Abstract
Poly(C)-binding proteins (PCBPs) are generally known as RNA-binding proteins that interact in a sequence-specific fashion with single-stranded poly(C). They can be divided into two groups: hnRNP K and PCBP1-4. These proteins are involved mainly in various posttranscriptional regulations (e.g., mRNA stabilization or translational activation/silencing). In this review, we summarize and discuss how PCBPs act as transcriptional regulators by binding to specific elements in gene promoters that interact with the RNA polymerase II transcription machinery. Transcriptional regulation of PCBPs might itself be regulated by their localization within the cell. For example, activation by p21-activated kinase 1 induces increased nuclear retention of PCBP1, as well as increased promoter activity. PCBPs can function as a signal-dependent and coordinated regulator of transcription in eukaryotic cells. We address the molecular mechanisms by which PCBPs binding to single- and double-stranded DNA mediates gene expression.
Keywords: Poly(C)-binding proteins, p21-activated kinase 1, DNA-binding proteins, Transcriptional regulation
The poly(C)-binding proteins (PCBPs) are characterized by high affinity for, and sequence-specific interaction with polycytosine, poly(C). In mammalian cells, these PCBPs belong to one of two subsets: hnRNP K/J, or the alpha-complex proteins (e.g., PCBP1-4) [1]. hnRNP K, PCBP1, and PCBP2 have been studied in the greatest detail. The latter two proteins are also known as αCP1 and αCP2, or hnRNPE1 and hnRNPE2 [2,3]. Recently, two other members of the αCP family were discovered: PCBP3 (αCP3) and PCBP4 (αCP4) [4].
PCBPs are expressed broadly in human and mouse tissues and demonstrate poly(C)-binding specificity [2,4,5]. All members of the PCBP family are related evolutionarily. The common feature of all PCBPs is the presence of three hnRNP K homology (KH) domains [1]; these are RNA-binding modules of about 70 amino acids in length. PCBP1 and PCBP2 share the highest level of amino acid sequence similarity (89%) [6]. PCBP3 is more divergent, and PCBP4 is the most distantly related (52% divergence from PCBP2 [4,7]. Members of this family perform multiple functions through their poly(C)-binding ability, including mRNA stabilization [8–10], translational silencing [11,12], and translational enhancement [9,13]. PCBP4 (MCG10) can induce apoptosis [14], and its expression can inhibit proliferation and tumorigenesis of lung cancer cells, both in vivo and in vitro, by delaying the progression of the cell cycle [15,16]. Perhaps most importantly, PCBPs function as signal-dependent and coordinated regulators of transcription in eukaryotic cells by binding to specific elements on gene promoters. This review focuses on the molecular mechanisms by which the interactions of poly(C)-binding proteins with single- and double-stranded DNA mediate gene expression.
Structure of PCBPs
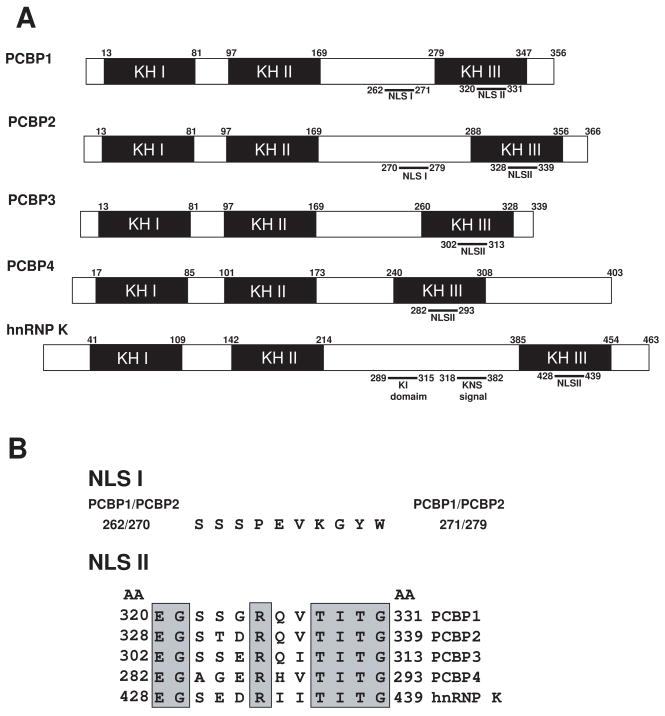
The PCBPs contain three KH domains, two consecutive KH domains at the amino terminus and a third KH domain at the carboxyl terminus, separated by an intervening sequence of variable length (Fig. 1A). The structure of each KH domain consists of three α-helices and β-strands arranged in the order β1-α1-α2-β2-β3-α3 (Fig. 1C) [17,18]. The three β-strands form an antiparallel β-sheet, with a spatial order β1-β3-β2; the three αhelices are packed against one side of the β-sheet [17,18]. The evolutionarily conserved Gly-X-X-Gly loop is located between α1 and α2; the variable loop is between β2 and β3.
Fig. 1.
A, Schematic diagram of domain structure of PCBPs. The five members of the PCBP family are shown. Numbers indicate the respective human sequence. KH domains I–III (shaded boxes); SH-3 binding motif (KI domain); hnRNP K nuclear shuttling signal (KNS signal). Adapted from Makeyev and Liebhaber (2002) [1]. B, Sequence alignments of PCBPs at NLS I and NLS II. NLS I shows perfect conservation between PCBP1 and PCBP2. There is no NLS I region present in PCBP3, PCBP4, or hnRNP K. Conserved amino acids of NLS II are shaded. Adapted from Chkheidze and Liebhaber (2003) [7]. C, Sequence alignments of KH domains from PCBP1-4 and hnRNP K. The GXXG motif and the three amino acids involved in hydrogen bonding with polycytosine are indicated in grey; the residues involved in hydrogen bonds with DNA bases (i.e., side-chain base hydrogen bonds) are labeled “S”. Adapted from Du et al. (2005) [17].
The PCBPs also carry a nuclear localization signal (NLS) sequence that mediates protein transport from the cytoplasm to the nucleus (Fig. 1B). The 10 amino acid segment of NLS I was mapped between the KH2 and KH3 domains and NLS II (12 amino acids) was localized at the KH3 domain (Fig. 1B) [7]. The predominantly nuclear PCBP1 and PCBP2 contain both NLS I and NLS II sequences [4,7], whereas PCBP3, PCBP4, and hnRNP K contain only NLS II. In addition, hnRNP K contains an hnRNP K-specific nuclear shuttling (KNS) domain located between KH II and III that promotes bidirectional transport through the nuclear pore complex [19]. hnRNP K also contains a K-protein-interactive (KI) domain located between KH II and III responsible for many of its known protein interactions [20].
The ability of PCBPs to recognize and bind poly(C) DNA and RNA sequences via their KH domains is critical for their function in mammalian cells. These interactions are mediated by a combination of several stabilizing forces, including hydrogen bonding, electrostatic interactions, van der Waals contacts, and shape complementarities. Specific recognition of the three cytosine residues is realized by a dense network of hydrogen bonds involved in the side chains of two conserved arginines and one glutamic acid (Fig. 1C) [17,18].
Localization of PCBPs
The diverse functions of PCBPs suggest that they act both in the cytoplasm (translation) and in the nucleus (transcription and splicing). Immunofluorescence studies revealed three distinct patterns of distribution: hnRNP K, PCBP1, and PCBP2 are predominantly localized to the nucleus, with specific enrichment of PCBP1 in the nuclear speckle [21]. In contrast, PCBP3 and PCBP4 are localized to the cytoplasm. PCBP2-KL and the PCBP2 splice variant are localized to both the cytoplasm and nucleus at significant levels [7]. Interestingly, although the NLS I sequences are conserved perfectly between PCBP1 and PCBP2 (Fig. 1B), PCBP1 is selectively concentrated in nuclear speckles, whereas PCBP2 is distributed more diffusely. At steady-state levels, PCBP1 is localized predominantly to the nucleus. Phosphorylation of p21-activated kinase 1 (Pak1) by mitogen induces phosphorylation of PCBP1, increasing its nuclear retention. In contrast, although at steady-state levels hnRNP K is also localized to the nulceoplasm, phosphorylation of hnRNP K by mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) induces its shift from the nucleus to the cytosol and cytoplasmic accumulation under physiological conditions [22].
Posttranslational modicafication of PCBPs
Posttranslational modifications of PCBPs are important for their ability to function as transcriptional factors that bind and regulate specific gene promoters. For example, mitogenic stimulation of human cells phosphorylates PCBP1 on threonines 60 and 127 in a Pak1-sensitive manner. This Pak1-dependent phosphorylation of PCBP1 reduces its binding and raises its translational inhibition of a differentiation-control element (DICE)-minigene [23].
ERK efficiently phosphorylates hnRNP K both in vitro and in vivo at serines 284 and 353. Phosphorylating hnRNP K induces its shift from the nucleus to the cytosol and regulates translation of 15-lipoxygenase mRNA via a DICE in the 3′ untranslated region (3′UTR) [22]. Src and Lck can tyrosine-phosphorylate the hnRNP K protein in vitro at Tyr230, Tyr234, and Tyr236 [24]. The KI domain of hnRNP K contains a serine (Ser302) that also acts as a site for protein kinase Cδ-mediated phosphorylation [25].
Arginine methyltransferase 1 is the only methyltransferase identified so far that methylates hnRNP K in vivo and in vitro. Tandem mass-spectrometric analyses of hnRNP K peptides show that both Arg296 and Arg299 are dimethylated; they are both asymmetric dimethylarginines [26,27]. Because both are located near the SH3-binding domains of hnRNP K, such methylation has the potential to regulate the interaction of hnRNP K with Src protein family members [26,27]. Arginine methylation of hnRNP K also enhances its affinity for p53. In contrast, inhibition of hnRNP K methylation attenuates the recruitment of p53 to the p21 promoter, and reduces p53 transcriptional activity, suggesting that arginine methylation of hnRNP K is a key element for p53 transcriptional activity [28]. hnRNP K can also be modified by the small ubiquitin-like modifier protein at a lysine residue [29].
Transcriptional regulation by PCBPs
PCBPs have been implicated in multiple aspects of transcriptional regulation (Table 1). For example, on binding, hnRNP K functions as a transcriptional activator for the SV40 early promoter [30], the pyrimidine-rich strand of the CT element in the human c-myc promoter [31], the neuronal nicotinic acetylcholine receptor gene [32], nonreceptor tyrosine kinase, the human SRC gene [33], the BRCA1 promoter [34] the basal promoter of the eIF4E promoter [35], and the proximal promoter of the mouse mu opioid receptor (MOR) [36–38]. In these cases, interaction activated transcription in vitro apparently by hnRNP K-dependent assembly of TFIID complexes at these promoters [39].
Table 1.
Examples of PCBPs involvement in transcriptional regulation
| PCBPs | Gene regulation | Gene | References |
|---|---|---|---|
| hnRNP K | Transcriptional activation | BRCA1 promoter | [34] |
| C-myc | [31] | ||
| Early promoter of SV40 | [30] | ||
| Eukaryotic translation initiation factor 4E | [35] | ||
| Human SRC gene | [33] | ||
| Mouse mu opioid receptor | [36] | ||
| Nicotinic acetylcholine receptor promoter | [32] | ||
| Transcriptional repression | Human thymidine kinase promoter | [41] | |
| CD43 gene promoter | [42] | ||
| PCBP1 | Transcriptional activation | BRCA1 promoter | [34] |
| Eukaryotic translation initiation factor 4E | [35] | ||
| Mouse mu opioid receptor | [36] | ||
| PCBP2 | Transcriptional activation | BRCA1 promoter | [34] |
| Mouse mu opioid receptor | [36] | ||
| PCBP3 | Transcriptional repression | Mouse mu opioid receptor | [36,45] |
Ritchie’s laboratory proposed a possible model for SRC transcriptional regulation by hnRNP K (Fig. 2A) [33]. hnRNP K recognizes and binds specifically to double-stranded polypurine:polypyrimidine sequences within TC1 and TC2, followed by strand separation facilitated by hnRNP K’s increased affinity for single-strand DNA. The resulting single-stranded bubble allows hnRNP K to bind TBP, recruit TFIID to the TC3 region, and aid in the assembly of a preinitiation complex.
Fig. 2.
A, Proposed model for transcriptional regulation of human SRC1A protooncogene by hnRNP K. hnRNP K recognizes and bind specifically to double-stranded polypurine:polypyrimidine sequences in the TC regions (TC1 and TC2), followed by strand separation facilitated by hnRNP K’s increased affinity for single-stranded DNA. The resulting single-stranded “bubble” encompasses the entire TC-tract region. The ability of hnRNP K to bind TBP recruits TFIID to the TC3 region and aids in the assembly of a preinitiation complex. Adapted from Ritchie et al. (2003) [33]. B, Proposed model for transcriptional regulation of mouse MOR by hnRNP K, αCP1, αCP2, αCP2-KL, and αCP3. The hnRNP K, αCP1, αCP2, αCP2-KL, and αCP3 bind to the single strand DNA element essential for activity of the MOR gene promoter and regulate its promoter activity at the transcriptional level.
In response to DNA damage, p53 and hnRNP K are recruited to the promoters of p53-responsive genes in a mutually dependent manner. By serving as a coactivator for p53, hnRNP K plays a key role in coordinating transcriptional responses to DNA damage [40]. However, hnRNP K can also act as a transcriptional repressor. It is a potent suppressor of human thymidine kinase-mediated gene activity. hnRNP K itself cannot bind to the human thymidine kinase promoter, but might repress the transcription by inhibiting the binding of hnRNP A1 and p38AUF of this promoter [41]. Single-stranded structures within the CD43 promoter could also play a major role in affecting CD43 repression. hnRNP K binds single-stranded DNA within the CD43 promoter and mediates its repression [42].
A single-stranded DNA element is also important for the MOR gene in neuronal cells (Fig. 2B) [43,44]. hnRNP K, PCBP1, PCBP2, PCBP2-KL, and PCBP3 all bind to the single-strand DNA of the mouse MOR gene promoter. hnRNP K, PCBP1, PCBP2, and PCBP2-KL activate the mouse MOR gene, whereas PCBP3 acts as a repressor [36]. PCBP3 also bound to the double-stranded poly(C) element essential for the MOR promoter and repressed the promoter activity at the transcriptional level, suggesting a novel function for PCBP3 as a transcriptional regulator [45]. It is likely that PCBPs function as transcriptional regulators on other specific genes as well.
Signal-dependent regulation of transcription and translation by PCBPs
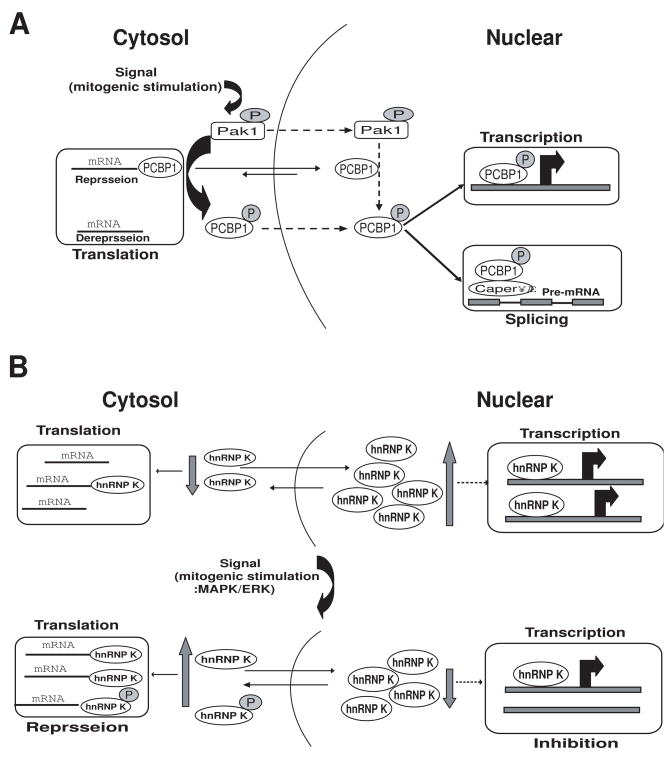
PCBP1 can act at multiple levels during gene expression: transcriptional activator, regulator of RNA splicing, and translational repressor [23,46]. Mitogenic stimulation of human cells phosphorylates PCBP1 on threonines 60 and 127 in a Pak1-sensitive manner. This Pak1-dependent phosphorylation releases PCBP1’s binding to, and translational inhibition of, a DICE-minigene. Pak1 activation also leads to increased nuclear retention of PCBP1, recruitment to the eIF4E promoter, and stimulation of eIF4E expression in a Pak1-sensitive manner. Moreover, mitogenic stimulation promotes Pak1- and PCBP1-dependent alternative splicing and exon inclusion from a CD44 minigene. The alternative splicing functions of PCBP1 are in turn mediated by its intrinsic interaction with Caper alpha, a U2 snRNP auxiliary factor-related protein previously implicated in RNA splicing. These findings establish the principle that a single coregulator can function as a signal-dependent and coordinated regulator of transcription, splicing, and translation [23] (Fig. 3A).
Fig. 3.
A, Proposed model for signal-dependent alterations in the nuclear and cytoplasmic activities of PCBP1. Upstream activators of the Pak1 pathway induce Pak1 kinase activity; active Pak1 has both cytoplasmic and nuclear functions. Pak1 phosphorylates cytoplasmic PCBP1, reducing its RNA-binding capabilities and thus releasing its translational repression of specific target mRNAs. Pak1 also phosphorylates PCBP1 in the nucleus (the mechanism by which phosphorylated PCBP1 moves from the cytoplasm to the nucleus is still not known). In the nucleus, PCBP1 is recruited to promoters on target genes and regulates their transcriptional activity. Phosphorylation also enhances PCBP1 binding to recently transcribed mRNA in the nucleus, influencing the splicing machinery via CAPER. Dashed lines represent the events that are not fully understood. Adapted from Meng et al., (2007) [23]. B, Proposed model for signal-dependent alterations of the nuclear and cytoplasmic activities of hnRNP K. MAPK/ERK efficiently phosphorylates hnRNP-K, increasing its accumulation in the cytoplasm and rendering the protein capable of regulating translation of mRNAs that have a DICE in the 3′UTR. Also, the quantity of hnRNP K in the can nucleus decrease, suggesting that the transcriptional activity of genes regulated by hnRNP K might also be changed. Dashed lines represent the events that are not fully understood. Adapted from Habelhah et al. (2001) [22].
As noted previously, the cytoplasmic accumulation of hnRNP K is also phosphorylation-dependent. MAPK/ERK efficiently phosphorylates hnRNP K both in vitro and in vivo at serines 284 and 353. Serum stimulation or constitutive activation of ERK kinase results in phosphorylation and cytoplasmic accumulation of hnRNP K. Mutation at ERK phosphoacceptor sites in hnRNP K abolishes the ability to accumulate in the cytoplasm and renders the protein incapable of regulating translation of mRNAs that have a DICE in the 3′UTR. Similarly, treatment with a pharmacological inhibitor of the ERK pathway abolishes cytoplasmic accumulation of hnRNP-K and attenuates inhibition of mRNA translation. These results establish the role of MAPK/ERK in the phosphorylation-dependent cellular distribution of hnRNP K. This mechanism could be required to silence mRNA translation (Fig. 3B), and cytosolic hnRNP K might decrease the transcriptional activity of specific genes.
Conclusion
PCBP genes translate into well-defined, polycytosine-specific, nucleic acid-binding proteins. All members of this protein family have three KH domains. They are involved mainly in various posttranscriptional regulations (e.g., mRNA stabilization or translational activation/silencing). These proteins clearly play important roles in gene expression at the transcriptional level via their ability to bind poly(C) regions. PCBP1 in particular acts at multiple levels in the expression process: as a translational repressor, a transcriptional coactivator, and as a regulator of RNA splicing through Pak1 kinase. Overall, PCBPs function as transcriptional regulators of specific genes and play key roles in coordinating transcriptional responses to environmental signals.
Acknowledgments
This work was supported by National Institutes of Health research grants DA000564, DA001583, DA011806, DA011190, DA007339, K05-DA070554, K05-DA00513 and K05-DA013926 and by the F. & A. Stark Fund of the Minnesota Medical Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Makeyev AV, Liebhaber SA. The poly(C)-binding proteins: a multiplicity of functions and a search for mechanisms. RNA. 2002;8:265–278. doi: 10.1017/s1355838202024627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leffers H, Dejgaard K, Celis JE. Characterisation of two major cellular poly(rC)-binding human proteins, each containing three K-homologous (KH) domains. Eur J Biochem. 1995;230:447–453. [PubMed] [Google Scholar]
- 3.Kiledjian M, Wang X, Liebhaber SA. Identification of two KH domain proteins in the alpha-globin mRNP stability complex. EMBO J. 1995;14:4357–4364. doi: 10.1002/j.1460-2075.1995.tb00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makeyev AV, Liebhaber SA. Identification of two novel mammalian genes establishes a subfamily of KH-domain RNA-binding proteins. Genomics. 2000;67:301–316. doi: 10.1006/geno.2000.6244. [DOI] [PubMed] [Google Scholar]
- 5.Makeyev AV, Chkheidze AN, Liebhaber SA. A set of highly conserved RNA-binding proteins, alphaCP-1 and alphaCP-2, implicated in mRNA stabilization, are coexpressed from an intronless gene and its intron-containing paralog. J Biol Chem. 1999;274:24849–24857. doi: 10.1074/jbc.274.35.24849. [DOI] [PubMed] [Google Scholar]
- 6.Tommerup N, Leffers H. Assignment of the human genes encoding 14,3-3 Eta (YWHAH) to 22q12, 14-3-3 zeta (YWHAZ) to 2p25.1-p25.2, and 14-3-3 beta (YWHAB) to 20q13.1 by in situ hybridization. Genomics. 1996;33:149–150. doi: 10.1006/geno.1996.0176. [DOI] [PubMed] [Google Scholar]
- 7.Chkheidze AN, Liebhaber SA. A novel set of nuclear localization signals determine distributions of the alphaCP RNA-binding proteins. Mol Cell Biol. 2003;23:8405–8415. doi: 10.1128/MCB.23.23.8405-8415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss IM, Liebhaber SA. Erythroid cell-specific mRNA stability elements in the alpha 2-globin 3′ nontranslated region. Mol Cell Biol. 1995;15:2457–2465. doi: 10.1128/mcb.15.5.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blyn LB, Towner JS, Semler BL, Ehrenfeld E. Requirement of poly(rC) binding protein 2 for translation of poliovirus RNA. J Virol. 1997;71:6243–6246. doi: 10.1128/jvi.71.8.6243-6246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gamarnik AV, Andino R. Two functional complexes formed by KH domain containing proteins with the 5′ noncoding region of poliovirus RNA. RNA. 1997;3:882–892. [PMC free article] [PubMed] [Google Scholar]
- 11.Collier B, Goobar-Larsson L, Sokolowski M, Schwartz S. Translational inhibition in vitro of human papillomavirus type 16 L2 mRNA mediated through interaction with heterogenous ribonucleoprotein K and poly(rC)-binding proteins 1 and 2. J Biol Chem. 1998;273:22648–22656. doi: 10.1074/jbc.273.35.22648. [DOI] [PubMed] [Google Scholar]
- 12.Ostareck DH, Ostareck-Lederer A, Shatsky IN, Hentze MW. Lipoxygenase mRNA silencing in erythroid differentiation: The 3′UTR regulatory complex controls 60S ribosomal subunit joining. Cell. 2001;104:281–290. doi: 10.1016/s0092-8674(01)00212-4. [DOI] [PubMed] [Google Scholar]
- 13.Andino R, Boddeker N, Silvera D, Gamarnik AV. Intracellular determinants of picornavirus replication. Trends Microbiol. 1999;7:76–82. doi: 10.1016/s0966-842x(98)01446-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J, Chen X. MCG10, a novel p53 target gene that encodes a KH domain RNA-binding protein, is capable of inducing apoptosis and cell cycle arrest in G(2)-M. Mol Cell Biol. 2000;20:5602–5618. doi: 10.1128/mcb.20.15.5602-5618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castano Z, Vergara-Irigaray N, Pajares MJ, Montuenga LM, Pio R. Expression of alpha CP-4 inhibits cell cycle progression and suppresses tumorigenicity of lung cancer cells. Int J Cancer. 2008;122:1512–1520. doi: 10.1002/ijc.23236. [DOI] [PubMed] [Google Scholar]
- 16.Pio R, Zudaire I, Pino I, Castano Z, Zabalegui N, Vicent S, Garcia-Amigot F, Odero MD, Lozano MD, Garcia-Foncillas J, Calasanz MJ, Montuenga LM. Alpha CP-4, encoded by a putative tumor suppressor gene at 3p21, but not its alternative splice variant alpha CP-4a, is underexpressed in lung cancer. Cancer Res. 2004;64:4171–4179. doi: 10.1158/0008-5472.CAN-03-2982. [DOI] [PubMed] [Google Scholar]
- 17.Du Z, Lee JK, Tjhen R, Li S, Pan H, Stroud RM, James TL. Crystal structure of the first KH domain of human poly(C)-binding protein-2 in complex with a C-rich strand of human telomeric DNA at 1.7 A. J Biol Chem. 2005;280:38823–38830. doi: 10.1074/jbc.M508183200. [DOI] [PubMed] [Google Scholar]
- 18.Sidiqi M, Wilce JA, Vivian JP, Porter CJ, Barker A, Leedman PJ, Wilce MC. Structure and RNA binding of the third KH domain of poly(C)-binding protein 1. Nucleic Acids Res. 2005;33:1213–1221. doi: 10.1093/nar/gki265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michael WM, Eder PS, Dreyfuss G. The K nuclear shuttling domain: a novel signal for nuclear import and nuclear export in the hnRNP K protein. Embo J. 1997;16:3587–3598. doi: 10.1093/emboj/16.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bomsztyk K, Denisenko O, Ostrowski J. hnRNP K: one protein multiple processes. Bioessays. 2004;26:629–638. doi: 10.1002/bies.20048. [DOI] [PubMed] [Google Scholar]
- 21.Berry AM, Flock KE, Loh HH, Ko JL. Molecular basis of cellular localization of poly C binding protein 1 in neuronal cells. Biochem Biophys Res Commun. 2006;349:1378–1386. doi: 10.1016/j.bbrc.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habelhah H, Shah K, Huang L, Ostareck-Lederer A, Burlingame AL, Shokat KM, Hentze MW, Ronai Z. ERK phosphorylation drives cytoplasmic accumulation of hnRNP-K and inhibition of mRNA translation. Nat Cell Biol. 2001;3:325–330. doi: 10.1038/35060131. [DOI] [PubMed] [Google Scholar]
- 23.Meng Q, Rayala SK, Gururaj AE, Talukder AH, O’Malley BW, Kumar R. Signaling-dependent and coordinated regulation of transcription, splicing, and translation resides in a single coregulator, PCBP1. Proc Natl Acad Sci U S A. 2007;104:5866–5871. doi: 10.1073/pnas.0701065104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostrowski J, Schullery DS, Denisenko ON, Higaki Y, Watts J, Aebersold R, Stempka L, Gschwendt M, Bomsztyk K. Role of tyrosine phosphorylation in the regulation of the interaction of heterogenous nuclear ribonucleoprotein K protein with its protein and RNA partners. J Biol Chem. 2000;275:3619–3628. doi: 10.1074/jbc.275.5.3619. [DOI] [PubMed] [Google Scholar]
- 25.Schullery DS, Ostrowski J, Denisenko ON, Stempka L, Shnyreva M, Suzuki H, Gschwendt M, Bomsztyk K. Regulated interaction of protein kinase Cdelta with the heterogeneous nuclear ribonucleoprotein K protein. J Biol Chem. 1999;274:15101–15109. doi: 10.1074/jbc.274.21.15101. [DOI] [PubMed] [Google Scholar]
- 26.Ostareck-Lederer A, Ostareck DH, Rucknagel KP, Schierhorn A, Moritz B, Huttelmaier S, Flach N, Handoko L, Wahle E. Asymmetric arginine dimethylation of heterogeneous nuclear ribonucleoprotein K by protein-arginine methyltransferase 1 inhibits its interaction with c-Src. J Biol Chem. 2006;281:11115–11125. doi: 10.1074/jbc.M513053200. [DOI] [PubMed] [Google Scholar]
- 27.Chiou YY, Lin WJ, Fu SL, Lin CH. Direct mass-spectrometric identification of Arg296 and Arg299 as the methylation sites of hnRNP K protein for methyltransferase PRMT1. Protein J. 2007;26:87–93. doi: 10.1007/s10930-006-9049-9. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Zhou X, Liu N, Wang C, Zhang L, Mo W, Hu G. Arginine methylation of hnRNP K enhances p53 transcriptional activity. FEBS Lett. 2008;582:1761–1765. doi: 10.1016/j.febslet.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 29.Li T, Evdokimov E, Shen RF, Chao CC, Tekle E, Wang T, Stadtman ER, Yang DC, Chock PB. Sumoylation of heterogeneous nuclear ribonucleoproteins, zinc finger proteins, and nuclear pore complex proteins: a proteomic analysis. Proc Natl Acad Sci U S A. 2004;101:8551–8556. doi: 10.1073/pnas.0402889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaillard C, Cabannes E, Strauss F. Identity of the RNA-binding protein K of hnRNP particles with protein H16, a sequence-specific single strand DNA-binding protein. Nucleic Acids Res. 1994;22:4183–4186. doi: 10.1093/nar/22.20.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michelotti GA, Michelotti EF, Pullner A, Duncan RC, Eick D, Levens D. Multiple single-stranded cis elements are associated with activated chromatin of the human c-myc gene in vivo. Mol Cell Biol. 1996;16:2656–2669. doi: 10.1128/mcb.16.6.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du Q, Melnikova IN, Gardner PD. Differential effects of heterogeneous nuclear ribonucleoprotein K on Sp1- and Sp3-mediated transcriptional activation of a neuronal nicotinic acetylcholine receptor promoter. J Biol Chem. 1998;273:19877–19883. doi: 10.1074/jbc.273.31.19877. [DOI] [PubMed] [Google Scholar]
- 33.Ritchie SA, Pasha MK, Batten DJ, Sharma RK, Olson DJ, Ross AR, Bonham K. Identification of the SRC pyrimidine-binding protein (SPy) as hnRNP K: implications in the regulation of SRC1A transcription. Nucleic Acids Res. 2003;31:1502–1513. doi: 10.1093/nar/gkg246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thakur S, Nakamura T, Calin G, Russo A, Tamburrino JF, Shimizu M, Baldassarre G, Battista S, Fusco A, Wassell RP, Dubois G, Alder H, Croce CM. Regulation of BRCA1 transcription by specific single-stranded DNA binding factors. Mol Cell Biol. 2003;23:3774–3787. doi: 10.1128/MCB.23.11.3774-3787.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynch M, Chen L, Ravitz MJ, Mehtani S, Korenblat K, Pazin MJ, Schmidt EV. hnRNP K binds a core polypyrimidine element in the eukaryotic translation initiation factor 4E (eIF4E) promoter, and its regulation of eIF4E contributes to neoplastic transformation. Mol Cell Biol. 2005;25:6436–6453. doi: 10.1128/MCB.25.15.6436-6453.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi HS, Song KY, Hwang CK, Kim CS, Law PY, Wei LN, Loh HH. A proteomic approach for identification of single-strand DNA-binding proteins involved in transcriptional regulation of mouse mu-opioid receptor gene. Mol Cell Proteomics. 2008;7:1517–1729. doi: 10.1074/mcp.M800052-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SS, Pandey KK, Choi HS, Kim SY, Law PY, Wei LN, Loh HH. Poly(C) binding protein family is a transcription factor in mu-opioid receptor gene expression. Mol Pharmacol. 2005;68:729–36. doi: 10.1124/mol.105.012245. [DOI] [PubMed] [Google Scholar]
- 38.Ko JL, Loh HH. Poly C binding protein, a single-stranded DNA binding protein, regulates mouse mu-opioid receptor gene expression. J Neurochem. 2005;93:749–761. doi: 10.1111/j.1471-4159.2005.03089.x. [DOI] [PubMed] [Google Scholar]
- 39.Michelotti EF, Michelotti GA, Aronsohn AI, Levens D. Heterogeneous nuclear ribonucleoprotein K is a transcription factor. Mol Cell Biol. 1996;16:2350–2360. doi: 10.1128/mcb.16.5.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moumen A, Masterson P, O’Connor MJ, Jackson SP. hnRNP K: an HDM2 target and transcriptional coactivator of p53 in response to DNA damage. Cell. 2005;123:1065–1078. doi: 10.1016/j.cell.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 41.Lau JS, Baumeister P, Kim E, Roy B, Hsieh TY, Lai M, Lee AS. Heterogeneous nuclear ribonucleoproteins as regulators of gene expression through interactions with the human thymidine kinase promoter. J Cell Biochem. 2000;79:395–406. doi: 10.1002/1097-4644(20001201)79:3<395::aid-jcb50>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 42.Da Silva N, Bharti A, Shelley CS. hnRNP-K and Pur(alpha) act together to repress the transcriptional activity of the CD43 gene promoter. Blood. 2002;100:3536–3544. doi: 10.1182/blood.V100.10.3536. [DOI] [PubMed] [Google Scholar]
- 43.Ko JL, Loh HH. Single-stranded DNA-binding complex involved in transcriptional regulation of mouse mu-opioid receptor gene. J Biol Chem. 2001;276:788–795. doi: 10.1074/jbc.M004279200. [DOI] [PubMed] [Google Scholar]
- 44.Malik AK, Flock KE, Godavarthi CL, Loh HH, Ko JL. Molecular basis underlying the poly C binding protein 1 as a regulator of the proximal promoter of mouse mu-opioid receptor gene. Brain Res. 2006;1112:33–45. doi: 10.1016/j.brainres.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Choi HS, Kim CS, Hwang CK, Song KY, Law PY, Wei LN, Loh HH. Novel function of the poly(C)-binding protein {alpha}CP3 as a transcriptional repressor of the mu opioid receptor gene. FASEB J. 2007;21:3963–3973. doi: 10.1096/fj.07-8561com. [DOI] [PubMed] [Google Scholar]
- 46.Lonard DM, O’Malley BW. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell. 2007;27:691–700. doi: 10.1016/j.molcel.2007.08.012. [DOI] [PubMed] [Google Scholar]