Abstract
Francisella tularensis causes the zoonosis tularemia in humans, and inhaled F. tularensis ssp. novicida induces lethal murine tularemia. Transcription of virulence factors in F. novicida is regulated by macrophage growth locus A (mglA), a global regulator required for bacterial replication in macrophages in vitro. We examined the infectivity and immunogenicity of attenuated F. novicida ΔmglA in the lung in vivo. Aerosolized ΔmglA caused replicative pulmonary infection that peaked at 7 days and was cleared thereafter, without clinical evidence of disease. In contrast, inhalation of wild type F. novicida resulted in more rapid bacterial replication and dissemination leading to death within 96 hours. Early containment of ΔmglA infection was partially dependent on myeloid differentiation factor 88 and interferon-γ but did not require B or T cells. However, lymphocytes were necessary for subsequent bacterial clearance. Infection with ΔmglA elicited specific IgG1-predominant antibodies and variable interferon-γ recall responses to wild type F. novicida. Inoculation of mice with aerosolized ΔmglA afforded no protection against a subsequent low-dose aerosol challenge with wild type F. novicida. These findings establish that inhalation of F. novicida ΔmglA results in replicative infection that elicits innate and adaptive immune responses but not protective immunity against invasive pneumonic tularemia.
Keywords: Francisella tularensis, Tularemia, Pneumonia, Immunology
1. Introduction
Francisella tularensis is a Gram-negative intracellular pathogen that causes the zoonosis tularemia. Although clinical manifestations of infection depend on the route of inoculation, pneumonia is the most lethal form of the disease. Infection can occur from human interaction with small mammals, in particular rodents and lagomorphs, as well as from the bites of blood-feeding arthropods [1]. In Europe, the majority of cases are caused by F. tularensis subspecies holarctica but the more virulent F. tularensis ssp. tularensis (type A) predominates throughout North America [1, 2]. Due to its low infectious dose and airborne transmissibility, F. tularensis is considered a potential bioweapon [3]. The pathogenicity of F. tularensis is not completely understood but its intracellular parasitism of macrophages involves escape from the phagosome prior to lysosomal fusion [4]. The organism also escapes neutrophil phagosomes after inhibiting the respiratory burst [5].
F. tularensis ssp. novicida is a rare cause of human disease but is highly lethal in mice [6-8]. A spontaneous mutant of F. novicida first discovered by Baron and Nano lacks macrophage growth locus A (mglA) [9], the positive regulatory gene of a 30kb pathogenicity island that is essential for intramacrophage growth [7] and the regulator of several other virulence genes [10]. The mglA protein is homologous to SspA, an Escherichia coli stringent starvation transcriptional regulatory protein [9]. Unlike the wild type organism, F. novicida ΔmglA does not escape the phagosome prior to lysosomal fusion [11]. The ΔmglA mutant fails to replicate in Acanthamoebae castellani, peritoneal macrophages, bone-marrow-derived macrophages or the J774 murine macrophage cell line [7, 9, 10], and is nonlethal in mice after intraperitoneal, subcutaneous, or intranasal challenge [7, 10]. However, it is not known if F. novicida ΔmglA replicates in the lungs or elicits an immune response that might be protective against invasive tularemia. Therefore, we aimed to characterize the host response to airborne infection with ΔmglA and determine if the organism has potential as a vaccine.
2. Material and methods
2.1. Bacterial strains and growth conditions
F. tularensis ssp. novicida U112 (wild type) and ΔmglA strains were kindly provided by Dr. Francis Nano (University of Victoria, Canada). Bacteria were grown from frozen glycerol stock in tryptic soy broth with 0.1% L-cysteine at 37 °C for 16-18 hours, isolated by centrifugation, washed twice in PBS, and suspended to the desired concentration estimated by optical density (OD), with an OD540nm of 0.200 yielding approximately 2 × 109 CFU/ml bacteria.
2.2. Animals
Specific-pathogen-free BALB/c, C57BL/6, and interferon (IFN)-γ-/- mice on a C57BL/6 background were obtained from Jackson Laboratories (Bar Harbor, ME). Rag2-/- mice on a C57BL/6 background were obtained from Taconic (Hudson, NY). Myeloid differentiation factor 88 (MyD88)-/- mice were obtained from S. Akira, Osaka, Japan [12, 13], backcrossed eight generations to C57BL/6 and bred in-house. All animals were housed in laminar flow cages and were permitted ad lib access to sterile food and water. Euthanasia was accomplished with intraperitoneal pentobarbital followed by exsanguination from cardiac puncture. The Institutional Animal Care and Use Committee of the University of Washington approved all experimental procedures.
2.3. Infection of animals
Mice were exposed to aerosolized bacteria using a snout-only inhalation system (In-Tox Products, Moriarty, NM). Aerosols were generated from UniHEART lo-flo or MiniHEART hi-flo nebulizers (Westmed, Tucson, AZ) driven at 40 psi. Airflow through the system was maintained for 10 minutes at 5 l/min for experiments performed with the UniHEART nebulizer and at 24 l/min for experiments performed with the MiniHEART nebulizer, followed by five minutes purge with air. Bacterial deposition in each experiment was determined from quantitative culture of the left lung from sentinel mice sacrificed immediately after infection. Animals were examined daily for illness or death. Ill animals that had ruffled fur, eye crusting, hunched posture, and lack of resistance to handling were euthanized.
2.4. Quantification of bacteria in animal tissues
At specific time points after infection mice were euthanized; the left lung, median hepatic lobe, and spleen each were homogenized in 1 ml sterile PBS and serial dilutions plated on tryptic soy agar with 0.1% L-cysteine. Colonies were counted after 3-5 days of incubation at 37 °C in humid air with 5% CO2.
2.5. Characterization of antibody response
To prepare antigen, 120 ml of 1 × 1011 CFU/ml F. novicida U112 in sterile PBS was lysed with 3 ml QIAamp ATL lysis buffer (Qiagen, Valencia, CA), diluted 10 fold, and 100 μL added to 96 well Nunc-Immuno Maxisorp plates (Nalge Nunc International, Rochester, NY) at 4 °C overnight. Plates were washed three times with wash buffer and blocked with 1% BSA in PBS with 0.05% sodium azide for two hours. After repeat washing, serially diluted serum from infected and uninfected animals was added to the plates for at least two hours. The initial dilution was 1:32 with twofold subsequent dilutions. After further washing, goat anti-mouse immunoglobulin conjugated to biotin or to HRP was added for two hours (polyvalent Ig, Zymed, South San Francisco, CA; IgG and IgG1, SouthernBiotech, Birmingham, AL; IgG2c, Bethyl Laboratories, Montgomery, TX). Streptavidin-HRP was added for 20 minutes for biotinylated antibody assays. Color development was obtained by adding substrate solution from a DuoSet ELISA kit (R&D Systems, Minneapolis, MN) and the reaction quenched with 1 M phosphoric acid. Plates were read at 405 nm using 570 nm for correction. A positive antibody titer was defined as the maximal dilution at which the OD of infected serum was greater than 0.05 and twice the OD of uninfected serum at a comparable dilution [14]. To confirm antibody specificity the procedure was repeated using 1 × 107 CFU/well heat killed Legionella pneumophila instead of lysed U112 as the capture antigen and goat anti-mouse polyvalent immunoglobulin conjugated to biotin for detection.
2.6. Characterization of splenocyte cytokine recall responses
Spleens from naïve or infected mice were placed in Dulbecco’s Modified Eagle’s Medium (DMEM) with FBS, penicillin, streptomycin, and glutamine (complete media); ruptured gently using a syringe plunger, and passed through a cell strainer. Ammonium chloride red cell lysis buffer was added and the splenocytes centrifuged for 5 minutes at 1200 rpm and resuspended in complete DMEM. Cells were added to a tissue culture plate at 1 × 106 cells/well and stimulated in triplicate with 1 × 108 CFU/well heat killed F. novicida U112; 50 ng/ml PMA and 2 μM ionomycin (positive control); or media (negative control). After 48 hours of incubation at 37 °C in humid air with 5% CO2 the cells were centrifuged and supernatants removed. IFN-γ and IL-4 were quantified in the supernatants by ELISA using DuoSet reagents (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
2.7. Statistical analyses
Combined data are reported as mean ± standard deviation. When data were normally distributed, comparisons between three or more groups were performed using one-way ANOVA, followed by Tukey’s post-test to identify significant differences between individual groups. When data did not follow a Gaussian distribution, comparisons between three or more groups were performed by log transformation and ANOVA with Tukey’s post-test. (GraphPad Prism 4.0, San Diego, CA). A p value ≤ 0.05 was considered significant.
3. Results
3.1. F. novicida ΔmglA replicates in the lungs after airborne infection
To determine whether mglA was required for replication and dissemination of F. novicida after aerosol challenge, lung, liver, and spleen were quantitatively cultured at serial time points after exposure of C57BL/6 mice to aerosolized U112 or ΔmglA (Fig. 1A). Regardless of deposition dose, U112 invariably replicated to 108 CFU/lung within 96 hours and disseminated in similar burdens to liver and spleen. In contrast, the ΔmglA mutant replicated to no higher than 106 CFU/lung in the first week after infection followed by progressive clearance of the organism over the subsequent two weeks (Fig. 1B). At deposition doses up to 104 CFU/lung, there was little or no dissemination of ΔmglA to liver or spleen. U112-infected mice inevitably suffered 100% mortality by 96 hours post-infection; ΔmglA-infected C57BL/6 mice survived at least eight weeks and showed no clinical signs of illness. A similar pattern of replication and clearance was identified in the lungs of ΔmglA-infected BALB/c mice. These animals were observed for 16 weeks without clinical evidence of illness.
Fig. 1.
U112 replicates aggressively in the lung within 72 hours whereas ΔmglA is initially replicative but contained after 7 days. A: 104 CFU/lung ΔmglA or U112 were deposited by aerosol in C57BL/6 mice. Animals were euthanized at 4, 24, and 72 or 96 hours post-infection. The left lung, median lobe of liver, and spleen were removed, homogenized, and quantitatively cultured. Data are displayed as mean ± SD. Similar dissemination and exponential replication after U112 infection was noted in four additional independent experiments, depositing 8, 102 (two experiments), or 103 CFU/lung. B: 104 (upper panel) or 103 (lower panel) CFU/lung ΔmglA were deposited by aerosol in C57BL/6 mice. Animals were euthanized at 7, 14, and (in the latter experiment) 21 days post-infection. The left lung was removed, homogenized, and quantitatively cultured. Bars indicate mean values.
3.2. Identification of host factors necessary for control of pneumonic ΔmglA infection
After observing that ΔmglA replicated in the lungs of mice for approximately seven days before containment was evident we sought to determine if a host immune response was involved in control of infection. MyD88-/- mice deficient in a key Toll-like receptor (TLR) signaling molecule, Rag2-/- mice lacking T and B cells, IFN-γ-/- mice, and wild type C57BL/6 controls were infected by aerosol with a deposition dose of 24 CFU/lung ΔmglA (Fig. 2). A low deposition dose was chosen to amplify differences in replication. At 7 and 14 days post-infection bacterial replication in the lungs of MyD88-/- mice was greater than in wild type mice. Increased pulmonary replication in IFN-γ-/- mice was also observed at 14 days compared to wild type controls. Notably, there was no heightened replication in lungs of Rag2-/- mice. Instead a trend towards enhanced clearance was identified. There were no significant differences in liver or spleen bacterial burdens at 7 or 14 days.
Fig. 2.
Early host defense against ΔmglA involves MyD88 and IFN-γ but is independent of T or B cells. 24 CFU/lung ΔmglA were deposited by aerosol in the lungs of wild type, MyD88-/-, IFN-γ-/-, and Rag2-/- C57BL/6 mice. Mice were euthanized at 7 and 14 days post-infection. The left lung, median lobe of liver, and spleen were removed, homogenized, and quantitatively cultured. Spleens of wild type mice were not cultured on day 14. Bars indicate mean values. The data shown are from one of two similar experiments with comparable deposition doses. A third experiment depositing 102 CFU/lung ΔmglA in wild type and Rag2-/- mice each treated with rabbit polyclonal Ig confirmed the enhanced clearance phenotype at 7 and 14 days in Rag2-/- mice. * indicates p<0.05, ** p<0.01, and *** p<0.001 between groups by ANOVA and Tukey’s post-test.
These findings prompted us to examine the course of infection over a longer duration in wild type and knockout mice. A modestly higher deposition dose was selected to maximize detection of differences late in infection. Therefore, 5 × 102 CFU/lung ΔmglA was deposited by aerosol in the lungs of MyD88-/-, Rag2-/-, IFN-γ-/-, and wild type C57BL/6 mice (Fig. 3). All mice remained clinically well and survived for four weeks, at which time they were euthanized. Organ cultures obtained four weeks following inoculation showed clearance of infection in wild type mice and in three of four MyD88-/- mice. However, persistent bacteria were detected in the lungs of three of four IFN-γ-/- and all Rag2-/- mice.
Fig. 3.
Clearance of pulmonary mglA infection requires T or B cells. 5 × 102 CFU/lung aerosolized ΔmglA was deposited in wild type, MyD88-/-, IFN-γ-/-, and Rag2-/- C57BL/6 mice. Animals were euthanized at four weeks post-infection. The left lung, median lobe of liver, and spleen were removed, homogenized, and quantitatively cultured. The data represent a single experiment. No significant differences were detected between groups by ANOVA and Tukey’s post-test.
3.3. ΔmglA-infected mice develop specific immune responses to F. novicida U112
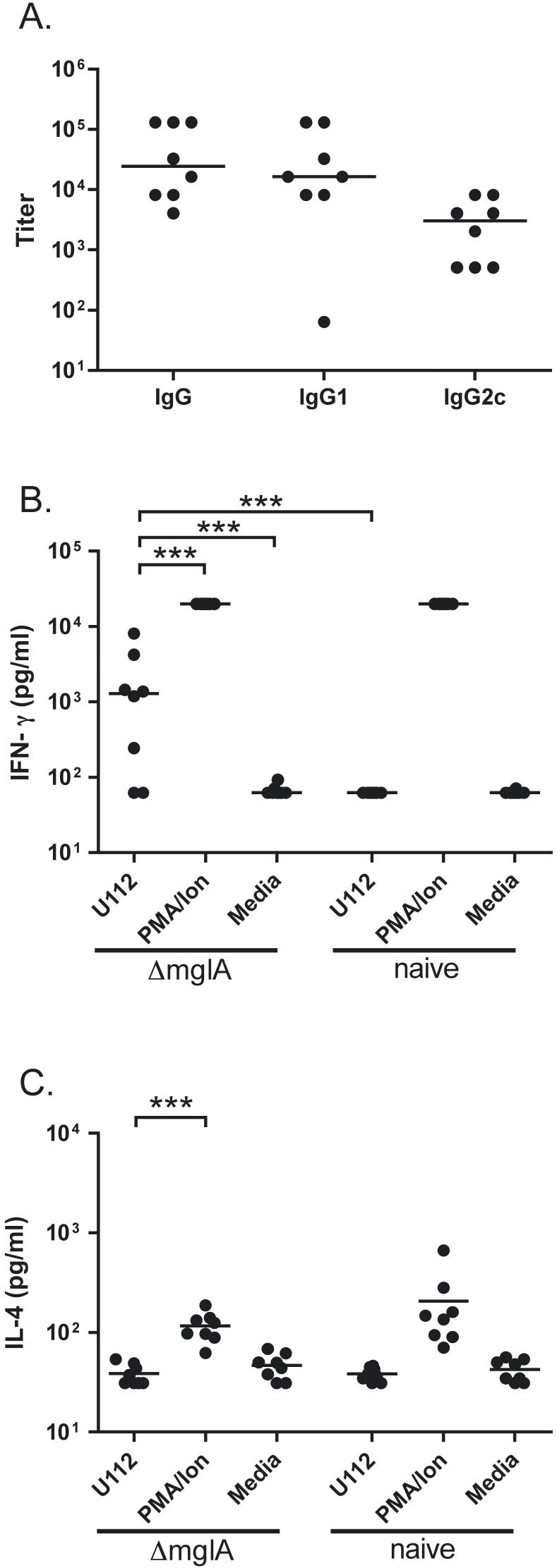
To determine whether infection with ΔmglA induced a specific antibody response, serum from infected animals was tested for antibodies to U112. In C57BL/6 mice infected by aerosol with 104 CFU/lung of ΔmglA, a pronounced IgG titer ranging from 1:4,096 to 1:131,072 was demonstrated on day 13 post-infection (Fig. 4A). The specificity of this response was confirmed by the absence of detectable antibody to heat killed Legionella pneumophila (not shown). The median IgG1 response was five fold greater than the median IgG2c titer. To measure T cell sensitization to ΔmglA infection, splenocytes were harvested from C57BL/6 mice infected 13 days previously with ΔmglA or from naïve mice. IFN-γ secretion in response to heat killed U112 was detected in supernatants of splenocytes from six of eight mice previously infected mice with ΔmglA versus zero of eight naïve mice (p<0.001) (Fig. 4B). IL-4 production by antigen-stimulated splenocytes was not detected in either group of mice (Fig. 4C).
Fig. 4.
F. novicida ΔmglA infection provokes antibody and splenocyte IFN-γ recall responses against U112. A: Serum from C57BL/6 mice 13 days post-aerosol deposition of 104 CFU/lung ΔmglA was assayed in a F. novicida U112 lysate ELISA using goat anti-mouse immunoglobulins conjugated to biotin or to HRP for detection. The data shown combine two independent aerosolization experiments each comprising four mice. B: IFN-γ production was measured in splenocytes harvested from mice 13 days following deposition of 105 CFU/lung ΔmglA or from naïve mice, then plated at 1 × 106 cells per well and stimulated with 1 × 108 CFU/well heat killed U112; 50 ng/ml PMA and 2 μM ionomycin; or media. The limits of detection of the assay were 62.5 pg/ml and 20,000 pg/ml. C: IL-4 production was measured in splenocytes harvested and stimulated in an identical fashion. The limits of detection of the assay were 31.25 pg/ml and 20,000 pg/ml. All cytokine measurements were performed in triplicate on samples from eight mice. Comparable IFN-γ measurements were obtained from two other independent experiments depositing 104 CFU/lung ΔmglA and comparing splenocyte recall responses between four infected and two naive mice. For all panels, bars indicate median values. For cytokine measurements, *** indicates p<0.001 between groups by log transformation and ANOVA with Tukey’s post-test. For clarity, only significant differences comparing U112-stimulated splenocytes from ΔmglA-infected mice are shown.
3.4. ΔmglA does not protect against subsequent F. novicida U112 infection
To determine whether the immune response induced by ΔmglA infection afforded any protection against infection with virulent U112, we conducted a series of experiments challenging mice with U112 at least one month after aerosol ΔmglA inoculation (Table 1). Both C57BL/6 and BALB/c mice were used in these experiments as BALB/c mice have been shown to be more responsive than C57BL/6 animals to immunization with F. tularensis LVS against type A F. tularensis [15, 16]. Despite escalating and serial deposition doses of ΔmglA and decreasing challenge doses of U112 there was no survival benefit in vaccinated animals. Furthermore, the lung burden of bacteria four days after infection with U112 did not differ between vaccinated and naïve mice (not shown).
Table 1.
Aerosolized ΔmglA does not protect against respiratory U112 infection
| Mouse strain | ΔmglA deposition dose (CFU/lung) | U112 challenge deposition dose (CFU/lung)a | Survival at 96 hoursd |
|---|---|---|---|
| C57BL/6 | 2 × 103 2 × 103 |
8 × 104 2 × 103 |
0/4 0/4 |
| BALB/c | 1 × 105 3 @ 1 × 105b |
6 × 102 35c |
0/4 0/4 |
Except where noted, U112 challenges were performed 6-8 weeks after ΔmglA infections.
Intervals between three successive ΔmglA infections were 8 and 4 weeks.
U112 challenge was performed 4 weeks after third ΔmglA infection.
Mice were observed for death or illness, and euthanized when moribund. Unvaccinated C57BL/6 mice challenged with 8 × 104 CFU/lung U112 and BALB/c mice challenged with 6 × 102 CFU/lung U112 had 100% mortality at 96 hours (n=4 per group).
4. Discussion
The major findings of this study are that F. novicida ΔmglA replicates within the lungs after airborne challenge of mice; that early control of this infection involves MyD88 and IFN-γ but does not require T or B cells; that subsequent bacterial clearance is lymphocyte-dependent; and that ΔmglA infection elicits specific immune responses but does not confer protection against wild type infection.
F. novicida is an attractive surrogate for highly virulent type A F. tularensis because it causes severe murine infection yet is generally non-pathogenic in humans [2, 6]. mglA exerts positive control over the Francisella Pathogenicity Island (FPI), including the expression of iglC, iglA, pdpA, and pdpD [7] and over other virulence genes [10]. Others have demonstrated that the ΔmglA mutant fails to escape the phagosome [11] and does not replicate in the J774 macrophage-like cell line, or peritoneal or bone-marrow-derived macrophages [7, 9, 10]. However, we found that F. novicida ΔmglA replicates in the lungs after aerosol challenge, before control of infection is established. The site of this in vivo replication is unknown. We have observed that the MH-S line of SV40-transformed murine alveolar macrophages does not permit intracellular replication in vitro (not shown), but parasitism of alveolar macrophages in vivo cannot be excluded. Alternatively, ΔmglA may replicate in dendritic or alveolar type II airway epithelial cells, both of which permit replication of F. tularensis LVS [17, 18]. That survival was not impaired in mice infected with ΔmglA, in contrast to the rapid lethality of U112, confirms the critical role of the mglA-regulated Francisella Pathogenicity Island in mediating virulence of the wild type organism.
We provide evidence that host immunity plays a role in controlling ΔmglA infection. TLRs 2, 4, and 9 recognize the bacterial components lipopeptide, lipopolysaccharide (LPS), and CpG-DNA, respectively, signal via the adaptor protein MyD88, and cause nuclear factor (NF)-κB activation and pro-inflammatory cytokine release [19]. Mice deficient in MyD88 are often highly susceptible to Gram-negative infections [13, 20] and MyD88 is essential in an intradermal model of LVS infection [21]. The present data show that early containment of aerosolized ΔmglA is partially dependent on MyD88, indicating that the mutant organism triggers an innate immune response. Rajaram et al. recently described heightened NF-κB activation and pro-inflammatory cytokine production in RAW 264.7 murine macrophages infected with ΔmglA compared with U112 that was dependent on activation of the serine/threonine kinase Akt [22]. Taken together, these findings suggest that the mglA-mediated pathogenicity of wild type F. novicida in the lung may be partly attributable to mitigated stimulation of the host innate immune system.
IFN-γ is critically important in regulating macrophage responses to intracellular pathogens [23] and it is known to be essential for control of LVS infection [24, 25]. Similarly, our data suggest that IFN-γ has an important role in containing ΔmglA replication within the first month of infection. In contrast, we identified differing roles for lymphocytes in the early and late phases of infection. T or B cells proved unnecessary for the early control of ΔmglA infection as Rag2-/- mice were at least as resistant as wild type mice at seven and 14 days after infection. However, four weeks after infection, wild type mice had no detectable bacteria in lung, liver, or spleen, whereas bacteria persisted in the lungs of Rag2-/- mice, indicating that T or B cells are required for eventual clearance of infection. It is known that NK cell-dependent IFN-γ production occurs in intranasal LVS infection [26] and others have noted an increase in the NK cell population in Rag2-/- mice on a BALB/c background with consequently increased IFN-γ production [27]. Extrapolating to C57BL/6 animals, an augmented NK cell response facilitating early bacterial containment but subsequently failing to clear the pathogen may conceivably account for our findings in Rag2-/- mice.
We also demonstrate clear evidence of the presence of an adaptive host immune response to the ΔmglA mutant. Initial replication is followed by progressive clearance of the organism after seven days in association with the development of specific antibody and cytokine recall responses. However, in contrast to intranasal infection with an iglC-deficient mutant of F. novicida [8], inhalation of F. novicida ΔmglA does not confer protection against wild type pulmonary infection. We have demonstrated that protection against inhaled U112 infection in this system is feasible by the administration of intranasal TLR4 agonists [31]. We speculate that the lack of protective immunity induced by ΔmglA infection may be explained by the variable magnitude of IFN-γ production by sensitized splenocytes. This may account for the lack of Th1 isotype switching manifested by an elevated IgG1:IgG2c ratio [28]. However, production of the Th2-type cytokine IL-4 by sensitized splenocytes was absent. Alternatively, the low level of IgG2 antibodies induced by ΔmglA infection may indicate insufficient opsonizing antibodies for Fc receptor mediated bacterial uptake, which others have shown promotes phagocytosis of F. novicida U112 [29]. IgG molecules are recognized by different Fcγ receptors, which trigger activating and inhibitory signaling pathways. IgG2 molecules in mice are pro-inflammatory and show greater activity than IgG1 [30]. Thus, the relatively low levels of IgG2c may reflect inadequate immune complex binding to activating Fcγ receptors and poor triggering of innate effector cells. It is possible that adjuvants could bolster the immune response to ΔmglA but this mutant may simply be too attenuated to serve as an effective live vaccine.
5. Acknowledgements
We thank Chris Wilson for supplying MyD88-/- mice, Ravi Iyer for technical assistance and Tina Guina for critical review of the manuscript. This work was supported by NIH awards 5T32HL07287 and 5U54AI057141.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].McLendon MK, Apicella MA, Allen LA. Francisella tularensis: taxonomy, genetics, and Immunopathogenesis of a potential agent of biowarfare. Annu Rev Microbiol. 2006;60:167–185. doi: 10.1146/annurev.micro.60.080805.142126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Titball RW, Johansson A, Forsman M. Will the enigma of Francisella tularensis virulence soon be solved? Trends Microbiol. 2003;11:118–123. doi: 10.1016/s0966-842x(03)00020-9. [DOI] [PubMed] [Google Scholar]
- [3].Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Layton M, Lillibridge SR, McDade JE, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Tonat K. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- [4].Santic M, Molmeret M, Abu Kwaik Y. Modulation of biogenesis of the Francisella tularensis subsp. novicida-containing phagosome in quiescent human macrophages and its maturation into a phagolysosome upon activation by IFN-γ. Cell Microbiol. 2005;7:957–967. doi: 10.1111/j.1462-5822.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- [5].McCaffrey RL, Allen LA. Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J Leukoc Biol. 2006;80:1224–1230. doi: 10.1189/jlb.0406287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hollis DG, Weaver RE, Steigerwalt AG, Wenger JD, Moss CW, Brenner DJ. Francisella philomiragia comb. nov. (formerly Yersinia philomiragia) and Francisella tularensis biogroup novicida (formerly Francisella novicida) associated with human disease. J Clin Microbiol. 1989;27:1601–1608. doi: 10.1128/jcm.27.7.1601-1608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lauriano CM, Barker JR, Yoon SS, Nano FE, Arulanandam BP, Hassett DJ, Klose KE. MglA regulates transcription of virulence factors necessary for Francisella tularensis intraamoebae and intramacrophage survival. Proc Natl Acad Sci U S A. 2004;101:4246–4249. doi: 10.1073/pnas.0307690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pammit MA, Raulie EK, Lauriano CM, Klose KE, Arulanandam BP. Intranasal vaccination with a defined attenuated Francisella novicida strain induces gamma interferon-dependent antibody-mediated protection against tularemia. Infect Immun. 2006;74:2063–2071. doi: 10.1128/IAI.74.4.2063-2071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Baron GS, Nano FE. MglA and MglB are required for the intramacrophage growth of Francisella novicida. Mol Microbiol. 1998;29:247–259. doi: 10.1046/j.1365-2958.1998.00926.x. [DOI] [PubMed] [Google Scholar]
- [10].Brotcke A, Weiss DS, Kim CC, Chain P, Malfatti S, Garcia E, Monack DM. Identification of MglA-regulated genes reveals novel virulence factors in Francisella tularensis. Infect Immun. 2006;74:6642–6655. doi: 10.1128/IAI.01250-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Santic M, Molmeret M, Klose KE, Jones S, Kwaik YA. The Francisella tularensis pathogenicity island protein IglC and its regulator MglA are essential for modulating phagosome biogenesis and subsequent bacterial escape into the cytoplasm. Cell Microbiol. 2005;7:969–979. doi: 10.1111/j.1462-5822.2005.00526.x. [DOI] [PubMed] [Google Scholar]
- [12].Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- [13].Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J Immunol. 2004;172:3377–3381. doi: 10.4049/jimmunol.172.6.3377. [DOI] [PubMed] [Google Scholar]
- [14].Rhinehart-Jones TR, Fortier AH, Elkins KL. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect Immun. 1994;62:3129–3137. doi: 10.1128/iai.62.8.3129-3137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen W, Shen H, Webb A, KuoLee R, Conlan JW. Tularemia in BALB/c and C57BL/6 mice vaccinated with Francisella tularensis LVS and challenged intradermally, or by aerosol with virulent isolates of the pathogen: protection varies depending on pathogen virulence, route of exposure, and host genetic background. Vaccine. 2003;21:3690–3700. doi: 10.1016/s0264-410x(03)00386-4. [DOI] [PubMed] [Google Scholar]
- [16].Wu TH, Hutt JA, Garrison KA, Berliba LS, Zhou Y, Lyons CR. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun. 2005;73:2644–2654. doi: 10.1128/IAI.73.5.2644-2654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bosio CM, Dow SW. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J Immunol. 2005;175:6792–6801. doi: 10.4049/jimmunol.175.10.6792. [DOI] [PubMed] [Google Scholar]
- [18].Hall JD, Craven RR, Fuller JR, Pickles RJ, Kawula TH. Francisella tularensis Replicates within Alveolar Type II Epithelial Cells In Vitro and In Vivo following Inhalation. Infect Immun. 2007;75:1034–1039. doi: 10.1128/IAI.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- [20].Hawn TR, Smith KD, Aderem A, Skerrett SJ. Myeloid differentiation primary response gene (88)- and toll-like receptor 2-deficient mice are susceptible to infection with aerosolized Legionella pneumophila. J Infect Dis. 2006;193:1693–1702. doi: 10.1086/504525. [DOI] [PubMed] [Google Scholar]
- [21].Collazo CM, Sher A, Meierovics AI, Elkins KL. Myeloid differentiation factor-88 (MyD88) is essential for control of primary in vivo Francisella tularensis LVS infection, but not for control of intra-macrophage bacterial replication. Microbes Infect. 2006;8:779–790. doi: 10.1016/j.micinf.2005.09.014. [DOI] [PubMed] [Google Scholar]
- [22].Rajaram MV, Ganesan LP, Parsa KV, Butchar JP, Gunn JS, Tridandapani S. Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J Immunol. 2006;177:6317–6324. doi: 10.4049/jimmunol.177.9.6317. [DOI] [PubMed] [Google Scholar]
- [23].Schroder K, Sweet MJ, Hume DA. Signal integration between IFN-γ and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–524. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- [24].Anthony LS, Ghadirian E, Nestel FP, Kongshavn PA. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb Pathog. 1989;7:421–428. doi: 10.1016/0882-4010(89)90022-3. [DOI] [PubMed] [Google Scholar]
- [25].Leiby DA, Fortier AH, Crawford RM, Schreiber RD, Nacy CA. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992;60:84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lopez MC, Duckett NS, Baron SD, Metzger DW. Early activation of NK cells after lung infection with the intracellular bacterium, Francisella tularensis LVS. Cell Immunol. 2004;232:75–85. doi: 10.1016/j.cellimm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- [27].Abou-Bacar A, Pfaff AW, Georges S, Letscher-Bru V, Filisetti D, Villard O, Antoni E, Klein JP, Candolfi E. Role of NK cells and gamma interferon in transplacental passage of Toxoplasma gondii in a mouse model of primary infection. Infect Immun. 2004;72:1397–1401. doi: 10.1128/IAI.72.3.1397-1401.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Finkelman FD, Holmes J, Katona IM, Urban JF, Jr., Beckmann MP, Park LS, chooley KA, Coffman RL, Mosmann TR, Paul WE. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- [29].Balagopal A, MacFarlane AS, Mohapatra N, Soni S, Gunn JS, Schlesinger LS. Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect Immun. 2006;74:5114–5125. doi: 10.1128/IAI.00795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- [31].Lembo A, Pelletier M, Iyer R, Timko M, Dudda JC, West TE, Wilson CB, Hajjar AM, Skerrett SJ. Administration of a synthetic TLR4 agonist protects mice from pneumonic tularemia. J, Immunol. 2008;180:7574–7581. doi: 10.4049/jimmunol.180.11.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]