Abstract
Background
Existing studies on the relationships between impairments and activities of daily living (ADLs) in nursing home residents have serious limitations. This study examines the relationships among admission impairments, including pain, depression, incontinence, balance, and falls, and follow-up ADLs, as well as the effect of the nursing home on follow-up ADLs of extended-stay nursing home residents.
Methods
This longitudinal cohort study consisted of 4,942 extended-stay residents who were admitted into 377 Minnesota nursing homes during 2004. General linear mixed models were used for all analyses, with 14 resident-level and 8 facility-level control variables.
Results
Incontinence and balance function at admission were significantly associated with increases in ADL dependence at follow-up. Individual nursing homes had independent effects on all three ADL models. Similar findings were found after facility-level control variables were added.
Conclusions
Incontinence predicts subsequent ADL functional levels. The relationship between balance dysfunction and subsequent ADL dependence could be causal. Future studies of the causal relationships between impairments and ADL should examine the effectiveness of impairment interventions on ADL as well as these relationships in different subgroups of nursing home residents.
Keywords: Nursing homes, Activities of daily living, Impairments, Incontinence, Falls
PAIN, depression, bowel and bladder incontinence, balance dysfunction, and falls are prevalent among nursing home residents, but their impact on activity of daily living (ADL) dependence is not well established (1–9). To date, studies that have examined the relationships between these impairments and ADL dependencies have serious limitations (10–20), because (i) most studies were conducted in community-dwelling populations, (ii) few specifically examined which factors predict individual ADL dependence (21), (iii) some failed to include important confounding variables that may simultaneously affect the predictor and outcome variables (eg, balance function and pain) (16, 18, 19), and (iv) none have accounted for the clustering of residents within a facility or have included a random nursing home effect to determine whether living in a particular facility will affect residents’ ADL dependence. Failure to account for this correlated data structure may have produced inefficient coefficient estimates in previous studies; that is, it is more likely to commit a Type II error where the false null hypothesis was not rejected.
This study addresses these limitations by examining which resident-level impairments at admission—pain, depression, bowel and bladder incontinence, balance dysfunction, and falls—predict 6-month follow-up ADL dependence and whether there is an independent nursing home effect on these individual ADLs at 6-month follow-up.
METHODS
Study Design and Data Sources
Data for this longitudinal cohort study of extended-stay nursing home residents in Minnesota were assembled from resident-level variables derived from the 2004 Minimum Data Set (MDS), nursing home characteristics from 2004 Minnesota state administrative data systems, and staffing levels from the 2004 Minnesota Department of Human Services Annual Facility Survey. The Institutional Review Board at the University of Minnesota approved this study.
Study Sample
Inclusion criteria required that the resident was aged 65 years or older at admission; admitted to a Minnesota nursing home in 2004; administered a MDS admission assessment and a follow-up assessment in the same facility approximately 6 months after the admission assessment; and not comatose, bedridden, quadriplegic, or on a feeding tube at baseline.
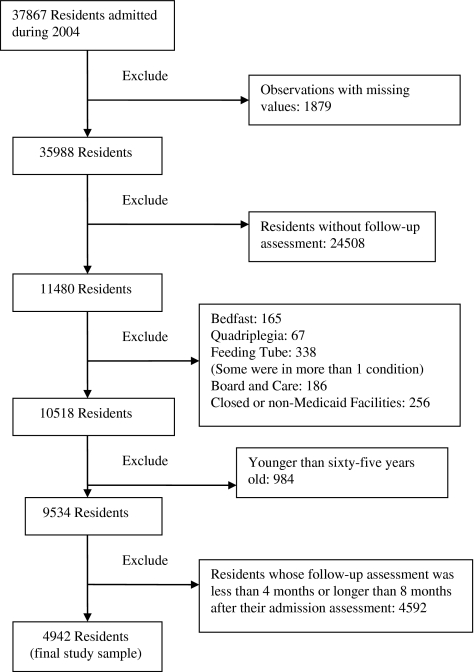
Figure 1 illustrates the participant selection process, which excluded 24,508 residents without follow-up assessments. Compared with the remaining 11,480 residents, the excluded residents were somewhat younger (mean age 77.8 vs 80.2, p < .001) and considerably more likely to have been admitted from an acute care hospital (87.9% vs 61.7%, p < .001). The excluded group also had a much lower proportion of cognitively impaired residents (40.9% vs 67.0%, p < .001) and had fewer residents with bowel and bladder incontinence, although they were likely to have more frequent and intense pain. Because of a quarterly MDS assessment requirement and a mandatory evaluation whenever a resident had a significant change in status, 4,592 residents were excluded because their length of follow-up was shorter than 4 months or longer than 8 months. Their demographics showed no significant difference from the final sample (N= 4,942), except that the final sample had a higher percentage of cognitively impaired residents (72.9% vs 64.2%, p < .001) and a lower proportion of residents with pain (52.5% vs 63.4%, p < .001). The final analytical file contains 4,942 residents with a length of follow-up between 4 and 8 months in 377 Minnesota nursing homes.
Figure 1.
Participant selection flow diagram.
Outcome Variables
An ordered loss among ADLs has been found in nursing home residents (22, 23):
Early-loss ADLs: dressing and personal hygiene.
Middle-loss ADLs: toileting, transfer, and locomotion.
Late-loss ADLs: bed mobility and eating.
We used personal hygiene, toileting, and eating as indicators of early, middle, and late ADL loss. In the MDS, each ADL task is scored from 0 (independent) to 4 (totally dependent). Each task was examined separately in regression models using the same predictor variables to assess whether different impairments may predict the ADLs that are lost in various stages.
Independent Variables
Pain was measured by the MDS Pain Scale, with a score ranging from 0 (no pain) to 3 (daily severe pain) (24). Depression was measured by the existence of a depression diagnosis in the MDS record. Bowel and bladder incontinence, each rated in MDS from 0 (continent) to 4 (incontinent), were entered separately as independent variables. Standing balance and sitting balance items in MDS were used to develop an overall balance scale, with a score ranging from 0 (good standing and sitting balance) to 5 (worst standing and sitting balance). Two MDS fall items, “fell in past 30 days” and “fell in past 31 to 180 days,” were included as separate independent variables.
Resident-Level Control Variables
All three ADL models included 14 resident-level control variables: age, gender, ethnicity, education, vision, cognition, restraint use, number of comorbidities, being admitted from a hospital, Medicare-reimbursed admission to the nursing home, unstable resident conditions, number of medications taken, previous nursing home admission, and length of follow-up (to account for the differences among residents in the 4- to 8-month follow-up period).
Cognition was measured by MDS Cognition Scale, with a score of 0 to 10 (25–27). Because of low restraint use, the five types of restraints were grouped into two variables—bedrail restraint and non-bedrail restraint—and were entered separately as control variables. Both were scored from 0 (not used) to 2 (used daily).
A total comorbidity score was calculated by adding the number of chronic conditions a resident had among 10 chronic conditions: diabetes mellitus, arthritis, hip fracture, congestive heart failure, peripheral vascular disease, osteoporosis, pathological bone fracture, cerebrovascular accident, Parkinson’s disease, and chronic obstructive pulmonary disease. The comorbidity scores ranged from 0 to 10.
Facility-Level Control Variables
Eight facility-level control variables were included in the second-phase analyses: facility profit status (profit, nonprofit, or public), location (Twin Cities metro, other metro, rural), facility size (total number of beds), hospital affiliation, licensed staffing levels (registered nurses and licensed practical nurses), unlicensed staffing levels (certified nursing assistants and medicine assistants), percentage of Medicare days, and nursing home community discharge rates. Percentage of Medicare days was calculated by dividing the number of Medicare-paid resident days per year by the number of resident days per year paid by all payment sources. Staffing levels were calculated by dividing the number of staffing hours per day by the total number of residents per day. The community discharge rate of each facility was calculated by dividing the number of residents who were discharged into community settings within the first 4 months by the number from the original cohort admitted into that facility (n = 37,867).
Statistical Analysis
All statistical analyses were conducted using SAS, Version 9.1 (SAS Institute, Inc., Cary, NC). The significance levels were set at .05. We used general linear mixed models (GLMMs) to conduct multivariate analyses and included a random nursing home effect to take into account the cluster-correlated data structure in the sample and produce more efficient fixed-effect estimates. Nursing home random effects also represented the combination of any unmeasured facility-level control variables that were not included in the model and allowed us to examine whether living in a particular nursing home affects a resident’s follow-up ADL.
The nursing home random effect was tested using likelihood ratio test statistics, calculated by subtracting the negative log likelihood of the reduced model (without nursing home effects) from the negative log likelihood of the full model (with nursing home effects). The resulting test statistic, the negative likelihood ratio, followed a mixture of chi-square (0) and chi-square (1) distributions (28). In this study, the likelihood ratio test statistics were compared with the critical levels of a chi-square (1) distribution, thus providing conservative p value estimates. Model details for nursing home random effects are shown in Appendix 1. Two series of analyses were conducted: In Series 1, baseline ADL, 7 resident-level independent variables, and 14 resident-level control variables were used as predictors; in Series 2, eight additional facility-level control variables were added to the Series 1 models.
RESULTS
Descriptive Statistics
The characteristics of the 377 nursing homes where the 4,942 participants resided are shown in Table 1, and Tables 2 and 3 show the demographics, impairment levels, and functional status of the study participants. The correlations among various predictor variables were generally low (tables not shown); thus, multicollinearity, a situation where there are high correlations between predictor variables, is not a concern in this study.
Table 1.
Characteristics of Minnesota Nursing Homes’ Sample in 2004 (n = 377)
| Characteristics | Number of Facilities | Percentage |
| Ownership | ||
| Government | 54 | 14.32 |
| For profit | 98 | 25.99 |
| Nonprofit | 225 | 59.68 |
| Hospital affiliation | ||
| Hospital based | 65 | 17.24 |
| Freestanding | 312 | 82.76 |
| Location | ||
| Twin Cities area | 116 | 30.77 |
| Other metro area | 51 | 13.53 |
| Rural | 210 | 55.70 |
| Characteristics | Mean (SD) | Range |
| Total bed size | 96.74 (57.36) | 24–559 |
| Number of participants per facility | 13.11 (9.48) | 1–88 |
| Licensed staffing level (hours per resident day) | 1.00 (0.23) | 0.37–2.06 |
| Unlicensed staffing level (hours per resident day) | 2.22 (0.34) | 0.43–3.83 |
| Percentage of Medicare days | 9.22% (4.71%) | 0.63%–34.00% |
| Community discharge rates | 38.38% (13.31%) | 0%–71.47% |
| Total ADL change score | −0.48 (2.39) | −9 to 7 |
Note: ADL = activity of daily living.
Table 2.
Basic Characteristics of Minnesota Nursing Home Residents Sample (N = 4,942)
| Characteristics | Mean (SD) or n (%) | Range |
| Age | 84.3 (7.6) | 65–106 |
| Gender | ||
| Male | 1,517 (30.7%) | |
| Female | 3,425 (69.3%) | |
| Race | ||
| White | 4,819 (97.5%) | |
| Non-White | 123 (2.5%) | |
| Education | ||
| No schooling | 59 (1.2%) | |
| 8th grade or less | 1,244 (25.2%) | |
| 9th–11th grade | 456 (9.2%) | |
| High school | 1,757 (35.6%) | |
| Technical or trade school | 365 (7.4%) | |
| Some college | 579 (11.7%) | |
| Bachelor’s degree | 350 (7.1%) | |
| Graduate degree | 132 (2.7%) | |
| Admission sources | ||
| Community (home, board and care facility, assistive living, and group home | 1,501(30.4%) | |
| Nursing homes | 668 (13.5%) | |
| Hospitals | 2,711 (54.5%) | |
| Other | 62 (1.3%) | |
| Length of follow-up* (days) | 172.8 (15.4) | 110–219 |
| Cognition (MDS Cognition Scale) | ||
| Intact to mild impairment | 1,342 (27.2%) | |
| Mild to moderate impairment | 1,790 (36.2%) | |
| Moderate to severe impairment | 1,631 (33.0%) | |
| Severe to very severe impairment | 179 (3.6%) | |
| Vision | ||
| Adequate | 3,382 (68.4%) | |
| Impaired | 935 (18.9%) | |
| Moderately impaired | 361 (7.3%) | |
| Highly impaired | 196 (4.0%) | |
| Severely impaired | 68 (1.4%) | |
| Number of comorbidities | 1.4 (1.1) | 0–7 |
| Number of medications | 9.4 (4.3) | 0–32 |
| Pain (MDS Pain Scale) | ||
| No pain | 2,346 (47.5%) | |
| Less than daily pain | 1,321 (26.7%) | |
| Mild/moderate daily pain | 1,080 (21.9%) | |
| Severe daily pain | 195 (4.0%) | |
| Balance dysfunction score | ||
| 0 | 245 (5.0%) | |
| 1 | 892 (18.1%) | |
| 2 | 2,427 (49.1%) | |
| 3 | 134 (2.7%) | |
| 4 | 870 (17.6%) | |
| 5 | 374 (7.6%) | |
| Depression | 1,683 (34.1%) | |
| Bowel incontinence | ||
| Continent | 3,293 (66.6%) | |
| Usually continent | 427 (8.6%) | |
| Occasionally incontinent | 342 (6.9%) | |
| Frequently incontinent | 453 (9.2%) | |
| Incontinent | 427 (8.6%) | |
| Bladder incontinence | ||
| Continent | 2,049 (41.5%) | |
| Usually continent | 444 (9.0%) | |
| Occasionally incontinent | 673 (13.6%) | |
| Frequently incontinent | 1,189 (24.1%) | |
| Incontinent | 587 (11.9%) | |
| Fall | ||
| In past 30 d | 2,005 (40.6%) | |
| In past 31–180 d | 662 (13.4%) | |
| Restraint use | ||
| Bedrail | ||
| Not used at all | 4,184 (84.7%) | |
| Used | 758 (15.3%) | |
| Non-bedrail | ||
| Not used at all | 4,815 (97.4%) | |
| Used | 127 (2.6%) | |
Notes: MDS = Minimum Data Set.
From admission assessment to follow-up assessment.
Table 3.
ADL Scores of Minnesota Nursing Home Residents at Admission and Follow-up Assessment (N = 4,942)
| Number (%) |
||
| Baseline | Follow-up | |
| Total ADL score | ||
| Totally independent | 286 (5.8) | 480 (9.7) |
| Totally dependent | 82 (1.7) | 128 (2.6) |
| Personal hygiene | ||
| Independent | 581 (11.8) | 767 (15.5) |
| Supervision | 502 (10.2) | 361 (7.3) |
| Limited assistance | 973 (19.7) | 796 (16.1) |
| Extensive assistance | 2,312 (46.8) | 2,287 (46.3) |
| Total dependence | 574 (11.6) | 731 (14.8) |
| Chi-square test* | Chi-square test statistic = 85.4 (p < .001) | |
| Toilet use | ||
| Independent | 766 (15.5) | 1,075 (21.8) |
| Supervision | 306 (6.2) | 204 (4.1) |
| Limited assistance | 844 (17.1) | 695 (14.1) |
| Extensive assistance | 2,462 (49.8) | 2,297 (46.5) |
| Total dependence | 564 (11.4) | 671 (13.6) |
| Chi-square test* | Chi-square test statistic = 101.7 (p < .001) | |
| Eating | ||
| Independent | 3,021 (61.1) | 2,864 (58.0) |
| Supervision | 1,033 (20.9) | 956 (19.3) |
| Limited assistance | 348 (7.0) | 413 (8.4) |
| Extensive assistance | 375 (7.6) | 463 (9.4) |
| Total dependence | 165 (3.3) | 246 (5.0) |
| Chi-square test* | Chi-square test statistic = 37.9 (p < .001) | |
Notes: ADL = activity of daily living.
Chi-square tests, two-tailed tests.
Effects of Impairments
Table 4 shows the GLMMs coefficients for the three ADL models. Bladder incontinence was associated with ADL declines in all three models, whereas bowel continence and balance dysfunction predicted worse toileting and personal hygiene. Pain, depression, and falls within the past month were not associated with any ADL decline. Follow-up hygiene dependence, an early-loss ADL, was predicted by bowel and bladder incontinence, balance dysfunction, and falls within 2–6 months. Toileting, a middle-loss ADL, was predicted by bowel and bladder incontinence and balance dysfunction. Eating, a late-loss ADL, was predicted only by bladder incontinence. These patterns were not changed by the addition of facility-level control variables into the models (Table 5). Cognition, admission from a hospital, and length of follow-up were significantly associated with all three ADL outcomes, but sociodemographic factors, including age, gender, race, and educational level, were not consistently associated with the outcomes. Few facility-level characteristics were significantly associated with ADL dependence at follow-up, and none were associated consistently across the outcomes (tables not shown).
Table 4.
GLMMs With Resident-Level Independent Variables and Control Variables*
| Hygiene | F Test | Toileting | F Test | Eating | F Test | |
| Baseline | .501 (0.015) | p < .001 | .520 (0.016) | p < .001 | .422 (0.017) | p < .001 |
| Pain = 0 | .113 (0.075) | p = .222 | .030 (0.080) | p = .544 | .042 (0.076) | p = .792 |
| Pain = 1 | .053 (0.075) | −.018 (0.080) | .016 (0.076) | |||
| Pain = 2 | .075 (0.075) | −.016 (0.081) | .010 (0.076) | |||
| Pain = 3 | 0 | 0 | 0 | |||
| Depression | .010 (0.030) | p = .742 | −.002 (0.032) | p = .954 | −.014 (0.031) | p = .649 |
| Bowel incontinence = 0 | −.112 (0.071) | p = .026 | −.143 (0.076) | p = .012 | −.202 (0.072) | p = .052 |
| Bowel incontinence = 1 | −.080 (0.081) | −.092 (0.087) | −.153 (0.082) | |||
| Bowel incontinence = 2 | −.002 (0.083) | −.063 (0.089) | −.108 (0.084) | |||
| Bowel incontinence = 3 | .045 (0.076) | .048 (0.081) | −.127 (0.077) | |||
| Bowel incontinence = 4 | 0 | 0 | 0 | |||
| Bladder incontinence = 0 | −.346 (0.062) | p < .001 | −.431 (0.067) | p < .001 | −.125 (0.063) | p = .013 |
| Bladder incontinence = 1 | −.319 (0.074) | −.277 (0.079) | −.116 (0.075) | |||
| Bladder incontinence = 2 | −.106 (0.068) | −.146 (0.073) | −.066 (0.069) | |||
| Bladder incontinence = 3 | −.076 (0.062) | −.090 (0.066) | .005 (0.062) | |||
| Bladder incontinence = 4 | 0 | 0 | 0 | |||
| Balance score = 0 | −.265 (0.086) | p = .002 | −.399 (0.092) | p < .001 | −.059 (0.086) | p = .220 |
| Balance score = 1 | −.236 (0.064) | −.379 (0.069) | −.046 (0.066) | |||
| Balance score = 2 | −.187 (0.058) | −.268 (0.062) | −.080 (0.059) | |||
| Balance score = 3 | −.195 (0.099) | −.237 (0.106) | −.030 (0.101) | |||
| Balance score = 4 | −.093 (0.060) | −.098 (0.065) | .024 (0.062) | |||
| Balance score = 5 | 0 | 0 | 0 | |||
| Fall within 30 d | .021 (0.030) | p = .477 | .016 (0.032) | p = .625 | −.017 (0.030) | p = .574 |
| Fall within 31–180 d | .097 (0.041) | p = .018 | .061 (0.044) | p = .167 | −.009 (0.041) | p = .825 |
Notes: All fixed effects were estimated with nursing home random intercept included in the models. Coefficients for resident-level control variables are not displayed. ADL = activity of daily living; GLMM = general linear mixed models.
Data are GLMM coefficient and its standard error. The sign indicates the direction of the effect. A negative sign indicates an ADL decline.
Values in bold denote significant findings.
Table 5.
GLMMs With Resident-Level Independent Variables, Control Variables, and Facility Factors*
| Hygiene | F Test | Toileting | F Test | Eating | F Test | |
| Baseline | .500 (0.015) | p < .001 | .518 (0.016) | p < .001 | .419 (0.017) | p < .001 |
| Pain = 0 | .115 (0.075) | p = .226 | .030 (0.080) | p = .555 | .047 (0.076) | P = .806 |
| Pain = 1 | .057 (0.075) | −.016 (0.080) | .024 (0.076) | |||
| Pain = 2 | .079 (0.075) | −.016 (0.081) | .015 (0.076) | |||
| Pain = 3 | 0 | 0 | 0 | |||
| Depression | .008 (0.030) | p = .779 | −.002 (0.032) | p = .947 | −.012 (0.031) | p = .697 |
| Bowel incontinence = 0 | −.112 (0.071) | p = .031 | −.141 (0.077) | p = .014 | −.196 (0.073) | p = .058 |
| Bowel incontinence = 1 | −.079 (0.081) | −.094 (0.087) | −.149 (0.082) | |||
| Bowel incontinence = 2 | −.004 (0.083) | −.061 (0.089) | −.096 (0.084) | |||
| Bowel incontinence = 3 | .041 (0.076) | .049 (0.082) | −.121 (0.077) | |||
| Bowel incontinence = 4 | 0 | 0 | 0 | |||
| Bladder incontinence = 0 | −.347 (0.062) | p < .001 | −.430 (0.067) | p < .001 | −.124 (0.063) | p = .015 |
| Bladder incontinence = 1 | −.318 (0.074) | −.271 (0.079) | −.116 (0.075) | |||
| Bladder incontinence = 2 | −.104 (0.068) | −.143 (0.073) | −.068 (0.069) | |||
| Bladder incontinence = 3 | −.074 (0.062) | −.084 (0.066) | .004 (0.062) | |||
| Bladder incontinence = 4 | 0 | 0 | 0 | |||
| Balance score = 0 | −.263 (0.086) | p = .002 | −.403 (0.092) | p < .001 | −.059 (0.086) | p = .230 |
| Balance score = 1 | −.237 (0.064) | −.380 (0.069) | −.046 (0.066) | |||
| Balance score = 2 | −.188 (0.058) | −.269 (0.062) | −.077 (0.059) | |||
| Balance score = 3 | −.194 (0.099) | −.235 (0.106) | −.030 (0.101) | |||
| Balance score = 4 | −.092 (0.060) | −.097 (0.065) | .027 (0.062) | |||
| Balance score = 5 | 0 | 0 | 0 | |||
| Fall within 30 d | .019 (0.030) | p = .524 | .012 (0.032) | p = .698 | −.018 (0.030) | p = .551 |
| Fall within 31–180 d | .094 (0.041) | p = .021 | .061 (0.044) | p = .168 | −.008 (0.041) | p = .846 |
Notes: All fixed effects were estimated with nursing home random intercept included in the models. Coefficients for resident- and facility-level control variables are not displayed. ADL = activity of daily living; GLMM = general linear mixed models.
Data are GLMM coefficient and its standard error. The sign indicates the direction of the effect. A negative sign indicates an ADL decline.
Values in bold denote significant findings.
Individual Effect of Nursing Homes
Table 6 shows the results of individual nursing home effects. The large magnitude of the likelihood ratio test statistic (T) does not represent the size of individual nursing home effects but is associated with very small p values. The statistically significant likelihood ratio tests for all three ADL equations indicated that living in a particular nursing home predicted a resident’s subsequent ADL dependence, independent of their impairments, even after controlling for specific facility characteristics.
Table 6.
Tests for Nursing Home Random Effect (N = 4,942)
| Series 1 | Series 2 | |
| Personal hygiene | ||
| Reduced model | 13,819.2 | 13,870.1 |
| Full model | 13,758.0 | 13,814.0 |
| Likelihood ratio | T* = 61.2 | T* = 56.1 |
| p value < .001 | p value < .001 | |
| Toileting | ||
| Reduced model | 14,455.9 | 14,518.3 |
| Full model | 14,437.3 | 14,499.3 |
| Likelihood ratio | T* = 18.6 | T* = 19.0 |
| p value < .001 | p value < .001 | |
| Eating | ||
| Reduced model | 13,896.9 | 13,951.8 |
| Full model | 13,884.5 | 13,940.4 |
| Likelihood ratio | T* = 12.4 | T* = 11.4 |
| p value < .001 | p value < .005 | |
Notes: Full model: with nursing home random effect. Reduced model: without nursing home random effect.
T = (negative log likelihood of reduced model) − (negative log likelihood of full model).
Examination of Floor and Ceiling Effects
The proportion of residents who, at baseline, were completely independent (ceiling) or completely dependent (floor) in eating (64.4%), toileting (26.9%), or personal hygiene (23.4%) can lead to challenges with model interpretation (floor and ceiling effects). Analyses were repeated after excluding residents who were completely independent or dependent in toileting and personal hygiene at baseline. After removing these residents, significant individual nursing home effects remained in both models. The effect sizes of the relationships between impairments and follow-up ADL remained similar; however, bowel incontinence became a nonsignificant predictor of subsequent ADL dependence, possibly because of reduced sample sizes. An analysis was also conducted excluding only those at the floor but leaving those at the ceiling in the models, with results very similar to the original findings (tables not shown). The floor or ceiling analysis was not conducted on eating function because more than 60% of residents were totally dependent in eating and excluding these residents would have greatly reduced the statistical power of the analysis.
DISCUSSION
This study found that bowel and bladder incontinence, along with balance dysfunction, were significant predictors of ADL decline at follow-up. Early-loss ADL was predicted by more impairments than was late-loss ADL. Contrary to previous studies, this study found that pain and depression were not associated with ADL decline at follow-up (13,20, 29–31). However, the relationships between incontinence and toileting function can be correlational, not causal. Our analyses showed that at baseline, residents who had more problems with incontinence had worse toileting function at follow-up. Still, many continent residents required extensive assistance with toileting, possibly for toilet transfer, commode set up, or catheter. In contrast to incontinence, balance dysfunction may directly impede a resident’s ability to complete personal hygiene and toileting independently and, thus, could be causally related to ADL decline at follow-up. To establish causal relationships between impairments and ADL in nursing home populations, future studies should examine the effectiveness of impairment interventions on ADL and assess whether these relationships are observed in different nursing home populations, such as residents with different levels of cognitive function.
In addition to impairment effects, significant individual nursing home effects were found for all three ADL measures. Most specific nursing home characteristics examined in this study did not significantly predict ADL decline at follow-up. Moreover, individual nursing home effects were still statistically significant after controlling for these facility-level factors. These results suggest that other important nursing home characteristics need to be identified and incorporated into assessments of quality and outcomes.
This study has limitations in its generalizability. The findings cannot be generalized to residents who were admitted for rehabilitation and who had a length of follow-up shorter than 4 months or longer than 8 months; to non-White nursing home populations because less than 3% of the sample is non-White; or beyond Minnesota. Future study should use a national sample of nursing home residents to assess whether our findings can be replicated, which would greatly improve the generalizability of these results.
The quality of MDS and its appropriateness for research use remain controversial, so this presents an additional limitation (32–34). The study also did not consider amount of rehabilitation as a control variable, and rehabilitation services that residents received during their stay in the facility may have affected their ADL decline at follow-up. However, we were uncertain whether MDS accurately reported the amount of rehabilitation residents received, so we did not control for this variable. Finally, because our participants were admitted throughout 2004, the staffing-level data obtained from the 2004 annual survey may not correspond exactly to the period between admission and follow-up for every participant.
GLMM assumes the dependent variables (ADLs) as continuous variables. Our analyses indicated that the residuals of all models were, in general, normally distributed, so they supported this underlying assumption of GLMMs. The alternative would be to use multinominal logistic regression with five-level dependent variables, but the interpretation of results would be cumbersome.
This study has several strengths. First, it examined the relationships between multiple important resident-level impairments and ADL decline at follow-up. It also controlled for many confounders that may simultaneously affect baseline impairments and ADL decline at follow-up. Finally, it is the first study of this type to incorporate a random nursing home effect to account for clustering of residents within facilities, which allowed us to determine whether unmeasured nursing home characteristics unique to each facility predict ADL decline at follow-up.
According to this study, incontinence and balance dysfunction significantly predict ADL declines at follow-up, so nursing homes can use continence and balance measures to identify residents who are at risk of ADL deterioration and implement rigorous rehabilitation protocols to improve, maintain, or at least delay the deterioration of ADL. However, a case-mix payment system, like the current nursing home prospective payment system, in which residents with higher ADL dependence are paid at higher rates, provides disincentives for nursing homes to treat residents’ ADL dysfunctions aggressively. A payment system that adjusts for the severity of ADL limitations but simultaneously rewards facilities for improving, maintaining, or delaying the deterioration of residents’ ADLs would create more desired incentives.
Although specific nursing home characteristics had very limited direct effects on ADL decline at follow-up, there was a significant nursing home effect after these facility-level factors were controlled for. The presence of such variations in nursing home effects provides support for an outcome-based nursing home payment system that may encourage nursing homes to improve their quality of care.
Acknowledgments
The authors thank Mark Woodhouse at Division of Health Policy and Management, University of Minnesota, for his assistance with data extraction and data management.
appendix 1
Statistical Model
Full model:
Reduced model:
where i = nursing homes; j = residents within each nursing home; Yij = follow-up ADLs of resident j in nursing home i; Xij1= the vector of resident-level covariates, Xij2 = the vector of facility-level covariates; βi = nursing home–specific random intercept for nursing home i; δij = random error term for resident j in nursing home i; 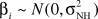 and
and 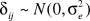 .
.
We test the following hypotheses: 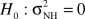 versus Ha:ONH>O.
versus Ha:ONH>O.
If 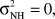 then βi ∼ N(0,0) = 0, then the random intercept model becomes a simple regression model.
then βi ∼ N(0,0) = 0, then the random intercept model becomes a simple regression model.
References
- 1.Ferrell BA, Ferrell BR, Osterweil D. Pain in the nursing home. J Am Geriatr Soc. 1990;38:409–414. doi: 10.1111/j.1532-5415.1990.tb03538.x. [DOI] [PubMed] [Google Scholar]
- 2.Sengstaken EA, King SA. The problems of pain and its detection among geriatric nursing home residents. J Am Geriatr Soc. 1993;41:541–544. doi: 10.1111/j.1532-5415.1993.tb01892.x. [DOI] [PubMed] [Google Scholar]
- 3.Jones RN, Marcantonio ER, Rabinowitz T. Prevalence and correlates of recognized depression in U.S. nursing homes. J Am Geriatr Soc. 2003;51:1404–1409. doi: 10.1046/j.1532-5415.2003.51458.x. [DOI] [PubMed] [Google Scholar]
- 4.Parmelee PA, Katz IR, Lawton MP. Depression among institutionalized aged: assessment and prevalence estimation. J Gerontol. 1989;44:M22–M29. doi: 10.1093/geronj/44.1.m22. [DOI] [PubMed] [Google Scholar]
- 5.Teresi J, Abrams R, Holmes D, Ramirez M, Eimicke J. Prevalence of depression and depression recognition in nursing homes. Soc Psychiatry Psychiatr Epidemiol. 2001;36:613–620. doi: 10.1007/s127-001-8202-7. [DOI] [PubMed] [Google Scholar]
- 6.Brandeis GH, Baumann MM, Hossain M, Morris JN, Resnick NM. The prevalence of potentially remediable urinary incontinence in frail older people: a study using the Minimum Data Set. J Am Geriatr Soc. 1997;45:179–184. doi: 10.1111/j.1532-5415.1997.tb04504.x. [Comment] [DOI] [PubMed] [Google Scholar]
- 7.Nelson R, Furner S, Jesudason V. Fecal incontinence in Wisconsin nursing homes: prevalence and associations. Dis Colon Rectum. 1998;41:1226–1229. doi: 10.1007/BF02258218. [DOI] [PubMed] [Google Scholar]
- 8.Ouslander JG, Kane RL, Abrass IB. Urinary incontinence in elderly nursing home patients. JAMA. 1982;248:1194–1198. [PubMed] [Google Scholar]
- 9.Rubenstein LZ, Josephson KR, Robbins AS. Falls in the nursing home. Ann Intern Med. 1994;121:442–451. doi: 10.7326/0003-4819-121-6-199409150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Stuck AE, Walthert JM, Nikolaus T, Bula CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48:445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 11.McCusker J, Kakuma R, Abrahamowicz M. Predictors of functional decline in hospitalized elderly patients: a systematic review. J Gerontol A Biol Sci Med Sci. 2002;57:M569–M577. doi: 10.1093/gerona/57.9.m569. [Comment] [DOI] [PubMed] [Google Scholar]
- 12.Mulrow CD, Gerety MB, Cornell JE, Lawrence VA, Kanten DN. The relationship between disease and function and perceived health in very frail elders. J Am Geriatr Soc. 1994;42:374–380. doi: 10.1111/j.1532-5415.1994.tb07484.x. [DOI] [PubMed] [Google Scholar]
- 13.Moseley CB. The impact of restraints on nursing home resident outcomes. Am J Med Qual. 1997;12:94–102. doi: 10.1177/0885713X9701200203. [DOI] [PubMed] [Google Scholar]
- 14.McConnell ES, Pieper CF, Sloane RJ, Branch LG. Effects of cognitive performance on change in physical function in long-stay nursing home residents. J Gerontol A Biol Sci Med Sci. 2002;57:M778–M784. doi: 10.1093/gerona/57.12.m778. [DOI] [PubMed] [Google Scholar]
- 15.Horowitz A. Vision impairment and functional disability among nursing home residents. Gerontologist. 1994;34:316–323. doi: 10.1093/geront/34.3.316. [DOI] [PubMed] [Google Scholar]
- 16.Gillen P, Spore D, Mor V, Freiberger W. Functional and residential status transitions among nursing home residents. J Gerontol A Biol Sci Med Sci. 1996;51:M29–M36. doi: 10.1093/gerona/51a.1.m29. [DOI] [PubMed] [Google Scholar]
- 17.Bean J, Kiely DK, Leveille SG, Morris J. Associating the onset of motor impairments with disability progression in nursing home residents. Am J Phys Med Rehabil. 2002;81:696, 704. doi: 10.1097/00002060-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Baigis J, Larson E, Haskey MY. Predictors of functional status in patients in a chronic-care facility. Clin Perform Qual Health Care. 1998;6:28–32. [PubMed] [Google Scholar]
- 19.Won A, Lapane K, Gambassi G, Bernabei R, Mor V, Lipsitz LA. Correlates and management of nonmalignant pain in the nursing home. SAGE Study Group. Systematic Assessment of Geriatric drug use via Epidemiology. J Am Geriatr Soc. 1999;47:936–942. doi: 10.1111/j.1532-5415.1999.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 20.Bean JF, Kiely DK, Cairns KD, Morris JN. Influence of poststroke urinary incontinence on disability: the nursing home setting. Am J Phys Med Rehabil. 2003;82:175–181. doi: 10.1097/01.PHM.0000052699.77091.21. [DOI] [PubMed] [Google Scholar]
- 21.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007;147:156–164. doi: 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- 22.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54:M546–M553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 23.Cohen-Mansfield J, Werner P, Reisberg B. Temporal order of cognitive and functional loss in a nursing home population. J Am Geriatr Soc. 1995;43:974–978. doi: 10.1111/j.1532-5415.1995.tb05560.x. [DOI] [PubMed] [Google Scholar]
- 24.Fries BE, Simon SE, Morris JN, Flodstrom C, Bookstein FL. Pain in U.S. nursing homes: validating a pain scale for the Minimum Data Set. Gerontologist. 2001;41:173–179. doi: 10.1093/geront/41.2.173. [Comment] [DOI] [PubMed] [Google Scholar]
- 25.Hartmaier SL, Sloane PD, Guess HA, Koch GG. The MDS Cognition Scale: a valid instrument for identifying and staging nursing home residents with dementia using the Minimum Data Set. J Am Geriatr Soc. 1994;42:1173–1179. doi: 10.1111/j.1532-5415.1994.tb06984.x. [Comment] [DOI] [PubMed] [Google Scholar]
- 26.Hartmaier SL, Sloane PD, Guess HA, Koch GG, Mitchell CM, Phillips CD. Validation of the Minimum Data Set Cognitive Performance Scale: agreement with the Mini-Mental State Examination. J Gerontol A Biol Sci Med Sci. 1995;50:M128–M133. doi: 10.1093/gerona/50a.2.m128. [DOI] [PubMed] [Google Scholar]
- 27.Gruber-Baldini AL, Zimmerman SI, Mortimore E, Magaziner J. The validity of the Minimum Data Set in measuring the cognitive impairment of persons admitted to nursing homes. J Am Geriatr Soc. 2000;48:1601–1606. doi: 10.1111/j.1532-5415.2000.tb03870.x. [Comment] [DOI] [PubMed] [Google Scholar]
- 28.Weiss ER. Modeling Longitudinal Data. New York, NY: Springer; 2005. [Google Scholar]
- 29.Mehta KM, Yaffe K, Covinsky KE. Cognitive impairment, depressive symptoms, and functional decline in older people. J Am Geriatr Soc. 2002;50:1045–1050. doi: 10.1046/j.1532-5415.2002.50259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Ronchi D, Bellini F, Berardi D, Serretti A, Ferrari B, Dalmonte E. Cognitive status, depressive symptoms, and health status as predictors of functional disability among elderly persons with low-to-moderate education: The Faenza Community Aging Study. Am J Geriatr Psychiatry. 2005;13:672–685. doi: 10.1176/appi.ajgp.13.8.672. [DOI] [PubMed] [Google Scholar]
- 31.Kaup BA, Loreck D, Gruber-Baldini AL, et al. Depression and its relationship to function and medical status, by dementia status, in nursing home admissions. Am J Geriatr Psychiatry. 2007;15:438–442. doi: 10.1097/JGP.0b013e31803c54f7. [DOI] [PubMed] [Google Scholar]
- 32.Hill-Westmoreland EE, Gruber-Baldini AL. Falls documentation in nursing homes: agreement between the Minimum Data Set and chart abstractions of medical and nursing documentation. J Am Geriatr Soc. 2005;53:268–273. doi: 10.1111/j.1532-5415.2005.53113.x. [DOI] [PubMed] [Google Scholar]
- 33.Crooks VC, Schnelle JF, Ouslander JP, McNees MP. Use of the Minimum Data Set to rate incontinence severity. J Am Geriatr Soc. 1995;43:1363–1369. doi: 10.1111/j.1532-5415.1995.tb06615.x. [DOI] [PubMed] [Google Scholar]
- 34.Lin WC, Lum TY, Mehr DR, Kane RL. Measuring pain presence and intensity in nursing home residents. J Am Med Dir Assoc. 2006;7:147–153. doi: 10.1016/j.jamda.2005.08.005. [DOI] [PubMed] [Google Scholar]