Abstract
The plasma membrane GABA transporter GAT1 is thought to mediate uptake of synaptically released GABA. In the primate dorsolateral prefrontal cortex (DLPFC), GAT1 expression changes significantly during development and in schizophrenia. The consequences of such changes, however, are not well understood because GAT1's role has not been investigated in primate neocortical circuits. We thus studied the effects of the GAT1 blocker 1,2,5,6-tetrahydro-1-[2-[[(diphenylmethylene)amino]oxy] ethyl]- 3-pyridinecarboxylic acid hydrochloride (NO711) on GABA transmission onto pyramidal neurons of monkey DLPFC. As in rat cortex, in monkey DLPFC NO711 did not substantially alter miniature GABA transmission, suggesting that GAT1 does not regulate single-synapse transmission. In rat cortical circuits, between-synapse GABA spillover produced by NO711 clearly prolongs the inhibitory postsynaptic currents, but whether NO711 also prolongs the inhibitory postsynaptic potentials (IPSPs) is unclear. Moreover, whether spillover differentially affects perisomatic versus dendritic inputs has not been examined. Here we found that NO711 prolonged the GABAA receptor-mediated IPSPs (GABAAR-IPSPs) evoked by stimulating perisomatic synapses. Dendritic, but not perisomatic, synapse stimulation often elicited a postsynaptic GABAB receptor-mediated IPSP that was enhanced by NO711. Blocking GABAB receptors revealed that NO711 prolonged the GABAAR-IPSPs evoked by stimulation of dendrite-targeting inputs. We conclude that a major functional role for GAT1 in primate cortical circuits is to prevent the effects of GABA spillover when multiple synapses are simultaneously active. Furthermore, we report that, at least in monkey DLPFC, GAT1 similarly restricts GABA spillover onto perisomatic or dendritic inputs, critically controlling the spatiotemporal specificity of inhibitory inputs onto proximal or distal compartments of the pyramidal cell membrane.
INTRODUCTION
The plasma membrane GABA transporter 1 (GAT1) is abundant in neocortex (Guastella et al. 1990) where it is localized in neuronal and glial membranes near synapses (Conti et al. 1998; Minelli et al. 1995). This location suggests that GAT1 regulates GABA transmission by the uptake of synaptically released GABA (Conti et al. 2004). GAT1 blockade actually prolongs the decay of inhibitory postsynaptic currents (IPSCs) evoked by multiple-synapse stimulation (Overstreet and Westbrook 2003), suggesting that GAT1 activity shortens the IPSC decay. However, miniature IPSCs (mIPSCs), which reflect single-synapse transmission, remain unchanged after GAT1 blockade (Isaacson et al. 1993; Overstreet and Westbrook 2003; Thompson and Gahwiler 1992) and in GAT1 knock-out mice (Bragina et al. 2008; Jensen et al. 2003).
The lack of effect of GAT1 blockade on mIPSCs indicates that GAT1 does not regulate transmission at single synapses and that prolongation by GAT1 block of IPSCs evoked by stimulating multiple synapses may result from between-synapse GABA spillover. Therefore GAT1 may help preserve synapse independence and the spatiotemporal specificity of inhibitory transmission, which is essential to maintain the distinct influence of different interneuron subtypes that provide nearby synaptic inputs onto the same membrane compartment (Klausberger and Somogyi 2008). For instance, although functionally diverse, parvalbumin- and cholecystokinin-containing basket cells both furnish perisomatic synapses onto pyramidal cells (Freund and Katona 2007). Similarly, multiple interneuron subtypes target dendrites (Somogyi et al. 1998).
The propensity for GAT1-regulated GABA spillover depends on the density of GABA synapses because GAT1 blockade does not affect IPSCs mediated by multiple synaptic contacts that are distant from each other (Overstreet and Westbrook 2003). Importantly, the density of GABA synapses is significantly lower in dendrites than in the perisomatic membrane of pyramidal cells (Andrasfalvy and Mody 2006; Megias et al. 2001; Papp et al. 2001). Therefore studies of synapse density suggest that GABA spillover may be less likely during activation of dendritic- than perisomatic-targeting inputs. Alternatively, GABA spillover may occur between nearby GABA synapses onto different neurons. If so, the probability of spillover may be more dependent on the density of axon terminals and dendrites in the neuropil, which determines the distance between GABA synapses onto different neurons. Thus, a lower GABA synapse density onto dendrites compared with soma of individual cells may be a less important determinant of spillover. Previous studies, however, have not determined whether GAT1 blockade has similar effects at dendritic versus perisomatic synaptic inputs.
It is well established that GABA spillover induced by GAT1 blockade increases the duration of IPSCs evoked by multiple synapse stimulation. However, whether GAT1 block also prolongs the inhibitory postsynaptic potential (IPSP) duration remains unclear. IPSPs typically outlast the underlying IPSCs because the IPSP decay is shaped by the cell's membrane properties (Koch et al. 1996). Thus IPSC prolongation by GAT1 block may not be sufficient to significantly prolong the IPSPs. An IPSP prolongation by GAT1 block may have important functional consequences because it would involve the effects of both the GABA-activated conductance and the associated change in membrane potential. Although hyperpolarizing IPSPs are inhibitory at peak and during decay, depolarizing IPSPs may inhibit at their peak but excite during decay (Bartos et al. 2007; Gulledge and Stuart 2003) or may be excitatory throughout their duration (Szabadics et al. 2006). Thus examining whether changes in the efficacy of GAT1-mediated uptake affect IPSP duration is important for understanding GAT1's role in cortical circuit function.
In the dorsolateral prefrontal cortex (DLPFC) of non-human primates, circuit maturation during adolescence is associated with a significant decrease in GAT1 levels in some GABA terminals (Cruz et al. 2003). Moreover, GAT1 mRNA and protein levels are reduced in the DLPFC of subjects with schizophrenia (Pierri et al. 1999; Volk et al. 2001). However, the consequences of such development- and disease-related changes in GAT1 levels, and thus of GABA uptake efficacy, are not well understood because the functional role of GAT1 has not been investigated in the human or non-human primate neocortex. One possibility suggested by anatomical studies is that GABA spillover is less significant in primate neocortex, which, compared with rodent neocortex, has lower density of inhibitory synapses in the neuropil, lower neuronal density and higher density of glial cells (DeFelipe et al. 2002; Herculano-Houzel et al. 2006, 2007; Sherwood et al. 2006). To determine whether GAT1-mediated uptake regulates GABA spillover in primate neocortical circuits, here we recorded from layer 3 pyramidal neurons of monkey DLPFC to test the effects of the GAT1 blocker 1,2,5,6-tetrahydro-1-[2-[[(diphenylmethylene) amino]oxy] ethyl]-3-pyridinecarboxylic acid hydrochloride (NO711). Specifically, we performed current-clamp recordings to determine whether GAT1 blockade affects IPSPs during miniature transmission or during stimulation of proximal (perisomatic) versus distal (dendritic) GABA synapses.
METHODS
Brain slice preparation
Experiments were performed in tissue obtained from ten female rhesus macaque monkeys (Macaca mulatta) and two male long-tailed macaque monkeys (M. fascicularis) supplied by the University of Pittsburgh Primate Research Center. Housing and experimental procedures were conducted in accordance with U.S. Department of Agriculture and National Institutes of Health guidelines and with approval of the University of Pittsburgh's Institutional Animal Care and Use Committee. All rhesus animals ≤45 mo of age were bred at this facility. All animals were experimentally naïve at the time of entry into this study.
Brain slices were prepared from five prepubertal rhesus monkeys 15–16 mo of age, four postpubertal rhesus monkeys 42–45 mo of age, one adult rhesus monkey 84 mo old, and two long-tailed monkeys 42–60 mo of age. Tissue blocks containing portions of DLPFC areas 9 and 46 were obtained from one or both hemispheres of each animal. Some of the animals were deeply anesthetized and perfused transcardially with a cold artificial cerebrospinal fluid (ACSF) solution of the following composition (in mM): 210.0 sucrose, 10.0 NaCl, 1.9 KCl, 1.2 Na2HPO4, 33.0 NaHCO3, 6.0 MgCl2, 1.0 CaCl2, 10.0 glucose, and 2.0 kynurenic acid; pH 7.3–7.4 when bubbled with 95% O2-5% CO2, and a DLPFC tissue block was rapidly prepared as previously described (Gonzalez-Burgos et al. 2004). For all other animals, an initial tissue block was removed from one hemisphere using a previously described surgical procedure (Gonzalez-Burgos et al. 2004), and then a second DLPFC tissue block was removed 1–2 wk later, following the transcardial cold ACSF perfusion procedure described in the preceding text. When two tissue blocks were removed per animal in separate surgical procedures, the locations of the blocks were off-set in the rostral-caudal axis, so that nonhomotopic portions of the DLPFC were studied from each hemisphere. Previous studies have shown that the first procedure does not alter the physiological or anatomical properties of the neurons and local circuits present in the tissue obtained in the second hemisphere (Gonzalez-Burgos et al. 2000).
Cortical slices (300–350 μm thick) were cut in the coronal plane using a vibrating microtome (VT1000S, Leica Microsystems, Nussloch, Germany) in ice-cold ACSF. Immediately after cutting, slices were transferred to an incubation chamber maintained at room temperature and filled with a solution containing (in mM) 126.0 NaCl, 2.0 KCl, 1.2 Na2HPO4, 10.0 glucose, 25.0 NaHCO3, 6.0 MgCl2, and 1.0 CaCl2, pH 7.3–7.4 when bubbled with 95% O2-5% CO2.
Electrophysiological recordings
For recording, slices were submerged in a chamber superfused at a rate of 2–3 ml/min with a solution containing (in mM) 126.0 NaCl, 2.5 KCl, 1.2 Na2HPO4, 25.0 Na2HCO3, 10.0 glucose, 2.0 CaCl2, 1.0 MgCl2, 0.02 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX), d,l-amino-5-phosphonopentanoic acid (AP5) 0.1, bubbled with 95% O2-5% CO2, and maintained at 30–32°C. In some experiments, gabazine or bicuculline methiodide (20 μM) was added to block GABAA receptors (GABAARs). Whole cell recordings were obtained from visually identified pyramidal neurons in layer 3 of DLPFC areas 9 and 46 using infrared differential interference contrast video microcoscopy in Olympus BX51 and BX61 microscopes (Olympus), or Zeiss FS Axioskop microscopes (Zeiss). Recording micropipettes pulled from borosilicate glass had a resistance of 3–5 MΩ when filled with a solution containing (in mM) 120.0 KCl, 10.0 NaCl, 0.2 EGTA, 10.0 HEPES, 4.0 MgATP, 0.3 NaGTP, 14.0 NaPhosphocreatine, and biocytin 0.5% (pH adjusted to 7.2–7.3). Assuming an intracellular bicarbonate concentration of 15 mM (Farrant and Kaila 2007), a permeability ratio PHCO3-/PCl- of 0.3 for GABAAR channels (Farrant and Kaila 2007) and using the Goldman-Hodkin-Katz equation, we estimated the reversal potential of the GABAAR-IPSP (EGABAA) to be near zero (−0.66 mV). On the other hand, the Nernst potential for K+ (EK+), which determines the reversal potential for GABABR-activated K+ currents (Luscher et al. 1997) was estimated at −102 mV. Recordings were performed using Multiclamp 200A or Multiclamp 200B amplifiers (Axon Instruments, Union City, CA) operating in current-clamp (bridge) mode. Signals were low-pass filtered at 4 kHz, digitized at 10 or 20 kHz, and stored on disk for off-line analysis. Data acquisition was performed using Power 1401 data-acquisition interface boards (Cambridge Electronic Design, Cambridge, UK) and Signal 3 software (Cambridge Electronic Design). Throughout the experiments, the series resistance was monitored, and if it exceeded 30 MΩ, recordings were excluded from data analysis.
Recording and analysis of mIPSPs
mIPSPs were recorded from layer 3 pyramidal neurons in slices obtained from postpubertal animals. To block action potentials thus focusing on IPSPs resulting from spontaneous GABA release at single synapses, the voltage-dependent Na+ channel blocker tetrodotoxin (1 μM) was added to a bath solution that otherwise had the same composition as that used to record IPSPs. The cells were recorded at −80 mV for 20 min and then NO711 (20 μM) or N,N,6-trimethyl-2-(4-methylphenyl)imidazo[1,2-a]pyridin e-3-acetamide (zolpidem, 1 μM) was applied for 15 min. For each cell, the mIPSPs were detected using MiniAnalysis (Synaptosoft, Decatur, GA). At least 300 nonoverlapping events were included to automatically generate an average mIPSP for each cell in control conditions and at the last 5 min of the NO711 or zolpidem application. The amplitude and the decay time constant of an exponential function fit to the 10–90% decay phase were determined for the average mIPSPs obtained for each cell.
Recording and analysis of IPSPs evoked by focal extracellular stimulation
Monosynaptic IPSPs were elicited by focal extracellular stimulation applied using theta-glass pipettes (tip diameter: 2–3 μm) filled with freshly oxygenated extracellular solution. Chlorinated silver wires placed inside each compartment of the theta glass were connected to a stimulus isolation unit (Model A350D-A, World Precision Instruments, Sarasota, FL) to apply bipolar stimulation. Stimulation electrodes were placed, to activate proximal inputs, ∼50–100 μm lateral to the soma of the recorded neurons or, to activate distal inputs, near the border between layers 1 and 2 (see Fig. 2A). Applying stimuli of 100 μs duration at a baseline frequency of 0.1 Hz, the current intensity (20–100 μA) and electrode position were adjusted to elicit IPSPs of the smallest possible amplitude without failures. Typically, eliciting IPSPs with distal stimulation required higher stimulation currents and produced smaller IPSP amplitudes (see results). Distal stimulation produced IPSPs with significantly slower 10–90% rise time (Fig. 2, B and C). In many experiments, IPSPs could be elicited with proximal stimulation, but distal stimulation failed to elicit an IPSP in the same neuron. In some individual experiments, IPSPs elicited by distal stimulation had a fast rise time, similar to that of IPSPs evoked in the same neuron by proximal stimulation. When all experiments that yielded both distal and proximal IPSPs were considered, independent of the individual IPSP rise times, statistical analysis demonstrated that distal stimulation was much more likely to elicit slow rising IPSPs (Fig. 2C). As illustrated in Fig. 2A, the distal dendritic tree of the recorded pyramidal neurons was typically well-preserved, displaying several branches intact in layer 1. We previously reported similar findings when studying dendritic spine density in the layer 1 portion of apical dendrites of recorded layer 3 pyramidal cells from monkey DLPFC (Gonzalez-Burgos et al. 2008). Studies from others also showed that in slices from monkey DLPFC, distal dendrites are also well preserved for many of the recorded layer 5 pyramidal neurons, which have significantly longer apical dendrites (Chang and Luebke 2007). Together these morphological findings indicate that a significant fraction of the distal apical dendrites is usually preserved in each neuron, providing a substrate for the activation of distal GABA synapses by distal stimulation. For analysis of the effects of the GAT1 inhibitor NO711, data were included only if both proximal and distal stimulation produced IPSPs in the same neuron and only if the distal IPSPs had a 10–90% rise time of ≥4.5 ms (approximately the mean of proximal IPSP rise time plus 2 SD). Unless specified otherwise, IPSPs were recorded at a somatic membrane potential of −70 to −75 mV, which was either the cells' resting membrane potential or was adjusted by current injection. IPSPs were recorded for ≥10 min in control conditions, before applying NO711 (20 μM) for 5 min and followed by ≥10 min of drug washout (which typically produced only a very small reversal of the effect, see Fig. 3F).
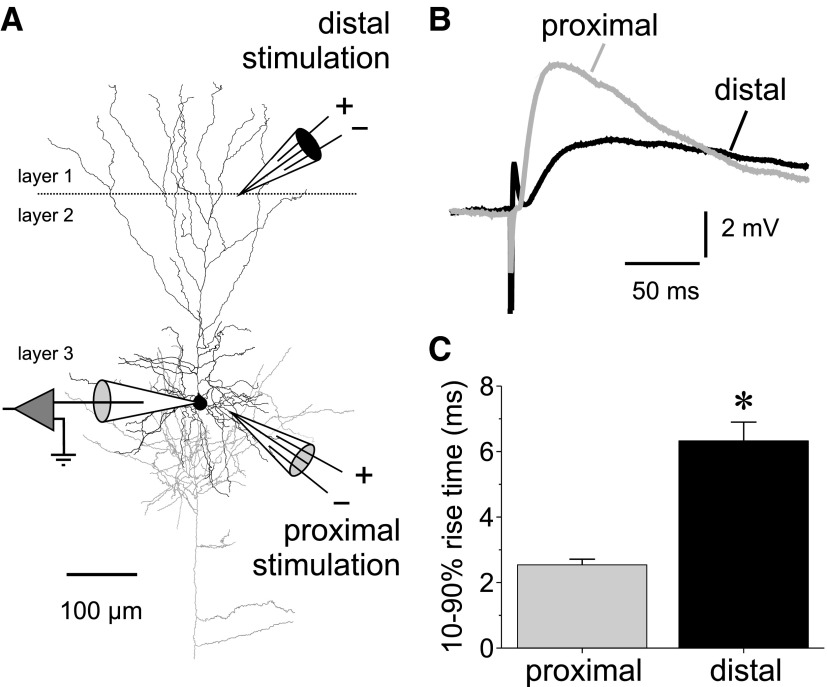
FIG. 2.
Stimulation of proximal and distal GABA synaptic inputs onto layer 3 pyramidal neurons. A: reconstruction of 1 of the cells recorded in this study showing the typical location of the stimulation electrodes. Distal stimulation was applied in near the layers 1/2 border. Proximal stimulation was applied 50–100 μm lateral to the soma of the recorded neuron. The pyramidal cell was reconstructed using Neurolucida (Microbrightfield, Williston VT), after staining to visualize the biocytin label was done as described previously (Gonzalez-Burgos et al. 2008). B: example average sweeps showing the differences in rising phase of IPSPs evoked in the same neuron by proximal vs. distal stimulation. C: summary graphs showing the statistically significant differences between the 10–90% rise time of IPSPs evoked by proximal and distal stimulation (rise time proximal IPSPs, 2.43 ± 0.21 ms, n = 30; distal IPSPs, 7.50 ± 0.87 ms, n = 24; independent samples t-test, t = 6.237, P < 0.00001).
FIG. 3.
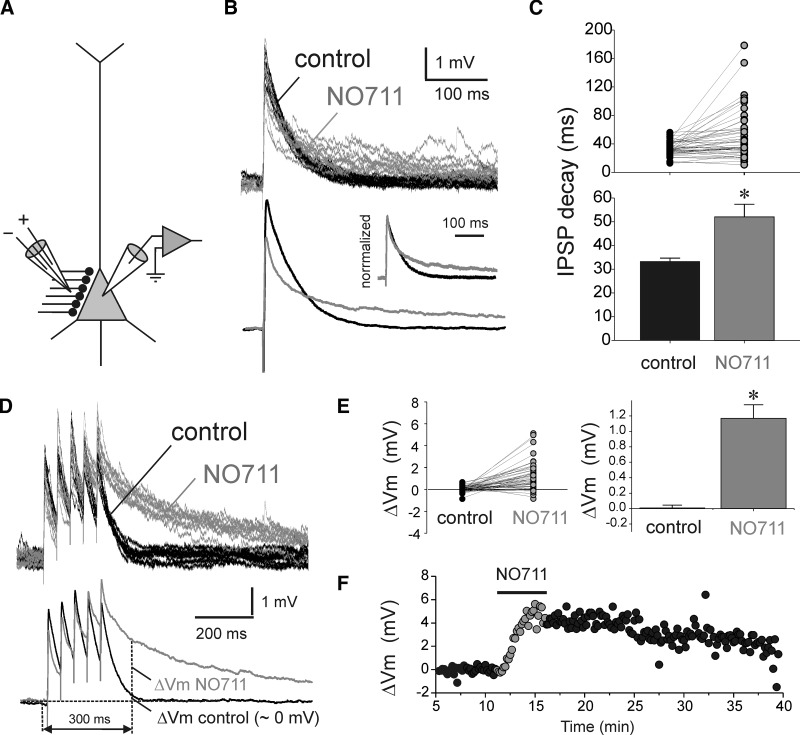
Effects of GAT1 blockade with NO711 on IPSPs evoked by stimulation of perisomatic-targeting inputs (psIPSPs). A: scheme showing the arrangement of the recording and stimulation electrodes. B: example experiment illustrating that NO711 application (20 μM) prolonged the decay and slightly decreased the amplitude of psIPSPs. Top: consecutive traces superimposed. Bottom: averages of traces recorded in control and NO711 conditions. Inset: the average traces normalized to the same peak amplitude and superimposed. C: summary graphs showing the statistically significant effects of NO711 (* = P < 0.05) on the decay time of psIPSPs. Shown at the time constants of single-exponential decay functions fit to the decay of the psIPSPs recorded from each neuron in control conditions and in the presence of NO711. D: example traces illustrating the effects of NO711 on the time course of psIPSP trains evoked by stimulation with input trains (5 stimuli at 20 Hz). Top: consecutive traces superimposed. Bottom: averages of traces recorded in control and NO711 conditions. E: summary graphs showing the statistically significant effects of NO711 (*, P < 0.05) on the psIPSP trains, measured as the difference ΔVm between the membrane potential 10 ms before and 300 ms after the onset of the stimulus train (see Fig. 3D). F: example of the time course of NO711 effects on psIPSPs, measured through the ΔVm values.
To determine the effects of NO711 on IPSPs, we averaged the last 20 consecutive control traces recorded before NO711 application and the last 20 consecutive traces before onset of NO711 washout. The average IPSPs were used to measure the peak amplitude and the decay in control and NO711 conditions. The changes in speed of IPSP decay induced by NO711 were measured fitting a single-exponential decay function. Although the decay kinetics of GABAAR-mediated currents and IPSCs recorded in voltage-clamp mode is typically best fit with double-exponential decay functions, in current-clamp experiments, the IPSP decay is shaped by the cells' membrane time constant. Consequently, we found that in most neurons the IPSP decay was well fit by a single-exponential function and that double-exponential decay functions did not improve the fit. The goal of this study was to determine if GAT1 block-induced spillover increases IPSP duration as opposed to examining if there are changes in complex kinetics of IPSC decay. Therefore the NO711 effects on the decay of single IPSPs were estimated by comparing single-exponential decay time constant. To determine the effects of NO711 during repetitive stimulation, trains of five stimuli at 20 Hz were applied every 10 s. The time course of the decay of the membrane potential at the end of the IPSP trains could not be well fit by single- or multiple-exponential decay functions. Therefore the effects of NO711 were determined measuring the difference, here named ΔVm, between the membrane potential 10 ms before and 300 ms after the onset of IPSP trains. For IPSPs evoked by perisomatic stimulation in control conditions, ΔVm fluctuated around zero, indicating that the membrane potential after perisomatic IPSP trains typically decayed to pretrain values by 300 ms posttrain onset. When dendritic IPSP trains displayed a posttrain hyperpolarizing potential, it typically peaked later than 300 ms posttrain onset, and therefore ΔVm was measured at the peak negative value posttrain, irrespective of time point. For the hyperpolarizing potential observed following single IPSPs, ΔVm was calculated similarly (peak hyperpolarizing Vm post-IPSP, minus Vm at 10 ms pre-IPSP).
Pharmacological compounds
Fast glutamate transmission was blocked with continuous bath application of 100 μM of d,l-AP5 and 20 μM CNQX, to block, respectively, NMDA and AMPA receptors. To block voltage-dependent sodium channels during mIPSP recordings we used tetrodotoxin (1 μM). To block GABAAR-mediated transmission, bicuculline methiodide or gabazine (20 μM) was added to the extracellular solution. To block GAT1-mediated GABA transport, we used NO711. NO711, also named NNC 711, is a partially lipophylic compound but has good solubility in water (up to ≥10 mM). GABAB receptors (GABABRs), were blocked with CGP35348. Zolpidem, was dissolved in dimethyl sulfoxide at 5 mM and then diluted to a final concentration of 1 μM. Dimethyl sulfoxide at its final concentration (0.002% vol/vol) did not produce any effect on the mIPSPs (data not shown). Zolpidem, CGP35348, and NO711 were obtained from Tocris Bioscience (Ellisville, MO). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Statistical data analysis
Data are expressed as means ± SE unless indicated otherwise. The statistical significance of the difference between group means was assessed using independent samples t-test, paired-samples t-test or two-way ANOVA, as indicated in each case. Pearson's χ2 test was employed to test differences in the proportion of NO711-sensitive versus NO711-insensitive IPSPs. Differences were considered significant when the P value for the statistical parameters was <0.05.
RESULTS
GAT1 block does not enhance miniature GABA transmission
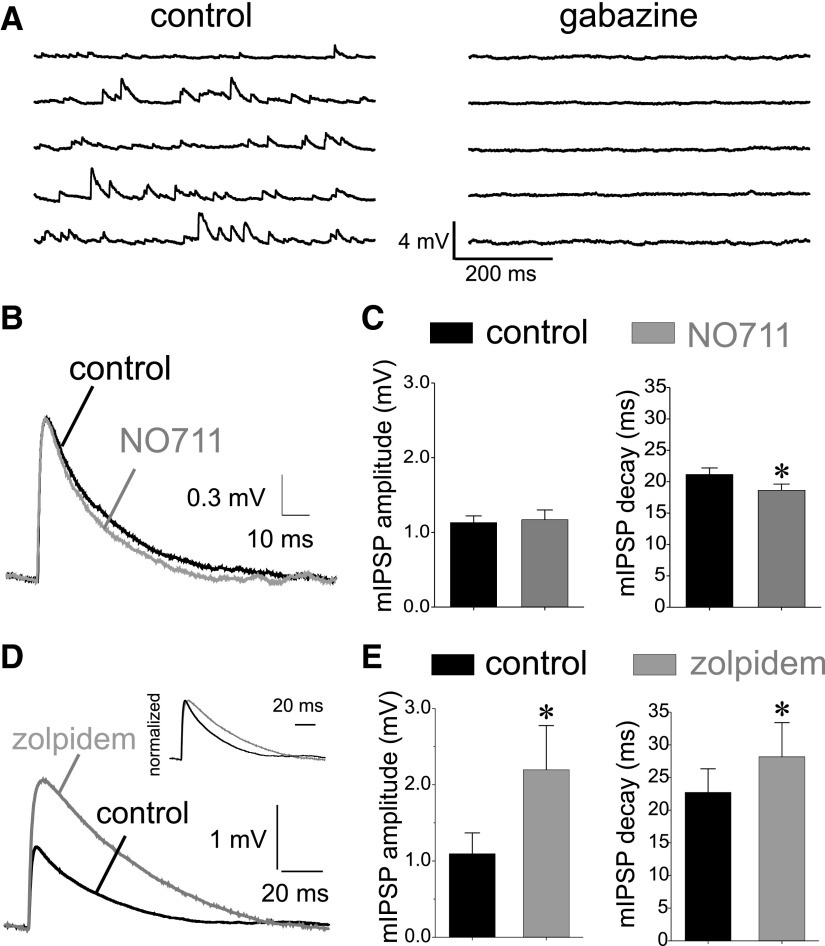
If GAT1-mediated uptake limits the amount of GABA available for synaptic receptor activation, then NO711 application should enhance miniature synaptic events (increase their size and/or duration). However, in rodent hippocampus and neocortex, GAT1 blockade does not affect the amplitude or duration of mIPSCs, suggesting that GAT1-mediated uptake does not affect transmission at single GABA synapses. To determine whether single-synapse GABA transmission is also independent of GAT1 activity in primate neocortical circuits, we studied miniature GABA transmission onto layer 3 pyramidal neurons of monkey DLPFC. mIPSPs were recorded first in control conditions and then in the presence of the GAT1-selective transport inhibitor NO711 (20 μM). This NO711 concentration is saturating for its effects on GAT1 (Borden 1996) but does not induce the GABAAR desensitization seen with higher (i.e., 100 μM) NO711 concentrations (Overstreet and Westbrook 2003; Overstreet et al. 2000). The depolarizing mIPSPs recorded at potentials between −75 and −70 mV using a high-chloride pipette solution (EGABAA ∼0 mV, see methods) were completely abolished by GABAAR antagonists (Fig. 1A).
FIG. 1.
Effects on miniature inhibitory postsynaptic potentials (mIPSPs) of applying the GABA transporter 1 (GAT1) blocker 1,2,5,6-tetrahydro-1-[2-[[(diphenylmethylene)amino]oxy] ethyl]-3-pyridinecarboxylic acid hydrochloride (NO711) or the benzodiazepine site agonist N,N,6-trimethyl-2-(4-methylphenyl)imidazo[1,2-a]pyridin e-3-acetamide (zolpidem). A: depolarizing mIPSPs recorded from layer 3 pyramidal neurons in control conditions (high chloride pipette solution), were completely abolished by applying the GABAA receptor (GABAAR) antagonist gabazine (10 μM). B: examples of average mIPSPs recorded in control conditions and following application of the GAT1 blocker NO711 (20 μM) show that NO711 did not significantly change the average mIPSP shape. C: summary graphs showing the absence of effects of NO711 on mIPSP amplitude (paired samples t-test t = 0.500, P = 0.627, n = 14) and a slight but significant acceleration of the mIPSP decay time constant (paired samples t-test, t = 3.046, P = 0.01, n = 14), which was correlated with a small NO711-induced acceleration of the cells' membrane time constant (see results). D: examples of average mIPSPs recorded in control conditions and following application of the benzodiazepine site agonist zolpidem (1 μM) show that zolpidem increased the mIPSP amplitude and prolonged mIPSP duration, as illustrated by the normalized traces in the inset. E: summary graphs showing the significant effects of zolpidem on the mIPSP amplitude (paired samples t-test, t = 3.554, P < 0.02, n = 8) and the mIPSP decay time constant (paired samples t-test, t = 2.849, P < 0.05, n = 8).
We determined the effects of GAT1 blockade on miniature transmission by obtaining average mIPSPs for each neuron in control and NO711 conditions (Fig. 1, B and C). A paired-samples t-test analysis revealed that NO711 application failed to significantly alter the amplitude or to prolong the duration of the average mIPSPs (Fig. 1, B and C). However, NO711 slightly (12%), but significantly accelerated the average mIPSP decay (paired samples t-test, t = 3.046, P = 0.01, n = 14). An acceleration of IPSP decay is contrary to the idea that GAT1 activity shortens the IPSP duration but is consistent with the possibility that NO711 decreases the cells' membrane time constant by enhancing a tonic GABA current after GAT1 block increases ambient GABA levels (Farrant and Nusser 2005). Indeed in several neurons, NO711 application produced a small depolarizing shift in the membrane potential (data not shown), consistent with the enhancement of a tonic GABA current, which in our experimental conditions should be depolarizing. This NO711-induced depolarization was not further studied and, when present, was compensated by current injection. We found that NO711 produced a small (19%) but significant acceleration of the membrane time constant in the same neurons (membrane time constant control: 21.3 ± 1.2 ms, membrane time constant NO711: 17.4 ± 0.9 ms, paired samples t-test, t = 3.70, P = 0.003, n = 14). Moreover, the acceleration of mIPSP decay by NO711 was strongly correlated with the acceleration of the membrane time constant in the same neurons (Pearson's correlation coefficient r = 0.67001, P = 0.008, n = 14) as expected if the mIPSP decay acceleration by NO711 was due to a decrease in the membrane time constant.
The absence of mIPSP enhancement by NO711 may be explained if the GABA concentration transient in the synaptic cleft saturates the synaptic GABAARs (Edwards 2007). GAT1 transporters have high affinity for GABA (Borden 1996) and transport GABA at a slow rate (Bicho and Grewer 2005; Mager et al. 1996). Therefore if the synaptic cleft GABA concentration transient saturates the GABAARs, it should largely saturate the GABA uptake capacity, making GAT1 block irrelevant. Whether the cleft GABA transient produces GABAAR saturation appears to be cell type- and synapse-specific (Hajos et al. 2000; Mozrzymas 2004; Szabadics et al. 2007). Therefore we determined the effects, on mIPSPs, of zolpidem, a compound that like other benzodiazepine site ligands increases the affinity of the GABAARs for GABA (Lavoie and Twyman 1996; Mozrzymas 2004) and does not enhance transmission if there is GABAAR saturation (Hajos et al. 2000; Perrais and Ropert 1999; Szabadics et al. 2007). As shown in Fig. 1, D and E, in monkey DLPFC layer 3 pyramidal neurons, zolpidem (1 μM) significantly increased both the amplitude and duration of mIPSPs (paired-samples t-test, mIPSP amplitude: t = 3.55, P < 0.01, n = 8 and mIPSP decay time constant: t = 2.85, P < 0.05, n = 8), a finding consistent with sub-saturating concentrations of synaptic cleft GABA. These results favor the conclusion that during single-synapse transmission, GAT1-mediated transport does not restrict the amount of neurotransmitter available to activate GABAARs even if the cleft GABA transient is sub-saturating.
GAT1 block prolongs the IPSPs elicited by stimulation of perisomatic-targeting inputs
Previous studies showed that when multiple synapses are stimulated, GAT1 blockade produces intersynaptic GABA spillover if the stimulated synapses are sufficiently close (Overstreet and Westbrook 2003). Because the density of GABA synapses is higher near the soma compared with more distal dendrites of single pyramidal neurons, the effects of GABA spillover may be larger for perisomatic-targeting versus dendrite-targeting inputs. To examine whether GAT1 blockade differentially affects perisomatic versus dendritic IPSPs, monosynaptic IPSPs were evoked by focal extracellular stimulation of proximal or distal inputs (Fig. 2A).
IPSPs evoked by proximal stimulation, or perisomatic IPSPs (psIPSPs), had rise times and decay kinetics very similar to those of unitary IPSPs elicited in monkey DLPFC pyramidal cells by perisomatic synapses from presynaptic fast-spiking basket cells and chandelier neurons (Gonzalez-Burgos et al. 2005). Whereas in our previous study we used near physiological chloride ion concentrations in the pipette solution (Gonzalez-Burgos et al. 2005), here the GABAAR-mediated IPSPs (GABAAR-IPSPs) were made depolarizing (EGABAA ∼0 mV) to improve the detection of small events. The similarities in GABAAR-IPSP kinetics therefore suggest that the depolarizing GABAAR-IPSPs evoked in this study, possibly due to their small size, did not cause more or less activation or inactivation of voltage-gated conductances than GABAAR-IPSPs recorded in physiological intracellular chloride conditions.
Compared with psIPSPs, IPSPs evoked by stimulation near the distal apical dendrite, or dendritic IPSPs (dIPSPs), had a significantly slower rise time (Fig. 2B). The IPSP rise time is a good indicator of distal versus proximal synapse location because it is determined by the degree of distance-dependent attenuation by filtering of IPSPs during propagation to the soma (Pouille and Scanziani 2004; Williams and Stuart 2003). Distal stimulation was much more likely to stimulate distal inputs, as indicated by the highly significant difference between the rise times of distally and proximally evoked IPSPs (Fig. 2C).
We first determined the effect of blocking GAT1-mediated uptake on psIPSPs elicited by proximal stimulation (Fig. 3A) at low frequency (0.1 Hz). We found that GABA transport block with 20 μM NO711 produced a significant prolongation of the psIPSPs (Fig. 3B). The magnitude of IPSP prolongation was determined by fitting an exponential decay function to the psIPSP decay (see methods) and comparing the decay time constant in control versus NO711 conditions. A pair-wise comparison revealed that NO711 increased by 57% the psIPSP decay time constant (Fig. 3C; paired samples t-test: t = 3.44, n = 45, P < 0.001). These results support the idea that when multiple perisomatic synapses are stimulated, GAT1 reduces spillover currents that otherwise significantly prolong the psIPSP duration. In addition to the change in IPSP duration, NO711 produced a small (22%, Fig. 3B) but statistically significant decrease in the psIPSP amplitude (control psIPSP amplitude: 5.01 ± 0.44 mV; NO711 psIPSP amplitude: 3.89 ± 0.43 mV, n = 45; paired samples t-test: t = 3.35, P < 0.002).
In most neurons (33 of 45, 74%), NO711 increased the psIPSP decay time constant (NO711-sensitive psIPSPs). In contrast, in a fraction of cells (12 of 45, 26%), NO711 application failed to increase the psIPSP decay by >5% (NO711-insensitive psIPSPs), as illustrated in Fig. 3C (top). NO711-insensitive psIPSPs may reflect a low probability of spillover due to stimulation of distant synapses (Overstreet and Westbrook 2003). Alternatively, NO711-sensitive psIPSPs could have been due to stimulation of a much larger number of inputs (Isaacson et al. 1993). However, before NO711 application, the peak amplitudes were small and were not different between NO711-sensitive (4.83 ± 0.49 mV, n = 33) and NO711-insensitive (5.57 ± 0.96 mV, n = 12) psIPSPs (independent samples t-test: t = 0.731, P = 0.468), suggesting that similarly small numbers of inputs were stimulated to elicit NO711-sensitive or -insensitive psIPSPs.
Further analysis of the psIPSP decay revealed that the decay time constant of psIPSPs recorded before NO711 application was not significantly different between NO711-sensitive psIPSPs (34.0 ± 1.7 ms, n = 33) and NO711-insensitive psIPSPs (31.2 ± 2.8 ms, n = 12; independent samples t-test: t = 0.858, P = 0.395). One possibility is that NO711-insensitive psIPSPs reflect cases in which spillover is absent or very small, for instance because the stimulated synapses are far apart. If this interpretation is correct, then the similar decay of NO711-sensitive and -insensitive psIPSPs recorded in control conditions suggests that GAT1 activity effectively prevents the effects of GABA spillover on psIPSP decay time.
GAT1-mediated uptake may be especially important during repetitive synaptic activity, because a higher release rate may increase the accumulation of GABA in the extracellular space compartment. We therefore examined the effects of NO711 during repetitive activation of proximal inputs to determine whether GAT1 regulates the time course of psIPSP trains. Every 10 s we applied trains of five stimuli at 20 Hz, a frequency that is within the range of firing rates of task-related activity of interneurons recorded in vivo from the neocortex of monkeys performing behavioral tasks (Constantinidis and Goldman-Rakic 2002; Mitchell et al. 2007; Wang et al. 2004). NO711 application (20 μM) prolonged the duration of each psIPSP in the train as well as the decay of the membrane potential at the end of the stimulus train (Fig. 3D). Exponential decay functions did not accurately fit the decay of individual psIPSPs or the posttrain potential (not shown). Therefore the effect of NO711 on psIPSP trains was estimated through the difference ΔVm between the pre- and posttrain membrane potential (Fig. 3, D and E). In control conditions, the membrane potential decayed back to its pretrain value by 300 ms posttrain (ΔVm control: 0.034 ± 0.035 mV). In contrast, in the presence of NO711, the neurons' posttrain membrane potential remained significantly depolarized (ΔVm NO711: 1.130 ± 0.183 mV; paired samples t-test: t = 5.79, n = 42, P < 0.0001). Although the effects of NO711 were typically visible shortly after the onset of bath application, reversal of the effect by washout was very slow (Fig. 3F), possibly due to the partially lipophylic nature of the compound (Borden 1996).
In many experiments with perisomatic stimulation, the effects of NO711 were tested both on single psIPSPs elicited at low stimulation frequency (0.1 Hz) and on psIPSP trains (20 Hz). This made it possible to compare the effects of NO711 on psIPSP trains, when stimulating inputs that produced NO711-sensitive versus NO711-insensitive single psIPSPs. If NO711-insensitive psIPSPs are due to stimulation of synapses lacking GAT1 transporters or expressing other GABA transporters, such as GAT3 (Keros and Hablitz 2005), then the psIPSP trains evoked by stimulation of the same inputs must also be NO711-insensitive. In contrast to this prediction, NO711 significantly prolonged the psIPSP trains in experiments in which single psIPSPs were NO711-insensitive (ΔVm control: −0.092 ± 0.100 mV; ΔVm NO711: 0.768 ± 0.434 mV; paired samples t-test: t = 1.833, n = 11, P < 0.05), although less so than in cases with NO711-sensitive single psIPSPs (ΔVm control: 0.045 ± 0.043 mV; ΔVm NO711: 1.365 ± 0.193 mV; paired samples t-test: t = 6.695, n = 29, P < 0.0001). By showing that NO711-insensitive inputs become NO711-sensitive in an activity-dependent manner, these results argue against the possibility that NO711-insensitive psIPSPs result from stimulating synapses lacking GAT1 transporters. These data suggest that in certain conditions the propensity for GABA spillover is very small or absent during low-frequency stimulation but becomes significant when the same group of synapses is activated repetitively. We found that NO711 produced a small but significant decrease in the cells' membrane time constant which slightly accelerated the mIPSP decay (see Fig. 1, B and C). Thus we cannot exclude the possibility that in some cases the IPSPs appeared to be NO711-insensitive because GAT1 block produced a very small IPSP prolongation that was obscured by the simultaneous decrease in membrane time constant.
Previous studies showed significant developmental changes through adolescence in GAT1 levels at some perisomatic synapses in monkey DLPFC (Cruz et al. 2003; Erickson and Lewis 2002). Because some of the present experiments were performed in slices from prepubertal monkeys (see methods), we determined whether the effects of GAT1 blockade on the psIPSPs were age-dependent. We found that blocking NO711 prolonged the psIPSPs in both age groups (prepubertal, psIPSP decay control: 34.3 ± 1.9 ms and psIPSP decay NO711: 55.9 ± 6.3 ms, n = 25; postpubertal, psIPSP decay control: 31.9 ± 2.3 ms and psIPSP decay NO711: 47.3 ± 8.9 ms, n = 20). Two-factor ANOVA revealed a significant effect of NO711 [F(1,43) = 14.2, P < 0.0005], no effect of age [F(1,43) = 0.817, P = 0.371], and no significant interaction between age and NO711 effect [F(1,43) = 0.408, P = 0.526]. In addition, we found that NO711 had significant effects on the psIPSP trains in neurons from both pre- and postpubertal animals (prepubertal, ΔVm control: −0.039 ± 0.049 mV and ΔVm NO711: 0.997 ± 0.235 mV, n = 28; postpubertal, ΔVm control: 0.071 ± 0.047 mV and ΔVm NO711: 1.385 ± 0.269 mV, n = 21). As with single psIPSPs, the effect of NO711 application was significant [F(1,43) = 13.6, P < 0.001], and we found no significant effect of age [F(1,43) = 0.0093, P = 0.924] and no significant age × NO711 effect interaction [F(1,43) = 0.0383, P = 0.844]. These data show that the effects on psIPSPs of GAT1-controlled spillover do not change significantly with age.
Complex regulation by GAT1-mediated uptake of IPSPs elicited by stimulation of dendrite-targeting inputs
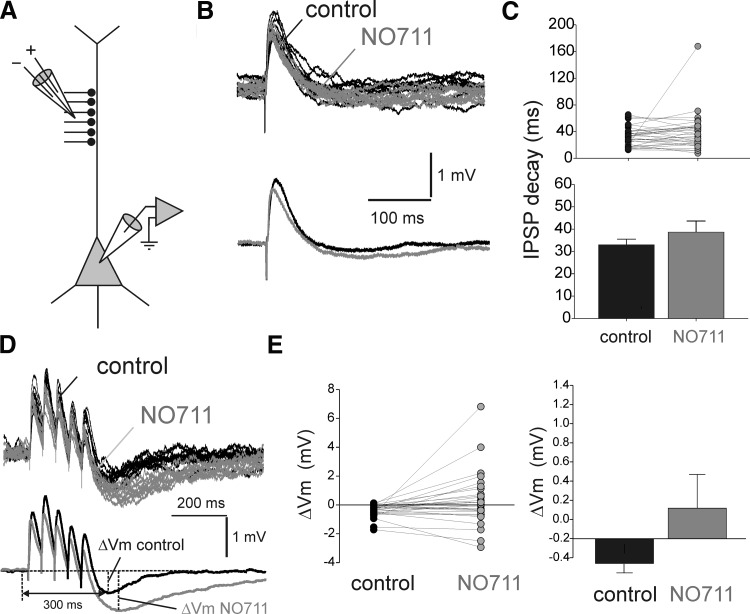
To examine the effects of GAT1-mediated uptake on dendritic IPSPs (dIPSPs), we applied NO711 during activation of dendrite-targeting inputs with focal stimulation of axons near the distal apical dendrite of the layer 3 pyramidal cells (Fig. 4A). Similar to psIPSPs, NO711 application slightly but significantly reduced the dIPSP amplitude (Fig. 4B) by 24.4% (control dIPSP amplitude: 2.17 ± 0.26 mV; NO711 dIPSP amplitude: 1.64 ± 0.25 mV, n = 32; paired samples t-test: t = 2.64, P < 0.02). However, in contrast to psIPSPs, GAT1 blockade did not significantly increase the dIPSP decay time (Fig. 4, B and C), as revealed by analysis of the exponential decay time constant (paired samples t-test: t = 1.12, P = 0.269, n = 32). NO711 application prolonged the dIPSP decay in 15 of 32 experiments but failed to increase the dIPSP decay time constant by >5% in 17 of 32 experiments (Fig. 4C). Interestingly, the proportion of NO711-insensitive IPSPs was significantly larger for dIPSPs than for psIPSPs (dIPSPs: 53%, 17 of 32; psIPSPs: 26%, 12 of 45; Pearson's χ2 test, P < 0.02). Although these data favor the conclusion that GABA spillover is less significant for dendritic than perisomatic IPSPs, the results of experiments with repetitive stimulation of distal GABA inputs revealed further complexity, as described in the following text.
FIG. 4.
Effects of GAT1 blockade with NO711 on IPSPs evoked by stimulation of dendrite-targeting inputs (dIPSPs). A: scheme showing the arrangement of the recording and stimulation electrodes. B: example experiment illustrating that NO711 application (20 μM) did not change the decay but slightly decreased the amplitude of dIPSPs. Top: consecutive traces superimposed. Bottom: averages of traces recorded in control and NO711 conditions. C: summary graphs showing the absence of statistically significant effects of NO711 on the decay time of dIPSPs. Shown at the time constants of single exponential decay functions fit to the decay of the dIPSPs recorded from each neuron in control conditions and in the presence of NO711. D: example traces illustrating the effects of NO711 on the time course of dIPSP trains evoked by stimulation with input trains (5 stimuli at 20 Hz). Top: consecutive traces superimposed. Bottom: averages of traces recorded in control and NO711 conditions. E: summary graphs showing the absence of statistically significant effects of NO711 on the dIPSP trains, measured as the difference ΔVm between the membrane potential 10 ms before and at the peak hyperpolarization after the onset of the stimulus train. Note that, typically, the hyperpolarization following dIPSP trains peaked later than 300 ms posttrain onset (see Fig. 4D).
Repetitive activity of dendrite-targeting inputs in control conditions induced, in many experiments, a hyperpolarizing potential after the end of the depolarizing dIPSP trains (Fig. 4D). Such hyperpolarization was not observed after the end of psIPSP trains in which the membrane potential decayed back to pretrain values at ∼300 ms posttrain onset (Fig. 3, D and E). NO711 application strongly increased the hyperpolarizing potential post dIPSP trains in 14 of 30 experiments, thus shortening the depolarization produced by dIPSP trains (Fig. 4D). However, the effects of NO711 were heterogeneous (Fig. 4E) and, consequently, the overall effect of NO711 on dIPSP trains was not statistically significant (ΔVm control: −0.285 ± 0.098 mV; ΔVm NO711: 0.318 ± 0.352; paired samples t-test: t = 1.816; P = 0.08, n = 30), in contrast to the significant prolongation of the posttrain depolarization in psIPSP trains (Fig. 3, D and E). These results suggest that the differences in the effects of GAT1 block on psIPSPs versus dIPSPs may be due, at least in part, to the presence of the hyperpolarizing potential in the latter. Therefore as described next, we characterized the mechanisms underlying the hyperpolarizing potential, to isolate it from the depolarizing GABAAR-IPSPs, and to compare the effects of GAT1 blockade on GABAAR-IPSPs elicited by stimulating dendritic- versus perisomatic-targeting inputs.
Stimulation of dendrite-targeting inputs produces a GABABR-mediated potential that is enhanced by GAT1 block
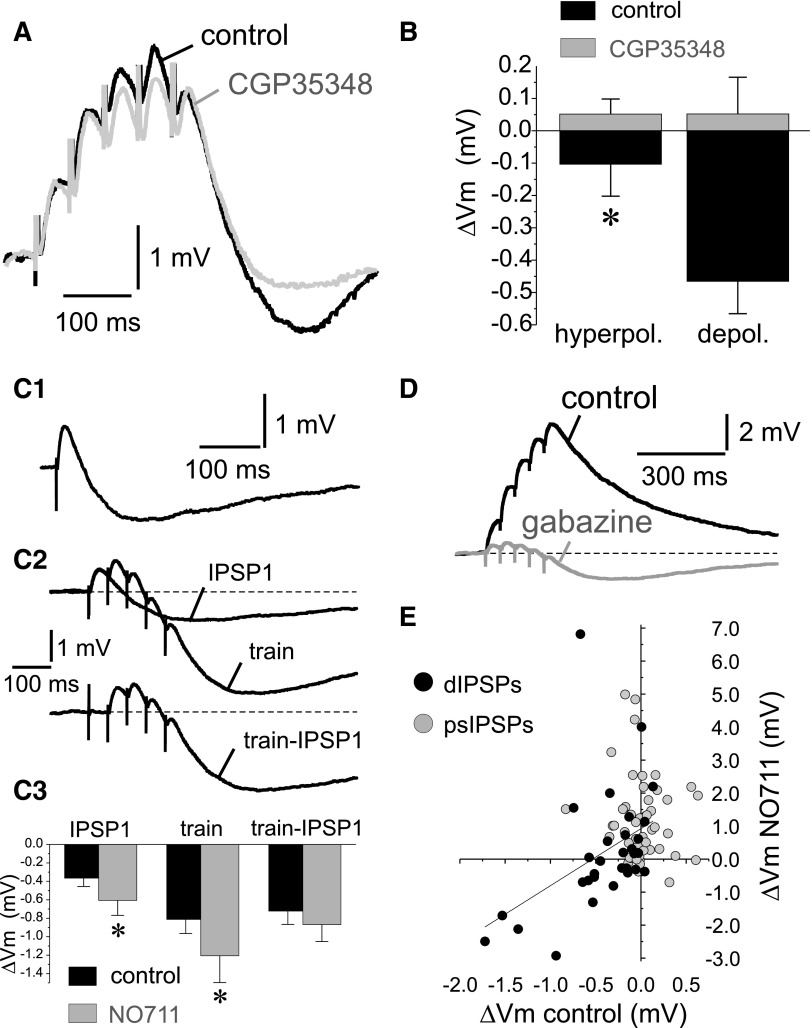
Throughout these experiments, the cells' somatic membrane potential was typically maintained at −70 to −75 mV, a range of values significantly more positive than the estimated K+ reversal potential (EK+ ∼ −100 mV, see methods). Because GABABRs produce hyperpolarizing IPSPs by activation of K+ channels (Luscher et al. 1997), we hypothesized that a K+ current activated by postsynaptic GABABRs could mediate the hyperpolarizing potential observed during stimulation of dendrite-targeting inputs. This hypothesis was tested first in experiments in which the posttrain hyperpolarizing potential was recorded in control conditions and then the GABABR antagonist CGP35348 (50 μM) was applied. We found that GABABR blockade abolished the hyperpolarizing potential (Fig. 5A), as revealed by a paired t-test comparison (control: −0.42 ± 0.11 mV; CGP35348: 0.03 ± 0.10 mV; n = 4, t = 2.865, P < 0.05). If a K+ current is involved in generating this potential, then increasing the K+ current driving force by membrane depolarization should enhance the hyperpolarizing potential amplitude. We found that the amplitude of the hyperpolarizing potential postdIPSP trains was significantly increased by membrane potential depolarization (Fig. 5B), consistent with its mediation by a K+ current. In contrast, when dendrite-targeting inputs were stimulated in the presence of CGP35348 (50 μM), no significant posttrain hyperpolarizing potential was observed at hyperpolarized potentials, nor after increasing the K+ current driving force with depolarization (Fig. 5B). The pharmacological and biophysical properties of the hyperpolarizing potential are therefore consistent with the idea that stimulation of dendrite-targeting inputs produced a GABAAR-IPSP and a K+ current- and GABAB-R mediated IPSP (GABABR-IPSP).
FIG. 5.
Stimulation of dendrite-targeting inputs elicits a GABABR-dIPSP. A: example traces showing that the GABABR antagonist 3-aminopropyl)(diethoxymethyl)phosphinic acid (CGP35348, 50 μM) markedly reduced the hyperpolarizing potential observed during the decay of dIPSP trains. B: in control conditions, the hyperpolarizing potential amplitude significantly increased (*, P < 0.05) following membrane depolarization (from −79.2 ± 3.3 to −61.7 ± 3.1 mV). In contrast, in the presence of CGP35348, membrane depolarization (from −77.8 ± 2.3 to −65.4 ± 1.5 mV) did not significantly change the posttrain potential. C1: example trace showing that in some experiments, single shock stimulation elicited GABABR-dIPSPs. C2: subtraction analysis demonstrated that typically the GABAB-dIPSP produced by stimulus trains was larger than that produced by single stimuli. C3: summary graphs showing the peak hyperpolarizing GABABR-dIPSP amplitude produced by single stimuli (IPSP1), by trains of 5 stimuli (train) and after subtraction from the trains of the GABABR-IPSP produced by a single stimulus (train –IPSP1). Two-way ANOVA indicated that there was a significant effect of stimulus [F(2,60) = 4.236, P < 0.02]. Bonferroni and Scheffe post hoc tests showed that the GABABR-dIPSP produced by trains was larger than with a single stimulus and that the GABABR-dIPSPs produced by a train or after subtracting the contribution of the first stimulus were not significantly different. In addition, ANOVA analysis indicated that the increase in the mean hyperpolarization post-dIPSP and post-dIPSP trains by NO711 did not reach significance [F(1,60) = 3.096, P = 0.083]. However, post hoc tests showed a significant effect of NO711 for IPSP1 and IPSP trains (P < 0.05). D: representative experiment showing that shortly after application of the GABAAR antagonist gabazine (10 μM), suppression of the GABAAR-dIPSPs revealed a hyperpolarizing GABABR-dIPSP component. E: plotting the ΔVm values measured in control and NO711 conditions revealed a significant correlation for dIPSPs (r = 0.4289, P < 0.02, n = 30) and an absence of correlation for psIPSPs (r = 0.1109, P = 0.443, n = 50).
Although the GABABR-IPSP was detectable mostly with repetitive stimulation of dendrite-targeting inputs, in some experiments (11 of 30), it was also observed during application of a single stimulus, following the decay of the GABAAR-dIPSP (Fig. 5C1). The presence of a GABABR-IPSP was not associated with weaker or stronger stimulation of dendrite-targeting inputs because the amplitude of the GABAAR-IPSP did not differ between responses with and without detectable GABABR-IPSP (GABAAR-IPSP without GABABR-IPSP: 2.05 ± 0.33 mV, n = 19; GABAAR-IPSP with GABABR-IPSP: 1.99 ± 0.43 mV, n = 11; independent samples t-test, t = 0.102, P = 0.919). Consistent with a postsynaptic GABABR-mediated response, the hyperpolarizing potential elicited by a single stimulus was long-lasting (with duration of ∼0.5 and ≤1.0 s). The GABABR-IPSP elicited by a single stimulus was typically smaller than that observed after the end of stimulus trains (Fig. 5C2). Because the GABABR-IPSP was long-lasting, part of the posttrain hyperpolarization was due to the GABABR-IPSP elicited by the first stimulus in the train (Fig. 5C2). To separate the contribution, to the posttrain GABABR-IPSP, of the first stimulus relative to subsequent stimuli in the trains, we subtracted from the dIPSP trains, traces with a single dIPSP recorded from the same neuron. Subtraction analysis showed that ∼80% of the posttrain GABABR-IPSP was elicited by GABA released by stimuli after the first in the train (Fig. 5C, 2 and 3). The GABABR-IPSP elicited by stimulation of dendrite-targeting inputs was typically increased by NO711 application (Fig. 5C3). These results suggest that activation of postsynaptic GABABRs probably was facilitated, or did not depress, by repetitive stimulation of dendrite-targeting inputs and by GAT1 blockade. GABA spillover may affect GABABR-IPSPs in a substantially different manner than GABAAR-IPSPs because GABABRs bind GABA with much higher affinity than GABAARs. Indeed many of the GABABRs in dendrites appear to be extrasynaptic (see discussion), suggesting that escape of GABA from the synaptic cleft followed by GAT1-controlled diffusion may represent a significant physiological source of GABABR activation (Scanziani 2000).
Due to their different reversal potentials in our experimental conditions, the GABAAR- and GABABR-mediated currents activated by stimulation of dendrite-targeting inputs should produce opposite effects on the decay of the pyramidal cell membrane potential at the end of dIPSP trains. Indeed blockade of GABABRs produced a depolarizing shift in the decay of membrane potential after the dIPSP trains (Fig. 5A) and application of GABAAR antagonists produced a hyperpolarizing shift (Fig. 5D). The opposing effects of the GABAA and GABAB currents suggest that the heterogeneity in the effects of NO711 on the decay of dIPSP trains (Fig. 4, D and E) could be due to variability in the relative amplitudes of the GABAAR- and GABABR-IPSPs produced before NO711 application. Consistent with this interpretation, a significant correlation was found (r = 0.4289, P < 0.02, n = 30) between the dIPSP ΔVm control and ΔVm NO711 values (Fig. 5E). This correlation indicated that GAT1 block enhanced both the GABAAR- and GABABR-dIPSPs, the increase in the GABABR-mediated component predominating if its amplitude in control conditions was more negative than approximately −0.5 mV (Fig. 5E). In contrast to the dIPSPs, no correlation was found (r = 0.1109, P = 0.443, n = 50) between ΔVm control and ΔVm NO711 values for psIPSPs, and NO711 induced a depolarizing change in ΔVm in most experiments (Fig. 5E). These results suggest that the presence of the GABABR-IPSP with stimulation of dendrite-targeting inputs interfered with the assessment of the effects of GAT1 block on the dendritic GABAAR-IPSPs. Because the GABABR-IPSP could be elicited by low-frequency stimulation (Fig. 5C1), it is likely that its presence precluded visualization of the NO711-induced prolongation of the GABAAR-IPSP, including in cases when a GABABR-IPSP could not be readily detected or produced a very small hyperpolarization that could be enhanced by NO711 (Fig. 4B).
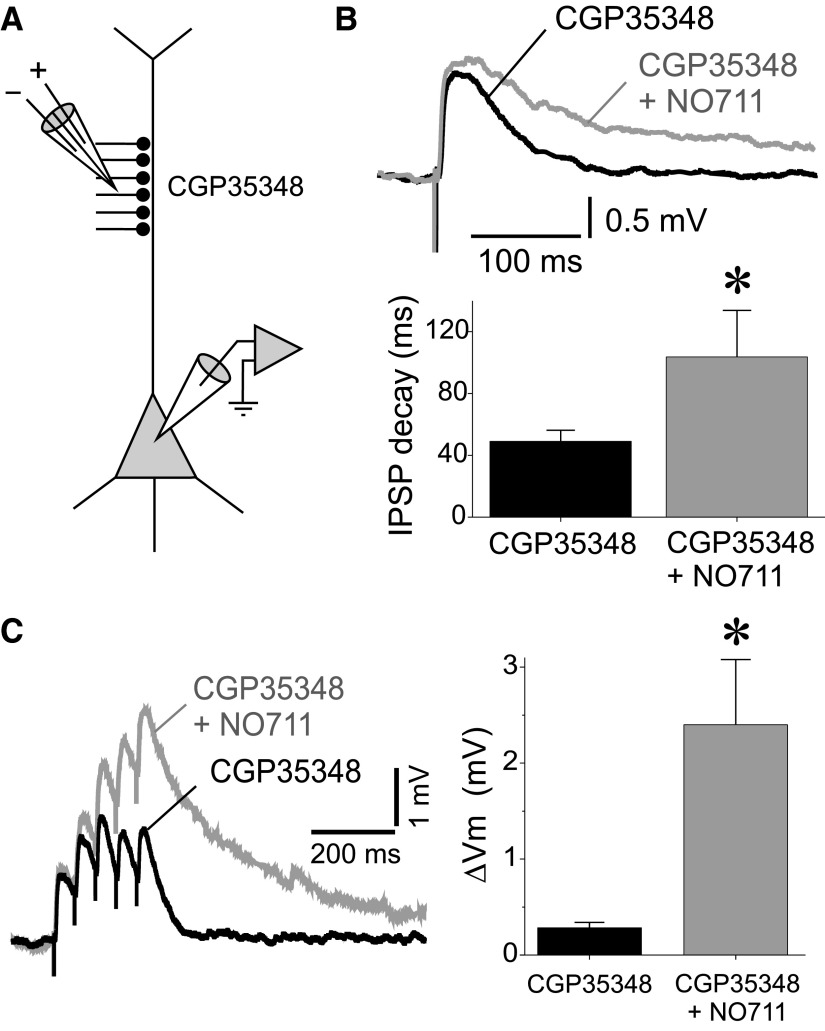
GAT1 block prolongs the GABAAR-IPSPs elicited by stimulation of dendrite-targeting inputs
If the lack of significant prolongation of dIPSPs by NO711 (Fig. 4, B–E) is indeed due to shunting or hyperpolarizing effects of the GABABR-dIPSP, then the GABAAR-dIPSP should be consistently prolonged by applying NO711 after GABABRs are blocked. To test this prediction, we recorded dIPSPs in the presence of the GABABR antagonist CGP35348 (Fig. 6A) and found that NO711 significantly prolonged the duration of the GABAAR-dIPSPs evoked at 0.1 Hz (Fig. 6B; GABAAR-dIPSP decay tau CGP35348: 52.7 ± 7.5 ms; GABAAR-dIPSP decay tau CGP35348+NO711: 125.0 ± 31.3 ms; paired samples t-test, t = 2.466, P < 0.05, n = 16). As in the case of GABAAR-psIPSPs, in some experiments, the GABAAR-dIPSPs recorded in the presence of CGP35348 were NO711-insensitive. The proportion of NO711-insensitive GABAAR-dIPSPs (31.2%, 5 of 16) was not significantly different (Pearson's χ2 test, P = 0.794) from the proportion of NO711-insensitive GABAAR-psIPSPs (26.7%, 12 of 45). If NO711-insensitive GABAAR-IPSPs reflect cases with low propensity for GABA spillover, these results suggest that the likelihood of GABA spillover is similar for psIPSPs and dIPSPs. The decay time constant of the GABAAR-dIPSPs before NO711 application was not different between NO711-sensitive (48.8 ± 7.45 ms, n = 11) and NO711-insensitive (61.3 ± 18.5 ms, n = 5; independent samples t-test: t = 0.759, P = 0.459) responses. These data suggest that, as for psIPSPs, GAT1 effectively prevents the effects of GABA spillover on dIPSP decay time.
FIG. 6.
Effects of GAT1 blockade on dIPSPs in the presence of the GABABR antagonist CGP35348. A: scheme showing the arrangement of the recording and stimulation electrodes. B: an example recording and summary graphs illustrating the prolongation of dIPSPs by NO711 (20 μM) in the presence of CGP35348 (50 μM). Shown are averages of 10 consecutive sweeps. The bar graphs display the time constants of single-exponential decay functions fit to the decay of the dIPSPs recorded from each neuron in the presence of CGP35348 and in the presence of CGP35348+NO711. C: an example recording and summary graphs illustrating the effects of NO711 (20 μM) on dIPSP trains recorded in the presence of CGP35348 (50 μM). The effect on dIPSP trains was measured as the difference ΔVm between the membrane potential measured 10 ms pre-IPSP train and at 300 ms after the onset of the stimulus train.
dIPSPs evoked by repetitive stimulation after GABABR blockade with CGP35348 did not display a posttrain hyperpolarizing potential (Fig. 6C). Furthermore, subsequent NO711 application strongly prolonged the decay of the membrane potential at the end of the depolarizing GABAAR-dIPSP train (Fig. 6C; ΔVm CGP35348: 0.283 ± 0.055 mV; ΔVm CGP35348+NO711: 2.400 ± 0.678; paired samples t-test: t = 3.333; P < 0.005, n = 16). These results indicate that when the shunting or hyperpolarizing effects of the GABABR-IPSPs were pharmacologically blocked, the NO711 effect was similar for dIPSPs and psIPSPs. With GABABRs blocked, NO711 increased significantly the posttrain ΔVm for inputs that produced NO711-insensitive single dIPSPs (ΔVm CGP35348: 0.17 ± 0.14 mV; ΔVm CGP35348+NO711: 1.27 ± 0.27 mV, P < 0.05, n = 5), although this effect was smaller than for inputs producing NO711-sensitive single dIPSPs (ΔVm CGP35348: 0.86 ± 0.43 mV; ΔVm CGP35348+NO711: 4.48 ± 1.32 mV, P < 0.005, n = 11). Thus similar to psIPSPs, for dendritic-targeting inputs GABA spillover may be negligible during low frequency activity but may become significant during repetitive activation of the same set of inputs.
In the absence of GABABR antagonists, NO711 decreased the amplitude of GABAAR-dIPSPs and GABAAR-psIPSPs (Figs. 3B and 4B). Interestingly, in the presence of CGP35348, the peak amplitude of the GABAAR-dIPSPs was not decreased by NO711 application (Fig. 6B; dIPSP amplitude CGP35348: 2.06 ± 0.44 mV; dIPSP amplitude CGP35348+NO711: 2.20 ± 0.49 mV; paired samples t-test: t = 0.8082, P = 0.438, n = 17). Similarly, in the presence of CGP35348 NO711 did not affect the GABAAR-psIPSP amplitude (psIPSP amplitude CGP35348: 3.72 ± 1.26 mV; psIPSP amplitude CGP35348+NO711: 4.65 ± 0.86 mV; paired samples t-test: t = 0.7631, P = 0.2501, n = 4), although in the same neurons NO711 significantly increased the posttrain ΔVm (ΔVm CGP35348: −0.082 ± 0.031 mV; ΔVm CGP35348+NO711: 2.801 ± 0.977 mV, 1-tail paired samples t-test: t = 2.9986, P < 0.05, n = 4). These data show that the decrease in GABAAR-IPSP amplitude by NO711 is GABABR-dependent.
One possibility is that the GABABR-dependent reduction in GABAAR-IPSP amplitude is mediated by presynaptic GABABRs that negatively control GABA release as shown in rat hippocampus (Buhl et al. 1995; Hefft et al. 2002; Neu et al. 2007; Price et al. 2008). GAT1 block may increase the transmitter available to activate such presynaptic GABABRs (Lei and McBain 2003). Whereas in certain synapses, presynaptic GABABRs are tonically activated by ambient GABA with uptake intact (Buhl et al. 1995; Lei and McBain 2003; Price et al. 2008), in other synapses, such tonic presynaptic receptor activation is not observed (Neu et al. 2007). Here we found that blockade of GABABRs in the absence of NO711 did not affect the amplitude of dIPSPs (dIPSP amplitude control: 1.16 ± 0.43 mV; dIPSP amplitude CGP35348: 1.26 ± 0.35 mV, t = 0.657, P = 0.539, paired samples t-test, n = 6). These data suggest that in monkey DLPFC GABABR-mediated regulation of GABAAR-IPSP amplitude, presumably via presynaptic mechanisms, is secondary to an increase in extracellular GABA levels by GAT1 block.
DISCUSSION
We determined the role of the GABA transporter GAT1 in regulating phasic GABA transmission in monkey DLPFC. We found that GAT1 block did not enhance miniature (single-synapse) GABA transmission but prolonged, most likely by increasing GABA spillover, IPSPs evoked by activating multiple synapses with extracellular axonal stimulation. Dendritic (but not perisomatic) stimulation produced a GABABR-IPSP that was enhanced by GAT1-mediated uptake. The GABAAR-IPSPs evoked by stimulation of perisomatic and dendritic GABA synapses were similarly prolonged by GAT1 block. In some experiments with either perisomatic or dendritic stimulation, the IPSPs were NO711-insensitive, suggesting a low propensity for GABA spillover. Because the proportion of NO711-insensitive IPSPs was similar for perisomatic and dendritic synapses, we conclude that at least in primate cortical circuits the propensity for spillover is similar for inputs onto proximal and distal compartments of the pyramidal cell membrane. Whether a similar situation is found in rat neocortex is not clear because no studies examined the role of GAT1-mediated uptake at dendrite-targeting inputs onto neocortical pyramidal neurons in rat brain. Finally, we found that GAT1 blockade produced a reduction of the GABAAR-IPSP amplitude that was abolished by application of a GABABR antagonist, which possibly blocked presynaptic GABABRs that negatively control GABA release.
Effects of GAT1 activity on miniature GABA transmission
Our results with mIPSP recordings argue against the idea that GABA uptake normally downregulates the IPSP amplitude or shortens the IPSP duration during GABAAR-mediated transmission at isolated synapses in monkey DLPFC. These data are consistent with findings from rat hippocampus showing that blockade of GABA uptake does not increase the amplitude nor the duration of single-synapse mIPSCs (Isaacson et al. 1993; Overstreet and Westbrook 2003; Thompson and Gahwiler 1992). Furthermore, our findings are unlikely to represent incomplete GAT1 block because NO711, a potent inhibitor with high efficiency at human and rat GAT1 homologs (Borden 1996), effectively blocks GAT1-mediated transport of endogenous GABA (Wu et al. 2007); we used a saturating NO711 concentration; and mIPSCs are also unchanged in GAT1 knock-out mice (Bragina et al. 2008; Jensen et al. 2003). Indeed we found that NO711 application slightly accelerated the mIPSP decay as well as the cells' membrane time constant (see results). These effects were strongly correlated, as expected if GAT1 blockade enhanced a tonic membrane conductance by increasing the ambient GABA levels that activate extrasynaptic GABAARs, as suggested elsewhere (Farrant and Nusser 2005). Investigating the control by GAT1 of GABAAR-mediated tonic currents was beyond the scope of the present study.
The absence of GAT1 effects on single-synapse transmission may be due to the predominantly extrasynaptic localization of this transporter (Vittellaro-Zuccarello et al. 2003). In addition, the kinetics of GABA transport by GAT1 is slow relative to the rapid kinetics of transmitter-receptor binding and channel gating (Bicho and Grewer 2005), probably contributing to the absence of regulation of single-synapse transmission. It is also possible that the synapses mediating mIPSPs by action potential-independent spontaneous GABA release lack GAT1 and thus differ from those mediating action potential-evoked release. However, previous studies ruled out this possibility by showing that IPSCs elicited by action potential-evoked release from single synapses are similarly NO711-insensitive (Overstreet and Westbrook 2003).
GAT1-mediated regulation of perisomatic versus dendritic IPSPs
In contrast to the absence of GAT1-mediated regulation of single-synapse transmission, blocking GAT1 typically prolonged GABAAR-IPSPs evoked by focal extracellular stimulation. Because the axons of GABA neurons typically make multiple synaptic contacts onto individual pyramidal cells, action potential-evoked IPSPs result from multiple-synapse stimulation, suggesting that IPSP prolongation produced by GAT1 blockade is due to between-synapse GABA spillover. Consistent with this interpretation, in GAT1 knock-out mice, IPSCs evoked by axonal stimulation exhibit significant prolongation without an increase in amplitude (Bragina et al. 2008). That GABA spillover slows the IPSC decay but does not increase the IPSC amplitude may be explained by the fact that the IPSC amplitude is determined by within-synapse GABA diffusion, which activates GABAARs much earlier than GABA diffusing between synapses (Barbour 2001).
We found that in some experiments, GABAAR-IPSPs evoked by extracellular axonal stimulation were NO711-insensitive, a finding also consistent with an absence of regulation of within-synapse GABAAR activation by GAT1. Our data suggest that such NO711-insensitive GABAAR-IPSPs were not due to stimulation of synapses lacking GAT1 because NO711 had significant effects when the probability of spillover was increased by repetitive stimulation of the same inputs. These findings may be explained if the NO711-insensitive IPSPs are mediated by distant synapses, and thus GABA spillover currents become significant only when, during repetitive stimulation, large amounts of GABA diffuse between synapses and can reach GABAARs more distant from the transmitter release sites.
Although GABA synapse density in the neuropil is thought to be lower in primate than rodent neocortex (DeFelipe et al. 2002), our data show that in monkey DLPFC GABA synapse density appears to be sufficient to produce significant spillover after GAT1 block. In addition, we found a similar proportion of NO711-insensitive psIPSPs and dIPSPs, suggesting a similar probability of spillover onto perisomatic and dendritic synapses. These data suggest that GABA synapse density in the neuropil is a more significant determinant of the probability of spillover than synapse density in the dendritic versus somatic membrane of individual pyramidal cells. Interestingly, prior to GAT1 block, NO711-sensitive GABAAR-IPSPs had similar duration than NO711-insensitive GABAAR-IPSPs, suggesting that GAT1 activity effectively prevents spillover.
We found that blocking GAT1 produced some differential effects at perisomatic versus dendritic GABA synaptic inputs. Specifically, stimulation of distal (but not proximal) synapses elicited, after the GABAAR-IPSP, a GABABR-IPSP that was enhanced by GAT1 block. That GABABR-IPSPs were preferentially evoked by distal synapse stimulation may be explained by the subcellular distribution of the GABABRs and K+ channels mediating the GABABR-IPSPs. Compared with the perisomatic compartment, distal pyramidal cell dendrites have higher density of GABABR subunits and Kir3.2 K+ channels (Kulik et al. 2006; Lopez-Bendito et al. 2002). Kir3.2 channels and GABABR subunits are co-clustered in the extrasynaptic membrane near dendritic GABA synapses (Kulik et al. 2006). An extrasynaptic localization suggests that GABABR activation requires GABA diffusion over a distance from the release sites. Consistent with this possibility, we found that GABABR-IPSPs were larger during repetitive activity and were enhanced by GAT1 blockade.
In rat neocortex, most interneuron subtypes elicit exclusively GABAAR-IPSPs. Similarly, we reported previously that in monkey DLPFC, perisomatic-targeting fast-spiking basket and chandelier neurons elicit in pyramidal cells IPSPs that are exclusively GABAAR-mediated (Gonzalez-Burgos et al. 2005). Cells in a third subtype of interneuron furnishing perisomatic inhibition, the nonfast-spiking basket neurons, signal exclusively through GABAARs, suggesting that perisomatic inhibition postsynaptically is purely GABAAR-mediated (Freund and Katona 2007). In contrast, rat neurogliaform cells (NGFCs) elicit dual IPSPs mediated by both GABAARs and GABABRs (Szabadics et al. 2007; Tamas et al. 2003). In human neocortex, GABABR-IPSPs were described previously (McCormick 1989) and recently identified to be mediated by NGFCs (Olah et al. 2007) as in the rat neocortex. Therefore the GABABR-dIPSPs recorded here may have been mediated by stimulating axons of NGFCs, which are present in monkey DLPFC (Povysheva et al. 2007; Zaitsev et al. 2008) and are known to target almost exclusively pyramidal cell dendritic spines and shafts (Tamas et al. 2003; Vida et al. 1998).
Some data, however, are not consistent with this interpretation. First, in rat neocortex, NGFCs make relatively proximal synapses (Szabadics et al. 2007; Tamas et al. 2003), which can be activated by perisomatic extracellular stimulation. Interestingly, however, in human neocortex NGFCs preferentially contact distal dendrites (Kisvarday et al. 1990). Second, in both rat and human neocortex, NGFC synapses display strong activity-dependent depression of GABA release with an extremely slow rate of recovery (Olah et al. 2007; Tamas et al. 2003). Thus in this study, NGFC-IPSPs should have been substantially or completely depressed by baseline stimulation. However, we found that GABABR-dIPSPs were stronger or were exclusively observed with repetitive stimulation, suggesting that some of the underlying inputs did not show significant depression. One possibility is that the GABABR-IPSPs evoked in this study were mediated by stimulating axons of non-NGFC subtypes. For instance, in rat hippocampus, NGFCs elicit GABABR-dIPSPs with strong depression (Price et al. 2008), but other interneuron subtypes produce GABABR-IPSPs that facilitate with repetitive stimulation (Thomson and Destexhe 1999). It is also possible that during repetitive stimulation and GAT1 blockade, GABABRs usually activated by NGFCs are activated by GABA released from other interneuron subtypes.
Functional implications
Our results suggest that in monkey DLPFC GAT1-mediated uptake restricts transmitter spillover at both perisomatic and dendritic GABA inputs onto pyramidal neurons. We also demonstrated that the effects of spillover induced by GAT1 blockade are sufficient to cause IPSP prolongation in addition to the IPSC prolongation found in previous studies. GAT1-mediated control of IPSP duration could be critical to the timing of GABA-mediated inhibition during network oscillations when interneurons of a given subtype show synchronized firing locked to a particular phase of the oscillation cycle (Klausberger and Somogyi 2008). Synchronous firing of multiple interneurons of the same subtype during oscillations would produce pooling of GABA released from multiple synapses, increasing the probability of spillover. Because the IPSP duration may be critical for the oscillation frequency (Kramer et al. 2008; Traub et al. 1996; Whittington et al. 1995), a deficit in GAT1 activity could alter the oscillation period, as shown in computational modeling studies (Vierling-Claassen et al. 2008). Moreover, IPSP prolongation may perturb the relation between inhibitory inputs from different interneuron subtypes during the oscillation cycle. For instance, IPSP prolongation may lead to overlapping effects of hyperpolarizing and depolarizing IPSPs that with GAT1 activity intact would have independent effects. Preserving IPSP duration from spillover-induced prolongation may thus be critical for independent cell type-specific inhibition during oscillations and therefore for cognitive functions that may depend on signal propagation based on oscillatory synchrony in neural circuits.
We found that GAT1 activity regulates the strength and duration of GABABR-IPSPs. Dendritic GABABR-IPSPs powerfully inhibit dendritic Ca2+ spikes (Perez-Garci et al. 2006) and spike backpropagation into dendrites (Leung and Peloquin 2006). Activation of the predominantly extrasynaptic dendritic GABABRs (Kulik et al. 2006), may be tightly regulated by GAT1-controlled GABA diffusion. Thus GAT1 activity modulation may be critical for the control by GABA of dendritic excitability, the timing of dendritic Ca2+ spike initiation and therefore of computations performed at pyramidal neuron dendrites to induce plasticity at glutamate synapses (Kampa et al. 2007). Interestingly, GAT1 activity can be regulated without changing the levels of GAT1 protein (Ortinski et al. 2006), for instance by phosphorylation-dependent internalization (Quick et al. 2004).
The effects of GAT1 block reported here were not different between psIPSPs recorded from neurons of pre- and postadolescent monkeys. However, because psIPSPs were evoked by stimulating axons of unknown source, some inputs may have been underrepresented, in particular the connections from chandelier neurons onto pyramidal cells, which display an adolescence-related decrease in GAT1 levels (Cruz et al. 2003). In our experimental conditions (EGABAA ∼0 mV), the GABAAR-IPSPs produced by chandelier cell connections would strongly depolarize the pyramidal cell axon near the spike initiation zone (Khirug et al. 2008) readily eliciting firing (Szabadics et al. 2006). Suprathreshold psIPSPs as those expected from chandelier cell axon stimulation were observed in some experiments (data not shown) but were not suitable to assess the effects of GAT1 block. Because chandelier cell synapses were most likely excluded from analysis, the absence of difference in the effects of NO711 on IPSPs from pre- and postadolescent animals is consistent with data showing that the overall density of GAT1-containing axon terminals does not change through adolescence in monkey DLPFC (Erickson and Lewis 2002). Furthermore, in monkey neocortex, the neuropil density of inhibitory synapses, which probably determines the propensity for spillover, appears to reach stable adult-like values early in development, well before adolescence begins (Rakic et al. 1986). Our findings thus support the idea that the decline in GAT1 levels seen during adolescence in chandelier cell axon cartridges (Cruz et al. 2003) is not observed at synapses from other GABA neurons in monkey DLPFC. The functional consequences of such a chandelier cell-specific adolescence-related decrease in GAT1 remain to be determined.
In the DLPFC of subjects with schizophrenia, GAT1 levels are decreased (Hashimoto et al. 2008) in a subset of GABA neurons (Volk et al. 2001). GAT1 reduction may increase spillover, disrupting GABA signaling and contributing to cortical circuit dysfunction in the illness (Vierling-Claassen et al. 2008). However, decreased GAT1 expression in schizophrenia co-occurs with a decrease in the mRNA for the GABA synthesis enzyme GAD67 (Hashimoto et al. 2008). GAD67 deficiency may reduce the concentration of GABA inside synaptic vesicles (Jin et al. 2003), decreasing the amount of GABA released and inhibitory synaptic strength but also decreasing the likelihood of spillover. Because long-term decreases in extracellular GABA reduce GAT1 expression (Bernstein and Quick 1999), GAT1 downregulation in schizophrenia may be a compensatory response (Lewis et al. 2005). Although GAT1 does not regulate within-synapse transmission when GABA release is normal, decreased GAT1 activity may be beneficial by helping restore synaptic strength if the amount of GABA released is reduced. To assess whether reduced GAT1 expression in schizophrenia is deleterious or beneficial, the role of GAT1 must be tested under conditions of decreased GABA synthesis and release.
GRANTS
This work was funded by National Institute of Mental Health Grants MH-51234 and MH-45156. Dr. Lewis currently receives research support from the Bristol-Myers Squibb Foundation, Merck, and Pfizer; he has served as a consultant for Bristol-Myers Squibb, Lilly, Merck, Neurogen, Pfizer, Hoffman-Roche, Sepracor, and Wyeth.
Acknowledgments
We thank O. Krimer for excellent assistance with neuron reconstruction.
Present addresses: A. V. Zaitsev, Dept. of Physiology, Trinity College, Dublin 2, Ireland; N. V. Povysheva, Dept. of Neuroscience, University of Pittsburgh, Pittsburgh, PA.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Andrasfalvy 2006.Andrasfalvy BK, Mody I. Differences between the scaling of miniature IPSCs and EPSCs recorded in the dendrites of CA1 mouse pyramidal neurons. J Physiol 576: 191–196, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour 2001.Barbour B An evaluation of synapse independence. J Neurosci 21: 7969–7984, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos 2007.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8: 45–56, 2007. [DOI] [PubMed] [Google Scholar]
- Bernstein 1999.Bernstein EM, Quick MW. Regulation of gamma-aminobutyric acid (GABA) transporters by extracellular GABA. J Biol Chem 274: 889–895, 1999. [DOI] [PubMed] [Google Scholar]
- Bicho 2005.Bicho A, Grewer C. Rapid substrate-induced charge movements of the GABA transporter GAT1. Biophys J 89: 211–231, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden 1996.Borden LA GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int 29: 335–356, 1996. [DOI] [PubMed] [Google Scholar]
- Bragina 2008.Bragina L, Marchionni I, Omrani A, Cozzi A, Pellegrini-Giampietro DE, Cherubini E, Conti F. GAT-1 regulates both tonic and phasic GABA(A) receptor-mediated inhibition in the cerebral cortex. J Neurochem 105: 1781–1793, 2008. [DOI] [PubMed] [Google Scholar]
- Buhl 1995.Buhl EH, Cobb SR, Halasy K, Somogyi P. Properties of unitary IPSPs evoked by anatomically identified basket cells in the rat hippocampus. Eur J Neurosci 7: 1989–2004, 1995. [DOI] [PubMed] [Google Scholar]
- Chang 2007.Chang YM, Luebke JI. Electrophysiological diversity of layer 5 pyramidal cells in the prefrontal cortex of the rhesus monkey: in vitro slice studies. J Neurophysiol 98: 2622–2632, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis 2002.Constantinidis C, Goldman-Rakic PS. Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J Neurophysiol 88: 3487–3497, 2002. [DOI] [PubMed] [Google Scholar]
- Conti 1998.Conti F, Melone M, De Biasi S, Minelli A, Brecha NC, Ducati A. Neuronal and glial localization of GAT-1, a high-affinity gamma-aminobutyric acid plasma membrane transporter, in human cerebral cortex: with a note on its distribution in monkey cortex. J Comp Neurol 396: 51–63, 1998. [DOI] [PubMed] [Google Scholar]
- Conti 2004.Conti F, Minelli A, Melone M. GABA transporters in the mammalian cerebral cortex: localization, development and pathological implications. Brain Res Brain Res Rev 45: 196–212, 2004. [DOI] [PubMed] [Google Scholar]
- Cruz 2003.Cruz DA, Eggan SM, Lewis DA. Postnatal development of pre- and postsynaptic GABA markers at chandelier cell connections with pyramidal neurons in monkey prefrontal cortex. J Comp Neurol 465: 385–400, 2003. [DOI] [PubMed] [Google Scholar]
- DeFelipe 2002.DeFelipe J, Alonso-Nanclares L, Arellano JI. Microstructure of the neocortex: comparative aspects. J Neurocytol 31: 299–316, 2002. [DOI] [PubMed] [Google Scholar]
- Edwards 2007.Edwards RH The neurotransmitter cycle and quantal size. Neuron 55: 835–858, 2007. [DOI] [PubMed] [Google Scholar]
- Erickson 2002.Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol 448: 186–202, 2002. [DOI] [PubMed] [Google Scholar]
- Farrant 2007.Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res 160: 59–87, 2007. [DOI] [PubMed] [Google Scholar]
- Farrant 2005.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6: 215–229, 2005. [DOI] [PubMed] [Google Scholar]
- Freund 2007.Freund TF, Katona I. Perisomatic inhibition. Neuron 56: 33–42, 2007. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos 2000.Gonzalez-Burgos G, Barrionuevo G, Lewis DA. Horizontal synaptic connections in monkey prefrontal cortex: an in vitro electrophysiological study. Cereb Cortex 10: 82–92, 2000. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos 2005.Gonzalez-Burgos G, Krimer LS, Povysheva NV, Barrionuevo G, Lewis DA. Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J Neurophysiol 93: 942–953, 2005. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos 2004.Gonzalez-Burgos G, Krimer LS, Urban NN, Barrionuevo G, Lewis DA. Synaptic efficacy during repetitive activation of excitatory inputs in primate dorsolateral prefrontal cortex. Cereb Cortex 14: 530–542, 2004. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos 2008.Gonzalez-Burgos G, Kroener S, Zaitsev AV, Povysheva NV, Krimer LS, Barrionuevo G, Lewis DA. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cereb Cortex 18: 626–637, 2008. [DOI] [PubMed] [Google Scholar]
- Guastella 1990.Guastella J, Nelson N, Nelson H, Czyzyk L, Keynan S, Miedel MC, Davidson N, Lester HA, Kanner BI. Cloning and expression of a rat brain GABA transporter. Science 249: 1303–1306, 1990. [DOI] [PubMed] [Google Scholar]
- Gulledge 2003.Gulledge AT, Stuart GJ. Excitatory actions of GABA in the cortex. Neuron 37: 299–309, 2003. [DOI] [PubMed] [Google Scholar]
- Hajos 2000.Hajos N, Nusser Z, Rancz EA, Freund TF, Mody I. Cell type- and synapse-specific variability in synaptic GABAA receptor occupancy. Eur J Neurosci 12: 810–818, 2000. [DOI] [PubMed] [Google Scholar]
- Hashimoto 2008.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry 13: 147–161, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft 2002.Hefft S, Kraushaar U, Geiger JR, Jonas P. Presynaptic short-term depression is maintained during regulation of transmitter release at a GABAergic synapse in rat hippocampus. J Physiol 539: 201–208, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel 2007.Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proc Natl Acad Sci USA 104: 3562–3567, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel 2006.Herculano-Houzel S, Mota B, Lent R. Cellular scaling rules for rodent brains. Proc Natl Acad Sci USA 103: 12138–12143, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson 1993.Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron 10: 165–175, 1993. [DOI] [PubMed] [Google Scholar]
- Jensen 2003.Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I. GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J Neurophysiol 90: 2690–2701, 2003. [DOI] [PubMed] [Google Scholar]
- Jin 2003.Jin H, Wu H, Osterhaus G, Wei J, Davis K, Sha D, Floor E, Hsu CC, Kopke RD, Wu JY. Demonstration of functional coupling between gamma -aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles. Proc Natl Acad Sci USA 100: 4293–4298, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa 2007.Kampa BM, Letzkus JJ, Stuart GJ. Dendritic mechanisms controlling spike-timing-dependent synaptic plasticity. Trends Neurosci 30: 456–463, 2007. [DOI] [PubMed] [Google Scholar]
- Keros 2005.Keros S, Hablitz JJ. Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. J Neurophysiol 94: 2073–2085, 2005. [DOI] [PubMed] [Google Scholar]
- Khirug 2008.Khirug S, Yamada J, Afzalov R, Voipio J, Khirough R, Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl- contransporter NKCC1. J Neurosci 28: 4635–4639, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisvarday 1990.Kisvarday ZF, Gulyas A, Beroukas D, North JB, Chubb IW, Somogyi P. Synapses, axonal and dendritic patterns of GABA-immunoreactive neurons in human cerebral cortex. Brain 113: 793–812, 1990. [DOI] [PubMed] [Google Scholar]
- Klausberger 2008.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321: 53–57, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch 1996.Koch C, Rapp M, Segev I. A brief history of time (constants). Cereb Cortex 6: 93–101, 1996. [DOI] [PubMed] [Google Scholar]
- Kramer 2008.Kramer MA, Roopun AK, Carracedo LM, Traub RD, Whittington MA and Kopell NJ. Rhythm generation through period concatenation in rat somatosensory cortex. PLoS Comput Biol 4: e1000169, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik 2006.Kulik A, Vida I, Fukazawa Y, Guetg N, Kasugai Y, Marker CL, Rigato F, Bettler B, Wickman K, Frotscher M, Shigemoto R. Compartment-dependent colocalization of Kir3.2-containing K+ channels and GABAB receptors in hippocampal pyramidal cells. J Neurosci 26: 4289–4297, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie 1996.Lavoie AM, Twyman RE. Direct evidence for diazepam modulation of GABAA receptor microscopic affinity. Neuropharmacology 35: 1383–1392, 1996. [DOI] [PubMed] [Google Scholar]
- Lei 2003.Lei S, McBain CJ. GABA B receptor modulation of excitatory and inhibitory synaptic transmission onto rat CA3 hippocampal interneurons. J Physiol 546: 439–453, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung 2006.Leung LS, Peloquin P. GABA(B) receptors inhibit backpropagating dendritic spikes in hippocampal CA1 pyramidal cells in vivo. Hippocampus 16: 388–407, 2006. [DOI] [PubMed] [Google Scholar]
- Lewis 2005.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6: 312–324, 2005. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito 2002.Lopez-Bendito G, Shigemoto R, Kulik A, Paulsen O, Fairen A, Lujan R. Expression and distribution of metabotropic GABA receptor subtypes GABABR1 and GABABR2 during rat neocortical development. Eur J Neurosci 15: 1766–1778, 2002. [DOI] [PubMed] [Google Scholar]
- Luscher 1997.Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19: 687–695, 1997. [DOI] [PubMed] [Google Scholar]
- Mager 1996.Mager S, Kleinberger-Doron N, Keshet GI, Davidson N, Kanner BI, Lester HA. Ion binding and permeation at the GABA transporter GAT1. J Neurosci 16: 5405–5414, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick 1989.McCormick DA GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol 62: 1018–1027, 1989. [DOI] [PubMed] [Google Scholar]
- Megias 2001.Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102: 527–540, 2001. [DOI] [PubMed] [Google Scholar]
- Minelli 1995.Minelli A, Brecha NC, Karschin C, DeBiasi S, Conti F. GAT-1, a high-affinity GABA plasma membrane transporter, is localized to neurons and astroglia in the cerebral cortex. J Neurosci 15: 7734–7746, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell 2007.Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55: 131–141, 2007. [DOI] [PubMed] [Google Scholar]
- Mozrzymas 2004.Mozrzymas JW Dynamism of GABA(A) receptor activation shapes the “personality” of inhibitory synapses. Neuropharmacology 47: 945–960, 2004. [DOI] [PubMed] [Google Scholar]
- Neu 2007.Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol 578: 233–247, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah 2007.Olah S, Komlosi G, Szabadics J, Varga C, Toth E, Barzo P, Tamas G. Output of neurogliaform cells to various neuron types in the human and rat cerebral cortex. Front Neural Circuits 1: 4, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski 2006.Ortinski PI, Turner JR, Barberis A, Motamedi G, Yasuda RP, Wolfe BB, Kellar KJ, Vicini S. Deletion of the GABA(A) receptor alpha1 subunit increases tonic GABA(A) receptor current: a role for GABA uptake transporters. J Neurosci 26: 9323–9331, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet 2000.Overstreet LS, Jones MV, Westbrook GL. Slow desensitization regulates the availability of synaptic GABA(A) receptors. J Neurosci 20: 7914–7921, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet 2003.Overstreet LS, Westbrook GL. Synapse density regulates independence at unitary inhibitory synapses. J Neurosci 23: 2618–2626, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp 2001.Papp E, Leinekugel X, Henze DA, Lee J, Buzsaki G. The apical shaft of CA1 pyramidal cells is under GABAergic interneuronal control. Neuroscience 102: 715–721, 2001. [DOI] [PubMed] [Google Scholar]
- Perez-Garci 2006.Perez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron 50: 603–616, 2006. [DOI] [PubMed] [Google Scholar]
- Perrais 1999.Perrais D, Ropert N. Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses. J Neurosci 19: 578–588, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierri 1999.Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry 156: 1709–1719, 1999. [DOI] [PubMed] [Google Scholar]
- Pouille 2004.Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature 429: 717–723, 2004. [DOI] [PubMed] [Google Scholar]
- Povysheva 2007.Povysheva NV, Zaitsev AV, Kroener S, Krimer OA, Rotaru DC, Gonzalez-Burgos G, Lewis DA, Krimer LS. Electrophysiological differences between neurogliaform cells from monkey and rat prefrontal cortex. J Neurophysiol 97: 1030–1039, 2007. [DOI] [PubMed] [Google Scholar]
- Price 2008.Price CJ, Scott R, Rusakov DA, Capogna M. GABA(B) receptor modulation of feedforward inhibition through hippocampal neurogliaform cells. J Neurosci 28: 6974–6982, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick 2004.Quick MW, Hu J, Wang D, Zhang HY. Regulation of a gamma-aminobutyric acid transporter by reciprocal tyrosine and serine phosphorylation. J Biol Chem 279: 15961–15967, 2004. [DOI] [PubMed] [Google Scholar]
- Rakic 1986.Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science 232: 232–235, 1986. [DOI] [PubMed] [Google Scholar]
- Scanziani 2000.Scanziani M GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron 25: 673–681, 2000. [DOI] [PubMed] [Google Scholar]
- Sherwood 2006.Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, Hof PR. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci USA 103: 13606–13611, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi 1998.Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Rev 26: 113–135, 1998. [DOI] [PubMed] [Google Scholar]
- Szabadics 2007.Szabadics J, Tamas G, Soltesz I. Different transmitter transients underlie presynaptic cell type specificity of GABAA,slow and GABAA,fast. Proc Natl Acad Sci USA 104: 14831–14836, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics 2006.Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311: 233–235, 2006. [DOI] [PubMed] [Google Scholar]
- Tamas 2003.Tamas G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science 299: 1902–1905, 2003. [DOI] [PubMed] [Google Scholar]
- Thompson 1992.Thompson SM, Gahwiler BH. Effects of the GABA uptake inhibitor tiagabine on inhibitory synaptic potentials in rat hippocampal slice cultures. J Neurophysiol 67: 1698–1701, 1992. [DOI] [PubMed] [Google Scholar]
- Thomson 1999.Thomson AM, Destexhe A. Dual intracellular recordings and computational models of slow inhibitory postsynaptic potentials in rat neocortical and hippocampal slices. Neuroscience 92: 1193–1215, 1999. [DOI] [PubMed] [Google Scholar]
- Traub 1996.Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol 493: 471–484, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida 1998.Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH. Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol 506: 755–773, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling-Claassen 2008.Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. J Neurophysiol 99: 2656–2671, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittellaro-Zuccarello 2003.Vittellaro-Zuccarello L, Calvaresi N, DeBiasi S. Expression of GABA transporters, GAT-1 and GAT-3, in the cerebral cortex and thalamus of the rat during postnatal development. Cell Tissue Res 313: 245–257, 2003. [DOI] [PubMed] [Google Scholar]
- Volk 2001.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry 158: 256–265, 2001. [DOI] [PubMed] [Google Scholar]
- Wang 2004.Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci USA 101: 1368–1373, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington 1995.Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373: 612–615, 1995. [DOI] [PubMed] [Google Scholar]
- Williams 2003.Williams SR, Stuart GJ. Voltage- and site-dependent control of the somatic impact of dendritic IPSPs. J Neurosci 23: 7358–7367, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu 2007.Wu Y, Wang W, Diez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron 56: 851–865, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev.Zaitsev AV, Povysheva NV, Gonzalez-Burgos G, Rotaru D, Fish KN, Krimer LS, Lewis DA. Interneuron diversity in layers 2–3 of monkey prefrontal cortex. Cereb Cortex doi: 10.1093/cercor/bhn198.2008. [DOI] [PMC free article] [PubMed]