Abstract
Loss of the mRNA-binding protein FMRP results in the most common inherited form of both mental retardation and autism spectrum disorders: fragile X syndrome (FXS). The leading FXS hypothesis proposes that metabotropic glutamate receptor (mGluR) signaling at the synapse controls FMRP function in the regulation of local protein translation to modulate synaptic transmission strength. In this study, we use the Drosophila FXS disease model to test the relationship between Drosophila FMRP (dFMRP) and the sole Drosophila mGluR (dmGluRA) in regulation of synaptic function, using two-electrode voltage-clamp recording at the glutamatergic neuromuscular junction (NMJ). Null dmGluRA mutants show minimal changes in basal synapse properties but pronounced defects during sustained high-frequency stimulation (HFS). The double null dfmr1;dmGluRA mutant shows repression of enhanced augmentation and delayed onset of premature long-term facilitation (LTF) and strongly reduces grossly elevated post-tetanic potentiation (PTP) phenotypes present in dmGluRA-null animals. Null dfmr1 mutants show features of synaptic hyperexcitability, including multiple transmission events in response to a single stimulus and cyclic modulation of transmission amplitude during prolonged HFS. The double null dfmr1;dmGluRA mutant shows amelioration of these defects but does not fully restore wildtype properties in dfmr1-null animals. These data suggest that dmGluRA functions in a negative feedback loop in which excess glutamate released during high-frequency transmission binds the glutamate receptor to dampen synaptic excitability, and dFMRP functions to suppress the translation of proteins regulating this synaptic excitability. Removal of the translational regulator partially compensates for loss of the receptor and, similarly, loss of the receptor weakly compensates for loss of the translational regulator.
INTRODUCTION
Fragile X syndrome (FXS) is a broad-spectrum neurological disease with symptoms including hyperactivity, hypersensitivity to sensory stimuli, and seizures (Koukoui and Chaudhuri 2007; Visootsak et al. 2005). FXS is commonly caused by an expanded 5′-CGG repeat region upstream of the fragile X mental retardation 1 (fmr1) gene, which causes hypermethylation and transcriptional silencing. The fmr1 product, FMRP, is an mRNA-binding protein that associates with polyribosomes and the RNA-induced silencing complex (RISC) and functions as a negative translational regulator (Garber et al. 2006). In mice, fmr1 knockout causes enhanced hippocampal long-term depression (LTD) (Huber et al. 2002), dependent on group I class 5 metabotropic glutamate receptor (mGluR5) signaling, which is sensitive to translational inhibitors (Huber et al. 2000; Koekkoek et al. 2005; Nosyreva and Huber 2006). Knockout (KO) mice also display depressed long-term potentiation (LTP) in the visual neocortex, dependent on mGluR5 activation (Wilson and Cox 2007). These results have given rise to the hypothesis that FMRP modulates synaptic transmission strength by regulating local protein synthesis downstream of mGluR signaling (Bear et al. 2004; Dolen et al. 2007; Pfeiffer and Huber 2006). This theory has been bolstered by findings of increased protein synthesis with mGluR stimulation in the absence of FMRP (Chuang et al. 2005; Hou et al. 2006; Koekkoek et al. 2005). Recently, rescue of several mouse fmr1 null phenotypes has been achieved by reducing mGluR signaling in the mGluR5 heterozygote background (Dolen et al. 2007), although without any assessment of neurotransmission itself. Here, we test this mGluR hypothesis by evaluating synaptic function in genetic mutants that completely eliminate mGluR signaling, FMRP function, or both in double homozygous null mutants.
Drosophila provides a powerful, simplified genetic model to test the mGluR theory of FXS, because the fly genome encodes a single fmr1 family member (dfmr1) and single mGluR (dmGluRA) (Bogdanik et al. 2004; Parmentier et al. 1996; Wan et al. 2000; Zhang et al. 2001). As in mice, dfmr1 mutants display synaptic structure defects and mistrafficking of ionotropic glutamate receptors (Gatto and Broadie 2008; Pan and Broadie 2007; Pan et al. 2004, 2008; Tessier and Broadie 2008; Zhang et al. 2001). Similarly, dmGluRA mutants display defective synaptic architecture, glutamate receptor trafficking, and activity-dependent synaptic plasticity (Bogdanik et al. 2004; Pan and Broadie 2007). In dfmr1;dmGluRA double mutants, synergisitic effects occur in the regulation of coordinated movement, sculpting of synaptic structure, and control of glutamate receptor trafficking (Pan and Broadie 2007; Pan et al. 2008). However, we have not yet addressed the hypothesized intersecting roles of dmGluRA signaling and dFMRP function in the modulation of synaptic functional plasticity.
In this study, we examine the relationship between dFMRP and dmGluRA in regulating neurotransmission at the glutamatergic neuromuscular junction (NMJ), a long-established system for studying the genetic foundations of synaptic plasticity. Null dfmr1 and dmGluRA mutants display largely normal basal transmission and short-term facilitation, but dfmr1;dmGluRA double mutants show increased facilitation. Single null mutant phenotypes are manifest during prolonged high-frequency stimulation (HFS). Null dmGluRA mutants display heightened augmentation, a dramatically lowered threshold for long-term facilitation, and greatly elevated post-tetanic potentiation. Importantly, removing dFMRP in dfmr1;dmGluRA double mutants partially alleviates all these defects, albeit with very weak reduction of the facilitation defects and strong suppression of the potentiation defect. Null dfmr1 mutants display an intriguing cyclic transmission amplitude periodicity during HFS trains and synaptic hyperexcitability during and following HFS. Importantly, removing dmGluRA in dfmr1;dmGluRA double mutants strongly reduces these defects. Taken together, these data suggest that loss of dFMRP translational inhibition partially alleviates the phenotypes resulting from loss of dmGluRA signaling and that loss of the receptor similarly partially corrects defects caused by impaired translation regulation. However, reduction of mutant phenotypes in both directions is mostly quite weak, showing that the convergence of dmGluRA and dFMRP function is limited and that other signaling pathways must interact with both glutamate receptor and translation regulator.
METHODS
Drosophila genetics
All Drosophila stocks were maintained at 25°C on standard food under standard conditions. The P-element imprecise excision deletion dmGluRA112b (hereafter called dmGluRA) is a null mutation of the sole functional Drosophila mGluR; the precise excision line from the same screen, dmGluRA2b (hereafter called control), was used as the genetic background control (Bogdanik et al. 2004; Pan and Broadie 2007). The P-element imprecise excision deletion dfmr13 (hereafter called dfmr1) is a dfmr1 null mutation (Dockendorff et al. 2002; Pan et al. 2008). The dfmr13 allele was backcrossed for six generations into the dmGluRA2b genetic background, so that this single genetic background control could be used for all conditions (Pan and Broadie 2007; Pan et al. 2008). A dfmr13;dmGluRA112b double homozygous null mutant (hereafter called dfmr1;dmGluRA) was generated using standard genetic techniques. Both single null mutants and the double null mutant are adult viable, and all genotypes have been repeatedly confirmed by sequencing, anti-dmGluRA/dFMRP immunocytochemistry and Western blot analyses (Pan and Broadie 2007; Pan et al. 2008).
Electrophysiology
Two-electrode voltage-clamp (TEVC) recordings were made from the wandering third-instar NMJ synapse as described previously (Long et al. 2008; Rohrbough et al. 1999; Trotta et al. 2004). Animals were dissected at 18°C in standard saline (in mM: 128 NaCl, 2 KCl, 4 MgCl2, 70 sucrose, 5 HEPES, pH 7.2) containing 0.15 mM Ca2+ unless otherwise indicated in individual experiments. All TEVC recordings were done at 18°C in anterior abdominal segments A3-4 at the muscle 6/7 NMJ with the muscle held at −60 mV using an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA). Intracellular recording electrodes were filled with 3 M KCl and typically had a resistance of 8–12 MOhms. Excitatory junction current (EJC) responses were evoked using a glass suction electrode on the severed motor nerve by application of brief stimuli (0.5 ms) from a S88 stimulator (Grass Instruments, Quincy, MA). Muscle 6 is innervated by two motor axons of differing physiological properties. In all experiments, both axons were stimulated in unison (suprathreshold) with stimulations at 150% of threshold. Acceptable recordings required a resting leak current of <25 nA for the entirety of the recording session, with no significant shifts in the leak current. Data acquisition was performed using pClamp8.0 (Axon Instruments) and Clampfit9.1 (Axon Instruments) software in all experiments.
Data analysis
Data analyses were performed using Prism software 4.0 (Graphpad, San Diego, CA). Appropriate statistical analyses were used as noted and included both one-way and two-way ANOVAs. Statistical significance was determined with appropriate posttests, most commonly a Bonferroni posttest, as indicated. Mean EJC amplitudes were determined by averaging all the stimulated responses that occurred within the designated period and stimulation condition, with the mean responses for each animal then averaged for all animals of each genetic line. For normalized comparisons of EJC amplitudes, the 0.5-Hz response amplitudes were first averaged before the high-frequency stimulation (HFS) condition. The response amplitudes from each genotype during the HFS period (At) and the basal 0.5-Hz stimulation period following HFS (potentiation period) were divided by the average of the initial basal amplitude (Ai) to determine the normalized value for each animal. The normalized values (At/Ai) were averaged to determine the mean normalized values for each genotype.
Hyperpotentiation was defined as a post-HFS response that was at least five times greater than the mean response amplitude (Ai) before the HFS train. The percentage of animals with hyperpotentiation was determined by identifying animals with at least one post-HFS stimulation normalized amplitude greater than five, and dividing this number by the n for that genetic condition. To determine the number of post-HFS stimulations defined as hyperpotentiated, the number of response amplitudes greater than five were counted for each animal within a given strain and averaged with all the other animals within that strain. Statistical significance for the average number of response amplitudes for each genotype was determined using one-way ANOVA and a Bonferroni post-test.
RESULTS
Basal Ca2+-dependent function largely normal in dfmr1 and dmGluRA mutants
Our previous work has shown that basal synaptic transmission amplitude is unaltered compared with genetic background control in the dmGluRA null mutant (Bogdanik et al. 2004); however, this finding has recently been challenged by a report of significantly elevated basal transmission amplitude in this mutant (Howlett et al. 2008). Our previous work has shown elevated presynaptic vesicle cycling and heightened transmission amplitude in the dfmr1 null mutant (Gatto and Broadie 2008; Zhang et al. 2001); however, this work was not pursued in the Ca2+ concentration range used in functional plasticity assays. In addition, we particularly wanted to assay the dfmr1;dmGluRA double null mutant, which has never before been subject to functional analyses. We therefore first performed assays of synaptic transmission strength at the wandering third-instar NMJ synapse over a range of external Ca2+ concentrations while suprathreshold stimulating the motor nerve at a basal frequency of 0.5 Hz. EJC recordings were made in the muscle clamped at −60 mV. The results of these basal analyses are shown in Fig. 1.
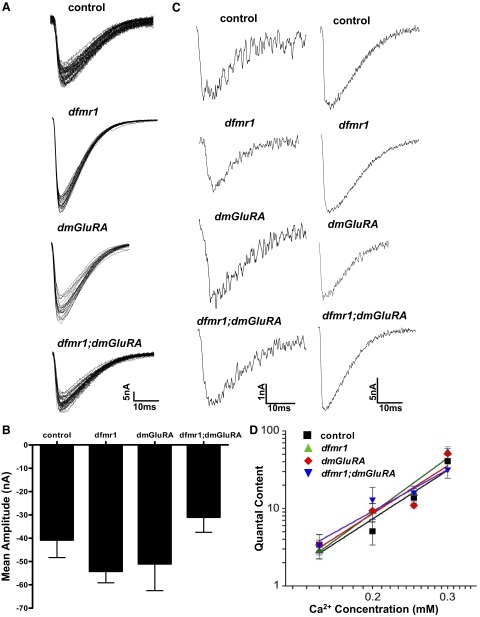
FIG. 1.
Basal synaptic transmission amplitude and calcium dependence. A: representative excitatory junction current (EJC) traces from the four genotypes used in this study: genetic background control (dmGluRA2b), dfmr1 null (dfmr13), dmGluRA null (dmGluRA112b), and the double homozygous null mutant (dfmr13/dfmr13; dmGluRA112b/dmGluRA112b). All recordings were made in wandering third instar from the muscle 6 neuromuscular junction (NMJ) synapse in 0.3 mM [Ca2+] with a stimulation frequency of 0.5 Hz. Scale bar, 5 nA and 10 ms. B: mean EJC amplitude of each genotype. There is no statistical significance between any of the genotypes. Sample sizes: control (n = 5), dfmr1 (n = 5), dmGluRA (n = 6), and dfmr1;dmGluRA (n = 7). C: representative single EJC traces of each genotype at 0.15 mM (left) and 0.25 mM (right) [Ca2+]. The scale bars are 1 nA and 10 ms (0.15 mM); 5 nA and 10 ms (0.25 mM). D: mean quantal content EJC responses for each genotype at four [Ca2+] concentrations: 0.15, 0.2, 0.25, and 0.3 mM. The fitted lines are on a log scale. Slopes: control, 3.56; dfmr1, 4.01; dmGluRA, 3.52; dfmr1;dmGluRA, 3.05.
During low-frequency stimulation, all genotypes display robust, high fidelity neurotransmission. Figure 1A shows representative traces of 20 superimposed EJC responses from the genetic background control (dmGluRA2b; see methods), single null mutant alleles of dfmr1 and dmGluRA, and the double null mutant dfmr1;dmGluRA. Both single mutants, dfmr1 and dmGluRA, showed a tendency toward elevated response amplitudes compared with control and especially compared with the dfmr1;dmGluRA double null (Fig. 1A). At 0.3 mM [Ca2+], mean amplitudes were 40.78 ± 7.54 (control), 54.2 ± 4.95 (dfmr1), 50.98 ± 11.53 nA (dmGluRA), and 31.01 ± 6.44 nA (dfmr1;dmGluRA). However, under these conditions, there were no statistically significant differences in EJC amplitude between any of the genotypes (one-way ANOVA, Kruskal-Wallis posttest P = 0.1504; Fig. 1B). The animal sample sizes were control (n = 5), dfmr1 (n = 5), dmGluRA (n = 6) and dfmr1;dmGluRA (n = 7). Synaptic transmission strength was next assayed over a range of Ca2+ concentrations of 0.15, 0.2, 0.25, and 0.3 mM. Figure 1C shows representative single EJC traces at 0.15 (left) and 0.25 mM (right) for all four genotypes. Typical responses were comparable under these basal stimulation conditions. There were no significant differences in EJC response amplitude between any of the genotypes studied, including control, dfmr1, dmGluRA, and dfmr1;dmGluRA double null (Fig. 1, C and D).
In a log plot, using the power function y = Axn, the relationship between transmission (quantal content) and external Ca2+ concentration was determined to assay the Ca2+ cooperativity for synaptic vesicle release (Fig. 1D). The theoretically predicted power relationship is 4.0. The measured slopes for this relationship were 3.56 (control), 4.01 (dfmr1), 3.52 (dmGluRA), and 3.05 (dfmr1;dmGluRA). The correlation fit (R) for these lines was 0.94–0.98 for all genotypes (Fig. 1D). Although these slopes differ, there is not a large change in the Ca2+ cooperativity of transmission. We conclude that, under these conditions of basal synaptic stimulation, there are no striking alterations in Ca2+-dependent EJC amplitude for either the dfmr1 or dmGluRA single null mutants or the dfmr1;dmGluRA double mutant.
Elevated short-term facilitation in the dfmr1;dmGluRA double null mutant
Although basal synaptic function is not strongly impacted by the absence of dFMRP, dmGluRA, or both proteins, we hypothesized that there may be critical requirements for these proteins under conditions of HFS. During stimuli trains >10 Hz, the NMJ synapse shows significant facilitation in extracellular Ca2+ concentrations <0.2 mM [Ca2+]; higher Ca2+ concentrations result in pronounced synaptic fatigue (Rohrbough et al. 1999; Zhong and Wu 1991). We therefore used 0.15 mM [Ca2+], beginning with 1 min of 0.5-Hz stimulation to establish basal EJC amplitude, followed by trials of short train stimulation at 20 Hz for each genotype to compare the level of transmission over the transient period of short-term facilitation (STF; <1 s) (Bogdanik et al. 2004). We assayed control, dfmr1, and dmGluRA single null mutants and the double null dfmr1;dmGluRA mutant. Normalization was done for each animal separately, with each HFS response normalized to the first event of the HFS train. After normalization of each of the trials, a mean normalization was determined for each animal and each genotype. Statistical analysis was completed using a two-way ANOVA with weighted means analysis, and a Bonferroni post-test. Representative traces and quantified facilitation curves are shown in Fig. 2.
FIG. 2.
Enhanced short-term facilitation in the dfmr1;dmGluRA double mutant. A: representative EJC traces during a 1-s, 20-Hz stimulation train in the genetic background control (top), dmfr1 single mutant null (2nd), dmGluRA single mutant null (3rd), and dmfr1;dmGluRA double null mutant (bottom). The dfmr1;dmGluRA double null shows strongly enhanced short-term facilitation compared with control, dfmr1, and dmGluRA. Scale bars: 5 nA, 15 ms. B: quantification of mean normalized EJC amplitudes for the 1st 20 responses of stimuli trains for each genotype. All responses were normalized to the first EJC amplitude of the 20-Hz stimulus train. Each animal was tested 5 times, and the mean of the normalized responses was averaged with all animals from that genotype. Facilitation is significantly enhanced in dfmr1;dmGluRA, with no significant difference between control, dfmr1, and dmGluRA. Sample sizes: control (n = 6), dfmr1 (n = 7), dmGluRA (n = 7), and dfmr1;dmGluRA (n = 7).
Under these conditions, the genetic background control, dfmr1, and dmGluRA single null mutants showed comparable levels of STF. Figure 2A shows representative traces of the 20 responses recorded to a 1-s, 20-Hz stimulus train for all genotypes. For the control, the average facilitation at the second stimulation of the train was 2.05 ± 0.25, and at the 20th stimulation, the facilitation was 6.11 ± 1.15 (n = 6; Fig. 2B). The dfmr1 null showed similar levels of facilitation with a normalized value of 2.12 ± 0.24 at the second stimulation and 6.07 ± 0.98 at the 20th stimulation (n = 7). Similarly, the dmGluRA null displayed 1.75 ± 0.22 and 5.66 ± 1.22 facilitation at these two time points (n = 7). Thus these three genotypes showed an indistinguishable STF profile under these conditions (Fig. 2B). In contrast, the dfmr1;dmGluRA double null manifested greatly elevated facilitation during the 20-Hz HFS. The representative trace in Fig. 2A shows elevated EJC response amplitudes and also the appearance of multiple peaks, which was not observed in the other genotypes. The quantified data reveal an average normalized value of 2.62 ± 0.42 (n = 7) at the second stimulation (Fig. 2B). At the end of the 1-s, 20-Hz stimulus train, the 20th stimulation displayed an average facilitation of 13.84 ± 3.01 (n = 7), more than twice the STF shown by either of the single mutants alone (Fig. 2B). These results indicate that STF under these conditions is normal in dfmr1 and dmGluRA mutants but that there is an unexpected synergistic interaction between dfmr1 and dmGluRA in the double mutant, leading to enhanced STF in dfmr1;dmGluRA.
Enhanced augmentation in dmGluRA and dfmr1;dmGluRA mutants
Our previous work has shown that dmGluRA null mutants display normal basal transmission but increased synaptic augmentation during maintained HFS (Bogdanik et al. 2004). A comparable analysis has not been performed in the dfmr1 null condition. We particularly wanted to assess whether the double null dfmr1;dmGluRA mutant might show a synergistic interaction or rescue the enhanced dmGluRA long-term augmentation toward control levels. We therefore applied prolonged exposure of 10-Hz stimuli trains to all genotypes to compare the level of transmission over the transient period of augmentation (>2 s, ≤1 min) (Bogdanik et al. 2004). To enable a direct comparison between genotypes, mean EJC amplitudes were first determined at the 0.5-Hz basal stimulation frequency (Ai) and normalized relative to the Ai amplitude at the given time following initiation of the 10-Hz HFS train (At). Stimulation artifacts were removed to better visualize augmentation. Representative traces and quantified augmentation curves are shown in Fig. 3.
FIG. 3.
Enhanced augmentation in dmGluRA modified in dfmr1;dmGluRA mutants. A: representative traces of EJC responses during a prolonged (1 min) 10-Hz stimulation train in the genetic background control (top), dmfr1 null (2nd), dmGluRA null (3rd), and dmfr1;dmGluRA double null (bottom). Arrows denote initiation of the high-frequency stimulation (HFS) train. Both dmGluRA and dfmr1;dmGluRA show a dramatic increase in augmentation during prolonged HFS. Scale bars 20 nA, 2 s. B: normalized EJC amplitudes during the 10-Hz stimulation train as a function of time (s) since HFS initiation. Quantification shows the 1st 30 s of 10-Hz HFS. C: mean EJC amplitudes for each genotype during 1 min of basal stimulation (0.5 Hz) and during HFS (10 Hz). Sample sizes: control (n = 7), dfmr1 (n = 9), dmGluRA (n = 8), and dfmr1;dmGluRA (n = 10). Significance indicated as P < 0.001 (***).
During prolonged 10-Hz stimulation, both control and dfmr1 null mutants maintain a relatively constant 2- to 4-fold increase in EJC response amplitude compared with basal transmission (Fig. 3A). There is an increase in EJC amplitude during the HFS period from 2.66 ± 0.39 and 2.53 ± 0.35 at t = 2 s to 3.52 ± 0.59 and 3.61 ± 0.63 at t = 30 s (n = 6, 9), respectively (Fig. 3B). Thus removal of dFMRP does not detectably alter synaptic transmission amplitudes during prolonged HFS. In sharp contrast, the profile of both the dmGluRA single null and dfmr1;dmGluRA double null exhibit a large increase in mean normalized response amplitude during the entire period of prolonged HFS (Fig. 3A). The normalized amplitude of dmGluRA increased from 3.36 ± 0.44 at t = 2 s, whereas the normalized amplitude at 30 s of HFS was 10.89 ± 2.39 (n = 10; Fig. 3B). Similarly, the double null mutant normalized amplitude at t = 2 s was 3.11 ± 0.47 and was 12.61 ± 2.72 at 30 s of HFS (n = 10; Fig. 3B). Thus removal of dmGluRA has a profound effect of transmission amplitudes during prolonged HFS, suggesting that excess glutamate release normally feedbacks through mGluR signaling to limit transmission strength.
Simultaneous removal of dFMRP does considerably modify the elevated augmentation of the dmGluRA null. During early HFS (<10 s), it is apparent that the single null dmGluRA mutant quickly reaches its augmentation peak amplitude (Fig. 3A). In contrast, the dfmr1;dmGluRA double mutant shows a drastically slower increase in response amplitude during the early HFS period, although the elevated augmentation is similar to the single mutant during later periods as HFS continues (Fig. 3A). This difference is apparent in the quantified data in Fig. 3B, comparing mean normalized EJC amplitudes at <10 s HFS. These data suggest that removal of dFMRP partially compensates for loss of mGluR signaling during the early phase of augmentation. Both the dmGluRA single null and dfmr1;dmGluRA double null show “bursts” of stepwise increases in response amplitudes followed by a gradual decrease in response amplitudes (Fig. 3B). These stepwise increases are suggestive of some “threshold” event, which is attained during HFS in both of these genotypes but not control and dfmr1 genotypes.
Mean response amplitudes for all four genotypes during pre-HFS at 0.5 Hz (1 min) and during 1 min of 10-Hz stimulation show that both dfmr1;dmGluRA and dmGluRA have much larger increases in response amplitude between LFS and HFS compared with control and dfmr1 (Fig. 3C). There are significant differences in HFS amplitudes between control and both dmGluRA and dfmr1;dmGluRA (P < 0.001 for both, n = 7, 8, and 10 for control, dmGluRA, and dfmr1;dmGluRA, respectively; Fig. 3C). Additionally, there are significant differences in HFS amplitudes between dfmr1 and both dmGluRA and dfmr1;dmGluRA (P < 0.001 for both comparisons, n = 9, 8, and 10 for dfmr1, dmGluRA, and dfmr1;dmGluRA, respectively; Fig. 3C). There are no significant differences between any other pre-HFS or HFS mean response amplitudes (Fig. 3C). Taken together, these results suggest a decrease in the mutant phenotype in dfmr1;dmGluRA compared with dmGluRA. However, the reduction of this phenotype in the double mutant is minimal, and therefore, there does not seem to be strong overlap in the requirements for the dmGluRA and dfmr1 pathways.
Hyperpotentiation in dmGluRA eliminated in dfmr1;dmGluRA double mutant
A prolonged HFS train >5 Hz induces post-tetanic potentiation (PTP) that persists for many minutes (Bogdanik et al. 2004; Rohrbough et al. 1999; Zhong and Wu 1991). The normal PTP that occurs at the wildtype NMJ synapse initially starts at ∼200% of basal amplitude and averages ∼160% potentiation over the first minute. We have previously shown that dmGluRA null synapses display significantly increased PTP (Bogdanik et al. 2004). We wanted to assay predicted changes in PTP in the dfmr1 null and to test possible interactions in the dfmr1;dmGluRA double null mutants. To better describe the observed phenotypes, we define here a state of hyperpotentiation as a mean EJC response >5 times (500%) the mean response amplitude of the animal before the HFS train (Ai). In these experiments, we stimulated each genotype for 1 min at 0.5 Hz to establish the basal Ai, followed by 1 min of 10-Hz HFS, and returned to the 0.5-Hz frequency for 2 min following the HFS. The results of this analysis are shown in Fig. 4.
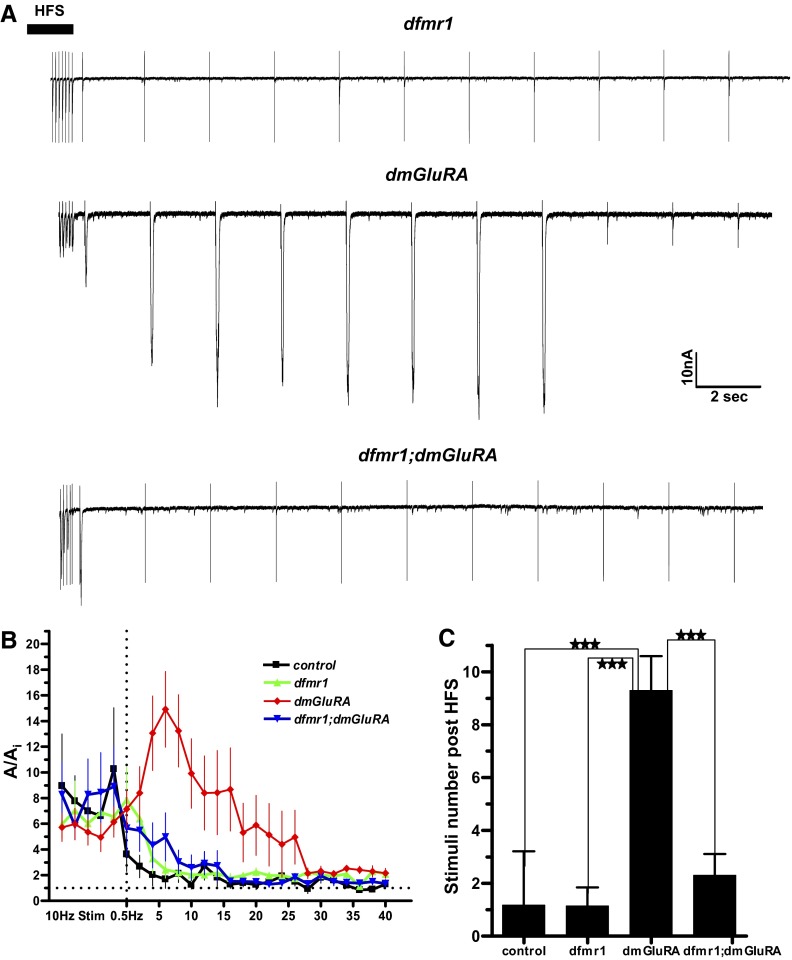
FIG. 4.
Hyperpotentiation in dmGluRA reduced in dfmr1;dmGluRA double mutant. A: representative traces of EJC responses during the PTP period after an HFS train (1 min at 10 Hz). The black bar denotes the end of HFS train. A hyper-elevated potentiation is manifest in the dmGluRA null, which is not present in control, the dfmr1 null or the dfmr1;dmGluRA double mutant. Scale bar: 10 nA, 2 s. B: quantification of normalized EJC amplitudes after the 10-Hz HFS train, highlighting PTP during the first 30 s of post-HFS 0.5-Hz stimulation. Sample sizes: control (n = 6), dfmr1 (n = 9), dmGluRA (n = 10), and dfmr1;dmGluRA (n = 10). C: quantification of the hyperpotentiation phenotypes defined as >5-fold increase from basal EJC amplitude (Ai). The dmGluRA null animals show significantly longer-lasting potentiation compared with all other genotypes. Significance indicated as P < 0.001 (***).
We focus here on the synaptic responses following the HFS train. Figure 4A shows representative traces at 0.5 Hz immediately at the end of the HFS period, as indicated. All four genotypes showed an extended period of low-level potentiation (>150% Ai) after the return to 0.5-Hz stimulation frequency. In addition, however, the dmGluRA null mutant exhibits a striking hyperpotentiation response amplitude, which was strongly reduced in the dfmr1;dmGluRA double null mutant (Fig. 4, A and B). The hyperpotentiated period is transient in the dmGluRA null mutant, persisting on average for ∼30 s following HFS (Fig. 4B). In the double mutant, this period of potentiated responses is rarely observed, and response amplitudes are comparable to the control and the dfmr1 single mutant (Fig. 4B). The number of post-tetanic stimulations with a normalized value >5 times Ai were 1.17 ± 0.83 (control), 1.14 ± 0.70 (dfmr1), 9.29 ± 1.30 (dmGluRA), and 2.30 ± 0.80 (dfmr1; dmGluRA; n = 6, 7, 7, and 10 for control, dfmr1, dmGluRA, and dfmr1;dmGluRA, respectively; Fig. 4C). There is a fourfold decrease in the duration of the hyperpotentiated responses in the double mutant compared with the dmGluRA single mutant. Thus only the null dmGluRA animals show a significantly increased number of hyperpotentiation responses (Fig. 4C).
These experiments show that removal of dFMRP can greatly reduce the grossly elevated PTP caused by a loss of dmGluRA. Although hyperpotentiation still occurs more frequently in dfmr1;dmGluRA mutant animals compared with control or dfmr1 animals, the hyperpotentiation lasts for a significantly shorter period and is significantly lower in mean amplitude than in dmGluRA mutants and is therefore more comparable in duration and degree to control and dfmr1. These results suggest that dFMRP and dmGluRA strongly interact in the regulation of synaptic potentiation.
Accelerated long-term facilitation in dmGluRA delayed in dfmr1;dmGluRA mutant
Previous work has described abrupt threshold LTF phenotypes in several mutants of critical neuronal genes (Mee et al. 2004; Poulain et al. 1994; Stern and Ganetzky 1989). These mutants either reduce voltage-gated K+ currents (hyperkinetic, frequenin) or increase Na+ currents (pumilio). During HFS, the resultant increase in neuron excitability drives these mutants to reach the LTF threshold more rapidly, with extended depolarization of the synaptic terminal. The heightened excitability triggers a greater Ca2+ influx, causing an increase in neurotransmitter release and abrupt increase in amplitude: LTF. We have shown previously that dmGluRA null mutants display a lowered excitability threshold when exposed to prolonged HFS, resulting in a much more rapid onset of LTF (Bogdanik et al. 2004). However, it is unknown how the loss of dFMRP impacts LTF threshold or whether the dfmr1;dmGluRA double mutant might accentuate or rescue the dmGluRA phenotype. We therefore assayed the onset and progression of LTF during prolonged 10-Hz HFS in all four genotypes. Representative traces are shown in Fig. 5.
FIG. 5.
Long-term facilitation in dmGluRA delayed in dfmr1;dmGluRA mutant. Representative EJC traces showing the appearance of long-term facilitation (LTF) during 10-Hz stimulus trains. A: the dfmr1 null mutant shows no LTF during a 1-min stimulus train. B: the dmGluRA null mutant always displays rapid, clean initiation of LTF, early in the HFS period. The bottom trace shows the LTF initiation phase (black bar) with higher temporal resolution. The dmGluRA null is the only genotype that increases its amplitude in a single step between two consecutive stimulations. C: the dfmr1;dmGluRA double null mutant displays an oscillating LTF phenotype. The double mutant shows multiple breakaway attempts before eventually maintaining the increased LTF response amplitude. The bottom trace shows the LTF initiation phase (black bar) with higher temporal resolution. Note the dramatic single step increase in amplitude of dmGluRA compared with the multiple rounds of dfmr1;dmGluRA to maintain the LTF threshold. Scale bar: 10 nA, 1 s; inset scale bars: 10 nA and 250 ms.
LTF is rarely observed at the wildtype NMJ synapse during HFS trains up to a minute. In controls, LTF is typically induced only by the application of drugs that increase neuronal excitability (Howlett et al. 2008; Jan and Jan 1978; Stern and Ganetzky 1989; Stern et al. 1990) or by much more prolonged periods of HFS (data not shown). Similarly, the dfmr1 null mutant rarely or never manifest early onset LTF but rather maintains a low level of augmentation for the duration of the HFS train (Fig. 5A). Thus dfmr1 alone is indistinguishable from control. In sharp contrast, both the dmGluRA null and the dfmr1;dmGluRA double null mutants show premature LTF, manifested as a sudden increase in EJC response amplitude during HFS (Fig. 5, B and C). In-depth analysis showed that the dmGluRA null transition between the modestly elevated augmentation level and dramatically elevated LTF level is very sharp, usually occurring in a single jump between two adjacent stimuli during the 10-Hz train (Fig. 5B, inset). In dmGluRA single mutants, this change is always unidirectional to the hyperfacilitated level, which persists for the length of the HFS and beyond (Fig. 5B). In contrast, the dfmr1;dmGluRA double mutant animals manifest a clear difficulty in surpassing the LTF threshold. Figure 5C shows the typical case in which the double mutant alternates between low- and high-amplitude states for a period of time before obtaining sustained LTF. Higher time resolution clearly shows that dfmr1;dmGluRA oscillates between the augmented and LTF states (Fig. 5C, inset). Thus removal of dFMRP results in the delay of onset of the premature LTF threshold caused by loss of mGluR signaling at the synapse.
The HFS period required before reach LTF threshold is greater in the control and dfmr1 null than in the dmGluRA null or the double null mutant. However, the onset of the LTF phenotype was not statistically significant between dmGluRA single null and dfmr1;dmGluRA (unpaired, 2-tailed t-test, P = 0.5684, 9,729 ± 2,467 ms; n = 8 and 11,840 ± 2,606 ms; n = 9, respectively). As above, these data suggest partial convergence of dfmr1 and dmGluRA pathways rather than an inclusive, linear pathway. Although dfmr1;dmGluRA still has a lower threshold for LTF compared with control and dfmr1, the fluctuation between low and high amplitudes before finally passing the LTF threshold is an indication of the return of this phenotype toward the wildtype condition.
Cyclic changes in EJC amplitude during HFS in dfmr1 and dfmr1;dmGluRA
The above results show significant changes in synaptic function in dmGluRA mutants during prolonged HFS, most often weakly modified by simultaneous removal of dFMRP in dfmr1;dmGluRA double null mutants. To this point, however, the dfmr1 null single mutant has resembled the control and has not shown any significant phenotypes when removed alone. Null dfmr1 phenotypes do appear at higher stimulation frequencies of prolonged duration. To expand on our understanding of the changes in excitability for each mutant, we increased the rate and number of stimuli applied to the NMJ synapse in an attempt to reach the postulated excitability threshold for all genotypes. We applied prolonged (1 min) 20-Hz stimuli trains while monitoring synaptic EJCs. Representative traces at this condition are shown in Fig. 6.
FIG. 6.
Cyclic EJC amplitude changes in dfmr1 and dfmr1;dmGluRA mutants. Prolonged stimulation at 20 Hz results in the manifestation of periodic alterations in EJC response amplitudes in the dfmr1 null mutant and dfmr1;dmGluRA double null mutant. Representative traces of genetic background control (top), dfmr1 (2nd), dmGluRA (3rd), and dfmr1;dmGluRA (bottom) after similar lengths of exposure to 20-Hz stimulation. A: the control always shows relatively stable maintained EJC amplitudes. B: most dfmr1 nulls display a characteristic cycling of EJC response amplitudes, from low to high transmission states, with a fairly regular periodicity. C: the dmGluRA null resembles the control, with stable maintenance of EJC amplitudes and no evidence of cycling, albeit with an elevated mean EJC amplitude. D: the dmfr1;dmGluRA double mutants are a mixed population; the smaller group (<25%) present significant cycling of response amplitudes compared with control and dmGluRA animals (top trace), but the larger group (>75%) are phenotypically similar to control and dmGluRA animals and show no cycling (bottom trace). Scale bars: 10 nA, 100 ms.
The wildtype synapse continues to follow well at 20 Hz, with EJC responses of fairly constant amplitude for the duration of the HFS train (Fig. 6A). In sharp contrast, the dfmr1 null mutant manifests a phenotype never before observed by us or, to our knowledge, by others: cyclic changes in EJC response amplitude (Fig. 6B). During prolonged 20-Hz HFS, dfmr1 mutants manifest periodic cycling of EJC response amplitudes, which begin with enhanced EJC amplitudes, followed by cycling between abnormally low and abnormally high values. Of the 16 dfmr1 null animals studied, 9 presented this EJC cycling phenotype (56%), whereas none of the control or dmGluRA animals showed any cycling under these conditions (Fig. 6C). As can be noted in Fig. 6B, during the lulls in EJC response to the HFS, complete loss of EJC response amplitude is not manifested but rather is greatly depressed, but persistent EJCs are recorded: there is no increased incidence of synaptic transmission failure. Additionally, a ramping up of response amplitude can often be seen during the larger response amplitude phases of the cycling rather than an immediate stepwise increase in response amplitude compared with the LTF threshold (cf. Fig. 5B). Thus this cycling phenotype must be caused by the modulation of neurotransmission strength at the synapse and not by a change in action potential propagation in the motor axons.
Simultaneous removal of dmGluRA provides clear reduction of this dfmr1 cyclic EJC amplitude phenotype. The dfmr1;dmGluRA double mutant does display the cyclic response amplitude defect during 20-Hz HFS (Fig. 6D). However, many fewer double mutant animals display the phenotype compared with dfmr1 single mutants (Fig. 6D); less than one half as many dfmr1;dmGluRA animals display this defect (23.5% in dfmr1;dmGluRA vs. 56% in dfmr1, n = 17 and 16 for dfmr1;dmGluRA and dfmr1, respectively). When the cycling is manifest, there is no significant difference in the average time from HFS onset to initiation of the cycling phenotype. The number of EJC events in a complete cycle seems greater in the double mutant (26.88 ± 3.75 for dfmr1;dmgluRA compared with 17.73 ± 1.82 for dfmr1), but the increase is not significant (1-way ANOVA, Bonferroni post-test). The average number of EJC events showing depressed amplitudes during the cycling is 12.98 ± 3.6 for dfmr1;dmGluRA (n = 4/17 animals) and 6.02 ± 0.3 for dfmr1 (n = 9/16 animals). The period of this depressed cycle is 301 ± 15 ms in the dfmr1 single null mutant compared with 649 ± 180 ms in the dfmr1;dmGluRA double mutant. Note that the elevated variability in the double mutant is caused, at least in part, by the small sample size, because few of the double mutants display any detectable cycling. The level of modulation from peak EJC to depressed EJC is greater in the dfmr1 single mutant compared with the dfmr1;dmGluRA double mutant (cf. Fig. 6, B and D). These results show that removal of dFMRP moves the synapse closer to an excitability threshold in which greatly elevated EJC amplitudes are manifest (i.e., similar to the LTF threshold) but that the defect is only clearly manifest at higher levels of HFS. Aspects of the phenotype are significantly reduced by loss of dmGluRA and co-removal of dmGluRA results in a large decrease in number of animals affected.
Increased synapse excitability in dfmr1, dmGluRA and dfmr1;dmGluRA mutants
Many dfmr1 null mutants also manifest a clear hyperactive state in which a single nerve stimulus results in multiple EJC responses. Such a phenotype is a well-established characteristic of membrane hyperexcitability mutants, such as the Shaker K+ channel mutant (Ganetzky and Wu 1986). We also previously showed a similar phenotype in dmGluRA null mutants, which, when exposed to prolonged HFS, showed asynchronous, bursting excitatory synaptic currents (Bogdanik et al. 2004). We therefore examined this hyperactivity phenotype in careful detail, focusing in particular on the dfmr1;dmGluRA double null mutant condition.
The wildtype NMJ synapse shows clean, single EJC responses to each stimulus in a 20-Hz stimulus train for the entire duration of the train (Fig. 7A). Each EJC event presents a smooth profile of relatively constant amplitude without the appearance of multiple response peaks. As noted previously, dfmr1 single mutants do not display augmentation, LTF, and PTP phenotypes, and seem well behaved in most regards compared with dmGluRA nulls. However, dfmr1 null mutants routinely display the aberrant profile of multiple, enlarged EJC events in response to a single stimulus during a prolonged HFS train (Fig. 7B). During 20-Hz stimulation trains, 88% of dfmr1 null mutants (n = 16) manifest this multiresponse profile starting early during the HFS train. For both dmGluRA and dfmr1;dmGluRA mutants, similar multiple EJC responses were apparent but appeared only immediately before the time of long-term facilitation onset (Fig. 7, C and D). Importantly, however, the multipeak characteristics of both dmGluRA and dfmr1;dmGluRA are always multiple, summating release events within a single EJC peak with a single return to baseline, whereas dfmr1 mutants show a different phenotype of multiple separate and complete EJC events (cf. Fig. 7, B with C and D). Indeed, in the 17 dfmr1;dmGluRA double mutant animals analyzed, there were only two isolated events, in separate animals, which showed the multiple EJC profile similar to the dfmr1 null. Thus the multiple discrete EJC events characteristic of dfmr1 are very much reduced by co-removal of dmGluRA. Control animals did not present this hyperexcitable phenotype during any of our studies.
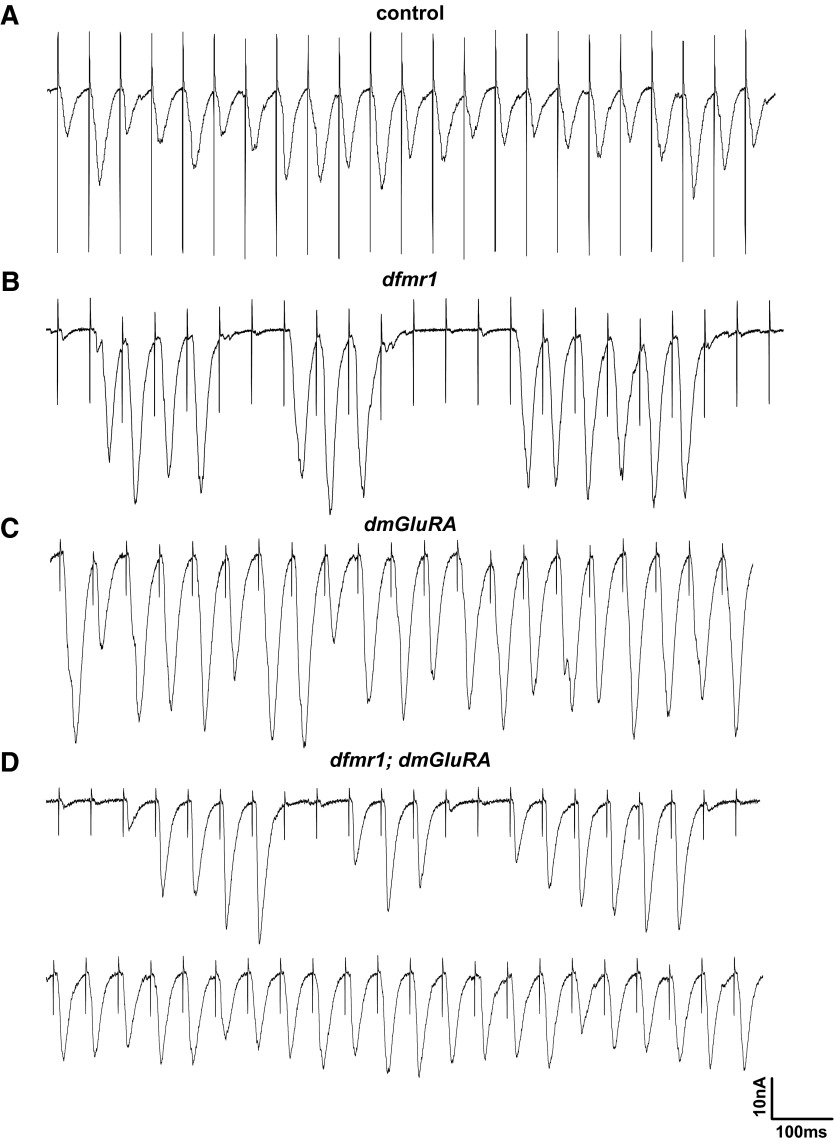
FIG. 7.
Hyperexcitability of dfmr1 null during HFS. Representative EJC traces during prolonged trains of 20-Hz stimulation. Multiple EJC responses per nerve stimulation event are observed in dfmr1, dmGluRA, and dfmr1;dmGluRA animals. A: the genetic background control shows clean, single EJC responses to every nerve stimulus. B: the dfmr1 null mutant often displays multiple EJC responses to a single stimulation; shown expanded in the bottom trace. The dfmr1 null shows multiple responses randomly during early portions of the 20-Hz stimulation train. Both dmGluRA (C) and dfmr1;dmGluRA (D) also manifest multiple EJC responses, which characteristically appear around the time of LTF initiation. Insets of each genotype highlight the difference between the asynchronous firing in dmGluRA and dfmr1;dmGluRA mutants, compared with separate multiple responses to a single stimulation in dfmr1. Scale bar: 10 nA, 50 ms; inset scale bars: 20 nA, 30 ms.
Null dfmr1 mutants also displayed a maintained hyperexcitabilty profile during basal (0.5 Hz) stimulation following the HFS train (Fig. 8). In control animals, the fidelity of synaptic transmission was always maintained both during the HFS train and following the HFS train (Fig. 8A). In sharp contrast, during the post-HFS period, dfmr1 mutants also presented a hyperexcitable state characterized by multiple, enlarged synaptic responses to a single nerve stimulus (Fig. 8B). This phenotype was never observed in the dmGluRA null (data not shown; indistinguishable from control in Fig. 8A). Importantly, the dfmr1;dmGluRA double mutant also never showed this hyperexcitable response defect, showing a strong reduction of the hyperexcitable state of the dfmr1 condition. This maintained hyperexcitable phenotype in the mutant was similar to the multiple responses per stimuli noted during HFS for dfmr1, in that each response was complete and separate from other EJCs occurring after a given stimuli. These results show that synaptic excitability is increased in the absence of dFMRP and that removal of mGluR signaling restores the dfmr1 mutant toward the normal, wildtype state of synaptic fidelity.
FIG. 8.
Hyperexcitability of dfmr1 null after prolonged HFS. Representative traces of control (top) and dfmr1 (bottom) during a 0.5-Hz stimulation period after 1 min of 20-Hz stimulation. A: the control shows small EJC responses to each stimulation event, with no spurious EJC events. B: multiple large EJC responses to a single stimulation are observed only in the dfmr1 null under these conditions. The bottom inset trace shows multiple separate EJC response to a single stimulation at a higher resolution. Scale bar: 10 nA, 500 ms; inset: 10 nA, 25 ms.
DISCUSSION
Drosophila is an excellent, simplified genetic system for comprehensively testing interactions between mGluR signaling and FMRP function in the nervous system. There is a single Drosophila homolog of the three member mammalian FMR gene family (dFMRP) (Wan et al. 2000; Zhang et al. 2001) and a single Drosophila homolog of the eight member mammalian mGluR family (dmGluRA) (Bogdanik et al. 2004; Parmentier et al. 1996). Antagonists of different mammalian mGluR classes have been shown to rescue several dfmr1 null phenotypes (McBride et al. 2005; Pan et al. 2008), suggesting that dmGluRA signaling does indeed have a mechanistic connection with dFMRP function. More importantly, the double mutant combination of the two Drosophila null alleles provides an excellent opportunity to test genetic relationships between all FMR family function and all mGluR signaling, particularly in the regulation of synapse development, function, and plasticity. Indeed, we have shown using double mutants that dFMRP and dmGluRA interact in the regulation of ionotropic glutamate receptor trafficking at the NMJ synapse (Pan and Broadie 2007), as well as in the modulation of movement behavior and the control of NMJ gross architecture and synaptic ultrastructure (Pan et al. 2008).
The goal of this study was to examine the key question of the role of dFMRP in synaptic transmission properties and to determine whether a genetic block in dmGluRA signaling would modulate functional defects caused by loss of dFMRP. All work was done in low external Ca2+ concentrations, as required to permit amplitude facilitation driven by high-frequency stimuli (Zucker and Regehr 2002). Our previous research has shown that dmGluRA is not required to maintain basal neurotransmission, but is critical for the regulation of activity-dependent synaptic plasticity processes, particularly in establishing the threshold for LTF and limiting the expression of PTP (Bogdanik et al. 2004). Null dmGluRA phenotypes resemble the consequences of applying K+ channel blockers to wildtype synapses, as well as mutants that either increase Na+ currents (pumilio) or decrease K+ currents (hyperkinetic, frequenin). Interestingly, the pumilio gene encodes an RNA-binding translational suppressor, like dFMRP, whose activity down-regulates the paralytic RNA encoding voltage-gated Na+ channels (Mee et al. 2004). The hyperkinetic gene encodes a K+ channel β subunit, and frequenin regulates the function of K+ channels (Poulain et al. 1994; Stern and Ganetzky 1989). Thus loss of dmGluRA generates synaptic phenotypes caused by increased neuronal membrane excitability. These comparisons suggest that the dmGluRA receptor monitors glutamate release, particularly during periods of high activity, to feedback and down-regulate the membrane excitability controlling Ca2+ influx and glutamate release, thus dictating neurotransmission strength. There are clear indications that dmGluRA interacts with dFMRP in synapse regulation (Pan and Broadie 2007; Pan et al. 2008), but dFMRP has not previously been shown to control synaptic excitability. This study aimed at discovering whether or not dFMRP and dmGluRA interact in the regulation of activity-dependent synaptic modulation, particularly through mechanisms of altered synaptic excitability.
We find no striking difference in basal transmission properties at low external Ca2+ concentrations in either dfmr1 or dmGluRA single mutants or the dfmr1;dmGluRA double mutant. There is a clear tendency of increased basal transmission strength, particularly in dfmr1, as well as shifts in the power relationship of Ca2+-dependent synaptic vesicle release. Together these changes explain the elevated dfmr1 synaptic strength and more rapid presynaptic vesicle cycle at higher Ca2+ concentrations (Gatto and Broadie 2008; Zhang et al. 2001). Nevertheless, under the low [Ca2+] conditions used here, we can infer that altered neurotransmission in response to HFS must be caused by activity-dependent changes in transmission probability in these genotypes. During short HFS trains, the single null mutants behave similar to control, but there is strongly elevated STF in the dfmr1;dmGluRA double null mutant. Similarly, our previous work on synaptic structure also has shown that some phenotypes interact in a synergistic fashion (Pan et al. 2008). The STF defect shows a clear interaction between dmGluRA-dependent glutamatergic signaling and the requirement for dFMRP function at the synapse, although it does not necessarily demonstrate that the two proteins work in the same pathway(s) in the manifestation of short-term changes in synapse function. It is possible that mutation of the two genes concurrently shows an overlapping function that is not evident otherwise.
The interaction between dmGluRA and dFMRP in long-term, activity-dependent changes in neurotransmission strength is more extensive and informative. During prolonged HFS, dmGluRA null mutants display a profound increase in augmentation, suggesting loss of a glutamate negative feedback loop to reign in glutamate release. Interestingly, removal of dFMRP in the dfmr1;dmGluRA double mutant modifies this defect during the early stages of HFS, extending the time it takes to elevate response amplitudes to the fully augmented level. However, as HFS continues, the full augmentation defect is expressed in the dfmr1;dmGluRA double mutant, indicating that other, dFMRP-independent pathways play a large role in the dmGluRA augmentation phenotype. Following prolonged HFS, wildtype synapses exhibit low-level, persistent PTP (Bogdanik et al. 2004; Zhong and Wu 1991), but dmGluRA mutants show grossly elevated PTP (>5-fold normalized amplitude). Importantly, removal of dFMRP in dfmr1;dmGluRA double mutants produces nearly complete loss of this potentiation defect. This restoration toward wildtype seems to indicate converging pathways for dFMRP and dmGluRA in the regulation of activity-dependent synaptic potentiation. The third plasticity defect apparent in dmGluRA nulls is the premature, sudden step-wise appearance of LTF. The dfmr1;dmGluRA animals retain this lowered LTF threshold phenotype, but aspects of the defect are reduced by co-removal of dFMRP. First, the time of LTF onset is longer in double mutants compared with dmGluRA (11.8 vs. 9.7 s). Second, the dmGluRA null always shows an abrupt, single step increase in response amplitude between two consecutive stimulations in the HFS train, whereas dfmr1;dmGluRA characteristically oscillates between augmented and LTF amplitudes multiple times before finally succumbing to LTF. These data suggest that removal of dFMRP alleviates the consequences of lost mGluR signaling, albeit insufficiently to block presentation of enhanced transmission phenotypes.
Taken together, the above results show clearly that removal of dFMRP can modulate defects caused by loss of dmGluRA. Removal of dFMRP by itself fails to present any defects in assayed forms of activity-dependent plasticity. However, we did identify two new phenotypes in the dfmr1 null synapse. First, during prolonged HFS, dfmr1 mutants fail to maintain consistent transmission amplitudes, but rather manifest striking and characteristic cycling of amplitudes between a low and high transmission state. This cycling presents with sudden, drastic changes in amplitude size in bursts of quite regular periodicity. To our knowledge, this is a novel phenotype without clear comparisons in the literature (Martinez et al. 2007). Second, dfmr1 mutants display multiple EJC events in response to single nerve stimuli during and after HFS. Similar hyperactivity is characteristic of Shaker K+ channel mutants (Tanouye and Ferrus 1985; Tanouye et al. 1981), and double mutation combinations with ether-a-go-go, acting synergistically to increase membrane excitability (Ganetzky and Wu 1983, 1985). Interestingly, synaptic hyperexcitability in Shaker, and also bang-senseless mutants, is rescued with loss of no action potential (nap), to reduce Na+ channels (Ganetzky and Wu 1982, 1983). The similar phenotypes of these mutants compared with dfmr1 suggests dFMRP regulates synaptic excitability, perhaps via regulating membrane excitability, providing a clear mechanistic relationship with mGluR-mediated negative feedback control (Bogdanik et al. 2004).
Consistent with this feedback loop, co-removal of mGluR signaling appreciably diminishes these dfmr1 defects. The dfmr1;dmGluRA double null still displays the EJC response amplitude cycling, and requires a similar duration of HFS prior to the onset of cycling. However, the cycling defect is present in far fewer dfmr1;dmGluRA animals compared with the dfmr1 single mutant, and the limited cycling manifest in the double mutants has a slower cycling period, showing partial alleviation of the phenotype in the double mutant. The threshold for manifestation of this intriguing synaptic modulation is clearly lowered by removal of dFMRP but raised again by co-removal of mGluR signaling, albeit not to wildtype levels. In dfmr1 mutants, multiple separate and distinct EJCs occur in response to a single stimulus during HFS. This hyperexcitability defect is effectively lowered in dfmr1;dmGluRA double mutants. Indeed, the hyperactive response was recorded only in two isolated incidents in two separate dfmr1;dmGluRA animals. In dfmr1 mutants, the hyperexcitable responsiveness persists following the HFS train during basal stimulation, but post-HFS hyperexcitability was never observed in dfmr1;dmGluRA animals. Thus co-removal of dmGluRA does indeed diminish the consequences of loss of dFMRP, only partially in the case of the cyclic transmission defect, but quite strongly to block dfmr1 hyperexcitability. Together, these data support the conclusion of a partial co-dependency of dmGluRA receptor signaling on dFMRP regulative function, and vice versa in a feedback loop, to modulate synapse properties critical for the maintenance of transmission fidelity and activity-dependent plasticity.
Our laboratory has previously shown both rescue and synergistic interactions of dfmr1 and dmGluRA null mutations in a range of synaptic mechanisms (Pan and Broadie 2007; Pan et al. 2008). However, a major, persistent limitation has been the lack of any functional data on synaptic transmission, a primary focus of FXS dysfunction. This crucial question has similarly not as yet been addressed in the mouse fmr1 KO model, despite evidence of rescue in other fmr1 defects (Dolen et al. 2007). Here we show that activity-dependent synaptic plasticity defects in dmGluRA nulls, including elevated augmentation, potentiation, and premature LTF, are each reduced by the co-removal of dFMRP. Similarly, the synaptic defects in dfmr1 nulls, including transmission amplitude cycling during HFS and multiple EJCs in response to a single stimulus, are decreased by the co-removal of dmGluRA, and hence loss of all mGluR signaling at the synapse. The striking exception to this trend is STF, which is somehow enhanced in the dfmr1;dmGluRA double null compared with both single mutants. These interactions clearly support the conclusion of a relationship between dFMRP function and dmGluRA signaling, but argue against a simple direct signaling cascade. Rather, dFMRP function is likely controlled by several converging signaling pathways, of which dmGluRA-mediated glutamatergic synaptic signaling is only one.
GRANTS
S. Repicky was supported by a postdoctoral training grant for the Program in Developmental Biology at Vanderbilt University. This work was supported by National Institute of General Medical Sciences Grant GM-54544 to K. Broadie.
Acknowledgments
We thank Dr. Jeff Rohrbough for advice on TEVC electrophysiology techniques and L. Coffee, C. Gatto, A. Long, J. Rohrbough, and C. Tessier for critical feedback on this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Bear et al. 2004.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci 27: 370–377, 2004. [DOI] [PubMed] [Google Scholar]
- Bogdanik et al. 2004.Bogdanik L, Mohrmann R, Ramaekers A, Bockaert J, Grau Y, Broadie K, Parmentier ML. The drosophila metabotropic glutamate receptor DmGluRA regulates activity-dependent synaptic facilitation and fine synaptic morphology. J Neurosci 24: 9105–9116, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang et al. 2005.Chuang SC, Zhao W, Bauchwitz R, Yang Q, Bianchi R, Wong RK. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci 25: 8048–8055, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockendorff et al. 2002.Dockendorff TC, Su HS, McBride SM, Yang Z, Choi CH, Siwicki KK, Sehgal A, Jongens TA. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron 34: 973–984, 2002. [DOI] [PubMed] [Google Scholar]
- Dolen et al. 2007.Dolen G, Osterweil E, Rao BSS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron 56: 955–962, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky and Wu 1982.Ganetzky B, Wu CF. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics 100: 597–614, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky and Wu 1983.Ganetzky B, Wu CF. Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenetics 1: 17–28, 1983. [DOI] [PubMed] [Google Scholar]
- Ganetzky and Wu 1985.Ganetzky B, Wu CF. Genes and membrane excitability in Drosophila. Trends Neurosci 8: 322–326, 1985. [Google Scholar]
- Ganetzky and Wu 1986.Ganetzky B, Wu CF. Neurogenetics of membrane excitability in Drosophila. Annu Rev Genet 20: 13–44, 1986. [DOI] [PubMed] [Google Scholar]
- Garber et al. 2006.Garber K, Smith KT, Reines D, Warren ST. Transcription, translation and fragile X syndrome. Curr Opin Genet Dev 16: 270–275, 2006. [DOI] [PubMed] [Google Scholar]
- Gatto and Broadie 2008.Gatto CL, Broadie K. Temporal requirements of the fragile X mental retardation protein in the regulation of synaptic structure. Development 135: 2637–2648, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou et al. 2006.Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron 51: 441–454, 2006. [DOI] [PubMed] [Google Scholar]
- Howlett et al. 2002.Howlett E, Lin C, Lavery W, Stern M. A PI3K-mediated negative feedback regulates Drosophila motor neuron excitability. PLoS Genet Epub Nov 28, 2008. [DOI] [PMC free article] [PubMed]
- Huber et al. 2002.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA 99: 7746–7750, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber et al. 2000.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288: 1254–1257, 2000. [DOI] [PubMed] [Google Scholar]
- Jan and Jan 1978.Jan YN, Jan LY. Genetic dissection of short-term and long-term facilitation at the drosophila neuromuscular junction. Proc Natl Acad Sci USA 75: 515–519, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koekkoek et al. 2005.Koekkoek SKE, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, Willemsen R, Ikeda T, Kakizawa S, Onodera K, Nelson DL, Mientjes E, Joosten M, DeSchutter E, Oostra BA, Ito M, DeZeeuw CI. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in fragile X syndrome. Neuron 47: 339–352, 2005. [DOI] [PubMed] [Google Scholar]
- Koukoui and Chaudhuri 2007.Koukoui SD, Chaudhuri A. Neuroanatomical, molecular genetic, and behavioral correlates of fragile X syndrome. Brain Res Rev 53: 27–38, 2007. [DOI] [PubMed] [Google Scholar]
- Long et al. 2008.Long AA, Kim E, Leung HT, Woodruff E III, An L, Doerge RW, Pak WL, Broadie K. Presynaptic calcium channel localization and calcium-dependent synaptic vesicle exocytosis regulated by Fuseless protein. J Neurosci 28: 3668–3682, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez et al. 2007.Martinez VG, Javadi CS, Ngo E, Ngo L, Lagow RD, Zhang B. Age-related changes in climbing behavior and neural circuit physiology in Drosophila. Dev Neurobiol 67: 778–791, 2007. [DOI] [PubMed] [Google Scholar]
- McBride et al. 2005.McBride SM, Choi CH, Wang Y, Liobelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, McDonald TV, Jongens TA. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a drosophila model of fragile X syndrome. Neuron 45: 753–764, 2005. [DOI] [PubMed] [Google Scholar]
- Mee et al. 2004.Mee CJ, Pym EC, Moffat KG, Baines RA. Regulation of neuronal excitability through pumilio-dependent control of a sodium channel gene. J Neurosci 24: 8695–8703, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva and Huber 2006.Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol 95: 3291–3295, 2006. [DOI] [PubMed] [Google Scholar]
- Pan and Broadie 2007.Pan L, Broadie K. Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A convergently regulate the synaptic ratio of ionotropic glutamate receptor subclasses. J Neurosci 27: 12378–12389, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan et al. 2008.Pan L, Woodruff E III, Liang P, Broadie K. Mechanistic relationships between Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A signaling. Mol Cell Neurosci 37: 747–760, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan et al. 2004.Pan L, Zhang YQ, Woodruff E, Broadic K. The Drosophila fragile X gene negatively regulates neuronal elaboration and synaptic differentiation. Curr Biol 14: 1863–1870, 2004. [DOI] [PubMed] [Google Scholar]
- Parmentier et al. 1996.Parmentier ML, Pin JP, Bockaert J, Grau Y. Cloning and functional expression of a Drosophila metabotropic glutamate receptor expressed in the embryonic CNS. J Neurosci 16: 6687–6694, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer and Huber 2006.Pfeiffer BE, Huber KM. Current advances in local protein synthesis and synaptic plasticity. J Neurosci 26: 7147–7150, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain et al. 1994.Poulain C, Ferrus A, Mallart A. Modulation of type A K+ current in Drosophila larval muscle by internal Ca2+; effects of the overexpression of frequenin. Pfluegers 427: 71–79, 1994. [DOI] [PubMed] [Google Scholar]
- Rohrbough et al. 1999.Rohrbough J, Pinto S, Mihalek RM, Tully T, Broadie K. latheo, a Drosophila gene involved in learning, regulates functional synaptic plasticity. Neuron 23: 55–70, 1999. [DOI] [PubMed] [Google Scholar]
- Stern and Ganetzky 1989.Stern M, Ganetzky B. Altered synaptic transmission in Drosophila hyperkinetic mutants. J Neurogenet 5: 215–228, 1989. [DOI] [PubMed] [Google Scholar]
- Stern et al. 1990.Stern M, Kreber R, Ganetzky B. Dosage effects of a Drosophila sodium channel gene on behavior and axonal excitability. Genetics 124: 133–143, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanouye and Ferrus 1985.Tanouye MA, Ferrus A. Action potentials in normal and Shaker mutant Drosophila. J Neurogenet 2: 253–271, 1985. [DOI] [PubMed] [Google Scholar]
- Tanouye and Ferrus 1981.Tanouye MA, Ferrus A, Fujita SC. Abnormal action potentials associated with the Shaker complex locus of Drosophila. Proc Natl Acad Sci USA 78: 6548–6552, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier and Broadie 2008.Tessier CR, Broadie K. Drosophila fragile X mental retardation protein developmentally regulates activity-dependent axon pruning. Development 135: 1547–1557, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta et al. 2004.Trotta N, Rodesch CK, Fergestad T, Broadie K. Cellular bases of activity-dependent paralysis in Drosophila stress-sensitive mutants. J Neurobiol 60: 328–347, 2004. [DOI] [PubMed] [Google Scholar]
- Visootsak et al. 2005.Visootsak J, Warren ST, Anido A, Graham JM Jr. Fragile X syndrome: an update and review for the primary pediatrician. Clin Pediatr 44: 371–381, 2005. [DOI] [PubMed] [Google Scholar]
- Wan et al. 2000.Wan L, Dockendorff TC, Jongens TA, Dreyfuss G. Characterization of dfmr1, a drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol 20: 8536–8547, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson and Cox 2007.Wilson BM, Cox CL. Absence of metabotropic glutamate receptor-mediated plasticity in the neocortex of fragile X mice. Proc Natl Acad Sci USA 104: 2454–2459, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. 2001.Zhang YQ, Bailey AM, Matthies H, Renden R, Smith M, Speese S, Rubin G, Broadie K. Drosophila fragile X-related gene regulated MAP1B homolog Futsch to control synaptic structure and function. Cell 107: 591–603, 2001. [DOI] [PubMed] [Google Scholar]
- Zhong and Wu 1991.Zhong Y, Wu CF. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science 251: 198–201, 1991. [DOI] [PubMed] [Google Scholar]
- Zucker and Regehr 2002.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol 64: 355–405, 2002. [DOI] [PubMed] [Google Scholar]