Abstract
Although the response of human cutaneous mechanoreceptors to controlled stimuli is well studied, it is not clear how these peripheral signals may be reflected in neuronal activity of the human CNS. We now test the hypothesis that individual neurons in the human thalamic principal somatic sensory nucleus [ventral caudal (Vc)] respond selectively to the optimal stimulus for one of the four mechanoreceptors. The optimal stimuli for particular mechanoreceptors were defined as follows: Pacinian corpuscles (PC), vibration at 128 Hz; rapidly adapting (RA), vibration at 32 or 64 Hz; slowly adapting type 1 (SA1), edge; slowly adapting type 2 (SA2), skin stretch. Nineteen neurons had a significant response to at least one optimal stimulus, and 17 had a significantly greater response to one stimulus than to the other three, including 7 PC-related, 7 RA-like, 3 SA1-like, and 2 SA2-like neurons. One of each of the SA1- and SA2-like thalamic neurons responded to vibration with firing rates that were lower than those to edge or stretch but not significantly. Except in the case of PC-related neurons, the receptive field (RF) sizes were larger for these thalamic neurons than for the corresponding mechanoreceptor. Von Frey thresholds were higher than those for the corresponding human RA and SA1 mechanoreceptors. These results suggest that there is a convergence of pathways transmitting input from multiple mechanoreceptors of one type on single thalamic neurons via the dorsal columns. They are also consistent with the presence of primate thalamic elements of modality and somatotopic isorepresentation.
INTRODUCTION
The stimulus that evokes the optimal response from a particular human cutaneous mechanoreceptor is well understood (Johansson and Vallbo 1983; Johnson et al. 2000). Slowly adapting type 1 (SA1) and type 2 (SA2) respond optimally to an edge stimulus and to a skin stretch stimulus, respectively (mechanoreceptor nomenclature as in Johnson 2001). Rapidly adapting (RA) and Pacinian corpuscles (PC) respond optimally to vibratory stimuli in the 30- to 60- and 100- to 300-Hz ranges, respectively. Similar responses have been shown in awake monkeys, except for the SA2 receptors, which occur only in humans (Johnson 2001; Vallbo and Johansson 1984).
In the ventral posterior thalamus of anesthetized marmosets and raccoons, neuronal response patterns have been compared with those of the mechanoreceptor classes (Hirai and Jones 1989; Warren et al. 1986; Zhang et al. 2001b), and each was found to be similar to that of the peripheral mechanoreceptors (Pubols and Pubols 1973). Studies of neuronal responses to cutaneous stimuli have also been reported in old world monkeys (Mountcastle et al. 1969; Sinclair et al. 1991). Comparison of these results with those in awake humans is complicated by anatomic differences between species and by the effect of anesthesia (Dougherty et al. 1997). To our knowledge, the responses of human neurons to optimal quantitative mechanical stimuli have not been recorded.
The response to manual stimuli neurons in the human thalamic principal sensory nucleus (ventral caudal, Vc) and the corresponding VP in old world monkeys has been studied previously. The responses of neurons in Vc and VP to manual cutaneous stimuli have been used to identify neurons with slowly adapting or rapidly adapting response patterns (Jones and Friedman 1982; Jones et al. 1982; Lenz et al. 1988). Microstimulation of this nucleus evokes sensations like those evoked by stimuli which activate cutaneous mechanoreceptors (Ohara et al. 2004). More than one mechanical or tingle sensation evoked was sometimes evoked by microstimulation at sites in Vc, even at sites with thresholds as low as 5 μA (Patel et al. 2006). Stimulation of sites where more than one sensation was evoked might result from convergence on single Vc neurons of input arising from more than one mechanoreceptor type.
We now test the hypothesis that the response of single neurons in Vc is significantly related to only one of the four optimal cutaneous mechanical stimuli. All of the four optimal stimuli were applied during recordings from each Vc neuron. Seventeen mechanoreceptor-like neurons were identified by a selective response to one stimulus, which was significantly greater than the response to each of the other three stimuli. Two nonselective mechanoreceptor neurons had a maximal response to one stimulus, which was not significantly greater than the response to another stimulus.
METHODS
These studies were carried out at the Johns Hopkins Hospital (2000–2005) during the physiologic exploration of the thalamus, which preceded implantation of deep brain stimulating (DBS) electrodes. The protocol was reviewed and approved annually by the Institutional Review Board of the Johns Hopkins University. All patients signed an informed consent for these studies. All surgical, electrophysiological, and psychophysical procedures have been previously described (Lee et al. 2005).
Intraoperative procedures
Thalamic exploration was performed as a stereotactic procedure using the Leksell frame. Patients had been off all medications that could be safely stopped for 18 h before the time of surgery. First, the frame coordinates of the anterior (AC) and posterior commissures (PC) were measured by MRI. These coordinates were used to estimate the location of Vc (Fig. 5), which was corroborated by microelectrode recordings and stimulation with the subject fully conscious.
FIG. 5.
Map of the estimated neuronal location by mechanoreceptor-like classification as in the inset. The calibration bar is 3 mm.
Search stimuli consisted of manual touch, tap, pressure, and stroking applied to the face, arm, and hand. Spontaneous activity was recorded first when a neuron was isolated. The neuron was studied to identify cutaneous sensory neurons responding to these stimuli and deep sensory neurons responding to pressure to muscles or ligaments and passive joint movement. The activity of neurons was also examined as patients carried out movements such as making a fist, flexing, or extending the wrist and elbow, pointing, etc. Microstimulation was carried out at intervals along each trajectory as previously described (Ohara and Lenz 2003).
The constant location of the electrode during the stimulation protocol was confirmed at each site. During recording, we compensated for movement of the electrode in the brain by making small movements of the electrode (<100 μm) to keep the size of the action potential constant. The receptive field, the projected field, and the size and shape of the action potential were checked for consistency during the studies at each site.
The anterior and inferior borders of Vc were identified by the location of the most anterior and the most inferior cutaneous sensory neurons in the region where deep or cutaneous sensory neurons were in the majority (Lee et al. 2005). As in all our previous studies, we used the atlas maps and nomenclature described by Hassler (Schaltenbrand and Bailey 1959). In each patient, the appropriate atlas map was positioned over the operative map of neuronal location by fitting the atlas map to the location of the ACPC line and the locations of sensory cells, In this way, we estimated location Vc and adjacent nuclei including ventral intermediate (Vim) and Vcpor (ventral caudal portae) (Fig. 5). In this paper, human Vc is equivalent to ventral posterior (VP) (including ventral posterior (VPM), ventral posterior lateral anterior (VPLa), and VPLp) in the nomenclature of Hirai and Jones (1989) for old world monkeys and equivalent to VP (including VPL and VPM) in the nomenclature of Carpenter for other primate species and raccoons (Parent 1996).
Quantitative cutaneous stimulation
We first examined cutaneous sensory neurons as identified by the manual stimuli described above. Thereafter, the receptive field (RF) was mapped in detail using a set of Frey hairs (calibrated yearly; North Coast Medical). A von Frey threshold for evoking a neuronal response was determined, and the RF was mapped using a hair with a weight (g) 10–20% higher than that of the threshold weight. Mechanical cutaneous stimuli were applied, including stretch, edge, and vibration. All these stimuli were applied within the RF at or adjacent to the center of the RF during intervals when visual and EMG examination showed no evidence of tremor or other movement. The absence of tremor during sensory stimulation was confirmed by visual inspection and by the EMG signals.
The RF was stretched along axes parallel [proximal-distal (P-D)] or perpendicular to the long axis of the limb [medio-lateral (M-L)] using surgical paper tapes (Micropore, 3M Healthcare, St. Paul, MN) attached to surface of the skin. These tapes were applied outside the RF to avoid direct stimulation of the RF. The RF was stimulated by a 1-in sharp edge of a plastic probe (Delrin), which was applied to the center of the RF, alternately parallel and perpendicular to the long axis of the limb.
For studies of each neuron, the hand was held fixed in a mold of malleable plastic, which was attached to the operating room table. Vibratory stimuli included either a computer-controlled Chubbuck stimulator (Chubbuck 1966) (13 neurons) held by a multijointed arm or precision tuning forks (Ragg, Sheffield, UK) (6 neurons). Vibratory stimuli using the Chubbuck started with an indentation (0.5 mm without vibration) followed by a period of vibration during the indentation (0.1 mm in amplitude). The tip of the Chubbuck stimulator probe was a flat, circular disc (diameter of 3 mm) that was placed on the skin at the center of the RF with a force of 50–100g. Tuning folks with a rectangular tip with a size ranging from 3 × 4 to 6 × 9 mm were applied manually to the center of the RF in the vibrating and nonvibrating conditions during different trials. We did not systematically attempt to stimulate hair, because no subject had facial hair and, to our knowledge, human hair mechanoreceptors have only been described on the whiskers (Nordin and Hagbarth 1989).
Data collection and analysis
The signals recorded on magnetic tape (Model 4000, Vetter, Rebersberg, PA) included the Chubbuck position signal and the foot pedal signal indicating the timing of stimulus control, the microelectrode signal, the EMGs, and an audio channel describing intraoperative events. Postoperatively, the spike train was digitized at 20 kHz, whereas the other signals were digitized at 200 Hz. Subsequent analyses of spike characteristics [e.g., firing rate and interstimulus interval (ISI)] were carried out using MATLAB (Mathworks, Natick, MA).
As a measure of phase locking in response to vibration, the percentage entrainment was calculated as the highest number of impulses in any continuous half-cycle of the cycle histogram, which was expressed as a percentage of the total number of impulses (Zhang et al. 2001a). The percentage entrainment has a minimum of 50% when there is no phase preference and a maximum of 100% when all spikes are in the same half-cycle (Zhang et al. 1996). The visual display of the dispersion of spikes relative to the cycle period of the vibration stimulus is the phase scatter graph shown in Figs. 1–4.
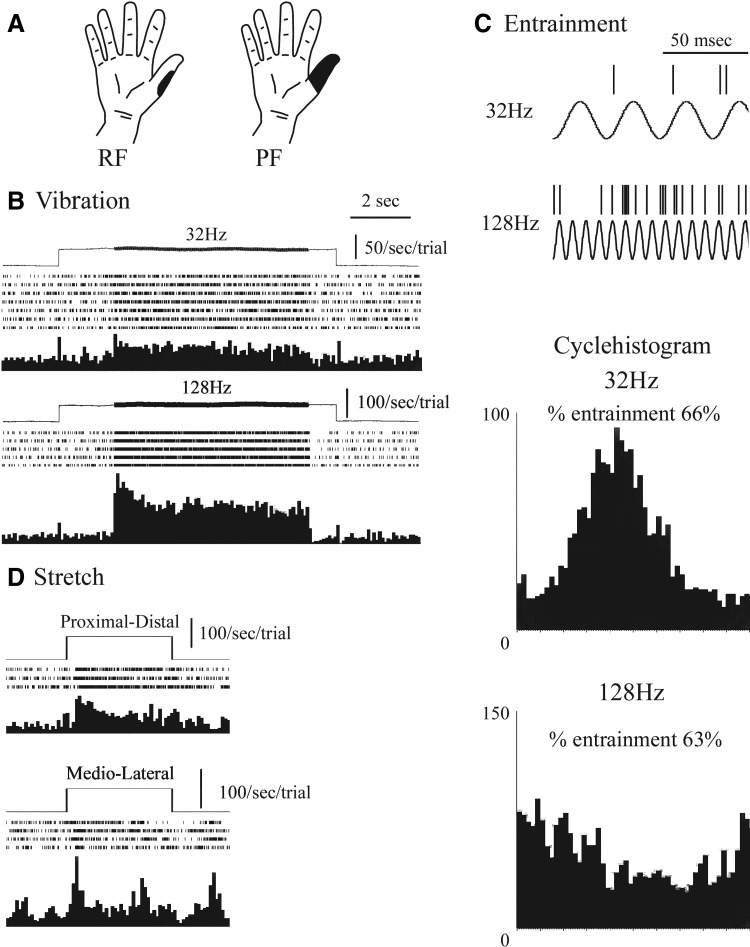
FIG. 1.
Physiologic characteristics of Pacinian corpuscles (PC)-related thalamic neurons. A: figurines of the receptive field (RF) and projected field (PF). B: response to step indentation and vibration at 32–128 Hz as measured by the Chubbuck stimulator (as labelled). C: entrainment of neuronal firing: top panel, spike trains that signal to the Chubbuck; middle and bottom panels cycle histograms, frequencies as indicated. D: skin stretch, directions as indicated.
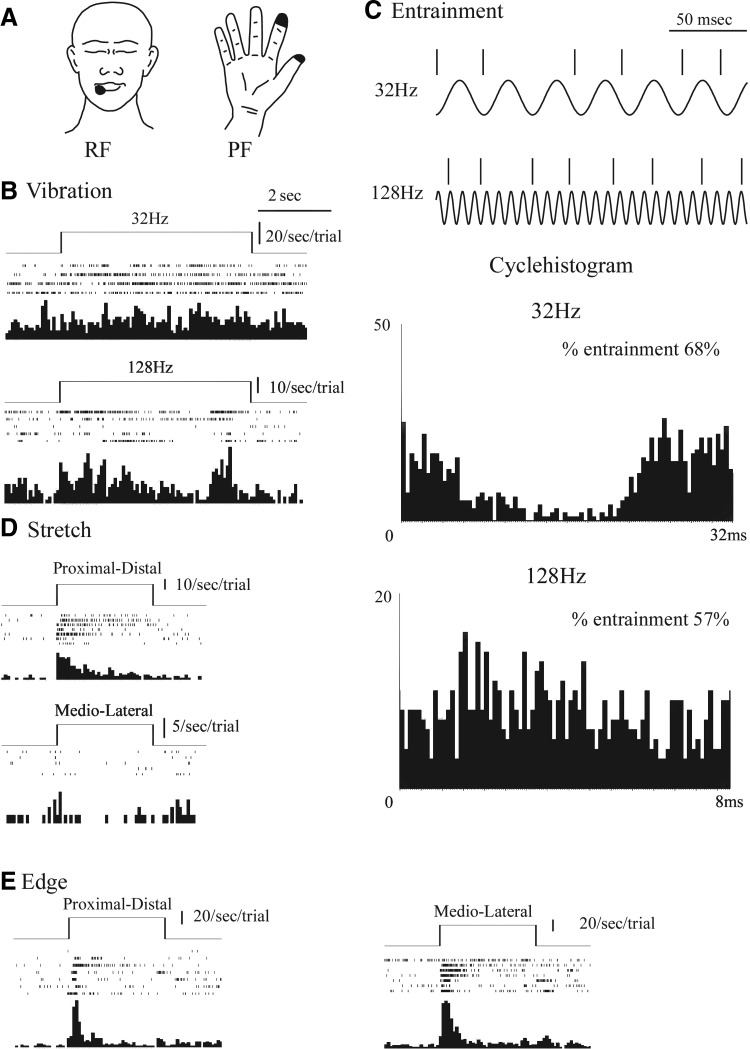
FIG. 4.
Physiologic characteristics of SA1-like neuron (neuron 3). Conventions as for Fig. 2.
Statistical analysis
The effect of different stimuli on firing rate was statistically compared by a two-way ANOVA by STIMULUS (different frequencies of vibration, stretch, or edge), and PRE-POST firing rate, e.g., Chubbuck vibration versus indenting (post-pre-stimulus onset). Post hoc testing of poststimulus onset firing rates by STIMULUS was carried out by Tukey's honestly significant difference (HSD). Prestimulus period was defined as 20 bins of 100 ms before the onset of the stimulus, i.e., 2 s before the stimulus onset. The poststimulus onset period has the same duration after the stimulus onset. For each period, the number of spikes in each of 20 bins was calculated and used as the dependent variable. The null hypothesis was rejected at P < 0.05 for all statistical tests.
The significance of phasic effects of the indentation, edge, or stretch stimuli was decided by assessing whether any bin was greater than baseline (mean + 3 SD). A phasic effect was identified if bins immediately following the stimulus onset or offset met this criterion and if the same effect could be seen in the majority of lines in the raster.
RESULTS
These results were obtained during awake thalamic surgery in patients with essential tremor (7 patients) or with cerebellar tremor, Parkinson's tremor, or chronic pain (1 patient each) (Koller et al. 2001). The neuron studied in the patient with chronic facial pain had a digital RF. These patients included six men (52–83 yr old) and four women (41–55 yr old). Surgery was carried out on a total of 12 thalami (6 on the left) in 10 patients, because both sides were studied in two patients. For each patient, between one and three trajectories were explored overall [1.7 ± 0.8 (SD)] and one to three trajectories traversed Vc (1.6 ± 0.8). A total of 30 neurons responsive to manual stimulation were studied along 13 trajectories, and 19 of these neurons responded to at least one of the optimal quantitative stimuli.
Rapidly adapting type neurons (PC-related and RA-like)
In these results, the fit between mechanoreceptor-like neurons and the corresponding mechanoreceptors was weakest for neurons possibly related to PC mechanoreceptors (see Characteristic activity of thalamic neurons). Therefore the term PC related is used for these neurons, whereas the others are designated as RA like, SA1 like, and SA2 like. Figure 1 shows an example of a thalamic PC-related neuron (neuron 18) showing a response to mechanical stimuli similar to that of a PC mechanoreceptor, with an RF on the thumb (D1; Fig. 1A). Significant differences in firing rate were found for this neuron by stimulus (ANOVA, F = 37.5, df = 3, P < 0.001), post-pre stimulus (F = 236.7, df = 1, P < 0.001), and interaction (F = 51.2, df = 3, P < 0.001). Figure 1B shows a response to vibratory stimuli at both 32 and 128 Hz. There is also a phasic response to indentation, consisting of a single bin at the onset of the indentation stimuli preceding both frequencies of vibration. Firing rates were significantly higher for the poststimulus interval versus prestimulus interval in the case of 128 Hz (P < 0.001), 32 Hz (P < 0.001), stretch, both M-L (P = 0.010) and P-D (P < 0.001, all HSD, Fig. 1D), and edge (data not shown).
When compared between stimulus type, the poststimulus firing rate was significantly higher for 128 Hz [154 ± 7/s (SE)] than for 32 Hz (58 ± 2/s), stretch stimuli, and both M-L (59 ± 8/s) and P-D (69 ± 6/s; P < 0.001, all HSD; Fig. 1D). The percentage entrainment (Fig. 1C) was not significantly different between 128 (64.6%) and 32 Hz (66.6%). The von Frey threshold was 6 g. The RF had an area of 300 mm squared on the proximal phalanx of the thumb (Fig. 1A).
A total of seven neurons had a response to 128 Hz, which was significantly greater than that for any other optimal stimulus (neuron numbers 8, 9, 14, 15, 16, 18, 19). The highest levels of entrainment were found at 128 Hz for cells 15 (59%) and 19 (59%) and at both 32 and 128 Hz for cells 14, 15, 17, 18, and 19. Phasic responses to an edge or Chubbuck indent stimulus were found for neurons 9, 15, and 19 and to a stretch stimulus for neuron 18. von Frey thresholds ordered by ascending neuron number were 4, 8, 10, 0.16, 0.16, 6, and 0.4 g. RFs ordered by ascending neuron number were as follows: perioral, 130 mm2; perioral, 200 mm2; D1, 150 mm2; D2, 400 mm2; D2, 400 mm2; D1, 300 mm2; D2, 400 mm2. These are smaller than PC afferents, which usually involve one whole finger (Table 1). Microstimulation adjacent to the recording sites evoked a deep, vibration sensation at site neuron 9 and a deep, touch, electric warm at site 18.
TABLE 1.
Summary of physiological characteristics of primate mechanoreceptors and thalamic mechanoreceptor-specific neurons
| Von Frey | Receptive Field | Response to Indentation | Frequency of Maximum Vibration Response | Amplitude of Maximum Vibration Response | Optimal Stimulus | |
|---|---|---|---|---|---|---|
| Thalamic SA1 | ≤0.5 g* | Volar aspect of phalanx* | Thresh < 0.2 mm plateau 0.5 mm,* 0.006–0.2 mm† | NA | NA | Step indentation |
| SA1 afferent | Half <0.1 and half 0.5 to 1.9 g‡ | Regular well-defined† <6 mm2 | Thresh 0.06 mm,** linear increment up to 1.5 mm†† | Nil | Nil | Edge contours of step indentation‡ |
| SA2 afferent (human only) | <25 mm2, obscure‡ than SAI adjacent to nailbed or skin folds‡‡ | Thresh 0.3 mm.** 4 times less sensitive than SAI** | Not well defined | Nil | Skin stretch† | |
| Thalamic RA | ≤0.5 g* | Fraction of Volar phalanx* | Plateaus between 0.1 and 0.3 mm | >100 Hz, may respond at <10 Hz* | Thresh <0.01 mm* | |
| RA afferent | <0.1 g‡ | Regular distinct 5–30 mm2 | Thresh 10−3 mm,** plateaus at 0.1–0.2 mm†† | 40–60 Hz‡ | Threshold ≥2 × 10−5 mm | Vibration 40–60 Hz |
| Thalamic PC | <0.1 g* | Larger than RA* | NA | >100 Hz* | Thresh ≥0.02 plateau = 0.08 mm* | |
| PC afferent | NA | Large (whole finger or palm), obscure, may involve the whole hand, rarely less than a phalanx | Thresh 10−2 mm,** sensitive to third derivative of displacement, not sustained indent | 200–300 Hz | Threshold ≥ 10−5 mm | Vibration 200–300 Hz |
Gybels and van Hees 1972. SA, slowly adapting; RA, rapidly adapting; PC, Pacinian corpusles; NA, not available; g, grams.
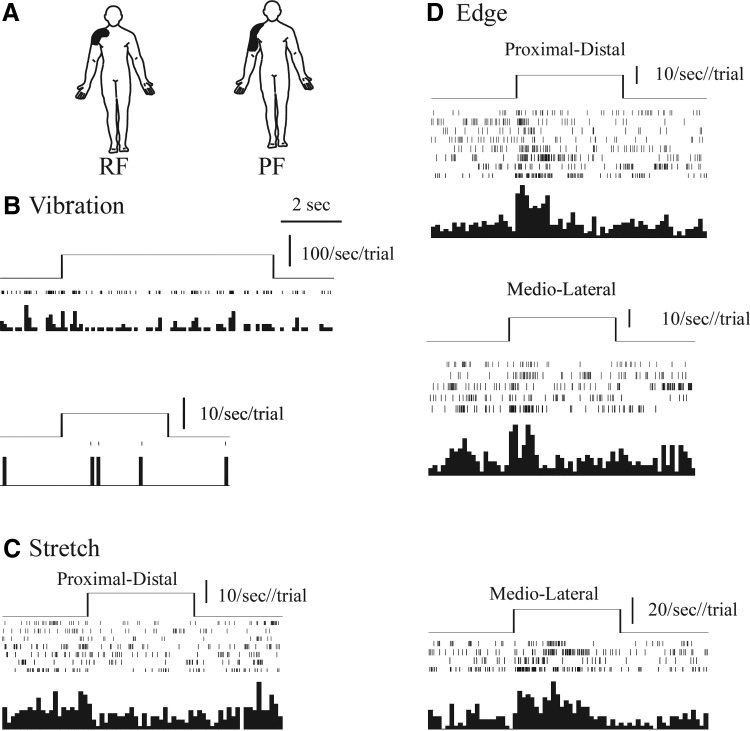
Figure 2 shows an example of a thalamic RA-like neuron. Significant differences in firing rate were found for this neuron by stimulus (ANOVA, F = 43.8, df = 9, P < 0.001), post-pre stimulus (F = 102.4, df = 1, P < 0.001), and the interaction (F = 10.2, df = 9, P < 0.001). There was a significant poststimulus onset increase of firing rate for 32 (P < 0.001, HSD) and 64 Hz (P < 0.001, HSD, Fig. 2B), but not for any other optimal stimulus. Significant phasic responses were found for both edge and stretch stimuli.
FIG. 2.
Physiologic characteristics of rapidly adapting (RA)-like neuron. Conventions as for Fig. 1, A–D. E: skin stretch proximal-distal (P-D) direction above stretch medio-lateral (M-L).
Among stimuli with a significant pre-post change, the poststimulus firing rate was significantly higher for 32 Hz (31 ± 2/s) than any other optimal stimulus. A significantly higher degree of entrainment was found at 32 (96%) than at 64 (71%) or 128 Hz (68%). The von Frey threshold was 100 g. Stimulation adjacent to neuron 11 evoked an unnatural, surface and deep, vibration and electric current sensation.
A total of seven neurons (1, 2, 6, 7, 11, 13, 17) showed a response to vibratory stimuli at 32 and/or 64 Hz that was significantly greater than that to any other optimal stimulus. These responses were largest at 32 (neurons 6, 11, 17) or 64 Hz (neuron 7) or both 32 and 64 Hz (neurons 1, 2, and 13). Six had a significant phasic response to edge/indentation stimuli (neurons 2, 6, 7, 11, and 17) or both edge and stretch (neuron 13). von Frey thresholds were 14.4, not determined (neuron 2), 6.0, 7.9, 100, 4, and 0.4 g in order by neuron number. Measured thresholds were all much larger than those for RA afferents (Table 1). By ascending order of neuronal numbers, the RFs had areas of 600 (D2), 100 (tip of D2), 600 (D2), 100 (tip of D3), 40 (distal D1), 150 (perioral), and 600 mm2 (D2). Three neurons had RFs much larger than those for RA mechanoreceptors. Microstimulation at or adjacent to the thalamic recording sites evoked deep vibration sensations at four sites (neurons 1, 6, 11, 13), whereas a surface, sharp, moving sensation was found once (neuron 2).
Slowly adapting type neurons (SA1- and SA2-like)
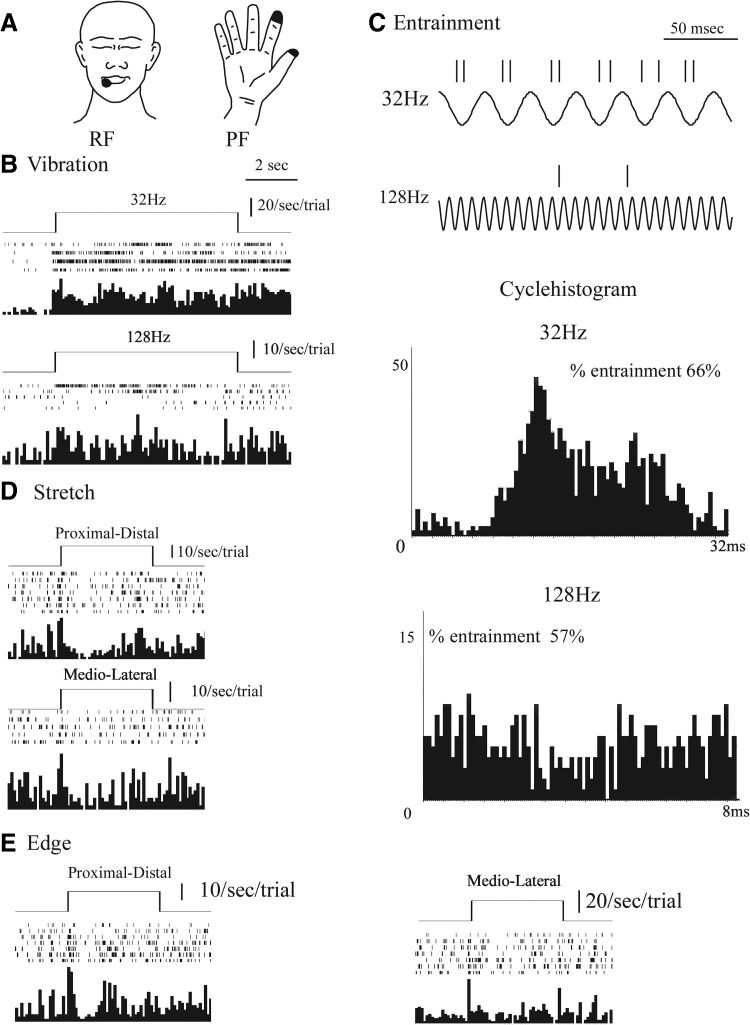
Figure 3 shows an example of a thalamic neuron (neuron 12) with SA1-like properties in a perioral RF. Overall, significant differences in firing rates were found for this neuron by stimulus (ANOVA by stimulus, P < 0.01, F = 8.2, df = 9), pre-post (P < 0.001, F = 50.3, df = 1, P < 0.001), and the interaction (F = 6.6, df = 9, P < 0.001). There was a significant pre-post increase of firing for the M-L edge (P < 0.001), P-D edge (P = 0.004), and P-D stretch (P = 0.029) but not for any other optimal stimulus. Poststimulus firing rate was significantly higher for the M-L edge (26 ± 6/s) than for all the other stimuli, with a significant pre-post difference, including P-D edge (12 ± 5). The von Frey threshold was 4 g. The perioral RF of this neuron had an area of 100 mm2.
FIG. 3.
Physiologic characteristics of slowly adapting type 1 (SA1)-like neuron. Conventions as for Fig. 2.
SA1-like characteristics were found for neurons 5, 10, and 12. Neurons 5 and 12 had a tonic plus phasic response to edge significantly greater than that to any other optimal stimulus. In the case of neuron 10, termed nonselective SA1 like, the response to edge was greater than that to any other optimal response except vibration at 32 and 128 Hz. The difference in the response between M-L and P-D stimulus orientations was only found for neuron 12.
The von Frey thresholds were not determined (neuron 5), 5.2, and 4.6 g, much greater than for SA1 mechanoreceptors (Table 1). These neurons had RFs on the palmar surface of D4 (600 mm2), the palmar distal phalanx of D3 (100 mm2), and the perioral area (100 mm2), all of which are much larger than human SA1 mechanoreceptors (Table 1). Stimulation produced an unnatural surface and deep vibration sensation in the case of two neurons (10 and 12) and an electric current sensation in the case of neuron 5. Therefore one of three neurons had evidence of convergent inputs from pathways transmitting input from SA1 with those from PC or RA mechanoreceptors (Johansson et al. 1982).
Figure 4 shows an example of a thalamic SA2-like neuron (neuron 3). Overall, there were significant differences in firing rate of this neuron by stimulus (ANOVA, F = 12.6, df = 7, P < 0.001), post-pre stimulus (F = 3.7, df = 1, P < 0.001), and interaction (F = 3.7, df = 7, P < 0.001). There was a significant pre-post increase in firing for M-L stretch (21 ± 2/s, P = 0.008) but not for any of the other optimal stimulus (range of 1–6 Hz). The RF was the skin overlying the deltoid with an area of ∼100 cm2. The von Frey threshold was 105 g. The sensation evoked by microstimulation was located in a PF on the shoulder and upper arm and was described as an unnatural, surface and deep, vibration, and electric current sensation.
This type of response was found for two neurons (3 and 4), both of which showed a significant response to stretch in either the M-L direction (neuron 3; Fig. 4), or both P-D and M-L (neuron 4). Neuron 4 also had a response to 32 Hz that was not significantly different from the response to stretch in either direction and therefore was termed a nonselective SA2-like neuron. The von Frey threshold was 11 g. The RF of neuron 4 was in the perioral area at the angle of the mouth (100 mm2). Stimulation at the site of neuron 4 evoked a sensation of vibration in an area of 100 mm2 at the angle of the mouth.
Therefore most neurons (17/19) responding to at least one optimal stimulus had a significantly greater response to one of these stimuli than to any other optimal stimulus. However, one SA1-like neuron (10) had a response to edge not significantly different from that to 32 and 128 Hz, and one SA2-like neuron (4) had a response to stretch not significantly different from that to 32 Hz.
This specificity of thalamic neuronal responses is greater than that expected at random by a combinatorial analysis. Specifically, any cell could be specific to one mechanoreceptor type in four ways, two mechanoreceptor types in six ways, three mechanoreceptors in four ways, and four mechanoreceptors in one way. Therefore the probability of having selectivity for one mechanoreceptor type for one neuron at random was 4/15 or P = 0.37. With these assumptions, these observations showed selectivity for one of the four mechanoreceptor types at random significantly more common than expected at random (17/19, P = 0.0001, Binomial). Therefore to a significant degree, thalamic neuronal responses were similar to those of a single mechanoreceptor. Neurons responding optimally to more than one optimal stimulus responded to either SA1 or SA2, and also responded to vibratory stimuli.
Relationship of recording to sensations evoked by stimulation result
Examples of nonoverlapping RFs and PFs were found for each of the mechanoreceptor-like neurons as follows: neuron 19 (PC related), neuron 13 (RA like), neurons 10 and 12 (S1 like), and neuron 4 (S2 like). If we assume conservatively that this overlap occurs with P = 0.5, the PF overlapped the RF (Figs. 1A and 4A) more commonly than expected at random (observed 14/19, P = 0.03, Binomial). The proportion of neurons with nonoverlapping fields was not significant between different mechanoreceptor-like neuronal types.
Among neurons with overlapping RFs and PFs, PFs were larger than RFs in all except one (RA-like neuron 17). This suggests that the thalamic psychophysical element subserving place specificity is the result of convergence of inputs arising from multiple mechanoreceptors of the same type (Patel et al. 2006).
The sensation evoked by stimulation at or adjacent to the recording site for each of these neurons was compared with the response evoked by intraneural microstimulation at recording sites for the corresponding mechanoreceptor axons as follows: PC and RA, vibration; SA1, pressure; SA2, stretch (Ohara et al. 2004; Torebjork et al. 1987). Specifically, vibration was evoked by stimulation at recording sites for one of seven PC-related neurons, vibration at four of seven sites for RA-like neurons, pressure for zero of three SA1-like neurons, and stretch for zero of two SA2-like neurons. Overall, the microstimulation evoked sensation at the recording site for any mechanoreceptor-like neuron was the same as the sensation evoked by intraneural stimulation of the corresponding mechanoreceptor less commonly than expected at random (5/19, P = 0.009, Binomial distribution). This suggests that the thalamic element of modality specificity is so small that stimulation at any site is likely to activate more than one thalamic element of modality representation.
Thalamic nuclear locations mechanoreceptor-like neurons
The locations of the neurons included in this analysis are shown in Fig. 5. These neurons do not seem to be found in different locations as a function of the mechanoreceptor response type, which is significantly related to neuronal activity. In particular, PC-related neurons do not seem to be located inferiorly (cf. Dykes et al. 1981).
DISCUSSION
This study describes the response properties of 19 human thalamic single neurons by their response to the four optimal stimuli, each of which optimally activates one of the four cutaneous mechanoreceptors. All but two of these neurons had a significantly greater response to one optimal stimulus than to any other and therefore were designated mechanoreceptor-like neurons. The RF sizes were usually larger for mechanoreceptor-like neurons than for the corresponding mechanoreceptor except in the case of PC-related neurons, which were equivalent or smaller than PC mechanoreceptors. von Frey thresholds were higher than those for the corresponding mechanoreceptor in the case of mechanoreceptors (RA and SA1) for which thresholds were available. These results may be the result of the convergence of pathways from multiple mechanoreceptors of a single type on neurons in the human thalamus and in the dorsal column nuclei (DCN) (Pubols and Pubols 1973).
Characteristic activity of thalamic neurons
These results show that PC-related responses are characterized by an optimal response to vibration at >100 Hz with significant entrainment, as in the human PC mechanoreceptors (Table 1). These neurons had von Frey thresholds that were substantially larger than those in marmosets (<0.1 g). Four of these PC-related neurons had relatively large RFs, involving the palmar surface of a digit, similar to PC mechanoreceptors (Johansson and Vallbo 1983; Johnson 2001). Overall, the behavior of these PC-related neurons was similar to that of marmoset thalamic neurons (Zhang et al. 2001a) and to that of human PC mechanoreceptors (Table 1). However, four of seven PC-related neurons had RFs that were smaller than PC mechanoreceptors and were located on the dorsal surface of the hand (2 neurons) or the perioral skin (2 neurons). Therefore these PC-related RFs are uncommon for the RFs of mechanoreceptors and suggest that simple convergence does not explain the results, despite the frequency response (Johansson and Vallbo 1979; Nordin and Hagbarth 1989).
RA-like neurons were defined by their frequency response, which was similar to the response of RA-selective neurons in the marmoset and monkey forebrain (Mountcastle et al. 1990; Warren et al. 1986). The present von Frey thresholds were >1.4 g in the case of all RA-like neurons, much greater than human RA mechanoreceptors (<0.1 g; Table 1) (Gybels and Van Hees 1972) or marmoset RA-specific neurons (<0.5 g; Table 1). The areas of the RFs for four RA-like neurons were comparable to the RFs of human RA mechanoreceptors (40–150 mm2), whereas the other three were much larger (600 mm2). These RFs suggest that some RA-like neurons receive convergent input from pathways arising from several RA mechanoreceptors. These thalamic neurons may mediate the passive or active perception of flutter or grating stimuli by analogy to forebrain neurons described in old world monkeys (Mountcastle et al. 1969; Sinclair et al. 1991).
SA1-like neurons responded to edge, consistent with both human SA1 afferents and marmoset SA1-specific thalamic neurons (Table 1). von Frey thresholds were 4 and 15 g, much greater than that for the human SA1 mechanoreceptors (Gybels and Van Hees 1972) or marmoset PC-specific thalamic neurons (Zhang et al. 2001a) (Table 1). RFs were 100 mm2 at the digital tip or in a perioral distribution, much greater than RF of the SA1 mechanoreceptor (Johansson and Vallbo 1983; Johnson et al. 2000), but comparable to the RFs of the marmoset thalamic neurons (Table 1). These SA1-like neuronal RFs strongly suggest that each thalamic neuron receives convergent input from pathways arising from several SA1 mechanoreceptors.
A maximal response to stretch was found for two neurons that were classified as SA2-like neurons (Johnson 2001); RFs were larger than those found for SA2 afferents. SA2-like neuron 3 and SA1-like neuron 12 showed a directional specificity to edge and stretch stimuli, respectively. Responses to directional edge or movement stimuli have been reported in monkey primary afferents (Phillips and Johnson 1981) and in the forebrain of old world monkeys and raccoons (Phillips and Johnson 1981; Pubols and LeRoy 1977). These results are consistent with psychophysical studies of the perception the direction of cutaneous stimuli (Essick et al. 1988, 1991).
Some prior studies of thalamic responses to optimal cutaneous mechanical stimuli were carried out in animals anesthetized by induction of ketamine and xylazine and maintenance on ketamine or induction and maintenance on flurane or pentothal (Warren et al. 1986; Zhang et al. 2001b). Neurons in the monkey thalamic VP have decreases in spontaneous and evoked firing in response to barbiturates, whereas inhalational agents at relevant concentrations may or may not decrease evoked responses (Dougherty et al. 1997). Therefore some of the differences between these results and those recorded in previous studies may be caused by anesthetic effects. Additionally, these differences may be caused by the physical properties of the skin between species, which may effect the evoked responses of primate mechanoreceptors (Pubols 1982).
Convergence of input arising from mechanoreceptors on mechanoreceptor-like neurons
Overall, some RA-like, SA1-like, and SA2-like neurons seem to receive convergent input arising from several mechanoreceptors of the corresponding type. This convergence was not found for PC-related neurons, which had RFs that were smaller than or equivalent to those in PC mechanoreceptors. Two nonselective mechanoreceptor-like neurons (10 and 3) showed responses to vibration not significantly different from the responses to edge or stretch, respectively. Therefore convergence of pathways arising from several mechanoreceptors of the type seems o be common among human thalamic mechanoreceptor-like neurons (Johansson and Vallbo 1983; Johansson et al. 1982).
Convergence of pathways arising from individual mechanoreceptors may give thalamic neurons greater potential for plasticity. Among 30 cells that responded to manual cutaneous stimuli, only 19 responded significantly to one of the optimal stimuli. The neurons that did not respond to quantitative stimuli may become responsive as a result of changes in the weight of different converging inputs (Smits et al. 1991). This mechanism might mediate the plasticity of neuronal responses to somatic sensory stimuli (Dykes and Lamour 1988), which can result from attention, learning, and memory processes or from nervous system injury (Bezdudnaya et al. 2006; Kaas 1991; Lenz et al. 1998; Rasmusson 1996).
Convergence of inputs arising from different types of mechanoreceptors may be consistent with the absence of anatomic clustering within Vc (Fig. 5). In particular, we did not observe clustering of PC-related neurons along the inferior aspect of Vc, as previously suggested (see Table 1 and Dykes et al. 1981; Zhang et al. 2001a). The small number of neurons studied quantitatively in any individual thalamus limits our confidence in identifying clusters (cf. Jones and Friedman 1982; Jones et al. 1982; Lenz et al. 1988; Patel et al. 2006).
Units of isorepresentation smaller than clusters are clearly above the resolution of this study. Nevertheless, the size of RFs in the thalamus of this study does suggest that there is convergence of inputs arising from multiple mechanoreceptors of the same type in the case of RA, SA1, and SA2 mechanoreceptors. These units of isorepresentation by modality can be interpreted in terms of the psychophysical elements of modality specificity shown by human thalamic recordings microstimulation (Lenz et al. 1988; Patel et al. 2006). The units of isorepresentation of somatopy for cutaneous modalities were larger than the RFs of SA1-like, SA2-like, or RA-like neurons whether measured by maps of PFs evoked by thalamic microstimulation in Vc or by maps of neuronal RFs neurons in Vc. Furthermore, microstimulation in Vc suggests that there is spatial convergence of input arising from multiple mechanoreceptors on neurons in the dorsal column nuclei or thalamus (Patel et al. 2006). These responses of mechanoreceptor-like thalamic neurons are strong evidence that individual neurons reflect the convergence in the thalamus of input arising from several mechanoreceptors of one type.
GRANTS
This work was supported by grants from Eli Lilly and National Institute of Neurological Disorders and Stroke Grants NS-383493 and NS-40059 to F. A. Lenz.
Acknowledgments
We thank L. H. Rowland for excellent technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Bezdudnaya et al. 2006.Bezdudnaya T, Cano M, Bereshpolova Y, Stoelzel CR, Alonso JM, Swadlow HA. Thalamic burst mode and inattention in the awake LGNd. Neuron 49: 421–432, 2006. [DOI] [PubMed] [Google Scholar]
- Chubbuck 1966.Chubbuck JG Small motion biological stimulator. Appl Phys Lab Tech Digest 5: 18–23, 1966. [Google Scholar]
- Dougherty et al. 1997.Dougherty PM, Li YJ, Lenz FA, Rowland L, Mittman S. Correlation of effects of general anesthetics on somatosensory neurons in the primate thalamus and cortical EEG power. J Neurophysiol 77: 1375–1392, 1997. [DOI] [PubMed] [Google Scholar]
- Dykes and Lamour 1988.Dykes R, Lamour Y. Neurons without demonstrable receptive fields outnumber neurons having receptive fields in samples from the somatosensory cortex of anesthetized or paralyzed cats and rats. Brain Res 440: 133–143, 1988. [DOI] [PubMed] [Google Scholar]
- Dykes et al. 1981.Dykes RW, Sur M, Merzenich MM, Kaas JH, Nelson RJ. Regional segregation of neurons responding to quickly adapting, slowly adapting, deep and pacinian receptors within thalamic ventroposterior lateral and ventroposterior inferior nuclei in the squirrel monkey. Neuroscience 6: 1687–1692, 1981. [DOI] [PubMed] [Google Scholar]
- Essick et al. 1988.Essick GK, Afferica T, Aldershof B, Nestor J, Kelly D, Whitsel B. Human perioral directional sensitivity. Exp Neurol 100: 506–523, 1988. [DOI] [PubMed] [Google Scholar]
- Essick et al. 1991.Essick GK, Bredehoeft KR, McLaughlin DF, Szaniszlo JA. Directional sensitivity along the upper limb in humans. Somatosens Mot Res 8: 13–22, 1991. [DOI] [PubMed] [Google Scholar]
- Gybels and Van Hees 1972.Gybels J, Van Hees J. Unit activity from mechanoreceptors in human peripheral nerve during intensity discrimination of touch. In: Neurophysiology Studied in Man, edited by Somjen GG. London: Excerpta Medica, 1972, p. 198–206.
- Hirai and Jones 1989.Hirai T, Jones EG. A new parcellation of the human thalamus on the basis of histochemical staining. Brain Res Rev 14: 1–34, 1989. [DOI] [PubMed] [Google Scholar]
- Johansson et al. 1982.Johansson RS, Landstrom U, Lundstrom R. Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to sinusoidal skin displacements. Brain Res 244: 17–25, 1982. [DOI] [PubMed] [Google Scholar]
- Johansson and Vallbo 1979.Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol 286: 283–300, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson and Vallbo 1983.Johansson RS, Vallbo AB. Tactile sensory coding in the glabrous skin of the human hand. Trends Neurosci 6: 27–32, 1983. [Google Scholar]
- Johnson 2001.Johnson KO The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol 11: 455–461, 2001. [DOI] [PubMed] [Google Scholar]
- Johnson et al. 2000.Johnson KO, Yoshioka T, Vega-Bermudez F. Tactile functions of mechanoreceptive afferents innervating the hand. J Clin Neurophysiol 17: 539–558, 2000. [DOI] [PubMed] [Google Scholar]
- Jones and Friedman 1982.Jones EG, Friedman DP. Projection pattern of functional components of thalamic ventrobasal complex on monkey somatosensory cortex. J Neurophysiol 48: 521–544, 1982. [DOI] [PubMed] [Google Scholar]
- Jones et al. 1982.Jones EG, Friedman DP, Hendry SH. Thalamic basis of place- and modality-specific columns in monkey somatosensory cortex: a correlative anatomical and physiological study. J Neurophysiol 48: 545–568, 1982. [DOI] [PubMed] [Google Scholar]
- Kaas 1991.Kaas JH Plasticity of sensory and motor maps in adult mammals. Annu Rev Neurosci 14: 137–167, 1991. [DOI] [PubMed] [Google Scholar]
- Koller et al. 2001.Koller WC, Lyons KE, Wilkinson SB, Troster AI, Pahwa R. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord 16: 464–468, 2001. [DOI] [PubMed] [Google Scholar]
- Lee et al. 2005.Lee JI, Ohara S, Dougherty PM, Lenz FA. Pain and temperature encoding in the human thalamic somatic sensory nucleus (ventral caudal): inhibition-related bursting evoked by somatic stimuli. J Neurophysiol 94: 1676–1687, 2005. [DOI] [PubMed] [Google Scholar]
- Lenz et al. 1988.Lenz FA, Dostrovsky JO, Tasker RR, Yamashiro K, Kwan HC, Murphy JT. Single-unit analysis of the human ventral thalamic nuclear group: somatosensory responses. J Neurophysiol 59: 299–316, 1988. [DOI] [PubMed] [Google Scholar]
- Lenz et al. 1998.Lenz FA, Garonzik IM, Zirh TA, Dougherty PM. Neuronal activity in the region of the thalamic principal sensory nucleus (ventralis caudalis) in patients with pain following amputations. Neuroscience 86: 1065–1081, 1998. [DOI] [PubMed] [Google Scholar]
- Mountcastle et al. 1990.Mountcastle VB, Steinmetz MA, Romo R. Frequency discrimination in the sense of flutter: psychophysical measurements correlated with postcentral events in behaving monkeys. J Neurosci 10: 3032–3044, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle et al. 1969.Mountcastle VB, Talbot WH, Sakata H, Hyvarinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol 32: 452–484, 1969. [DOI] [PubMed] [Google Scholar]
- Nordin and Hagbarth 1989.Nordin M, Hagbarth KE. Mechanoreceptive units in the human infra-orbital nerve. Acta Physiol Scand 135: 149–161, 1989. [DOI] [PubMed] [Google Scholar]
- Ohara and Lenz 2003.Ohara S, Lenz FA. Medial lateral extent of thermal and pain sensations evoked by microstimulation in somatic sensory nuclei of human thalamus. J Neurophysiol 90: 2367–2377, 2003. [DOI] [PubMed] [Google Scholar]
- Ohara et al. 2004.Ohara S, Weiss N, Lenz FA. Microstimulation in the region of the human thalamic principal somatic sensory nucleus evokes sensations like those of mechanical stimulation and movement. J Neurophysiol 91: 736–745, 2004. [DOI] [PubMed] [Google Scholar]
- Parent 1996.Parent A Carpenter's Human Neuroanatomy. Media, PA: William and Wilkins, 1996.
- Patel et al. 2006.Patel S, Ohara S, Dougherty PM, Gracely RH, Lenz FA. Psychophysical elements of place and modality specificity in the thalamic somatic sensory nucleus (ventral caudal, vc) of awake humans. J Neurophysiol 95: 646–659, 2006. [DOI] [PubMed] [Google Scholar]
- Phillips and Johnson 1981.Phillips JR, Johnson KO. Tactile spatial resolution. II. Neural representation of bars, edges, and gratings in monkey primary afferents. J Neurophysiol 46: 1192–1203, 1981. [DOI] [PubMed] [Google Scholar]
- Pubols 1982.Pubols BH Factors affecting cutaneous mechanoreceptor response. II. Changes in mechanical properties of skin with repeated stimulation. J Neurophysiol 47: 530–542, 1982. [DOI] [PubMed] [Google Scholar]
- Pubols and LeRoy 1977.Pubols LM, LeRoy RF. Orientation detectors in the primary somatosensory neocortex of the raccoon. Brain Res 129: 61–74, 1977. [DOI] [PubMed] [Google Scholar]
- Pubols and Pubols 1973.Pubols LM, Pubols BH Jr. Modality composition and functional characteristics of dorsal column mechanoreceptive afferent fibers innervating the raccoon's forepaw. J Neurophysiol 36: 1023–1037, 1973. [DOI] [PubMed] [Google Scholar]
- Rasmusson 1996.Rasmusson DD Changes in the response properties of neurons in the ventroposterior lateral thalamic nucleus of the raccoon after peripheral deafferentation. J Neurophysiol 75: 2441–2450, 1996. [DOI] [PubMed] [Google Scholar]
- Schaltenbrand and Bailey 1959.Schaltenbrand G, Bailey P. Introduction to stereotaxis with an atlas of the human brain. Stuttgart, Germany: Thieme, 1959.
- Sinclair et al. 1991.Sinclair RJ, Sathian K, Burton H. Neuronal responses in ventroposterolateral nucleus of thalamus in monkeys (Macaca mulatta) during active touch of gratings. Somatosens Mot Res 8: 293–300, 1991. [DOI] [PubMed] [Google Scholar]
- Smits et al. 1991.Smits E, Gordon DC, Witte S, Rasmusson DD, Zarzecki P. Synaptic potentials evoked by convergent somatosensory and corticocortical inputs in raccoon somatosensory cortex: substrates for plasticity. J Neurophysiol 66: 688–695, 1991. [DOI] [PubMed] [Google Scholar]
- Torebjork et al. 1987.Torebjork E, Vallbo AB, Ochoa J. Intraneural microstimulation in man: its relation to specificity of tactile sensations. Brain 110: 1509–1529, 1987. [DOI] [PubMed] [Google Scholar]
- Vallbo and Johansson 1984.Vallbo AB, Johansson RS. Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum Neurobiol 3: 3–14, 1984. [PubMed] [Google Scholar]
- Warren et al. 1986.Warren S, Kelahan AM, Pubols BH Jr. The somatosensory thalamus of the raccoon: properties of single neurons responsive to light mechanical stimulation in the forepaw. J Neurosci 6: 308–317, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. 2001a.Zhang HQ, Murray GM, Coleman GT, Turman AB, Zhang SP, Rowe MJ. Functional characteristics of the parallel SI- and SII-projecting neurons of the thalamic ventral posterior nucleus in the marmoset. J Neurophysiol 85: 1805–1822, 2001a. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 1996.Zhang HQ, Murray GM, Turman AB, Mackie PD, Coleman GT, Rowe MJ. Parallel processing in cerebral cortex of the marmoset monkey: effect of reversible SI inactivation on tactile responses in SII. J Neurophysiol 76: 3633–3655, 1996. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 2001b.Zhang HQ, Zachariah MK, Coleman GT, Rowe MJ. Hierarchical equivalence of somatosensory areas I and II for tactile processing in the cerebral cortex of the marmoset monkey. J Neurophysiol 85: 1823–1835, 2001b. [DOI] [PubMed] [Google Scholar]