Abstract
Midbrain dopamine neuron activity results from the integration of the responses to metabo- and ionotropic receptors with the postsynaptic excitability of these intrinsic pacemakers. Interestingly, intrinsic pacemaker rate varies greatly between individual dopamine neurons and is subject to short- and long-term regulation. Here responses of substantia nigra dopamine neurons to defined dynamic-clamp stimuli were measured to quantify the impact of cell-to-cell variation in intrinsic pacemaker rate. Then this approach was repeated in single dopamine neurons in which pacemaker rate was altered by activation of muscarinic receptors or current injection. These experiments revealed a dramatic exponential dependence on pacemaker interval for the responses to voltage-gated A-type K+ channels, voltage-independent cation channels and ionotropic synapses. Likewise, responses to native metabotropic (GABAb and mGluR1) inhibitory synapses depended steeply on pacemaker interval. These results show that observed variations in dopamine neuron pacemaker rate are functionally significant because they produce a >10-fold difference in responses to diverse stimuli. Both the magnitude and the mathematical form of the relationship between pacemaker interval and responses were not previously anticipated.
INTRODUCTION
Dopamine (DA) neuron activity in vivo displays a variety of patterns including bursts and regular rhythmic activity (Grace and Bunney 1984a,b; Hyland et al. 2002; Wilson et al. 1977). These patterns arise from the interaction of iono- and metabotropic receptor responses with the intrinsic excitability of these neurons. Unlike most neurons in the brain, DA neurons spontaneously produce repetitive regular activity after isolation or reduction of synaptic input (i.e., by generating brain slices) (Hainsworth et al. 1991; Kita et al. 1986). In fact, this intrinsic pacemaker activity is highly variable between individual DA neurons and subject to short- and long-term regulation (Dai and Tepper 1998; Franz et al. 2000; Hahn et al. 2003, 2006; Liss et al. 2001; Yang et al. 2001). Because DA neurons don't control timing (i.e., the role of many pacemakers), their pacemaker properties may influence computation. Specifically, there must be some effect of the variations in intrinsic excitability that produce pacemaker rate differences (Hahn et al. 2003, 2006; Liss et al. 2001) on the efficacy of synapses, modulators, and drugs (e.g., near the maximal firing rate, responses will be reduced). Yet the impact of variation in intrinsic pacemaker activity has not been measured. Furthermore, it is unclear whether all DA neuron responses share the same dependence on pacemaker rate.
Determining the relationship between the DA neuron responses and their varied intrinsic pacemaker rates is technically challenging with standard approaches. First, synapses are usually studied in isolation by preventing pacemaker activity (i.e., in hyperpolarized cells), whereas the intrinsic pacemaker mechanism is often studied in the absence of synaptic activity. Second, because each DA neuron is regulated by a large variety of synapses, it is difficult to stimulate an identical input on individual DA neurons with varied intrinsic properties. These difficulties can be bypassed with the dynamic clamp, a method that allows the investigator to acutely and specifically add virtual channels to a single neuron (Prinz et al. 2004). The dynamic clamp has already been applied to study Kv4.3 channels in cultured DA neurons and episodically activated synaptic channels in other neurons (Desai and Walcott 2006; Fernandez and White 2008; Hahn et al. 2006; Kullmann and Horn 2006). Thus the dynamic clamp could be used to directly quantify how variations in DA neuron pacemaker rate found in substantia nigra brain slices affect the response to experimentally defined stimuli.
This study begins by using virtual Kv4.3 channels as a defined stimulus because native Kv4.3 channels mediate acute and long-term modulation of DA neuron excitability induced by GDNF and D2 autoreceptors, respectively (Hahn et al. 2003, 2006; Yang et al. 2001). After determining that pacemaker diversity between DA neurons has a dramatic impact, the effect of pacemaker rate is shown to apply to individual DA neurons in which activity is altered by current injection or activation of native muscarinic receptors. This steep exponential relationship is also conserved for responses to virtual voltage-independent cation channels, virtual ionotropic synapses, and native metabotropic synapses. Hence known variations in pacemaker rate are functionally significant because they alter DA neuron responses to a wide variety of stimuli by >10-fold.
METHODS
Brain slices
Reagents were from Sigma-Aldrich if not stated otherwise. All experiments were conducted in accordance with protocols approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Sprague Dawley rats (postnatal days 14–21, Hilltop Labs) were anesthetized with isoflurane and decapitated. The brain was removed and placed into ice-cold, 95% O2-5% CO2-saturated, sucrose-based artificial cerebrospinal fluid (s-ACSF, containing in mM: 87 NaCl, 75 sucrose, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 0.5 CaCl2, 7.0 MgSO4, 25 glucose, 0.15 ascorbic acid, and 1 kynurenic acid, pH 7.4). Coronal midbrain slices (250 μm) were cut on a vibratome (Vibratome 3000, Vibratome). The slices were incubated in s-ACSF at room temperature for ≥1 h before the experiments.
Patch clamping
Substantia nigra pars compacta DA neurons were identified based on location, morphology and characteristic electrophysiological properties (Dai and Tepper 1998; Grace and Bunney 1983; Lacey et al. 1989; Neuhoff et al. 2002; Paxinos and Watson 2005). Whole cell recordings were performed at 30–32°C using an AM Systems 2400 amplifier. This patch-clamp amplifier is set up for fast true current-clamp recording, which is required for dynamic clamp. The resistance of patch-clamp electrodes was 2–4 MΩ. The pipette solution (containing, in mM: 120 potassium gluconate, 20 KCl, 10 HEPES, 2 MgCl2, 0.1 EGTA, and 1.2 ATP, pH 7.3) was chosen so that pacemaker activity recorded initially in the on-cell configuration (Perkins 2006) was not perturbed following breaking into the whole cell configuration. Over the course of experiments (≤30 min after changing to whole cell configuration), no differences in mean interspike interval (ISI) and its coefficient of variation were detected (n = 8; data not shown). Oxygenated standard ACSF (containing, in mM: 124 NaCl, 4 KCl, 25.7 NaHCO3, 1.25 NaH2PO4, 2.45 CaCl2, 1.2 MgSO4, 11 glucose, and 0.15 ascorbic acid, pH 7.4) was superfused over the slice at 1–2 ml/min. Inclusion of blockers of AMPA, N-methyl-d-aspartate (NMDA), and GABAA receptors did not affect pacemaker activity, showing that background synaptic activity was not significant (n = 11; data not shown).
Dynamic clamp
The dynamic-clamp setup and the virtual DA neuron Kv4.3 A-type K+ conductance, the nonspecific cation conductance, and implementation of virtual synaptic conductances have been described previously (Hahn et al. 2006; Kullmann and Horn 2006; Kullmann et al. 2004). In brief, the equation for A-type K conductance was IA(V,t) = gA m(V,t) h(V,t) (V –Erev), with Erev = −84 mV, dm(V,t)/dt = [m∞(V) –m(V,t)]/τm(V), m∞(V) = 1/{1 + exp[–(V + 24.8)/13.9]}, τm(V) = 2 –(1.6/{1 + exp[–(V + 20)/15]}), dh(V,t)/dt = [h∞(V) –h(V,t)]/τh(V); h∞(V) = 1/{1 + exp[(V + 78.7)/9.2]}, and τh(V) = 28 –(9.4/{1 + exp[−(V –2)/16]}). The equations for the synaptic conductance were Isyn(t) = gsyn(t) (V –Erev) and gsyn(t) = k [exp(−t/τrise) − exp(−t/τfall)], with τrise = 1 ms, τrise = 5 ms, and Erev = 0 mV for excitation and −65 mV for inhibition.
Stimulation of native synapses
Field stimulation of the substantia nigra pars reticulata was induced with square-wave pulses (10–60 V, 100 μs) through a bipolar stainless-steel stimulating electrode placed 200–1000 μm away from the recorded neuron (Mereu et al. 1991). The stimulator was triggered by the same computer generated random 5-Hz protocol used for dynamic-clamp application of virtual synaptic conductances.
Drug application
Where indicated, ionotropic receptor inhibitors were superfused over the slice: 50 μM picrotoxin (a GABAA receptor antagonist), 50 μM d-AP5 (an NMDA-receptor antagonist), and 10 μM 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX; an AMPA/kainate receptor antagonist). To block metabotropic inhibitory receptors, 30 μM CGP35348 (a GABAb receptor antagonist) and 100 μM 1-aminoindan-1,5-dicarboxylic acid (AIDA; an mGluR1 receptor antagonist) were superfused over the slice. Muscarine was added by superperfusion at a final concentration of 1 μM.
Data analysis
Data are displayed as mean with bars indicating SE. Linear and nonlinear regression fits, along with 95% confidence intervals (displayed as - - -), were calculated with Graphpad Prism. Mean baseline and altered steady-state ISIs were calculated from ≥10 values. The initial change in ISI (ΔISIi) equaled the difference between the first ISI after changing firing (e.g., after addition of A-conductance) and baseline ISI (ISIB), while steady-state ΔISI was calculated as the absolute difference of ISIB and new steady-state ISI (e.g., from the last 20 s of the period after ISI is altered).
RESULTS
Relationship between pacemaker interval and the response to A-type K+ channels
Spontaneous pacemaker activity of substantia nigra pars compacta DA neurons was recorded in rat brain slices. As described in methods, these measurements were not affected by the recording configuration or background synaptic activity. We began our analysis by using the dynamic clamp to add 100 nS of Kv4.3 A-type K+ channel conductance (gKA) to DA neurons with diverse intrinsic pacemaker rates. Kv4.3 channels were chosen because both their gating and expression are controlled in DA neurons and this channel has already been implemented in the dynamic clamp (Hahn et al. 2006; Yang et al. 2001). Thus the dynamic clamp could be used to quantify whether the impact of such changes in Kv4.3 activity would be significantly affected by the known variation in pacemaker rate found among individual DA neurons.
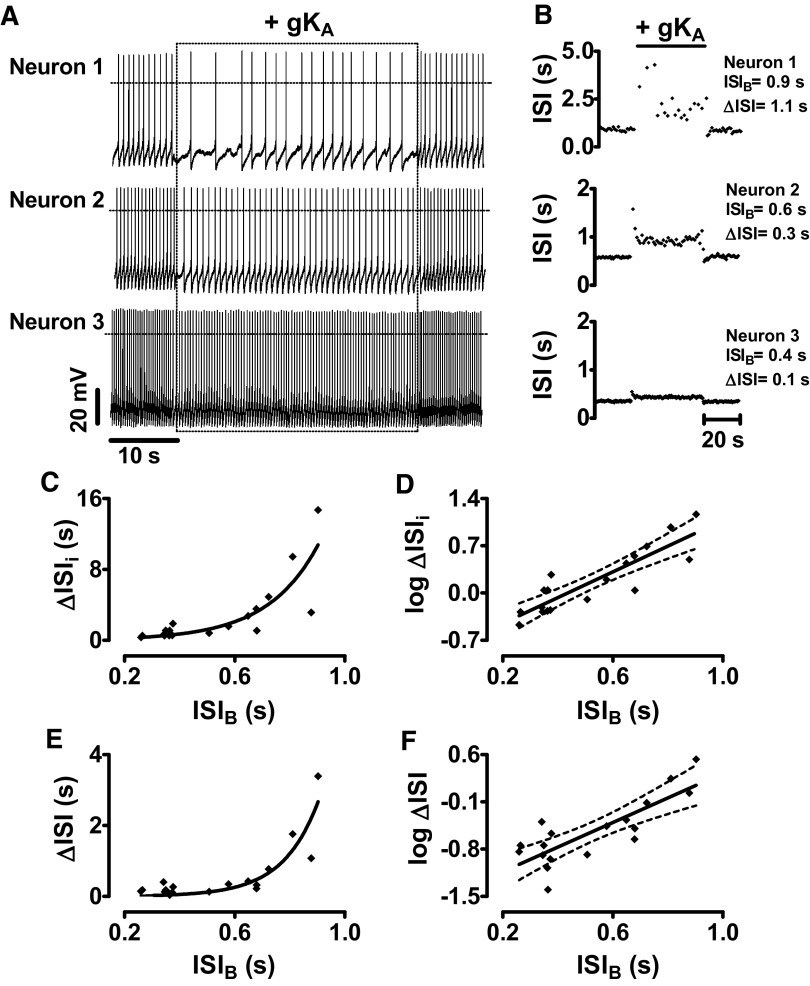
Addition of virtual A-type channels elicited an initial increase in the ISI, which was then followed by a period of adaptation before a new steady-state pacemaker rate was established (Fig. 1, A and B). However, the size of the response depended dramatically on baseline pacemaker interval (Fig. 1, A and B; note the varying y-axis scales in B). Linear and semi-log plots show that both the initial and steady-state changes in ISI (ΔISIi and ΔISI) increased exponentially with baseline ISI (ISIB; Fig. 1, C–F). The similarity in the relationships for the initial and sustained changes in firing implies that the effect of pacemaker interval on the response to A-type channels is independent of adaptation. Furthermore, the fit over a wide range of intervals implies that this relationship does not reflect a simple saturation effect. Most importantly, the diversity of pacemaker rates among individual DA neurons is associated with dramatic variation in the response to a change in A-type channel activity.
FIG. 1.
Dopamine (DA) neuron responses to A-type channels exponentially increase with pacemaker interval. A: dynamic-clamp experiments showing the effect of adding 100 nS A-type potassium conductance (gKA) for 40 s to 3 DA neurons with different intrinsic pacemaker rates. - - -, 0 mV. B: interspike interval (ISI)/time plot for the recordings shown in A. Note the different y-axis scales. C: initial ΔISI (ΔISIi) plotted vs. the intrinsic pacemaker interval for 18 individual neurons. The line is a fit to ΔISI = a + exp(k*ISIB), with a = 0.0081, k = 5.42. D: semi-log plot of the data in C. Slope of linear regression equaled 1.9, r2 = 0.78. E: steady-state ΔISI plotted versus the intrinsic ISI for the same neurons in C. The line is a fit to ΔISI = a + exp(k*ISIB), with a = 0.0024, k = 7.755. F: semi-log display of E. Slope of linear regression: 1.82, r2 = 0.66. Dashed lines in D and F show 95% confidence intervals. Note that log values are base 10.
Pacemaker interval effect is evident in individual DA neurons
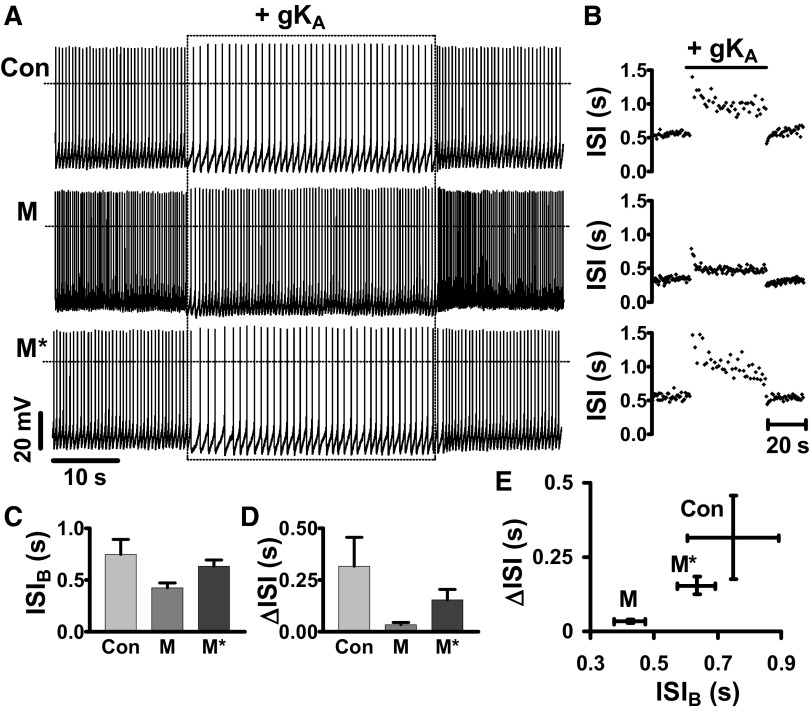
To determine whether the pacemaker interval effect is an intrinsic property of individual DA neurons, the effect of adding virtual A-type channels was tested under control conditions and after increasing baseline firing rate (ISIB) by activating endogenous muscarinic receptors with 1 μM muscarine (Lacey et al. 1990) (Fig. 2, A–C; compare ISIB for Con and M). ISI-time plots from a single DA neuron show that activation of muscarinic receptors decreased the response to 100 nS gKA (Fig. 2B). This trend is evident in the change in ΔISI: muscarinic receptor activation is accompanied by a decrease in the effect of A-type channels (Fig. 2D).
FIG. 2.
Muscarinic receptors reduce the effect of A-type channels by decreasing pacemaker interval. A: the effect of 100 nS gKA (Con) is reduced by 1 μM muscarine bath application (M), but this effect is reversed by resetting ISIB to the premuscarine value with bias current (M*). B: ISI vs. time plots for data in A. C: mean ISIB for control (Con), during muscarine application (M), and after adjusting firing rate in muscarine (M*). D: quantification of the effects of muscarine and the compensating current injection on ΔISI. E: ΔISI vs. ISIB plot. n = 3 for C–E.
This could reflect a specific effect of muscarinic receptors or be a more general consequence of the change in firing rate. To differentiate between these possibilities, the firing rate was reset toward premuscarine levels by injecting negative bias current and then the same dynamic clamp stimulus was applied again (Fig. 2A; compare M to M*). In the continued presence of muscarine, current injection that tended to reverse the muscarinic effect on firing (Fig. 2, A–C) also tended to reverse the change in ΔISI (Fig. 2, B, D, and E). Thus the change in sensitivity to virtual A-type channels induced by native muscarinic receptors was simply due to the change in pacemaker interval.
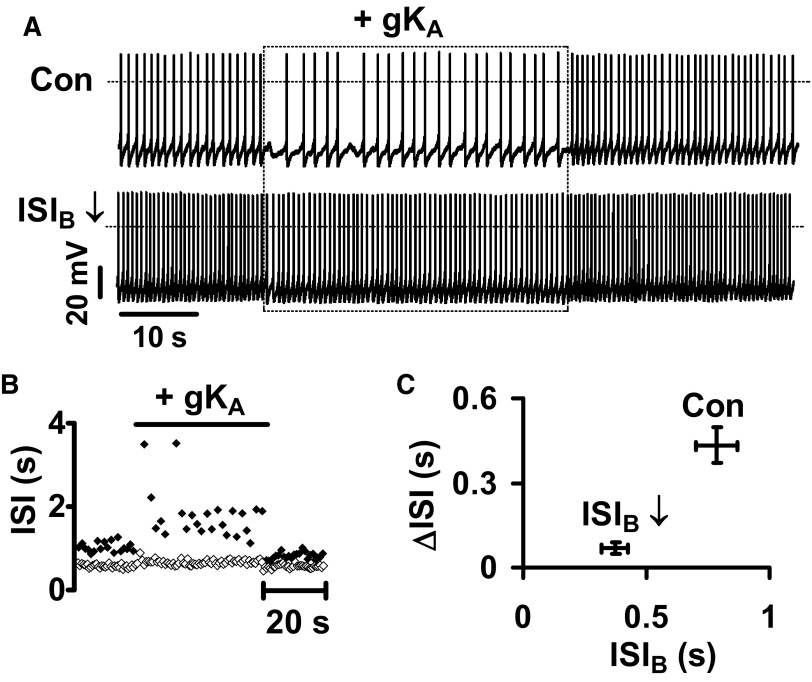
This conclusion is further supported by examining the effect of increasing baseline firing rate (ISI↓) with a 10-pA bias current (Fig. 3A). Even though the basis of the decrease in interval was different (i.e., muscarinic receptors were not involved), the response to virtual A-type channels again was smaller when the pacemaker interval was shortened (Fig. 3, A–C). Therefore the pacemaker interval dependence of the sensitivity to virtual A-type channels is preserved regardless of how firing rate is altered. Furthermore, this relationship, which was first detected by comparing the responses in different DA neurons (Fig. 1), is an intrinsic property of each DA neuron.
FIG. 3.
Pacemaker regulation applies to electrically driven changes in the pacemaker rate of individual DA neurons. A: injection of 50 nS gKA into a spontaneously pacing DA neuron before (Con) and after increasing its firing rate with +10 pA bias current (ISI↓). B: ISI plotted vs. time for A. ⧫, control; ◊, ISI↓. C: ΔISI vs. ISIB plot for 4 DA neurons.
Exponential dependence on pacemaker interval applies to voltage-independent cation channels
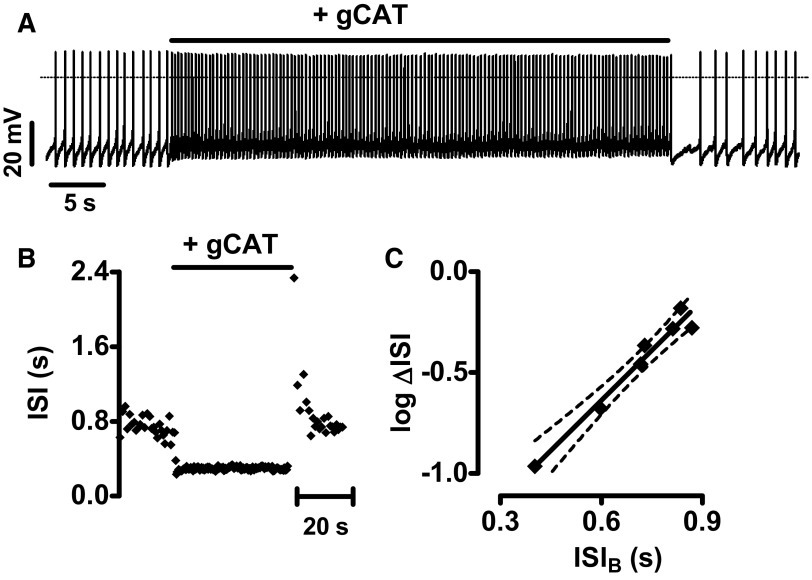
To test whether the pacemaker control of the effect of A-type voltage-gated K+ channels applies to other channels, the response to a nonselective voltage-insensitive cation conductance (gCAT) was examined. For this purpose, a 1-nS conductance with a reversal potential (Erev) of 0 mV was added to DA neurons with the dynamic clamp, resulting in increased firing (Fig. 4, A and B). Plotting the effect of virtual cation channels on ΔISI of seven spontaneously firing DA neurons shows that the response retains the exponential dependence on baseline pacemaker interval (Fig. 4C). Therefore the exponential pacemaker interval relationship applies to channels whether they are voltage-sensitive or insensitive and whether they are excitatory or inhibitory.
FIG. 4.
Pacemaker control of the response to nonselective cation conductance (gCAT). A: dynamic clamp was used to add 1 nS of gCAT for 60 s to a spontaneously firing DA neuron. B: ISI vs. time plot for A. C: relation of log(ΔISI) to ISIB plotted for 7 cells. Slope: 1.65, r2 = 0.96. - - -, 95% confidence intervals.
DA neuron pacemaker control of responses to virtual excitatory and inhibitory synapses
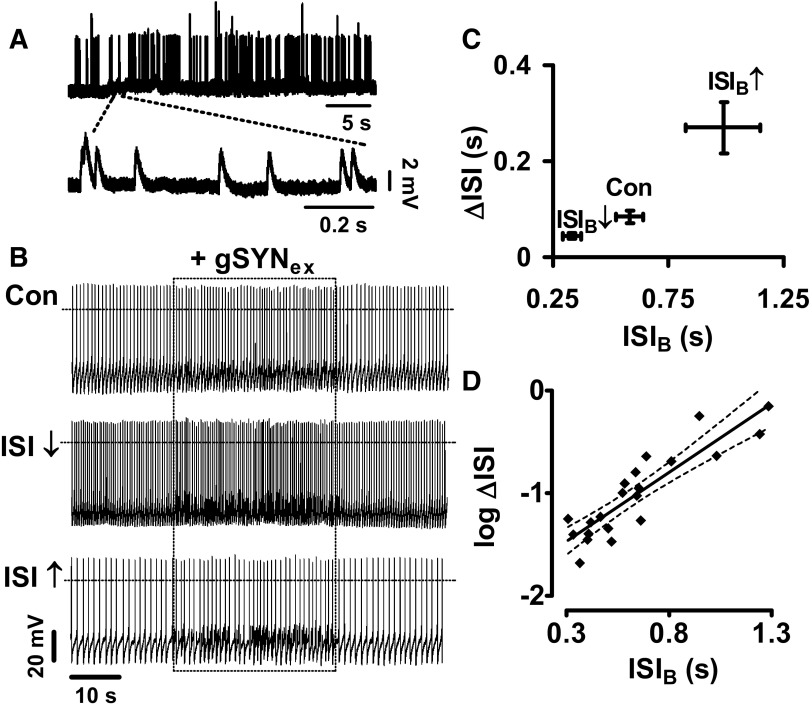
Because acute responses depend on pacemaker interval (Fig. 1, C and D), the effect of episodically active synapses could also be steeply dependent on pacemaker interval. To explore this hypothesis, virtual excitatory synaptic input was applied to DA neurons via the dynamic clamp. Specifically, a template was used in which a 5-nS postsynaptic nonselective cation conductance (gSYNex, Erev = 0 mV) was active at a mean frequency of 5 Hz. Furthermore, the template emulated randomly timed synaptic activity to ensure that no phase-cycle bias would be introduced. Turning off the pacemaker by hyperpolarization to −90 mV, which also provides more driving force for the synaptic conductance, showed that this template elicits a 40-s-long barrage of small excitatory postsynaptic potentials (Fig. 5A). The dynamic-clamp template was then used to excite spontaneously active DA neurons (Fig. 5B, Con). Subsequently, mean baseline firing rate was altered with positive or negative bias current (10 pA), and the identical noisy excitatory barrage was repeated in the same neuron (Fig. 5B, ISI↓ and ISI↑). Quantification shows that the response to virtual excitatory synapses increased when ISIB was increased and decreased when ISIB was lowered (Fig. 5C). Furthermore, analysis of individual responses shows that ΔISI increased exponentially with ISIB, the baseline interspike interval (Fig. 5D). The slope in this plot was less than in earlier experiments, but the 95% confidence intervals show that this difference was not statistically significant. Therefore the efficacy of excitatory ionotropic synapses is subject to exponential pacemaker interval relationship.
FIG. 5.
Pacemaker control of the response to virtual excitatory synaptic input. A: dynamic-clamp gSYNex template (Erev = 0 mV, g = 5 nS, 5 Hz) applied to a DA neuron hyperpolarized to −90 mV. Note that even with the large driving force produced by the holding potential, synaptic responses are small. B: the same gSYNex template applied to a spontaneously pacing DA neuron the firing rate of which was altered by bias current: Con, no bias current; ISI↓, Ihold = +10 pA; ISI↑, Ihold = −10 pA. C: ΔISI vs. ISIB plot (n = 3). D: the relation of log(ΔISI) and ISIB is preserved with virtual excitatory synapses: Slope = 1.34, r2 = 0.80. 95% confidence interval (- - -) indicate that this slope is not significantly different from in Fig. 1. Data are from 22 recordings of 10 DA neurons.
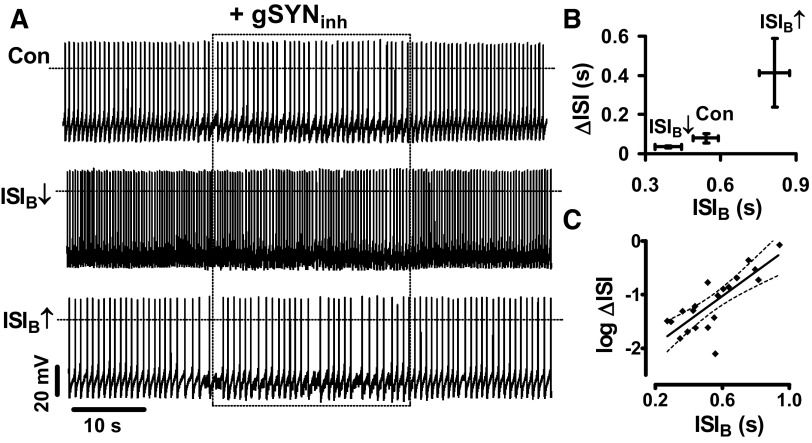
The effect of episodic inhibition was then examined. For these experiments, the template of virtual synaptic activity was changed to a hyperpolarizing voltage independent conductance (Erev = −65 mV) so that the episodic inhibitory template slowed spontaneous pacemaker activity (Fig. 6A, Con). As in the previous experiment, the effect of the template was determined after DA neuron firing interval was altered with positive and negative current injection. In three neurons in which all three conditions were tested (i.e., as in Fig. 6A), increasing ISIB amplified the effect of the virtual inhibitory synapse while decreasing ISIB diminished the perturbation of pacemaking (Fig. 6B). Plotting individual ΔISI values versus baseline ISI from all experiments shows that the impact of inhibitory virtual synapses was exponentially related to pacemaker interval (Fig. 6C). Hence the pacemaker control of synaptic efficacy applies to both excitatory and inhibitory virtual synapses.
FIG. 6.
Pacemaker control of the response to virtual inhibitory synaptic input (gSYNinh). A: the dynamic-clamp template shown in Fig. 5A was altered by changing Erev to −65 mV to generate inhibitory stimulation of spontaneously firing DA neurons. Con, control; ISI↓, ISIB is decreased by 10 pA of bias current; ISI↑, ISI is increased by −10 pA of bias current. B: quantification of ΔISI by the inhibitory synaptic template (n = 3). C: Log(ΔISI) vs. ISIB plot for 21 single experiments performed on 10 spontaneously firing DA neurons treated with gSYNinh. Slope: 2.2, r2 = 0.62. - - -, 95% confidence intervals.
Pacemaker control of the efficacy of native metabotropic synapses
The dynamic clamp can easily emulate simple ionotropic synapses, but applying this approach to complex metabotropic receptor signaling is difficult. Furthermore, the flow of current from the dynamic clamp occurs through the recording pipette, while native transmitter receptors are distributed over the cell body and dendrites. Therefore to address these limitations, the effect of pacemaker interval on responses to native metabotropic synapses was examined.
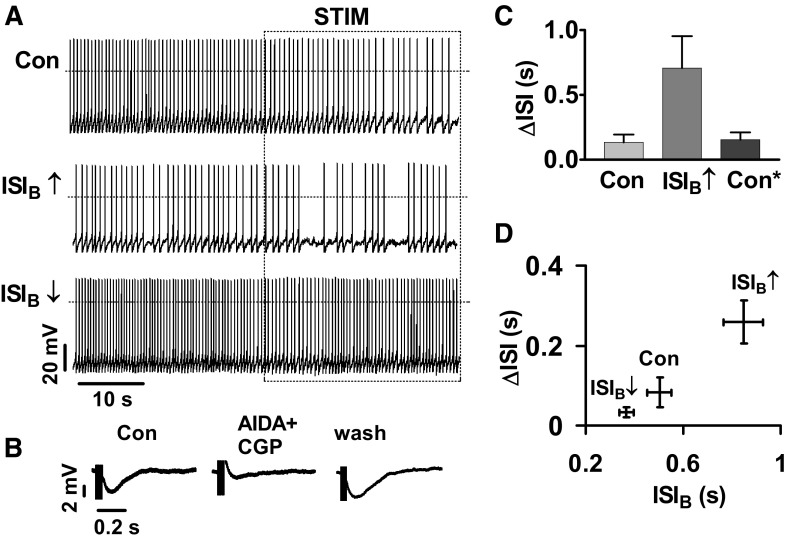
For these experiments, the slice was bathed in blockers of ionotropic glutamate and GABA receptors (CNQX, 2-amino-5-phosphonovaleric acid, and picrotoxin). Then a region of the pars reticulata was subjected to extracellular field stimulation (STIM) at 5 Hz. The timing of this stimulation was controlled by the same randomly distributed template used in dynamic-clamp experiments to ensure that phase biases were not introduced. As expected from previous studies, this resulted in slowing of DA neuron pacemaker activity (Fig. 7A, Con). Current-clamp recordings revealed that this stimulation evoked an inhibitory response that was altered by the metabotropic GABAb receptor antagonist CGP35348 (CGP) (data not shown) and nearly abolished by a combination of CGP35348 and the metabotropic glutamate receptor (mGluR1) antagonist AIDA (Fig. 7B). Thus pars reticulata electrical stimulation activated known native metabotropic inhibitory synapses (Fiorillo and Williams 1998; Johnson et al. 1992). Because previous experiments predicted that the DA neuron pacemaker interval effect should not depend on the specific mechanisms stimulated, the contributions of other receptors were not studied. Rather the change in the metabotropic response to pars reticulata stimulation was measured before and after slowing pacemaker rate by injecting −10 pA of bias current. (Fig. 7A, ISI↑).
FIG. 7.
Pacemaker control of the response to native inhibitory metabotropic synapses. A: stimulation of native inhibitory synapses (STIM) slows DA neuron firing. The same stimulus was applied under control conditions (Con) and after changing firing rate (ISI↓ or ISI↑) with bias current injection. Ionotropic glutamate and GABA receptors were blocked. B: inhibitory postsynaptic potential evoked by electrical field stimulation (Control, Con) is reversibly blocked by a combination of GABAb and mGluR1 receptor antagonists (AIDA+CGP). The DA neuron was current clamped to −55 mV. C: increasing ISIB reversibly increased ΔISI induced by native synapses (n = 5). Con* refers to the resetting of bias current to 0. D: the dependence of ΔISI evoked by metabotropic synapse stimulation on ISIB (n = 6).
The ∼1.7-fold increase in pacemaker interval caused by current injection increased the synaptic responses ∼5.5-fold (Fig. 7, A and C). Furthermore, this effect was reversible (Fig. 7C, Con*), showing that the increase in synaptic efficacy is directly dependent on firing rate. Finally, pacemaker interval was reduced by injecting 10 pA of current (Fig. 7A, ISI↓). As expected for the pacemaker interval effect seen in dynamic-clamp experiments, the increased firing rate reduced the synaptic response (Fig. 7, A and D). Indeed changing pacemaker intervals ∼2.3-fold produced a nearly ninefold change in the impact of metabotropic inhibitory synaptic stimulation (compare ISI↑ to ISI↓ in Fig. 7D). Thus DA neuron pacemaker diversity is functionally significant because the response to native metabotropic synapses depends steeply on pacemaker interval.
DISCUSSION
Previous studies have separately examined DA neuron synaptic and pacemaker mechanisms, but the effect of pacemaker rate on DA neuron responses had not been quantified. Therefore the significance of pacemaker rate diversity and regulation for responses to changes in voltage-gated channels (e.g., Kv4.3 gating and expression regulation by GDNF and D2 autoreceptors, respectively) (Hahn et al. 2003, 2006; Yang et al. 2001) and synaptic conductances were not known. In this study, the impact of DA neuron pacemaker rate on responses to dynamic clamp-defined stimuli and metabotropic synapses was determined. First principles suggested that there would be some effect of pacemaker rate, but the finding that known variations in DA neuron pacemaker rate change responses dramatically (>10-fold) regardless of whether they are excitatory, inhibitory, voltage dependent or independent, or sustained or episodic, was not anticipated previously. This exponential effect, which was statistically indistinguishable in all experiments (compare 95% confidence intervals in semilog plots), is expected for homoclinic bifurcation pacemaker models at the transition between repetitive firing and electrical silence, but it is not clear that the relationship applies to small perturbations of ongoing activity that never actually silence the neuron (G. B. Ermentrout, personal communication). Therefore both the large magnitude and the mathematical form of the dependence on DA neuron pacemaker interval were not predicted.
Does the DA neuron pacemaker interval relationship, which was discovered in brain slice recordings, apply in the intact brain? Regular pacemaker activity represents only a subset of the activity patterns produced by DA neurons in vivo (Hyland et al. 2002). However, because the channels that underlie pacemaker activity are present, the more complex and irregular activity seen in vivo is due to interaction of pacemaker mechanisms with ongoing synaptic activity. The data presented in this study suggest that the exponential pacemaker relationship applies to responses to changes in Kv4.3 A-type channel activity, which are induced by GDNF and the antipsychotic drug haloperidol (Hahn et al. 2003, 2006; Yang et al. 2001), metabotropic synapses (e.g., mediated by GABAb and mGluR1 receptors) (Fiorillo and Williams 1998; Johnson et al. 1992) and ionotropic excitatory and inhibitory synapses that are electrotonically close to the soma (i.e., the site of current injection by the dynamic clamp in this study). On the other hand, the response to distal excitatory synapses that evoke bursts may be more complex. Given that the basis of bursts is not understood, this issue will require more experimentation. However, evoked bursts are not the only relevant change in activity for DA neurons: for example, aversion is linked to inhibition of ongoing activity (Schultz 2007). Therefore it is likely that the steep exponential dependence on pacemaker interval applies to many physiological stimuli in vivo.
Because experiments were performed using immature rats, the results presented here do not reveal whether the role of the pacemaker changes during development. It is known that DA neuron mean firing rate increases with age (Tepper et al. 1990), but it is not known whether this reflects a change in background synaptic activity or a remodeling of the pacemaker mechanism itself. A developmental change in background synaptic activity alone would imply that the properties described here apply to the adult. Likewise, a shift in intrinsic pacemaker rate with development would not necessarily alter the exponential dependence on pacemaker interval. With these two scenarios, pacemaker control of synaptic efficacy could have wide ranging consequences. A high pacemaker rate would serve to make DA neuron activity more autonomous by limiting the impact of many stimuli. Such neurons would produce a tonic unregulated background release of DA. In contrast, a low pacemaker rate would ensure maximal detection of changes in synaptic input resulting in large phasic changes in DA release. Likewise, pacemaker control of synaptic efficacy could also be relevant for antipsychotic drugs that decrease pacemaker rate: the observed approximately two- to threefold increases in pacemaker interval (Hahn et al. 2003, 2006) might have functional benefit because the resultant approximately four- to ninefold increase in synaptic efficacy could compensate for deficits in information processing and attentional functioning associated with schizophrenia (Nuechterlein and Dawson 1984).
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-53050.
Acknowledgments
We thank Drs. G. Bard Ermentrout (University of Pittsburgh), Paul Shepard (University of Maryland), and Carmen Canavier (Louisiana State University) for comments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Dai and Tepper 1998.Dai M, Tepper JM. Do silent dopaminergic neurons exist in rat substantia nigra in vivo? Neuroscience 85: 1089–1099, 1998. [DOI] [PubMed] [Google Scholar]
- Desai and Walcott 2006.Desai NS, Walcott EC. Synaptic bombardement modulates muscarinic effects in forelimb motor cortex. J Neurosci 26: 2215–2226, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez and White 2008.Fernandez FR, White JA. Artificial synaptic conductances reduce subthreshold oscillations and periodic firing in stellate cells of the entorhinal cortex. J Neurosci 28: 3790–3803, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo and Williams 1998.Fiorillo CD, Williams JT. Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature 394: 78–82, 1998. [DOI] [PubMed] [Google Scholar]
- Franz et al. 2000.Franz O, Liss B, Neu A, Roeper J. Single cell mRNA of HCN1 correlates with a fast gating phenotype of hyperpolarization-activated cyclic nucleotide gated ion channels (Ih) in central neurons. Eur J Neurosci 12: 2685–2693, 2000. [DOI] [PubMed] [Google Scholar]
- Grace and Bunney 1983.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons. II. Action potential generating mechanisms and morphological correlates. Neuroscience 10: 317–331, 1983. [DOI] [PubMed] [Google Scholar]
- Grace and Bunney 1984a.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 4: 2866–2876, 1984a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace and Bunney 1984b.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4: 2877–2890, 1984b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn et al. 2006.Hahn J, Kullmann PH, Horn JP, Levitan ES. D2 autoreceptors chronically enhance dopamine neuron pacemaker activity. J Neurosci 26: 5240–5247, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn et al. 2003.Hahn J, Tse TE, Levitan ES. Long-term K+ channel-mediated dampening of dopamine neuron excitability by the antipsychotic drug haloperidol. J Neurosci 23: 10859–10866, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth et al. 1991.Hainsworth AH, Roper J, Kapoor R, Ashcroft FM. Identification and electrophysiology of isolated pars compacta neurons from guinea pig substantia nigra. Neuroscience 43: 81–93, 1991. [DOI] [PubMed] [Google Scholar]
- Hyland et al. 2002.Hyland BI, Reynolds, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience, 114: 475–492, 2002. [DOI] [PubMed] [Google Scholar]
- Johnson et al. 1992.Johnson SW, Mercuri NB, North RA. 5-hydroxytryptamine 1B receptors block the GABAB synaptic potential in rat dopamine neurons. J Neurosci 12: 2000–2006, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita et al. 1986.Kita T, Kita H, Kitai ST. Electrical properties of rat substantia nigra compacta neurons in an in vitro slice preparation. Brain Res 372: 21–30, 1986. [DOI] [PubMed] [Google Scholar]
- Kullmann and Horn 2006.Kullmann PH, Horn JP. Excitatory muscarinic modulation strengthens virtual nicotinic synapses on sympathetic neurons and thereby enhances synaptic gain. J Neurophysiol 96: 3104–3113, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann et al. 2004.Kullmann PH, Wheeler DW, Beacom J, Horn JP. Implementation of a fast 16-Bit dynamic clamp using LabVIEW-RT. J Neurophysiol 91: 542–554, 2004. [DOI] [PubMed] [Google Scholar]
- Lacey et al. 1990.Lacey MG, Calabresi P, North RA. Muscarine depolarizes rat substantia nigra zona compacta and ventral tegmental neurons in vitro through M1-like receptors. J Pharmacol Exp Ther 253: 395–400, 1990. [PubMed] [Google Scholar]
- Lacey et al. 1989.Lacey MG, Mercuri NB, North RA. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci 9: 1233–1241, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss et al. 2001.Liss B, Franz O, Sewing S, Bruns R, Neuhoff H, Roeper J. Tuning pacemaker frequency of individual dopaminergic neurons by Kv4.3L and Kchip3.1 transcription. EMBO J. 20: 5715–5724, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu et al. 1991.Mereu G, Costa E, Armstrong DM, Vicini S. Glutamate receptor subtypes mediate excitatory synaptic currents of dopamine neurons in midbrain slices. J Neurosci 11: 1359–1366, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff et al. 2002.Neuhoff H, Neu A, Liss B, Roeper J. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci 22: 1290–1302, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein and Dawson 1984.Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull 10: 160–203, 1984. [DOI] [PubMed] [Google Scholar]
- Paxinos and Watson 2005.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Burlington, MA: Elsevier Academic, 2005.
- Perkins 2006.Perkins KL Cell-attached voltage-clamp and current-clamp recordings and stimulation techniques in brain slices. J Neurosci Methods 154: 1–18, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz et al. 2004.Prinz AA, Abbott LF, Marder E. The dynamic clamp comes of age. Trends Neurosci 27: 218–224, 2004. [DOI] [PubMed] [Google Scholar]
- Schultz 2007.Schultz W Behavioral dopamine signals. Trends Neurosci 30: 203–210, 2007. [DOI] [PubMed] [Google Scholar]
- Tepper et al. 1990.Tepper JM, Trent F, Nakamura S. Postnatal development of the electrical activity of rat nigrostriatal dopaminergic neurons. Brain Res Dev Brain Res 54: 21–33, 1990. [DOI] [PubMed] [Google Scholar]
- Wilson et al. 1977.Wilson CJ, Young SJ, Groves PM. Statistical properties of neuronal spike trains in the substantia nigra: cell types and their interactions. Brain Res 136: 243–260, 1977. [DOI] [PubMed] [Google Scholar]
- Yang et al. 2001.Yang F, Feng L, Zheng F, Johnson SW, Du J, Shen L, Wu CP, Lu B. GDNF acutely modulates excitability and A-type K (+) channels in midbrain dopaminergic neurons. Nat Neurosci 4: 1071–1078, 2001. [DOI] [PubMed] [Google Scholar]