Abstract
Human upper airway and facial muscles support breathing, swallowing, speech, mastication, and facial expression, but their endurance performance in sustained contractions is poorly understood. The muscular fatigue typically associated with task failure during sustained contractions has both central and intramuscular causes, with the contribution of each believed to be task dependent. Previously we failed to show central fatigue in the nasal dilator muscles of subjects that performed intermittent maximal voluntary contractions (MVCs). Here we test the hypothesis that central mechanisms contribute to the fatigue of submaximal, sustained contractions in nasal dilator muscles. Nasal dilator muscle force and EMG activities were recorded in 11 subjects that performed submaximal contractions (20, 35, and 65% MVC) until force dropped to ≤90% of the target force for ≥3 s, which we defined as task failure. MVC and twitch forces (the latter obtained by applying supramaximal shocks to the facial nerve) were recorded before the trial and at several time points over the first 10 min of recovery. The time to task failure was inversely related to contraction intensity. MVC force was depressed by roughly 30% at task failure in all three trials, but recovered within 2 min. Twitch force fell by 30–44% depending on contraction intensity and remained depressed after 10 min of recovery, consistent with low-frequency fatigue. Average EMG activity increased with time, but never exceeded 75% of the maximal, pretrial level despite task failure. EMG mean power frequency declined by 20–25% in all trials, suggesting reduced action potential conduction velocity at task failure. In contrast, the maximal evoked potential did not change significantly in any of the tasks, indicating that the EMG deficit at task failure was due largely to mechanisms proximal to the neuromuscular junction. Additional experiments using the interpolated twitch technique suggest that subjects can produce about 92% of the maximal evocable force with this muscle, which is not a large enough deficit to explain the entire shortfall in the EMG at task failure. These data show that the nervous system fails to fully activate the nasal dilator muscles during sustained, submaximal contractions; putative mechanisms are discussed.
INTRODUCTION
Muscles of the vertebrate upper airway and face participate in diverse functions such as breathing, swallowing, speaking, deglutition, and facial expression. Although there have been many recent studies on the control of these muscles during each of these behaviors, we know comparatively little about their physiological properties, including their endurance performance in sustained contractions. With regard to breathing, it is clear that human subjects show considerable upper airway muscle activity during both wakefulness and sleep. In wakefulness, one can record both tonically discharging motor units and units that discharge during the inspiratory phase, but are largely silent during the expiratory phase (“phasic units”) (Saboisky et al. 2006). Interestingly, as subjects enter non-REM sleep, the majority of the phasic motor units drop out, whereas tonically discharging units continue to discharge action potentials at rates that are the same as those recorded in wakefulness (10–40 Hz) (Bailey et al. 2007). This predominance of tonic discharge in upper airway motoneurons suggests that sustained contractions of upper airway muscles are common in human subjects and that they are important for protecting the upper airway during sleep (Remmers et al. 1978; White 2006) and exercise (Connel and Fregosi 1993; Fregosi and Lansing 1995; Fuller et al. 1995; Sullivan et al. 1996; Williams et al. 2000). Consequently, understanding the mechanisms of task failure induced by sustained, submaximal contractions in human upper airway muscles is functionally relevant.
Part of the reason for our poor understanding of the physiological properties of upper airway and facial muscles stems from the fact that they are small, may or may not have tendon attachments, and both the muscles and the muscle nerves can be difficult to access, particularly in human subjects. We have previously used the human nasal dilator muscles as a representative upper airway muscle and, with this model, have been able to measure voluntary and respiratory-related force and electromyogram (EMG) activities (Connel and Fregosi 1993; DelloRusso et al. 2002; Fuller et al. 1995; Sullivan et al. 1996), evoked muscle twitch forces with facial nerve stimulation (DelloRusso et al. 2002; Fuller et al. 1995), and the contractile properties of individual motor units (Mateika et al. 1998).
Previously, we examined the fatigability of human nasal dilator muscles during intermittent maximal voluntary contraction (MVC) maneuvers (DelloRusso et al. 2002). We found that task failure was due to intramuscular mechanisms; central mechanisms did not appear to play a role because the average EMG and maximal evoked potentials (MEPs) were unaltered, with the exception of a small decline in EMG at the endurance limit. These observations are consistent with earlier work showing that the task failure associated with maximal, intermittent contractions of hand (Liu et al. 2005) and limb (Taylor et al. 2000) muscles is due primarily to intramuscular mechanisms. In contrast, the average EMG at task failure is well below maximal levels during sustained, submaximal contractions of human limb (Todd et al. 2003) and hand muscles (Carpentier et al. 2001; Fuglevand et al. 1993; Kalmar and Cafarelli 2004). These observations suggest that task failure induced by sustained contractions is due at least in part to inadequate central activation of the muscle. Accordingly, the purpose of the present study was to test the hypothesis that neural factors contribute to task failure of the nasal dilator muscles during sustained, submaximal contractions.
METHODS
Subjects
The Human Subjects Committee at the University of Arizona College of Medicine approved all procedures and all subjects provided written informed consent. Five men and 6 women (ages 20–27 yr) participated in the study. All subjects were healthy, with no history of lung disease, allergies, rhinitis, cleft palate, or deviation of the nasal septum. The ability to voluntarily activate or “flare” the nasal dilator muscles with sufficient skill to complete the protocol (see following text) was a criterion for inclusion in the study. We brought 22 subjects into the laboratory and each was instrumented, as described in the following text, and asked to perform the maneuvers. Of these 22 subjects, 12 were initially considered to have good ability to control nasal dilator muscle force with visual feedback. One of these 12 subsequently dropped out after the second training session, leaving us with 11 subjects.
Measurement of voluntary force, EMG, and evoked twitch force
The anatomy of the human nasal musculature was described in our recent report (DelloRusso et al. 2002). Briefly, the nasal muscles are small “skin muscles,” with fibers that originate on cartilage or bone but insert into the skin layers that overlie the nose. There are two pairs of nasal dilator muscles and they both act to flare the nares: the dilator alaris posterior, which arises from the margin of the nasal notch of the maxilla and from the lesser alar cartilages, and inserts into the skin near the margin of the nostril; and the dilator alaris anterior, a delicate fasciculus passing from the greater alar cartilage to the integument near the margin of the nostril.
We have recently developed and described in detail a model that allows us to reliably measure force and the EMG of the nasal dilator muscles during voluntary and respiratory-related contractions (DelloRusso et al. 2002; Fuller et al. 1995). Briefly, subjects wear a custom-designed headpiece that is mounted via the forehead and upper lip. The headpiece holds a micromanipulator that contains a steel rod with a button force compression transducer (Model 13/2446–06; Sensotec, Columbus, OH) mounted on its tip. The voltage output of the transducer is linear from 0 to 4.9 N and is calibrated by hanging weights on the button. The transducer is adjusted until it is almost perpendicular to the external nares and then pushed tightly against the nares. Our previous studies demonstrate that voluntary flaring of the nares against the transducer results in a linear relationship between force and average EMG, with high test–retest reliability (DelloRusso et al. 2002; Fuller et al. 1995), and that our measurements are sensitive enough to measure the force produced by single nasal dilator muscle motor units (Mateika et al. 1998). However, because the nasal dilator muscles are skin muscles, there is variable compliance in the system, which will influence absolute measurements of force (Fuller et al. 1995). Accordingly, we routinely express force as a percentage of the MVC force, as well as in absolute terms.
EMG activities of both right and left nasal dilator muscles were recorded with 2-mm silver/silver-chloride disc electrodes (Grass Instruments, Braintree, MA). Subsequent analysis showed that the activities recorded from the left and right nasal dilator muscles did not differ, so we report data on the left nasal dilator muscle, which is the same side used for the measurement of muscle force. The EMG activities were amplified and filtered (10–1,000 Hz) with Grass Model 7P511 amplifiers, which were connected in series to a circuit that rectified and RC-filtered the EMG with a time constant of 100 ms (Coulborn Instruments, Allentown, PA), resulting in a moving-time average, which we will refer to as the integrated EMG (iEMG). Muscle force and iEMG were recorded continuously on a Grass polygraph (Model 7). Unprocessed EMG activities and force were also recorded on videocassette recorder tapes, following pulse-code modulation (Vetter, Reading, PA), for subsequent analog to digital conversion and computer-assisted data analysis.
Because the facial motor nerve that innervates the nasal dilator muscles is superficially located, we were also able to produce evoked contractions by exciting the nerve electrically through 4-mm surface electrodes with a stimulator (Grass Model S44) and constant-current stimulus isolation unit (Grass PSIU). This procedure allowed us to obtain the MEP from the EMG recording, together with the muscle twitch force. The facial nerve was stimulated with single 0.1-ms square-wave pulses, with the voltage set 20–40% above the level that elicited the maximal twitch force. As demonstrated previously (DelloRusso et al. 2002; Fuller et al. 1995), contractile speeds measured with this technique agree well with published data for human limb and diaphragm muscles.
Experimental protocol 1: sustained contractions to task failure
Each subject performed four to five training sessions to practice flaring the nares and holding the force at a target level (see following text). Each subject then completed three trials on different days and in random order. The trials consisted of sustained contractions corresponding to 20, 35, and 65% of the MVC force. Since there are no other data on sustained, submaximal contraction forces in facial muscles, we needed another muscle in which to make comparisons. We chose the first dorsal interosseus (FDI) because it is commonly used and, like the nasal dilator muscle, is very small and very close to the skin surface. We chose our contraction intensities based on the work of Fuglevand et al. (1993). As here, they had subjects do sustained contractions of the FDI to task failure at 20, 35, and 65% of the MVC force. Studies examining the recruitment of FDI motor units during isometric contractions show that 27 of 38 motor units (70% of the sample) were recruited at forces ≤20% MVC force, 33/38 (87%) were recruited at forces <35% MVC force, and all units were recruited at ≥61% MVC force (Moritz et al. 2005). Although we do not know how nasal dilator muscle motor units are recruited during fatiguing contractions, we are assuming that the facial motor unit pool behaves at least somewhat similarly to the FDI pool. Thus the force levels that we chose provide two comparisons where recruitment of motor units is incomplete, but where the endurance capacity is still quite different, as well as a third comparison where the force level exceeds the upper limit of motor unit recruitment and results in relatively rapid task failure (see following text).
Experimental protocol 1: submaximal contractions sustained to task failure
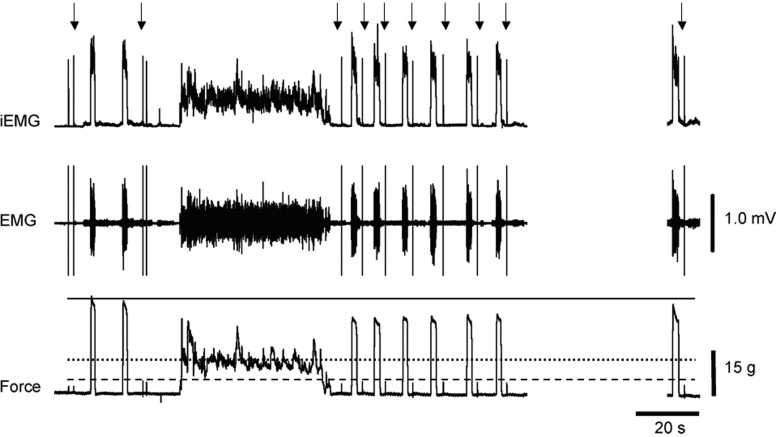
On the day of an experiment, subjects reported to the laboratory between 8:00 am and 4:00 pm and were instructed to refrain from caffeinated beverages and alcohol for ≥8 h prior to their scheduled start time. The subject sat in a comfortable chair, was instrumented, and then sat quietly for about 10 min until the experiment commenced. As shown in Fig. 1, the investigator began the experiment by applying two supramaximal stimuli to the facial nerve, each separated by about 2–5 s, to obtain MEPs and twitch forces. The subject then produced two MVCs, each separated by 7 to 10 s. Subjects were instructed to slowly bring force up to the maximal volitional level and to maintain it for 2–3 s once this maximal level was reached. About 5–10 s later another supramaximal stimulus was applied to the facial nerve. After another 10-s period, the subject was told to focus on the target line displayed on a computer monitor and, when an investigator said “Begin,” the subject raised the force to the target level by flaring the nares. The subject was instructed to maintain force as long as possible and was given verbal encouragement throughout the trial. Task failure was reached when force dropped to 90% of the target for ≥3 s. At this point, the subject was instructed to stop and a series of six supramaximal stimuli were delivered to the facial nerve, each followed by an MVC maneuver. Each stimulus–MVC pair was completed within an approximately 10-s interval (Fig. 1). This stimulus–MVC sequence was repeated during the last 10-s epoch of each of the next 4 min (minutes 2–5). The experiment ended with a final stimulus–MVC sequence 10 min after the endurance limit was reached.
FIG. 1.
Representative experiment during a fatigue trial with the target set at 35% maximal voluntary contraction (MVC) force. Electromyogram (EMG) rectified and integrated EMG (iEMG) and force tracings for the entire experiment are shown. The solid horizontal line on the force tracing demarcates the prefatigue MVC force; the dotted horizontal line on the force tracing represents the target force. Arrows above the iEMG tracing indicate the artifact that accompanied each supramaximal stimulus delivered to the facial nerve. The twitch forces f can be seen on the force tracing, with the peak potentiated twitch amplitude demarcated by the dashed horizontal line. The protocol began with 2 stimulations, followed by 2 MVC maneuvers, and then 2 more stimulations of the facial nerve. The subject then attempted to hold the target force for as long as possible. As soon as the endurance limit was reached, a series of 6 stimulation–MVC sequences were completed, each about 10 s apart. We then repeated this sequence at the 2nd, 3rd, 4th, 5th, and 10th minutes of recovery; note that data for the 2nd–5th minutes of recovery has been omitted, but maneuvers completed at minute 10 of recovery are included (maneuver following the break in record). Calibration values for EMG and force are shown on the far right.
Experimental protocol 2: twitch interpolation as a test of central activation
We restudied seven of the subjects to determine whether they were capable of fully activating the nasal dilator muscles during voluntary contractions. Subjects performed fifteen 10-s-long MVCs, each separated by a 10-s rest. Interpolated twitches were obtained during the fifth, tenth, and fifteenth contractions. We did not evoke interpolated twitches during the sustained, submaximal contractions because controlling volitional force with these muscles is difficult even in the absence of such distracting maneuvers. As a result, we decided not to interrupt the nasal dilator muscle contractions with interpolated twitches because our primary focus was to obtain unique and unblemished data on time to task failure and the corresponding changes in twitch force, EMG activity, EMG mean power frequency, and MEPs during and after each task.
Data analysis and statistics
Force and EMG recordings were sampled (Force, 2,000 Hz; EMG, 5,000 Hz) and analyzed digitally using commercially available hardware and software (Spike2, Cambridge Electronics Design, London, UK). All pre- and posttask failure MVCs were measured as described previously (DelloRusso et al. 2002) and the average data are reported. We calculated changes in the average EMG and in the mean power frequency of the EMG in each 10% epoch of each trial. This was done by dividing the time to task failure into 10 equivalent epochs. The total area of the iEMG occurring in each epoch was divided by the epoch duration to derive the average EMG occurring in each epoch. This value was expressed as a percentage of the average EMG activity measured during the first two pretest MVC maneuvers. To examine the rate of rise of the EMG and rate of decline of mean power frequency, we also examined these variables as a function of the absolute time to task failure and computed the slopes using linear regression analysis.
A fast Fourier transformation of the unprocessed EMG occurring in each 10% epoch was also performed and the power spectrum and mean power frequency were obtained using all data falling between 10 and 650 Hz (DelloRusso et al. 2002). The time to task failure varied greatly across trials and subjects, with 36 s the minimum value across all three tasks and all subjects. Therefore we used a block size of 3,072 ms, with the block taken from the center portion of each 10% epoch of each trial. This allowed us to capture equivalent segments of data with the same resolution across all trials and all subjects. Given that the sampling rate of the EMG was 5,000 Hz, the resolution in the frequency domain was 1.63 Hz. Since we used only one block per epoch, windowing of the data was unnecessary.
Twitch forces were analyzed for peak force (Po), the time to peak force (TTP), and the one-half relaxation time (1/2 RT). The MEP accompanying each twitch was analyzed for peak-to-peak amplitude, duration, and area. We did not measure conduction velocity directly because we are not confident in measurement of the distance between stimulating and recording electrodes on the face and nose, respectively, because these structures have complex contours. Instead, we measured conduction time, defined as the interval between the onset of the stimulus artifact and the onset of the MEP (Fig. 3B). Because during each experiment all electrodes were securely anchored, changes in conduction time must reflect changes in conduction velocity along the facial nerve. Because the stimulating electrodes were on the facial nerve and the recording electrodes on the nasal dilator muscles, our measurement of conduction time represents conduction along both nerve and sarcolemma (Almeida et al. 2008). However, we did not observe any changes in the duration of the MEP, suggesting that the increase in conduction time in the 20% MVC trial (Fig. 5) was the result of changes in facial nerve conduction, rather than conduction along the sarcolemmal membrane (see results).
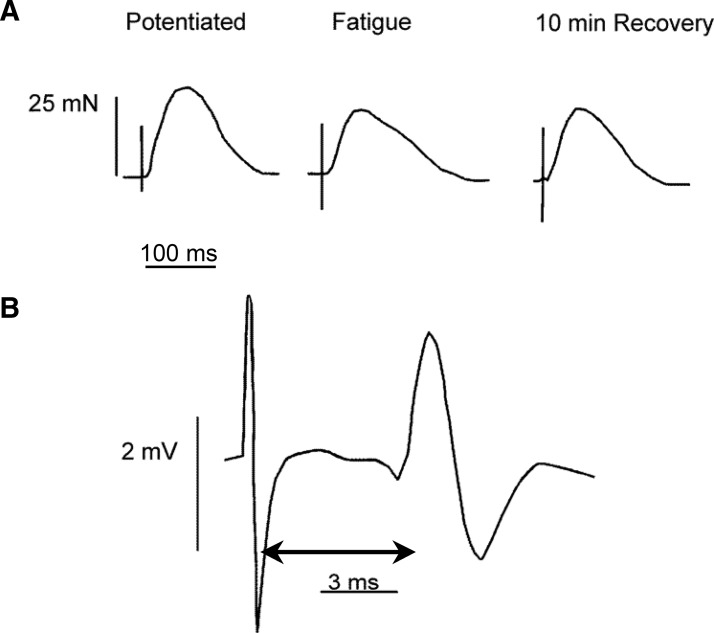
FIG. 3.
A: representative twitch forces from one subject. The potentiated twitch was obtained shortly after the subject performed 2 successive MVCs (see methods and Fig. 1). Task failure refers to the first twitch obtained after the subject reached the endurance limit and stopped contracting the muscle. In this example twitch force was reduced by about 25% at task failure and by 14.5% 10 min into the recovery period. B: representative maximal-evoked potential (MEP) from the same subject, measured during the potentiated twitch. The 2-sided arrow demonstrates how we measured conduction time (time from onset of the stimulus artifact, to the first negative deflection in the MEP).
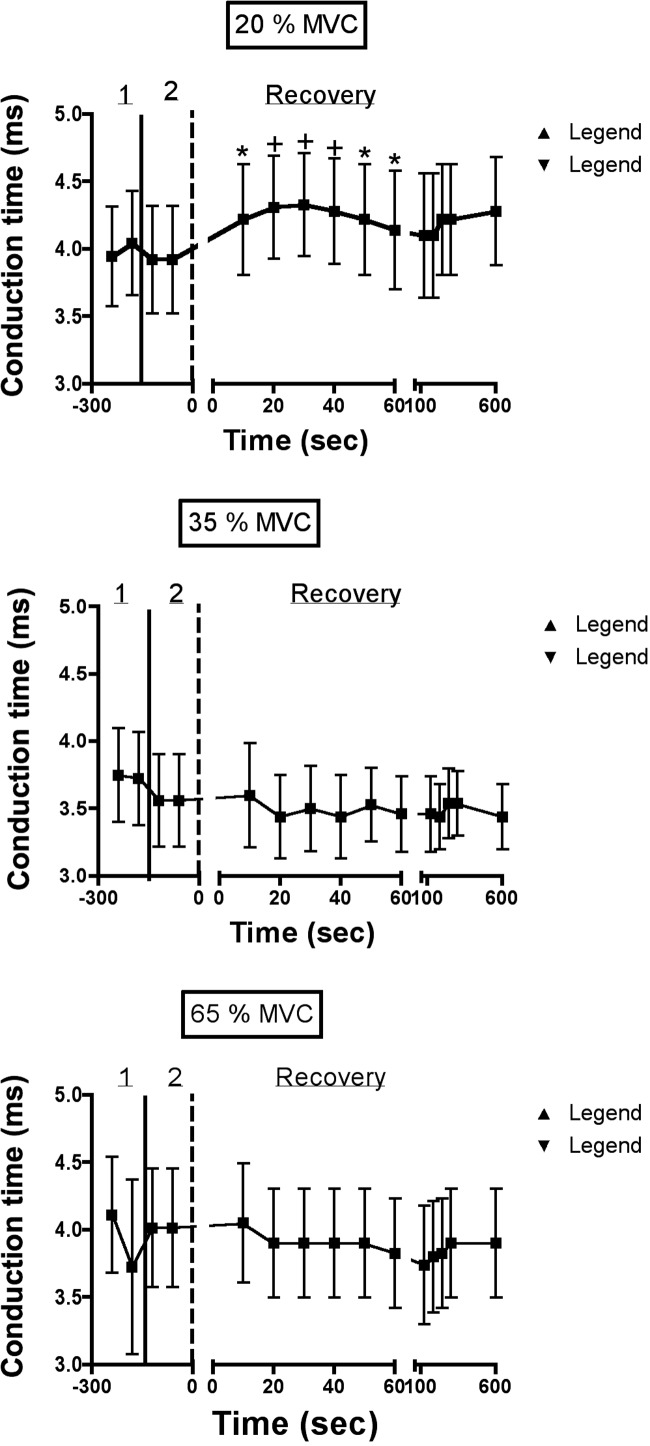
FIG. 5.
Average changes in conduction time of the MEP for each experimental trial (see Fig. 3B for definition of conduction time). Figure conventions are identical to those used in Fig. 4. Conduction time increased slightly when fatigue was induced by a contraction intensity of 20% MVC, but recovered to prefatigue levels within 1 min. There were no changes in conduction time during the 35 and 65% MVC trials. *P < 0.05; †P < 0.01.
We used the potentiated, pretrial twitch force and MEP values as the “baseline” measures in each subject. As shown in Table 1 and in Figs. 1 and 4, the twitches obtained just after the first two MVC maneuvers (“potentiated twitches”) were greater than those obtained before the first two MVC maneuvers. This postactivation potentiation has been observed in all types of contractions and in a variety of muscles (Baudry and Duchateau 2004) and, because of this, the contractile properties obtained from the potentiated twitch are the most relevant when comparisons are made before and after contractile activity.
TABLE 1.
Average nasal dilator muscle contractile properties, MEP area, and MVC force obtained during the 20% MVC test
| Property | Po, mN | TTP, ms | 1/2 RT, ms | MEP Area, mV·s−4 | MVC Force, mN |
|---|---|---|---|---|---|
| Initial value | 20.1 ± 3.7 | 42.2 ± 3.0 | 53.3 ± 5.4 | 45.4 ± 10.0 | 246 ± 15.0 |
| Potentiated | 30.0 ± 3.6** | 47.1 ± 4.4 | 43.7 ± 3.4 | 43.6 ± 10.3 | 253 ± 13.5 |
| Tlim | 19.4 ± 3.8* | 51.3 ± 2.1 | 52.6 ± 6.1 | 42.9 ± 8.8 | 188 ± 17.8** |
| 10 min post-Tlim | 17.0 ± 3.0** | 39.0 ± 3.4 | 45.7 ± 5.8 | 44.6 ± 9.6 | 254 ± 16.0 |
Values are means ± SE. Values obtained before the fatigue trial, at the endurance limit (Tlim), and following 10 min of recovery are reported. Values obtained during the 35 and 65% MVC tests were not significantly different from the values reported here. Asterisks indicate significant difference from pretest values (
P < 0.05,
P < 0.01) compared with initial values. Tlim, time limit of the fatigue task; Po, peak twitch force; TTP, time to peak twitch force; 1/2 RT, one half twitch relaxation time; MEP, maximal evoked potential recorded from the nasal dilator muscles following supramaximal stimulation of the facial nerve; MVC, maximal voluntary contraction force.
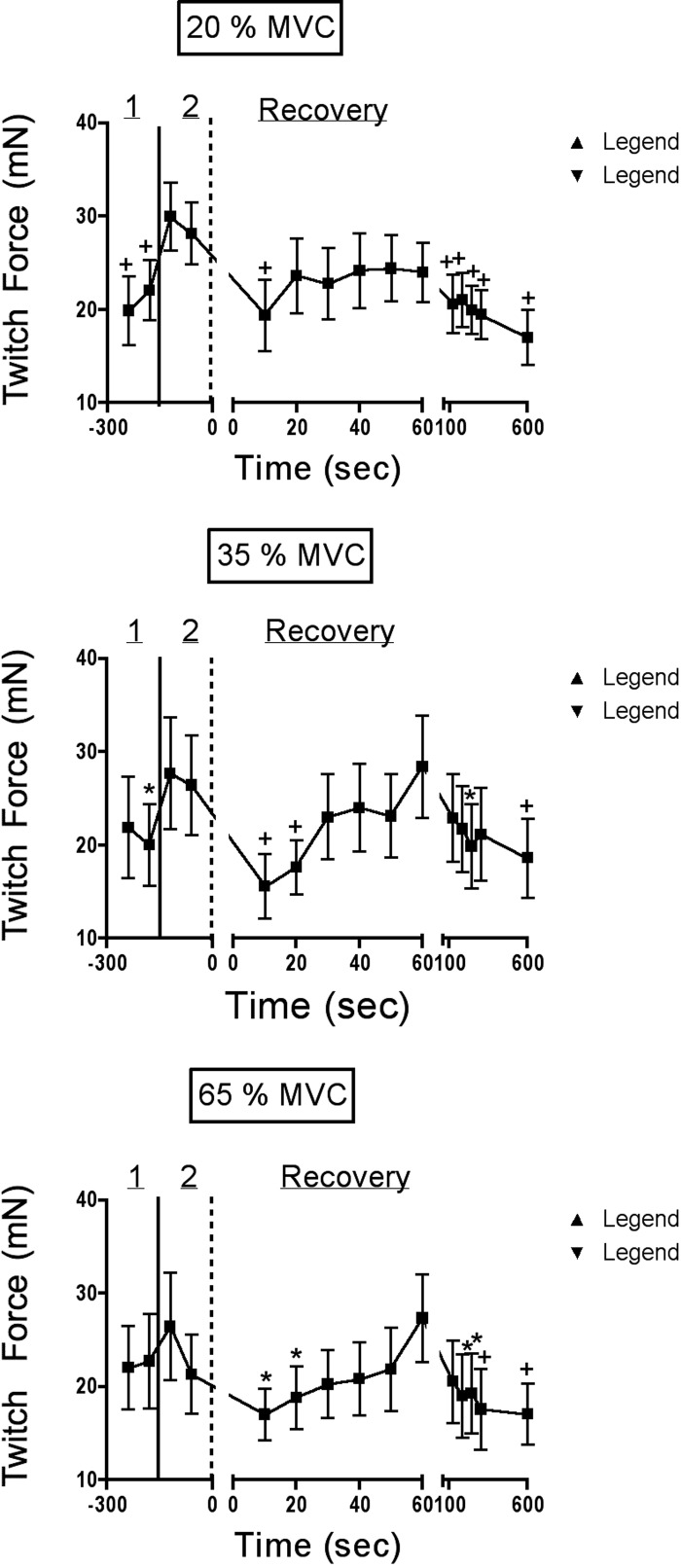
FIG. 4.
Average twitch force data for each of the experimental trials. Period 1 refers to the time when the first 2 nerve stimulations were applied; period 2 contains the potentiated twitches (those obtained after the first 2 MVC maneuvers; refer to Fig. 1). Recovery twitches are shown to the right of the dashed vertical line. Twitch force fell significantly at the endurance limit, began to recover quickly in the 1st minute of recovery, but remained depressed for ≥10 min in all trials. For this statistical analysis, the first potentiated twitch served as the control point and all other points were compared with this value with Dunnett's test (see methods). *P < 0.05; +P < 0.01.
Interpolated twitch amplitude was measured by first magnifying force and iEMG tracings using the Spike2 software program and manually placing horizontal cursors at the onset and peak of the interpolated twitch. Interpolated twitch onset was delineated by placing a vertical cursor at the stimulus artifact on the EMG and extending it to the force tracing (see Fig. 8). Interpolated twitch amplitude was expressed as a percentage of the corresponding MVC force [(twitch force/MVC force) × 100].
FIG. 8.
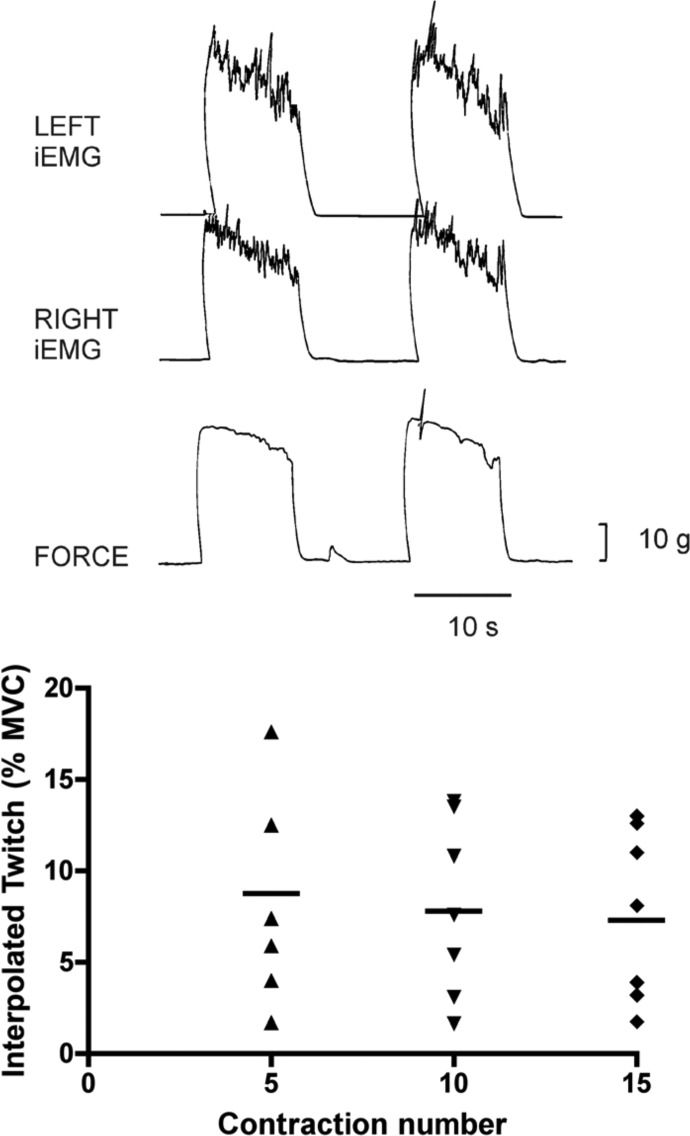
Top: representative tracings showing the influence of supramaximal stimulation of the left facial nerve during ongoing MVC maneuvers that were sustained for 10 s. Each of 7 subjects performed 15, 10-s MVCs each separated by 10 s of rest. Facial nerve stimulation was delivered during the 5th, 10th, and 15th contractions. The increase in force evoked by stimulation was used to estimate the percentage voluntary activation, as explained in methods. The top 2 traces are rectified and integrated EMG recordings (iEMG) from the left and right nasal dilator muscles and the bottom trace is nasal dilator muscle force. Bottom: interpolated twitch values obtained during the 5th, 10th, and 15th contractions, expressed as a percentage of the MVC force, for each of 7 subjects. Horizontal lines depict the mean value, which remained the same even though MVC force fell by an average of 30% by the 15th MVC. See text for discussion.
In all experiments, repeated-measures ANOVA and the Bonferroni post hoc procedure were used to establish the statistical significance of differences. The slopes and SE estimates obtained from linear regression analyses at each of the three contraction intensities were also compared with ANOVA and Bonferroni post hoc analysis. In all cases a value of P < 0.05 served as the threshold for defining statistical significance.
RESULTS
Time to task failure
As expected, time to task failure was inversely related to target force in all subjects and averaged 207.5 ± 36 (mean ± SE), 121.6 ± 42, and 39.4 ± 6 s when contractions were sustained at 20, 35, and 65% of maximal force, respectively. The average time to task failure in the three trials were significantly different from one another.
Voluntary and evoked (twitch) forces before and after sustained contractions
Figure 1 shows force (and EMG) recordings obtained before, during, and after a typical trial at 35% of the MVC force in a representative subject. The MVC force was reduced at the endurance limit but rose progressively throughout recovery. In this subject complete recovery was achieved within 10 min. In contrast, twitch force was still below the pretrial, potentiated level 10 min after the point of task failure, despite recovery of the MVC force.
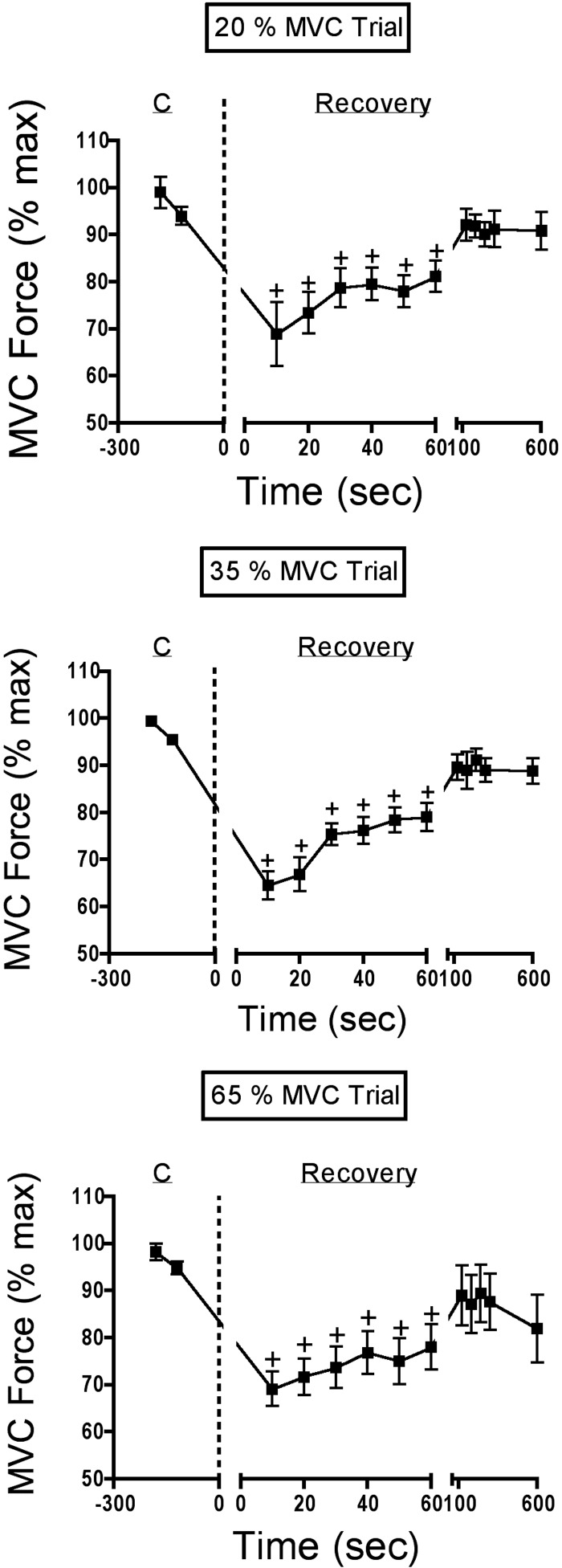
The average MVC force data for all trials ares shown in Fig. 2. Here we report MVC force as a percentage of the maximal value (see Fig. 1), but for comparative purposes we also include the absolute MVC force values, measured at selected time points, in Table 1. Note that in all three trials MVC force was reduced by about 30% at task failure, remained significantly depressed in the first minute of recovery, but recovered fully by the second minute.
FIG. 2.
Average data for MVC force for each of the experimental trials. The 2 MVCs just before the onset of the task are shown, followed by the MVC maneuvers recorded during the recovery period (refer to Fig. 1). MVC force was decreased significantly throughout the 1st minute in all 3 trials, but recovery was complete in all trials by the 2nd minute of recovery. C: control period. †, different than control (P < 0.01).
Twitch forces, evoked by supramaximal stimulation of the facial nerve in one subject, are shown in Fig. 3A. The twitch forces obtained in this subject are representative of the average responses, demonstrating that twitch force is significantly reduced at task failure and that this reduction lasts for several minutes into the recovery period. Average twitch force data for all subjects are shown in Fig. 4 and average data for contractile properties, obtained at select time points, are shown in Table 1. In each panel of Fig. 4, periods 1 and 2 refer to values obtained before and after potentiation of the twitch by the intervening MVC maneuvers (see Table 1 and Figs. 1 and 3A). At task failure, twitch force was depressed by the same amount in all three trials (Fig. 4). The depression persisted for the first 10–20 s of recovery, then returned to the baseline level over the next 1–3 min, depending on the contraction intensity (Fig. 4). However, in all trials the twitch force was again significantly depressed at 4–5 and at 10 min of recovery. Contractile properties did not change significantly at any point in any of the trials (Table 1).
We also measured changes in the peak-to-peak amplitude, area, and conduction time of the MEP, recorded during facial nerve stimulation. A representative MEP is shown in Fig. 3B, with the method used to estimate conduction time also illustrated. We did not observe any significant changes in peak-to-peak amplitude, area, or duration of the MEP at any time point in any of the conditions. Average values for MEP area at select time points are given in Table 1. We did, however, observe significant changes in facial nerve conduction time following the 20% MVC trial (Fig. 5). Note that conduction time increased slightly but significantly immediately after task failure, but recovered within 1 min. Similar changes were not observed in the 35 and 65% MVC trials.
EMG and mean power frequency before and after sustained contractions
Analysis of the average EMG and the mean power frequency as a function of absolute endurance time is shown in Fig. 6. The rate of rise of EMG activity and the rate of decline of mean power frequency were significantly greater in the 65 compared with the 35 and 20% MVC tasks. Although the slopes in the 20 and 35% MVC tasks appear to differ, these trends were not significant. Results of this analysis were qualitatively identical when the curves were fit with polynomial functions instead of linear ones.
FIG. 6.
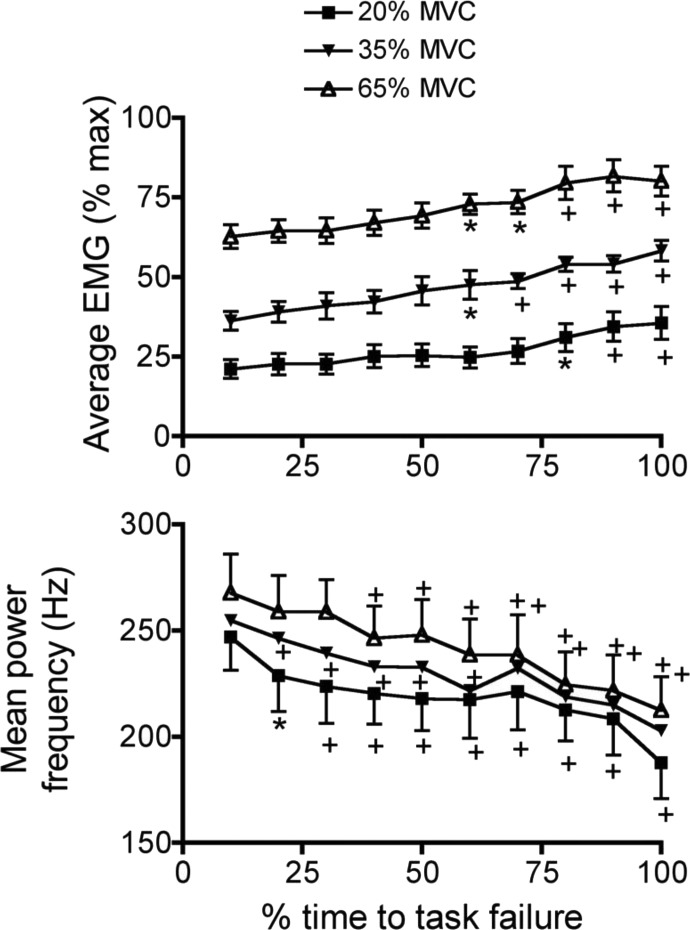
Change in average EMG (top) and mean power frequency (bottom) as a function of time to task failure. Average EMG rose progressively throughout the trial, but never exceeded 75–80% of the maximal value, even at the endurance limit. The rate of increase in the EMG was significantly greater in the 65% MVC task compared with the 2 lower-intensity tasks. EMG mean power frequency (bottom) declined progressively throughout the trial and the rate of decline was significantly greater in the 65% MVC task compared with that of the 2 lower-intensity tasks. *, slope in the 65% MVC trial is different from the slopes obtained at 20 and 35% MVC trials (P < 0.05).
When expressed as a percentage of time to task failure, average EMG rose monotonically in all three trials, with significant increases observed at 60–100% of the time to task failure (Fig. 7, top), although average EMG never reached the maximal level. The maximal EMG activity recorded throughout the recovery period was not significantly different from the maximal values recorded before the trials in any of the subjects (data not shown; example shown in Fig. 1). The mean power frequency of the EMG fell significantly as a function of time to task failure in all three trials (Fig. 7, bottom). Significant reductions in mean power commenced as early as 20% of time to task failure in the 20 and 35% MVC trials and at 40% time to task failure in the 65% MVC trial. Mean power frequency continued to decline as the trial progressed and, at task failure, was reduced by an average of 23, 19, and 19% in the 20, 35, and 65% MVC trials, respectively.
FIG. 7.
Change in average EMG (top) and mean power frequency (bottom) as a function time for task failure, which is normalized and divided into 10% intervals, as described in methods. Average EMG rose progressively throughout the trial, but never exceeded about 75% of the maximal value, even at task failure. EMG mean power frequency declined progressively throughout the trial, although the magnitude of the drop was similar at all 3 effort intensities. For these statistical analyses, the data point at 10% time to task failure served as the control point within each of the 3 experimental trials and all subsequent points were compared with this value with Dunnett's test (see methods). *P < 0.05; +P < 0.01.
Interpolated twitches during intermittent MVCs
MVC force dropped to 83, 76, and 68% MVC by the fifth, tenth, and fifteenth contractions, respectively (Fig. 8). The roughly 30% drop in MVC force by the fifteenth contraction was similar to the reduction in force at task failure in the 20, 35, and 65% MVC trials (Fig. 2). The average interpolated twitch force was about 8% of MVC force at all three measurement intervals, indicating that progressively worsening fatigue did not significantly alter the magnitude of voluntary activation (Fig. 8). Thus in the nasal dilator muscles voluntary activation averages about 92% of maximal evocable force and does not change significantly as fatigue intensifies.
DISCUSSION
Summary
Our goal was to test the hypothesis that central mechanisms contribute to the task failure associated with sustained contractions in a human upper airway muscle. Our main finding is that, although the average EMG increased with time, it never exceeded 75–80% of the maximal, pretrial level despite task failure. We also showed a steady and significant decline in the mean power frequency of the EMG throughout the task as well as a sustained reduction in twitch force despite fairly rapid (∼2 min) recovery of the MVC force. The MEP did not change significantly in any of the tasks, suggesting that the failure to maximally drive the muscles at task failure was due to mechanisms proximal to the neuromuscular junction. The rate of rise of EMG activity was significantly higher at 65% MVC compared with the trials performed at 20 and 35% MVC. These data indicate a more rapid increase in motor unit discharge rate at 65% MVC and the failure to optimize rate modulation and/or recruitment at the two lower-intensity tasks (see following text). Taken together, these observations suggest that the volitional motor system fails to fully activate the nasal dilator muscles when well-motivated, healthy subjects are asked to perform sustained, submaximal contractions at mild, moderate, and high intensity; putative mechanisms are discussed in the following text.
Time to task failure during sustained contractions
As anticipated, time to task failure was inversely and linearly related to the force requirement of each task. The same holds true for human hand muscle contractions performed at identical contraction intensities (Fuglevand et al. 1993). Previous studies have estimated the time to task failure in human genioglossus and masseter muscles during sustained, quasi-isometric contractions at intensities ranging from 30 to 100% of the MVC force. Time to task failure ranged from about 3 min at 30% MVC to about 30 s at 80% MVC, depending on the muscle and the characteristics of the subjects (Blumen et al. 2002, 2004; Maton et al. 1992; Mortimore et al. 1999; Scardella et al. 1993); these values are well within the range reported here for the nasal dilator muscles. This simple observation suggests that whatever the mechanism of task failure, the endurance limit of human upper airway and facial muscles, as in hand muscles, is closely related to the contraction intensity, at least over the range of intensities studied herein.
Maximal voluntary contraction force
MVC force was depressed at the endurance limit but recovered within 60 s at all three target force levels. The 30–40% decline in MVC force measured immediately after the point of task failure was similar at all three contraction intensities, although there was a trend for a larger reduction at the lower-intensity levels. This is similar to findings in small hand muscles, where MVC force at the endurance limit fell by 40% at an intensity of 20% MVC, compared with 30 and 19% reductions at 35 and 65% MVC, respectively (Fuglevand et al. 1993). In the present study, MVC force remained depressed for the first minute of recovery in all three trials, but thereafter was not significantly different from baseline MVC force. In the study by Fuglevand et al. (1993), MVC force was still significantly depressed 10 min into the recovery period at 20, 35, and 65% MVC. Thus following a sustained submaximal, quasi-isometric contraction protocol, human nasal dilator muscles regain their ability to produce MVC force more quickly than hand muscles do.
Evoked twitch force
The change in twitch force amplitude evoked by supramaximal stimulation of the facial nerve followed a biphasic trajectory following the termination of all three tasks. Twitch force was reduced significantly at task failure, showed some recovery over the subsequent minute, and then declined again over the last 5–10 min of the recovery phase, even as MVC force fully recovered within 1 min. These data are consistent with low-frequency fatigue, a phenomenon believed to be due to impaired calcium kinetics within the sarcolemma (Westerblad et al. 1993). We also found evidence for low-frequency fatigue during an intermittent maximal contraction protocol in our previous study of nasal dilator muscles (DelloRusso et al. 2002). This differs from findings in small hand muscles, wherein both twitch force and MVC force declined in parallel after fatiguing, sustained contractions, with both remaining depressed well into the recovery period (Fuglevand et al. 1993). Thus low-frequency fatigue appears to be a phenomenon underlying force failure in the nasal dilator muscles with both sustained and intermittent contractions (DelloRusso et al. 2002). It should be noted that contractile speed and relaxation times did not change in parallel with the decline in peak twitch force; again, this is consistent with previous data in this muscle (DelloRusso et al. 2002).
EMG changes
“Central fatigue” has been defined as “that component of overall muscle fatigue dependent on a progressive failure to drive motoneurons (and muscle fibers) voluntarily…” (Gandevia 2001). Although the surface EMG is an imperfect index of changes in central neural drive during sustained contractions, it is considered to be adequate in the absence of a concomitant change in the MEP (Gandevia 2001), as observed herein. With this in mind, one of the most remarkable features of this study is that the average EMG never exceeded 75–80% of the maximal, pretrial level despite task failure, suggesting an inability to maximally drive motoneurons (i.e., central fatigue). The deficit in average EMG was inversely related to contraction intensity, with much larger deficits in the low-intensity tasks (e.g., a 70% deficit during contractions sustained at 20% MVC). Similar EMG deficits have been observed previously in hand and limb muscles of subjects performing submaximal contractions at intensities within the range used here (Bigland-Ritchie et al. 1986; Fuglevand et al. 1993; Lind and Petrofsky 1979; Loscher et al. 1996a; Petrofsky et al. 1982; Sacco et al. 1997; Yoon et al. 2008). Although technical issues such as amplitude cancellation could explain some of the deficit, the contribution is expected to be small. Indeed, modeling studies show that when EMG recordings are normalized as a percentage of the maximum level recorded during MVC maneuvers, as done here, cancellation has no significant effect on EMG quantification (see Fig. 3B in Keenan et al. 2005).
The rate of rise of EMG activity was significantly higher in the 65 compared with the 35 and 20% MVC tasks (Fig. 6). The simplest interpretation of these data is that the rate of increase of motor unit discharge rate was higher during the 65% MVC task compared with the lower-intensity tasks. We say this because the motor unit pool for most muscles is fully recruited at roughly 60% of the MVC (Enoka and Duchateau 2008; Moritz et al. 2005). Although we do not know how individual nasal dilator muscle motor units are recruited during fatiguing contractions, it seems safe to assume, for the sake of argument, that the entire motor unit pool was recruited during the 65% MVC task (see methods). That said, the increase in EMG in the 65% MVC trial would have to be the result of discharge rate modulation. Importantly, changes in rate modulation did not reach maximal levels, as indicated by the 20–25% deficit in EMG at task failure. Although there is a trend for the 20 and 35% MVC EMG time slopes to differ from one another, the trend was not significant, indicating that the rate of rise of recruitment and rate modulation in these two tasks was similar.
Since the MEP did not change with task failure in our subjects, the activation failure occurred proximal to the neuromuscular junction at all three exercise intensities. Using the twitch interpolation method, several investigators have documented central fatigue during sustained, submaximal isometric contractions of limb and hand muscles, an effect that is particularly robust at lower contraction intensities (de Ruiter et al. 2004; Eichelberger and Bilodeau 2007; Gandevia 2001; Loscher et al. 1996a; Maton et al. 1992; Sacco et al. 1997). In our study of central activation of nasal dilator muscles (Fig. 8), MVC force dropped to 83, 76, and 68% MVC by the fifth, tenth, and fifteenth contraction, respectively. The roughly 30% drop in MVC force by the fifteenth contraction was similar to the reduction in force at task failure for the 20, 35, and 65% MVC trials (Fig. 2). The average interpolated twitch amplitude was about 8% of the MVC force at all three time points, indicating that progressive reductions in MVC force were not associated with changes in voluntary activation. This deficit occurred despite vigorous encouragement and the subjects' belief that they were exerting maximal effort to maintain the target force. Thus in the nasal dilator muscles voluntary activation averages about 92% of the maximal evocable force and does not change significantly as fatigue intensifies. This level of voluntary activation is within the range obtained in elbow flexor muscles (Gandevia et al. 1996).
Although we cannot quantify the proportion of the EMG deficit due to CNS mechanisms, it was likely substantial, particularly in the low-intensity tasks. For example, deficits in the surface EMG show good correspondence with the magnitude of change in central activation during submaximal fatiguing contractions of biceps brachii (Kalmar and Cafarelli 1999; Sacco et al. 1997) and vastus lateralis muscles (de Ruiter et al. 2004). Thus if we assume an 8–10% deficit in central activation during the sustained submaximal contraction tasks, the EMG activity at task failure during the 65% MVC task would have approached 100% of maximum if the subjects could in fact fully activate the muscle; this suggests that task failure under these conditions was explained in part by a failure of central activation, although we are unable to quantify its exact magnitude or locus. In contrast, this level of central activation failure cannot explain the very large EMG deficits observed in the 20 and 35% MVC tasks. Nevertheless, we cannot rule out the possibility that central activation failure was higher in these submaximal contraction tasks compared with what we measured during the MVCs.
Other factors that could explain the EMG deficit during sustained, submaximal contractions that we did not test—and that would be extremely difficult to examine in human subjects—include intrinsic adaptation of facial motoneurons (Magarinos-Ascone et al. 1999), inhibition from thin fiber, nonmyelinated axons sensitive to chemicals released by the contracting muscle (Garland and Kaufman 1995), or failure of recurrent inhibition to decline, as it does during fatiguing contractions of the soleus muscle (Loscher et al. 1996b). Although withdrawal of excitatory input from spindles and tendon organs plays a role in limb muscle fatigue (Enoka and Duchateau 2008; Klass et al. 2008), the nasal dilator muscles have no tendon organs and a paucity of spindles, if any (Bowden and Mahran 1956; Kubota and Masegi 1972); accordingly, this factor would have no role or a minimal one in our system.
Changes in EMG mean power frequency
The rate of decline of mean power frequency was significantly higher in the 65 compared with the 35 and 20% MVC tasks. When examined as a function of the percentage of time to task failure, the average drop in mean power averaged 20–25% in all three conditions. The drop in mean power, regardless of how it is evaluated, suggests that the shortfall in the EMG at task failure (i.e., the neural drive to the muscle) may have been underestimated because a decline in the mean power is typically associated with an increase in the interference EMG (Bigland-Ritchie et al. 1981; Fuglevand 1995; Fuglevand et al. 1989; Moritani et al. 1986). Observations in a variety of human skeletal muscles suggest that at least some of the decline in EMG mean power is the result of a slowing of muscle fiber conduction velocity (Bigland-Ritchie et al. 1981; Krogh-Lund and Jorgensen 1992; Lindstrom et al. 1970; Moritani et al. 1986; Vollestad 1997), although we found no significant changes in the duration of the MEP. Fatigue induced by submaximal contractions of the genioglossus, masseter, and temporalis muscles is also associated with declines in the mean power frequency of the EMG (Blumen et al. 2004; Maton et al. 1992; Svensson et al. 2001; Tortopidis et al. 1999), with the percentage reduction variable but within the range that we observed here (25–30% reduction, Fig. 6). As discussed in our previous report (DelloRusso et al. 2002), the mean power measured in the human nasal dilator muscles is higher than that recorded in other human muscles, including the masseter (Maton et al. 1992; Svensson et al. 2001). This is due mostly to the fact that the fibers insert into the skin, and thus are close to the recording electrodes, and also because the interelectrode distance is short (Bilodeau et al. 1990; De la Barrera and Milner 1994). Task failure at the end of the 20% MVC task was associated with a slight but significant reduction in the rate of action potential conduction along the facial nerve. This is probably not due to a dropping out of fast motor units because, if this were so, a decrease in MEP amplitude would have been observed, but MEP was unchanged. It is possible that sustained voluntary activation decreased the rate of neurotransmitter release secondary to intracellular depletion of calcium, which is in turn the result of calcium channel inactivation (“habituation”). Whether this occurs in skeletal muscle during voluntary, fatiguing contractions will require further investigation.
Conclusions
The endurance capacity of the nasal dilator muscles during sustained, submaximal contractions compares well to hand, pharyngeal, and other facial muscles despite several differences in anatomy and function. Endurance capacity of the nasal dilator muscles depends importantly on central mechanisms because subjects could not maximize neural drive to the muscle despite task failure. However, we emphasize that, although central fatigue must be present, we were unable to quantify its magnitude or where it originates (e.g., suprabulbar motor centers, brain stem interneurons, facial motoneurons). The persistent posttask depression of twitch force despite rapid recovery of MVC force suggests that this task also evoked low-frequency fatigue.
GRANTS
This work was supported by United States Public Health Service Grants HL-51056, HL-56876, and HL-68162.
Acknowledgments
We thank E. Essif, P. Suchdev, and J. C. Reeder for expert technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Almeida 2008.Almeida S, Riddell MC, Cafarelli E. Slower conduction velocity and motor unit discharge frequency are associated with muscle fatigue during isometric exercise in type 1 diabetes mellitus. Muscle Nerve 37: 231–240, 2008. [DOI] [PubMed] [Google Scholar]
- Bailey 2007.Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol 98: 3284–3291, 2007. [DOI] [PubMed] [Google Scholar]
- Baudry 2004.Baudry S, Duchateau J. Postactivation potentiation in human muscle is not related to the type of maximal conditioning contraction. Muscle Nerve 30: 328–336, 2004. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie 1981.Bigland-Ritchie B, Donovan EF, Roussos CS. Conduction velocity and EMG power spectrum changes in fatigue of sustained maximal efforts. J Appl Physiol 51: 1300–1305, 1981. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie 1986.Bigland-Ritchie B, Furbush F, Woods JJ. Fatigue of intermittent submaximal voluntary contractions: central and peripheral factors. J Appl Physiol 61: 421–429, 1986. [DOI] [PubMed] [Google Scholar]
- Bilodeau 1990.Bilodeau M, Arsenault AB, Gravel D, Bourbonnais D. The influence of an increase in the level of force on the EMG power spectrum of elbow extensors. Eur J Appl Physiol Occup Physiol 61: 461–466, 1990. [DOI] [PubMed] [Google Scholar]
- Blumen 2002.Blumen MB, Perez de La Sota A, Quera-Salva MA, Frachet B, Chabolle F, Lofaso F. Genioglossal electromyogram during maintained contraction in normal humans. Eur J Appl Physiol 88: 170–177, 2002. [DOI] [PubMed] [Google Scholar]
- Blumen 2004.Blumen MB, Perez de La Sota A, Quera-Salva MA, Frachet B, Chabolle F, Lofaso F. Tongue mechanical characteristics and genioglossus muscle EMG in obstructive sleep apnoea patients. Respir Physiol Neurobiol 140: 155–164, 2004. [DOI] [PubMed] [Google Scholar]
- Bowden 1956.Bowden RE, Mahran ZY. The functional significance of the pattern of innervation of the muscle quadratus labii superioris of the rabbit, cat and rat. J Anat 90: 217–227, 1956. [PMC free article] [PubMed] [Google Scholar]
- Carpentier 2001.Carpentier A, Duchateau J, Hainaut K. Motor unit behaviour and contractile changes during fatigue in the human first dorsal interosseus. J Physiol 534: 903–912, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connel 1993.Connel DC, Fregosi RF. Influence of nasal airflow and resistance on nasal dilator muscle activities during exercise. J Appl Physiol 74: 2529–2536, 1993. [DOI] [PubMed] [Google Scholar]
- De la Barrera 1994.De la Barrera EJ, Milner TE. The effects of skinfold thickness on the selectivity of surface EMG. Electroencephalogr Clin Neurophysiol 93: 91–99, 1994. [DOI] [PubMed] [Google Scholar]
- DelloRusso 2002.DelloRusso C, Khurana N, Rankin L, Sullivan J, Fregosi RF. Mechanisms of force failure during repetitive maximal efforts in a human upper airway muscle. Muscle Nerve 26: 94–100, 2002. [DOI] [PubMed] [Google Scholar]
- de Ruiter 2004.de Ruiter CJ, Elzinga MJ, Verdijk PW, van Mechelen W, de Haan A. Voluntary drive-dependent changes in vastus lateralis motor unit firing rates during a sustained isometric contraction at 50% of maximum knee extension force. Pflügers Arch 447: 436–444, 2004. [DOI] [PubMed] [Google Scholar]
- Eichelberger 2007.Eichelberger TD, Bilodeau M. Central fatigue of the first dorsal interosseous muscle during low-force and high-force sustained submaximal contractions. Clin Physiol Funct Imaging 27: 298–304, 2007. [DOI] [PubMed] [Google Scholar]
- Enoka 2008.Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol 586: 11–23, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi 1995.Fregosi RF, Lansing RW. Neural drive to nasal dilator muscles: influence of exercise intensity and oronasal flow partitioning. J Appl Physiol 79: 1330–1337, 1995. [DOI] [PubMed] [Google Scholar]
- Fuglevand 1995.Fuglevand AJ The role of the sarcolemma action potential in fatigue. Adv Exp Med Biol 384: 101–108, 1995. [DOI] [PubMed] [Google Scholar]
- Fuglevand 1989.Fuglevand AJ, Winter DA, Patla AE, Stashuk D. Effect of increased motor unit action potential duration on the amplitude and mean power frequency of the electromyogram. In: Proceedings of the 11th Annual International Conference of IEEE, Engineering in Medicine and Biology Society, 1989, Seattle, WA. Piscataway, NJ: IEEE, 1989, p. 953–954.
- Fuglevand 1993.Fuglevand AJ, Zackowski KM, Huey KA, Enoka RM. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol 460: 549–572, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller 1995.Fuller D, Sullivan J, Essif E, Personius K, Fregosi RF. Measurement of the EMG–force relationship in a human upper airway muscle. J Appl Physiol 79: 270–278, 1995. [DOI] [PubMed] [Google Scholar]
- Gandevia 2001.Gandevia SC Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. [DOI] [PubMed] [Google Scholar]
- Gandevia 1996.Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol 490: 529–536, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland 1995.Garland SJ, Kaufman MP. Role of muscle afferents in the inhibition of motoneurons during fatigue. Adv Exp Med Biol 384: 271–278, 1995. [DOI] [PubMed] [Google Scholar]
- Kalmar 1999.Kalmar JM, Cafarelli E. Effects of caffeine on neuromuscular function. J Appl Physiol 87: 801–808, 1999. [DOI] [PubMed] [Google Scholar]
- Kalmar 2004.Kalmar JM, Cafarelli E. Central fatigue and transcranial magnetic stimulation: effect of caffeine and the confound of peripheral transmission failure. J Neurosci Methods 138: 15–-26, 2004. [DOI] [PubMed] [Google Scholar]
- Keenan 2005.Keenan KG, Farina D, Maluf KS, Merletti R, Enoka RM. Influence of amplitude cancellation on the simulated surface electromyogram. J Appl Physiol 98: 120–−131, 2005. [DOI] [PubMed] [Google Scholar]
- Klass 2008.Klass M, Levenez M, Enoka RM, Duchateau J. Spinal mechanisms contribute to differences in the time to failure of submaximal fatiguing contractions performed with different loads. J Neurophysiol 99: 1096–1104, 2008. [DOI] [PubMed] [Google Scholar]
- Krogh-Lund 1992.Krogh-Lund C, Jorgensen K. Modification of myo-electric power spectrum in fatigue from 15% maximal voluntary contraction of human elbow flexor muscles, to limit of endurance: reflection of conduction velocity variation and/or centrally mediated mechanisms? Eur J Appl Physiol Occup Physiol 64: 359–370, 1992. [DOI] [PubMed] [Google Scholar]
- Kubota 1972.Kubota K, Masegi T. Muscle spindle distribution in snout musculature of the Japanese shrew-mole. Anat Rec 172: 703–709, 1972. [DOI] [PubMed] [Google Scholar]
- Lind 1979.Lind AR, Petrofsky JS. Amplitude of the surface electromyogram during fatiguing isometric contractions. Muscle Nerve 2: 257–264, 1979. [DOI] [PubMed] [Google Scholar]
- Lindstrom 1970.Lindstrom L, Magnusson R, Petersen I. Muscular fatigue and action potential conduction velocity changes studied with frequency analysis of EMG signals. Electromyography 10: 341–356, 1970. [PubMed] [Google Scholar]
- Liu 2005.Liu JZ, Zhang L, Yao B, Sahgal V, Yue GH. Fatigue induced by intermittent maximal voluntary contractions is associated with significant losses in muscle output but limited reductions in functional MRI-measured brain activation level. Brain Res 1040: 44–54, 2005. [DOI] [PubMed] [Google Scholar]
- Loscher 1996a.Loscher WN, Cresswell AG, Thorstensson A. Central fatigue during a long-lasting submaximal contraction of the triceps surae. Exp Brain Res 108: 305–314, 1996a. [DOI] [PubMed] [Google Scholar]
- Loscher 1996b.Loscher WN, Cresswell AG, Thorstensson A. Recurrent inhibition of soleus alpha-motoneurons during a sustained submaximal plantar flexion. Electroencephalogr Clin Neurophysiol 101: 334–338, 1996b. [DOI] [PubMed] [Google Scholar]
- Magarinos-Ascone 1999.Magarinos-Ascone C, Nunez A, Delgado-Garcia JM. Different discharge properties of rat facial nucleus motoneurons. Neuroscience 94: 879–886, 1999. [DOI] [PubMed] [Google Scholar]
- Mateika 1998.Mateika JH, Essif EG, Dellorusso C, Fregosi RF. Contractile properties of human nasal dilator motor units. J Neurophysiol 79: 371–378, 1998. [DOI] [PubMed] [Google Scholar]
- Maton 1992.Maton B, Rendell J, Gentil M, Gay T. Masticatory muscle fatigue: endurance times and spectral changes in the electromyogram during the production of sustained bite forces. Arch Oral Biol 37: 521–529, 1992. [DOI] [PubMed] [Google Scholar]
- Moritani 1986.Moritani T, Muro M, Nagata A. Intramuscular and surface electromyogram changes during muscle fatigue. J Appl Physiol 60: 1179–1185, 1986. [DOI] [PubMed] [Google Scholar]
- Moritz 2005.Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol 93: 2449–2459, 2005. [DOI] [PubMed] [Google Scholar]
- Mortimore 1999.Mortimore IL, Fiddes P, Stephens S, Douglas NJ. Tongue protrusion force and fatigability in male and female subjects. Eur Respir J 14: 191–195, 1999. [DOI] [PubMed] [Google Scholar]
- Petrofsky 1982.Petrofsky JS, Glaser RM, Phillips CA, Lind AR, Williams C. Evaluation of amplitude and frequency components of the surface EMG as an index of muscle fatigue. Ergonomics 25: 213–223, 1982. [DOI] [PubMed] [Google Scholar]
- Remmers 1978.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44: 931–938, 1978. [DOI] [PubMed] [Google Scholar]
- Saboisky 2006.Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol 95: 2213–2221, 2006. [DOI] [PubMed] [Google Scholar]
- Sacco 1997.Sacco P, Thickbroom GW, Thompson ML, Mastaglia FL. Changes in corticomotor excitation and inhibition during prolonged submaximal muscle contractions. Muscle Nerve 20: 1158–1166, 1997. [DOI] [PubMed] [Google Scholar]
- Scardella 1993.Scardella AT, Krawciw N, Petrozzino JJ, Co MA, Santiago TV, Edelman NH. Strength and endurance characteristics of the normal human genioglossus. Am Rev Respir Dis 148: 179–184, 1993. [DOI] [PubMed] [Google Scholar]
- Sullivan 1996.Sullivan J, Fuller D, Fregosi RF. Control of nasal dilator muscle activities during exercise: role of nasopharyngeal afferents. J Appl Physiol 80: 1520–1527, 1996. [DOI] [PubMed] [Google Scholar]
- Svensson 2001.Svensson P, Burgaard A, Schlosser S. Fatigue and pain in human jaw muscles during a sustained, low-intensity clenching task. Arch Oral Biol 46: 773–777, 2001. [DOI] [PubMed] [Google Scholar]
- Taylor 2000.Taylor JL, Allen GM, Butler JE, Gandevia SC. Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol 89: 305–313, 2000. [DOI] [PubMed] [Google Scholar]
- Todd 2003.Todd G, Petersen NT, Taylor JL, Gandevia SC. The effect of a contralateral contraction on maximal voluntary activation and central fatigue in elbow flexor muscles. Exp Brain Res 150: 308–313, 2003. [DOI] [PubMed] [Google Scholar]
- Tortopidis 1999.Tortopidis D, Lyons MF, Baxendale RH. Bite force, endurance and masseter muscle fatigue in healthy edentulous subjects and those with TMD. J Oral Rehabil 26: 321–328, 1999. [DOI] [PubMed] [Google Scholar]
- Vollestad 1997.Vollestad NK Measurement of human muscle fatigue. J Neurosci Methods 74: 219–227, 1997. [DOI] [PubMed] [Google Scholar]
- Westerblad 1993.Westerblad H, Duty S, Allen DG. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol 75: 382–388, 1993. [DOI] [PubMed] [Google Scholar]
- White 2006.White DP The pathogenesis of obstructive sleep apnea: advances in the past 100 years. Am J Respir Cell Mol Biol 34: 1–6, 2006. [DOI] [PubMed] [Google Scholar]
- Williams 2000.Williams JS, Janssen PL, Fuller DD, Fregosi RF. Influence of posture and breathing route on neural drive to upper airway dilator muscles during exercise. J Appl Physiol 89: 590–598, 2000. [DOI] [PubMed] [Google Scholar]
- Yoon 2008.Yoon T, De-Lap BS, Griffith EE, Hunter SK. Age-related muscle fatigue after a low-force fatiguing contraction is explained by central fatigue. Muscle Nerve 37: 457–466, 2008. [DOI] [PubMed] [Google Scholar]