Abstract
The pulvinar region of the thalamus has repeatedly been linked with the control of attention. However, the functions of the pulvinar remain poorly characterized, both in human and in nonhuman primates. In a functional MRI study, we examined the relative contributions to activity in the human posterior pulvinar made by visual drive (the presence of an unattended visual stimulus) and attention (covert spatial attention to the stimulus). In an event-related design, large optic flow stimuli were presented to the left and/or right of a central fixation point. When unattended, the stimuli robustly activated two regions of the pulvinar, one medial and one dorsal with respect to the lateral geniculate. The activity in both regions shows a strong contralateral bias, suggesting retinotopic organization. Primate physiology suggests that the two regions could be two portions of the same double map of the visual field. In our paradigm, attending to the stimulus enhanced the response by about 20%. Thus attention is not necessary to activate the human pulvinar and the degree of attentional enhancement matches, but does not exceed, that seen in the cortical regions with which the posterior pulvinar connects.
INTRODUCTION
The thalamic pulvinar nuclei have widespread connections with the cerebral cortex. The posterior portion of the pulvinar connects with the occipital cortex and posterior parietal cortex (PPC) and it has been associated with vision and visual attention. In macaques, visually responsive neurons are common (Bender 1981, 1982; Benevento and Miller 1981; Petersen et al. 1985; Robinson et al. 1991), even in anesthetized preparations (e.g., Bender 1981, 1982). However, the literature on the function of the macaque pulvinar has consistently emphasized a key role in attention (for reviews, see Grieve et al. 2000; Robinson and Petersen 1992; Shipp 2004). For example, Petersen et al. (1987) showed that the pulvinar response to a flash is about 40% greater if the flash is a target for a saccade than if it is not and suggested that the pulvinar may be involved in switching attention. Similarly, human functional magnetic resonance imaging (fMRI) work has suggested that the human pulvinar is involved in task switching in the visual domain (Yantis et al. 2002).
A similar emphasis on attention is seen in the limited literature that exists on the human pulvinar. In an fMRI experiment, Kastner et al. (2004) reported an absence of pulvinar responses during passive viewing of flickering checkerboards, but found activity in a dorsal portion of the posterior pulvinar when participants were required to attend to the stimuli. In a positron emission tomography study, Villeneuve et al. (2005) also used attention to the stimulus to demonstrate visual responses in the pulvinar, although there was no comparison between attended and unattended stimuli.
In a previous fMRI study (Cotton and Smith 2007), we identified a region of the inferior (ventral) pulvinar that shows a retinotopic organization, at least to the extent that there is a strong bias in favor of contralateral stimuli. This region is some 8 mm more ventral than the dorsal region identified in the study of Kastner et al. (2004). Based on knowledge of anatomical connections in primate pulvinar, it is probable that the inferior area connects most strongly with occipital visual areas, whereas the dorsal region may connect more strongly with the PPC (Shipp 2003). If so, this might lead to expectations of stronger attention effects in the dorsal than in the ventral region, since the PPC is widely associated with attentional control. In a series of experiments, we showed that inferior pulvinar responses to a prominent visual motion stimulus were present both with and without attention to the stimulus. However, the responses were strongest when the stimulus was attended, suggesting that both passive visual drive and attention contribute to inferior pulvinar activity. Activity in the dorsal pulvinar was generally absent.
In the present study, we explicitly compare the contributions of visual drive and spatial attention, to test the hypothesis that the pulvinar has a special role in attention, and we do so in both dorsal and ventral portions of the visual pulvinar. In experiment 1, to obtain generalizable results, we present data from a group of 16 subjects. In experiment 2, to avoid confusion between the LGN and pulvinar. we repeat the experiment on two individuals in whom the two areas have been distinguished anatomically.
METHODS
Experiment 1
PARTICIPANTS.
Sixteen healthy female student volunteers (ages 18–22 yr) took part. All had normal or corrected-to-normal vision. They were screened according to standard procedures and informed consent was obtained. They were paid for their time. The project was approved by the relevant local ethics committee.
DATA ACQUISITION.
MRI images were obtained with a 3-Tesla Siemens Magnetom Trio scanner and a standard Siemens eight-channel array head coil. Anatomical (T1-weighted) images of the whole brain were obtained at the start of each scanning session (magnetization-prepared rapid gradient echo [MP-RAGE] sequence: 160 axial slices, in-plane resolution 256 × 256, 1-mm isotropic voxels, repetition time [TR] = 1,830 ms, echo time [TE] = 4.43 ms, flip angle = 11°, bandwidth = 130 Hz/pixel). This was followed by six functional scan runs. The functional data were acquired with a gradient echo, echoplanar sequence (TR = 1,500 ms, 17 contiguous axial slices centered on the thalamus, interleaved acquisition order, 3-mm isotropic voxels, field of view [FOV] = 192 × 192 mm, flip angle = 75°, TE = 36 ms, bandwidth = 1,202 Hz/pixel). Each consisted of 190 acquisition volumes and lasted 4 min 45 s. An event-related fMRI design with a variable interstimulus interval was used.
VISUAL STIMULATION AND TASKS.
Computer-generated visual stimuli were presented via a liquid crystal display projector on a screen at the end of the scanner bore and were viewed via a mirror mounted on the head coil. A central fixation point was present continuously. Targets were presented for 3 s and were centered at an eccentricity of 9° to either the right or the left of fixation. Each consisted of a large, circular patch (12° diameter) of 150 moving white dots on a dark background. Each dot subtended 0.5° and moved at a speed of 12°/s. Local motion directions were controlled, to create a global pattern of optic flow. The stimulus was based on that of Morrone et al. (2000), in which optic flow varies over time, smoothly changing between expansion/contraction and rotation, via intermediate spiral motions. This stimulus was chosen because it was effective in eliciting pulvinar responses in our previous study (Cotton and Smith 2007). The strength of the motion percept was manipulated by assigning random directions to a proportion of the dots (noise dots). During the first 1,500 ms of each trial, the signal strength was 60%. Midway through each presentation (i.e., after 1,500 ms), the proportion of noise dots abruptly increased (typically to 75%) or decreased (typically to 45%), causing the perception of global motion to either strengthen or weaken.
Each 3-s presentation formed one trial of the event-related design. Between trials, the screen was blank apart from the fixation spot. The intertrial interval (ITI) was varied according to a Poisson distribution (Hagberg et al. 2001) with an average ITI of 6 s. There were 32 trials in each scan run (192 trials total per participant across the 6 scan runs). There were four trial types, each presented eight times in each scan run (48 times total per participant). The order of trials was determined such that each trial type was preceded equally often by each of the four trial types, including itself.
On each trial, participants performed one of three tasks, as directed by a cue at fixation. At 1 s before the onset of each trial, a small arrow appeared pointing left, right, or up from the fixation point. Left and right indicated that the participant should maintain central fixation, attend to the random-dot pattern to the left or right of fixation, respectively, and report (via button presses) the direction of the change in global motion strength of the pattern. Increases and decreases of motion strength were equally probable and the direction of change was selected pseudorandomly on each trial. An arrow pointing up indicated that the participant should ignore the motion stimuli and report the direction of a small change in brightness of the fixation spot that occurred midway through the trial. Thus in these trials the motion stimuli were not attended.
In two of the trial types, two motion stimuli were present, one to each side of fixation. The participant was cued to attend and judge the stimulus on the left (condition A) or right (condition B) of fixation. Both stimuli underwent a change in motion strength and the direction of change was determined independently for each stimulus. Thus attending to the wrong side would result in chance performance. The luminance change was present in the fixation spot even when motion was attended. In the other two trial types, only one motion stimulus was present, either to the left (condition C) or to the right (condition D) of fixation. In these trials, the cue always indicated that the participant should attend centrally and judge the brightness change at fixation. The motion stimulus underwent a change in motion strength even though it was unattended. The four trial types are summarized in Table 1.
TABLE 1.
Summary of the four stimulus conditions and associated tasks
| Condition | Motion Stimuli | Task |
|---|---|---|
| A | Left and right | Motion (left) |
| B | Left and right | Motion (right) |
| C | Left only | Brightness (central) |
| D | Right only | Brightness (central) |
These four trial types were designed to allow various different statistical contrasts to be performed between pairs of trial types, for different purposes (see results). Conditions A and B allow effects of spatial attention to be examined, whereas conditions C and D allow responses to unattended stimuli to be documented.
Prior to the experiment, each participant viewed a practice sequence outside the scanner. The responses were used to adjust the magnitude of the change in motion strength and the change in fixation-spot brightness, such that close to 75% of responses were correct. Participants also had a short trial run in the scanner.
DATA ANALYSIS.
The data were analyzed using BrainVoyager QX (version 1.4; Brain Innovation, Maastricht, The Netherlands). The first four volumes of every functional run were discarded. Functional data were corrected for head motion and filtered with a temporal high-pass filter of 0.014 Hz. The data were spatially normalized across participants by transforming each data set into a standard (Talairach) space.
The data were analyzed both individually and as a group. Each event type was modeled separately, by convolving the event timings with a canonical hemodynamic impulse response function formed from two gamma functions. As well as the four models so created, the analysis included six nuisance regressors derived from the head-motion data. Correction for serial autocorrelations was made using the AR(1) method. To minimize blurring of activity between the pulvinar and the nearby lateral geniculate nucleus (LGN), no spatial smoothing was applied. For each individual participant, various statistical contrasts among the four trial types were performed. In addition, a second-level, random-effects group analysis was conducted on the data from all 16 participants and the same contrasts were examined. Based on the resulting activation maps, regions of interest (ROIs) were defined in Talairach space for the LGN and two regions of the pulvinar (see results). Mean effect sizes for these ROIs (in terms of percentage signal change) were then calculated from the beta values obtained in the first-level analysis.
Experiment 2: individual analysis with anatomical localization
A concern with the group analysis of experiment 1 is that the inferior pulvinar is adjacent to the LGN and so individual difference in the location of the LGN, errors in normalization, or errors in coregistration could all lead to blurring of the LGN response in the nearby pulvinar.
We therefore repeated our experiment in two individuals in whom the LGN had been identified anatomically, using smaller (2-mm isotropic) functional voxels to reduce integration of different responses within voxels and paying close attention to the accuracy of coregistration between the functional data and the anatomical scan.
PARTICIPANTS.
Two participants (female, mean age 28 yr) took part. Neither had taken part in experiment 1. Both were experienced MRI participants. One (CM) is one of the authors.
DATA ACQUISITION.
The LGN was identified by means of proton density scans (Devlin et al. 2007; Fujita et al. 2001). With appropriately chosen scan parameters, the LGN appears as lighter than the surrounding tissue and, in some cases, its characteristic shape can be seen. Each participant underwent a proton density scan in a separate session, prior to the functional scan. The scan parameters were based on those of Devlin et al. (2007). A long repetition time (TR) of 6 s was combined with a short echo time (TE) of 9 ms. In all, 48 coronal slices (2-mm slice thickness, 1-mm in-plane resolution) including the entire thalamus were acquired. The scan was repeated three times and the results averaged, to improve the signal-to-noise ratio. The LGN was then located by visual inspection of the results.
Functional data were acquired over two 1-h sessions on different days. Anatomical (T1-weighted) images of the whole brain were obtained at the start of each session (MP-RAGE, as in experiment 1). This was followed by nine functional scan runs. The functional data were acquired with a gradient echo, echoplanar sequence (TR = 2,000 ms, 22 contiguous axial slices centered on the thalamus, interleaved acquisition order, 2-mm isotropic voxels, FOV = 128 × 128 mm, oversampling to prevent phase wrap, flip angle = 80°, TE = 40 ms). Each scan consisted of 144 acquisition volumes and lasted 4 min 48 s. Eighteen such runs were conducted over the two sessions, giving 576 trials in total (144 per condition). This was sufficient to obtain reliable results in a single participant, despite the use of small voxels. To aid accurate coregistration, an echo planar image (EPI) covering the whole brain was also obtained in each session.
VISUAL STIMULATION AND TASKS.
The stimuli, conditions, and tasks were the same as those in experiment 1.
DATA ANALYSIS.
The functional data were modeled in a general linear model (GLM) in a way similar to that in experiment 1, in a separate single-subject analysis for each participant. Coregistration between the functional and anatomical data was performed via an intermediate stage using a whole-brain EPI volume. The registration was then visually inspected and adjusted manually as required, to achieve perfect registration in the thalamus. For each condition, quantitative estimates were obtained for three ROIs, as in experiment 1. The location of the LGN ROI was based on the proton density scan. The LGN was identified by visual inspection in each hemisphere and the corresponding location was found in the three-dimensional anatomical scan used for coregistration. Thus the LGN ROI was determined with certainty in each case. The two pulvinar ROIs were specified in a way similar to that in experiment 1, but with care to exclude the LGN by a safe margin. Locations of the centers of the various ROIs are shown in Table 2.
TABLE 2.
Talairach coordinates (xyz) for regions of interest (ROIs) in experiment 2
| ROI | CM Left | CM Right | AS Left | AS Right |
|---|---|---|---|---|
| LGN | −22−26−2 | 23−270 | −21−24−1 | 19−252 |
| Inferior pulvinar | −13−322 | 15−284 | −14−262 | 12−255 |
| Dorsal pulvinar | −21−279 | 24−2610 | −24−288 | 21−279 |
RESULTS
Experiment 1
BEHAVIORAL DATA.
The mean percentage correct response rate, averaged across the 16 participants, was 75.4%, in line with the desired level. This indicates that task difficulty was set appropriately. The SD of the participant means was 17.4%.
IMAGING DATA.
We first report statistical results (activation maps) based on independent analysis of voxels and then quantitative data based on ROIs.
EFFECTS OF PASSIVE VISUAL DRIVE.
The presence of contralaterally organized visual activity arising from an unattended visual stimulus was assessed by contrasting conditions C and D. In both these conditions, attention was diverted from the stimulus by a demanding task at fixation, so observed activity can be assumed to relate to passive visual drive. Contrasting right and left stimuli will isolate activity that is specific to the contralateral hemifield and will eliminate any activity that is not spatially specific.
Figure 1 shows activity related to an unattended, contralateral motion stimulus in the right (orange/yellow) and left (blue/green) thalamus. Figure 1, A and B shows the results from the random-effects group analysis as color overlays on coronal and axial slices through the LGN and pulvinar. The anatomical template used for these two panels is that obtained by spatially normalizing the 16 brains and then averaging them and it can be seen that the similarity of the transformed brains (indexed by degree of blur) is good in the vicinity of the thalamus. Clear and strong contralateral activity is apparent in the posterior/lateral thalamus, bilaterally. The activity peaks at [−22, −25, 3] and [22, −27, 1] for left and right thalamus, respectively (all coordinates are in Talairach space). Both the anatomical location assessed visually and the coordinates are consistent with a location in the LGN. Strong, contralateral LGN activity is of course expected. In the right thalamus, there are strong signs that the activity extends medially into the portion of the inferior pulvinar identified as visually responsive and contralaterally organized in our previous work (Cotton and Smith 2007). In the left thalamus, only the LGN is significantly active in this analysis. Note that we use the term “inferior pulvinar” simply to mean the inferior part of the pulvinar, not to mean the anatomical structure sometimes called inferior pulvinar (or PI).
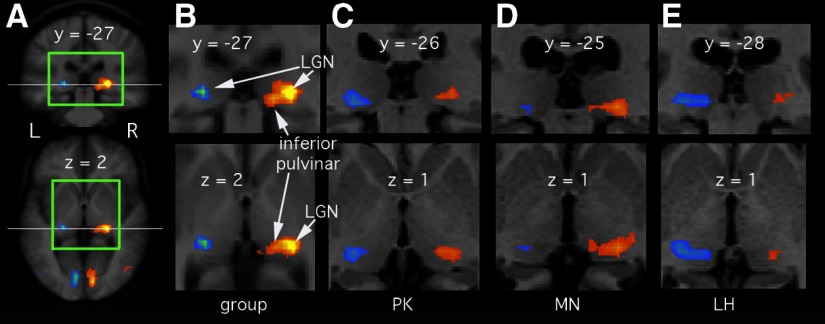
FIG. 1.
Responses in the lateral geniculate nucleus (LGN) and pulvinar to viewing an unattended contralateral visual stimulus. Brain slices are in neurological convention (left on the left). All slice locations identified are in Talairach coordinates. A: results of a random-effects group analysis (n = 16). A coronal (top) and an axial (bottom) slice through the thalamus are shown. These are taken from the brain volume created by spatially normalizing the 16 brains and then averaging them, thus showing the level of spatial resolution in the group analysis. The horizontal white line in each slice indicates the plane of the other slice. The colored overlay shows differential activity for the contrast C > D (referring to the conditions in the text). Activity arising from the left visual hemifield is shown in orange/yellow and that from the right hemifield in blue/green. Activation is thresholded at P < 0.01, corrected (false discovery rate [FDR]). B: the same group results taken from the region bounded by green in A, with the locations of the LGN and inferior pulvinar indicated. C–E: indicative results from 3 individual analyses in the same format but thresholded at P < 0.001, uncorrected. These brain slices are from the scan of each individual's own brain, after spatial normalization, not the template used for A and B.
Inspection of the individual participants' results revealed that LGN activity was detectable in many individual cases (23 of 32 hemispheres). In addition, in some cases (9 of 32) activity could also be seen more medially, in the inferior pulvinar. Three such cases are illustrated in Fig. 1, C–E. Participant PK shows this, although only modestly, in both hemispheres. MN shows it clearly on the right and LH shows it clearly on the left. The fact that inferior pulvinar is active in individual participants helps to discount the possibility that pulvinar activity in the group analysis is not real but reflects variability in the location of the LGN. The fact that the left pulvinar is sometimes active (5 of 32) suggests that the left–right asymmetry in the group analysis (Fig. 1B) is not real but reflects sensitivity limitations.
EFFECTS OF ATTENDING TO A CONTRALATERAL STIMULUS.
The effect of attention to a visual stimulus was assessed in two ways. First, attending to a stimulus on the left (or right) was contrasted with attending to the central fixation spot with an unattended stimulus present on the left (or right). For the right thalamus, condition A was contrasted with condition C; for the left thalamus, B was contrasted with D. This approach revealed clear effects of attention bilaterally in the group analysis, shown in Fig. 2 A. Attention-related activity was centered on the LGN, confirming previous findings of attentional modulation in the human LGN (O'Connor et al. 2002). From there, it appears to extend both medially and dorsally into the pulvinar, particularly on the left, although it is difficult to be sure that this is not just blurring of activity in the LGN. (Note that because of the contrasts used, only the side marked with crosshairs in each image reflects attention effects alone.) In the left thalamus, a second active region can be seen (top left) at a much more dorsal location [−18 −27, 11]. This appears discontinuous with the activity in the LGN and inferior pulvinar in this analysis, but not in other analyses (e.g., the right thalamus in the same panel).
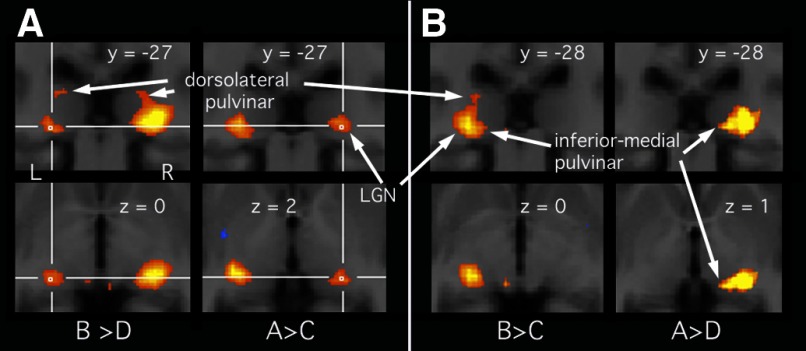
FIG. 2.
A: attentional modulation of the response to a contralateral stimulus, from the group analysis (thresholded at P < 0.01, FDR). As in Fig. 1, the top row shows coronal slices and the bottom row shows axial slices, confined to the region bounded in green in Fig. 1A. Attention-related responses come from different statistical contrasts for the 2 hemispheres and are indicated with crosshairs (the larger response on the other side of the brain in each case reflects visual drive). The contrast between conditions used to produce the images is indicated below each coronal/axial image pair. B: responses to an attended contralateral stimulus, from the group analysis (thresholded at P < 0.01, FDR). Again, different contrasts, indicated below each image pair, were used for isolating activity in the left and right thalamus (left and right image pair, respectively).
Inspection of the results for individual subjects also showed that the LGN is differentially active in a few cases, although in many cases it was not. There was no individual with unambiguous (i.e., clearly not arising in LGN) attentional modulation in the pulvinar (but see the following text).
In a second approach to examining effects of attention, conditions A and B were contrasted. Both conditions had a stimulus on each side and the only difference between them was which stimulus was attended. The group analysis showed no significant differential activity, either in the LGN or in any part of the pulvinar. This was also reflected in most of the individual analyses.
Thus the two statistical contrasts in the voxelwise group analysis that lend themselves to assessing effects of attention provide only weak evidence for such an effect in the pulvinar in one case and none in the other case.
RESPONSES TO AN ATTENDED CONTRALATERAL VISUAL STIMULUS.
The above-cited analyses show strong evidence for pulvinar activity during passive viewing of a motion stimulus, as shown previously, and weaker evidence for effects of attention. If both factors are influential, the largest differential activations should be obtained when the response to an attended stimulus is compared with no stimulus. To test this, we contrasted condition A with condition D for the right thalamus and B with C for the left. The group results, shown in Fig. 2B, show the expected strong LGN activity. This extends into both the inferior/medial portion of the pulvinar (most clearly in the right thalamus) and also the dorsolateral portion (most clearly in the left thalamus).
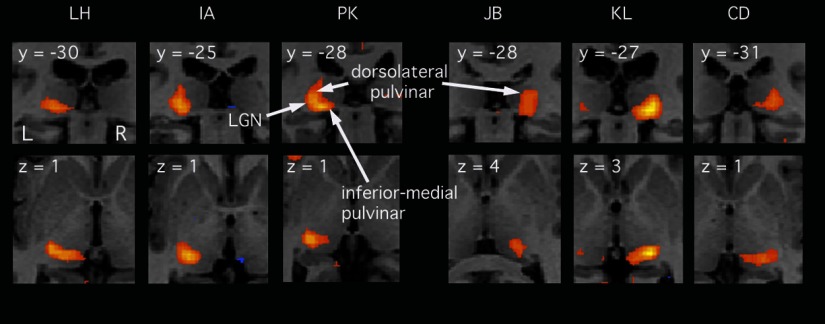
As before, it is necessary to distinguish pulvinar activity from LGN activity in individual brains because of the spatial blurring inherent in any group analysis. Based on the contrasts used for Fig. 2B, pulvinar activity was evident in many individuals. Figure 3 illustrates results from six of them, in a format similar to that of previous figures. Three show results from the contrast used to show activity in the left thalamus and the other three show the right thalamus. All six show activity at the expected location of the LGN. Five (all except JB) show activity in the inferior pulvinar. Similarly five (all except LH) show activity in the dorsal pulvinar, typically extending almost to the top of the pulvinar, some 10 mm above the LGN. The characteristic and repeatable pattern of extension of activity from the LGN in two directions (dorsally and medially), particularly clear in PK (coronal slice, top row), rules out the explanation that activity is simply spreading in all directions from the LGN due to inherent blood oxygenation level–dependent (BOLD) spread.
FIG. 3.
Responses to an attended contralateral stimulus, for each of 6 individual participants (thresholded at P < 0.001, uncorrected). Each slice is from the relevant individual's anatomical scan, after spatial normalization. The top row shows coronal slices; the bottom row shows axial slices and each slice location is identified. The first 3 cases (left) show responses in the left thalamus, as revealed in a statistical contrast between conditions B and C (see text), and can be compared directly to the group data in the left column of Fig. 2B. The last 3 cases show responses in the right thalamus (condition A contrasted with condition D) and can be compared directly to the right column of Fig. 2B.
FEATURE-BASED ATTENTION.
All the comparisons described earlier relate to spatial attention. However, it is now well documented that attention may also be directed to a particular image attribute or “feature,” in this case motion, irrespective of the spatial location at which it occurs. Attention to motion anywhere in the visual field increases sensitivity to similar motion at all other locations, both in single neurons in the macaque middle temporal (MT) cortical area (Treue and Trujillo 1999) and in human MT measured with fMRI (Saenz et al. 2002). To test whether feature-based attention might occur in the pulvinar, we used the same contrasts used earlier for responses to an attended contralateral visual stimulus, but inspected the opposite hemisphere. For the left pulvinar, condition A was contrasted with condition D. For the right pulvinar, condition B was contrasted with condition C. In each case, this compares an unattended contralateral motion stimulus across two attention conditions: attention to ipsilateral motion and attention to a central luminance task. Feature-based attention is expected to lead to a greater response in the former case. No significant effects were found. This is illustrated by the lack of activity in Fig. 2B for the hemisphere/contrast combinations described.
In summary, an attended contralateral optic flow stimulus activates two distinct regions of the posterior pulvinar. One is the inferior-medial portion, identified in our previous work (Cotton and Smith 2007). The other is a dorsolateral portion, located about 6–10 mm above the LGN. Effects of attention are modest in both regions at the level of voxelwise statistical contrasts.
QUANTITATIVE MEASUREMENTS.
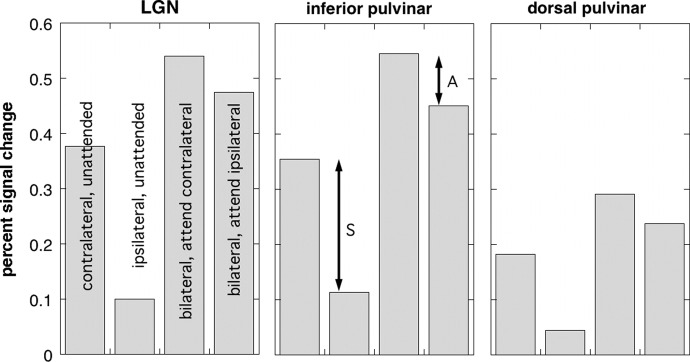
For each of the four conditions, mean response magnitudes were estimated for each of three ROIs in each hemisphere. These ROIs were defined on the template brain used for spatial normalization, based on anatomical criteria, the location of activity in the group analysis and (in the case of the LGN) previously published coordinates. The diameter of each ROI was 6 mm. The LGN ROI was centered at [±24 −22 0]. The inferior pulvinar ROI was centered more medially and posteriorly at [±17 −29 2] and the dorsal pulvinar ROI was located more dorsally and posteriorly at [±20 −27 8]. Within each ROI, mean activation was estimated as the beta value from the relevant regressor in the GLM analysis, converted to percentage signal change from baseline, and averaged across all functional voxels in the ROI and across the 16 participants. Finally, the response magnitudes were combined across hemispheres to give separate estimates of contralateral and ipsilateral visual drive and attention.
Figure 4 shows the results of this analysis. The left panel shows the LGN. The first two bars show the responses to contralateral and ipsilateral stimuli when attention is at fixation (derived from conditions C and D). As expected, there is a strong contralateral preference, giving rise to the significant voxelwise left versus right difference in the LGN seen in Fig. 1B. The difference between the two is highly significant (t = 10.1, df = 15, P < 0.0001). The other two bars show responses to bilateral stimulation (conditions A and B), with either the contralateral or ipsilateral stimulus attended. A smaller but again significant contralateral bias is evident, consistent with modulation by spatial attention (t = 4.1, df = 15, P < 0.001). The response to an unattended stimulus is greater when the participants attend to ipsilateral motion (fourth bar) then when they attend to luminance at fixation (first bar). The difference is statistically significant (t = 3.57, df = 15, P < 0.005). This might reflect feature-based attention. The presence of feature-based attention would help to explain the lack of significant difference at the single-voxel level between conditions A and B, which tests only for spatial attention and is compromised if a response decrement due to lack of spatial attention is offset by feature-based attentional enhancement. However, given that contralateral specificity is not perfect (response to unattended ipsilateral stimulus is nonzero), an alternative explanation is that the enhancement is unrelated to attention but simply reflects the presence of two motion stimuli rather than one motion stimulus. It is therefore unsafe to attribute the difference to feature-based attention, particularly since motion is not a stimulus attribute that is thought to be represented in the LGN.
FIG. 4.
Mean activations, expressed in terms of percentage signal change, for each of the 4 conditions in each of 3 regions of interest (ROIs). Responses from left and right thalamus are grouped in terms of contralateral and ipsilateral stimulus locations. Each bar represents the mean across 32 hemispheres in 16 participants: all cases were included, irrespective of whether statistically significant activity was present. The 4 conditions are identified in the LGN plot. The arrow marked “S” indicates the effect of the spatial location of the stimulus and that marked “A” indicates the differential effect of spatial attention.
The center panel shows the corresponding results for the inferior pulvinar ROI. They are similar in all respects to the LGN results. Thus a strong contralateral bias in terms of passive (unattended) visual drive is confirmed, shown as the arrow marked “S.” This is in line with our previous work. In addition, a modest effect of spatial attention is evident, shown by the arrow marked “A.” Both differences are significant (t = 7.9, df = 15, P < 0.0001 and t = 6.5, df = 15, P < 0.0001, respectively). Thus the data suggest that in the inferior pulvinar, as in the LGN, effects of spatial attention do occur but the presence/absence of a visual stimulus modulates activity much more strongly than whether attention is directed to the stimulus. A significant difference also exists between the first and fourth bars (t = 2.3, df = 15, P < 0.05). Again, this could be due to feature-based attention, but could simply reflect the presence of two motion stimuli. The former is a little more likely in the pulvinar than the LGN because motion is represented in the pulvinar, although such a conclusion would nonetheless be unsafe.
The right panel shows results for the superior pulvinar. Responses are smaller than those in the LGN and inferior pulvinar, but show a similar pattern across the conditions. The difference between contralateral and ipsilateral is again significant for both unilateral unattended stimuli (t = 10.3, df = 15, P < 0.0001) and for the effect of attention (t = 5.0, df = 15, P < 0.0001). The difference between the first and fourth bars does not reach significance. Thus we demonstrate the existence of a visually responsive region in the dorsal portion of the posterior pulvinar and we show that, like the LGN and inferior pulvinar, it is contralaterally organized. Again, effects of attention are more modest than effects of stimulus location. The relationship of this region to the dorsal pulvinar region identified by Kastner et al. (2004) will be considered in the discussion.
Experiment 2
The similarity of results between the LGN and the pulvinar in experiment 1 raises concerns that the spatial blurring that is inherent in a group analysis might have lead us to mistake LGN activity for pulvinar activity. We do not think that this is the case, for several reasons. First, the location of the LGN is fairly consistent across brains. Second, spatial normalization is anchored at a cental point, the anterior commissure, and so distortions in central regions such as the thalamus are expected to be much less than in the cortex. Third, pulvinar activity was evident in some of the individual brains, discounting an explanation in terms of blurring by spatial normalization. Fourth, the pulvinar regions we have defined are removed from the LGN by some 8–10 mm (center-to-center) which is substantially more than the estimated point spread of gradient echo BOLD at 3 Tesla (e.g., Engel et al. 1997). Nonetheless, experiment 2 was conducted to rule out the possibility that LGN activity might account for the activity we have attributed to the pulvinar, by anatomically identifying the LGN in two participants.
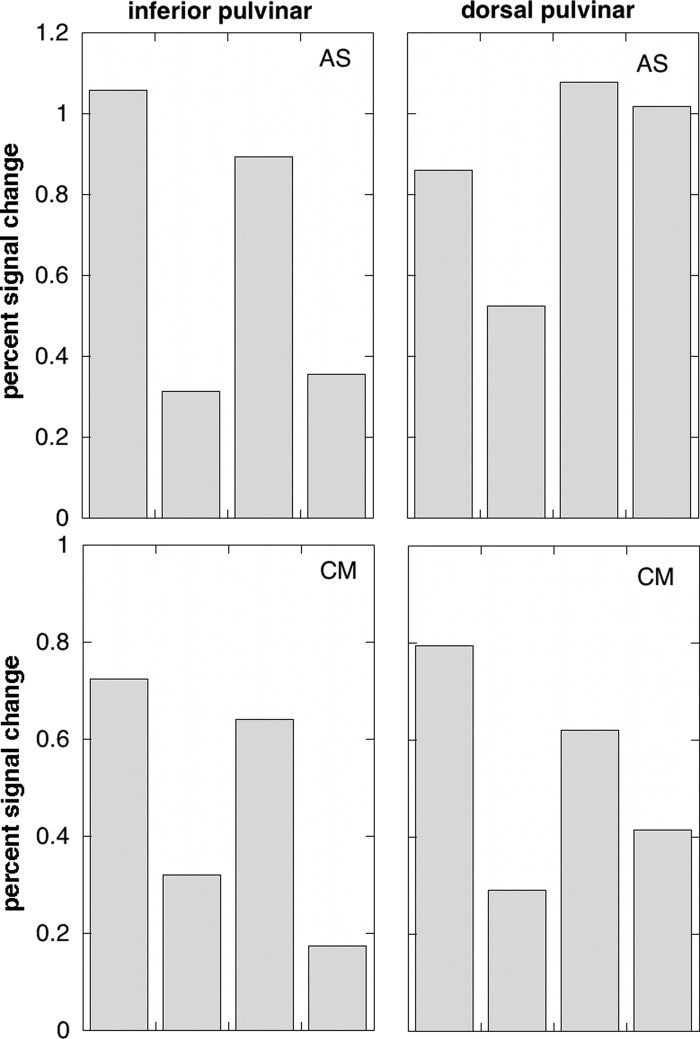
Figure 5 shows images taken from the proton density scans showing the location of the LGN. This was clearly visible in every case. Quantitative results for the main experiment are shown for each participant in Fig. 6, in the same format as that of the group data in Fig. 4. The results were in line with those of experiment 1. In both inferior and dorsal pulvinar, there is again a clear response to an unattended visual stimulus, with a strong contralateral bias. With the possible exception of the dorsal pulvinar in AS (top right) there is also a clear contralateral attention bias when stimuli are present bilaterally.
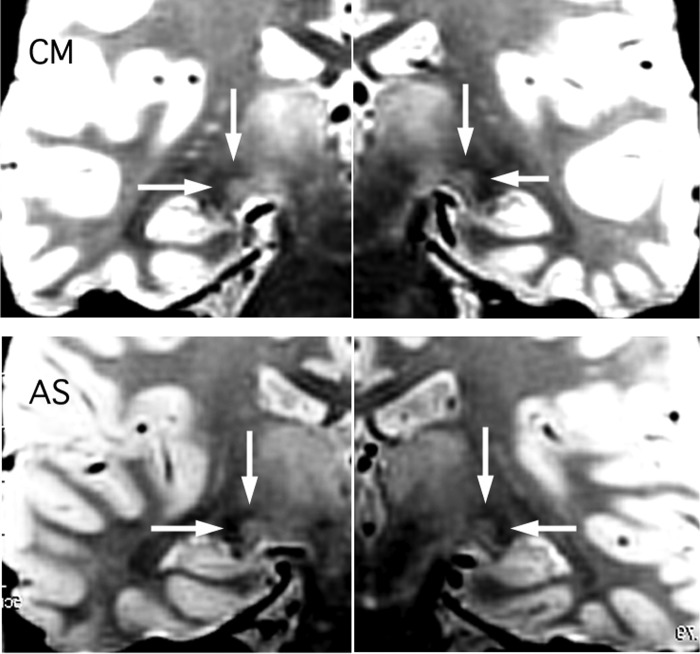
FIG. 5.
Coronal slices through the LGN from proton density scans carried out on the 2 participants in experiment 2. The LGN appears as a light gray region and is located at the intersection of the arrows in each case. Each image is constructed from 2 half images because the right and left LGN appeared in different slices.
FIG. 6.
Activations in the inferior pulvinar (left panels) and dorsal pulvinar (right panels) from experiment 2. Results are shown separately for the 2 participants (top and bottom) The key to the 4 conditions is the same as for Fig. 4 and, again, results from left and right thalamus are combined in terms of contralateral and ipsilateral stimulus locations. The ROIs were determined by reference to the location of the LGN, determined anatomically, and are shown in Fig. 5.
With only two participants, magnitudes of the responses cannot be generalized with accuracy and Fig. 4 provides a better quantitative guide. However, the results serve to show that the general similarity in response properties between the LGN and the two pulvinar regions does not reflect mistakenly including part of the LGN response in the pulvinar ROIs, since this possibility is definitively ruled out in these two individuals.
DISCUSSION
Visual activity in the human pulvinar
We have reported visual activity in two different portions of the pulvinar, referred to here as inferior pulvinar and dorsal pulvinar. The inferior area is the same as that identified in our earlier work (Cotton and Smith 2007). The mean Talairach coordinates quoted in that study are close to those reported here and we have confirmed the strong contralateral bias reported previously. However, we show here that there is also a region of the dorsal pulvinar that has a strong preference for contralateral stimuli and responds even when attention is engaged elsewhere. The dorsal area has a location similar to that reported by Kastner et al. (2004), raising the possibility that it is the same subregion, but the properties we document are sufficiently different from those reported by Kastner et al. (2004) to leave considerable doubt. We return to this question in the final section. Either way, it is clear that contralateral visual drive occurs in part of the dorsal pulvinar in the absence of attention.
Effects of attention
We have documented the contribution of visual attention to activity within the human pulvinar complex and compared it directly to that of visual drive. The result (summarized in Fig. 4) is clear: provided an appropriate stimulus is selected, the mere presence of the stimulus is sufficient to give a robust response, in both inferior and dorsal portions of the pulvinar, and attending to the stimulus adds to the response relatively modestly (21% enhancement in inferior pulvinar and 22% in dorsal pulvinar). Attentional enhancement (13%) was also evident in the LGN, as previously shown (O'Connor et al. 2002).
Although the effect of attention on pulvinar responses is substantial and of interest in its own right, it is of limited assistance in determining whether the pulvinar has a special role in attention (e.g., Robinson and Petersen 1992; Shipp 2004). The degree of attentional modulation is in line with that seen with fMRI in the visual cortical regions with which the pulvinar connects (e.g., Gandhi et al. 1999; Somers et al. 1999; Watanabe et al. 1998). In all human visual cortical areas studied, the principal effect of attention is to increase the response elicited by an unattended stimulus, typically by 10–40%, depending on the visual area and the study. This is mirrored by comparable (if somewhat smaller) effects in single neurons of the macaque visual system (e.g., McAdams and Maunsell 1999; Motter 1993). One physiological study (Bender and Youakim 2001) reported attentional modulation in the pulvinar and the level found (≤26%, in line with our result) was comparable to that found in V2 and V4 in the same study (but much greater than that found in the LGN). There is no evidence that attention has a greater modulatory effect on sensory responses in the pulvinar than elsewhere.
Having said this, the extent of attentional modulation of visual responses is not necessarily the best test for a central role in attention. Shipp (2004) takes the view that “attention is a matter of organizing multiple brain centers to act in concert on the task at hand.” He suggests that the pulvinar may act as a hub for coordinating activity across multiple cortical visual maps. It is not obvious what the pulvinar population response measured with fMRI (or, indeed, single-unit responses) should look like under such a hypothesis. This depends on the mechanism of coordination. If coordination occurs through facilitation, increased visual responses might be expected in the presence of attention, but not necessarily to a greater extent than in the posterior cortical areas with which the visual pulvinar connects. It is far from clear that intrapulvinar circuitry is performing a simple integration of inputs. The overlapping representations of visual space throughout the visual hierarchy (Shipp 2003) may mean that the local processing capacity of the pulvinar is more complex than that in other subcortical structures. If coordination occurs, for example, by modifying synchronization of firing then it is not clear even that an increased BOLD response is expected. The connections of the pulvinar are indeed exceptionally well suited to a coordinating function. Nothing in our results contradicts the notion of a special role in coordinating visual attention.
Relation to primate neurophysiology
The retinotopically organized portion of the macaque pulvinar has been described in detail (Bender 1981). Two separate maps of visual space are present, but one largely surrounds the other so that they form a single block of retinotopic tissue. The first map is located in the inferior pulvinar, at the same dorsoventral level as the LGN, broadly between the LGN and the medial geniculate (MGN). The whole of the contralateral hemifield is represented, although there is no ipsilateral representation. The second map extends from the first, laterally and posteriorly, into the lateral division of the pulvinar. Again, only the contralateral hemifield is represented. The lower and upper quadrants are represented in the dorsal and ventral portions of the lateral pulvinar, respectively.
Given the similar results we have obtained in the dorsal and ventral pulvinar, a parsimonious explanation of our results is that although the two regions appear separate, they are in fact different portions of the same map or maps of visual space. If the arrangement is the same as that in macaques, the primary map is compact and entirely inferior and, taken alone, does not support such an interpretation. However, the second map has a different shape and might do so. The data reported by Bender (1981) show that the dorsoventral extent of the map is about 5 mm in macaques. The organization of the second map is such that the horizontal meridian is represented along the dorsal and ventral margins of the retinotopic region. Our stimuli, being located on the horizontal meridian, would therefore be expected to stimulate the dorsal and ventral extremes of the region, as well as activating a third zone in the primary map. Especially when allowing for the fact that the human thalamus is bigger than the macaque thalamus, the 6 mm or so that vertically separates our two pulvinar regions is not too great for them to reflect two parts of the same (secondary) map.
We may therefore posit that our inferior pulvinar region contains much of the primary map together with the ventral extremity of the second map, and that our dorsal region consists mainly of the dorsal part of the second map. This, of course, assumes that the human and macaque pulvinar regions are similarly organized. If so, there must be a middle portion of the second (and perhaps first) map that is not included in either of our regions. This is perhaps too close to the LGN to be separated from it with fMRI and the “safe” positioning of our two pulvinar ROIs means that we capture only the two extremities. The notion that the primary map is largely confined to the inferior region is consistent with the fact that activation is stronger in the inferior region (Fig. 4) and more frequently seen in individual participants. It may also explain why we saw only the inferior region in our earlier study (Cotton and Smith 2007). On the last point, another contributory factor may be that the stimuli were smaller in that study (5° in two experiments and 1.5° in the third).
An alternative hypothesis that may be derived from primate neurophysiology is that our inferior pulvinar region corresponds to one or both of the retinotopic regions described earlier, whereas our dorsal pulvinar region corresponds to a distinct region: the visually active portion of the dorsomedial pulvinar (Pdm) identified by Petersen et al. (1985). Pdm shows a loose retinotopic organization, but receptive fields are often very large and may straddle the vertical meridian, so retinotopic organization should be harder to discern with fMRI than in the inferior pulvinar. Attentional modulation is reportedly stronger in Pdm than that in inferior and lateral pulvinar. Moreover, injection of a γ-aminobutyric acid agonist into Pdm impairs performance in an attentional task (Petersen et al. 1987).
On this second hypothesis, we would expect to find two differences between our dorsal and ventral pulvinar regions. First, we should find less difference between contralateral and ipsilateral stimuli in the dorsal than in the ventral region. Second, greater effects of attention should be apparent in the dorsal than in the ventral region. Neither of these predictions is borne out and so this interpretation appears unlikely. The lateral location of the dorsal activity also argues against it and in favor of an interpretation in terms of two portions of the same visual representation.
In macaque pulvinar, a third visual representation has been identified (Standage and Benevento 1982), in addition to the two retinotopic regions discussed earlier. It was termed VP3 by Shipp (2003) and is located more medially than the first two visual regions. It receives a projection from MT and it has no clear retinotopic organization. In view of the MT input, we might expect to see this region in our experiments, since moving-dot stimuli have been shown many times to give a strong response in MT. However, we see no activity at the expected location of VP3, either in the group analysis or in individual data.
Relation to previous fMRI studies
The activation of dorsal pulvinar reported here differs from the findings of Kastner et al. (2004). There are two interpretations of this. One is that their area and ours are the same, but the different experimental designs give different results; the other is that they are different regions of the pulvinar. The coordinates are sufficiently similar for it to be possible that they are the same. In this case, it is possible that they failed to activate the region by passive viewing and failed to detect contralateral organization because their stimuli did not drive neurons as well as our stimuli did. Their stimuli were flickering checkerboards, which we find less effective than dot-motion patterns. They were closer to the fovea and extended less far into the periphery than did ours, making it less likely that they would detect contralateral specificity if it exists, especially given that receptive fields can be quite large in the pulvinar.
The second, perhaps more likely, interpretation is that their area corresponds to the Pdm of Petersen et al. (1987), whereas ours corresponds to the dorsal (lower quadrant) portion of the lateral map, as discussed in the previous section. This would explain why our results emphasize passive visual stimulation and retinotopic organization, whereas their results emphasize attention. It would require that their region be located more medially than ours. Although distant from the medial surface, their activation does appear to be slightly more medial—and indeed slightly more dorsal—than ours (compare Figs. 2 and 3 with their Fig. 7). Moreover, homology with Pdm was the authors' own favored interpretation.
Conclusion
The likely interpretation of our data is that all parts of the pulvinar that were activated in our study reflect portions of two nested retinotopic maps similar to those found in primate pulvinar. When the contributions of visual drive and attention are compared, no evidence of a special role in attention is revealed. Instead, the balance is similar to that found in the cortical regions with which the pulvinar connects: an unattended visual stimulus generates a strong response and this is modestly enhanced by attending to the stimulus.
GRANTS
This work was supported by Biotechnology and Biological Sciences Research Council Grant BBS/B/16399 to A. T. Smith.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Bender 1981.Bender D Retinotopic organization of macaque pulvinar. J Neurophysiol 46: 672–693, 1981. [DOI] [PubMed] [Google Scholar]
- Bender 1982.Bender D Receptive-field properties of neurons in the macaque inferior pulvinar. J Neurophysiol 48: 1–17, 1982. [DOI] [PubMed] [Google Scholar]
- Bender and Youakim 2001.Bender DB, Youakim M. Effect of attentive fixation in macaque thalamus and cortex. J Neurophysiol 85: 219–234, 2001. [DOI] [PubMed] [Google Scholar]
- Benevento and Miller 1981.Benevento L, Miller J. Visual responses of single neurons in the caudal lateral pulvinar of the macaque monkey. J Neurosci 1: 1268–1278, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton and Smith 2007.Cotton PL, Smith AT. Contralateral visual hemifield representations in the human pulvinar nucleus. J Neurophysiol 98: 1600–1609, 2007. [DOI] [PubMed] [Google Scholar]
- Devlin et al. 2007.Devlin JT, Sillery EL, Hall DA, Hobden P, Behrens TEJ, Nunes RG, Clare S, Matthews PM, Moore DR, Johansen-Berg H. Reliable identification of the auditory thalamus using multi-modal structural analyses. Neuroimage 30: 1112–1120, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel et al. 1997.Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex 7: 181–192, 1997. [DOI] [PubMed] [Google Scholar]
- Fujita et al. 2001.Fujita N, Tanaka H, Takanashi M, Hirabuli N, Abe K, Yoshimura H, Nakamura H. Lateral geniculate nucleus: anatomical and functional identification by use of MR imaging. Am J Radiol 22: 1710–1726, 2001. [PMC free article] [PubMed] [Google Scholar]
- Gandhi et al. 1999.Gandhi S, Heeger D, Boynton G. Spatial attention affects brain activity in human primary visual cortex. Proc Natl Acad Sci USA 96: 3314–3319, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve et al. 2000.Grieve KL, Acuna C, Cudeiro J. The primate pulvinar nuclei: vision and action. Trends Neurosci 23: 35–39, 2000. [DOI] [PubMed] [Google Scholar]
- Hagberg et al. 2001.Hagberg G, Zito G, Patria F, Sanes J. Improved detection of event-related functional MRI signals using probability functions. Neuroimage 14: 1193–1205, 2001. [DOI] [PubMed] [Google Scholar]
- Kastner et al. 2004.Kastner S, O'Connor DH, Fukui MM, Fehd HM, Herwig U, Pinsk MA. Functional imaging of the human lateral geniculate nucleus and pulvinar. J Neurophysiol 91: 438–448, 2004. [DOI] [PubMed] [Google Scholar]
- McAdams and Maunsell 1999.McAdams C, Maunsell J. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci 19: 431–441, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone et al. 2000.Morrone MC, Tosetti M, Montanaro D, Fiorentini A, Cioni G, Burr DC. A cortical area that responds specifically to optic flow, revealed by fMRI. Nat Neurosci 3: 1322–1328, 2000. [DOI] [PubMed] [Google Scholar]
- Motter 1993.Motter BC Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol 70: 909–919, 1993. [DOI] [PubMed] [Google Scholar]
- O'Connor et al. 2002.O'Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci 5: 1203–1209, 2002. [DOI] [PubMed] [Google Scholar]
- Petersen et al. 1987.Petersen S, Robinson D, Morris J. Contributions of the pulvinar to visual spatial attention. Neuropsychologia 25: 97–105, 1987. [DOI] [PubMed] [Google Scholar]
- Petersen et al. 1985.Petersen SE, Robinson DL, Keys W. Pulvinar nuclei of the behaving rhesus monkey: visual responses and their modulation. J Neurophysiol 54: 867–886, 1985. [DOI] [PubMed] [Google Scholar]
- Robinson et al. 1991.Robinson D, McClurkin J, Kertzman C, Petersen S. Visual responses of pulvinar and collicular neurons during eye movements of awake, trained macaques. J Neurophysiol 66: 485–495, 1991. [DOI] [PubMed] [Google Scholar]
- Robinson and Petersen 1992.Robinson DL, Petersen SE. The pulvinar and visual salience. Trends Neurosci 15: 127–132, 1992. [DOI] [PubMed] [Google Scholar]
- Saenz et al. 2002.Saenz M, Buracas GT, Boynton GM. Global effects of feature-based attention in human visual cortex. Nat Neurosci 5: 631–632, 2002. [DOI] [PubMed] [Google Scholar]
- Shipp 2003.Shipp S The functional logic of cortico-pulvinar connections. Philos Trans R Soc Lond B Biol Sci 358: 1605–1624, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp 2004.Shipp S The brain circuitry of attention. Trends Cogn Sci 8: 223–230, 2004. [DOI] [PubMed] [Google Scholar]
- Somers et al. 1999.Somers D, Dale A, Seiffert A, Tootell R. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci USA 96: 1663–1668, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standage and Benevento 1982.Standage G, Benevento L. The organization of connections between the pulvinar and visual area MT in the macaque monkey. Brain Res 262: 288–294, 1982. [DOI] [PubMed] [Google Scholar]
- Treue and Trujillo 1999.Treue S, Trujillo JCM. Feature-based attention influences motion processing gain in macaque visual cortex. Nature 399: 575–579, 1999. [DOI] [PubMed] [Google Scholar]
- Villeneuve et al. 2005.Villeneuve MY, Kupers R, Gjedde A, Ptito M, Casanova C. Pattern-motion selectivity in the human pulvinar. Neuroimage 28: 474–480, 2005. [DOI] [PubMed] [Google Scholar]
- Watanabe et al. 1998.Watanabe T, Harner AM, Miyauchi S, Sasaki Y, Nielsen M, Palomo D, Mukai I. Task-dependent influences of attention on the activation of human primary visual cortex. Proc Natl Acad Sci USA 95: 11489–11492, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis et al. 2002.Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci 5: 995–1002, 2002. [DOI] [PubMed] [Google Scholar]