Abstract
Noradrenaline is released throughout the forebrain from locus coeruleus (LC) projections in close temporal proximity to emotional and goal-directed events. To examine interactive influences of these processes on LC neuronal activity, we used a task where Pavlovian and operant processes vary and can be easily identified. We recorded 69 single LC neurons from two monkeys performing a task where cues indicate the progression through schedules of one, two, or three operant trials. Pavlovian responses and phasic LC activations occur following the appearance of conditioned visual cues (54/69 neurons), especially those at the beginning of new schedules, whether the current trial will be rewarded (single trial schedule) or not (2 or 3 trial schedules), and after visual imperative signals eliciting the operant response (64/69 neurons), whether the current trial will be rewarded or not. The modulation of LC responses seems to be relatively independent of attention or motivation, because the responses do not covary with operant performance in the task. The magnitude of LC responses across the schedules varied in close relation to the intensity of Pavlovian behavior but these responses were also modulated by operant processes. Our conclusion is that LC activation occurs when task-relevant stimuli evoke a conditioned instinctive (Pavlovian) response, with the strength of the activation also being modulated by goal-directed processes. Thus locus coeruleus neurons broadcast information about stimulus-elicited primitive and goal-directed behaviors to forebrain structures important for executive functions and emotions.
INTRODUCTION
The noradrenergic system seems to be strongly related to normal emotional and cognitive functions including learning and memory, attention, and decision making, all functions that are critical to surviving in a rapidly changing environment (Arnsten 2000; Aston-Jones and Cohen 2005; Bouret and Sara 2005; McGaughy et al. 2008; Sara 1985, 2000, 2008; Sterpenich et al. 2006; Yu and Dayan 2005). Noradrenergic innervation in the forebrain arises almost entirely from the locus coeruleus (LC).
LC neurons are transiently activated when salient stimuli appear. The strength of activation is correlated with the strength of autonomic, orienting, or approach behaviors occurring in response to the stimuli (Abercrombie and Jacobs 1987; Aston-Jones and Bloom 1981; Grant et al. 1988; Vankov et al. 1995). These spontaneous responses to salient stimuli are part of the animal's innate behavioral repertoire and can be regarded as instinctive.
LC neurons are also activated in cognitive tasks on the appearance of task-relevant stimuli (Aston-Jones et al. 1994; Sara and Segal 1991). It has been suggested that the activations to both salient stimuli that induce orienting and to relevant stimuli in cognitive tasks might be related to the need to alter (increase or decrease) or shift attention (Aston-Jones et al. 1994; Bouret and Sara 2005). Aston-Jones and colleagues also suggested a role in decision-making, when LC activation precedes an operant response (Clayton et al. 2004; Rajkowski et al. 2004). However, it is not clear whether LC activation is related either to goal-directed processes, such as selective attention or decision making in cognitive tasks, or to more general underlying instinctive processes, such as those known to trigger LC activation outside of these tasks, or both.
To investigate how the joint influences of both goal-directed and instinctive behaviors affect LC activity, we recorded single unit activity from the LC in monkeys performing a visually cued reward schedule task in which goal-directed and instinctive behaviors were operationally defined by operant and Pavlovian responses, respectively. We measured the error rates and reaction times of the operant responses (bar release), which, as expected for a goal-directed response, varied as a function of task conditions and expected reward. We also measured a Pavlovian response, lipping of the reward spout, that spontaneously occurs when a fluid reward is delivered and also occurred as a conditioned response to salient stimuli such as informative visual cues and imperative signals (Go signal) in our experiments. The Pavlovian behavior in this task was strongest in first trials, whereas the best operant performance occurred in rewarded trials. This dissociation provided a means to compare LC responses with these two different types of behavioral response. We found that LC neuronal responses occurred in close proximity to stimuli eliciting lipping, and the modulation of the neuronal responses was close to, but not entirely overlapping, with the amount of the lipping.
METHODS
Animals
Two male rhesus monkeys, monkey D (9.5 kg) and monkey K (4.5 kg), were used. The experimental procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the NIMH Animal Care and Use Committee.
Behavior
Each monkey squatted in a primate chair positioned in front of a monitor on which visual stimuli were displayed. A touch sensitive bar was mounted on the chair at the level of the monkey's hands. Liquid rewards were delivered from a tube positioned between the monkey's lips, with care to keep the tube away from the teeth. With this placement of the reward tube, the monkeys displayed very little protrusion of the tongue. The tube was equipped with a force transducer to monitor the movement of the lips (referred to as lipping). Before each experiment, the amplitude of the signal evoked by delivering of a drop of water through the spout was checked to ensure that it accurately matched the observed lipping response.
OPERANT TASK.
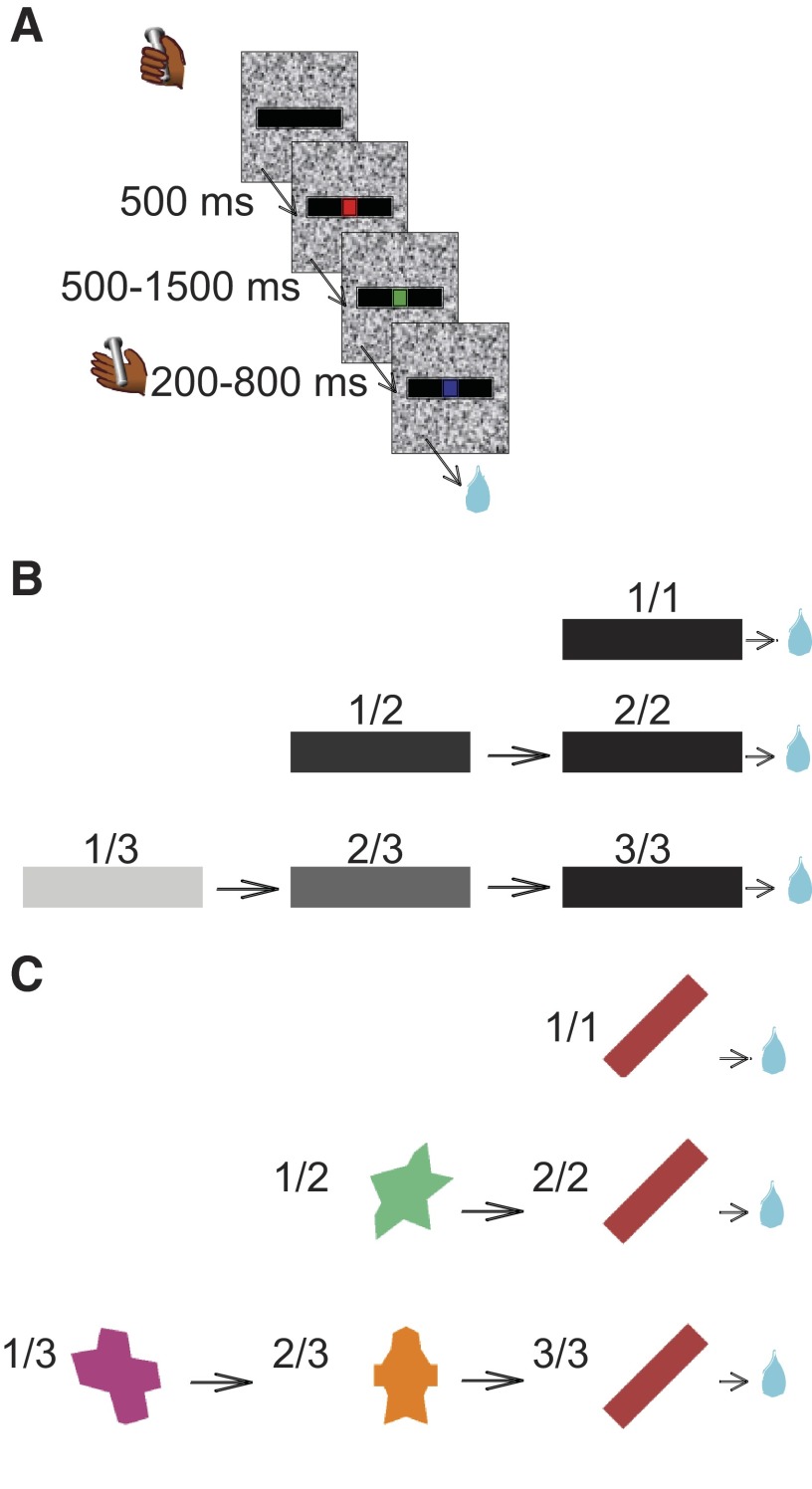
Each monkey was initially trained to perform a sequential color discrimination task, in which it was rewarded for detecting when a target, consisting of a small dot, changed from red to green (Fig. 1 A). Each trial began when the monkey touched the touch bar. A visual cue (black bar) appeared, followed 500 ms later by a red target (wait signal) in the center of the cue. After a random interval of 500–1,500 ms, the target turned green (go signal). If the monkey released the touch bar 200–800 ms after the green target appeared, the target turned blue (OK signal), and a drop of water was delivered 250–350 ms later as a reward. The temporal uncertainty in the duration of the red signal was included so that the monkeys had to pay attention to the red–green transition to obtain a reward. If the monkey released the bar before the go signal appeared, or after the go signal disappeared, an error was registered. Each monkey was first trained to achieve 80% correct performance on this single trial task (∼3 wk).
FIG. 1.
Behavioral tasks. A: sequential color-discrimination trial. A trial was initiated when the monkey touched a bar. A cue appeared (black bar). Colored dots, red (Wait), green (Go), and blue (OK, after correct bar release), appeared in sequence. B: trial sequences in the reward schedule task. The rectangular cue's brightness changed with the schedule state, in proportion to the schedule fraction. A black cue was always used when the trial was to be rewarded. The brightness of the cue changed at the beginning of each trial. To move on to the next schedule state, the monkey had to complete the current trial. After an incorrect trial, the same cue was repeated until the trial was performed correctly. C: Pavlovian schedule task and cues. In the Pavlovian schedule task, cues also indicated how many trials had to be completed before obtaining the reward. In contrast to the operant task, there was no color discrimination or operant response; the blue dot appeared 2.5 s after cue onset.
The visually cued reward schedule task was started. In this task, the monkey was required to perform randomly chosen schedules of one, two, or three color discrimination trials to earn a reward (Fig. 1B). The schedule states will be referred as i/j, with i being the current trial and j the current schedule. A horizontal bar was used as a cue. The task could be run in two conditions: valid and random cue. In the valid cue condition, cues indicate the progression through the current reward schedule. Cues changed brightness as the schedule progressed, becoming darker as the rewarded trial approached. Thus each cue appearing on the first trial of a schedule has a different brightness, and this set will be referred to as first cues; the same cue (a black bar) appears in all last trials (the rewarded trials at the end of each schedule), and this set will be referred to as rewarded cues, even though its members are physically identical.
In the random cue condition, the cues had no systematic relation to progression through reward schedules. The same cues as in the valid condition were used, but the one presented in each trial was chosen randomly. The animal still had to perform one, two, or three trials to get a reward, but the cue no longer indicated which trial or schedule was currently being presented. In the random cue condition, the number of trials that have passed since the last reward (the postreward trial number) could be counted.
The valid and random cue conditions were run in blocks with ≥120 trials each. Switching between conditions occurred abruptly without explicit signaling. In both conditions, reaction times and error rates were measured and taken as indices of operant motivation. No explicit punishment was given for an error in either condition, but the monkey had to perform a correct trial to move on in the schedule or receive the reward. That is, the monkey had to repeat the same trial in the schedule until the trial was completed correctly.
PAVLOVIAN TASK.
A Pavlovian version of the reward schedule task was introduced several months after the beginning of the recording experiments. First, the monkeys were trained in a simple classical conditioning task using two stimuli that were different from the usual bars (an orange star and a blue circle). These were presented in random order, with an intertrial interval of ∼10 s. Each stimulus was presented for 2.5 s and the orange star (CS+) was immediately followed by the delivery of a rewarding drop of water. Nothing happened at the end of the trials with the blue circle (CS−). The touch bar was removed to habituate the animals to the absence of operant contingency.
Once each monkey had displayed a reliable differential lipping pattern by responding strongly and specifically to the CS+, the Pavlovian reward schedule task was introduced (monkey K: 4 sessions; monkey D: 10 sessions). The Pavlovian reward schedule task differs from the operant reward schedule task by the absence of red/green fixation points, the lack of an operant contingency, and different visual cues. Cues in the Pavlovian task were geometrical shapes of various colors, without any obvious relationship between physical attributes (shape and color) and progression through the schedules (Fig. 1C). The temporal parameters were chosen to approximate those of the operant version. Each trial started with the presentation of the cue, followed after 2 s by the appearance of the blue fixation spot to indicate imminent trial completion. No action was required. The end of the trial was signaled by the disappearance of the cue and the delivery of the reward in the last trials of a schedule. During a typical recording session, blocks of operant reward schedules were interleaved with Pavlovian schedules. Neurons were usually recorded in the operant version first.
Electrophysiology
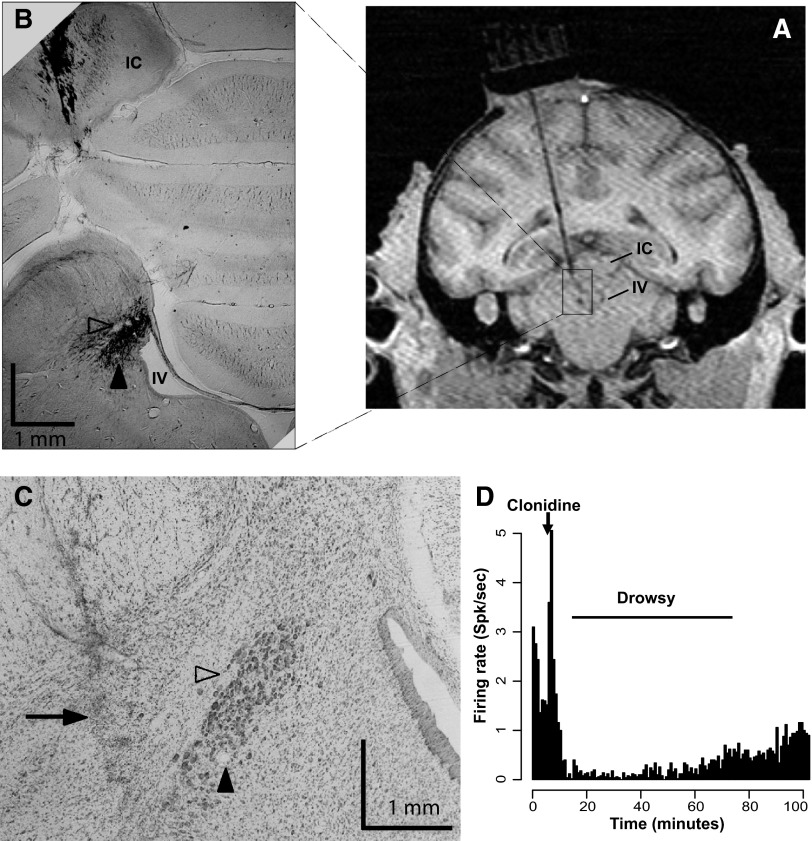
After initial behavioral training, an MR image at 1.5 T was obtained to determine the placement of the recording well. Although the LC itself was not visible on the MR image, its location could be established using landmarks, especially the anterior part of the cerebellum, the inferior colliculus, and the fourth ventricle (Fig. 2). A sterile surgical procedure was carried out under general isoflurane anesthesia in a fully equipped and staffed surgical suite to place the recording well and head fixation post. The well was positioned at the level of the interaural line, with an angle of ∼10° in the coronal plane (Fig. 2A).
FIG. 2.
Recording localization and pharmacological characterization. A: MR image (1.5 T) of monkey D with an electrode at 1 site where locus coeruleus (LC) neurons were identified by physiological criteria. Structures are labeled on the side contralateral to the recordings to avoid obscuring the electrode track. The electrode goes through the inferior colliculus (IC) and terminates in the region known to contain the LC, lateral from the 4th ventricule (IV). B: brain section from the same monkey, stained for tyrosine hydroxylase (1.25×). The LC appears in black laterally from the 4th ventricle (labeled IV). Tracks directed toward the LC are visible in the IC. Arowheads point to electrolytically made microlesions in the LC. C: brain section stained with thionine (5×), 3 sections anterior form the one shown in B. Arrowheads indicate the microlesions shown in B. An electrode track (arrow) can be seen 1 mm lateral from the microlesions. D: pharmacological test of a presumptive LC neuron. Clonidine (20 μg/kg, ip) was injected at the time represented by the labeled arrow. Following the brief injection-induced activation of the cell, there was a long-lasting inhibition that disappears over 10s of minutes.
Electrophysiological recordings were made with tungsten microelectrodes (UEWLEHSM3PNM, FHC, Bowdoin, ME). The electrode was positioned using a stereotaxic plastic insert (Crist Instruments) with holes 1 mm apart in a rectangular grid. The electrode was inserted through a guide tube that extended ventrally to the dorsal part of the inferior colliculus. The position of the LC was mapped using previously established electrophysiological criteria (Bouret and Sara 2004; Grant et al. 1988): low rate of spontaneous activity (<4 Hz), broad waveforms (>0.6 ms for the initial peak), and a characteristic burst-pause response to brief auditory or tactile stimuli (e.g., tapping on the chamber wall). In addition, if during the session the monkeys became drowsy, the firing rate of the neurons decreased sharply.
After the LC had been located, MR scans were obtained with the electrode at one of the LC recording sites; the position of the recording sites where LC neurons were identified was consistent with the expected position of the LC based on a stereotaxic atlas (Paxinos et al. 2000) (Fig. 2, A–C). The α-2 receptor antagonist clonidine has been shown to reversibly inhibit the spontaneous activity of noradrenergic neurons (Grant et al. 1988). Therefore as a last control, several cells were tested by giving the monkey a systemic dose of clonidine. Neuronal activity was monitored for a few minutes, and clonidine (20 μg/kg, im) was injected subcutaneously. All neurons tentatively identified as LC neurons based on the previously defined criteria displayed a prolonged decrease in activity after clonidine injection lasting 10s of minutes (Fig. 2D). All of the recorded neurons were found in a region covered by two adjacent grid holes and at depths that varied by no more than 1 mm.
Histology
Electrolytic microlesions (20 μA, 30 s) were made in the brain of one monkey (monkey K). Five days later, the monkey was deeply anesthetized with pentobarbital sodium and perfused through the heart with saline and 10% formalin fixative. The brain was sectioned into 50-μm slices in the coronal plane and stained with thionine (1/5 section). Sections around the LC were treated for tyrosine hydroxilaze (TH) immunochemistry (Simmons et al. 2008). The sites of guide tube and electrode tracks and microlesions were identified on the sections under microscopic examination.
Data analysis
LIPPING BEHAVIOR.
At the beginning of each recording session, the sensitivity of the strain gauge on the reward spout was adjusted so that the signal evoked by lipping to the reward had a peak value of ∼500 mV. The lipping signal was monitored continuously and digitized at 1 kHz. Only sessions for which data were collected both in valid cue and random cue conditions were used (n = 16 for monkey D, n = 20 for monkey K).
For each trial, the lipping latency of cue and go responses was estimated by identifying the first of three successive windows in which the signal displayed a consistent increase in voltage of ≥100 mV. In addition, the power of the lipping signal 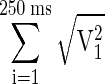 , where V is the voltage) was calculated for each trial in four windows of 250 ms: immediately before cue onset, after cue onset, immediately before bar release, and after bar release. For each monkey, the position of the cue elicited lipping window was adjusted to start at the shortest average lipping latency of the six schedule states (600 ms for monkey D, 250 ms for monkey K). These measures were converted to a standard scale, the z-score, by subtracting the mean of the data across all trials and conditions from each measurement and dividing by the SD in both valid and random cue conditions. Data from each monkey were analyzed separately using ANOVAs and t-tests on these standardized values. The same procedure was used to analyze lipping responses to cues in the Pavlovian reward schedule task, in which mean lipping latencies were similar to those in the operant task (monkey D: 676 ms; monkey K: 277 ms).
, where V is the voltage) was calculated for each trial in four windows of 250 ms: immediately before cue onset, after cue onset, immediately before bar release, and after bar release. For each monkey, the position of the cue elicited lipping window was adjusted to start at the shortest average lipping latency of the six schedule states (600 ms for monkey D, 250 ms for monkey K). These measures were converted to a standard scale, the z-score, by subtracting the mean of the data across all trials and conditions from each measurement and dividing by the SD in both valid and random cue conditions. Data from each monkey were analyzed separately using ANOVAs and t-tests on these standardized values. The same procedure was used to analyze lipping responses to cues in the Pavlovian reward schedule task, in which mean lipping latencies were similar to those in the operant task (monkey D: 676 ms; monkey K: 277 ms).
SINGLE UNIT ACTIVITY.
All data analyses were performed in the R statistical computing environment (Team RDC 2004). Following initial inspection of the data, we focused on two periods where phasic LC activations occurred consistently: at cue onset and between the go signal and the operant response.
The latencies of phasic responses were determined by using a series of χ2 tests (Ravel and Richmond 2006). Our recording data were sampled at 1-ms time resolution, which establishes a natural bin size. All of the spikes were counted across trials in two chosen windows: 500 ms for the background and 100 ms for the test windows. For cue responses, the background window spanned between 500 and 0 ms before cue onset. The 100-ms test window was moved in 15-ms increments, from 0 to 450 ms after the cue appearance. For each 100-ms test window, a χ2 test was used to determine whether the proportions of filled to empty 1-ms bins in the 100-ms test interval was significantly different from the proportion in the 500-ms background window. The response latency was taken to be the middle of the first of four consecutive 100-ms test intervals showing a significant difference (P < 0.05) in the spike count between the test and background window. The time of response termination was defined as the middle of the last window showing a significant difference. Response duration was defined as the difference between response latency and response termination. For go responses, a 500-ms period before the go signal was used as a background window and the beginning of the 100-ms test window was moved in 15-ms steps starting 400 before the bar release (operant response) and ending at bar release. The procedure worked well for all of the tested neurons, providing latencies that matched those we would have chosen by inspection. A neuron was classified as responsive to an event when a significant response latency could be detected in at least one of the six states.
For population analysis, spike counts in the cue response window (0–500 ms after cue onset) and in the go response window (250-0 ms before bar release) were converted to z-scores for each neuron as described above for the lipping. Standardized data from all neurons were pooled into valid and random cue conditions, respectively.
RESULTS
Behavior
We made measurements related to two aspects of the behavior in the reward schedule task: bar release and lip movement (lipping). Bar release is a classic operant response, for which we measured the error rates and reaction times. For lipping, we measured the movement of the reward spout, which was placed between the lips. We show below that lipping meets criteria for a Pavlovian response.
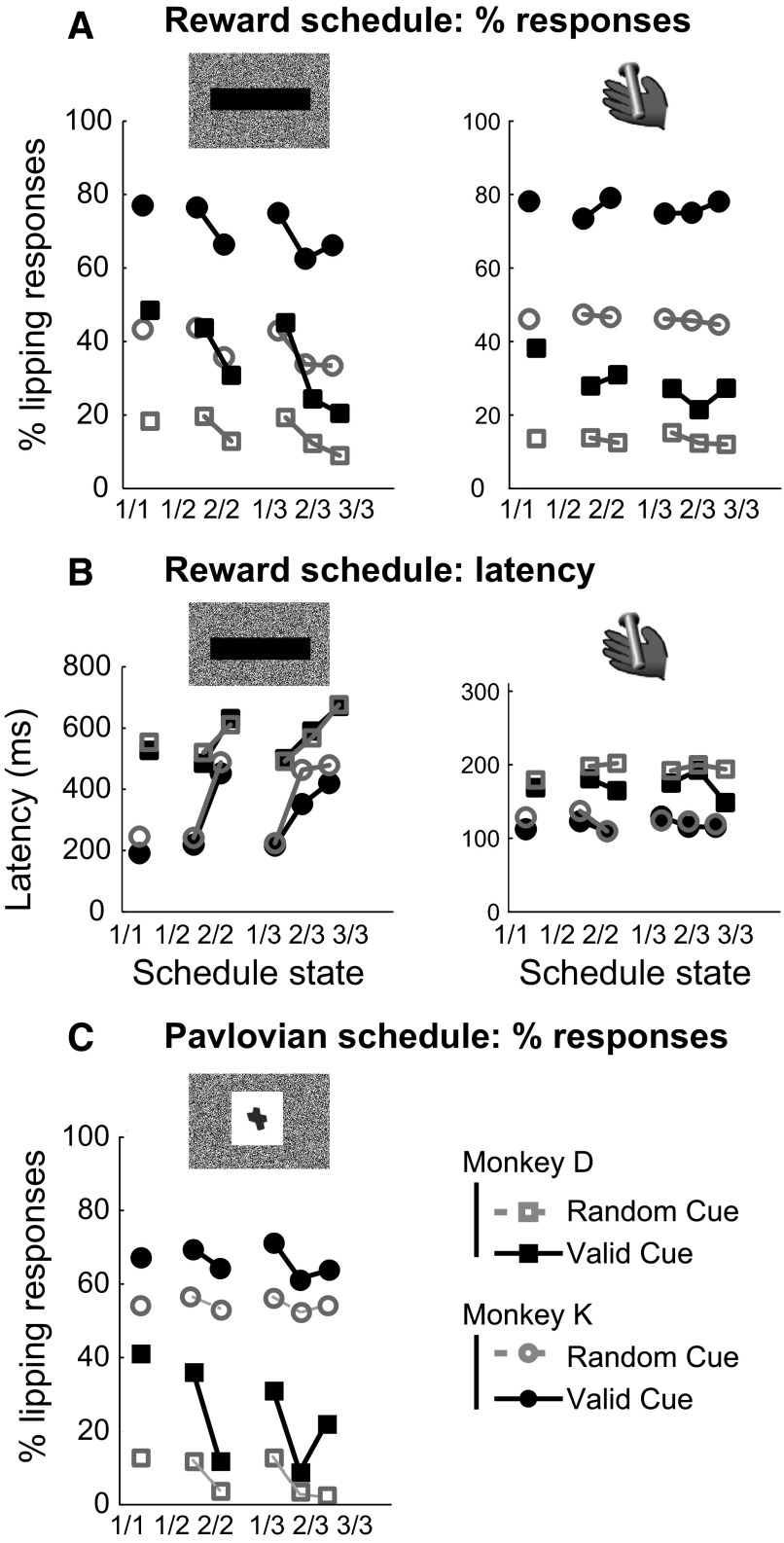
In the valid cue condition, the error rates varied as a function of the schedules (Fig. 3 A). When the cues indicated progression through the schedules, error rates were proportional to the number of trials remaining before the reward: they were small in all rewarded (last) trials of the schedules and increased with the number of trials remaining before the reward (Fig. 3A). The reaction times showed similar effects (Fig. 3B). These findings are consistent with previous studies indicating that the monkeys use the cues to adjust their motivation in relation to the number of trials remaining before reward in the valid cue condition.
FIG. 3.
Operant behavior in the reward schedule task. A: error rates for the 6 schedule states (fractions on x-axis) in valid cue (black solid line, filled symbols) and random cue (gray broken line, open symbols) conditions. The data were averaged over all the sessions for monkey K (circles) and monkey D (squares). The error rates decreased as the number of trials remaining before the reward decreased in the valid cue condition. In the random cue condition, the schedules were still in effect, but the cue displayed in any trial was chosen randomly. Both the factors of “schedule state” and “condition” were significant, and there was a significant interaction [2-way ANOVA, monkey D: F(5) = 5.1, P = 5 × 10−8, r2 = 12% and F(1) = 6.9, P = 0.009, r2 = 1.2% for schedule state and condition, respectively, and F(5,456) = 9.1, P = 3 × 10−8, r2 = 7.9%, for the interaction; monkey K: F(5) = 6.2, P = 1.4 × 10−5, r2 = 5.6% and F(1) = 12.8, P = 0.0004, r2 = 2.3%, F(5,486) = 5.1, P = 0.0001, r2 = 4.6%]. In the random cue condition, the error rates were indistinguishable across schedule states. In the valid cue condition, there were significantly fewer errors in rewarded trials than in unrewarded trials (Tukey test, P < 0.01). B: reaction times of the operant response in the 6 schedule states. In the valid cue condition, the reaction times get shorter as monkeys approach the reward. In the random cue condition, reaction times were indistinguishable across schedule states. There was a significant effect of schedule state, condition, and a significant interaction between the 2 factors [monkey D: F(5) = 14.5, P = 3 × 10−13, r2 = 12% and F(1) = 8, P = 0.005, r2 = 1.3% for schedule state and condition, respectively, and F(5,456) = 11.8, P = 1 × 10−10, r2 = 9.9%, for the interaction; monkey K: F(5) = 3, P = 0.01, r2 = 2.8% and F(1) = 14.8, P = 0.0001, r2 = 2.8%, F(5,486) = 2.5, P = 0.03, r2 = 2.3%].
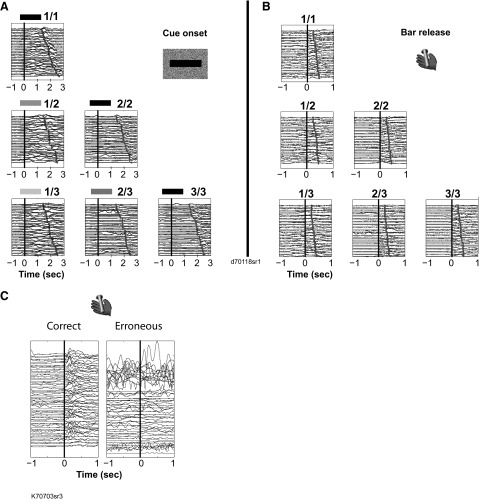
Lipping responses occurred mostly at two times: after the onset of a cue (Fig. 4 A) and during the bar release but before the trial outcome (Fig. 4B). There was no systematic lipping around erroneous bar releases or when monkeys released the bar between trials (Fig. 4C). The cue-related lipping was more frequent in the valid cue than the random cue condition (Fig. 5 A, left). It was significantly more frequent in first trials than nonfirst trials in both conditions (Fig. 5A, left). The same pattern was observed using latencies (Fig. 5B, left) and response magnitudes. One monkey (monkey D) was tested in a version of the task where the intertrial interval varied randomly between 1 and 8 s to investigate whether the intensity of the lipping in the first trials of schedules might have been related to the temporal proximity of the previous reward. Lipping was still stronger in first trials than in nonfirst trials [t(2,704) = 2.7, P = 0.007], and there was no correlation between the duration of the intertrial interval and the magnitude of the lipping response [t-test: t(2,726) = 1.4, r2 = 0.0007, P = 0.16]. Thus the strength of the lipping response to cues in first trials of each schedule seems related to the beginnings of schedules.
FIG. 4.
Example of lipping behavior in the reward schedule task. A: lipping signal around cue onset in the 6 schedule states, indicated by corresponding fraction and cue for a representative session in the valid cue condition. Each line is the signal (in mV, for 1 trial) from the strain gauge attached to the sipper tube positioned between the monkey's lips. On the y-axis, traces are offset by 250 mV for clarity of viewing. Traces are aligned on cue onset (black vertical line, t = 0) for each trial, sorted in increasing latencies between cue onset and onset of the blue fixation spot (gray dot). Time around cue onset is in seconds. The monkey showed a strong, reliable lipping response after each 1st cues of a schedule, irrespectively of cue brightness. B: lipping signal around bar release (t = 0, vertical line). Gray dots indicate trial outcome (cue off, with or without reward delivery). Other display conventions as in A. In 1st states (1/1, 1/2, and 1/3), the cue-elicited response is visible before bar release. The monkey displays a small but significant lipping response at the time of the bar release. This response is more pronounced in rewarded trials. Sustained (unconditioned) lipping occurs after reward delivery in 1/1, 2/2, and 3/3 states. C: lipping signal around correct (left) and erroneous (right) bar release (t = 0, vertical line). In this session, the monkey made numerous erroneous bar releases, which, in contrast to correct bar releases, are not associated with lipping responses.
FIG. 5.
Pavlovian responses in the reward schedule task. A: percentage of trials with lipping responses to cues (left) and bar release (right). The percentage of trials with lipping responses to cues is higher in first trials [in valid cue condition, monkey D: 46 vs. 25% χ2(1) = 179, P < 1 × 10−10; monkey K: 76 vs. 65%, χ2(1) = 70, P < 1 × 10−10; in random cue: monkey D: 19 vs. 11% χ2(1) = 87, P < 1 × 10−10; monkey K: 43 vs. 34%, χ2(1) = 57, P < 1 × 10−10]. At bar release, the proportion of lipping responses is significantly higher in rewarded than in nonrewarded trials [monkey D: 32 vs. 25%, χ2(1) = 22, P = 5 × 10−6; monkey K: 78 vs. 74%, χ2(1) = 12, P = 0.0006]. For both cue onset and bar release, more trials had lipping in the valid cue than in the random cue condition [cues: monkey D: 36 vs. 15%, χ2(1) = 621, P < 1 × 10−10; monkey K: 71 vs. 39%, χ2(1) = 1,123, P < 1 × 10−10; bar release: monkey D: 29 vs. 13%, χ2(1) = 418, P < 1 × 10−10; monkey K: 76 vs. 46%, χ2(1) = 1,054, P < 1 × 10−10]. B: latency of lipping responses. At cue onset, lipping latencies were shortest in 1st trials (504 and 208 ms for monkeys D and K, respectively) and longer in subsequent trials [628 and 408 ms, t(979) = 5, P = 8 †× 10−8 and t(2,359) = 17, P < 1 × 10−10 for monkeys D and K, respectively). Latencies of response to bar release were shorter (134 ± 3 and 120 ± 1 ms for monkeys D and K, respectively) and indistinguishable across schedule states. C: lipping responses to cues in the Pavlovian schedule task. As in the reward schedule task, more trials had lipping in the valid cue than in the random cue condition [monkey D: 22 vs. 7%, χ2(1) = 180, P < 1 × 10−10; monkey K: 65 vs. 53%, χ2(1) = 31, P = 2.4 × 10−8]. The percentage of trials with lipping responses to cues is higher in 1st trials [in valid cue condition: monkey D: 35 vs. 10%, χ2(1) = 118, P < 1 × 10−10; monkey K: 69 vs. 62%, χ2(1) = 8, P = 0.005].
The lipping behavior around bar release was different. In the valid cue condition, the lipping occurred more frequently in rewarded than in unrewarded trials (Fig. 5A, right). In the random cue condition, the lipping around bar release was significantly weaker and showed less contrast across schedule states than in the valid cue condition. Lipping responses at bar release had shorter latencies than those after the cue (Fig. 5B).
To investigate whether cue-related lipping depended on the requirement that the animals were planning an action, i.e., going to make an operant responses, here a bar release, the monkeys were exposed to a Pavlovian version of the reward schedule task. In this version, in which there was no operant requirement, a different set of cues was used to indicate progression through schedules of one, two, or three trials (Fig. 1C). As in the operant version, the lipping responses were more frequent in valid than in random cue conditions. In both conditions, lipping was more frequent in first trials (Fig. 5C).
In summary, for the operant behavior, error rates and reaction times were affected by cues in the valid cue condition, where both were smallest in rewarded compared with unrewarded trials. The lipping responses occurred after the cues and at time of the operant bar release. They were stronger in valid cue than in random cue condition. Cue-evoked lipping was stronger in first trials of a schedule in both conditions. Bar release–evoked lipping was stronger in rewarded trials, only in the valid cue condition.
LC activity
Eighty-two single units were recorded from the LC (52 from monkey D, 30 from monkey K), of which 78 (51 from monkey D, 27 from monkey K) were recorded during the reward schedule task, 69 during the valid cue condition, and 48 during the random cue condition. Twelve neurons were recorded in both the Pavlovian and operant schedule tasks and four more in the Pavlovian task only. Only single unit recordings were considered for the analysis. These had a median number of 161 trials per condition (IQR = 121:192 trials). Overall, the mean firing rate of these neurons was 2.0 ± 0.1 spikes/s (averaged over the entire recording session).
LC responses: timing, intensity, and selectivity
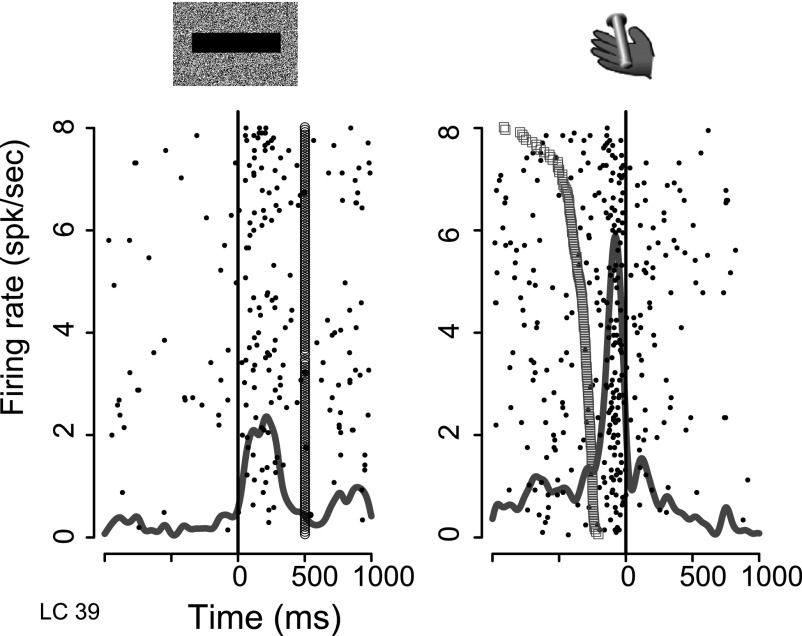
Phasic responses occurred around two events of the task: at cue onset and after the go signal, beginning shortly before the bar release (Fig. 6). These neurons did not respond before an erroneous bar release (before the go signal or after the maximum allowed reaction time) or before bar release between trials.
FIG. 6.
Activity of an LC unit. Raster and overlaid spike density displays of neural activity. Each row of dots shows spike times in 1 trial. The continuous gray line shows the spike density, representing the average firing per millisecond per trial over all trials. Left: aligned on cue onset (t = 0, black vertical line). Trials are displayed in chronological order (1st at top). The line of open circles at t = 500 ms indicates the time when the red fixation point appeared. Right: activity aligned on operant response (bar release). The open squares show the time when the green spot (go signal) appears. Trials are sorted by reaction time (longest on top).
The proportion of neurons responding and the response latencies were similar for the two monkeys, so the data were pooled. Neurons were first screened using a sliding window procedure to identify times at which firing probability increased compared with baseline in each of the six schedule states (response latency; see methods for further details). Neurons displaying a significant response in at least one of the six states were classified as responding to the event of interest. In the valid cue condition, 54 of 69 LC neurons (78%) responded to cues, with a median latency of 110 ms (IQR = 54:189 ms). Median response latencies were indistinguishable across schedule states or between first/nonfirst or rewarded/unrewarded trials (Kruskal-Wallis, P > 0.05). In the random cue condition, the proportion of neurons responding to cues was significantly smaller [21/48; 44%, χ2(1) = 13, P = 0.0003], and the median response latency was significantly longer (210 ms; IQR = 88:327 ms, Wilcoxon, P = 0.009).
The proportion of neurons activated just before bar release in valid and random cue conditions was large, 64/69 and 40/48, respectively. Both the number of neurons and the latencies (median = −140 vs. −125 ms) were indistinguishable [numbers of neurons, χ2(1) = 1.7, P = 0.2; latency: Wilcoxon, P = 0.3].
Response duration was estimated with the same procedure used to determine response latency (see methods). Duration of responses to cues (median = 105 ms, IQR = 75:180 ms) and responses before bar release (median = 105 ms, IQR = 75:151 ms) were indistinguishable (Wilcoxon, P = 0.7). There was no correlation between durations of cue and bar release responses [r2 = 0.01, F(1,37) = 1.5, P = 0.2].
Cue-related responses were significantly weaker than bar release responses (Fig. 7 A), and response magnitudes in the valid and random cue conditions were indistinguishable at both times. Response selectivity was identified using a six-level state factor in an ANOVA. Responses to cues were more selective than bar release responses, especially in the valid cue condition (Fig. 7B). Thus, although LC responses were stronger before bar release than after cue onset, cue responses were more strongly modulated across the schedule states than bar release responses.
FIG. 7.
Magnitude and schedule sensitivity of LC responses. A: mean response magnitude of LC neurons showing a significant response to cues (left) and bar release (right) in the reward sxchedule task. Error bars indicate SE. Response magnitude is the firing rate (in spikes/s) during response windows (500 ms after cue onset or 250 ms before bar release). Responses were stronger at bar release than at cue onset, with no difference between valid cue and random cue conditions [2-way ANOVA, effect of stimulus: F(1) = 23.3, P = 3.0 × 10−6, r2 = 12%; effect of condition: F(1) = 0.005, P = 0.09, r2 = 0.2%; interaction: F(1,175) = 0.02, P = 0.96, r2 = 0%]. B: the sensitivity to schedule states was quantified for each cell by measuring the variance accounted for by a 6-level schedule state factor on response magnitude using an ANOVA. Mean and SE across the population of responding neurons are shown. For both responses to cues (left) and bar release (right), responses are more selective to state in the valid cue than in the random cue condition. [2-way ANOVA, significant effect of stimulus: F(1) = 11.5, P = 0.008, r2 = 5.9%; significant effect of condition: F(1) = 7.6, P = 0.006, r2 = 3.9% and no significant interaction: F(1,175) = 0.4, P = 0.5, r2 = 0.2%].
Categories of LC responses
CUE RESPONSES.
There were 54 neurons that responded to one or more cues (based on the screening completed using response latency analysis). From inspection, it appeared that the cue-responding neurons fell into four categories: those where the responses 1) were indistinguishable across all schedule states, 2) distinguished between first and nonfirst trials in the schedule, 3) distinguished between rewarded and unrewarded trials, and 4) showed idiosyncratic response patterns across the six schedule states. To test whether the cue-elicited responses were selective across states, the responses for each neuron was tested with a six-level one-way ANOVA, where the six levels coded the six schedule states. Thirty-six of 54 (67%) neurons in the valid cue condition and 10/21 (48%) in the random cue condition showed significant cue selectivity (P < 0.05).
To evaluate whether this cue selectivity arose because there was first–nonfirst or reward–no-reward selectivity, the responses were subjected to two additional ANOVAs: a two-level ANOVA where the levels coded whether the trial was a first trial or not and a two-level ANOVA where the levels coded whether the trial was rewarded or not. If the first–nonfirst test was significant, the one-way, two-level ANOVA model was tested against the six-level ANOVA model (anova function in R). This latter procedure determines whether the extra degrees of freedom in the six-level ANOVA are justified. If the difference was not significant, the simpler model, i.e., the two-level ANOVA, was preferred and the neuron was classified as first–nonfirst.
This procedure identified 18 first–nonfirst neurons (50% of the sample) in the valid cue condition, of which 15 had larger responses in the first trials (an example, Fig. 8; population summary, Fig. 9 A). There were 10 first–nonfirst neurons in the random cue condition. The proportion of cue selective first–nonfirst neurons in the valid cue condition was not significantly different from in the random cue condition [10/21 vs. 18/36; χ2(1) = 1.6, P = 0.2]. Using a similar procedure, three neurons with reward selective cue responses were identified.
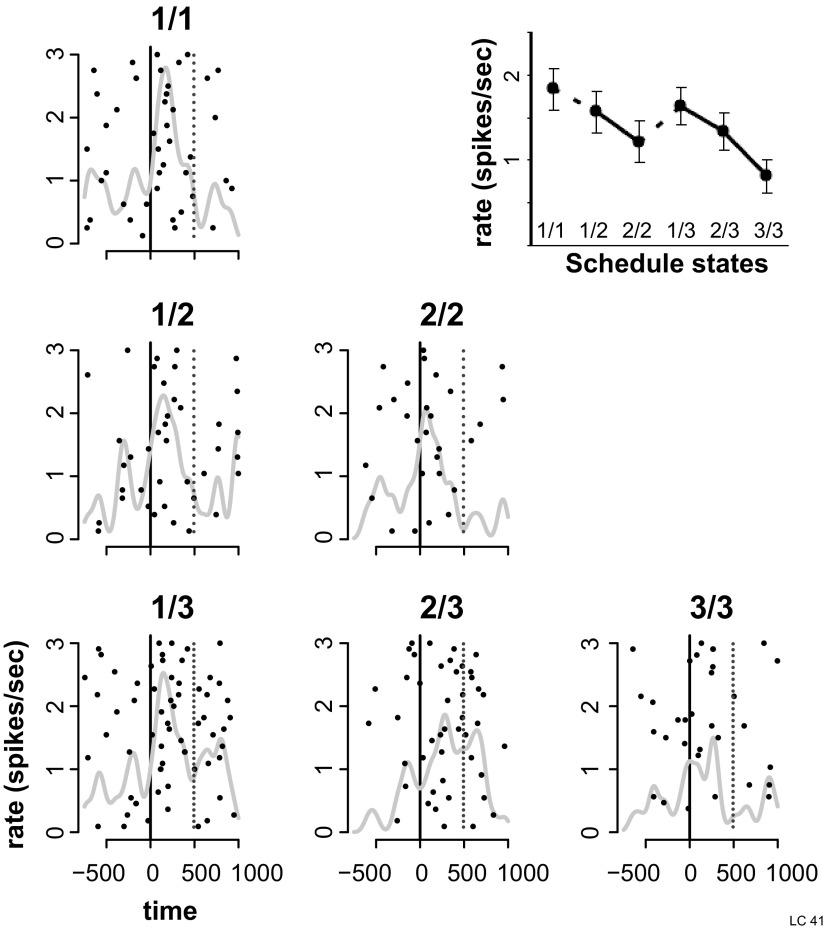
FIG. 8.
Responses to cues, example of cell showing a 1st–non-1st effect. Activity of an LC neuron around cue onset in the valid cue condition of the reward schedule task. Plotting conventions as in Fig. 6. Responses are shown in the 6 schedule states, indicated by fractions. Inset: mean and SE of responses across the 6 schedule states. The response was significantly stronger in trials indicating the beginning of a schedule (1/1, 1/2, and 1/3) than in subsequent trials. Such response patterns were classified as 1st–non-1st effect.
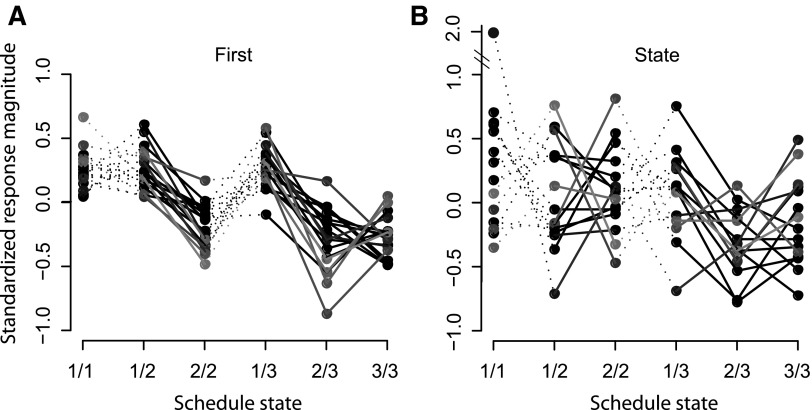
FIG. 9.
Average standardized responses of neurons showing 1st–non-1st and state responses to cues. Selective neurons were classified as 1st–non-1st, reward–no-reward, or state as a function of their response pattern across schedule states. A: mean standardized responses (z scores) across schedule states for the 18 neurons classified as 1st for their response to cues. Gray levels were attributed randomly to each neuron to facilitate viewing. Responses of the 3 cells for which response magnitudes were higher in non-1st than in 1st states were multiplied by −1 for graphical purposes. For neurons classified as 1st–non-1st, the amount of variance explained by the 6-level schedule state factor in a 1-way ANOVA was not significantly different form the variance explained by the 2-level (1st–non-1st) factor in another ANOVA. B: mean standardized responses of the 15 neurons classified as state for their response to cues. The responses show an idiosyncratic pattern, and the amount of variance explained by the 6-level ANOVA was significantly higher than the variance explained by either of the 1-level ANOVAs for 1st–non-1st and reward–no-reward effects.
For 15 neurons, the six-level ANOVA was preferred, i.e., it was significantly better than either two-level ANOVA, so the neurons were classified as state selective in that the cue elicited response patterns across schedule states were idiosyncratic (population summary, Fig. 9B; example, Fig. 10).
FIG. 10.
Responses to cues, example of cell showing a state effect. Activity of an LC neuron around cue onset in the valid cue condition of the reward schedule task. Responses are stronger in 1st trials but, in addition, the activation is significantly stronger for the cue indicating the 1 trial schedule (which is both 1st and rewarded). The response to the cue indicating the 2nd trial of 3 trials schedules, which is neither 1st nor rewarded, is the weakest. The 6-level state factor accounts for significantly more variance than either 1st or reward factors. This cell was classified as displaying a state effect.
Although response latencies (latency at which firing rate was significantly different from baseline) were indistinguishable across schedule states, we conducted an additional analysis to determine the time at which LC neurons started to discriminate among schedule states. Discrimination latencies (latency at which firing rate was significantly different across the six schedule states, median = 140 ms, IQR = 70:220 ms) were calculated for the 36 cue-selective neurons in the valid cue condition, and these were indistinguishable from response latencies (median = 118 ms, IQR = 61:185 ms). Thus the cue-related firing was modulated according to schedule state from the beginning of the response.
BAR RELEASE RESPONSES.
In the valid cue condition, 15/64 (23%) bar release–responding neurons discriminated across schedule states, with 8 of these being classified as reward selective (distinguishing between rewarded and unrewarded trials only). The responses were weaker in rewarded trials for five of eight neurons. The responses of six and two neurons were classified as first–nonfirst selective in the valid and random cue conditions, respectively, with five of six and one of two neurons showing stronger responses in first than in nonfirst trials, respectively. There was only one neuron in the valid cue condition classified as state selective.
POPULATION RESPONSES.
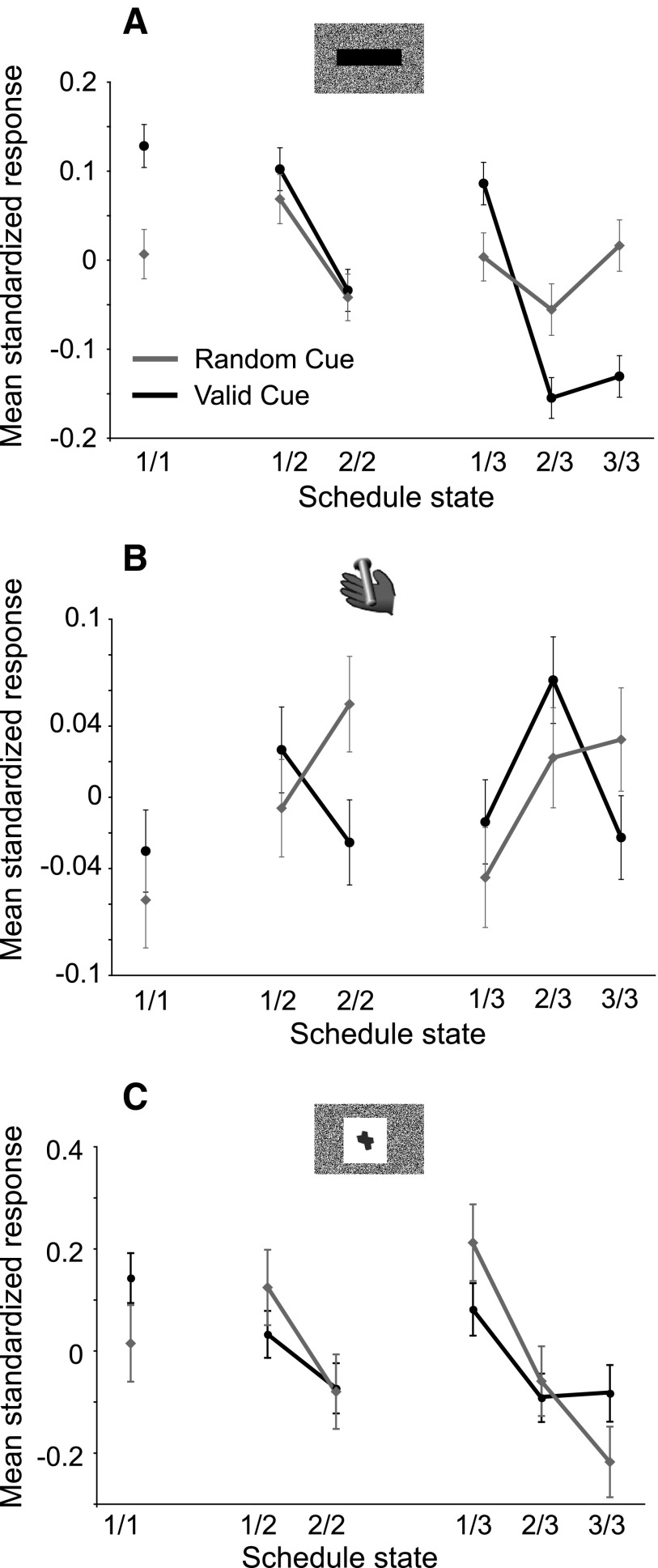
The numbers of neurons with different types of selectivity are summarized in Fig. 11. To study the responses of the whole population further, the responses of every neuron were converted to z-scores (see methods), and the data from all of the recorded neurons were pooled. These standardized responses were compared across the six schedule states, between first and nonfirst, and between rewarded and unrewarded states (Fig. 12). In the valid cue condition, the population of LC neurons showed a significant effect of first–nonfirst [1-way, 2-level ANOVA, F(1) = 120, P = <1 × 10−10, r2 = 1%] and state factors [1-way, 6-level ANOVA, F(5) = 27, P = <1 × 10−10 r2 = 1.3%] on responses to cues (Fig. 12A), with the six-level (state) model significantly different from the two-level (1st) ANOVA model (ANOVA, P < 0.001). A post hoc analysis performed on the six-level model showed that LC responses to cues are strongest and indistinguishable in the first states (1/1, 1/2, 1/3), weaker for 2/2 cues, and even weaker in 2/3 and 3/3 states (Tukey test, all P < 0.05; cf. Fig. 12A). This pattern in the population analysis was not seen in the cell-by-cell analysis.
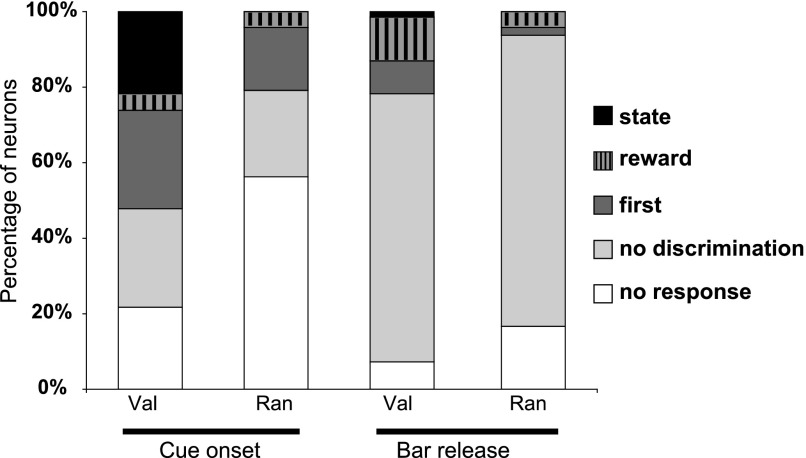
FIG. 11.
Categories of LC responses. Percentage of the different categories of LC neurons in valid (n = 69 neurons) and random cue conditions (n = 48 neurons) for responses to cues and before bar release. For each bar, the proportion of unresponsive neurons is shown in white. Responding neurons were broken down into nondiscriminative neurons (light gray) and neurons presenting a significant discrimination across the 6 schedule states (6-level ANOVA). For responses to cues, the proportion of responding neurons was significantly higher in valid cue than in random cue conditions. In the valid cue condition, most responding neurons discriminated across schedule states, with an equivalent proportion of neurons classified as 1st–non-1st and state. In the random cue condition, the proportion of selective neurons was smaller than in the valid cue condition, and virtually all of them were classified as 1st. Most LC neurons responded before the operant response, with no significant difference between the proportion of responding neurons in valid cue and random cue conditions [χ2(1) = 1.7, P = 0.2]. Compared with cue responses, only a small proportion of neurons responding before bar release discriminated across the 6 schedule states and about one half of the selective bar-release neurons showed a reward–no-reward effect in the valid cue condition.
FIG. 12.
Population responses of LC neurons. A: in the reward schedule task, at cue onset, the population response is stronger in 1st trials vs. subsequent trials. In the valid cue condition, the population response also shows a state effect. In the random cue condition, neurons only distinguish between 1st and non-1st cues (1st effect). B: at bar release, differences across schedule states were smaller. In the valid cue condition, the response magnitude of the population was significantly weaker in rewarded trials (1/1, 2/2, and 3/3) compared with nonrewarded trials (1/2, 2/3, and 3/3). In the random cue condition, responses were weaker in 1st compared with non-1st trials. C: population responses to cues in the Pavlovian schedule task. Although the variance in this task is larger than in the reward schedule task (fewer neurons were recorded), these cells also displayed significantly stronger responses to cues in 1st compared with non-1st cues. This 1st effect was significant in both valid and random cue conditions.
In the random cue condition, standardized LC responses to cues also had a significant effect of the factor first–nonfirst [F(1) = 5.8, P = 0.02, r2 = 0.1%] and of the factor state [F(5) = 2.6, P = 0.02, r2 = 0.1%], with no significant difference between the two-level and the six-level models (ANOVA, P > 0.05). In other words, in the random conditions, a first–nonfirst effect is sufficient to explain the variance in the cue responses, with responses in first trials significantly stronger than responses in nonfirst trials (Tukey test, P < 0.05).
The bar release population response displayed a different pattern (Fig. 12B). In the valid cue condition, there was a significant effect of reward–no-reward [F(1) = 7.2, P = 0.007, r2 = 0.1%] and state [F(5) = 2.6, P = 0.02, r2 = 0.1%], with no difference between the two-level (reward–no-reward) and the six-level (state) ANOVA model (ANOVA, P > 0.05). In this situation, LC responses are simply stronger in unrewarded than in rewarded trials (Tukey test, P < 0.05). In the random cue condition, bar release LC responses presented a significant effect of first–nonfirst [F(1) = 10, P = 0.001, r2 = 0.1%] and state [F(5) = 2.5 P = 0.03, r2 = 0.1%] factors, with no significant difference between the two models (ANOVA, P > 0.05). Thus in the random cue condition, LC neurons simply show stronger responses in nonfirst trials than in first trials (Tukey test, P < 0.05). Overall, what is shown is that there are weak, but significant, effects in the average taken across the whole population without regard to individual neuronal response selectivity.
Correlation between LC responses and lipping: trial by trial analysis
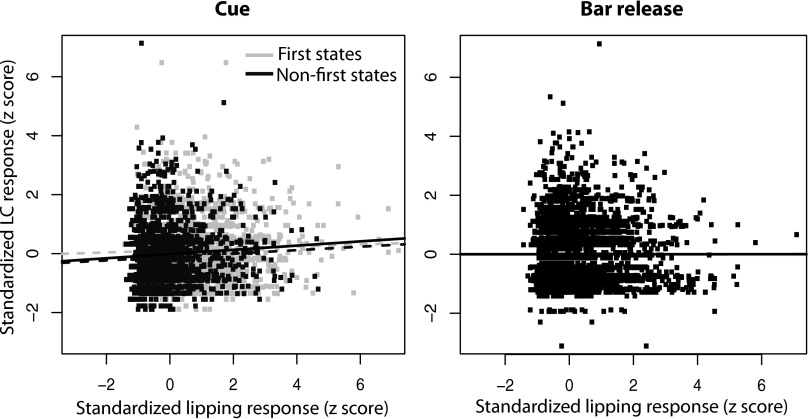
The variations in magnitude of LC responses to cues across schedule states present a similar pattern to that of the lipping behavior (stronger in 1st states). We compared LC responses and lipping on a trial-by-trial basis using linear regression for 28 neurons (18 from monkey D and 10 from monkey K) for which there were sufficient lipping data. The data for each neuron were standardized by converting them to z-scores for the neuronal and lipping data on a cell-by-cell basis (see methods). For cue-related responses (Fig. 13 A), the correlation between the magnitude of neuronal and lipping responses was significant, although very small. The correlation was still present even after trials were broken down into first and nonfirst (P < 0.005). At bar release (Fig. 13B), there was no significant correlation between LC and lipping responses. This provides additional mild additional evidence that LC responses to cues are related to intensity of Pavlovian processes.
FIG. 13.
Correlation between LC and lipping responses. The magnitude of lipping and LC responses in each trial was standardized using a z-score procedure within each session, and we tested the strength of the correlation between these 2 variables. For cue-related activity (left), there was a significant positive correlation between the magnitude of neuronal and lipping responses [black line, slope = 0.07, adjusted r2 = 0.07, F(1,4507) = 32.2; P = 1.5 × 10−8]. If only 1st trials were selected (light gray), this correlation was weaker but still significant [light gray broken line, slope = 0.03, adjusted r2 = 0.001, F(1,2242) = 4.06; P = 0.04]. This correlation was also significant if only non-1st was included [dark gray, slope = 0.06, adjusted r2 = 0.003, F(1,2263) = 7.4; P = 0.006]. At bar release (right), no significant correlation was found between lipping and LC activity (P > 0.05).
Pavlovian schedule task
Sixteen neurons were recorded in the valid cue condition of the Pavlovian schedule task and 9 of these in the random cue condition, also. Neuronal responses occurred at cue onset but not at the outcome of the trials. In the valid cue condition, 13 neurons (81%) responded to cues (median latency = 145 ms, IQR = 80:235 ms). Five of them showed selectivity, with four of these classified as first–nonfirst and one as state. In the random cue condition, five (56%) neurons responded to cues (median latency = 95 ms, IQR = 50:170 ms). Two of these were classified as first–nonfirst, with the other three being nonselective. In the valid cue condition, the population (again using responses converted to z-scores) showed strong first–nonfirst selectivity [F(1) = 16.8, P = 4.1 × 10−5, r2 = 0.7%] and state factors [F(5) = 3.9, P = 0.0016, r2 = 0.8%], with no difference between these two ANOVA models [ANOVA, F(4) = 0.6, P = 0.64; Fig. 12C). Again, as for the operant reward schedule task, the random cue condition also showed significant first–nonfirst selectivity [1st: F(1) = 15.5, P = 8.5 × 10−5, r2 = 1.4%; state: F(5) = 4.3, P = 0.0007, r2 = 1.9%; no difference between state and 1st ANOVA models; ANOVA, F(4) = 1.5, P = 0.2]. Thus in both valid and random Cue conditions, LC firing after cue onset was stronger in first rather than in nonfirst trials.
DISCUSSION
Our study of LC single neuronal activity showed that these neurons are activated in close relation to a Pavlovian response, lipping, and that this activity is modulated by a cognitive factor, knowledge of where the current trial is in the reward schedules (cf. Fig. 9).
Operant and instinctive behaviors in the reward schedules
The bar release in the reward schedules is an operant response, and, as in earlier studies, the monkeys failed to release the bar at the correct time in proportion of the number of trials remaining before the rewarded trial (Bowman et al. 1996). The other behavior, lipping, shows characteristics of a Pavlovian conditioned response. First, it is an innate behavior that occurs spontaneously when the reward (the unconditioned stimulus) is delivered. Second, it is related to task events that predict the reward in the absence of any causal relation between behavioral response and reward. That is, monkeys get the reward whether or not lipping occurs. In earlier studies conducted in this laboratory, licking responses were monitored in monkeys performing the same reward schedule task with the spout being positioned outside of the monkey's mouth (Sugase-Miyamoto and Richmond 2005). In those conditions, cue-evoked licking was stronger in rewarded trials of the schedules. Because the monkeys had to extend their tongues out of their mouths to get the primary reward, the licking response to cues could have been learned, at least in part, through an operant mechanism. The need to protrude the tongue to obtain the reward is a plausible explanation to account for the similarity between licking and bar release patterns in these conditions (higher in rewarded trials), whereas the cue-evoked lipping pattern reported here (higher in 1st trials of a schedule) occurs when the tube is located between the monkey's lips. Last, the pattern of lipping responses to cues was similar in the operant and Pavlovian schedule tasks. Thus the conditioned lipping meets requirements to be classified as a Pavlovian response.
In each trial of the valid cue condition, the monkeys use the information provided by the cue to adjust their operant responses, which were strongest in rewarded trials. Pavlovian responses to cues, however, were strongest in first trials. Although the lipping responses to cues seem to be triggered by the stimulus, the intensity of these responses seems mostly controlled by the context in which the trials begin (valid or random cue condition, beginning or continuation of a schedule). The lipping response to a cue probably does not require identification of the stimulus; it might reflect how much information the stimulus provides (i.e., the amount of uncertainty that it removes), as a function of the context. In both our operant and Pavlovian tasks, a valid first cue has all of the information needed to know how many trials must be completed to obtain the reward. Subsequent cues carry no additional information (assuming prior knowledge of the schedule structure) and, in line with an information hypothesis, evoke smaller responses. In the random cue condition, the first cues indicate only that a schedule is starting but not how many trials will be needed to obtain the reward, i.e., they carry less information, and the lipping is weaker.
Relation between LC and behavioral responses
Just as previous work has shown that LC neurons are not activated by simple motor responses (Aston-Jones and Cohen 2005; Bouret and Sara 2005; Yamamoto and Ozawa 1989), the activation of LC neurons before bar release in our task cannot be solely related to the action per se; it does not happen with bar releases occurring between trials, nor with erroneous bar releases. The LC neuronal responses in this task seem closely related to the occurrence of conditioned lipping. The activation of LC neurons is not a correlate of simple lip movement, however, because it did not occur at reward delivery. The latency of bar release–evoked lipping is significantly shorter than that of cue-evoked lipping. This is compatible with the idea that the bar release–related lipping is triggered by an event occurring before the movement itself, perhaps the green go signal, as is the case for the LC response (Aston-Jones and Cohen 2005; Bouret and Sara 2005).
Had we monitored other behavioral parameters, we would have expected to find autonomic reactivity at the same times that we observed lipping, given the autonomic changes described both in response to significant cues and operant responses in other tasks (Amiez et al. 2003; Braesicke et al. 2005; Collet et al. 1999). Thus the LC responses here that occur in proximity to conditioned events are compatible with coactivation of LC responses and events reflecting sympathetic activation (Abercrombie and Jacobs 1987; Aston-Jones et al. 1996) or emotional processes in humans (Sterpenich et al. 2006). Together, these data reaffirm the previously observed strong relation between the activation of LC neurons and instinctive or autonomic responses. These autonomic responses are often used as means to infer the presence of emotional reactions. Our results raise the possibility that LC responses are related to an underlying emotional reaction in a cognitive task.
Earlier studies showed that LC responses and levels of attention were correlated, when attention was measured by reaction times and error rates in a vigilance task (Aston-Jones et al. 1994). Here, however, the pattern of LC responses cannot simply arise as a correlate of the level of attention defined in this manner because in the 1/1 trials, LC neurons responded strongly to the appearance of the cue, whereas in the 2/2 and 3/3 trials, the neurons did not respond as strongly, even though error rates and reaction times were the same and smallest (cf. Figs. 5A and 12A). One can reach the same conclusion by observing that in the 1/1, 1/2, and 1/3 states where the LC neuronal responses are the same and the reaction times and error rates differ. LC responses to cues were largest in first trials, both in valid cue and random cue conditions, and thus were better correlated with Pavlovian behavior than with level of attention, as measured by operant responsiveness. It would be difficult to observe this dissociation if the task used here did not allow dissociation of operant responsiveness and Pavlovian responses.
Although LC responses share several common features with Pavlovian responses, they cannot be simply looked at as mere neuronal correlates of lipping. Indeed, the intensity of lipping and LC responses at bar release did not show the correlation observed for responses to cues. In addition, LC responses (but not lipping) were stronger before bar release than at cue onset. These data suggest that goal-directed processes taking place around the operant response also have a significant influence on the intensity of LC responses.
In summary, although the triggering of LC responses is closely related to Pavlovian behavior, the magnitude of these responses is modulated by operant processes, an interaction that could only be shown by using a task in which the effects of these classes of behaviors can be simultaneously observed and yet easily separated.
Conditions of LC activation: changes in state?
The LC activation in this task seems to be related to changes in a reward-contingent state induced by behaviorally relevant stimuli. These stimuli include cues, which signal the beginning of a trial, and the green go signal, which signals that it is time to act to progress toward a reward. The activation seems more closely related to the behavioral responses, which presumably reflect acknowledgment of the state change than to the signals themselves. Neuronal responses to cues are stronger in first trials. It might be suggested that first cues, because they carry more information, evoke larger state changes. Bar release responses in LC tend to be stronger in unrewarded trials, where the correct operant response is harder to trigger, as indicated by higher error rates in these conditions. Maybe the slightly stronger LC activation prior to bar release in unrewarded trials can be related to this increased difficulty in triggering the response, which could be looked at as a more profound change from inaction to action. This general hypothesis is further supported by recent experiments showing pupil dilation presumably reflecting LC activation in time with perceptual changes in perceptual rivalry paradigms (Einhauser et al. 2008). This idea is compatible with previously proposed hypotheses underlining the contribution of the noradrenergic system in various types of perceptual or attentional shifts (Bouret and Sara 2005; Corbetta et al. 2008; Dayan and Yu 2006; Yu and Dayan 2005). These data enable a finer characterization, both qualitatively and quantitatively, of behavioral processes underlying the activation of LC neurons.
Relation with activity of other structures in the reward schedule task
Responses of LC neurons in this task are similar in many ways to those displayed by dopaminergic neurons (Ravel and Richmond 2006). They mostly differ in two ways: first, although LC activation after the go signal is better aligned with the operant response, the activation of dopaminergic neurons is better aligned with stimulus onset. Second, dopaminergic neurons, but not LC neurons, responded at the outcome of the trials.
The latency of responses to cues in the LC are in the middle of the distribution (∼110 ms) compared with neurons in other structures (78 ms in TE, 144 ms in perirhinal cortex (Liu and Richmond 2000), 113 ms in the amygdala (Sugase-Miyamoto and Richmond 2005), 112 ms in mesencephalic dopaminergic neurons (Ravel and Richmond 2006), and ∼200 ms in the orbitofrontal cortex (Simmons et al. 2007). This is similar to earlier work in the rat showing that neither frontal cortex nor amygdala responses had shorter latencies than LC in a go–nogo task (Bouret and Sara 2004, 2005). Thus, although LC neurons receive strong inputs from the amygdala (Bouret et al. 2003; Van Bockstaele et al. 1996), as well as orbitofrontal and cingulate cortices (Aston-Jones and Cohen 2005), cue responses in this task are probably not triggered, or at least not solely, by any of these telencephalic structures. The strong correlation between LC and Pavlovian responses, and probably with other instinctive and emotional responses, suggests that LC activation could be triggered by inputs from the brain stem.
The modulation of cue responses must depend on contextual information available before the cue onset because both lipping and LC neuronal responses were stronger in first trials in valid and random cue conditions. Precue anticipatory activity that could play a role in this difference in first–nonfirst LC neuronal activity has been seen in the amygdala and orbitofrontal cortex, where activity is stronger before first cues than before nonfirst cues (Simmons et al. 2007; Sugase-Miyamoto and Richmond 2005). This activity could enhance responses to first cues by specifically increasing the activation by a nondiscriminatory input (indistinguishable across schedule states), therefore accounting for the rapid and discriminative LC responses. A similar modulatory influence could account for the small but significant modulation of LC responses before bar release, because both amygdala and orbitofrontal neurons also displayed reward-predicting responses before bar release.
The activation of LC, a phylogenetically old structure, seems to be especially related to primitive behavioral reactions that occur when an animal detects a significant change in its environment. Overall, LC neurons are activated when task-relevant stimuli induce a conditioned instinctive response. These responses are modulated by contextual information and operant processes, which we speculate might arise from forebrain structures such as the amygdala and orbitofrontal cortex. The integration of these primitive reactions and cognitive goal-directed influences is reflected in the output of LC neurons that can influence its forebrain targets, perhaps promoting the rapid behavioral flexibility that norepinephrine is supposed to enhance.
GRANTS
This work was supported by the Intramural Research Program of the National Institute of Mental Health. S. Bouret was partially supported by the Fondation Fyssen.
Acknowledgments
We thank S. Ravel, J. Simmons, T. Minamimoto, and G. Lacamera for helpful comments. This work was written as part of B. J. Richmond's official duties as a U.S. Government employee.
The views expressed in this article do not necessarily represent the views of the National Institute of Mental Heath, National Institutes of Health, U.S. Dept of Health and Human Services or the U.S. Government.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Abercrombie and Jacobs 1987.Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli. J Neurosci 7: 2837–2843, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez et al. 2003.Amiez C, Procyk E, Honore J, Sequeira H, Joseph JP. Reward anticipation, cognition, and electrodermal activity in the conditioned monkey. Exp Brain Res 149: 267–275, 2003. [DOI] [PubMed] [Google Scholar]
- Arnsten 2000.Arnsten AF Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plast 7: 133–146, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones and Bloom 1981.Aston-Jones G, Bloom FE. Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1: 887–900, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones and Cohen 2005.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28: 403–450, 2005. [DOI] [PubMed] [Google Scholar]
- Aston-Jones et al. 1994.Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci 14: 4467–4480, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones et al. 1996.Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. Role of the locus coeruleus in emotional activation. Prog Brain Res 107: 379–402, 1996. [DOI] [PubMed] [Google Scholar]
- Bouret et al. 2003.Bouret S, Duvel A, Onat S, Sara SJ. Phasic activation of locus ceruleus neurons by the central nucleus of the amygdala. J Neurosci 23: 3491–3497, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret and Sara 2004.Bouret S, Sara SJ. Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur J Neurosci 20: 791–802, 2004. [DOI] [PubMed] [Google Scholar]
- Bouret and Sara 2005.Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci 28: 574–582, 2005. [DOI] [PubMed] [Google Scholar]
- Bowman et al. 1996.Bowman EM, Aigner TG, Richmond BJ. Neural signals in the monkey ventral striatum related to motivation for juice and cocaine rewards. J Neurophysiol 75: 1061–1073, 1996. [DOI] [PubMed] [Google Scholar]
- Braesicke et al. 2005.Braesicke K, Parkinson JA, Reekie Y, Man MS, Hopewell L, Pears A, Crofts H, Schnell CR, Roberts AC. Autonomic arousal in an appetitive context in primates: a behavioural and neural analysis. Eur J Neurosci 21: 1733–1740, 2005. [DOI] [PubMed] [Google Scholar]
- Clayton et al. 2004.Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G. Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. J Neurosci 24: 9914–9920, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet et al. 1999.Collet C, Dittmar A, Vernet-Maury E. Programming or inhibiting action: evidence for differential autonomic nervous system response patterns. Int J Psychophysiol 32: 261–276, 1999. [DOI] [PubMed] [Google Scholar]
- Corbetta et al. 2008.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron 58: 306–324, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan and Yu 2006.Dayan P, Yu AJ. Phasic norepinephrine: a neural interrupt signal for unexpected events. Network 17: 335–350, 2006. [DOI] [PubMed] [Google Scholar]
- Einhauser et al. 2008.Einhauser W, Stout J, Koch C, Carter O. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proc Natl Acad Sci USA 105: 1704–1709, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant et al. 1988.Grant SJ, Aston-Jones G, Redmond DE Jr. Responses of primate locus coeruleus neurons to simple and complex sensory stimuli. Brain Res Bull 21: 401–410, 1988. [DOI] [PubMed] [Google Scholar]
- Liu and Richmond 2000.Liu Z, Richmond BJ. Response differences in monkey TE and perirhinal cortex: stimulus association related to reward schedules. J Neurophysiol 83: 1677–1692, 2000. [DOI] [PubMed] [Google Scholar]
- McGaughy et al. 2008.McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience 153: 63–71, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos et al. 2000.Paxinos G, Huang XF, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press, 2000, p. 1
- Rajkowski et al. 2004.Rajkowski J, Majczynski H, Clayton E, Aston-Jones G. Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J Neurophysiol 92: 361–371, 2004. [DOI] [PubMed] [Google Scholar]
- Ravel and Richmond 2006.Ravel S, Richmond BJ. Dopamine neuronal responses in monkeys performing visually cued reward schedules. Eur J Neurosci 24: 277–290, 2006. [DOI] [PubMed] [Google Scholar]
- Sara 1985.Sara SJ Noradrenergic modulation of selective attention: its role in memory retrieval. Ann NY Acad Sci 444: 178–193, 1985. [DOI] [PubMed] [Google Scholar]
- Sara 2000.Sara SJ Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem 7: 73–84, 2000. [DOI] [PubMed] [Google Scholar]
- Sara 2008.Sara SJ The Locus Coeruleus and noradrenergic modulation of cognition. Nat Neurosci Rev In press. [DOI] [PubMed]
- Sara and Segal 1991.Sara SJ, Segal M. Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog Brain Res 88: 571–585, 1991. [DOI] [PubMed] [Google Scholar]
- Simmons et al. 2007.Simmons JM, Ravel S, Shidara M, Richmond BJ. A comparison of reward-contingent neuronal activity in monkey orbitofrontal cortex and ventral striatum: guiding actions toward rewards. Ann NY Acad Sci 1121: 376–394, 2007. [DOI] [PubMed] [Google Scholar]
- Simmons et al. 2008.Simmons JM, Saad ZS, Lizak MJ, Ortiz M, Koretsky AP, Richmond BJ. Mapping prefrontal circuits in vivo with manganese-enhanced magnetic resonance imaging in monkeys. J Neurosci 28: 7637–7647, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterpenich et al. 2006.Sterpenich V, D'Argembeau A, Desseilles M, Balteau E, Albouy G, Vandewalle G, Degueldre C, Luxen A, Collette F, Maquet P. The locus ceruleus is involved in the successful retrieval of emotional memories in humans. J Neurosci 26: 7416–7423, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugase-Miyamoto and Richmond 2005.Sugase-Miyamoto Y, Richmond BJ. Neuronal signals in the monkey basolateral amygdala during reward schedules. J Neurosci 25: 11071–11083, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele et al. 1996.Van Bockstaele EJ, Chan J, Pickel VM. Input from central nucleus of the amygdala efferents to pericoerulear dendrites, some of which contain tyrosine hydroxylase immunoreactivity. J Neurosci Res 45: 289–302, 1996. [DOI] [PubMed] [Google Scholar]
- Vankov et al. 1995.Vankov A, Herve-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci 7: 1180–1187, 1995. [DOI] [PubMed] [Google Scholar]
- Yamamoto and Ozawa 1989.Yamamoto K, Ozawa N. Increased firing of locus coeruleus neurons associated with preparatory set in rats. Neurosci Lett 106: 112–118, 1989. [DOI] [PubMed] [Google Scholar]
- Yu and Dayan 2005.Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron 46: 681–692, 2005. [DOI] [PubMed] [Google Scholar]