Abstract
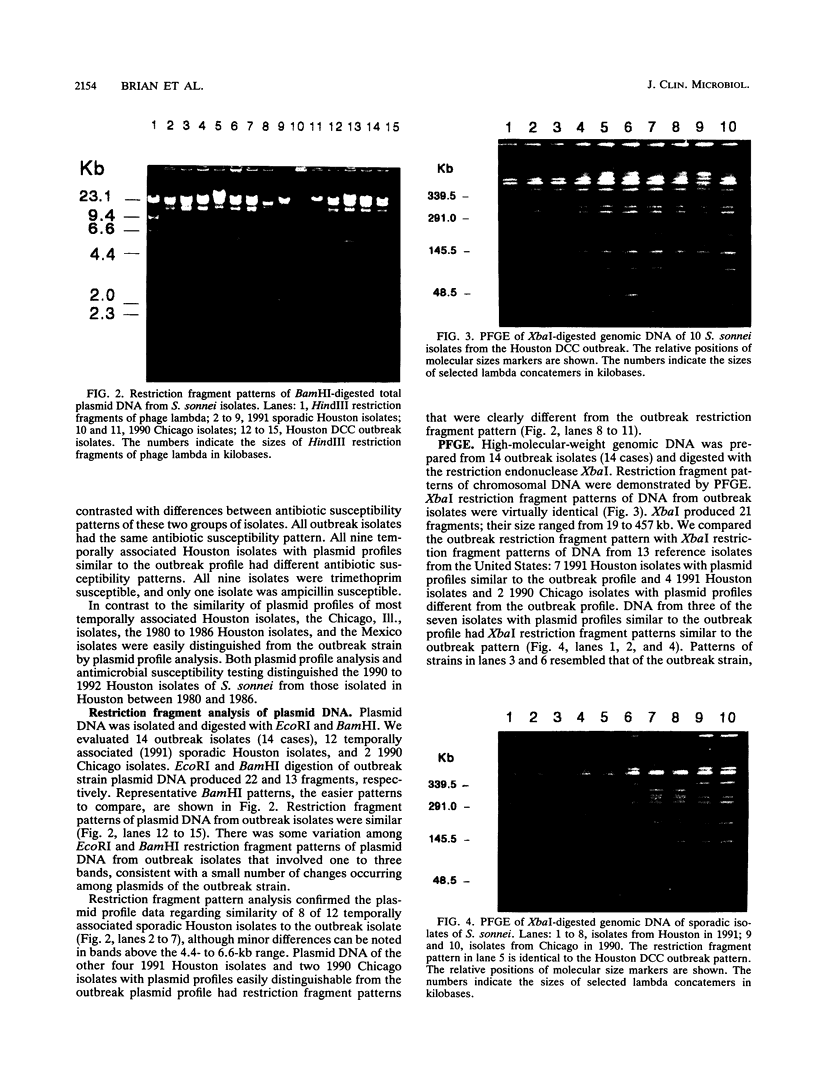
Outbreaks of diarrhea in child day-care centers (DCC) are common. This study was undertaken to evaluate the molecular epidemiology of an outbreak of diarrhea due to Shigella sonnei. This outbreak involved 25 of 52 (48%) DCC children and 14 of 132 (11%) teachers and household contacts. S. sonnei isolates from nine children and five contacts were characterized by antimicrobial susceptibility, plasmid content, plasmid DNA restriction fragment pattern, and pulsed-field gel electrophoresis (PFGE) of total genomic DNA; 33 isolates from Houston, Tex., Chicago, Ill., and Mexico City, Mexico, also were studied. All outbreak isolates were resistant to ampicillin and trimethoprim-sulfamethoxazole and shared five to six plasmids ranging from 3.3 to 70 MDa. A total of 8 of 12 temporally associated nonoutbreak Houston isolates had plasmid profiles and restriction fragment patterns similar to those of the outbreak strain, despite possessing different antibiotic susceptibility patterns. PFGE demonstrated identical DNA patterns among outbreak isolates and similar or identical patterns among temporally associated sporadic Houston isolates with plasmid profiles similar to that of the outbreak strain. All other nonoutbreak strains from Houston, Chicago, and Mexico had plasmid profiles, restriction fragment patterns, and PFGE patterns different from those of the outbreak strain. DCC outbreak isolates could be distinguished from most sporadic isolates by antimicrobial susceptibility testing, but plasmid analysis and PFGE could not differentiate common-source isolates from sporadic isolates in the same location during the same time period, indicating that isolates present in the community were genetically similar to those producing outbreaks in the DCC.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. J., Singh K. V., Murray B. E., Erlich J. Molecular epidemiology of Shigella infection in Central Australia. Epidemiol Infect. 1990 Aug;105(1):51–57. doi: 10.1017/s0950268800047634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordillo M. E., Reeve G. R., Pappas J., Mathewson J. J., DuPont H. L., Murray B. E. Molecular characterization of strains of enteroinvasive Escherichia coli O143, including isolates from a large outbreak in Houston, Texas. J Clin Microbiol. 1992 Apr;30(4):889–893. doi: 10.1128/jcm.30.4.889-893.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa-Ahumada M., Swaminathan B., Hunter S. B., Cameron D. N., Kiehlbauch J. A., Wachsmuth I. K., Strockbine N. A. Restriction fragment length polymorphisms in rRNA operons for subtyping Shigella sonnei. J Clin Microbiol. 1991 Nov;29(11):2380–2384. doi: 10.1128/jcm.29.11.2380-2384.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huovinen P. Trimethoprim resistance. Antimicrob Agents Chemother. 1987 Oct;31(10):1451–1456. doi: 10.1128/aac.31.10.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin C. M., Ryan K. J., Chipowsky S., Storm A., McCombie S. Molecular epidemiology of Shigella sonnei in Pima County, Arizona: evidence for a Mexico-related plasmid. J Infect Dis. 1990 Apr;161(4):797–800. doi: 10.1093/infdis/161.4.797. [DOI] [PubMed] [Google Scholar]
- Litwin C. M., Storm A. L., Chipowsky S., Ryan K. J. Molecular epidemiology of Shigella infections: plasmid profiles, serotype correlation, and restriction endonuclease analysis. J Clin Microbiol. 1991 Jan;29(1):104–108. doi: 10.1128/jcm.29.1.104-108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A. G., Singh K. V., Murray B. E. DNA fingerprinting of Enterococcus faecium by pulsed-field gel electrophoresis may be a useful epidemiologic tool. J Clin Microbiol. 1991 Dec;29(12):2752–2757. doi: 10.1128/jcm.29.12.2752-2757.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E. Resistance of Shigella, Salmonella, and other selected enteric pathogens to antimicrobial agents. Rev Infect Dis. 1986 May-Jun;8 (Suppl 2):S172–S181. doi: 10.1093/clinids/8.supplement_2.s172. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990 Sep;28(9):2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering L. K., Bartlett A. V., Woodward W. E. Acute infectious diarrhea among children in day care: epidemiology and control. Rev Infect Dis. 1986 Jul-Aug;8(4):539–547. doi: 10.1093/clinids/8.4.539. [DOI] [PubMed] [Google Scholar]
- Prado D., Murray B. E., Cleary T. G., Pickering L. K. Limitations of using the plasmid pattern as an epidemiological tool for clinical isolates of Shigella sonnei. J Infect Dis. 1987 Feb;155(2):314–316. doi: 10.1093/infdis/155.2.314. [DOI] [PubMed] [Google Scholar]
- Soldati L., Piffaretti J. C. Molecular typing of Shigella strains using pulsed field gel electrophoresis and genome hybridization with insertion sequences. Res Microbiol. 1991 Jun;142(5):489–498. doi: 10.1016/0923-2508(91)90182-a. [DOI] [PubMed] [Google Scholar]
- Staat M. A., Morrow A. L., Reves R. R., Bartlett A. V., Pickering L. K. Diarrhea in children newly enrolled in day-care centers in Houston. Pediatr Infect Dis J. 1991 Apr;10(4):282–286. doi: 10.1097/00006454-199104000-00003. [DOI] [PubMed] [Google Scholar]
- Tacket C. O., Cohen M. L. Shigellosis in day care centers: use of plasmid analysis to assess control measures. Pediatr Infect Dis. 1983 Mar-Apr;2(2):127–130. [PubMed] [Google Scholar]
- Tauxe R. V., Johnson K. E., Boase J. C., Helgerson S. D., Blake P. A. Control of day care shigellosis: a trial of convalescent day care in isolation. Am J Public Health. 1986 Jun;76(6):627–630. doi: 10.2105/ajph.76.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauxe R. V., Puhr N. D., Wells J. G., Hargrett-Bean N., Blake P. A. Antimicrobial resistance of Shigella isolates in the USA: the importance of international travelers. J Infect Dis. 1990 Nov;162(5):1107–1111. doi: 10.1093/infdis/162.5.1107. [DOI] [PubMed] [Google Scholar]
- Weissman J. B., Schmerler A., Weiler P., Filice G., Godbey N., Hansen I. The role of preschool children and day-care centers in the spread of shigellosis in urban communities. J Pediatr. 1974 Jun;84(6):797–802. doi: 10.1016/s0022-3476(74)80750-x. [DOI] [PubMed] [Google Scholar]
- Yagupsky P., Loeffelholz M., Bell K., Menegus M. A. Use of multiple markers for investigation of an epidemic of Shigella sonnei infections in Monroe County, New York. J Clin Microbiol. 1991 Dec;29(12):2850–2855. doi: 10.1128/jcm.29.12.2850-2855.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]