Abstract
The plant hormone ethylene is an important signal in plant growth responses to environmental cues. In vegetative growth, ethylene is generally considered as a regulator of cell expansion, but a role in the control of meristem growth has also been suggested based on pharmacological experiments and ethylene-overproducing mutants. In this study, we used transgenic ethylene-insensitive and ethylene-overproducing hybrid aspen (Populus tremula × tremuloides) in combination with experiments using an ethylene perception inhibitor [1-methylcyclopropene (1-MCP)] to demonstrate that endogenous ethylene produced in response to leaning stimulates cell division in the cambial meristem. This ethylene-controlled growth gives rise to the eccentricity of Populus stems that is formed in association with tension wood.
Keywords: plant hormones, secondary xylem, tension wood, vascular cambium, wood development
The vascular cambium is the meristem that produces secondary xylem and phloem by periclinal cell divisions, and it is responsible for wood production and stem diameter growth in trees. Cambial growth rate along the trunk is correlated with leaf biomass and crown structure (1). Superimposed on this intrinsic control, cambial growth is also strongly influenced by environment, where mechanical and gravitational loads imposed by wind and leaning are important (2). Wind sway induces increased diameter growth that protects against stem breakage (3), whereas a static lean results in a localized growth response known as tension wood (TW) in dicotyledonous angiosperms. TW is formed on the upper side of the leaning stem, resulting in characteristic asymmetric growth, and it serves to correct the stem position (4). In addition to the striking increase in cambial cell divisions, TW has an altered anatomy, and the fibers form an additional inner, cellulose-rich, gelatinous secondary cell wall layer (G layer) (5).
Experiments with ethylene applications have revealed that this volatile plant hormone has the potential to both inhibit and stimulate growth (6). Its synthesis from S-adenosylmethionine through the action of 1-aminocyclopropane-1-carboxylate (ACC) synthase (ACS) and ACC oxidase (ACO) is triggered in vegetative tissues in response to many environmental cues. To date, concepts of ethylene function in vegetative growth have mainly implicated its role in primary tissue and cell expansion (6). However, ethylene biosynthesis increases during TW formation because of an asymmetric induction of ACO (7, 8), and application of ethylene has been shown to stimulate cambial growth both in trees and herbaceous species (9, 10). Additional observations also support a potential role for ethylene in cell division. Applied ethylene stimulated endoreduplication in cucumber hypocotyls and growth of the intercalary meristem in deepwater rice (11–13). Moreover, the ethylene-overproducing Arabidopsis mutant eto1 exhibits aberrant cell divisions in the quiescent center of the root (14). However, experiments where ethylene homeostasis is artificially manipulated by any means (pharmacological, transgenic, or mutant approaches) are likely to suffer from aberrant compartmentalization and/or concentration of the hormone, and they do not prove its function under natural conditions. Therefore, conclusive evidence supporting a causal link between endogenous ethylene and the stimulation of meristematic growth has been lacking.
The most attractive approach to establishing the function of endogenous ethylene is to block ethylene perception. Ethylene is perceived by a family of membrane-bound receptors (15). The first receptor identified, ETHYLENE RESPONSE 1 (ETR1), was revealed in a mutant screen for an aberrant triple-response phenotype in etiolated Arabidopsis seedlings (16, 17). In the etr1 mutant, ethylene binding to the receptor is lost, and because of the dominant nature of the mutant alleles, this renders the plant insensitive to ethylene (18). In species less amenable to mutant screens, including trees, heterologous expression of the Arabidopsis etr1-1 mutant allele has been used to construct ethylene-insensitive plants to explore the function of endogenous ethylene (19–22).
We have used a transgenic approach to generate ethylene-overproducing and ethylene-insensitive Populus trees. Together with experiments using the ethylene perception inhibitor 1-methylcyclopropene (1-MCP) (23), our results demonstrate that ethylene stimulates cambial growth by acting through ethylene receptors, and that endogenous ethylene produced in leaning trees is a key regulator for the asymmetrical cambial growth in the TW response. Our results establish a causal link between endogenous ethylene and the stimulation of meristem growth and, indeed, wood production.
Results
Production and Selection of Trees with Reduced Ethylene Sensitivity.
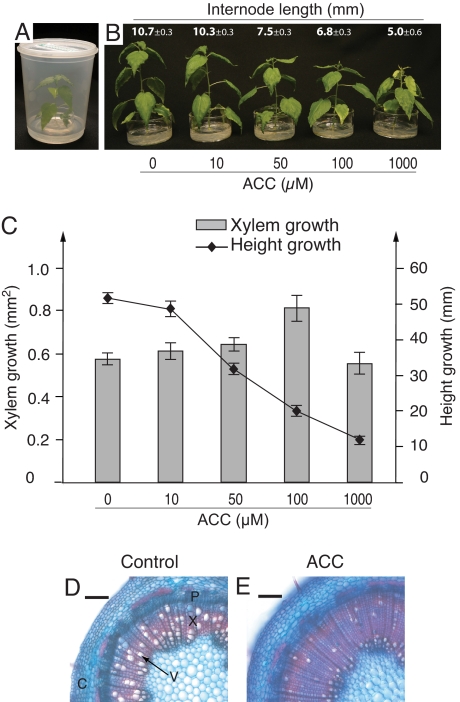
To investigate the role of ethylene in wood formation, we expressed the etr1-1 mutant allele of the Arabidopsis ethylene receptor ETR1 (16, 17) in Populus tremula × tremuloides trees. In addition to the constitutive cauliflower mosaic virus 35S promoter (35S), we used a second promoter, pLMX5, identified for the purpose of directing gene expression to the vascular tissues. Its expression pattern was visualized in transgenic lines expressing β-glucuronidase (GUS) driven by pLMX5 and showed GUS staining preferentially in the cambial meristem, developing wood cells, and developing phloem fibers (Fig. S1). GUS staining was not observed in the apical meristems. In total, 15 35S lines and 9 pLMX5 lines expressing Atetr1-1 were regenerated and screened for ethylene insensitivity. This was performed in an in vitro tree culture system, which is time- and space-efficient compared with greenhouse experiments and, more importantly, facilitates treatments with ACC and 1-MCP (Fig. 1A). ACC was added to the medium when the trees had grown to 5 cm in height. The youngest internode with a complete vascular cambium at the time of treatment was identified and used as a reference internode to measure xylem growth and fiber/vessel element morphology. This procedure confirmed that all wood properties measured were laid down under the influence of the ACC treatment. Populus trees grown in vitro developed normally, produced wood from a vascular cambium, and exhibited asymmetric growth in response to leaning.
Fig. 1.
Applied ACC stimulates xylem growth, inhibits height growth, and decreases xylem cell size in wild-type Populus. (A) The trees were grown in vitro and treated with water or different concentrations of ACC added to the medium (the small 5-cm-diameter cup inside the jar). (B and C) Height growth and internode length were progressively reduced with increasing concentrations of ACC. Xylem growth (measured as xylem area) was stimulated up to a concentration of 100 μM (mean ± SE, n = 6 independent trees). (D and E) ACC treatment induced a xylem with smaller vessels. The micrographs show a typical cross-section of a tree treated with water (D) and 100 μM ACC (E). (Scale bar: 100 μm.) C, cortex; P, phloem; X, xylem; V, vessel. ACC treatments were done when the trees had reached a height of 5 cm, and trees were sampled 12 days after the treatment. Height growth was calculated from final height minus the height at treatment. Internode length was measured on fully elongated internodes formed under the influence of ACC. Xylem growth and cross-section measurements were from the reference internode, in which all xylem was formed under the influence of ACC.
To establish a suitable ACC treatment, we conducted dose–response experiments in which 2 well-established ethylene responses were assayed: height growth inhibition and secondary xylem growth stimulation. Height growth was inhibited progressively with increasing ACC concentration (Fig. 1 B and C). This was a result of shorter internodes rather than internode number, and the number of leaves was not affected (Fig. 1B). Secondary xylem growth was stimulated up to 100 μM ACC (Fig. 1C). This concentration is comparable to the endogenous ACC concentrations measured in developing wood of leaning Populus trees (8) and was used in all subsequent experiments. ACC treatments with 100 μM also caused altered xylem anatomy, as seen on transverse sections (Fig. 1 D and E), with smaller vessel diameter as the most striking effect. This was confirmed by measuring the morphology of single-vessel elements and fibers in xylem macerates, which showed that the cell width was decreased in both cell types (Table 1). These data support the deduction that the ACC-stimulated increase in xylem diameter was due to an increase in the number of xylem cells rather than an increase in their radial width; hence, a stimulation of cambial cell division.
Table 1.
Applied ACC reduce length and diameter of xylem cells
| Cell type | Treatment | Length, μm | Diameter, μm | Tip growth, μm |
|---|---|---|---|---|
| Fiber | Water | 360 ± 8 | 18.4 ± 0.4 | 95.7 ± 4.9 |
| ACC | 326 ± 11* | 17.2 ± 0.3* | 106.9 ± 7.0 | |
| Vessel element | Water | 264 ± 7 | 33.3 ± 0.9 | |
| ACC | 219 ± 9*** | 27.8 ± 0.7*** |
Measurements of macerated xylem demonstrated that the ACC treatment significantly inhibited the diameter and length of vessel elements and fibers. Intrusive tip growth, measured as fiber length minus vessel length, was not affected by ACC. For each tree, 20 vessels and 30 fibers were measured, and a mean value for each tree was calculated. These mean values from individual trees were used to calculate an overall mean ± SE of between 8 and 15 independent trees. ACC treatments were done when the trees had reached a height of 5 cm, and trees were sampled 12 days after the treatment. Xylem cell measurements were all from the reference internode, in which all xylem was formed under the influence of ACC. Asterisks indicate statistically significant differences (Student's t test) between water control and ACC treated trees. *, P < 0.05;
***, P < 0.001.
A decrease in both fiber and vessel element length after ACC treatment was also observed (Table 1). The length of a vessel element reflects the length of the cambial initial, and stimulated cambial growth rate (diameter growth) gives rise to shorter daughter initials (24). Therefore, the shorter vessel element length observed after ACC treatment may be due to its stimulation of cambial growth rate. The length of wood fibers is in addition to the cambial initial length also determined by intrusive tip growth (24). We did not observe a statistically significant effect of ACC on fiber tip growth (Table 1), suggesting that any apparent decrease in fiber or vessel element length is best explained by its shorter initial lengths, and is therefore a secondary effect of ACC-stimulated cambial growth rate.
To assess the transgenic lines expressing Atetr1-1 for ethylene insensitivity, ACC effects on xylem anatomy (as observed from hand-cut sections; Fig. S2) together with height growth were observed. According to these criteria, most lines that were regenerated from independent transformation events showed reduced ACC response to various degrees. For both promoter constructs, we selected 2 lines showing low response to ACC for both xylem anatomy and height growth. These lines were 35S lines 1E and 3A, and pLMX5 lines 1 and 6 (hereafter denoted ethylene-insensitive lines). The expression of Atetr1-1 in these lines was verified by real-time quantitative PCR (qPCR), and neither promoter was induced by ACC (Fig. S3).
Applied ACC and Ethylene Stimulate Cambial Cell Division and Inhibit Height Growth and Wood Cell Expansion by Acting Through the Ethylene Receptor.
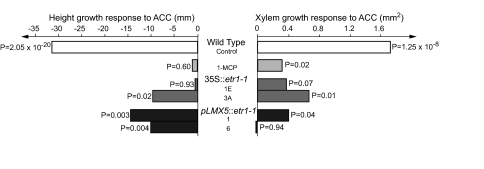
The selected ethylene-insensitive lines were used to establish phenotypes that are induced by ACC through the ethylene receptor. We also established a method to apply 1-MCP to trees cultured in vitro to serve as a positive control for the transgenic ethylene-insensitive trees. This structural analog of ethylene is a highly specific inhibitor of ethylene perception (23). Under in vitro growth conditions, transgenic and 1-MCP-treated wild-type trees developed normally, consistent with the concept that ethylene is not produced in significant amounts under optimized growth conditions (22). We found that blocking ethylene perception reduced or nullified the ACC-induced effects on height growth, xylem growth (Fig. 2), and xylem cell width (Table S1) compared with wild-type trees.
Fig. 2.
Populus trees expressing Atetr1-1 or treated with 1-MCP have reduced sensitivity to ACC-induced xylem growth and height growth inhibition. Wild-type control trees, transgenic trees expressing Atetr1-1 under the 35S (lines 1E and 3A) or LMX5 (lines 1 and 6) promoters, and wild-type trees treated with 1-MCP were grown in vitro and treated with either water or 100 μM ACC. The ACC effects on height and xylem growth were nullified, or much reduced, in transgenic and 1-MCP-treated trees, demonstrating their ethylene insensitivity for these processes. Treatments were done when the trees had reached a height of 5 cm, and trees were sampled 12 days after the treatment. Xylem growth was measured as xylem area in the reference internode, in which all xylem was formed under the influence of ACC. The growth response was calculated as the mean difference between water- and ACC-treated trees within each genotype (or within 1-MCP-treated trees), based on 6 independent trees per treatment and genotype. A P value was calculated by Student's t test for each comparison between water- and ACC -treated trees.
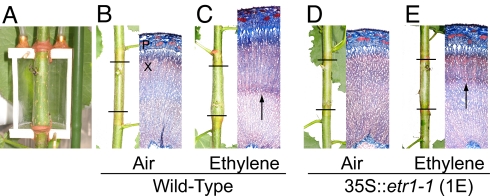
In complementary greenhouse experiments, airtight flow-through chambers fixed to the stems of approximately 2-m-tall greenhouse-grown trees were used to deliver a continuous ethylene exposure (2 ppm) to a restricted part of the stem. Such specific exposure to stem tissues will avoid any potential secondary effects with ACC treatment to whole plants in the in vitro experiments. Treatment with ethylene for 2 weeks stimulated cambial growth and reduced vessel diameter in a manner similar to that observed in ACC-treated trees cultured in vitro, and these effects were reduced in the ethylene-insensitive lines tested (Fig. 3). Taken together, our results demonstrate that applied ACC and ethylene are acting through ETR1 type signaling to inhibit height growth, stimulate cell division in the cambial meristem, and inhibit radial expansion of fibers and vessel elements.
Fig. 3.
Populus trees expressing Atetr1-1 have reduced sensitivity to ethylene applied locally to the stem. (A) Ethylene (2 ppm) was applied for 14 days by a flow-through cuvette to a restricted stem section of approximately 2-m-tall trees. (B and C) Ethylene stimulated cambial growth and wood formation in the wild type. (D and E) This response was reduced by the expression of the dominant-negative mutant allele Atetr1-1. Horizontal lines indicate the position of the cuvette (63 mm long). The experiment was performed on 3 independent trees of each genotype (wild-type, 35S::etr1-1 lines 1E and 3A). The picture shows a typical response from wild-type and ethylene-insensitive trees. The arrows indicate the approximate position after which wood was formed under the influence of ethylene. Field of width of transverse cross sections is 2 mm.
Overexpression of ACO Stimulates Xylem Growth.
Elevated rates of ethylene production in leaning tree stems involve strong induction of ACO activity at the TW side of the stem and accumulation of ACC at the opposite side, suggesting that ACO has a regulatory role for ethylene biosynthesis in TW-forming tissues (8). Northern blot analysis indicated that PttACO1 was the major ACO induced and was abundant in developing xylem tissues. Here, we analyzed the expression of all Populus ACSs and ACOs (8 and 7 family members, respectively) in developing xylem and phloem/cambium tissues from upright and leaned greenhouse-grown trees with qPCR and gene-specific primers. We confirmed the strong expression of PttACO1 in developing xylem, and we also revealed some other, less-abundant ACOs expressed in Populus stem tissues (Fig. S4). ACS expression was generally more prominent in phloem/cambium tissue fractions, with PttACS1 being the most abundant transcript.
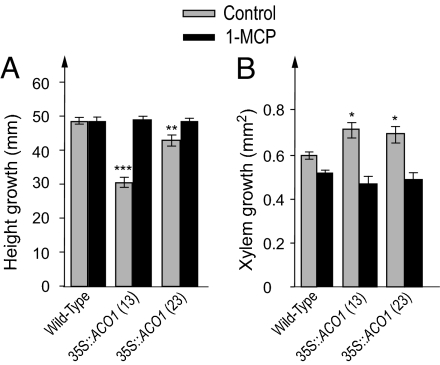
To perturb endogenous ethylene production, we constructed transgenic trees expressing PttACO1 under the 35S promoter. Five independent lines were screened for PttACO1 expression and ACO enzyme activity assays. Two lines (13 and 23) showing the highest ACO activity and confirmed to overexpress PttACO1 (Fig. S5) were selected for characterization in vitro. When compared with wild-type trees, both lines overexpressing PttACO1 exhibited increased xylem growth and decreased height growth (Fig. 4). These phenotypes were blocked by 1-MCP, demonstrating that they were indeed due to elevated endogenous ethylene production (Fig. 4). This observation confirmed that ethylene produced in planta stimulates cambial cell division and inhibits height growth in Populus. The data also strongly support the concept that ACO has a function in regulating ethylene biosynthesis in Populus stems (8).
Fig. 4.
Populus trees overexpressing PttACO1 show increased xylem growth and inhibited height growth. Wild-type and transgenic trees overexpressing PttACO1 (lines 13 and 23) were grown in vitro and either left untreated (control) or treated with 1-MCP. (A and B) The PttACO1 overexpression results in an inhibition of height growth (A) and a stimulation of xylem growth (B) compared with wild-type trees. This difference between wild-type and transgenic trees was nullified in the 1-MCP-treated trees, demonstrating that it was an effect of ethylene overproduction (mean ± SE, n = 5–7 independent trees). Asterisks indicate statistically significant differences (Student's t test) between wild-type and transgenic control (untreated) trees. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Height growth for both control and 1-MCP-treated trees was calculated from final height minus the height when 1-MCP treatment was done (5 cm). Xylem growth was measured in the reference internode, in which all xylem was formed after the tree had reached a height of 5 cm.
Endogenous Ethylene Induced by Leaning Stimulates Cambial Cell Division and Xylem Growth.
To test whether endogenous ethylene production induced in leaning trees mediates enhances cambial growth that leads to the eccentricity of xylem seen in association with TW, ethylene-insensitive trees (transgenic and 1-MCP-treated wild type) were tilted during in vitro culture. Both approaches to disrupt the trees' capacity to perceive ethylene decreased the degree of xylem eccentricity when compared with wild-type trees (Fig. 5). Any difference in other attributes of the TW response could not be detected. The inhibition of cambial cell division in response to leaning demonstrates that ethylene is indeed a key regulator in the TW response and, more importantly, that endogenous ethylene stimulates meristematic activity in the vascular cambium of plants.
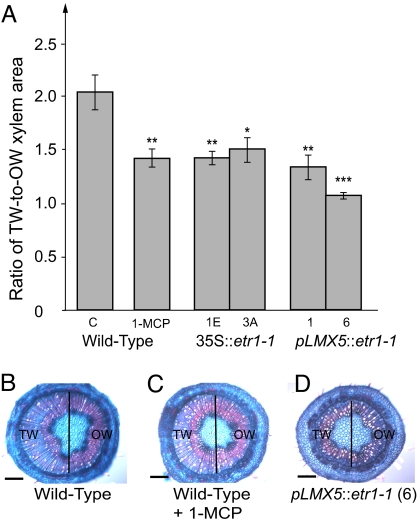
Fig. 5.
Eccentric xylem formed in the TW response is inhibited in ethylene-insensitive Populus trees. Wild-type control trees, transgenic trees expressing the dominant-negative mutant allele Atetr1-1 under the 35S (lines 1E and 3A) or LMX5 (lines 1 and 6) promoters, and 1-MCP-treated trees were grown in vitro and leaned to 30° for 12 days to induce the TW response. Xylem area was measured in the TW and opposite wood halves of the xylem in a defined internode, and these areas are expressed as a ratio to illustrate asymmetric growth. (A) The stimulation of xylem growth at the TW side was reduced in ethylene-insensitive trees (mean ± SE of 5 independent trees). Asterisks indicate statistically significant differences (Student's t test) between wild-type control trees and ethylene-insensitive trees *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B–D) Cross-sections taken from wild type (B), wild type treated with 1-MCP (C), and transgenic tree pLMX5::etr1-1 (line 6) (D). The vertical lines across cross-sections indicate division between measured TW and opposite wood xylem areas. C indicates control; TW, tension wood half; OW, opposite wood half. (Scale bar: 200 μm.)
Discussion
Ethylene is an endogenous regulator of meristem growth. We have demonstrated that both applied and ectopically produced ethylene stimulates xylem growth by means of cambial cell division, and that endogenous ethylene mediates growth in the vascular cambium in response to leaning. The inhibition of this response in trees where ethylene perception was reduced (transgenic and 1-MCP-treated wild type) further showed that it was mediated through ethylene receptors. Application experiments have also demonstrated a potential for ethylene to stimulate endoreduplication in hypocotyls of Arabidopsis and cucumber (11–13), cell division activity, and the induction of cell cycle-related genes in adventitious root formation and in the intercalary meristem of deepwater rice (25, 26). In line with these observations, overproduction of ethylene in Arabidopsis roots due to the loss of function of ETO1 [encoding a BTB/POZ protein that regulates ACS5 stability, and hence elevated ethylene biosynthesis, through ubiquitin E3 ligase complex (27)] not only inhibited root elongation but also induced additional cell division in the quiescent center, and a function for endogenous ethylene in postembryonic stem cell division was postulated (14). However, conclusive interpretation of the role of endogenous ethylene requires experiments where its perception or biosynthesis is blocked. Therefore, our finding that endogenous ethylene stimulates meristematic growth extends current concepts of ethylene regulation in vegetative growth to include not only cell expansion but also aspects of cell division.
The role of other hormones in TW associated growth is not resolved. In addition to ethylene, both auxin and gibberellins (GAs) are known stimulators of cambial cell division (1, 28). It is plausible that ethylene is a primary response to leaning, potentiating growth responses that are regulated through downstream interactions with other phytohormones. In deepwater rice and Rumex palustris, for example, the induction of ethylene upon submergence stimulated elongation growth by acting through inhibition of abscisic acid biosynthesis, which in turn led to a stimulation of GA biosynthesis (25, 29–31). Considering the recent demonstration of ethylene-induced auxin biosynthesis in Arabidopsis roots (32–34), together with the long-standing idea that ethylene interacts with auxin polar transport both in herbaceous plants and trees (35, 36), auxin could be a joint mediator that stimulates cambial growth upon leaning. However, measurements of indole-3-acetic acid (IAA) across TW-forming tissues in Populus trees showed no change in IAA compared with upright trees, despite increased cell division activity (4), suggesting that ethylene does not act through an increase in IAA levels per se. Interestingly, GA was recently demonstrated to induce all aspects of TW response when applied to woody stem tissues (37). Endogenous GAs in upright trees, however, are present mainly in cambial derivatives in the stage of expansion, with only low levels in the cambial meristem, suggesting a role for GAs in cell expansion rather than cell division (38). However, an altered balance of GAs during the TW response cannot be excluded.
Do Responses to Gravitational and Mechanical Loads Converge Through Induced Ethylene?
Similar to the leaning response, cambial cell division and stem diameter growth are stimulated by mechanical load imposed by, for example, wind or the weight of branches in both trees and herbaceous species (2, 3, 39, 40). This response, along with height growth inhibition, is described by the concept of thigmomorphogenesis and is a major factor shaping plant form in nature (40, 41). Mechanical load induces ethylene biosynthesis, and thigmomorphogenic responses in herbs as well as in trees can be mimicked by applied ethylene (Fig. 2) (2, 9, 41). These observations suggest a common primary role for ethylene in cambial growth responses induced by both leaning and mechanical loads. It is a matter of long debate whether reaction wood (a collective name for TW and its corresponding phenomenon in gymnosperms, compression wood) is a gravitational or a mechanical response. Experimental treatments normally induce both cues, and experiments excluding the influence of the gravity vector have yielded conflicting results (42, 43). However, it is beyond doubt that leaning and reaction wood formation have additional components besides purely mechanical ones; reaction wood is always induced unilaterally and develops at the side of the stem, which creates a force that counters its displacement, independent of tension or compression forces (43). Little is known about gravitational sensing in secondary stems, but the hypothesis holds that there may be a convergence of this signaling with that derived from mechanical perturbations—both acting through the induction of ethylene, and hence a stimulation of cambial cell division and diameter growth (2). In nature, any mechanical load will also include a gravitational stimulus, and vice versa. As such, ethylene biosynthesis in stems and internodes in planta will be a rule rather than an exception. In conclusion, we have demonstrated that endogenous ethylene mediates growth in the vascular cambium in response to leaning, and we propose that it has a similar function in thigmomorphogenic responses.
Materials and Methods
Plant Material and Growth Conditions.
Plant material was hybrid aspen (Populus tremula L. × tremuloides Michx.; clone T89). For the in vitro experiments, trees were grown in clear polypropylene containers (height, 14 cm; diameter, 10 cm) with OS140+ODS140 gas-exchange spore filters (Combiness) on Murashige and Skoog medium (2.2 g·L−1, pH 5.6) with Phytagel P8169 solidifying agent (2.7 g·L−1; Sigma–Aldrich). Temperature was 22/18 °C (light/dark), photoperiod was 16 h, and light intensity was 90 μmol·m−2·s−1. Containers were tilted to 30° to induce TW. For greenhouse experiments, trees were grown in a mixture of peat, sand, and Vermiculite (6:2:1, vol/vol/vol) at 20/18 °C (light/dark), photoperiod was 18 h, and light intensity was a maximum 400 μmol·m−2·s−1 from natural daylight (controlled by curtains), supplemented when required with high-pressure sodium lamps. Trees were fertilized with 2.5 g·L−1 (wt/vol) of Osmocote Exact Hi-Start (N/P/K 15:4:8; Scotts).
Treatments with ACC, Ethylene, and 1-MCP.
For in vitro experiments, distilled and autoclaved water, with and without ACC (Sigma–Aldrich), was supplied on the medium surface and allowed to diffuse in to create an appropriate concentration. Gaseous 1-MCP (30 ppm; EthylBloc; AgroFresh) was generated according to the manufacturer's instructions and injected twice a day into the growth containers throughout the experiment. For greenhouse experiments, synthetic air with 350 ppm carbon dioxide, with and without 2 ppm ethylene (The Linde Group, AGA Finland), was delivered with a flow rate of 20 μL/s to a length of 63 mm of internode 42 (counting from top) by using flow-through, handmade cuvettes (Bahtijor Rasulov, Institute of Molecular and Cell Biology, Tartu University, Tartu, Estonia). Air pressure was controlled by a self-adjusting valve in the gas bottle followed by a rotameter to control volumetric flow, and flow rate was controlled by a clamp valve attached to each tube leading to every individual cuvette.
Vector Constructions and Plant Transformation.
The LMX5 promoter was derived from the EST clone A055P19 (AI164126) of the Umeå Plant Science Centre Populus EST database. A 1.8-kb genomic fragment (DJ416317) was cloned with the GenomeWalker Universal Kit (Clontech) upstream of the EST clone. Two subsequently amplified fragments were cloned into pGEM-T Easy vector (Promega) and sequenced. The translational start codon was predicted based on the best BLASTX hit in the Arabidopsis proteome (Fig. S6), and the 1.8-kb upstream sequence was cloned by PCR from genomic DNA by using primers with BglII and SalI sites (Table S2) and with the HF2-Taq according to the supplier's recommendations (Clontech). The PCR product was BglII-SalI-digested and cloned into the binary vector pPCV812 (44) to result in a transcriptional GUS reporter gene construct pLMX5::GUS.
The Gateway technology (Invitrogen) was chosen to construct a binary vector harboring LMX5 promoter. The Gateway conversion cassette with reading frame B (catalog no. 11828029; Invitrogen) was amplified by PCR to generate unique BclI and SacI sites by using cassette-specific primers (Table S2). The GUS gene from the previously constructed pLMX5::GUS fusion (in pPCV812) was excised by BamHI-SacI digestion after the PCR product of cassette with reading frame B was BclI-SacI-digested and cloned into the binary vector pPCV812 with the LMX5 promoter. Thus, a Gateway-compatible pLMX5 destination vector was created to readily clone target genes that are regulated by this promoter.
To generate Populus trees insensitive to ethylene, DNA was extracted with DNeasy Plant Mini Kit (Qiagen) from Arabidopsis etr1-1 mutant plants (16), and a full-length genomic copy of the etr1-1 mutant allele was amplified by PCR using gene-specific primers (Table S2) containing the attB1 and attB2 sites for the Gateway recombination reaction. The amplified attB-PCR product was cloned into the Gateway donor vector pDonor207 after the created entry clone with etr1-1 insert was further transferred into the pLMX5 destination vector to create an expression vector with pLMX5::etr1-1 fusion in pPCV812 binary vector for plant transformation. For the 35S::etr1-1 construct, the same entry clone (with etr1-1 insert) was cloned into the pK2GW7 (45) binary vector to generate an expression vector for plant transformations. Intact etr1-1 insert from entry clone was confirmed by sequencing. All of the procedures were conducted according to the supplier's instructions (Invitrogen).
To generate trees overexpressing ACO1 under the 35S promoter, a full-length PttACO1 (AY167040) cDNA clone (8) was HindIII-SmaI-digested from the pBluescript SK+ vector and cloned into the pPCV702 (46) binary vector.
All of the binary vectors were transformed into Agrobacterium strain GV3101 pmp90RK, and hybrid aspen was transformed and regenerated according to Nilsson et al. (47).
GUS Expression and ACO Activity Assay.
GUS expression was visualized according to Regan et al. (48). ACO activity was measured according to Andersson-Gunnerås et al. (8).
Transcript Profiling.
Total RNA was extracted from aliquots of homogenized tissues with the RNeasy Plant Mini Kit (Qiagen) according to the supplier's instructions. The extracted total RNA was DNase-treated (Turbo DNA-free Kit; Ambion) and quantified with a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies). cDNA synthesis was conducted with an Iscript cDNA Synthesis kit according to the supplier's instructions (Bio-Rad) with up to 1 μg of total RNA. Real-time qPCR on synthesized cDNA was done by using SYBR Green mastermix (SYBR Green PCR Kit; Bio-Rad) with 0.2 μM of each primer, 1:80 dilution of the cDNA as a template, and 0.25 pmol of Fluorescent Calibration Dye (Bio-Rad) in a total volume of 25 μL. PCRs were performed with a 96-well Bio-Rad iCycler iQ Real-Time PCR Detection System. (For detailed information, see SI Methods.) All of the primer sequences are given in Table S3.
Anatomical Analyses.
The xylem area of in vitro-grown trees was measured on transverse hand sections stained with safranin/alcian blue. Fiber and vessel morphology was measured in macerated xylem [maceration according to Siedlecka et al. (24) with a Zeiss Axioplan 2 microscope and Axiovision software (AxioVs40 V4.5.0.0)]. Cryomicrotome sections from the air/ethylene-treated stems of greenhouse-grown trees were stained with 1% safrinin/astra blue, dried with a series of alcohol washes, and permanently embedded in slides with Canada balsam and examined with a Leica DMLB microscope equipped with a Leica DC300 digital camera.
Supplementary Material
Acknowledgments.
We thank Elliot Meyerowitz (California Institute of Technology, Pasadena, CA) for supplying seeds of the etr1-1 mutant; Drs. Hannes Kollist and Bahtijor Rasulov for technical advice and help in ethylene treatments of the Populus stems; Marja Tomell, Pekka Lönnqvist, and Leena Laakso for producing and nursing the plant material; Kjell Olofsson, Tuomas Puukko, Eija Rinne, and Åsa Sundberg for technical help in laboratory analyses; and Drs. Totte Niittylä and Ron Sederoff for valuable comments on the manuscript. The work was funded by the Academy of Finland and the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning (FORMAS) bilateral research program Wood Wisdom, by FORMAS excellence center FUNCFIBER, and by FORMAS research grant 229-2005-870 (to B.S.), and by the Finnish Centre of Excellence program 2000-2005 and 2006-2011 and by Finnish Funding Agency for Technology and Innovation project FuncWood (to J.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811660106/DCSupplemental.
References
- 1.Uggla C, Mellerowicz EJ, Sundberg B. Indole-3-acetic acid controls cambial growth in Scots pine by positional signaling. Plant Physiol. 1998;117:113–121. doi: 10.1104/pp.117.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Telewski FW. A unified hypothesis of mechanoperception in plants. Am J Bot. 2006;93:1466–1476. doi: 10.3732/ajb.93.10.1466. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs M. The effect of wind sway on the form and development of Pinus radiata D. Don. Aust J Bot. 1954;2:35–51. [Google Scholar]
- 4.Hellgren JM, Olofsson K, Sundberg B. Patterns of auxin distribution during gravitational induction of reaction wood in poplar and pine. Plant Physiol. 2004;135:212–220. doi: 10.1104/pp.104.038927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson-Gunnerås S, et al. Biosynthesis of cellulose-enriched tension wood in Populus: Global analysis of transcripts and metabolites identifies biochemical and developmental regulators in secondary wall biosynthesis. Plant J. 2006;45:144–165. doi: 10.1111/j.1365-313X.2005.02584.x. [DOI] [PubMed] [Google Scholar]
- 6.Pierik R, Tholen D, Poorter H, Visser EJ, Voesenek LA. The Janus face of ethylene: Growth inhibition and stimulation. Trends Plant Sci. 2006;11:176–183. doi: 10.1016/j.tplants.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Du S, Yamamoto F. Ethylene evolution changes in the stems of Metasequoia glyptostroboides and Aesculus turbinata seedlings in relation to gravity-induced reaction wood formation. Trees Struct Funct. 2003;17:522–528. [Google Scholar]
- 8.Andersson-Gunnerås S, et al. Asymmetric expression of a poplar ACC oxidase controls ethylene production during gravitational induction of tension wood. Plant J. 2003;34:339–349. doi: 10.1046/j.1365-313x.2003.01727.x. [DOI] [PubMed] [Google Scholar]
- 9.Biro RL, Hunt ER, Jr, Erner Y, Jaffe MJ. Thigmomorphogenesis: Changes in cell division and elongation in the internodes of mechanically-perturbed or ethrel-treated bean plants. Ann Bot. 1980;45:655–664. [Google Scholar]
- 10.Little CHA, Pharis RP. In: Plant Stems: Physiology and Functional Morphology. Gartner BL, editor. San Diego: Academic; 1995. pp. 281–319. [Google Scholar]
- 11.Gendreau E, Orbovic V, Höfte H, Traas J. Gibberellin and ethylene control endoreduplication levels in the Arabidopsis thaliana hypocotyl. Planta. 1999;209:513–516. doi: 10.1007/PL00008123. [DOI] [PubMed] [Google Scholar]
- 12.Dan H, Imaseki H, Wasteneys GO, Kazama H. Ethylene stimulates endoreduplication but inhibits cytokinesis in cucumber hypocotyl epidermis. Plant Physiol. 2003;133:1726–1731. doi: 10.1104/pp.103.025783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazama H, Dan H, Imaseki H, Wasteneys GO. Transient exposure to ethylene stimulates cell division and alters the fate and polarity of hypocotyl epidermal cells. Plant Physiol. 2004;134:1614–1623. doi: 10.1104/pp.103.031088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortega-Martinez O, Pernas M, Carol RJ, Dolan L. Ethylene modulates stem cell division in the Arabidopsis thaliana root. Science. 2007;317:507–510. doi: 10.1126/science.1143409. [DOI] [PubMed] [Google Scholar]
- 15.Alonso JM, Stepanova AN. The ethylene signaling pathway. Science. 2004;306:1513–1515. doi: 10.1126/science.1104812. [DOI] [PubMed] [Google Scholar]
- 16.Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;141:1086–1087. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- 17.Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: Similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- 18.Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–271. doi: 10.1016/s0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson JQ, et al. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat Biotechnol. 1997;15:444–447. doi: 10.1038/nbt0597-444. [DOI] [PubMed] [Google Scholar]
- 20.Pierik R, Visser EJW, De Kroon H, Voesenek LA. Ethylene is required in tobacco to successfully compete with proximate neighbours. Plant Cell Environ. 2003;26:1229–1234. [Google Scholar]
- 21.Vahala J, Ruonala R, Keinänen M, Tuominen H, Kangasjärvi J. Ethylene insensitivity modulates ozone-induced cell death in birch. Plant Physiol. 2003;132:185–195. doi: 10.1104/pp.102.018887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tholen D, Voesenek LAC, Poorter H. Ethylene insensitivity does not increase leaf area or relative growth rate in Arabidopsis, Nicotiana tabacum, and Petunia x hybrida. Plant Physiol. 2004;134:1803–1812. doi: 10.1104/pp.103.034389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sisler EC, Serek M. Inhibitors of ethylene responses in plants at the receptor level: Recent developments. Physiol Plant. 1997;100:577–582. [Google Scholar]
- 24.Siedlecka A, et al. Pectin methyl esterase inhibits intrusive and symplastic cell growth in developing wood cells of Populus. Plant Physiol. 2008;146:554–565. doi: 10.1104/pp.107.111963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kende H, van der Knaap E, Cho HT. Deepwater rice: A model plant to study stem elongation. Plant Physiol. 1998;118:1105–1110. doi: 10.1104/pp.118.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorbiecke R, Sauter M. Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol. 1999;119:21–30. doi: 10.1104/pp.119.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang KL, Yoshida H, Lurin C, Ecker JR. Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature. 2004;428:945–950. doi: 10.1038/nature02516. [DOI] [PubMed] [Google Scholar]
- 28.Björklund S, Antti H, Uddestrand I, Moritz T, Sundberg B. Cross-talk between gibberellin and auxin in development of Populus wood: Gibberellin stimulates polar auxin transport and has a common transcriptome with auxin. Plant J. 2007;52:499–511. doi: 10.1111/j.1365-313X.2007.03250.x. [DOI] [PubMed] [Google Scholar]
- 29.Cox MC, et al. The roles of ethylene, auxin, abscisic acid, and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiol. 2004;136:2948–2960. doi: 10.1104/pp.104.049197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benschop JJ, et al. Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. Plant J. 2005;44:756–768. doi: 10.1111/j.1365-313X.2005.02563.x. [DOI] [PubMed] [Google Scholar]
- 31.Voesenek LA, Colmer TD, Pierik R, Millenaar FF, Peeters AJ. How plants cope with complete submergence. New Phytol. 2006;170:213–226. doi: 10.1111/j.1469-8137.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- 32.Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell. 2007;19:2169–2185. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swarup R, et al. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell. 2007;19:2186–2196. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruzicka K, et al. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell. 2007;19:2197–2212. doi: 10.1105/tpc.107.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suttle JC. Effect of ethylene treatment on polar IAA transport, net IAA uptake and specific binding of N-1-naphthylphthalamic acid in tissues and microsomes isolated from etiolated pea epicotyls. Plant Physiol. 1988;88:795–799. doi: 10.1104/pp.88.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eklund L, Little CHA. Laterally applied Ethrel causes local increases in radial growth and indole-3-acetic acid concentration in Abies balsamea shoots. Tree Physiol. 1996;16:509–513. doi: 10.1093/treephys/16.5.509. [DOI] [PubMed] [Google Scholar]
- 37.Funada R, et al. Gibberellin-induced formation of tension wood in angiosperm trees. Planta. 2008;227:1409–1414. doi: 10.1007/s00425-008-0712-6. [DOI] [PubMed] [Google Scholar]
- 38.Israelsson M, Sundberg B, Moritz T. Tissue-specific localization of gibberellins and expression of gibberellin-biosynthetic and signaling genes in wood-forming tissues in aspen. Plant J. 2005;44:494–504. doi: 10.1111/j.1365-313X.2005.02547.x. [DOI] [PubMed] [Google Scholar]
- 39.Ko JH, Han KH, Park S, Yang J. Plant body weight-induced secondary growth in Arabidopsis and its transcription phenotype revealed by whole-transcriptome profiling. Plant Physiol. 2004;135:1069–1083. doi: 10.1104/pp.104.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaffe MJ. Morphogenetic responses of plants to mechanical stimuli or stress. BioSciences. 1980;30:239–243. [Google Scholar]
- 41.Braam J. In touch: Plant responses to mechanical stimuli. New Phytol. 2005;165:373–389. doi: 10.1111/j.1469-8137.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 42.Kwon M, Bedgara DL, Piastuchb W, Davina LB, Lewis NG. Induced compression wood formation in Douglas fir (Pseudotsuga menziesii) in microgravity. Phytochemistry. 2001;57:847–857. doi: 10.1016/s0031-9422(01)00145-5. [DOI] [PubMed] [Google Scholar]
- 43.Timell TE. Compression Wood in Gymnosperms. Vol 2. Heidelberg: Springer; 1986. pp. 983–1262. [Google Scholar]
- 44.Koncz C, et al. In: Plant Molecular Biology Manual. Gelvin SB, Schilperoort RA, Verma DPS, editors. Vol B2. Dordrecht, The Netherlands: Kluwer; 1994. pp. 1–22. [Google Scholar]
- 45.Karimi M, Inze D, Depicker A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002;7:193–195. doi: 10.1016/s1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]
- 46.Koncz C, et al. High-frequency T-DNA-mediated gene tagging in plants. Proc Natl Acad Sci USA. 1989;86:8467–8471. doi: 10.1073/pnas.86.21.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nilsson O, et al. Spatial pattern of cauliflower mosaic virus 35S promoter-luciferase expression in transgenic hybrid aspen trees monitored by enzymatic assay and non-destructive imaging. Transgenic Res. 1992;1:209–220. [Google Scholar]
- 48.Regan S, Bouquin V, Tuominen H, Sundberg B. Accurate and high resolution in situ hybridization analysis of gene expression in secondary stem tissues. Plant J. 1999;19:363–369. doi: 10.1046/j.1365-313x.1999.00536.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.