Abstract
A Pavlovian-conditioning procedure may produce modifications in multiple behavioral responses. As an example, conditioning may result in the elicitation of a specific somatomotor conditioned response (CR) and, in addition, other motor and visceral CRs. In the mollusk Hermissenda conditioning produces two conditioned responses: foot-shortening and decreased locomotion. The neural circuitry supporting ciliary locomotion is well characterized, although the neural circuit underlying foot-shortening is poorly understood. Here we describe efferent neurons in the pedal ganglion that produce contraction or extension of specific regions of the foot in semi-intact preparations. Synaptic connections between polysensory type Ib and type Is interneurons and identified foot contractile efferent neurons were examined. Type Ib and type Is interneurons receive synaptic input from the visual, graviceptive, and somatosensory systems. Depolarization of type Ib interneurons evoked spikes in identified tail and lateral foot contractile efferent neurons. Mechanical displacement of the statocyst evoked complex excitatory postsynaptic potentials (EPSPs) and spikes recorded from type Ib and type Is interneurons and complex EPSPs and spikes in identified foot contractile efferent neurons. Depolarization of type Ib interneurons in semi-intact preparations produced contraction and shortening along the rostrocaudal axis of the foot. Depolarization of Is interneurons in semi-intact preparations produced contraction of the anterior region of the foot. Taken collectively, the results suggest that type Ib and type Is polysensory interneurons may contribute to the neural circuit underlying the foot-shortening CR in Hermissenda.
INTRODUCTION
Pavlovian-conditioning studies in diverse species have shown that learning involves the acquisition of multiple conditioned responses (CRs) produced by the same conditioning procedure (Ayers and Powell 2002; Black and Toledo 1972; Gantt 1960; Godsil et al. 2000; Konorski 1967;Powell 1999; Prokasy 1984; Schneiderman 1972; Weinberger and Diamond 1987). As an example, the acquisition of a somatomotor CR also results in the development of concomitant visceral CRs. The development of multiple responses with classical conditioning raises a number of issues concerning the CR complex. Do the different CRs develop independently or are there interactions between components of the underlying network? If acquisition rates are different for the multiple CRs, does learning in one response pathway contribute to the development of the other response? Are there mechanistic differences between the CRs with respect to initial acquisition or retention? The results of lesion studies suggest that the development of somatomotor CRs involves different areas of the brain than the visceral CRs that are concomitantly acquired (Buchanan and Powell 1982; Kao and Powell 1988; Lavond et al. 1984). However, the issue concerning independent or dependent development of multiple CRs is further complicated by the differential effects of trace versus delayed conditioning procedures (Green and Woodruff-Pak 2000; McLaughlin et al. 2002). Many of the questions concerning mechanistic processes associated with the synaptic interactions between conditioned stimulus (CS) and unconditioned stimulus (US) pathways supporting multiple CRs are effectively addressed in less complex nervous systems where the circuitry is more characterized.
In the nudibranch mollusk Hermissenda crassicornis Pavlovian conditioning results in the acquisition of two different CRs. The CS elicits foot-shortening and inhibition of forward locomotion (Crow and Alkon 1978; Lederhendler et al. 1986). An analysis of acquisition of the two CRs suggested that the responses are supported by distinct efferent pathways and develop independently (Matzel et al. 1990). The neural circuit contributing to turning behavior in Hermissenda has been examined (Goh and Alkon 1984) and recent studies have identified the neural circuit underlying ciliary locomotion (Crow and Tian 2000, 2002a,b, 2003a, 2004). However, little is known about the neural circuit mediating graviceptive elicited foot-shortening. In this study using semi-intact preparations, we have identified and characterized efferent neurons that innervate the tail and midlateral regions of the foot. We show that identified lateral foot contractile, tail contractile, and dorsal ciliary efferent neurons receive synaptic input from polysensory type Ib interneurons. Depolarization of Ib interneurons in semi-intact preparations evoked contraction along the rostrocaudal axis of the foot. We provide evidence that identified Is interneurons project to efferent neurons innervating the anterior foot. The Is interneurons are polysensory; receiving synaptic input from the graviceptive, visual, and somatosensory systems. The strong synaptic input from the graviceptive sensory system to Ib and Is interneurons and their synaptic projections to efferent neurons innervating different regions of the foot suggest that type Ib and type Is interneurons may contribute to the circuit generating the foot-shortening CR.
METHODS
Animals
Adult Hermissenda crassicornis were used in the experiments. The animals were obtained from Sea Life Supply (Sand City, CA) and maintained in closed artificial seawater (ASW) aquaria at 14°C on a 12-h light/dark cycle. All electrophysiological procedures were conducted during the light phase of the light/dark cycle.
Intracellular recordings
Simultaneous intracellular recordings from identified Ib and Is interneurons and efferent neurons were collected from partially split-foot semi-intact preparations (see following text). Studies examining synaptic connections between the graviceptive system and interneurons and the visual system and interneurons included both isolated nervous systems and semi-intact preparations. Experiments involving recordings from efferent neurons were conducted in semi-intact preparations. Anatomical and electrophysiological criteria were used to identify type Ib and type Is interneurons as described previously for type I and type II interneurons (Crow and Tian 2000). The location of Ib and Is somas in the cerebropleural ganglion relative to reliable landmarks such as type I interneurons provided a general anatomical identification. Type Ib and type Is interneurons were then physiologically identified by mechanically stimulating statocyst hair cells. Displacement of the statocyst elicited complex postsynaptic potentials (PSPs) and spikes in type Ib and type Is interneurons and identified efferent neurons. The procedure for mechanical displacement of the statocyst is similar to the methods described in previously published reports (Alkon and Bak 1973; Detwiler and Alkon 1973). Briefly, mechanical stimulation of hair cells was produced by displacement of the statocyst provided by a glass probe attached to the end of a ceramic bimorph fixed to a micromanipulator. A train of 0.2- to 1.0-mA pulses (100–200 Hz, 0.2–0.5 ms in duration) applied to the ceramic bimorph resulted in a 7- to 11-μm displacement of the statocyst and oscillation of the statoconia within the lumen of the statocyst. Surgical desheathing of a small area of the cerebropleural and ventral and dorsal pedal ganglion was conducted to expose the cell bodies of interneurons and efferent neurons. In semi-intact preparations pedal efferent neurons were identified by verifying foot contractions produced by depolarization with extrinsic current.
The partially desheathed circumesophageal nervous systems were pinned to a silicone elastomer (Sylgard, Dow Chemical) stage in a recording chamber filled with ASW of the following composition (in mM): 460 NaCl, 10 KCl, 10 CaCl2, and 55 MgCl2, buffered with 10 mM HEPES and brought to pH 7.46 with dilute NaOH. The ASW in the recording chamber was monitored by a thermistor and held at 15 ± 0.5°C. Illumination of the preparation was provided by a tungsten–halogen incandescent lamp attached to a fiber-optic bundle mounted underneath the recording chamber. Maximum light intensity (10−4 W/cm2) was attenuated with neutral density filters expressed in negative log units. Interneurons and efferent neurons were impaled with microelectrodes filled with 4 M KAc. Microelectrodes were connected to the two headstages of an Axoclamp 2A (Axon Instruments, Foster City, CA). Standard intracellular recording and stimulation techniques were used. Single spikes elicited by brief extrinsic current pulses and trains of action potentials elicited by current steps were applied in the dark through a bridge circuit. Electrophysiological data were digitized with a CED power 1401 (Cambridge Electronic Design [CED], Cambridge, UK) and stored on a computer hard drive. Digitized data were analyzed and prepared for figures using Spike2 software (CED).
Semi-intact preparations
Semi-intact anterior split-foot Hermissenda were prepared by cooling the animals in ASW to between 0 and 1°C, followed by isolation of the circumesophageal nervous system from the buccal crest and body, leaving intact pedal nerves P1 and P2. The partially split foot was positioned ventral side up adjacent to the isolated circumesophageal nervous system pinned to the elevated central stage in the recording chamber. For recordings from neurons on the ventral surface, the left pedal ganglion was rotated about 150° to provide for visualization of neuronal cell bodies. The exposed nervous system and foot were imaged in visible light by a 45-W tungsten–halogen light source projected by a light guide to the central stage of the recording chamber. A Leica DFC280 Digital Camera (Leica Microsystems, Wetzlar, Germany) was used to record foot movement elicited by current stimulation of identified pedal efferent neurons. The camera was mounted on a stereomicroscope (Wild M5A) and connected to a computer. The camera was configured at 30 consecutive frames (multiple-image capture) with a cumulative interval of 50 ms. The exposure time was set at 500 ms. Pictures were computer processed with Image-Pro-Express (Version 5.1.0.12 for Windows 2000/XP Professional, Media Cybernetics).
Interneuron labeling
Identified Is interneurons were impaled with microelectrodes whose tips contained 2% Lucifer yellow in 0.2 M LiCl (electrode resistance 100–150 MΩ). A hyperpolarizing current (0.5–1 nA) was applied for 1 h to iontophorese Lucifer yellow. The nervous system remained in the recording chamber for an additional 1 h followed by overnight fixation with 4% paraformaldehyde in ASW. Nervous systems were rinsed three times (10-min interval) in 0.1 M phosphate-buffered saline, dehydrated in ethanol, cleared in methyl salicylate, mounted, and viewed under a fluorescent microscope. Images were collected using a laser scanning confocal microscope (BioRad Radiance 2100). The sampling steps were set at 2 μM and the z-stack of 65 sections was merged to generate the final image of labeled Is interneurons.
RESULTS
Graviceptive stimulation produces reflexive contraction of foot muscles that results in a change in the overall configuration of the foot. A change in foot configuration underlies both the clinging response elicited by graviceptive stimulation and the foot-shortening CR evoked by the CS following Pavlovian conditioning (Lederhendler et al. 1986). Rotation applied as an unconditioned stimulus (US) in the Pavlovian-conditioning paradigm elicits a decrease in the length of the foot. The clinging response in Hermissenda is expressed when animals increase contact of the foot with the underlying substrate in response to seawater turbulence produced by agitation (Alkon 1974). In this study we have identified pedal neurons in semi-intact preparations that innervate contractions or extension of different regions of the foot and body wall that produce changes in foot configuration when stimulated with extrinsic current.
Foot contractile pedal neurons
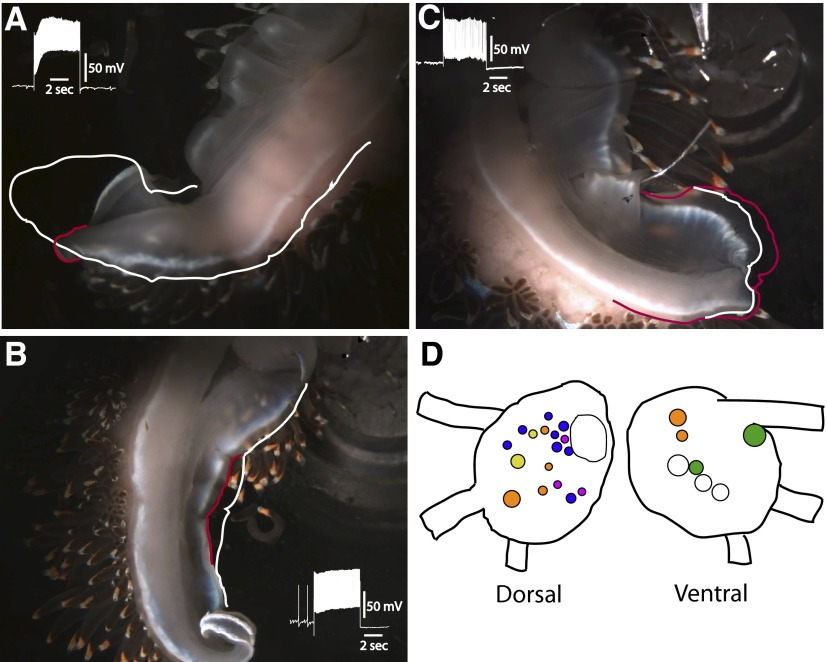
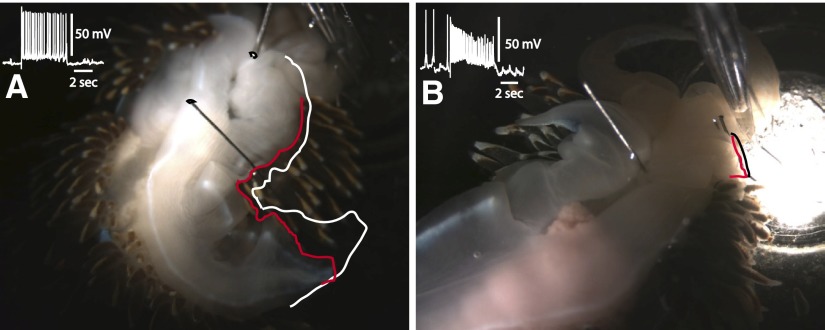
The general anatomical organization of the pedal-motor system in Hermissenda consists of efferent neurons whose cell bodies are located on the dorsal and ventral surfaces of the pedal ganglia, with axonal projections in nerves P1 and P2. Previous research has shown that neurons with axons in P1 innervate the posterior three fourths of the foot, whereas neurons with axons in P2 innervate the anterior region of the foot (Richards and Farley 1987). In general, many dorsal pedal neurons have axons in P1 and ventral pedal neurons have axons projecting to the periphery in P2. In this study, several types of efferent neurons were identified that altered foot configuration when depolarized with extrinsic current. Depolarization of identified efferent neurons produced tail contraction (TC), lateral foot contraction (LFC), anterior foot contraction (VC), or tail extension (TE). In all, 75 semi-intact preparations were used in the studies. The surgical procedures used for the generation of semi-intact preparations involved cutting the pedal nerves innervating the right side of the foot, leaving intact left P1 and P2. This procedure resulted in an ipsilateral denervation and hemiplegia of the foot muscles on the right side and normal innervation of the left side of the foot. An example of tail contraction evoked by stimulation of a TC efferent neuron with a 5-s current pulse is shown in Fig. 1A. The slight turning of the tail region during contraction is the result of innervation of the muscles on the left side of the foot moving the flaccid denervated right-side tail region during current depolarization (Fig. 1A). The white tracing around the perimeter of the posterior region of the foot indicates foot position immediately before the onset of the current pulse. The contracted or shortened region of the tail is outlined in red and indicates the position of the tail at the end of the current pulse. The inset of Fig. 1A shows an example of depolarization of the TC neuron produced by the 5-s current step that resulted in the tail contraction shown in Fig. 1A. The white outline extending to the middle region of the foot indicates that the midregion of the foot did not contract during current stimulation of the TC neuron. An example of midfoot contraction produced by current depolarization of the LFC efferent neuron is shown in Fig. 1B. The white outline drawn on the ipsilateral foot indicates the position of the foot immediately before current depolarization of the LFC efferent neuron. The contracted midregion of the lateral foot is shown by the red line in the photograph taken at the end of the current pulse. Contraction of the lateral foot was restricted to the midregion and did not include the posterior region near the tail or the anterior part of the foot as shown in the photograph. The inset is an example of a recording from the LFC efferent neuron during current depolarization that produced the midfoot contraction shown in Fig. 1B. An example of tail extension evoked by stimulation of a TE efferent neuron is shown in Fig. 1C. In the photograph, the red outline shows the position of the posterior foot at the end of the depolarizing current pulse. The position of the tail before current stimulation of the TE neuron is indicated by the white line (Fig. 1C). The primary extension of the posterior foot was restricted to the ipsilateral region of the tail and did not involve the denervated contralateral side of the posterior tail. In addition, the ipsilateral midfoot and anterior region did not contract or extend during the current pulse (Fig. 1C). The inset shows an example of depolarization of the TE neuron that elicited the tail extension shown in Fig. 1C.
FIG. 1.
Photographs of representative semi-intact preparations before stimulation and at the termination of a 5-s depolarizing current pulse delivered to (A) a tail contractile efferent neuron (TC), (B) a lateral foot contractile efferent neuron (LFC), and (C) a tail extension efferent neuron (TE). The white outline indicates the position of the foot immediately before stimulation in A, B, and C. The red outline shows the position of the foot and the end of the current pulse in A, B, and C. The insets are recordings of spike activity in the efferent neurons immediately before and during current stimulation. In B, note that the anterior ipsilateral and posterior ipsilateral regions of the foot did not contract. In C, note that the anterior and middle regions of the foot did not contract or extend during the current pulse. D: dorsal and ventral surface maps of the pedal ganglion showing the locations of neuronal somata of ciliary efferent neurons (orange), tail contractile efferent neurons (yellow), lateral foot contractile efferent neurons (blue), anterior foot contractile efferent neurons (green), and tail extension efferent neurons (purple). The noncolored cells indicated in the drawings are useful landmarks for anatomical identification of efferent neurons. The large noncolor labeled cell in the left pedal ganglia is LP1 (Jerussi and Alkon 1981).
A diagram of the dorsal and ventral surfaces of the left pedal ganglion, indicating the location of identified efferent neurons generating the different contractions and extension of the foot, is shown in Fig. 1D. In addition, the location of identified ciliary efferent neurons and previously identified anterior foot contraction efferent neurons is shown in the diagram of the pedal ganglia.
Ib interneurons
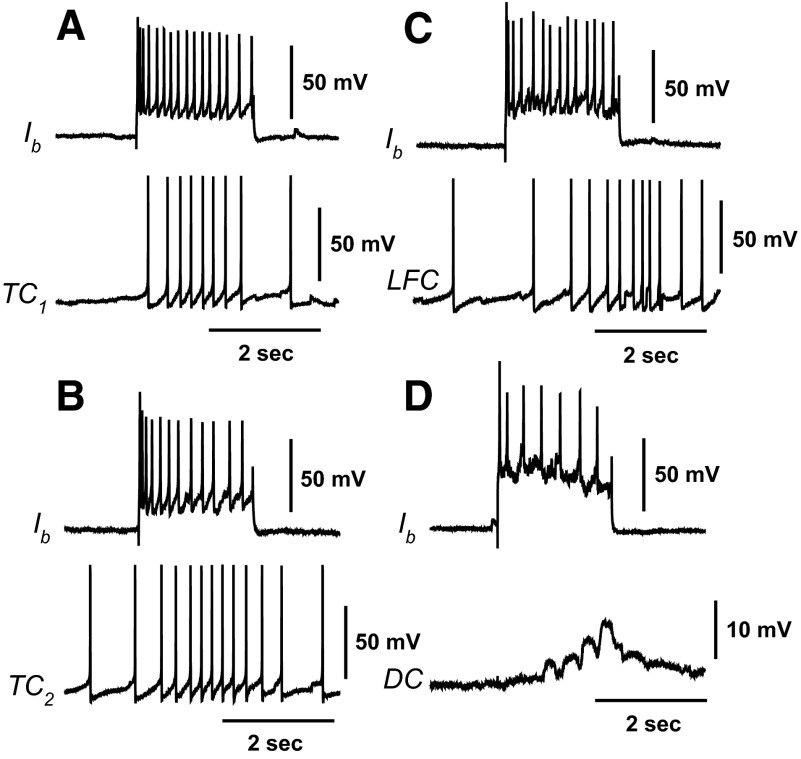
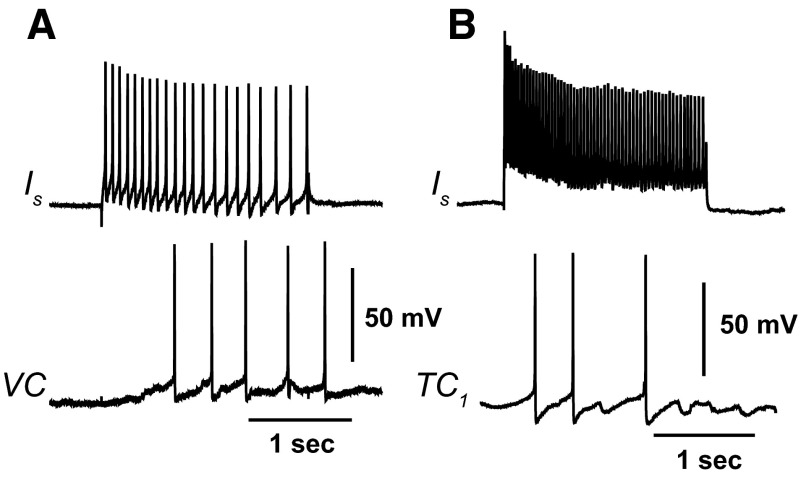
Identified interneurons that project to both ventral contractile (VC) efferent neurons and VP1 ciliary efferent neurons have been characterized in previous studies of the neural circuitry underlying ciliary locomotion and anterior foot contraction (Crow and Tian 2004). Here we examined synaptic connections between identified type Ib interneurons and the recently identified efferent neurons producing contraction of different regions of the foot. Representative examples of simultaneous recordings from Ib interneurons and identified efferent neurons in semi-intact preparations are shown in Fig. 2. Type Ib interneurons project to several efferent neurons contributing to foot contraction and ciliary locomotion. Depolarizing current stimulation of an Ib interneuron elicited spikes recorded from an identified tail contractile (TC1) neuron (Fig. 2A). As shown in Fig. 2B, depolarization of an Ib interneuron evoked spikes recorded from a different identified tail contractile neuron (TC2) located in the rostral region of the pedal ganglion. Depolarization of an Ib interneuron also evoked spikes recorded from an identified lateral foot contractile (LFC) efferent neuron (Fig. 2C). Consistent with previous results showing synaptic connections between VP1 ciliary efferent neurons and Ib interneurons, Ib depolarization evoked a complex excitatory postsynaptic potential (EPSP) recorded from a ciliary efferent neuron located on the dorsal pedal ganglion (DC) (Fig. 2D).
FIG. 2.
Polysensory type Ib interneurons project to pedal neurons innervating foot contraction and activation of foot cilia. Simultaneous recordings from Ib interneurons and efferent neurons in semi-intact preparations. A: depolarization of a type Ib interneuron with a 2-s current pulse evoked spikes recorded from a contralateral tail contractile (TC1) efferent neuron. B: depolarization of a type Ib interneuron with a 2-s current pulse evoked spikes recorded from a different contralateral tail contractile (TC2) efferent neuron located in the rostral region of the pedal ganglion. C: depolarization of an Ib interneuron with a 2-s depolarizing current pulse evoked spikes recorded from a lateral foot contractile (LFC) efferent neuron that produced foot narrowing. D: depolarization of an Ib interneuron evoked a complex excitatory postsynaptic potential (EPSP) recorded from a dorsal ciliary (DC) efferent neuron.
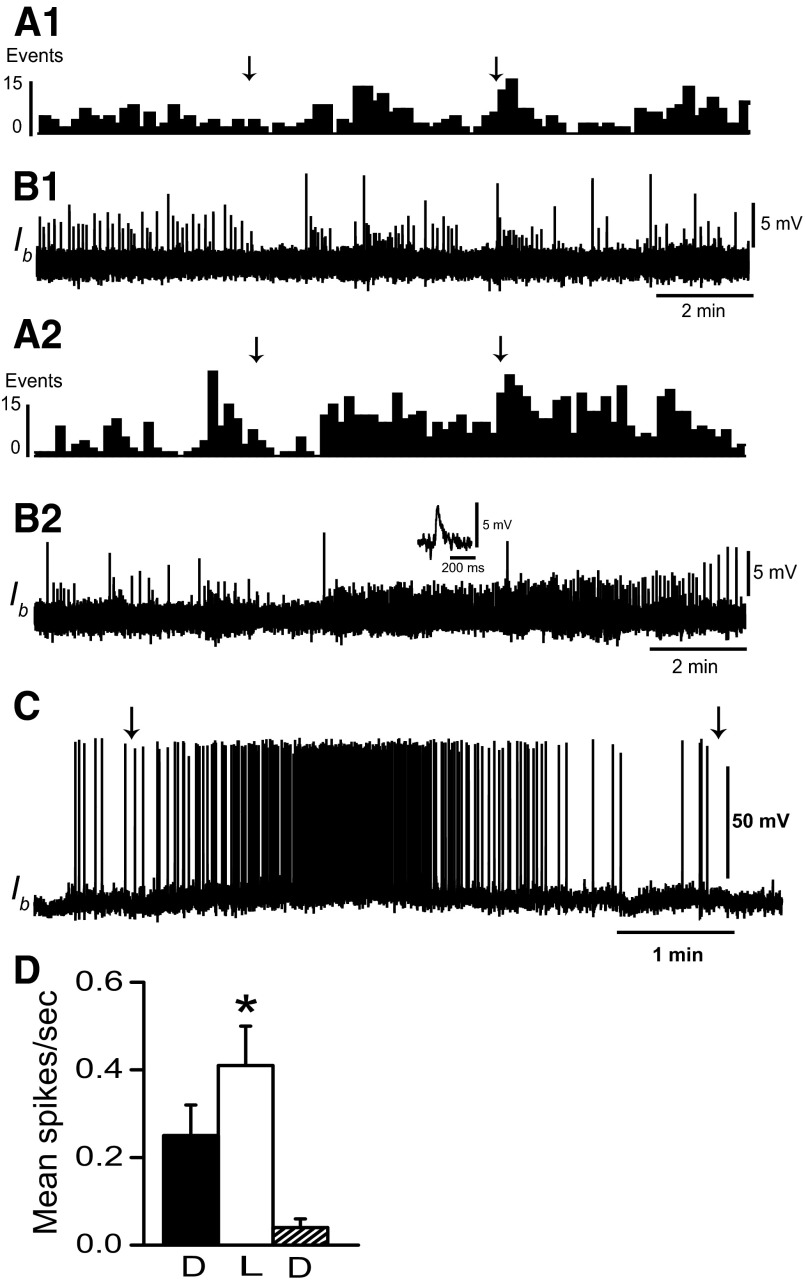
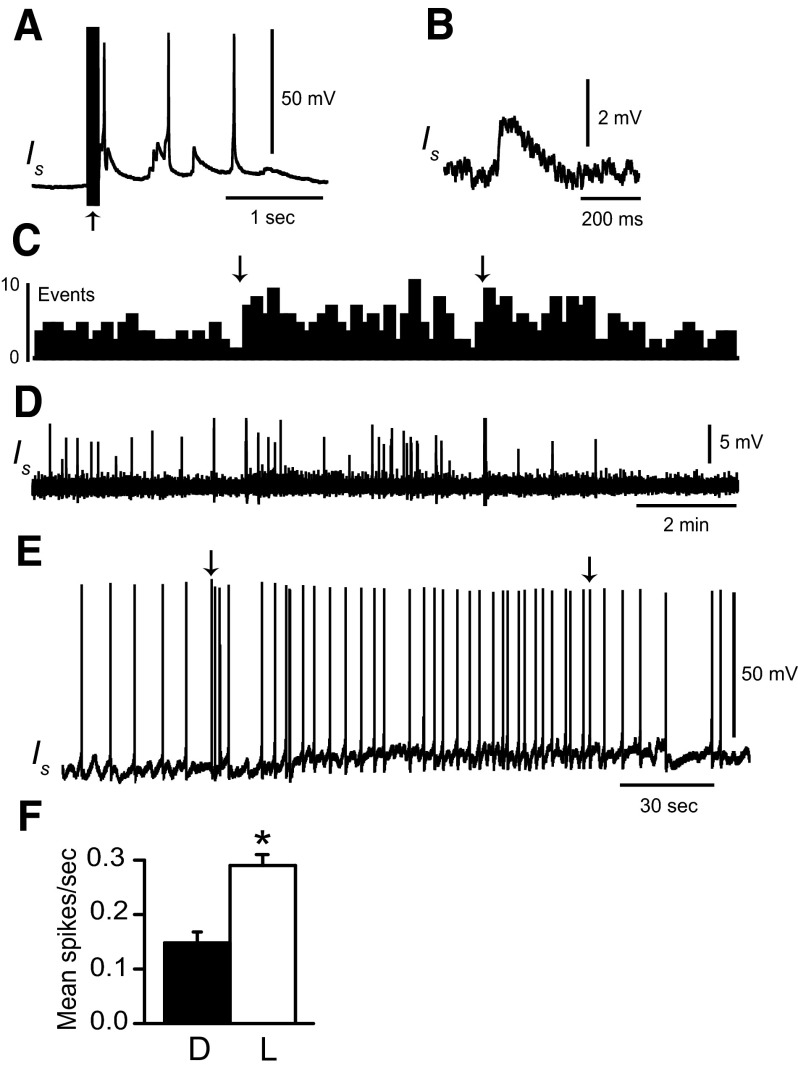
Type Ie and type Ii interneurons in the circuit supporting ciliary locomotion are polysensory. We have previously shown that type Ib interneurons receive synaptic input from ipsilateral rostral and lateral statocyst hair cells (Crow and Tian 2004). We further examined sensory projections to type Ib interneurons by recording EPSPs and spikes elicited during illumination of the eyes. Dark-adapted type Ib interneurons typically do not spontaneously generate spikes. As shown in the histograms of EPSPs in Fig. 3, light evoked an increase in EPSP frequency recorded from type Ib interneurons relative to dark-adapted baseline activity. EPSP frequency depended on light intensity. Unattenuated light evoked more Ib EPSPs (Fig. 3, A2 and B2) compared with light-attenuated 1 log unit (Fig. 3, A1 and B1). The primary effect of light was to increase the frequency of EPSPs with amplitudes between 2 and 5 mV. An example is shown in the inset of Fig. 3B2. Unattenuated light (5 min) evoked an increase in the frequency of 2- to 5-mV EPSPs (mean = 0.38/s) compared with 5 min in the dark immediately before light (mean = 0.17/s). EPSPs with amplitudes >5 mV occurred less frequently in the light (mean = 0.007/s) and dark (mean = 0.02/s). Current depolarization of Ib interneurons sufficient to produce spikes in the dark revealed a modest increase in spike activity measured during 5-min periods of illumination (Fig. 3C). Analysis of the group data in Fig. 3D from Ib interneurons depolarized with steady current (n = 5) revealed that following 12 min of dark adaptation (mean spike activity in the dark = 0.25 ± 0.07 spikes/s), a 5-min exposure to light resulted in a modest increase in spike activity (mean spike activity = 0.41 ± 0.09 spikes/s). Following the termination of illumination the average spike activity during a 5-min period in the dark was 0.04 ± 0.02 spikes/s. Statistical analysis revealed a significant overall difference in the mean spike activity of type Ib interneurons between light-adapted and the two dark-adapted conditions [F(2,4) = 5.3; P < 0.03]. As a control to rule out potential synaptic input to Ib interneurons from extrinsic light responsive neurons or intrinsic light responses of Ib cells, we examined spike activity in the dark and in light during conditions of current depolarization in preparations where the eyes were ablated (n = 5). Analysis of Ib spike activity from ablated preparations did not reveal statistically significant differences between dark-adapted conditions and illumination (t4 = 0.42; NS). These results suggest that type Ib interneurons receive synaptic input from the visual system that is typically not sufficient to produce spike activity unless synaptic input from other sensory systems provides sufficient depolarization to bring the cells to threshold for spike generation. Results of our analysis of synaptic input to Ib interneurons from the visual system and the identified connections between Ib interneurons and efferent neurons are consistent with results indicating that light typically does not elicit foot-shortening (Lederhendler et al. 1986).
FIG. 3.
Light evokes EPSPs in type Ib interneurons. A1: histogram (10-s bin width) of EPSPs recorded from a dark-adapted type Ib interneuron, during 5 min of illumination of the photoreceptors and during 5 min in the dark following the offset of light. B1: intracellular recording of type Ib interneuron EPSPs contributing to the histogram in A1. The arrows above the histogram indicate the onset and offset of light-attenuated 1 log unit. A2: histogram of EPSPs recorded from the same dark-adapted type Ib interneuron as shown in A1 during 5 min of unattenuated light and 5 min in the dark following light offset. B2: EPSPs recorded from the type Ib interneuron contributing to the histogram in A2. The inset shows an example of an EPSP evoked by illumination of the photoreceptors. EPSP frequency increased as light intensity increased. Resting membrane potential of the Ib interneuron was 64–65 mV. C: light response of a type Ib interneuron depolarized to spike threshold prior to the onset of a 5-min period of illumination. D: group summary data (n = 5) of mean type Ib interneuron spike activity recorded during current depolarization in the dark (D), during 5 min of illumination of the photoreceptors (L) and during a 5-min period in the dark immediately following illumination (D). The arrows in C indicate the onset and offset of light that was attenuated 1 log unit; *P < 0.03.
Is interneurons
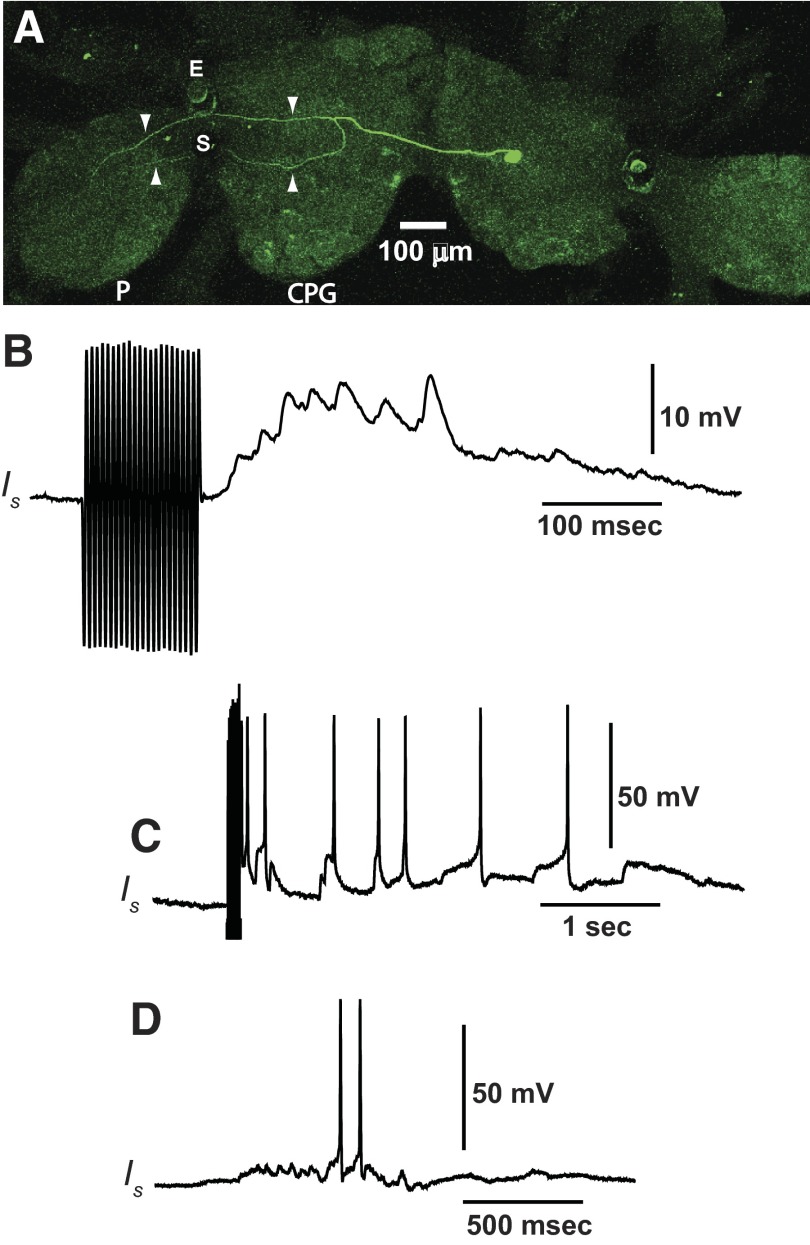
In this study we have identified an additional interneuron type that receives synaptic input from the graviceptive system and has synaptic projections to identified efferent neurons. Recordings from newly identified type Is interneurons showed that they project to efferent neurons that innervate primarily the anterior region of the foot. Labeling of Is interneurons with Lucifer yellow (n = 5 preparations) revealed a bifurcation of the primary axonal process in the contralateral cerebropleural ganglion and two axonal processes that project to different regions of the contralateral pedal ganglion. A representative example of a Lucifer-labeled Is interneuron is shown in Fig. 4A. The fluorescent image from a confocal scan of the circumesophageal nervous system shows a labeled Is soma (green) and a bifurcating axonal process indicated by white arrowheads in the contralateral cerebropleural ganglion and pedal ganglion (Fig. 4A). We examined synaptic input to Is interneurons from different sensory systems. Type Is interneurons receive synaptic input from the graviceptive and somatosensory systems. As shown in Fig. 4B depolarization of hair cells by mechanical displacement of the statocyst evoked a complex EPSP recorded from an Is interneuron. A larger displacement of the statocyst in a different preparation evoked a train of spikes recorded from an Is interneuron (Fig. 4C). In a previous study identified polysensory interneurons in the cerebropleural ganglion were shown to receive somatosensory input from the foot and/or body wall (Tian et al. 2006). We examined whether polysensory Is interneurons also received somatosensory input from the foot by recording spike activity in Is cells evoked by cutaneous stimulation of the foot. As shown in Fig. 4D, flicking a von Frey hair with a pressure of 3.14 g/mm2 across the midregion of the foot evoked a depolarization and spike activity recorded in an Is interneuron. We next examined synaptic input to Is interneurons from the visual system. An example of EPSPs recorded from a dark-adapted Is interneuron and during illumination of the photoreceptors is shown in Fig. 5. Statocyst hair cell activation was used to assist in the identification of the Is interneurons (Fig. 5A). Stimulation of the eyes with light-attenuated 1 log unit increased the frequency of EPSPs with amplitudes between 1.5 and 3.2 mV (Fig. 5B), as indicated by the histogram and EPSP recordings from an Is interneuron shown in Fig. 5, C and D. The mean frequency of 1.5- to 3.2-mV EPSPs in the dark was, respectively, 0.15 and 0.27/s during the 5-min period of light. EPSPs with amplitudes >3.2 mV occurred less frequently in both light (mean = 0.07/s) and dark (mean = 0.05/s). The average spontaneous spike activity of Is interneurons in dark-adapted preparations was 0.002 ± 0.0007 spikes/s (n = 18). Spontaneous spike activity recorded from Is interneurons during a 5-min period of illumination of the photoreceptors did not change significantly from dark-adapted activity. However, as shown in the example of Fig. 5E, current depolarization of type Is interneurons that exceeded spike threshold under dark-adapted conditions revealed a modest increase in spike activity detected during illumination. Analysis of the group summary data in Fig. 5F (n = 5) collected during current depolarization showed a statistically significant increase in Is spike activity (t4 = 6.7; P < 0.003) during illumination (mean = 0.29 ± 0.02 spikes/s) relative to baseline spike activity recorded in the dark (mean = 0.14 ± 0.02 spikes/s).
FIG. 4.
A: Lucifer yellow labeling of an Is interneuron. The primary axonal process crosses in the cerebropleural commissure to the contralateral cerebropleural ganglion where the process bifurcates (white arrowheads) before projecting to the pedal ganglion. The 2 processes are indicated by the white arrowheads in the pedal ganglion. P, pedal ganglion; CPG, cerebropleural ganglion; E, eye; S, statocyst. Type Is interneurons are polysensory. B: statocyst hair cell activation produced by the movement of a glass rod attached to a ceramic bimorph evoked a complex EPSP recorded in an Is interneuron (B) and spikes recorded from a different Is interneuron (C). The artifact preceding the recordings in B and C is from the pulses applied to the bimorph. Is interneurons also exhibit responses to cutaneous stimulation of the foot. D: spikes elicited in an identified Is interneuron in response to mechanical stimulation of the foot delivered with a calibrated von Frey hair (von Frey hair pressure = 3.14 g/mm2).
FIG. 5.
Light evokes EPSPs in type Is interneurons. A: statocyst hair cell activation evoked a depolarization and spikes recorded from an Is interneuron. Hair cell synaptic input was used as one of the criteria for Is identification. The arrowhead beneath the stimulus artifact in A denotes bimorph stimulation. B: an example of an EPSP recorded from the Is interneuron evoked by illumination of the photoreceptors. C: histogram of EPSPs (10-s bin width) recorded from a dark-adapted type Is interneuron, during 5 min of light-attenuated 1 log unit and during 5 min in the dark following light offset. The arrows above the histogram indicate the onset and offset of light. D: type Is interneuron EPSPs contributing to the histogram in C. Resting membrane potential of the Is interneuron was 60–61 mV. E: light response of a type Is interneuron depolarized to evoke spikes prior to the onset of illumination. Arrows indicate the onset and offset of light-attenuated 1 log unit. F: group summary data of mean spike activity of type Is interneurons (n = 5) depolarized to evoke spike activity in the dark (5 min) and during light (5 min). The light was attenuated 1 log unit from maximum intensity; *P < 0.003.
As shown in Fig. 6A, depolarization of an identified Is interneuron evoked spikes recorded from a ventral contractile (VC) efferent neuron that innervates the anterior region of the foot. Depolarization of a different Is interneuron also evoked spikes recorded from a tail contractile (TC1) neuron (Fig. 6B).
FIG. 6.
Type Is interneurons project to identified efferent neurons. Simultaneous recordings from Is interneurons and efferent neurons in semi-intact preparations. A: depolarizing current stimulation of an Is interneuron evoked spikes recorded from an identified ventral contractile (VC) efferent neuron. B: depolarizing current stimulation of a different Is interneuron elicited a weak response in an identified TC1 efferent neuron.
Depolarization of Ib and Is interneurons in semi-intact preparations
Since type Ib interneurons have synaptic connections with foot contractile efferent neurons we examined the effect of current depolarization of Ib interneurons on contraction of the foot in semi-intact preparations (n = 12 preparations). Depolarization of contralateral type Ib interneurons produced contraction of the ipsilateral tail and lateral foot along the rostrocaudal axis of the foot. A representative example of the configuration of the foot immediately before current stimulation of an Ib interneuron is indicated by the white outline in Fig. 7A. The position of the ipsilateral foot at the end of the current pulse is indicated by the red outline in the photograph shown in Fig. 7A. The 5-s current depolarization of the Ib interneuron that evoked the foot contraction is shown in the inset of Fig. 7A. Current depolarization of Is interneurons in semi-intact preparations (n = 6) evoked a small contraction of the anterior region of the foot. The black line in the photograph of Fig. 7B indicates the position of the ipsilateral anterior foot immediately before current stimulation of the Is interneuron. Anterior foot position at the end of the current pulse is indicated by the red line in the photograph. The current depolarization of the Is interneuron that evoked movement of the anterior foot is shown in the inset of Fig. 7B.
FIG. 7.
A: photograph of a representative semi-intact preparation before stimulation and at the termination of a 5-s depolarizing current pulse delivered to a type Ib interneuron. The white outline of the ipsilateral perimeter of the foot shows the foot immediately before stimulation and the red outline, the position of the foot at the end of the current pulse. Type Ib depolarization elicited a contraction and shortening of the rostral–caudal axis of the ipsilateral foot. The inset depicts spike activity of the Ib interneuron immediately before and during the 5-s current pulse. B: photograph of a representative semi-intact preparation before stimulation and at the termination of a 5-s depolarizing current pulse delivered to a type Is interneuron. The outline of the anterior foot before stimulation is shown in black and, at the end of the current pulse, the anterior foot position is indicated by the red line. The inset shows the spike activity of the Is interneuron immediately before and during the 5-s current pulse.
Visual input to foot contractile efferent neurons
Our results indicate that type Ib and type Is interneurons receive synaptic input from the visual system. Visual input to identified foot contractile efferent neurons was examined by measuring changes in spike activity of identified efferent neurons in semi-intact preparations during illumination of photoreceptors relative to dark-adapted baseline activity. Analysis of the group summary data for recordings in TC efferent neurons (n = 8) did not reveal statistically significant changes in spike activity during a 5-min presentation of light (mean = 0.87 ± 0.24 spikes/s) compared with a 5-min period in the dark (mean = 0.89 ± 0.25 spikes/s) immediately preceding the light (t7 = 0.9; NS). Although TC efferent neurons may not exhibit changes in spike activity expressed over a 5-min period of illumination, they may show transient “on” responses to illumination of the photoreceptors following dark-adapted conditions. We examined changes in spike activity of identified TC efferent neurons during two consecutive 5-s epochs following the onset of light compared with the average spike activity recorded during two 5-s periods in the dark immediately preceding light onset. Results of the ANOVA did not support statistically significant differences in spike activity relative to dark-adapted prelight baseline activity in either 5-s period following the onset of light-attenuated 1 log unit (n = 7) [F(2,6) = 1.6, NS] or bright light (0 attenuation) (n = 5) [F(2,4) = 1.8; NS].
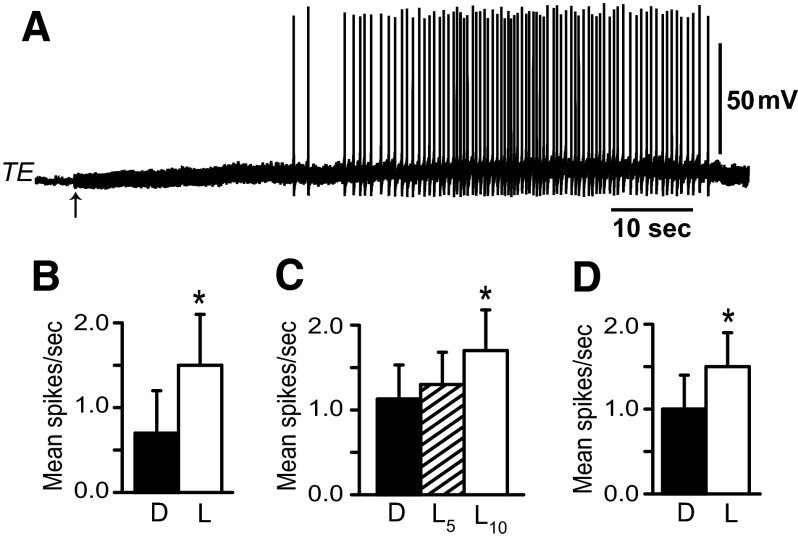
Examination of light-evoked spike activity in identified VC efferent neurons (n = 7) during the first two 5-s epochs following the onset of light (mean = 0.40 ± 0.183 and 0.40 ± 0.15 spikes/s) compared with activity in the dark immediately before light (mean = 0.46 ± 0.18 spikes/s) also did not reveal statistically significant changes [F(2,6) = 0.7; NS]. In contrast to the results of illumination on the spike activity of TC and VC neurons, both TE efferent neurons and LFC efferent neurons exhibited increased spike activity evoked by illumination of the photoreceptors. As shown in the recording in Fig. 8A, light elicited an increase in the spike activity of an identified TE efferent neuron. Short-latency “on” responses were not characteristic of light-evoked activity of TE efferent neurons. Analysis of the group data shown in Fig. 8B revealed that mean spike activity of TE efferent neurons during light (mean = 1.5 ± 0.6 spikes/s) was significantly greater than the corresponding period in the dark (mean = 0.7 ± 0.5 spikes/s) (t4 = 3.4; P < 0.025). In contrast to TE efferent neurons, recordings of spike activity in LFC efferent neurons (n = 10) exhibited “on” responses to illumination of the photoreceptors. Statistical analysis of the group data shown in Fig. 8C revealed an overall significant difference in spike activity in light compared with dark-adapted baseline activity [F(2,9) = 4.3; P < 0.03]. Paired comparisons (Tukey test) showed that the first 5-s epoch after light onset was not statistically different (mean = 1.3 ± 0.38 spikes/s) compared with the dark immediately preceding light (mean = 1.13 ± 0.4 spikes/s). However, the second 5-s epoch after light onset (mean = 1.7 ± 0.48 spikes/s) was significantly different from baseline (P < 0.05). In addition, the mean activity of LFC efferent neurons during a 2-min light presentation (Fig. 8D) (mean = 1.48 ± 0.4 spikes/s) was significantly greater than the equivalent period in the dark before the presentation of light (mean = 1.05 ± 0.4 spikes/s) (t9 = 2.57; P < 0.025).
FIG. 8.
A: recording from a tail extension (TE) efferent neuron showing an increase in spike activity elicited by light-attenuated 1 log unit. The arrow indicates the onset of light. B: group data of mean spike activity recorded from tail extension efferent neurons (n = 5) immediately before light onset (D) and during the presentation of light-attenuated 1 log unit (L); *P < 0.025. C: group data of mean spike activity recorded from lateral foot contractile (LFC) efferent neurons (n = 10) averaged from two 5-s epochs in the dark (D) immediately preceding light onset, average spike activity recorded during the first 5-s epoch in light (L5), and the second 5-s epoch in light (L10). Illumination of the photoreceptors elicited an “on” response in LFC efferent neurons. Light-attenuated 1 log unit from maximum intensity; *P < 0.05. D: group data depicting mean spike activity of LFC efferent neurons (n = 10) in the dark before light onset (D) and during a 2-min presentation of light-attenuated 1 log unit; *P < 0.025.
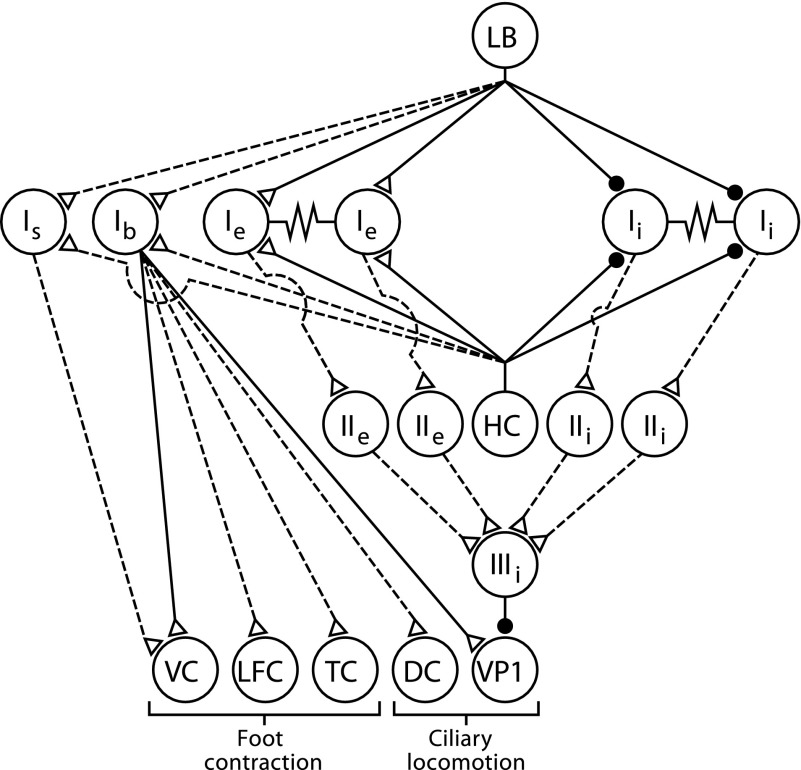
A diagram summarizing the synaptic connections between interneurons and efferent neurons identified in this study and previously identified components of the ciliary locomotion circuitry is shown in Fig. 9. As previously noted (Tian and Crow 2004) there is little overlap between the components of the graviceptive foot contraction circuit and the light-elicited ciliary locomotion circuit. However, type Ib interneurons that have synaptic connections with foot contractile efferent neurons also project to efferent neurons that activate cilia on the anterior and posterior foot.
FIG. 9.
Neural circuit supporting ciliary locomotion and foot contraction. Diagram of the sensory neurons, interneurons, efferent neurons, and synaptic connections. The synaptic connections from only one identified photoreceptor [lateral B (LB)] to the type I interneurons is shown. However, each photoreceptor within the eye forms monosynaptic connections with different aggregates of type Ie and type Ii interneurons (Crow and Tian 2000). Statocyst hair cells (HCs) form monosynaptic connections with type I interneurons (Akaike and Alkon 1980). The synaptic connection between type IIIi inhibitory interneurons and ciliary efferent neurons (VP1) are monosynaptic. Type Ib and type Is polysensory interneurons receive input from the visual and graviceptive sensory systems and project to identified foot contractile efferent neurons. Ventral contractile (VC), lateral foot contractile (LFC), tail contractile (TC), and ciliary efferent neurons on the dorsal surface of the pedal ganglia (DC). Filled circles, inhibitory synapses; open triangles, excitatory synapses; solid lines, monosynaptic connections; dashed lines, polysynaptic or synaptic connections where potential monosynaptic inputs have not been tested.
DISCUSSION
Neural circuit supporting ciliary locomotion
Of the two behaviors that have been studied in Hermissenda, the neural circuit controlling ciliary locomotion and its modulation by the visual system is more extensively characterized (see Fig. 9). The neural circuit supporting ciliary locomotion consists of polysynaptic projections from type I interneurons to type IIIi inhibitory interneurons that form monosynaptic inhibitory connections with ciliary efferent neurons (Crow and Tian 2003a, 2004). Both type Ie and Ii interneurons excite type IIIi inhibitory interneurons, resulting in increased inhibition of ciliary efferent neuron spike activity. The visual system modulates ciliary locomotion by the monosynaptic connections from photoreceptors to aggregates of type I interneurons that result in light-evoked inhibition of Ii interneurons and light-evoked excitation of Ie interneurons (Akaike and Alkon 1980; Crow and Tian 2000, 2002a,b). The net effect of illumination is an inhibition of excitatory synaptic input from type I interneurons to type IIIi interneurons that produces a decrease in inhibitory synaptic input to ciliary efferent neurons and an increase in the spike frequency of ciliary efferent neurons. A second interneuronal projection in the circuit is from the type Ib interneurons that form monosynaptic connections with ciliary efferent neurons (Crow and Tian 2004). However, Ib interneurons contribute more to the modulation of ciliary efferent neuron activity produced by the graviceptive sensory system than the visual system. Current stimulation of statocyst hair cells produces complex EPSPs in type Ib interneurons and increased Ib spike activity (Crow and Tian 2004). Mechanical activation of the statocyst with bimorph stimulation also evokes EPSPs and spikes recorded in Ib interneurons. Here we show that light evokes EPSPs in type Ib interneurons, but not spikes. However, depolarization of Ib interneurons to a potential that generates spike activity before and during the presentation of light revealed a modest light-evoked increase in Ib spike activity. This suggests that type Ib interneurons receive synaptic input from the visual system that under normal conditions is not sufficient to generate spikes since Ib interneurons are typically not spontaneously active. Therefore Ib interneurons do not make major contributions to the neural circuit underlying visually modulated ciliary locomotion, even though Ib interneurons form monosynaptic connections with identified ciliary efferent neurons. However, Ib interneurons contribute to the activity of the graviceptive circuitry since statocyst hair cell activation generates both EPSPs and spikes in Ib interneurons and efferent neurons. Here we have shown that Ib interneurons project to efferent neurons that innervate different regions of the foot. Taken collectively, the results suggest that light modulation of ciliary locomotion and its modification by Pavlovian conditioning involves circuit components that may be distinct from the graviceptive system that contributes to foot contraction.
Is interneurons
Type Is interneurons are polysensory and receive synaptic input from the graviceptive, somatosensory, and visual systems. Bimorph stimulation of the statocyst evoked EPSPs and spikes recorded from identified Is interneurons and cutaneous stimulation of the foot evoked EPSPs and spikes in Is interneurons. Type Is interneurons do not typically generate spikes in response to illumination, although light elicited an increase in EPSP frequency recorded from Is interneurons. However, current depolarization of Is interneurons sufficient to generate spikes revealed a modest increase in spike activity elicited during illumination compared with dark-adapted baseline activity. Here we show that type Is interneurons also project to VC efferent neurons that innervate the anterior region of the foot and current stimulation of Is interneurons in semi-intact preparations produced anterior foot contraction.
Neural circuitry contributing to foot-shortening
In the present study we have identified efferent neurons that innervate different areas of the foot that result in specific changes in foot configuration. The movements evoked by stimulation involve tail contraction, lateral foot contraction, contraction of the midfoot region, and tail extension. Efferent neurons innervating the anterior region of the foot have been previously identified (Crow and Tian 2003a, 2004). Interneuronal synaptic projections to efferent neurons have also been characterized in the present study. Current depolarization of Ib interneurons evoked spikes in TC, LFC, and DC efferent neurons and contraction of the rostrocaudal axis of the foot, which is similar to hair cell–mediated foot-shortening.
The absence of light-evoked spike activity in Ib and Is interneurons would suggest that foot contraction efferent neurons identified in this study may not respond to illumination. This observation is consistent with previous research showing that light does not elicit the clinging reflex (Alkon 1974) or evoke foot-shortening prior to conditioning (Lederhendler et al. 1986; Matzel et al. 1990). However, an identified putative motor neuron (MN1) was shown to increase spike activity in response to light stimulation of the eyes, activation of statocyst hair cells, interneuron depolarization, and current depolarization of type A photoreceptors (Goh and Alkon 1984). Current depolarization of MN1 also produced turning of the ipsilateral posterior foot in restrained semi-intact preparations (Goh and Alkon 1984). Taken collectively, the results of the Goh and Alkon (1984) study suggest that MN1 does not contribute to graviceptive-elicited foot-shortening. In addition, there are differences between MN1 and TC efferent neurons identified in this study regarding their response to illumination. Statistically significant changes in spike frequency of MN1 elicited by light in nonconditioned animals occurred primarily during the first 5 s of illumination (Goh et al. 1985). We found that TC efferent neurons exhibited a modest, but nonstatistically significant, increase in spike activity during the first 5 s of illumination (see results). However, spike activity had returned to dark-adapted baseline levels during the second 5 s of light, which is also consistent with the previous report of MN1 spike activity (Goh et al. 1985). Moreover, average spike activity recorded from identified TC efferent neurons during 5 min of light was not significantly different from dark-adapted baseline activity collected 5 min immediately before light or 5 min of dark-adaptation after the termination of light. In addition, we did not observe reliable evidence for post-illumination spike bursting of TC efferent neurons, as was reported in the previous study of MN1 (Goh and Alkon 1984). Our findings indicate that stimulation of the graviceptive system makes the major contribution to contraction of different regions of the foot through polysynaptic interneuronal projects to foot contractile efferent neurons. With the exception of TE and LFC efferent neurons, light does not elicit foot contractions or produce significant changes in the spike activity of TC or VC efferent neurons. However, both TE and LFC efferent neurons may contribute to light-elicited increased foot length observed before conditioning (Lederhendler et al. 1986). Our analysis of the foot contraction circuitry is consistent with the behavioral observations showing that light does not elicit foot-shortening prior to conditioning (Lederhendler et al. 1986; Matzel et al. 1990).
Circuitry supporting the CR complex
The acquisition of multiple conditioned responses produced by pairings of a single CS with a single US is characteristic of many Pavlovian-conditioning preparations. Somatomotor CRs such as eyeblink, nictitating membrane, or leg flexion, are accompanied by concomitant conditioned visceral changes involving heart rate, blood pressure, skin conductance, and pupillary diameter. Visceral CRs are typically rapidly acquired and occur earlier in training than the more slowly acquired somatomotor responses (for reviews see Cohen and Randall 1984; Weinberger and Diamond 1987). The same Pavlovian-conditioning procedure in Hermissenda produces two different behavioral CRs: CS-elicited inhibition of locomotion and foot-shortening (Crow and Alkon 1978; Lederhendler et al. 1986). The two CRs develop at different rates and may involve different efferent pathways, suggesting that CS-elicited foot-shortening and light-elicited inhibition of locomotion may develop independently (Matzel et al. 1990). The two behaviors are quite dissimilar since graviceptive-evoked foot-shortening is muscular and phasic and light modulation of ciliary locomotion is nonmuscular and tonic. However, motor networks in diverse species have been shown to contribute to the generation of multiple behaviors (for reviews see Briggman and Kristan Jr 2008; Morton and Chiel 1994). There is also evidence that dissimilar behaviors may be generated from the same neural network (Popescu and Frost 2002). To date, analyses of the neural circuitry supporting foot-shortening and ciliary locomotion and its modification by conditioning suggest that CS- and US-elicited inhibition of ciliary locomotion and US-elicited foot-shortening involve different interneuronal circuit components (Crow and Tian 2003b, 2004). Results of the present study are consistent with the hypothesis that US-evoked foot-shortening and US-reduced locomotion emerge independently (Matzel et al. 1990). Previous research has identified intrinsic changes with Pavlovian conditioning in two cell types: photoreceptors and type I interneurons (Crow and Alkon 1980; Crow and Tian 2003b; Farley et al. 1990). Neural correlates of Pavlovian conditioning—identified in the neural circuit supporting ciliary locomotion and CS-elicited inhibition of ciliary efferent neuron activity—are now well documented (Crow and Tian 2003b). However, modification of the neural circuit responsible for CS-elicited foot-shortening is not documented. As shown in the present study, with the exception of LFC efferent neurons and TE efferent neurons, light has little effect on the activity of the circuit activated by the graviceptive system that elicits foot contractions. However, it is intriguing to speculate that this circuit can be reconfigured by conditioning to produce CS-elicited foot-shortening and changes in intrinsic excitability and synaptic strength in circuit components such as the Ib and Is interneurons that do not typically exhibit responses to the CS in nonconditioned preparations.
GRANTS
This research was supported by National Institute of Mental Health Grant MH-58698.
Acknowledgments
We thank D. Parker for assistance with the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Akaike and Alkon 1980.Akaike T, Alkon DL. Sensory convergence on central visual neurons in Hermissenda. J Neurophysiol 44: 501–513, 1980. [DOI] [PubMed] [Google Scholar]
- Alkon 1974.Alkon DL Associative training of Hermissenda. J Gen Physiol 64: 70–84, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon and Bak 1973.Alkon DL, Bak A. Hair cell generator potentials. J Gen Physiol 61: 619–637, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers and Powell 2002.Ayers ED, Powell DA. Multiple response measures during classical conditioning. J Neurosci Methods 114: 33–38, 2002. [DOI] [PubMed] [Google Scholar]
- Black and de Toledo 1972.Black AH, de Toledo L. The relationship among classically conditioned responses: heart rate and skeletal behavior. In: Classical Conditioning II: Current Research and Theory, edited by Black AH, Prokasy WF. New York: Appleton-Century-Crofts, 1972, p. 290–311.
- Briggman and Kristan 2008.Briggman KL, Kristan WB Jr. Multifunctional pattern-generating circuits. Annu Rev Neurosci 31: 271–294, 2008. [DOI] [PubMed] [Google Scholar]
- Buchanan and Powell 1982.Buchanan SL, Powell DA. Cingulate cortex: its role in Pavlovian conditioning. J Comp Physiol Psychol 96: 855–774, 1982. [DOI] [PubMed] [Google Scholar]
- Cohen and Randall 1984.Cohen DH, Randall DC. Classical conditioning of cardiovascular responses. Annu Rev Physiol 46: 187–197, 1984. [DOI] [PubMed] [Google Scholar]
- Crow and Alkon 1978.Crow T, Alkon DL. Retention of an associative behavioral change in Hermissenda. Science 201: 1239–1241, 1978. [DOI] [PubMed] [Google Scholar]
- Crow and Alkon 1980.Crow T, Alkon DL. Associative behavioral modification in Hermissenda: cellular correlates. Science 209: 412–414, 1980. [DOI] [PubMed] [Google Scholar]
- Crow and Tian 2000.Crow T, Tian LM. Monosynaptic connections between identified A and B photoreceptors and interneurons in Hermissenda: evidence for labeled-lines. J Neurophysiol 84: 367–375, 2000. [DOI] [PubMed] [Google Scholar]
- Crow and Tian 2002a.Crow T, Tian LM. Morphological characteristics and central projections of two types of interneurons in the visual pathway of Hermissenda. J Neurophysiol 87: 322–332, 2002a. [DOI] [PubMed] [Google Scholar]
- Crow and Tian 2002b.Crow T, Tian LM. Facilitation of monosynaptic and complex PSPs in type I interneurons of conditioned Hermissenda. J Neurosci 22: 7818–7824, 2002b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow and Tian 2003a.Crow T, Tian LM. Interneuronal projections to identified cilia-activating pedal neurons in Hermissenda. J Neurophysiol 84: 2420–2429, 2003a. [DOI] [PubMed] [Google Scholar]
- Crow and Tian 2003b.Crow T, Tian LM. Neural correlates of Pavlovian conditioning in components of the neural network supporting ciliary locomotion in Hermissenda. Learn Mem 10: 209–216, 2003b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow and Tian 2004.Crow T, Tian LM. Statocyst hair cell activation of identified interneurons and foot contraction motor neurons in Hermissenda. J Neurophysiol 92: 2874–2883, 2004. [DOI] [PubMed] [Google Scholar]
- Detwiler and Alkon 1973.Detwiler PB, Alkon DL. Hair cell interactions in the statocyst of Hermissenda. J Gen Physiol 62: 618–642, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley et al. 1990.Farley J, Richards WG, Grover LM. Associative learning changes intrinsic to Hermissenda type A photoreceptors. Behav Neurosci 104: 135–152, 1990. [DOI] [PubMed] [Google Scholar]
- Gantt 1960.Gantt WH Cardiovascular component of the conditioned reflex to pain, foods and other stimuli. Physiol Rev 40: 266–291, 1960. [PubMed] [Google Scholar]
- Godsil et al. 2000.Godsil BP, Quinn JJ, Fanselow MS. Body temperature as a conditional response measure for Pavlovian fear conditioning. Learn Mem 7: 353–356, 2000. [DOI] [PubMed] [Google Scholar]
- Goh and Alkon 1984.Goh Y, Alkon DL. Sensory, interneuronal, and motor interactions within Hermissenda visual pathway. J Neurophysiol 52: 156–169, 1984. [DOI] [PubMed] [Google Scholar]
- Goh et al. 1985.Goh Y, Lederhendler I, Alkon DL. Input and output changes of an identified neural pathway are correlated with associative learning in Hermissenda. J Neurosci 5: 536–543, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green and Woodruff-Pak 2000.Green JT, Woodruff-Pak DS. Eyeblink classical conditioning: hippocampal formation is for neutral stimulus associations as cerebellum is for association-response. Psychol Bull 126: 138–158, 2000. [DOI] [PubMed] [Google Scholar]
- Jerussi and Alkon 1981.Jerussi TP, Alkon DL. Ocular and extraocular responses of identifiable neurons in pedal ganglia of Hermissenda crassicornis. J Neurophysiol 46: 659–671, 1981. [DOI] [PubMed] [Google Scholar]
- Kao and Powell 1988.Kao KT, Powell DA. Lesions in the substantia nigra retard Pavlovian eye-blink but not heart rate conditioning in the rabbit. Behav Neurosci 102: 515–525, 1988. [DOI] [PubMed] [Google Scholar]
- Konorski 1967.Konorski J Integrative Activity of the Brain. Chicago, IL: Univ. of Chicago Press, 1967.
- Lavond et al. 1984.Lavond DG, Lincoln JS, McCormick DA, Thompson RF. Effect of bilateral lesions of the dentate and interpositus cerebellar nuclei on conditioning of heart rate and nictitating membrane eyelid responses in the rabbit. Brain Res 305: 233–320, 1984. [DOI] [PubMed] [Google Scholar]
- Lederhendler et al. 1986.Lederhendler II, Gart S, Alkon DL. Classical conditioning of Hermissenda: origin of a new response. J Neurosci 6: 1325–1331, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel et al. 1990.Matzel LD, Schreurs BG, Alkon DL. Pavlovian conditioning of distinct components of Hermissenda's response to rotation. Behav Neurol Biol 54: 131–145, 1990. [DOI] [PubMed] [Google Scholar]
- McLaughlin et al. 2002.McLaughlin J, Skaggs H, Churchwell J, Powell DA. Medial prefrontal cortex and Pavlovian conditioning: trace versus delay conditioning. Behav Neurosci 116: 37–47, 2002. [PubMed] [Google Scholar]
- Morton and Chiel 1994.Morton DW, Chiel HJ. Neural architectures for adaptive behavior. Trends Neurosci 17: 413–430, 1994. [DOI] [PubMed] [Google Scholar]
- Popescu and Frost 2002.Popescu IR, Frost WN. Highly dissimilar behaviors mediated by a multifunctional network in the marine mollusk, Tritonia diomedea. J Neurosci 22: 1985–1993, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell 1999.Powell DA A behavioral stages model of classical (Pavlovian) conditioning: application to cognitive aging. Neurosci Biobehav Rev 23: 797–816, 1999. [DOI] [PubMed] [Google Scholar]
- Prokasy 1984.Prokasy WF Acquisition of skeletal conditioned responses in Pavlovian conditioning. Psychophysiology 21: 1–13, 1984.6701237 [Google Scholar]
- Richards and Farley 1987.Richards WG, Farley J. Motor correlates of phototaxis and associative learning. Brain Res Bull 19: 174–189, 1987. [DOI] [PubMed] [Google Scholar]
- Schneiderman 1972.Schneiderman N Response system divergences in aversive classical conditioning. In: Classical Conditioning II: Current Theory and Research, edited by Black AH, Prokasy WF. New York: Appleton-Century-Crofts, 1972, p. 341–376.
- Tian et al. 2006.Tian L-M, Kawai R, Crow T. Serotonin-immunoreactive CPT interneurons in Hermissenda: Identification of sensory input and motor projections. J Neurophysiol 96: 327–335, 2006. [DOI] [PubMed] [Google Scholar]
- Weinberger and Diamond 1987.Weinberger NM, Diamond DM. Physiological plasticity in auditory cortex: rapid induction by learning. Prog Neurobiol 29: 1–55, 1987. [DOI] [PubMed] [Google Scholar]