to obtain the descriptive measures listed in Table 1. For the calculation of per-whisk summary measures (Table 1, B and D) the point of minimum protraction between two successive protraction–retraction phases was taken as the separation boundary between consecutive whisks. The clips were also examined to determine whether contacts with the target surface, in the first-contact whisk, occurred on one side or both sides of the snout. Two subsets were identified for further analysis: 1) the unilateral set (n = 25) in which contact, during the first-contact whisk, was on one side only, and 2) the bilateral set (n = 35) in which there were whisker–surface contacts on both sides of the snout in the first-contact whisk. Further details concerning our tracking and data analysis methods are provided in the Supplement, including raw data (whisker trajectories) for two exemplar clips (Supplemental Fig. S4).
TABLE 1.
Definitions of whisking, contact-related, and head movement measures
| Name | Unit | Description |
|---|---|---|
| A. Whisker movement measures per video frame (overhead view) | ||
| Angular position | degrees | Angle from the whisker shaft to the head midline, such that forward movement (protraction) causes an increase in angle |
| Mean angular position | degrees | Mean angular position across all five tracked whiskers |
| Velocity | deg/ms | Rate of change of the mean angular position |
| Spread | degrees | Difference between the largest and smallest instantaneous angular positions of the five tracked whiskers |
| B. Summary whisker movement measures per whisk (or per specified portion of a whisk) | ||
| Minimum and maximum protraction | degrees | Minimum and maximum values of the mean angular position. Minimum protraction is also sometimes referred to as peak retraction or the whisking “set point” |
| Mean, minimum, and maximum spread | degrees | Summary measures of spread |
| Mean protraction and retraction velocities | deg/ms | Summary measures of velocity calculated separately for the protraction and retraction phases of the whisk |
| Whisk duration | ms | Time from the previous minimum protraction to the next |
| C. Measures relating to the initial contact with the wall within a whisk | ||
| Number of contacts on tracked whiskers | 2–5 | Total number of tracked whiskers making contact with the wall over the course of the whisk |
| Angular velocity at contact | deg/ms | Instantaneous angular velocity of the whisker making first contact calculated from the two frames preceding the contact |
| Angular position at contact | degrees | Mean angular position in the frame immediately prior to the first surface contact |
| Contact duration | ms | Time from initial contact to the first contacting whisker becoming detached from the surface (measured for the most rostral whisker if two whiskers make simultaneous first contact) |
| Time from contact to maximum protraction | ms | Time from the initial contact to maximum protraction averaged across all five tracked whiskers |
| D. Head position measures per whisk | ||
| Snout elevation | mm | Mean distance from the snout tip to the floor (vertical view) over the course of the whisk |
| Snout vertical velocity | mm/ms | Absolute value of the rate of change in snout elevation |
| Head orientation | degrees | Mean angle of the head relative to the wall (overhead view), with zero being perpendicular and all angles positive, over the course of the whisk |
| Head angular velocity | deg/ms | Absolute value of the rate of change in head orientation |
| Distance to wall | mm | Mean length of the perpendicular from the snout tip to the wall (overhead view) over the course of the whisk |
| Velocity toward wall | mm/ms | Rate of change in distance to wall |
| Velocity along wall | mm/ms | Rate of change of head position parallel to the wall and floor (overhead view) |
3D whisker-tip trajectory reconstruction
To better evaluate the contribution of head movements to the patterns of whisker movement observed in the overhead view, four representative clips were selected for 3D reconstruction of whisker-tip trajectories. Tracking and trajectory reconstruction, using a stereo correspondence algorithm, were performed as described in the Supplemental Material.
Statistical considerations
The primary focus of this investigation was on contrasts within triplets of consecutive whisks; data were therefore pooled across animals with the video clip of each tracked whisking episode taken as the fundamental unit for analysis. Except where otherwise specified the results presented were calculated for the 60 selected clips in the overhead tracking data set. MANOVA was used to test for overall differences between whisk types in relation to each of the main classes of data, with univariate ANOVAs and appropriate post hoc tests used to examine differences on specific variables. An alpha-level of 0.05 (two-tailed) was used for statistical tests corrected for multiple comparisons, when required, using a Bonferroni correction. Significant results are summarized in the following text and figures, with SE bars included in graphs where appropriate. Further details of statistical analyses, including tables of all univariate ANOVA tests, including means, SDs, F-values, and effect sizes, are provided in the Supplemental Material.
RESULTS
Our presentation of results is structured as follows. In the next section we provide a principal components analysis (PCA) of our whisker position data and show that the two main components of these data, determined algorithmically, correspond with the theoretically meaningful measures of the mean angular position of the whiskers and whisker spread. Next, in Variation across whisk types in summary measures of whisker control, whisker–surface contacts, and head position, we quantitatively characterize the whisking behavior of the rat during exploration of an unexpected surface by analyzing variation in summary measures of whisk control (Table 1B), surface contact (Table 1C), and head movement (Table 1D). Armed with a good understanding of the differences between the three whisk types, A closer look at whisker spread then considers whisker spread in more detail, evaluating several alternatives to the hypothesis that spread is controlled using differential whisker velocities and in anticipation of surface contacts. Finding that anticipatory control of whisker velocity is supported by the available data, Sensory consequences of whisker control then examines whether such control is consistent with a “maximizing contact” strategy. We then turn our attention to the “minimal impingement” aspect of our active touch hypothesis and consider whether this is supported by our new data concerning the timing of cessation of protraction following contact. Finally, we look at the duration of whisker–surface contacts and explore the relationship of this important variable to several elements of whisker control, particularly the angular velocity of the whiskers during the retraction phase.
Principal components of whisker configurations
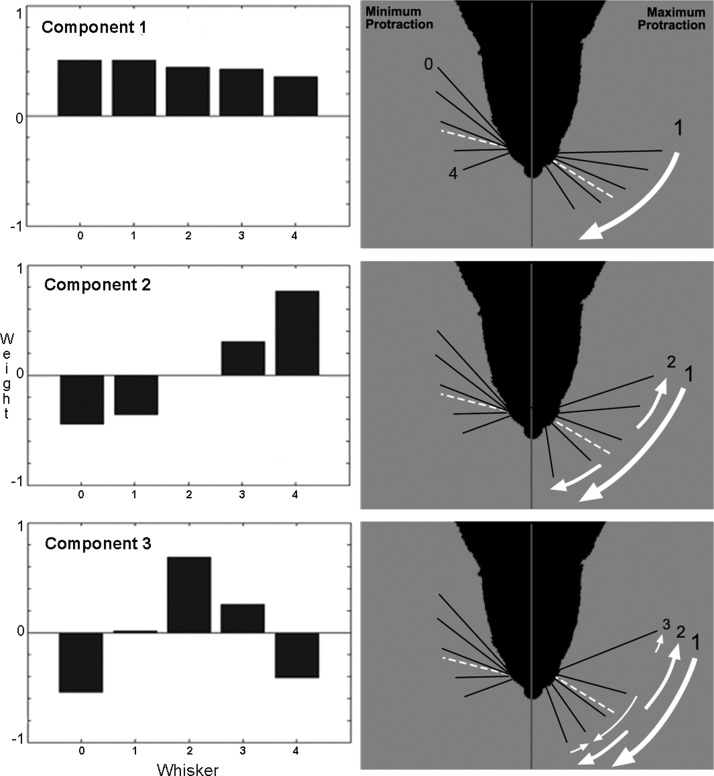
To better characterize the nature of rat whisking control a PCA analysis was run on the raw whisker angular position data from all five tracked whiskers and across all three specified whisk types (precontact, first contact, second contact). Three components were found to be present in this data set, as illustrated in Fig. 1.
Component 1 explained 80.4% (precontact whisk), 76.8% (first contact), and 70.1% (second contact) of the variance in whisker positions (Fig. 1, top) and was extremely well correlated (r > 0.99) with the mean angular position across all whisk types. Thus as might be expected since whisker movement is strongly synchronized, the average protraction angle of the whisker field strongly predicts the positions of all the tracked whiskers.
Component 2 explained 13.4% (precontact whisk), 15.5% (first contact), and 20.2% (second contact) of the variance in whisker positions (Fig. 1, center) and was well correlated with whisker spread (precontact: r = 0.90; first contact: r = 0.91; second contact: r = 0.92). Therefore the overall change in the angular separation of the whiskers constitutes the second most important parameter for describing the spatial arrangement of the whiskers at any time during the whisk.
Component 3 explained 3.1% (precontact whisk), 3.8% (first contact), and 4.6% (second contact) of the variance in whisker positions (Fig. 1, bottom), but was not significantly correlated with any of the measured whisking parameters. Component 3 is best described as the middle whisker columns (1–3), tending to be more protracted than the two outer columns (0 and 4). Although the proportion of variance explained is small, this component was present in every whisk type, suggesting it is unlikely to be an artifact of the measurement/analysis process (in contrast, components 4 and 5 did not have a consistent shape across whisk types and so are probably not meaningful).
FIG. 1.
The principal components of rat whisker configurations. The first 3 principal components account for 70–80% (component 1, top), 13–20% (component 2, center), and 3–5% (component 3, bottom) of the variance in whisker angles seen in the overhead view. Here the histograms on the left indicate the weighting on each tracked whisker (0: most caudal; 4: most rostral) for each component. The images on the right illustrate the components graphically and cumulatively (top: component 1; center: 1 + 2; bottom: 1 + 2 + 3). Each image shows the whiskers at minimum protraction on the left-hand side of the rat snout and at maximum protraction, as determined by the effects of the principal components (white arrows), on the right-hand side. Dotted white lines indicate the mean angular position. Note that the first 2 components are highly correlated with mean angular position and whisker spread, respectively, and together account for 89–93% of the variance in observed whisker positions.
In summary, then, although the whiskers largely move as a group (70–80% of variance captured by their mean angular position), there is important variation in relative whisker positions (13–20%) that is well characterized by the measure of whisker spread. Using these two components together can account for 90–94% of all the variance in the whisker position data seen in the overhead view. In other words, given just these two values for each frame we could reconstruct the actual angular positions of all five tracked whiskers to this level of accuracy.
Having established mean angular position and spread as the two most informative parameters for describing whisker configurations it is useful to briefly consider the phase relationship of the two measures over the course of the whisk cycle. In Supplemental Fig. S5 we show, using cross-covariance analysis, that in the precontact and first-contact whisks, spread peaks reliably 0–6 ms before angular position. However, this relationship breaks down somewhat in the second-contact whisk, where we see a much weaker coupling between the two measures with out-of-phase relationships (>10-ms difference between the angular position and spread peaks) in 26 (43%) of the 60 whisks. The temporal relationship between spread and angular position seen in the precontact and first-contact whisks is consistent with observations by Sachdev et al. (2002), made in head-fixed animals whisking in air, who described the spread as being maximal at, or close to, maximum protraction. That this relationship is much less in evidence for the second-contact whisk is consistent with the hypothesis, explored in the following text, that active control mechanisms may be influencing whisker spread when the rat is able to anticipate contact with a vertical surface.
Variation across whisk types in summary measures of whisker control, whisker–surface contacts, and head position
VARIATION IN WHISKER CONTROL ACROSS WHISK TYPES.
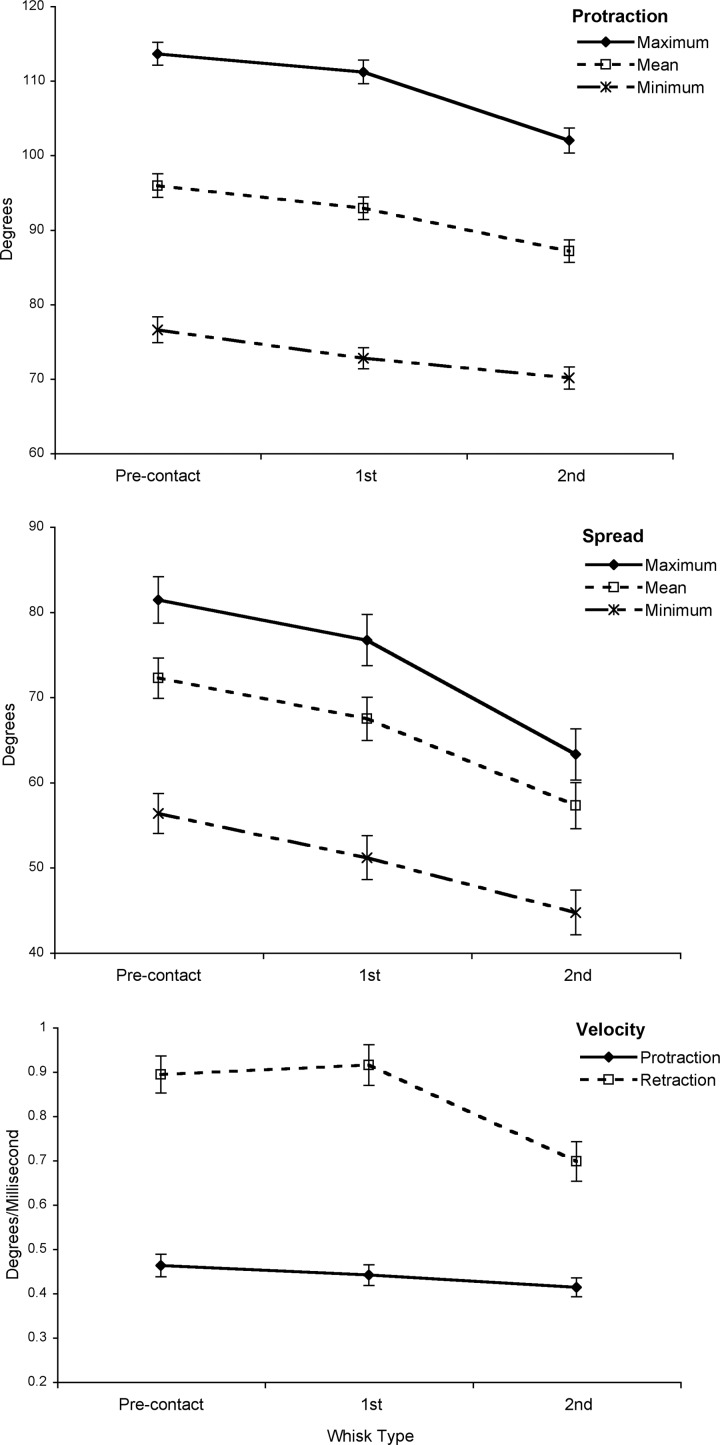
To begin our investigation of changes in whisk control following unexpected surface contacts a MANOVA was conducted using whisk type as a three-way within-subjects factor and the summary whisker movement measures (Table 1B) maximum protraction angle, minimum protraction angle, mean spread, mean protraction velocity, mean retraction velocity, and whisk duration as dependant variables. This analysis revealed a significant multivariate difference between whisk types (P < 0.001). To determine which aspects of whisking were contributing to this result, separate three-way univariate ANOVAs were then carried out on each of these six variables as detailed in Supplemental Table S1. All of the analyzed variables showed reductions between the precontact and second-contact whisk, with the first-contact whisk being, on the whole, more similar to the precontact whisk than to the second-contact whisk (as indicated by post hoc tests). Two parameters, in particular, stood out as showing large changes by the second-contact whisk—the mean spread and the mean retraction velocity. These measures fell by 21% (P < 0.001) and 22% (P < 0.001), respectively, over the course of the three whisks. Smaller but still significant changes also occurred in minimum and maximum protraction angles of the whisk (7 and 10%, P < 0.001). Figure 2 provides graphs illustrating each of the significant trends in whisk protraction angle, spread, and velocity. Examples of the reduction in overall spread are also provided in Supplemental Video S1 and in the sequence of snapshots in Fig. 3. Comparison of clips with bilateral and unilateral first contacts found a significant interaction effect between whisk type and whether the initial contact was on one side or both sides (P < 0.001; see Supplemental Fig. S6), indicating that the reduction in spread across whisks was more pronounced on occasions where there were bilateral surface contacts.
FIG. 2.
Changes in whisk protraction, spread, and velocity on encountering an unexpected vertical surface. From top to bottom: maximum, minimum, and mean protraction (degrees); maximum, minimum, and mean spread (degrees); and mean protraction and retraction velocities for precontact, 1st-contact, and 2nd-contact whisks. All of these measures, except protraction velocity, are reduced significantly by the 2nd-contact whisk.
FIG. 3.

Snapshots of whiskers at maximum protraction for 3 consecutive whisks. From bottom to top: precontact, 1st contact, and 2nd contact. Whisker spread is significantly reduced by the 2nd-contact whisk. These snapshots are taken from the clip provided as Supplemental Video S1.
VARIATION IN WHISKER–SURFACE CONTACT ACROSS WHISK TYPES.
We next examine the relationship between whisker movement and surface contact for the two contact whisks. Four variables that have potentially important sensory consequences—and therefore deserve attention—are the number of whisker contacts, the angular velocity and angular position of the whisker at the point of contact, and the duration of whisker–surface contact (see Table 1C for definitions). Further, since we previously proposed “minimal impingement” as a general characteristic of exploratory whisking (Mitchinson et al. 2007) we will also examine the time from contact to maximum protraction, predicting that control of whisker movement following contact will show rapid cessation of protraction in both the first- and second-contact whisks. Two limitations of the analyzed data should be made clear: First, our measure of the number of whisker contacts is the number of tracked whiskers touching the vertical surface during the whisk cycle of interest and not the total number of contacting whiskers (which is difficult to measure from video recordings). Second, contact duration was calculated for only the first whisker to touch the surface, although this is also generally the last whisker to leave the surface. Furthermore, in 25 (42%) of the second-contact whisks contact continued beyond the end of the whisk cycle. Contact duration measures for these whisks were therefore calculated as the time from the initial contact, in that whisk, to the end of the whisk cycle.
A MANOVA for the preceding contact-related measures again showed a large difference between whisk types (P < 0.001). Subsequent univariate analyses (Supplemental Table S2) found that there were more contacts on tracked whiskers in the second-contact whisk (+14%, P = 0.001), that second contacts occurred at smaller angular positions (−9%, P < 0.001), and that the mean duration of contacts was markedly longer in second-contact whisks compared with first-contact whisks (+40%, P < 0.001). Mean time from contact to maximum protraction was found to be <15 ms in both whisks (14.3 and 11.3 ms for the first- and second-contact whisks, respectively), consistent with the “minimal impingement” hypothesis. The findings of more contacts, longer contact durations, and rapid cessation of protraction will each be subsequently examined further in Sensory consequences of whisker control. To conclude the current section, however, we briefly consider the impact of head movements on whisking control and on whisker–surface contacts.
VARIATION IN HEAD POSITION AND MOVEMENT ACROSS WHISK TYPES.
Although our primary focus here is on changes in whisker movements it is important to consider whether there may be consistent patterns in the positioning and movement of the rat head during exploratory whisking. This matters both because head movement can influence the sensory consequences of whisking (movement toward a surface, for instance, will increase bending of any contacting whiskers) and because head/body movement is likely to be an important element of the overall control strategy used by the rat to position its whiskers. The head position and movement parameters listed in Table 1D were selected for quantitative analysis as being most relevant to understanding whisker movement and positioning with respect to a vertical surface. An initial MANOVA again showed a strongly significant difference between whisk types (P < 0.001), whereas subsequent univariate ANOVAs (Supplemental Table S3) showed that the mean distance to the wall and the mean velocity toward the wall both decreased by >50% (P < 0.001) by the second-contact whisk. Meanwhile, the vertical velocity of the snout (a useful indicator of the change in head tilt) increased 56% by the second-contact whisk (P = 0.021). By including measures of head rotation and movement along the wall (parallel to the floor), we can estimate that, during a typical approximately 100-ms whisk cycle, each rat moved 9–12 mm horizontally, raised or lowered its snout by 6–10 mm, and rotated its head through 5–6°. In other words, these animals were rarely stationary and often moved at quite significant speeds while exploring nearby surfaces with their whiskers.
EFFECTS OF HEAD MOVEMENT ON CONTACT VELOCITY.
The above-cited findings suggest that the rat's head and body movements during whisking may be of sufficient magnitude to influence the nature of whisker deflections. These impacts could arise either by changing the effective speed of the whiskers as they meet the contacted surface, by changing the duration of contact, or by causing movement of the whisker shaft as it “sticks” or “slips” (Ritt et al. 2008) across a surface. Here we consider the likely impact of head movement on the effective velocity of the initial contact; effects during contacts will be briefly considered in Sensory consequences of whisker control.
Because the surfaces in our arena are flat, the vast majority of whisker–surface contacts in our data set were observed to begin at or near the tip of the whisker. We therefore make the simplifying assumption that the velocity at the whisker tip is a good approximation for the velocity at the point of contact on the whisker shaft. Using measurements of the lengths of contacting whiskers estimated over three successive video frames, and the formula
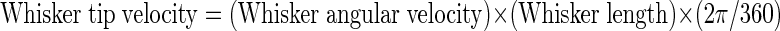 |
we estimate that the mean velocity at the tip, immediately prior to contact, was 0.44 mm/ms in both the first- and second-contact whisks (SD 0.32 and 0.38, respectively). Mean head angular velocity of about 6°, in both contact whisk types, suggests that the effective tip velocity could increase or decrease by ±5% as the result of head rotation in a typical whisk. Combining whisker tip and head translation velocities (which were predominantly positive in both whisks) suggests that movement toward the wall increased the effective tip velocity at contact by around 27% to 0.60 mm/ms (SD 0.34) in the first-contact whisk and by 13% to 0.55 mm/ms (SD 0.38) in the second. Overall, then, we can conclude that head movements contributed significantly to the velocity of whisker–surface impacts.
A closer look at whisker spread
In the previous two sections, whisker spread was first identified as the second largest component of whisking behavior, and then as one of the two whisker movement parameters (the other being retraction velocity, which we will subsequently consider) that change most significantly when a rat explores an unexpected vertical surface. We propose the hypothesis that variation in observed whisker spread is at least partly the consequence of differential control of whisker velocity and that significant changes in spread arise in response to surface contacts and in anticipation of future contacts. Evidence in support of this view will be provided in this section by evaluating, and presenting evidence against, three alternative explanations of the data presented so far: 1) that variations in apparent spread occur primarily as the consequence of head movements (particularly head tilt) and thus do not arise from differences in how the whiskers themselves are controlled; 2) that variation in spread across whisks arises as the result of correlated changes in other whisker control parameters and can be reduced or eliminated by controlling for these covariates; and 3) that changes in spread occur only after surface contacts and not prior to, and in anticipation of, such contacts.
CAN APPARENT CHANGES IN SPREAD BE EXPLAINED BY HEAD MOVEMENT?
As previously noted, a change in the orientation of the whisking plane can substantially affect the apparent spacing between whiskers observed in the overhead view; thus the differences in whisker spread that we have identified could have arisen partly, or wholly, as the consequence of changes in head position. To investigate this possibility we performed three analyses.
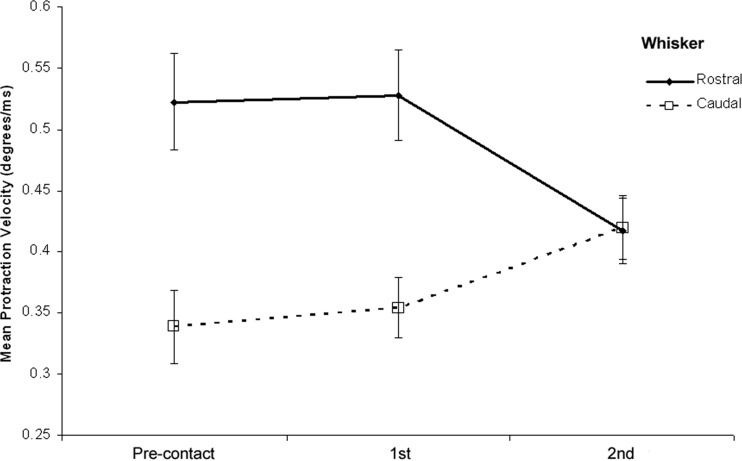
First, we looked separately at the protraction velocities for the most rostral and caudal tracked whiskers across each of the whisk types. We know from Fig. 2 that the whiskers are, overall, moving slightly more slowly in the second-contact whisk, although for spread to be reduced in this whisk the most caudal whiskers will have to match their velocities more closely to those of the rostral ones. This is true, of course, only if the changes in spread are the consequence of differential control of whisker velocities. If changes in observed spread are the result of head movement, then we should expect to see corresponding changes in whisker velocity, but all whiskers should be affected equally as they move in approximately parallel trajectories (see, e.g., Bermejo et al. 2002 and Fig. 6). In other words, head-movement and whisker-movement explanations of spread make quite different predictions concerning individual whisker velocities. A 3 × 2 ANOVA of the relevant whisker velocity data is provided in Supplemental Table S4 and the key results are summarized in Fig. 4. Here we see that although the more rostral whiskers did protract faster than the most caudal ones overall (P < 0.001), there was a significant interaction (P < 0.001) such that by the second-contact whisk, both rostral and caudal whiskers were moving at approximately the same speed and the most caudal whiskers were moving faster than in the preceding whisks. Since this result cannot be explained by head movement we therefore conclude that a significant part of the observed variance in whisker spread is due to the way in which the whiskers themselves are controlled.
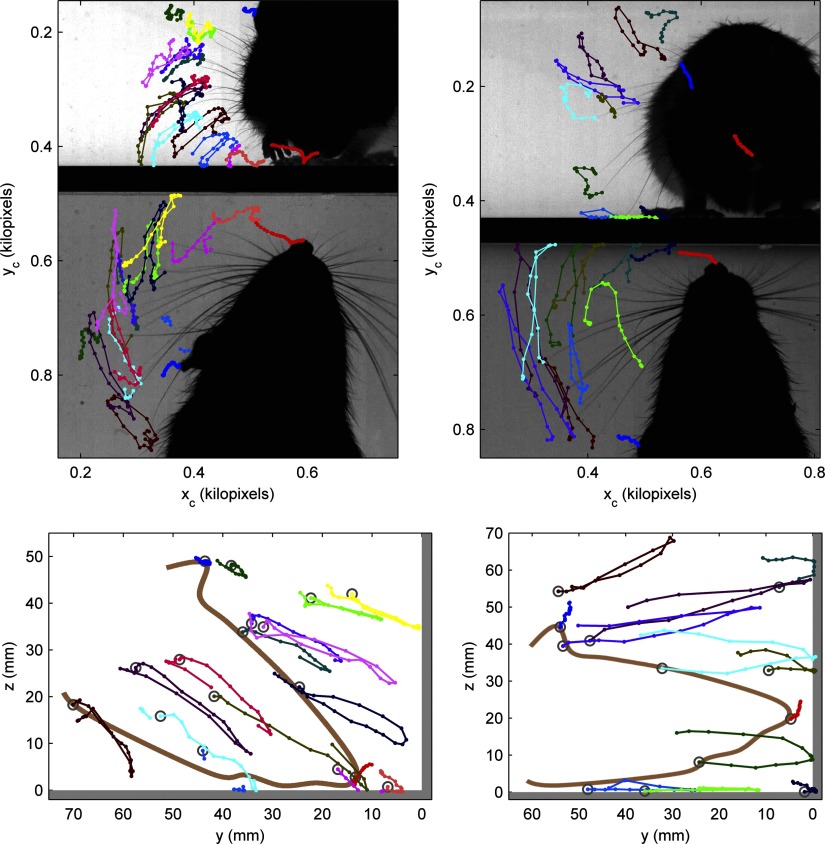
FIG. 6.
Whisker tip trajectories for a 1st-contact (left) and a 2nd-contact whisk (right). Top: a high-speed video frame from each clip with whisker tip trajectories overlaid in both the side-on (mirror) and overhead views. Colored lines show trajectories of individual whiskers matched across the 2 views using a stereo correspondence algorithm. The tip of the snout (red) and of one ear (blue) were also tracked in the both whisks. Bottom: reconstruction of whisker tip trajectories in a rotated view showing movement in a plane perpendicular to both the floor and the end wall (surfaces shown by thick gray outlines). Axes show distance to wall and height above the floor in millimeters. The approximate shape and position of the head are illustrated by the brown outline. The video clips from which the trajectories were reconstructed are provided as Supplemental Videos S2 and S3 with tracking overlaid.
FIG. 4.
Changes in the protraction velocities of the most rostral and most caudal tracked whiskers on encountering an unexpected surface. In the precontact whisk the most rostral whisker moves significantly faster than the most caudal one; however, the protraction velocities converge by the 2nd-contact whisk, consistent with a substantial reduction in whisker spread in that whisk.
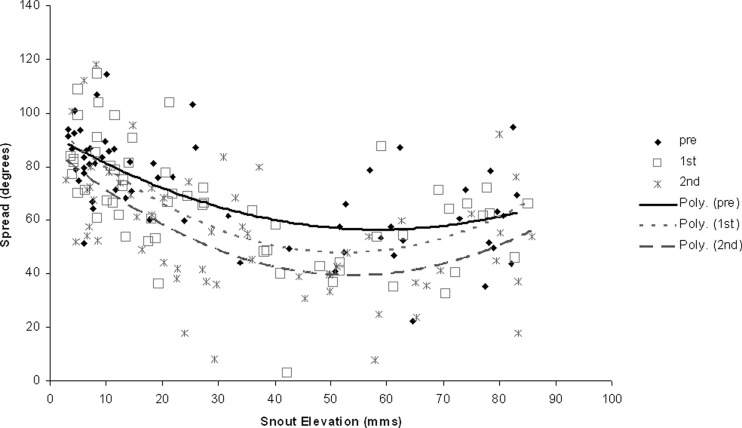
Second, to investigate the possible effects of changes in head position on observed spread we plotted a scattergram, shown in Fig. 5, of snout elevation (used as an indicator of degree head tilt) versus mean spread for all three whisk types in all 60 clips. We then fitted separate polynomial curves to this distribution for each whisk type. Two observations arise from inspecting these figures. First, and most important, it can be seen that the curve for the second-contact whisk data lies beneath that for the other two types and does not overlap them at any point. This confirms that the observed reduction in spread in that whisk type, compared with the others, is relatively independent of this measure of head position. Second, it is noteworthy that all three curves in Fig. 5 are mildly U-shaped, with the largest spread values occurring when the snout is near the floor or the ceiling and the smallest when the snout is at medium height. Since the same five whiskers are tracked in all frames, this is the opposite of what one would expect if spread, as recorded in the overhead camera, was changing solely, or primarily, as the result of changes in head tilt. In that case, spread should be maximal somewhere in the central range of elevations, where whisker motion is parallel with the viewing plane of the camera, and should be smallest at the extremes, where the whisking plane angles away from, or toward, the camera. That the range of spread values for different snout elevations shows the opposite trend implies that tracking in the overhead view may be causing us to underestimate, rather than overestimate, the extent to which spread is varying within the plane of whisker movement at different head tilts.
FIG. 5.
Scattergram of whisker spread against snout elevation with best-fit curves for different whisk types. The plot shows that whisker spread (y-axis) is reduced in the 2nd-contact whisk across the full range of snout elevation (x-axis values). Curve fitting used 2nd-order polynomials. That the best-fit curves are mildly U-shaped suggests that tracking in the overhead view may lead to underestimation, rather than overestimation, of the extent to which spread varies within the plane of the whiskers.
Finally, we looked at some illustrative data that compare whisker spread, as measured in the overhead view, with an alternative estimate of relative whisker spacing calculated within a coordinate frame defined by the whiskers themselves. Specifically, two representative clips were chosen for tracking in both overhead and end-on views and 3D trajectories calculated for a subset of whisker tips as described in the Supplemental Material. For both clips we then computed a per-frame, head-invariant spread measure as the average distance between all pairs of tracked whisker tip positions. A comparison between this new estimate of whisker spacing and the original viewpoint-dependent spread measure, for both whisking episodes, is shown in Supplemental Fig. S7. These graphs show a reasonable match between the two measures (Pearson's r correlations of 0.72 and 0.54 for the two clips) and a similar reduction in spread over the three whisk types, confirming that spread, as measured in the overhead view, captures a significant portion of the variance of a measure of whisker spacing that is independent of head movement.
ARE APPARENT CHANGES IN SPREAD THE RESULT OF CORRELATED CHANGES IN OTHER WHISK PARAMETERS?
In a multivariate analysis it is important to control for the possible effects of covariates on a measure of interest. In this case, when calculated across all whisk types (i.e., n = 180), whisker spread was found to have a significant, though relatively small, positive correlation with maximum protraction (r = +0.18, P = 0.015) and a nonsignificant but positive correlation with minimum protraction (r = +0.14, P = 0.068) (correlations with other measured whisking parameters were negligible). These relationships are potentially important because we know that both maximum and minimum protractions are reduced on the second-contact whisk (Fig. 2). To establish whether variation in spread is independent of these other differences between whisk types, a univariate ANOVA (see Supplemental Table S5) was performed using values of mean spread calculated solely within the arc x to y degrees of the protraction phase, where x = max (minimum protraction) and y = min (maximum protraction), and where x and y were calculated separately within each clip across all three whisk types. After controlling for differences in minimum and maximum protractions in this way, mean spread was still found to decrease substantially by the second-contact whisk (P < 0.001) and by 17.9% compared with the precontact whisk, which is a similar reduction to that seen overall in the protraction phase (16.5%). We therefore conclude that the variation in spread seen across whisk types is not explained by differences in the protraction start and end positions.
DO CHANGES IN SPREAD OCCUR PRIOR TO SURFACE CONTACTS?
Differences in observed spread between precontact and contact whisks could occur throughout the whisk cycle or could arise, primarily or wholly, as the result of changes in whisker movement taking place after the whiskers have touched the surface. To determine whether there were significant changes in whisker spread prior to any surface contact, an analysis (see Table S5) was conducted in which mean spread was calculated solely within the arc x to z degrees of the protraction phase where x = max (minimum protraction) (as above) and z = min (mean angular position prior to contact). Again, as in the previous test, mean spread still showed a significant decrease (−14.3%, P < 0.001) by the second-contact whisk, confirming that there is a significant reduction in whisker spacing prior to surface contact. In this context it is also worth looking briefly at differences in spread in the retraction phase of the whisk. Again, and as expected, this showed a significant reduction across the whisk types (P < 0.001); however, notably, here the reduction in spread began in the first-contact whisk (−14.1% compared with precontact). This result suggests that, following the initial contact, whisker control begins to adapt almost immediately to provide reduced whisker spread during further exploration of the contacted surface.
Sensory consequences of whisker control
The active touch hypothesis for rat whisking postulates that the control of whisker positioning is regulated on a moment-to-moment basis to provide the animal with better or more task-relevant tactile sensory signals. In this final subsection we examine three aspects of whisker–surface interactions—the quantity of whisker–surface contacts, the amount of bending during contacts, and the duration of contacts—and attempt to pinpoint some of the elements of whisker control that could be actively regulated to modify each of them.
INCREASING THE QUANTITY OF SURFACE CONTACTS BY CONTROLLING WHISKER SPREAD AND HEAD TILT.
Reducing spread through differential control of whisker movement brings the whisker tips closer together and should allow the rat to increase the density of sensory signals from targeted regions of space where objects or surfaces are expected. To verify this “maximizing contact” effect we calculated correlations between the number of contacts on tracked whiskers and a range of potential predictor variables for the combined set of 120 first- and second-contact whisks; a stepwise multiple linear regression was then performed using the most promising candidate predictors. This analysis showed that the number of contacts increased both with the inverse mean spread (lower values of spread are most discriminative), which showed a part correlation of +0.202 (P = 0.019) after removing the affects of other predictors, and with increasing snout elevation (part correlation +0.195, P = 0.024). Further details of these analyses, together with residual plots confirming that the relationships were approximately linear, are provided in the Supplemental Material. We conclude that the rat can increase the number of surface contacts by reducing whisker spread. The increase in contacts due to greater snout elevation remains to be explained and is briefly considered next.
Figure 6 shows the whisker tip trajectories for a first-contact whisk (left) and a second-contact whisk (right), calculated using tracking data from both camera views using our 3D trajectory reconstruction algorithm, and then rotated (in the bottom half of the figure) so that the trajectories can be observed in a plane perpendicular to both the wall and the arena. For illustrative purposes the tracked video clips used to generate these trajectories are also provided as Supplemental Videos S2 and S3. The left panel shows that during a whisk with low elevation many whiskers, and particularly the more caudal ones, make contact with the floor and would do so even if the rat were closer to the wall. Increased floor contact and reduced wall contact are likely simply because the rat's head tilts down toward the floor at a significant angle. For the whisk with higher snout elevation, shown in the right panel, whisker movement is close to being perpendicular to the wall, and the head position near horizontal, so we can expect, and do observe, many more wall contacts and few floor contacts. What happens for still higher elevations? When the head tilts above the horizontal this will bring some of the longer more caudal whiskers closer to the wall, increasing the likelihood that these whiskers will touch even as some of the dorsal whiskers rotate away from the wall (and may begin to touch the ceiling). We can thus conclude that tilting the head upward is, overall, a useful “maximizing contact” strategy for exploring walls, whereas tilting downward is clearly favorable for whisking during floor traversal.
CONTROLLING BENDING AGAINST SURFACES THROUGH RAPID CESSATION OF PROTRACTION FOLLOWING CONTACT.
In our previous study (Mitchinson et al. 2007) we found that, following a unilateral surface contact, the whiskers on that side of the snout cease protraction on average 13 ms after the initial touch. In the current study we used a larger sample size (60 vs. 22 clips), a higher frame rate (500 vs. 250 frames/s), and measured the average time from contact to maximum protraction across all five tracked whiskers. Performing this analysis for data from the first-contact whisk produced a mean time to maximum protraction of 14.3 ms—i.e., a result that is well within the expected margin of error of our previous investigation (±2 ms). When the same calculation was performed for the second-contact whisk, interestingly, the time to maximum protraction fell to 11.3 ms, a latency that was marginally faster than that for the first contact, although this result should be treated with some caution due to the relatively high P value (0.038, which exceeds the Bonferroni corrected alpha of 0.01). To further test the robustness of these estimates we separately examined the time to maximum protraction for each of the tracked whisker columns. As shown in Supplemental Fig. S9, the difference between first- and second-contact whisks was found consistently across all columns, whereas there was also an interesting trend for the more rostral whiskers to reach maximum protraction earlier than the more caudal ones by up to 5 ms. Time to maximum protraction was also compared for whisker columns that did, and did not, make contact with the wall; these were found to be very similar (P = 0.974), indicating that the contacts themselves were not significantly distorting this measure. An analysis by contact type (bilateral vs. unilateral; see Supplemental Fig. S10) also found no significant effect on the timing of maximum protraction (P = 0.095). Overall then we can conclude that rapid cessation of protraction is a general and consistent feature of whisker–surface contacts during exploratory whisking, which may possibly be more pronounced (faster) in subsequent whisks that in the initial contact, and whose effect will be to reduce bending of the whiskers against the surface compared with nonmodulated whisks.
CONTACT DURATION—A FURTHER CANDIDATE FOR ACTIVE CONTROL?
On first inspection, the finding that whisker–surface contacts have much longer duration appears to be inconsistent with the rapid cessation of protraction and reduced head velocity toward the wall in the second-contact whisk. However, the likely explanation for these longer contact times is easily found by reviewing Fig. 2—here we saw that whisk retraction velocity was at a significantly slower pace on the second-contact whisk than on the first. To understand this further, we next divided the retraction phase into two halves and found that the decrease in retraction velocity in the second-contact whisk was much more evident in the first half of the retraction phase (−54%, P < 0.001) than in the second half (−9%, P = 0.237). This establishes that slower retraction occurs during the period of the whisk immediately following peak protraction and is thus most likely to influence contact duration. To confirm that retraction velocity was genuinely slower following the second contact, and not simply that drag on the contacting whiskers created the appearance of slower retraction, we looked at retraction velocity on the most caudal tracked whisker, since this rarely contacts the vertical surface (only three contacts in the 60 second-contact whisks). For this whisker, too, velocity during the first half of the retraction phase was also considerably slower on the second-contact whisk (−49% compared with −47% for the most rostral whisker, both P < 0.001). Details of each of these analyses are provided in Supplemental Table S6.
To further establish which control factors most influence contact duration we performed a stepwise multiple linear regression using head- and whisker-movement parameters that were likely predictors of duration. The dependent variable was contact duration measured for the 95 first- and second-contact whisks in which contact with the surface ceased before the end of the whisk cycle (60 first-contact and 35 second-contact whisks). This analysis showed that contact duration was most strongly predicted by time from contact to maximum protraction (part correlation of r = +0.487), retraction velocity (r = −0.293), snout vertical velocity (r = −0.246), and snout elevation (r = +0.183). Further details of this analysis, including residual plots confirming that the relationships were approximately linear, are provided in the Supplemental Material. The high positive correlation with the time from contact to maximum protraction confirms the importance of controlling protraction cessation, using sensory feedback, to the tactile experience of the rat. The negative correlation with retraction velocity shows that this parameter is a further important element of whisking strategy in determining the duration of whisker–surface contact signals. We leave to the discussion consideration of why retraction velocity is reduced in the second-contact whisk and whether increasing contact duration through this mechanism should be considered as an additional active touch strategy.
DISCUSSION
Sensory signals are generally ambiguous, sometimes entirely meaningless, in the absence of knowledge of how the sensor that generated those signals was controlled. This is particularly true of touch, where signals are obtained only through physical contact and where the trajectory of the sensor with respect to the surface codetermines, with the surface properties of the object, the nature of the signals that are obtained. Increasing evidence, both in this study and in other studies, indicates that the sensor (whisker) trajectories of the rat vibrissal system are carefully controlled, modified on the basis of recent sensory experience and in anticipation of future experience, and directed at obtaining high-quality, task-relevant information. This type of active sensing control is also apparent in human fingertip touch (Chapman 1994; Lederman and Klatzky 1993; Smith et al. 2002), suggesting that the rat whisker system can be a useful model in which to investigate “sensorimotor contingencies” (O'Regan and Noe 2001) similar to those underlying our own tactile experience of the world. In the following, our new findings in relation to active touch sensing in the rat are summarized and evaluated, beginning with the evidence that whisker spread is actively controlled, then turning to the active touch sensing strategies that appear to be used by the rat.
Control of whisker spread
WHISKER SPREAD EXPLAINS A SIGNIFICANT PORTION OF WHISKING VARIANCE.
Although changes in the horizontal spacing of whiskers have been previously noted (Sachdev 2002), this is the first study to have quantified the contribution of these changes to the overall observed whisking pattern. We found that 13–19% of the variance in whisker angular position data for freely moving animals, tracked from overhead, can be accounted for in terms of one summary parameter—the “spread” or angle of arc between the rostral-most and caudal-most tracked whisker. Further, together with the mean angular rotation of the whisker field these two parameters can account for ≤93% of the variance seen in the overhead view.
WHISKER SPREAD VARIES ACROSS DIFFERENT WHISK TYPES.
It is important to be clear that we regard the spread parameter as simply a descriptive measure that usefully summarizes some of the observed changes in whisking behavior. We have no direct evidence that the rat brain encodes spread as a specific control parameter (any more than it encodes amplitude, frequency, or set point as control parameters, all of which are also descriptive concepts). That the spacing between whiskers changes with time is consistent with, but does not necessarily require, active control of underlying mechanisms. Passive, rather than active, control (such as might be provided by purely mechanical properties of the vibrissal system) would imply that whisker spread should vary across the whisk cycle in a predictable and consistent manner across all types of whisk. To obtain evidence that observed changes in spread are not simply passive we needed to demonstrate variability across different whisking contexts; therefore we investigated whether anticipation of a proximal vertical surface could change the pattern of spread changes over the course of a whisk cycle. Our data demonstrate such variability. Specifically, we have shown that in the second-contact whisk, whisker spread and angular position can become decoupled, that spread is typically reduced compared with preceding whisk cycles, and most reduced following bilateral whisker–surface contacts.
EXPLAINING THE OBSERVED VARIANCE IN SPREAD ACROSS WHISK TYPES.
Observed spread thus appears to vary across whisking contexts, but what is the source of this variation? In our analysis we tested and provided evidence against several alternative explanations, concluding that observed changes in spread were not simply the consequence of head movements, changes in other aspects of whisker control, or the result of the whiskers bending against the contacted surface. Most tellingly, perhaps, we found evidence of differences in whisker velocity indicating that the most rostral whiskers move substantially faster than the most caudal ones in precontact and first-contact whisks, but not in second-contact whisks. This result is difficult to explain other than by assuming some capacity for differential control of the whiskers themselves.
COULD CHANGES IN WHISKER SPREAD INVOLVE CONTROL OF THE WHISKING MUSCULATURE?
Although we have ruled out the most plausible alternatives, based on the current data we cannot directly test the hypothesis that the apparent changes in whisker spread involved control of the whisking musculature. Results from other laboratories (Berg and Kleinfeld 2003; Dorfl 1982; Hill et al. 2008; Klein and Rhoades 1985; Wineski 1985), however, show that the rat does have sufficient degrees of freedom of whisker control to effect some differential movement of either individual whiskers or whisker columns. Moreover, some divergent movement of the whiskers has been observed in animals trained to make texture discriminations (Carvell and Simons 1990) and in head-fixed animals trained to whisk for reward (Sachdev et al. 2002). That the rat has the capacity to focus its whisker field toward a target has also been suggested before in the context of the “foveal whisking” behavior described by Berg and Kleinfeld (2003). In the following we briefly compare some of the observations made in that study with our current results.
WHISKING MODULATION AND WHISKING MODES.
Berg and Kleinfeld (2003) used high-speed videography and electromyographic recording of the whisking musculature to investigate whisking behavior in rats that were trained to explore a maze to obtain food rewards. They described two general modes of whisking behavior that were distinguished both by their spectral properties (whisk amplitude and frequency) and by differential patterns of activation in the whisking musculature. The first mode, termed “exploratory whisking,” consisted of bouts of relatively large amplitude whisks occurring at a frequency 5–15 Hz. In the second, less frequent “foveal whisking” mode, rats exhibited bouts of relatively small amplitude but high-frequency (15–25 Hz) whisking. During general maze traversal, while the animals searched for a food resource, they exhibited exploratory whisking; however, the animals shifted to the foveal mode when required to “crane their necks” across a gap to reach a food tube.
The changes in whisking control during exploration of surfaces described herein are more subtle than the marked switch from one whisking mode to another described by Berg and Kleinfeld (2003). Indeed, in the current data, the frequency and velocity of the whisker movement changed relatively little on surface contact and, instead, we saw differences in some less well studied whisking parameters such as the whisker spread and the mean retraction velocity. We consider, then, that the whisking patterns we have observed here fall within the general class of “exploratory whisking” described by Berg and Kleinfeld (2003), and we suggest that, within this mode, the rat has the capacity to modulate whisking control on a per-whisk basis, and, to some degree, per-whisker (or whisker column) basis. It is worth noting that Berg and Kleinfeld (2003) described foveal whisking as involving the vibrissae being “clustered in front of the head in a relatively dense pattern.” Although this clustering is not precisely quantified it does seem consistent with what we are calling a change in whisker spread. If differential use of the whisking musculature can bring about the substantial changes in whisker movement seen following the transition to foveal whisking, it seems reasonable to suppose that similar but subtler changes in muscular control could also underpin the reduction in whisker spread we have observed when rats explore a proximal surface.
Active touch sensing in the rat
We have previously proposed (Mitchinson et al. 2007) that rat whisking uses active control strategies that serve to increase the number of whiskers contacting surfaces of interest (“maximizing contact”) while controlling the amount of bending against those surfaces (“minimizing impingement”). In the current study, the reduction in whisker spread and associated changes in head tilt, found in the second-contact whisk, are consistent with both strategies because they allow an increased number of whiskers to make contact with the vertical wall without requiring that the whiskers necessarily press harder against that surface. Several other aspects of whisking and head control that might also be considered to be part of the rat's active sensing strategy are considered in the following text.
MINIMIZING IMPINGEMENT BY CONTROLLING CESSATION OF PROTRACTION.
Consistent with our earlier findings (Mitchinson et al. 2007), the current study found that whisker protraction ceased rapidly following an initial contact with a surface (mean of 14.30 ms from contact to maximum protraction in the first-contact whisk). In the case of unilateral contacts, we previously found a difference between the ipsilateral (to the contact) whisker field, where protraction stopped soon after contact, and the contralateral field (where there was no contact) where it did not. From this we inferred the existence of a fast sensory feedback loop controlling the timing of whisker protraction to implement a “minimal impingement” strategy and ensure that contacts were made with a relatively “light touch.” Our new data suggest that rapid cessation of protraction occurs for both unilateral and bilateral contacts and may even be quicker on the second-contact whisk (mean latency 11.25 ms). The latter finding, if supported by future studies, would imply some additional element of anticipatory control. The literature on classical conditioning shows that the latency of a reflex response, such as the rabbit eyeblink, is significantly reduced when the animal can anticipate the timing of the unconditioned stimulus (Gormezano et al. 1983). Thus similarly, the rat's ability to anticipate a forthcoming surface contact could influence the control circuitry underlying the proposed whisking sensory feedback loop, enabling it to respond more rapidly when an expected whisker deflection takes place.
CONTROLLING THE DURATION OF WHISKER SURFACE CONTACTS.
An unanticipated finding of the current study was that the duration of contacts with surfaces was generally much longer in the second-contact whisk. Duration of contact was found to be best predicted by two whisking control parameters—the time from contact to maximum protraction (discussed earlier) and the retraction velocity (especially in the first half of the retraction phase). These two parameters oppose each other, but, by their interplay, it would appear that the rat could control the duration of contact, the speed at which the whisker is drawn across the surface, and the amount of bending in the whisker shaft. We think it is possible that the slower retraction during the second-contact whisk can be understood as an active touch strategy aimed at prolonging contact and thereby aiding the extraction of information about surface characteristics such as texture. Thus perhaps, the first-contact whisk could be thought of as locating the surface in space and the second as discerning more details concerning the nature of that surface. However, it is important to consider that there may be alternative explanations of reduced retraction velocity on the second-contact whisk that are not directly concerned with contact duration or with the sensory consequences of this contact. For instance, one possibility is that there might be compensatory mechanisms within the whisker pattern generator that act to reduce whisk retraction velocity following early cessation of protraction due to surface contact. Such a mechanism might conceivably operate to prevent a strong mismatch in phase between the left and right whisker fields, since it can be generally observed that the two fields have a strong tendency to return to synchronized movement following perturbation. Evaluation of this alternative will require a better understanding of the coupled motor pattern generators that generate whisker movements in the two fields and of their modulation by sensory signals. Future research on this topic should also benefit from the investigation of generative computational and robotic models of whisker geometry, musculature, and neural control systems (e.g., Hill et al. 2008; Mitchinson et al. 2006; Pearson et al. 2007).
COMBINING HEAD AND WHISKER MOVEMENT TO MAKE EXPLORATION MORE EFFECTIVE.
Although going somewhat beyond the current data, we now propose the following hypothesis, consistent with the above-cited active sensing strategies that could serve as a further simplifying principle for understanding whisking control in exploring animals.
The rat appears to control its whiskers so that the spacing between the whiskers is reduced relative to the surface of interest. Thus if this is a vertical surface we see reduced spread in the overhead view (as demonstrated by our data); if a horizontal surface (e.g., the floor) we see reduced spread in the end-on view (consistent with our informal observations of video recordings but remaining to be demonstrated quantitatively). A strong version of this hypothesis would suggest that the rat also seeks to increase spacing parallel to the surface of interest, to simultaneously explore as much of that surface as possible. Thus when proceeding across the floor the whiskers appear relatively spread out when viewed from above and directed at the area of the floor around and immediately in front of the animal in its direction of motion (see, e.g., Fig. 7, left). In contrast, when investigating a vertical wall, the whiskers appear close together in the overhead view and much more widely separated in the end-on view (see, e.g., Fig. 7, right). These changes in whisker spacing are likely brought about partly through differential control of the whiskers and partly through controlled positioning of the head with respect to the surface of interest. Thus obtaining a better understanding of the interaction between head movements and whisking movements will be important to be able to fully characterize the active touch sensing strategies of the rat.
FIG. 7.
Control of whisker spread and head position in active touch. Snapshots consistent with the hypothesis that the rat uses it body, neck, and whisker musculature so as to reduce whisker spread perpendicular to a surface of interest, while increasing spread parallel to that surface. Thus when the rat is moving across the floor, spread is reduced in the end-on view (left top) and increased in the overhead view (left bottom), whereas when exploring a wall, 2 whisks later, spread is increased in the end-on view (right top) and reduced in the overhead view (right bottom).
GRANTS
This research was supported by European Union Integrating Cognition, Emotion, and Autonomy Grant IST- 027819 and Biomimetic Technology for Vibrissal Action Touch Grant ICT-215910. R. A. Grant was funded by a doctoral training grant from the United Kingdom Engineering and Physical Sciences Research Council.
Supplementary Material
Acknowledgments
We thank P. Redgrave for scientific advice and M. Simkins, L. Hetherington, A. Ham, and M. Benn for technical support.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Aloimonos et al. 1988.Aloimonos JY, Weiss I, Bandopadhay A. Active vision. Int J Comput Vision 1: 333–356, 1988. [Google Scholar]
- Ballard 1991.Ballard DH Animate vision. Artif Intell 48: 57–96, 1991. [Google Scholar]
- Berg and Kleinfeld 2003.Berg RW, Kleinfeld D. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J Neurophysiol 89: 104–117, 2003. [DOI] [PubMed] [Google Scholar]
- Bermejo et al. 2002.Bermejo R, Vyas A, Zeigler HP. Topography of rodent whisking. I. Two-dimensional monitoring of whisker movements. Somatosens Mot Res 19: 341–346, 2002. [DOI] [PubMed] [Google Scholar]
- Carvell and Simons 1990.Carvell GE, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci 10: 2638–2648, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman 1994.Chapman CE Active versus passive touch: factors influencing the transmission of somatosensory signals to primary somatosensory cortex. Can J Physiol Pharmacol 72: 558–570, 1994. [DOI] [PubMed] [Google Scholar]
- Dorfl 1982.Dorfl J The musculature of the mystacial vibrissae of the white-mouse. J Anat 135: 147–154, 1982. [PMC free article] [PubMed] [Google Scholar]
- Etienne et al. 1996.Etienne AS, Maurer R, Seguinot V. Path integration in mammals and its interaction with visual landmarks. J Exp Biol 199: 201–209, 1996. [DOI] [PubMed] [Google Scholar]
- Gao et al. 2001.Gao P, Bermejo R, Zeigler HP. Whisker deafferentation and rodent whisking patterns: behavioral evidence for a central pattern generator. J Neurosci 21: 5374–5380, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson 1962.Gibson JJ Observations on active touch. Psych Rev 69: 477–491, 1962. [DOI] [PubMed] [Google Scholar]
- Gormezano et al. 1983.Gormezano I, Kehoe EJ, Marshall BS. Twenty years of classical conditioning research with the rabbit. In: Progress in Psychobiology and Physiological Psychology, edited by Sprague JM, Epstein AN. New York: Academic Press, 1983, p. 197–275.
- Gustafson and Felbain-Keramidas 1977.Gustafson JW, Felbain-Keramidas SL. Behavioral and neural approaches to the function of the mystacial vibrissae. Psychol Bull 84: 477–488, 1977. [PubMed] [Google Scholar]
- Hartmann 2001.Hartmann MJ Active sensing capabilities of the rat whisker system. Auton Robots 11: 249–254, 2001. [Google Scholar]
- Hetherington et al. 2000.Hetherington L, Benn M, Coffey PJ, Lund RD. Sensory capacity of the Royal College of Surgeons rat. Invest Ophthalmol Vis Sci 41: 3979–3983, 2000. [PubMed] [Google Scholar]
- Hill et al. 2008.Hill DN, Bermejo R, Zeigler HP, Kleinfeld D. Biomechanics of the vibrissa motor plant in rat: rhythmic whisking consists of triphasic neuromuscular activity. J Neurosci 28: 3438–3455, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein and Rhoades 1985.Klein BG, Rhoades RW. Representation of whisker follicle intrinsic musculature in the facial motor nucleus of the rat. J Comp Neurol 232: 55–69, 1985. [DOI] [PubMed] [Google Scholar]
- Knutsen et al. 2008.Knutsen PM, Biess A, Ahissar E. Vibrissal kinematics in 3D: tight coupling of azimuth, elevation, and torsion across different whisking modes. Neuron 59: 35–42, 2008. [DOI] [PubMed] [Google Scholar]
- Knutsen et al. 2005.Knutsen PM, Derdikman D, Ahissar E. Tracking whisker and head movements in unrestrained behaving rodents. J Neurophysiol 93: 2294–2301, 2005. [DOI] [PubMed] [Google Scholar]
- Lederman and Klatzky 1993.Lederman SJ, Klatzky RL. Extracting object properties through haptic exploration. Acta Psychol (Amst) 84: 29–40, 1993. [DOI] [PubMed] [Google Scholar]
- Lungarella et al. 2005.Lungarella M, Pegors T, Bulwinkle D, Sporns O. Methods for quantifying the informational structure of sensory and motor data. Neuroinformatics 3: 243–262, 2005. [DOI] [PubMed] [Google Scholar]
- Mitchinson et al. 2007.Mitchinson B, Martin CJ, Grant RA, Prescott TJ. Feedback control in active sensing: rat exploratory whisking is modulated by environmental contact. Proc Biol Sci 274: 1035–1041, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchinson et al. 2006.Mitchinson B, Pearson M, Melhuish C, Prescott TJ. A model of sensorimotor coordination in the rat whisker system. In: From Animals to Animats 9: Proceedings of the 9th International Conference on Simulation of Adaptive Behaviour, SAB 2006, Rome, Italy, September 25–29, 2006, edited by Nolfi S, Baldassarre G, Calabretta R, Hallam J, Marocco D, Miglino O, Meyer J-A, Parisi D. Berlin: Springer-Verlag, 2006, p. 77–88.
- O'Regan and Noe 2001.O'Regan JK, Noe A. A sensorimotor account of vision and visual consciousness. Behav Brain Sci 24: 939–1031, 2001. [DOI] [PubMed] [Google Scholar]
- Pearson et al. 2007.Pearson MJ, Pipe AG, Melhuish C, Mitchinson B, Prescott TJ. Whiskerbot: a robotic active touch system modeled on the rat whisker sensory system. Adapt Behav 15: 223–240, 2007. [Google Scholar]
- Ritt et al. 2008.Ritt JT, Andermann ML, Moore CI. Embodied information processing: vibrissa mechanics and texture features shape micromotions in actively sensing rats. Neuron 57: 599–613, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev et al. 2003.Sachdev RN, Berg RW, Champney G, Kleinfeld D, Ebner FF. Unilateral vibrissa contact: changes in amplitude but not timing of rhythmic whisking. Somatosens Mot Res 20: 163–169, 2003. [DOI] [PubMed] [Google Scholar]
- Sachdev et al. 2002.Sachdev RN, Sato T, Ebner FF. Divergent movement of adjacent whiskers. J Neurophysiol 87: 1440–1448, 2002. [DOI] [PubMed] [Google Scholar]
- Sellien et al. 2005.Sellien H, Eshenroder DS, Ebner FF. Comparison of bilateral whisker movement in freely exploring and head-fixed adult rats. Somatosens Mot Res 22: 97–114, 2005. [DOI] [PubMed] [Google Scholar]
- Smith et al. 2002.Smith AM, Gosselin G, Houde B. Deployment of fingertip forces in tactile exploration. Exp Brain Res 147: 209–218, 2002. [DOI] [PubMed] [Google Scholar]
- Stuttgen et al. 2006.Stuttgen MC, Ruter J, Schwarz C. Two psychophysical channels of whisker deflection in rats align with two neuronal classes of primary afferents. J Neurosci 26: 7933–7941, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towal and Hartmann 2006.Towal RB, Hartmann MJ. Right-left asymmetries in the whisking behavior of rats anticipate head movements. J Neurosci 26: 8838–8846, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towal and Hartmann 2008.Towal RB, Hartmann MJ. Variability in velocity profiles during free-air whisking behavior of unrestrained rats. J Neurophysiol 100: 740–752, 2008. [DOI] [PubMed] [Google Scholar]
- Vincent 1912.Vincent SB The function of the vibrissae in the behaviour of the white rat. Behav Monogr 1: 1–82, 1912. [Google Scholar]
- Voigts et al. 2008.Voigts J, Sakmann B, Celikel T. Unsupervised whisker tracking in unrestrained behaving animals. J Neurophysiol 100: 504–752, 2008. [DOI] [PubMed] [Google Scholar]
- Wineski 1985.Wineski LE Facial morphology and vibrissal movement in the golden hamster. J Morphol 183: 199–217, 1985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.