Abstract
Background
Other than several rare, highly penetrant familial syndromes, genetic risk factors for sporadic pancreatic cancer are largely unknown. ABO blood type is an inherited characteristic that in previous small studies has been associated with the risk of gastrointestinal malignancies.
Methods
We separately examined the relationship between ABO blood type and the risk of incident pancreatic cancer in two large, independent, prospective cohort studies (the Nurses’ Health Study and Health Professionals Follow-up Study) that collected blood group data on 107 503 US health professionals. Hazard ratios for pancreatic cancer by ABO blood type were calculated using Cox proportional hazards models with adjustment for other known risk factors, including age, tobacco use, body mass index, physical activity, and history of diabetes mellitus. All statistical tests were two-sided.
Results
During 927 995 person-years of follow-up, 316 participants developed pancreatic cancer. ABO blood type was associated with the risk of developing pancreatic cancer (P = .004; log-rank test). Compared with participants with blood group O, those with blood groups A, AB, or B were more likely to develop pancreatic cancer (adjusted hazard ratios for incident pancreatic cancer were 1.32 [95% confidence interval {CI} = 1.02 to 1.72], 1.51 [95% CI = 1.02 to 2.23], and 1.72 [95% CI = 1.25 to 2.38], respectively). The association between blood type and pancreatic cancer risk was nearly identical in the two cohorts (Pinteraction = .97). Overall, 17% of the pancreatic cancer cases were attributable to inheriting a non-O blood group (blood group A, B, or AB). The age-adjusted incidence rates for pancreatic cancer per 100 000 person-years were 27 (95% CI = 23 to 33) for participants with blood type O, 36 (95% CI = 26 to 50) for those with blood type A, 41 (95% CI = 31 to 56) for those with blood type AB, and 46 (95% CI = 32 to 68) for those with blood type B.
Conclusions
In two large, independent populations, ABO blood type was statistically significantly associated with the risk of pancreatic cancer. Further studies are necessary to define the mechanisms by which ABO blood type or closely linked genetic variants may influence pancreatic cancer risk.
CONTEXT AND CAVEATS
Prior knowledge
The genetic determinants of sporadic pancreatic cancer were unknown. Previous studies had suggested that blood type (A, B, AB, or O) might be associated with various malignancies, including pancreatic cancer.
Study design
The association of blood type and risk of pancreatic cancer was studied in the context of two prospective cohort studies. Cox proportional hazards models were used to calculate the risk of pancreatic cancer by blood type after adjustment for other known risk factors.
Contributions
This study found that there was a slightly increased risk of pancreatic cancer in those with blood groups A, AB, or B relative to those with blood group O, such that approximately 17% of pancreatic cancers were attributable to a non-O blood group.
Implications
Additional studies will be necessary to determine the biological basis for the association of blood group and pancreatic cancer risk.
Limitations
This study could not determine whether the ABO gene itself or genetic variations closely linked to this gene are the basis of the observed associations.
From the Editors
Pancreatic cancer is the fourth leading cause of cancer-related mortality in the United States (1). Tobacco use and obesity are two modifiable risk factors for this disease (2,3), and a history of pancreatic cancer in a first-degree relative increases the lifetime risk by 1.5- to 3.0-fold (4,5). Pancreatic cancer is also associated with several genetic syndromes, including hereditary breast and ovarian cancer, familial atypical multiple mole melanoma syndrome (FAMMM), Peutz–Jeghers syndrome, and hereditary pancreatitis (6,7). However, these genetic syndromes and other established risk factors account only for a minority of pancreatic cancer cases; the predisposing environmental and genetic factors for most patients are unknown.
Studies conducted several decades ago suggested a link between inherited human blood group antigens and the risk of various malignancies, including pancreatic cancer (8–14). However, studies of pancreatic cancer were limited by their retrospective design and small case numbers. Human blood group antigens are glycoproteins expressed on the surface of red blood cells and several other tissue types, including cells from the gastrointestinal tract. The sugar residues of these glycoproteins are attached to a protein backbone, the H antigen, by a glycosyltransferase encoded for by the ABO gene on chromosome 9q34 (15). In laboratory investigations, patient-derived pancreatic cancer cells express blood group antigens on their cell surface and have different patterns of expression than cells in adjacent normal pancreatic ducts (16–19), suggesting that modifications to glycosyltransferase specificity occur during pancreatic tumorigenesis. Alterations in surface glycoconjugates may lead to modifications in intercellular adhesion, membrane signaling, and immunosurveillance, which could have important implications for tumor development and spread (20–22). Nevertheless, the association between ABO blood type and the risk of pancreatic cancer has not been rigorously and prospectively evaluated.
We therefore examined the relationship between ABO blood type and pancreatic cancer risk in two large and independent prospective cohort studies of male and female health professionals. In both cohorts, blood group status was recorded before the diagnosis of pancreatic cancer, thus avoiding potential biases inherent in retrospective analyses.
Subjects and Methods
Study Population
The Nurses’ Health Study (NHS) began in 1976, when 121 700 female nurses between 30 and 55 years of age completed a baseline questionnaire about risk factors for cancer and cardiovascular disease. Subsequently, participants have completed a self-administered, mailed questionnaire biennially to update information on their lifestyle, medical history, and diet. The Health Professionals Follow-up Study (HPFS) began in 1986 when 51 529 US male dentists, veterinarians, pharmacists, optometrists, osteopathic physicians, and podiatrists, aged 40–75 years, completed a baseline questionnaire on diet, lifestyle, and medical conditions, including a history of cancer. This information was then updated with biennial questionnaires. In 1996, questionnaires in both cohorts were expanded to include ABO blood type and Rh type. For this analysis of ABO blood type and the subsequent risk of pancreatic cancer, we included all participants who provided information on ABO blood type for the 1996 questionnaire. A total of 107 503 subjects were included in this analysis, 77 360 female nurses and 30 143 male health professionals. Of these subjects, 92% also reported Rh type on the 1996 questionnaire. The Human Research Committee at the Brigham and Women's Hospital (Boston, MA) approved this study, and all participants provided written informed consent.
Assessment of ABO Blood Group and Covariates
On the 1996 questionnaire in both the NHS and HPFS, participants were asked their blood type (A, B, AB, O, or unknown) and their Rh factor (positive, negative, or unknown). We did not serologically confirm the ABO blood type reported by all study participants. However, we obtained laboratory confirmation of the reported blood type in a subsample of 98 subjects who underwent serologic testing as part of their medical care; 96 of these subjects also had self-reported and serologically tested Rh factor. Self-reported ABO blood type matched the results of serologic testing in 91% of participants, whereas self-reported Rh type matched the serologic results in 98% of participants. The results of this validation did not materially differ between the two cohort studies; concordance of self-reported and serologically confirmed ABO blood type was 93% for NHS and 90% for HPFS, and concordance of self-reported and serologically confirmed Rh type was 100% for NHS and 96% for HPFS.
Data for the other covariates in this analysis were obtained from the 1996 questionnaire or if data from 1996 were not available, from the most recent previous questionnaire. We used body mass index (BMI, weight in kilograms/[height in meters]2) as a measure of total adiposity. As previously described and validated (23,24), participants were asked to average the time spent per week in eight different activities over the previous year and a weekly physical activity level was derived by multiplying the time spent in each activity per week by its typical energy expenditure requirements expressed in metabolic equivalents. Participants were also asked about their smoking status (current, past, or never) and history of diabetes mellitus (yes or no). Participants in the NHS were asked on the 1996 questionnaire if there was a family history of pancreatic cancer in a parent or sibling; this information was not obtained from HPFS participants. Of the 144 082 participants who returned the 1996 questionnaires in the NHS and HPFS, 75% provided their ABO blood group. The clinical characteristics mentioned above were highly similar for participants who provided their blood type on the 1996 questionnaires and those who did not.
Identification of Pancreatic Cancer Cases
In both cohorts, when a participant (or the next of kin for decedents) reported a diagnosis of pancreatic cancer on a follow-up questionnaire, we asked permission to obtain their hospital records and pathology reports. We searched the National Death Index to identify deaths among nonrespondents and to ascertain pancreatic cancer diagnoses. More than 98% of deaths in these cohorts have been identified in this way (25). Study physicians at the NHS and HPFS who were blinded to the exposure data reviewed medical records and assigned cancer diagnoses and causes of death. In this study, 96% of the pancreatic cancer cases were histologically confirmed on medical record review.
Statistical Analyses
The primary exposure was a participant's self-reported ABO blood type. We determined participant characteristics, including age, sex, race/ethnicity, BMI, physical activity, smoking status, history of diabetes mellitus, and family history of pancreatic cancer in a first-degree relative, for those within each ABO blood group. We used Cox proportional hazards models to calculate adjusted hazard ratios and 95% confidence intervals (95% CIs) for incident pancreatic cancer. Follow-up time was calculated from the date of the 1996 questionnaire to the date of pancreatic cancer diagnosis, death from another cause, or the end of the follow-up (June 30, 2005, in NHS, and January 31, 2005, in HPFS), whichever came first. The proportionality of hazards assumption was satisfied by P values greater than .05 by the Wald test for time-dependent variables, which were the cross-products of ABO blood type and time.
We evaluated effect modification by investigating Cox proportional hazards models after stratifying by other known risk factors for pancreatic cancer, including age (above or below the combined cohort median), sex (male or female), BMI (above or below the combined cohort median), physical activity (above or below the combined cohort median), and smoking status (never, past, or current). Tests of interaction between ABO blood group and potential effect modifiers were assessed by entering into the model the cross-product of ABO blood group and the dichotomized covariate. Incidence rates of pancreatic cancer for participants with a specific ABO blood group were calculated by dividing the number of incident cases by the number of person-years of follow-up. The pancreatic cancer–free survival by ABO blood group was compared using the log-rank test and cumulative pure incidence curves, which plot 1 − Kaplan–Meier survival rate (26). The population attributable risk (PAR) for pancreatic cancer due to non-O blood groups was calculated using the following equation: PAR = Pd ([HR − 1]/HR), where Pd = prevalence of exposure among pancreatic cancer cases and HR = multivariable-adjusted hazard ratio calculated by Cox proportional hazards models (27,28). All statistical analyses were performed using the SAS 9.1 statistical package (SAS Institute, Cary, NC), and all P values were derived from two-sided tests.
Results
On the 1996 questionnaires, 107 503 participants in the NHS and HPFS (77 360 female nurses and 30 143 male health professionals) provided information on their ABO blood type and were eligible for this analysis. In this study, the mean follow-up time was 8.6 years. Baseline characteristics of participants in this study were highly similar across the four ABO blood groups (Table 1). The frequency distributions of ABO blood groups were nearly identical among participants in the NHS and HPFS, and the distributions in these cohorts were similar to those for whites living in the United States as recorded in population registries (29,30) (Table 2).
Table 1.
Baseline characteristics of study subjects according to ABO blood type*
| Blood group |
||||
| Characteristic | O | A | AB | B |
| No. of subjects | ||||
| NHS | 33 258 | 27 514 | 6150 | 10 438 |
| HPFS | 12 962 | 11 153 | 2314 | 3714 |
| Total | 46 220 | 38 667 | 8464 | 14 152 |
| Age | ||||
| Median (years) | 62 | 62 | 64 | 62 |
| <60 years (%) | 38.8 | 40.0 | 32.6 | 40.4 |
| 60–69.9 years (%) | 38.9 | 38.2 | 39.8 | 38.6 |
| ≥70 years (%) | 22.2 | 21.8 | 27.6 | 21.0 |
| Female (%) | 72.0 | 71.1 | 72.7 | 73.8 |
| White (%) | 97.1 | 97.9 | 96.3 | 96.2 |
| BMI | ||||
| Median (kg/m2) | 25.8 | 25.7 | 25.6 | 25.7 |
| <25 kg/m2 (%) | 42.5 | 42.8 | 43.9 | 42.2 |
| 25–29.9 kg/m2 (%) | 37.8 | 38.0 | 37.3 | 38.2 |
| ≥30 kg/m2 (%) | 19.7 | 19.3 | 18.9 | 19.6 |
| Physical activity level | ||||
| Median (MET-h/wk) | 13.4 | 13.7 | 13.1 | 13.4 |
| <10 MET-h/wk (%) | 42.1 | 41.2 | 43.2 | 42.2 |
| 10–19.9 MET-h/wk (%) | 19.9 | 20.9 | 19.0 | 20.1 |
| ≥20 MET-h/wk (%) | 38.0 | 37.8 | 37.8 | 37.7 |
| Smoking status (%) | ||||
| Never | 45.0 | 46.2 | 44.5 | 45.0 |
| Past | 44.4 | 43.7 | 44.1 | 44.2 |
| Current | 10.6 | 10.2 | 11.3 | 10.8 |
| Diabetes mellitus (%) | 6.7 | 6.7 | 7.9 | 7.1 |
| Family history of pancreatic cancer (%)† | 2.5 | 2.5 | 3.0 | 2.9 |
NHS = Nurses’ Health Study; HPFS = Health Professionals Follow-up Study; BMI = body mass index; MET-h/wk = metabolic equivalent task hours per week.
History of pancreatic cancer in a participant's mother, father, or sibling; data available only for Nurses’ Health Study participants.
Table 2.
Distribution of ABO blood group and Rh type among subjects from the NHS, HPFS, and US white population*
| ABO blood group | NHS (%) | HPFS (%) | Total study population (%) | US white population† (%) |
| O | 43 | 43 | 43 | 45 |
| A | 36 | 37 | 36 | 40 |
| AB | 8 | 8 | 8 | 4 |
| B | 13 | 12 | 13 | 11 |
| Rh negative | 22 | 21 | 22 | 17 |
| Rh positive | 78 | 79 | 78 | 83 |
NHS = Nurses’ Health Study; HPFS = Health Professionals Follow-up Study.
Data from references (29,30).
Between 1996 and 2005, 316 participants (199 female nurses and 117 male health professionals) developed pancreatic cancer. The risk of developing pancreatic cancer differed statistically significantly by ABO blood type in each of the two cohorts (Table 3), and this association between blood type and pancreatic cancer risk was highly similar between the two independent populations (Pinteraction = .97). For the combined study population, compared with participants with blood group O, those with blood groups A, AB, or B were more likely to develop pancreatic cancer (adjusted hazard ratios for incident pancreatic cancer, 1.32 [95% CI = 1.02 to 1.72], 1.51 [95% CI = 1.02 to 2.23], and 1.72 [95% CI = 1.25 to 2.38], respectively) (Table 3). The age-adjusted risks of pancreatic cancer associated with blood groups A, B, and AB were not substantially altered by multivariable adjustment for other known or suspected risk factors for the disease. In addition, the hazard ratios were not substantially altered by including only the 96% of pancreatic cancer cases that were pathologically confirmed on medical record review.
Table 3.
Age-adjusted and multivariable-adjusted hazard ratios and 95% confidence intervals for pancreatic cancer by ABO blood type*
| ABO blood type |
||||
| Cohort | O | A | AB | B |
| NHS | ||||
| No. of cases/No. of person-years | 70/289 174 | 71/238 774 | 22/53 134 | 36/90 750 |
| Age-adjusted HR (95% CI) | 1.00 (referent) | 1.24 (0.89 to 1.72) | 1.58 (0.98 to 2.55) | 1.65 (1.11 to 2.47) |
| Multivariable-adjusted HR† (95% CI) | 1.00 (referent) | 1.25 (0.90 to 1.73) | 1.57 (0.97 to 2.53) | 1.65 (1.10 to 2.47) |
| HPFS | ||||
| No. of cases/No. of person-years | 39/110 109 | 47/95 073 | 11/19 362 | 20/31 619 |
| Age-adjusted HR (95% CI) | 1.00 (referent) | 1.44 (0.94 to 2.21) | 1.43 (0.73 to 2.79) | 1.85 (1.08 to 3.18) |
| Multivariable-adjusted HR† (95% CI) | 1.00 (referent) | 1.45 (0.95 to 2.22) | 1.40 (0.72 to 2.74) | 1.84 (1.07 to 3.15) |
| Total study population | ||||
| No. of cases/No. of person-years | 109/399 283 | 118/333 847 | 33/72 496 | 56/122 369 |
| Age-adjusted HR (95% CI) | 1.00 (referent) | 1.32 (1.01 to 1.71) | 1.52 (1.03 to 2.24) | 1.71 (1.24 to 2.36) |
| Multivariable-adjusted HR‡ (95% CI) | 1.00 (referent) | 1.32 (1.02 to 1.72) | 1.51 (1.02 to 2.23) | 1.72 (1.25 to 2.38) |
NHS = Nurses’ Health Study; HR = hazard ratio; CI = confidence interval; HPFS = Health Professionals Follow-up Study; MET-h/wk = metabolic equivalent task hours per week.
Adjusted for age (years), body mass index (kg/m2), level of physical activity (MET-h/wk), smoking status (current, past, or never), and history of diabetes mellitus. NHS analysis also adjusted for history of pancreatic cancer in a participant's mother, father, or sibling.
Adjusted for age (years), body mass index (kg/m2), level of physical activity (MET-h/wk), smoking status (current, past, or never), history of diabetes mellitus, and prospective cohort (NHS, HPFS, which also adjusts for sex).
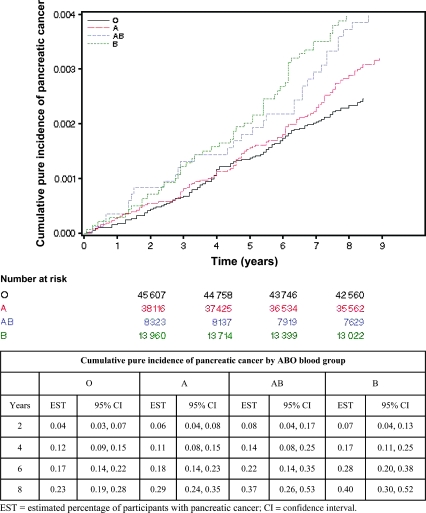
During a combined 927 995 person-years years of follow-up, the cumulative incidence of pancreatic cancer was statistically significantly different among the four ABO blood groups (P = .004, log-rank test) (Figure 1). The age-adjusted incidence rates for pancreatic cancer per 100 000 person-years were 27 (95% CI = 23 to 33) for participants with blood type O, 36 (95% CI = 26 to 50) for those with blood type A, 41 (95% CI = 31 to 56) for those with blood type AB, and 46 (95% CI = 32 to 68) for those with blood type B. The absolute, age-adjusted rate for pancreatic cancer over the 9 years of follow-up was 0.24% for participants with blood type O, 0.31% for those with blood type A, 0.36% for those with blood type AB, and 0.41% for those with blood type B. The age at diagnosis was not statistically significantly different among participants with blood groups O, A, AB, and B (mean ages, 71.6, 71.0, 72.5, and 71.6 years, respectively; P = .78).
Figure 1.
Cumulative pure incidence curves for pancreatic cancer by ABO blood group. Number of participants at risk, percentage of participants diagnosed with pancreatic cancer, and 95% confidence intervals are shown for selected times.
We also examined the risk of pancreatic cancer in the combined cohorts associated with the presence of at least one A or B blood group allele. Compared with participants who reported blood group O, those who reported any blood group antigen A (blood group A or AB) had an adjusted hazard ratio of 1.36 (95% CI = 1.06 to 1.74), whereas those who reported any blood group antigen B (blood group B or AB) had an adjusted hazard ratio of 1.64 (95% CI = 1.24 to 2.17). By contrast, we found no association between Rh type and pancreatic cancer risk; compared with participants who were Rh positive, those who were Rh negative had an adjusted hazard ratio of 1.13 (95% CI = 0.86 to 1.48).
We assessed whether the association between ABO blood group and pancreatic cancer risk differed according to strata of other known or suspected risk factors for this malignancy. As shown in Table 4, the association between ABO blood group and the subsequent risk of pancreatic cancer was not modified to a statistically significant extent by age, BMI, level of physical activity, or smoking status.
Table 4.
Stratified analyses of pancreatic cancer by ABO blood type*
| ABO blood type HR† (95% confidence interval) |
||||||
| Characteristic | No. of cases | O | A | AB | B | Pinteraction |
| Age (years)‡ | .24 | |||||
| <62 | 71 | 1.00 (referent) | 1.43 (0.81 to 2.51) | 2.57 (1.09 to 5.06) | 1.85 (0.93 to 3.67) | |
| ≥62 | 245 | 1.00 (referent) | 1.28 (0.96 to 1.72) | 1.35 (0.86 to 2.12) | 1.68 (1.16 to 2.42) | |
| Body mass index (kg/m2)‡ | .72 | |||||
| <25.7 | 149 | 1.00 (referent) | 1.37 (0.94 to 2.00) | 1.43 (0.80 to 2.54) | 1.64 (1.01 to 2.64) | |
| ≥25.7 | 167 | 1.00 (referent) | 1.28 (0.89 to 1.83) | 1.59 (0.94 to 2.70) | 1.80 (1.17 to 2.79) | |
| Physical activity (MET-h/wk)‡ | .59 | |||||
| <13.5 | 166 | 1.00 (referent) | 1.28 (0.90 to 1.83) | 1.34 (0.77 to 2.33) | 1.68 (1.08 to 2.61) | |
| ≥13.5 | 150 | 1.00 (referent) | 1.37 (0.94 to 2.00) | 1.72 (0.99 to 2.98) | 1.77 (1.10 to 2.84) | |
| Smoking status | .28 | |||||
| Never | 126 | 1.00 (referent) | 1.04 (0.67 to 1.59) | 1.89 (1.08 to 3.30) | 1.87 (1.15 to 3.05) | |
| Past or current | 190 | 1.00 (referent) | 1.53 (1.10 to 2.12) | 1.25 (0.72 to 2.17) | 1.62 (1.02 to 2.48) | |
MET-h/wk = metabolic equivalent task hours per week.
Adjusted for age (years), body mass index (kg/m2), level of physical activity (MET-h/wk), smoking status (current, past, never), history of diabetes mellitus, and prospective cohort (NHS, HPFS, which also adjusts for sex), and excluding the stratification variable.
Dichotomized at the medians of age, body mass index, and physical activity.
To estimate the burden of pancreatic cancer due to ABO blood group status among white men and women (i.e., those of primarily European descent) in the United States, we calculated the PAR for participants who reported having blood groups A, AB, or B (i.e. non-O blood groups). Participants with non-O blood group had an adjusted hazard ratio for pancreatic cancer of 1.44 (95% CI = 1.14 to 1.82). Based on this hazard ratio and the estimated prevalence of blood types A, AB, and B in the combined study population, we estimated that 17% of all pancreatic cancers were attributable to the inheritance of a non-O blood group.
Discussion
In two large, independent prospective cohorts, we observed a statistically significantly elevated risk for incident pancreatic cancer among participants with blood group antigens A or B compared with those with blood group O. The highest risk was observed for participants with blood group B, and intermediate risks were observed for those with blood groups A and AB. The association between blood group and pancreatic cancer risk was not statistically significantly modified by other known risk factors for pancreatic cancer, including age, sex, smoking status, BMI, or physical activity. Within the combined cohorts, 17% of all cases of pancreatic cancer were attributable to inheriting a non-O blood group (blood groups A, B, or AB).
Studies of familial pancreatic cancer have identified several uncommon yet highly penetrant genetic variants involved in pancreatic tumorigenesis. Among patients with familial pancreatic cancer, germline mutations have been identified in BRCA2 (hereditary breast and ovarian cancer syndrome), LKB1 (Peutz–Jeghers syndrome), p16/CDKN2A (FAMMM), and PRSS1 (hereditary pancreatitis) (6,7). Nonetheless, studies suggest that a substantial proportion of pancreatic cancer is the result of more common but only partially penetrant, inherited susceptibilities (7).
For several decades, a role for ABO blood group antigens in the development of cancer has been suspected, with early studies noting an association between blood type A and incident gastric cancer (8–10). However, an association between blood type and pancreatic cancer risk has not been consistently observed. Several retrospective case–control studies performed in the 1960s and 1970s suggested an increased risk of pancreatic cancer in subjects with blood group A (11,12,31), relative to subjects with blood group O or B. A more recently conducted case–control study noted an increased proportion of blood group B among pancreatic cancer case subjects compared with a randomly selected group of patients admitted with nonmalignant diseases and a randomly selected group of blood donors from the same hospital (relative risks, 1.5 and 1.7, P < .025 for both comparisons) (14). The lack of a consistent association between blood group and pancreatic cancer risk across these studies likely stems from small case numbers, retrospective data collection, heterogeneous pathology review, and the use of poorly matched control populations. In this study, blood group status was recorded prospectively before the diagnosis of pancreatic cancer in a uniform, contemporary patient population, eliminating some of the problems inherent in the retrospective case–control studies described above.
The ABO blood groups are defined by carbohydrate moieties displayed on the surface of red blood cells and attached to a protein backbone, known as the H antigen. Three variant alleles (A, B, and O) of a single gene on chromosomes 9q34, the ABO gene, determine a person's blood type by encoding three glycosyltransferases with different substrate specificities (15). As a group, glycosyltransferases catalyze the transfer of sugar moieties from activated donor molecules to specific acceptor molecules, such as the H antigen. The A and B alleles differ at eight nucleotides, leading to four amino acid substitutions and altered enzyme specificity for substrate (32). Consequently, the A and B glycosyltransferases attach N-acetylgalactosamine and D-galactose, respectively, to the H antigen backbone. In contrast, the O allele encodes a nonfunctional glycosyltransferase, and therefore, the H antigen remains unmodified.
In addition to their expression on the surface of red blood cells, the ABO antigens are highly expressed on the surface of epithelial cells of the gastrointestinal, bronchopulmonary, and urogenital tracts (21). Pathology studies have demonstrated the deletion and the novel expression of A, B, and H antigens on the surface of pancreatic cancer cells compared with surrounding normal ductal cells (16–19), suggesting that alterations in glycosyltransferase specificity may occur during pancreatic tumorigenesis. Glycosyltransferase specificity has broad implications, beyond defining ABO blood type (22). Glycoconjugates are important mediators of intercellular adhesion and membrane signaling, two processes integral to malignant progression and spread (21). In addition, these surface molecules are recognized by the host immune response and may have a role in facilitating immunosurveillance for malignant cells (20,33).
Alterations in the host inflammatory state due to ABO blood group antigens may provide a further mechanism to explain an association between blood type and pancreatic cancer risk. A growing body of evidence has demonstrated a link between chronic inflammatory states and cancer, including pancreatic ductal carcinoma (34–36). Two recent genome-wide association studies have noted an association between single nucleotide polymorphisms (SNPs) at the ABO locus and plasma markers of inflammation (37,38). In an analysis of 496 032 SNPs among 1200 participants of the InCHIANTI study (37), two SNPs at the ABO locus were associated with serum levels of tumor necrosis factor-alpha, an inflammatory cytokine known to modulate rates of pancreatic ductal cell apoptosis (35,37). In a second, independent study of 336 108 SNPs in 6578 women participating in the Women's Health Study (38), an SNP at the ABO locus was found to be statistically significantly associated with plasma levels of soluble intercellular adhesion molecule 1, which is a marker of inflammation associated with the risk of incident diabetes (39,40) and myocardial infarction (41). These results raise the possibility that blood group antigens may alter the systemic inflammatory response and thus suggest a possible mechanism to explain the association between blood type and pancreatic cancer risk.
Furthermore, an interaction between chronic Helicobacter pylori infection of the stomach and blood group antigens has been noted, whereby H. pylori bacterial adhesion, colonization, and the host inflammatory response vary depending on the differential expression of blood group antigens on the cell surface of the gastric mucosa (42–44). It has been hypothesized that the interaction of H. pylori with surface glycoconjugates may, in part, explain the observed differences in gastric cancer risk by blood group (43). Recently, H. pylori infection has also been associated with pancreatic cancer risk (45,46), and chronic infection by H. pylori is intriguing as a possible intermediary mechanism for the association of blood type and pancreatic cancer risk.
Although glycoconjugate-specific effects are plausible mechanisms for the observed association between ABO blood type and pancreatic cancer risk, the ABO gene locus may instead act as a marker of allelic variants in nearby genes on chromosome 9q34 and may not be directly involved in pancreatic carcinogenesis. In either case, our data would suggest that chromosome 9q34 may be an important region for inherited susceptibility to sporadic pancreatic cancer.
Our study has several possible limitations. First, blood group in this study was self-reported, making it susceptible to measurement error due to inaccurate reporting. However, because all participants were health care professionals, the accuracy of the reports is likely to be high. We also performed a validation study and found that greater than 90% of participants correctly reported their blood type, indicating that measurement error was unlikely to have materially influenced our results. In a separate validation study of 276 non–health care professionals treated at an outpatient clinic, 97% correctly reported their ABO blood group (47).
Second, our study population was mostly composed of white participants, who were all health-care professionals, which somewhat limits the generalizability of our results. Other risk factors for pancreatic cancer have not been found to differ substantially by race or ethnicity, and the distribution of blood types among the health professionals in our study was similar to that of the general US population. Nonetheless, further investigations that include more diverse study populations are warranted. Finally, we cannot rule out the presence of residual confounding by other predisposing factors for pancreatic cancer. It is reassuring that the age-adjusted hazard ratios for pancreatic cancer by blood type did not change substantially when other known risk factors for pancreatic cancer were included in the multivariable models. In addition, the risk of detecting a false association due to population stratification was low, given the prospective cohort design, the primarily non-Hispanic European-American ancestry of the study population, and the paucity of evidence for variation in pancreatic cancer risk in the ancestral population (48,49).
The prospective design of this study avoided recall or selection bias and the need for proxy interviews (50). Further strengths of this study included prospective covariate information ascertained at the time of initiation of follow-up, physician record review to establish pancreatic cancer diagnoses, high follow-up rates in both cohorts, and a large number of participants with information on ABO blood group.
To date, there is only a limited understanding of pancreatic cancer pathogenesis. Our results in two large, independent prospective cohorts suggest that ABO blood group status may represent a common, partially penetrant, inherited susceptibility for pancreatic cancer. Additional investigation is necessary to further confirm these findings and to determine the potential mechanisms by which ABO antigens may influence pancreatic cancer risk. In particular, the potential influence of blood group antigens on processes of inflammation deserves attention.
Funding
National Cancer Institute, National Institutes of Health (P01 CA87969, P01 CA55075, P50 CA127003, R01 CA124908, T32 CA09001); Lustgarten Foundation for Pancreatic Cancer Research.
Footnotes
The study sponsors had no role in the design of the study; collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs CS, Colditz GA, Stampfer MJ, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med. 1996;156(19):2255–2260. [PubMed] [Google Scholar]
- 3.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 4.Schenk M, Schwartz AG, O’Neal E, et al. Familial risk of pancreatic cancer. J Natl Cancer Inst. 2001;93(8):640–644. doi: 10.1093/jnci/93.8.640. [DOI] [PubMed] [Google Scholar]
- 5.McWilliams RR, Rabe KG, Olswold C, De Andrade M, Petersen GM. Risk of malignancy in first-degree relatives of patients with pancreatic carcinoma. Cancer. 2005;104(2):388–394. doi: 10.1002/cncr.21166. [DOI] [PubMed] [Google Scholar]
- 6.Lochan R, Daly AK, Reeves HL, Charnley RM. Genetic susceptibility in pancreatic ductal adenocarcinoma. Br J Surg. 2008;95(1):22–32. doi: 10.1002/bjs.6049. [DOI] [PubMed] [Google Scholar]
- 7.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20(10):1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 8.Aird I, Bentall HH, Roberts JA. A relationship between cancer of stomach and the ABO blood groups. Br Med J. 1953;1(4814):799–801. doi: 10.1136/bmj.1.4814.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoskins LC, Loux HA, Britten A, Zamcheck N. Distribution of ABO blood groups in patients with pernicious anemia, gastric carcinoma and gastric carcinoma associated with pernicious anemia. N Engl J Med. 1965;273(12):633–637. doi: 10.1056/NEJM196509162731204. [DOI] [PubMed] [Google Scholar]
- 10.Marcus DM. The ABO and Lewis blood-group system. Immunochemistry, genetics and relation to human disease. N Engl J Med. 1969;280(18):994–1006. doi: 10.1056/NEJM196905012801806. [DOI] [PubMed] [Google Scholar]
- 11.Vogel F. Controversy in human genetics. ABO blood groups and disease. Am J Hum Genet. 1970;22(4):464–475. [PMC free article] [PubMed] [Google Scholar]
- 12.Newell GR, Gordon JE, Monlezun AP, Horwitz JS. ABO blood groups and cancer. J Natl Cancer Inst. 1974;52(5):1425–1430. doi: 10.1093/jnci/52.5.1425. [DOI] [PubMed] [Google Scholar]
- 13.Vioque J, Walker AM. Pancreatic cancer and ABO blood types: a study of cases and controls. Med Clin (Barc) 1991;96(20):761–764. [PubMed] [Google Scholar]
- 14.Annese V, Minervini M, Gabbrielli A, Gambassi G, Manna R. ABO blood groups and cancer of the pancreas. Int J Pancreatol. 1990;6(2):81–88. doi: 10.1007/BF02933042. [DOI] [PubMed] [Google Scholar]
- 15.Reid ME, Mohandas N. Red blood cell blood group antigens: structure and function. Semin Hematol. 2004;41(2):93–117. doi: 10.1053/j.seminhematol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Itzkowitz SH, Yuan M, Ferrell LD, et al. Cancer-associated alterations of blood group antigen expression in the human pancreas. J Natl Cancer Inst. 1987;79(3):425–434. [PubMed] [Google Scholar]
- 17.Uchida E, Tempero MA, Burnett DA, Steplewski Z, Pour PM. Correlative studies on antigenicity of pancreatic cancer and blood group types. Cancer Detect Prev Suppl. 1987;1:145–148. [PubMed] [Google Scholar]
- 18.Pour PM, Tempero MM, Takasaki H, et al. Expression of blood group-related antigens ABH, Lewis A, Lewis B, Lewis X, Lewis Y, and CA 19-9 in pancreatic cancer cells in comparison with the patient's blood group type. Cancer Res. 1988;48(19):5422–5426. [PubMed] [Google Scholar]
- 19.Schuessler MH, Pintado S, Welt S, et al. Blood group and blood-group-related antigens in normal pancreas and pancreas cancer: enhanced expression of precursor type 1, Tn and sialyl-Tn in pancreas cancer. Int J Cancer. 1991;47(2):180–187. doi: 10.1002/ijc.2910470204. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Zhang HS, Cordon-Cardo C, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: II. Blood group-related antigens. Int J Cancer. 1997;73(1):50–56. doi: 10.1002/(sici)1097-0215(19970926)73:1<50::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim Biophys Acta. 1999;1473(1):247–266. doi: 10.1016/s0304-4165(99)00183-x. [DOI] [PubMed] [Google Scholar]
- 22.Roseman S. Reflections on glycobiology. J Biol Chem. 2001;276(45):41527–41542. doi: 10.1074/jbc.R100053200. [DOI] [PubMed] [Google Scholar]
- 23.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7(1):81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 25.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Wacholder S, Benichou J, Heineman EF, Hartge P, Hoover RN. Attributable risk: advantages of a broad definition of exposure. Am J Epidemiol. 1994;140(4):303–309. doi: 10.1093/oxfordjournals.aje.a117252. [DOI] [PubMed] [Google Scholar]
- 28.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88(1):15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garratty G, Glynn SA, McEntire R. ABO and Rh(D) phenotype frequencies of different racial/ethnic groups in the United States. Transfusion. 2004;44(5):703–706. doi: 10.1111/j.1537-2995.2004.03338.x. [DOI] [PubMed] [Google Scholar]
- 30.Mourant A, Kopec AC, Domantewska-Sobczak K. The Distribution of the Human Blood Groups and Other Polymorphisms. London: Oxford University Press; 1976. [Google Scholar]
- 31.Aird I, Lee DR, Roberts JA. ABO blood groups and cancer of oesophagus, cancer of pancreas, and pituitary adenoma. Br Med J. 1960;1(5180):1163–1166. doi: 10.1136/bmj.1.5180.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yip SP. Single-tube multiplex PCR-SSCP analysis distinguishes 7 common ABO alleles and readily identifies new alleles. Blood. 2000;95(4):1487–1492. [PubMed] [Google Scholar]
- 33.Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines [Review] Adv Exp Med Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 34.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89(6):442–446. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 35.Garcea G, Dennison AR, Steward WP, Berry DP. Role of inflammation in pancreatic carcinogenesis and the implications for future therapy. Pancreatology. 2005;5(6):514–529. doi: 10.1159/000087493. [DOI] [PubMed] [Google Scholar]
- 36.Guerra C, Schuhmacher AJ, Canamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11(3):291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Melzer D, Perry JR, Hernandez D, et al. A genome-wide association study identifies protein quantitative trait loci (pQTLs) PLoS Genet. 2008;4(5) doi: 10.1371/journal.pgen.1000072. e1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pare G, Chasman DI, Kellogg M, et al. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet. 2008;4(7) doi: 10.1371/journal.pgen.1000118. e1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291(16):1978–1986. doi: 10.1001/jama.291.16.1978. [DOI] [PubMed] [Google Scholar]
- 40.Song Y, Manson JE, Tinker L, et al. Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes. 2007;56(7):1898–1904. doi: 10.2337/db07-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351(9096):88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 42.Boren T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262(5141):1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 43.Suerbaum S, Josenhans C. Helicobacter pylori evolution and phenotypic diversification in a changing host. Nat Rev Microbiol. 2007;5(6):441–452. doi: 10.1038/nrmicro1658. [DOI] [PubMed] [Google Scholar]
- 44.Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134(1):306–323. doi: 10.1053/j.gastro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Raderer M, Wrba F, Kornek G, et al. Association between Helicobacter pylori infection and pancreatic cancer. Oncology. 1998;55(1):16–19. doi: 10.1159/000011830. [DOI] [PubMed] [Google Scholar]
- 46.Stolzenberg-Solomon RZ, Blaser MJ, Limburg PJ, et al. Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J Natl Cancer Inst. 2001;93(12):937–941. doi: 10.1093/jnci/93.12.937. [DOI] [PubMed] [Google Scholar]
- 47.Ito H, Matsuo K, Saito T, et al. Valid responses to ABO blood type question in self-reporting questionnaire. Asian Pac J Cancer Prev. 2001;2(4):315–317. [PubMed] [Google Scholar]
- 48.Wacholder S, Rothman N, Caporaso N. Population stratification in epidemiologic studies of common genetic variants and cancer: quantification of bias. J Natl Cancer Inst. 2000;92(14):1151–1158. doi: 10.1093/jnci/92.14.1151. [DOI] [PubMed] [Google Scholar]
- 49.Shibuya K, Mathers CD, Boschi-Pinto C, Lopez AD, Murray CJ. Global and regional estimates of cancer mortality and incidence by site, II: results for the global burden of disease 2000. BMC Cancer. 2002;2:37. doi: 10.1186/1471-2407-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langholz B, Rothman N, Wacholder S, Thomas DC. Cohort studies for characterizing measured genes. J Natl Cancer Inst Monogr. 1999;(26):39–42. doi: 10.1093/oxfordjournals.jncimonographs.a024224. [DOI] [PubMed] [Google Scholar]