Abstract
Growth hormone (GH) signaling influences longevity in mice, with decreased GH signaling associated with longer life span and increased GH signaling with shortened life span. A proposed mechanism through which GH signaling influences life span postulates that decreased GH signaling lowers metabolic rate, thus slowing aging by decreasing production of damaging free radicals. The influence of altered GH signaling on metabolism was tested by monitoring oxygen consumption (VO2), respiratory quotient (RQ), and heat production in long-lived GH receptor knockout (GHRKO) and Ames dwarf mice, and short-lived bovine GH-overexpressing transgenic (bGH TG) mice. Intriguingly, both GHRKO and Ames dwarf mice have increased VO2 and heat per gram body weight, and decreased RQ, whereas bGH TG mice have decreased VO2 and heat per gram body weight and increased RQ. In conclusion, decreased GH signaling associates with increased metabolism per body weight and may beneficially affect mitochondrial flexibility by increasing the capacity for fat oxidation; generally, GH excess produces opposite metabolic effects.
Keywords: Metabolism, Altered growth hormone signaling, Thyroid hormone, Oxygen consumption, Respiratory quotient
MAMMALIAN aging is a complex process that has been studied for centuries yet still is not completely understood. Recent discoveries in genetic mutant animals have provided biogerontologists with some new clues. Evidence from long-lived mutant mice suggests that deficiencies in growth hormone (GH) and insulin-like growth factor 1 (IGF-1) signaling confer a longevity advantage over genetically normal animals. Further, mice genetically engineered to overexpress GH display increased GH and IGF-1 signaling and are short-lived. In addition to life span, genetically induced alterations in the GH signaling pathway can affect many physiological parameters in mice, including body weight, organ weight, size, adiposity, onset of puberty, fertility, hormone levels (eg, insulin, IGF-1, triiodothyronine [T3], and thyroxine [T4]), body temperature, and presumably metabolic rate (1).
The effects of altered GH signaling on metabolism are also not fully understood. Previous evidence suggests reduced GH signaling in mice would lead to reduced oxygen consumption (VO2), body core temperature, and mitochondrial reactive oxygen species (ROS) production (1,2). In addition, it was previously reported that the GH receptor knockout (GHRKO) mouse is mildly hypothyroid (3), presumably resulting in a reduced metabolic rate. These physiological characteristics suggest possible mechanisms of extended longevity as they could result in decreased production of ROS, which in turn causes oxidative damage to cellular structures including DNA, and may contribute to aging (4,5). However, several potentially confounding factors, including age and sex, were not fully investigated in these experiments, leaving a number of questions unanswered. In fact, VO2 was increased in 10-week-old male GHRKO mice fed either a high- or a low-fat diet as determined by 6 hours of indirect calorimetry (6). These counterintuitive findings emphasize the difficulty in defining the role of GH in the control of mammalian metabolism. Furthermore, the results of the present study indicate that the effects of altered GH signaling on metabolism are more complex than previously thought.
Additional evidence for the relationship between GH and metabolism is derived from studies of caloric restriction (CR). CR remains the only nongenetic intervention known to consistently increase life span in mice and many other organisms (7,8). The mechanisms through which CR increases life span are unknown, but reduced GH and IGF-1 signaling are among hallmarks of CR in rodents (7,9). In addition, CR is known to decrease ROS, a byproduct of oxidative metabolism (10,11). Surprisingly, the mass-specific and lean mass-specific metabolic rates of normal mice on CR are increased when compared with mice on higher calorie diets after 2 months (10). Also, rats under CR for 4.5 months had no significant change in VO2 (12). This evidence is incongruent with findings in poikilothermic species, in which reducing ambient temperature and thus metabolic rate extends life (13); with the “rate of living” theory of aging, which postulates that an increased metabolic rate should result in an increased rate of aging (14); and with the corresponding hypothesis that a decreased metabolic rate is a mechanism through which CR extends life span.
The objective of this study was to investigate the impact of altered GH signaling on metabolism in adult male mice by using two types of GH signaling-deficient mice, the GHRKO and Ames dwarf (Prop1df) mice, and one type of GH-overexpressing mouse, the bovine GH transgenic (bGH TG). Using indirect calorimetry, we examined the following parameters: VO2, respiratory quotient (RQ, VCO2/VO2), and heat production (calories/h). In GHRKO and bGH TG mice, serum levels of thyroid hormones T3 and T4 were also measured.
METHODS
Animals
GHRKO (−/−) and normal (+/−) mice were derived from GHRKO animals (15) kindly provided by Dr. J. J. Kopchick (Ohio University, Athens, OH) and produced in our breeding colony by mating −/− males to +/− females. Ames dwarf mice were derived from a closed breeding colony at Southern Illinois University. Ames dwarf mice were produced by mating heterozygous carriers of the df (Prop-1df) mutation (df/+) or (df/df) males with (df/+) females. Normal (?/+) siblings of Ames dwarfs were used as controls. Both GHRKO and Ames dwarf mice were on a heterogeneous genetic background. PEPCK bGH mice overexpressing bGH were originally developed by microinjection of fertilized eggs with a gene construct consisting of the rat PEPCK promoter fused with the bGH gene (16). bGH TG mice for this experiment were produced in our colony derived from animals kindly provided by Dr. T. Wagner and J. S. Yun (Ohio University, Athens, OH) by mating male transgenic mice with normal (C57BL6/J × C3H/J F1) hybrid females. Normal siblings of bGH TG mice were used as controls for this experiment. Animals were housed under temperature- and light-controlled conditions (21°C–23°C and 12-hour light/12-hour darkness cycle) and were fed Lab Diet Formula 5001 (23.4% protein, 4.5% fat, 5.8% fiber; (Ralston Purina Co., St. Louis, MO). All animal protocols for this study were approved by the Animal Care and Use Committee of Southern Illinois University.
Indirect Calorimetry
For indirect calorimetry, we used adult (7–12 months old) male long-lived GHRKO and Ames dwarf mice, short-lived bGH TG mice, and their respective normal siblings (n = 8 per phenotype). Indirect calorimetry was performed using the AccuScan Instruments, Inc. PhysioScan Metabolic System (Columbus, OH). This system uses zirconia and infrared sensors to monitor oxygen (O2) and carbon dioxide (CO2), respectively, inside respiratory chambers in which individual mice were tested. All comparisons are based on animals studied simultaneously in eight different chambers connected to the same O2 and CO2 sensors in an effort to minimize the effect of environmental variations and calibration on data. After a 24-hour acclimation period, mice were monitored in the metabolic chambers for 24 hours with ad-libitum access to standard chow (Laboratory Diet 5001) and water, and then for a second 24-hour period without food. Gas samples were collected and analyzed every 5 minutes per animal, and the data were averaged for each hour. Output parameters include VO2 (mL/kg/min), RQ (VCO2/VO2), and heat production (calories/h). To correct for problems related to calibration of gas sensors, all RQ values were multiplied by a constant coefficient (1.07). The absolute values of calculated RQ in the pilot studies were used to determine the coefficient value. The application of this constant coefficient has no effect on overall differences between the results or on their statistical significance.
Thyroid Hormone Levels
Following isoflurane (Butler Animal Health Supply, Dublin, OH) anesthesia, blood was collected via cardiac puncture from (5–12 months old) male GHRKO, bGH TG mice, and their respective normal counterparts (n = 5–8 per phenotype). Ames dwarf mice were excluded from thyroid hormone measurements due to hereditary deficiency in thyroid-stimulating hormone, resulting in barely detectable serum T3 and T4 (1). Mice were in fed condition at time of sacrifice. Blood was centrifuged at 9,279 g for 15 minutes at 4°C, and serum was collected. Serum total T3 and total T4 were measured by radioimmunoassay (RIA) using Coat-A-Count total T3 and T4 kits (Diagnostic Products Corp., Los Angeles, CA). All T3 and T4 measurements were performed in duplicate samples. The sensitivity of these assays were as follows: T3, 7 ng/dL; T4, 0.25 μg/dL. All samples were processed in the same assay and the intra-assay coefficients of variation were 5.4% and 6.2% for T3 and T4, respectively.
Statistical Analyses
Heat and VO2 measurements were averaged over each hour of the 24-hour period from each group (n = 8) and analyzed by two-factor repeated measures analysis of variance. Average VO2 and heat per gram measurements represent the daily average from each group of mice and were analyzed by unpaired Student’s t test for comparison of mutant and normal. RQ and thyroid hormone data were analyzed by unpaired Student’s t test for comparison of mutant and normal. Graphs and statistics were generated with GraphPad Prism 4 (GraphPad Software, La Jolla, CA) and SPSS 14.0 for Windows (SPSS Inc., Chicago, IL).
RESULTS
Calorimetry
Oxygen consumption.—
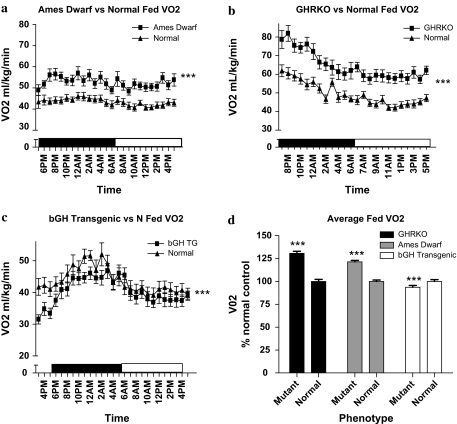
VO2 is the volume of oxygen consumed by an organism over a certain period of time, in this case expressed as mL/kg/min. The VO2 for all mice displayed the expected diurnal rhythm of increased VO2 during the dark period, when the animals are normally more active and feeding, compared with the light period (p < .05). Throughout the 24-hour measurement, the Ames dwarf mice showed increased VO2 compared with normal controls during both the fed (Figure 1a) and fasted (supplemental figures) periods (p < .0001). The GHRKO mice also showed a significantly increased VO2 compared with normal controls on both fed (Figure 1b) and fasted (supplemental figures) days (p < .0001). Conversely, the bGH TG mice showed decreased VO2 when compared with their normal controls on fed days (p < .0001; Figure 1c), and no significant difference on the fasted day (supplemental figures). Average VO2 from all three mutants and their normal controls in fed conditions (expressed as percent differences) shows that both the long-lived GHRKO and Ames dwarf mice have increased average VO2 compared with their corresponding controls, whereas the short-lived bGH TG mice have decreased average VO2 compared with their normal controls (Figure 1d). Summary area under the curve data are presented in Table 1.
Figure 1.
VO2 (mL/kg/Min) plotted at 1-hour intervals of male Ames dwarf (a), GHRKO (b), and bGH TG (c) mice and their normal siblings (n = 8–10 per phenotype). VO2 in the long-lived Ames dwarf (F = 235.5) and GHRKO (F = 326.5) mice was significantly increased compared with their normal siblings, whereas the bGH TG (F = 26.40) mice showed decreased VO2 compared with their normal siblings. The bar graph (d) illustrates daily VO2 from all mice with all time points compiled into daily averages and expressed as percent of normal control. Data reported are means ± standard error of the mean. Note: bGH TG = bovine GH-overexpressing transgenic; F = F statistic; GHRKO = growth hormone receptor knockout; VO2 = oxygen consumption; ***, significantly different (p < .001).
Table 1.
Twenty-Four-Hour VO2 Area Under Curve
| VO2 Area Under Curve | GHRKO | Ames Dwarf | bGH TG |
| Mutant | 1,486 ± 22.85, n = 8 | 1,217 ± 42.13, n = 8 | 957.5 ± 36.57, n = 8 |
| Normal | 1,142 ± 13.06, n = 8 | 997.5 ± 24.93, n = 8 | 1,014 ± 24.49, n = 8 |
| p value | <.0001 | .0005 | .2217 |
Note: bGH TG = bovine GH-overexpressing transgenic; GHRKO = growth hormone receptor knockout; VO2 = oxygen consumption.
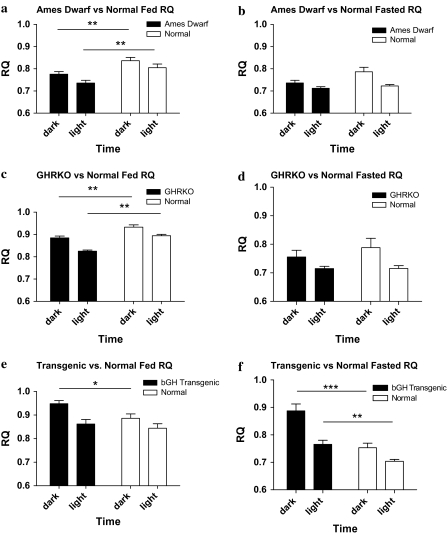
Respiratory quotient.—
RQ is a dimensionless ratio comparing the volume of carbon dioxide an organism produces over a given time (VCO2) to VO2 (RQ = VCO2/VO2), which gives an estimate of the daily transition between fat (RQ = 0.7) and carbohydrate (RQ = 1) oxidation. The RQ varies inversely with lipid oxidation. A higher fasting RQ, which indicates lowered fat oxidation, is linked to body weight gain, metabolic inflexibility, and insulin resistance (17–20). All mice displayed the diurnal rhythm of a higher RQ during the dark period compared with the RQ during the light period as expected from the feeding pattern of these nocturnal animals. The Ames dwarf mice had a significantly decreased RQ compared with their normal controls during both the dark and the light periods on the fed day of measurement (Figure 2a; p < .01); however, no statistically significant differences were observed in the RQ on the fasted day (Figure 2b). The GHRKO mice also displayed a decreased RQ in both the dark and the light periods compared with their normal controls on the fed day (Figure 2c; p < .01) and showed nonsignificant decreases on the fasted day (Figure 2d). In contrast, bGH TG mice showed significant increases in their RQ during the dark period of the fed day (Figure 2e; p < .01), and during both the dark and the light period of the fasted day (Figure 2f; p < .001, p <.01, dark and light, respectively). Notably, the bGH TG mice showed a delayed response to fasting by maintaining an elevated RQ 12 hours into the fasting period (Figure 2e and f), suggesting metabolic inflexibility.
Figure 2.
RQ values plotted as 12-hour averages representing either dark or light periods on both fed and fasted days (n = 8–10 per phenotype). All mice showed a diurnal rhythm of higher RQ during the dark period and lower RQ during the light period. Both the Ames dwarf (p = .0064 dark, p = .0048 light) and GHRKO (p = .0039 dark, p < .0001 light) mice showed significantly decreased RQ during both the dark and the light period on the fed day (a and c) but showed nonsignificant differences on the fasted days (b and d) compared with their normal siblings. In contrast, the bGH TG mice showed a significantly increased RQ compared with their normal siblings during the dark period on the fed day (p = .0240) (e) and during both periods on the fasted day (p = .0010 dark, .0039 light) (f). Data reported are means ± standard error of the mean. Note: GHRKO = growth hormone receptor knockout; RQ = respiratory quotient; *p < .05, **p < .01, ***p < .001.
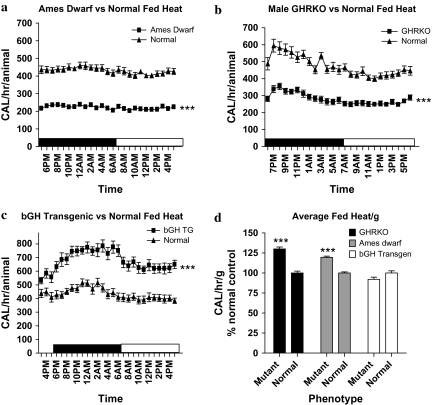
Heat.—
Heat production (calories/h) is a measure of caloric output and is directly proportional to metabolic rate. All mice once again displayed the expected diurnal rhythm with increased heat production during the dark period (p < .05). Heat production per animal was decreased on both fed and fasted days in Ames dwarf (Figure 3a; p < .0001) and GHRKO mice (Figure 3b; p < .0001), whereas bGH TG mice showed increased heat production on both days (Figure 3c; p < .0001), compared with their normal controls. However, when heat production was expressed on a per gram basis, GHRKO and Ames dwarf mice both showed increased heat compared with their normal siblings (Figure 3d; p < .001), whereas bGH TG mice showed a nonsignificant reduction in heat production. Summary area under the curve data are presented in Table 2.
Figure 3.
Heat (calories/h) production per mouse plotted at 1-hour intervals of male Ames dwarf (a), GHRKO (b), and bGH TG (c) mice and their normal siblings (n = 8–10 per phenotype). Heat production per mouse was decreased in the long-lived Ames dwarf (F = 2774) and GHRKO (F = 999.3) mice compared with their normal siblings; however, the bGH TG (F = 633.8) mice displayed increased heat production per mouse compared with their normal siblings. In contrast, daily average heat production per gram (calories/h/g) tissue expressed as percent normal (d) showed increased heat production in both the long-lived Ames dwarf and GHRKO mice, whereas bGH TG mice displayed numerically decreased heat per gram compared with their normal siblings. Data reported are means ± standard error of the mean. Note: bGH TG = bovine GH-overexpressing transgenic; F = F statistic; GHRKO = growth hormone receptor knockout; ***, significantly different (p < .001).
Table 2.
Twenty-Four-Hour Heat per Gram Area Under Curve
| Heat per Gram Area Under Curve | GHRKO | Ames Dwarf | bGH TG |
| Mutant | 433.4 ± 7.241, n = 8 | 336.4 ± 11.70, n = 8 | 291.6 ± 10.85, n = 8 |
| Normal | 335.5 ± 3.603, n = 8 | 279.0 ± 7.255, n = 8 | 308.6 ± 7.839, n = 8 |
| p value | <.0001 | .0010 | .2252 |
Note: bGH TG = bovine GH-overexpressing transgenic; GHRKO = growth hormone receptor knockout.
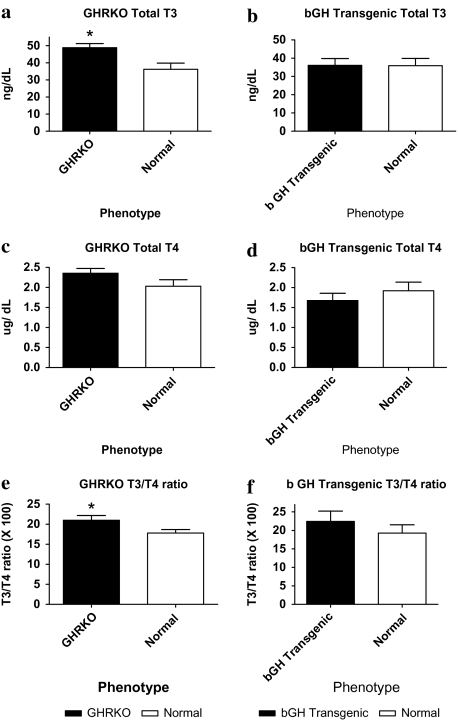
Thyroid Hormone Levels
The thyroid gland is present in all vertebrates and is essential for the coordinated development and control of numerous functions. In homeothermic species, thyroid hormones play a crucial role in temperature homeostasis and metabolic control. In mice, the thyroid hormones T3 and T4 are involved in many biologic processes and are known to increase metabolic rate and thermogenesis (21). Total T3 was increased in GHRKO mice compared with normal (Figure 4a; p < .05), whereas bGH TG mice showed no significant change in T3 levels (Figure 4b). T4 levels were not significantly altered in either GHRKO or bGH TG mice (Figure 4c and d), and the T3/T4 ratio was significantly increased in GHRKO mice compared with normal controls (Figure 4e; p < .05). bGH TG mice had no significant alteration in T3/T4 ratio (Figure 4f).
Figure 4.
Thyroid hormone triiodothyronine (T3) levels (n = 8–10 per phenotype) in GHRKO (a) mice were elevated compared with their normal siblings (p = .0158), whereas bGH TG (b) mice showed no difference. Thyroxine (T4) levels were not significantly altered in either the GHRKO (c) or the bGH TG (d) mice when compared with their normal siblings. GHRKO mice (e) displayed a significantly elevated T3/T4 ratio compared with normal mice (p = .0442), whereas bGH TG (f) mice showed no significant difference. Data reported are means ± standard error of the mean.
Note: bGH TG = bovine GH-overexpressing transgenic; GHRKO = growth hormone receptor knockout; *p < .05.
DISCUSSION
GH signaling plays an important role in the growth, development, and maintenance of almost every tissue in the body. In mice, reductions in GH signaling result in prolonged life span, whereas massive increases in GH signaling cause early death (1,22). The purpose of this study was to investigate the adjustments in metabolism that accompany the physiological changes that are associated with altered GH signaling and may be related to differences in life span. Here we have shown that both long-lived Ames dwarf and GHRKO mice have increased VO2, decreased RQ, and increased heat per gram body weight, compared with normal controls, whereas short-lived bGH TG mice have reduced VO2, increased RQ, and a nonsignificant trend for decreased heat per gram body weight compared with control animals. In addition, we have shown that GHRKO mice have increased T3 levels and T3/T4 ratio, whereas bGH TG mice had no significant change in thyroid hormone levels compared with normal controls.
Although VO2 and heat production in relation to absolute body weight were increased in Ames dwarf and GHRKO mice and decreased in bGH TG, when these parameters were expressed in terms of “metabolic body weight” (g2/3 or g3/4; data not shown) (23,24), the phenotypic differences previously seen were eliminated. The interpretation of these scaling exponents as applied in this study is complicated by known differences in body composition in these mice that allometric scaling does not account for. For example, GHRKO mice have a roughly 36% increase in percent body fat, a 92% increase in relative brain weight, a 30% reduction in relative liver weight, and a nonsignificant reduction in percent lean body mass (25,26). With reductions in relative size of skeletal muscle and liver, two major oxygen-consuming organs in the rodent (27), the actual metabolic body weight may be smaller than a simple scaling exponent would predict. These presumptions are consistent with our previously published dual-energy x-ray absorptiometry (DXA) measurements of body composition of GHRKO mice of the same age and sex as used in this experiment, which show that the lean tissue percentage reported as fat-free mass minus bone tissue is 35% in GHRKO mice and 36% in normal controls (25). These measured values of functional metabolic body mass show that the allometric scaling exponents err in opposite ways for the mutant and normal mice, overestimating the observed metabolic body weight in GHRKO mice and underestimating metabolic body weight in normal animals. When the actual measurements of metabolic body weight are used, significantly increased VO2 is observed in GHRKO mice compared with normal mice (heat production showed similar results). Increased VO2 per lean body mass was also seen in GHRKO mice during 6-hour measurements (6). Further, the increases in VO2 are of the same magnitude (1.3-fold) as those observed when expressing VO2 and heat per gram (as shown in figures), indicating that in comparisons of GHRKO with normal mice the isometric analysis more accurately corresponds to metabolic rate per functional metabolic mass than the allometric scaling exponent estimate.
Unfortunately, we do not have DXA measurements of lean tissue percent reported as fat-free mass minus bone tissue for the other animals used in this experiment, the Ames dwarf and bGH TG mice. However, we know that age-matched male Ames dwarf mice have roughly 20% body fat, whereas normal controls have about 35% (28). Once again, we expect that allometric scaling will not account for the differences in body composition induced by the Ames dwarf mutation. We have no densitometric analysis of the bGH TG mice, but we know that these mutants are significantly leaner than their normal counterparts (26), indicating a high percent functional metabolic mass. Because these animals have greater overall body weight, we once again anticipate that an allometric scaling exponent will underestimate the actual metabolically functional mass. Without densitometric measurements, there are no satisfactory alternatives to the representation of actual metabolic rate in terms of body weight, as shown in the figures.
The results of this study have yielded two novel conclusions. First, the notion that reduced levels of GH and IGF-1 are expected to reduce VO2 (2, reviewed in Bartke [1] in mice is not always correct. In fact, in the present study, the opposite is seen in 24-hour measurements in both GHRKO and Ames dwarf mice. Additionally, increased levels of GH, IGF-1, and insulin as seen in the bGH TG mice are associated with decreased VO2. Importantly, the data reported in Benedict and Lee (2) were obtained in Snell dwarf mice (Pit1 rather that Prop1 mutants) on a different genetic background. The “severity” of phenotype in these mutants depends on genetic background, with sexual maturation and fertility being good examples (29,30). Second, although the levels of thyroid hormones T3 and T4 were previously found to be decreased in 4- to 5-month-old female GHRKO mice (3), we found significant increases in T3 and T3/T4 ratio when measuring these hormones in 7- to 12-month-old male GHRKO mice. These results show that male Ames dwarf and GHRKO mice, although both long lived, exhibit opposite alterations in thyroid hormone levels, with Ames dwarf mice having decreased T3 levels and GHRKO mice having elevated T3 levels. Perhaps, male GHRKO mice have increased T3 as a compensatory mechanism to stimulate metabolic rate in the absence of GH. Interestingly, the Ames dwarf mouse lacks the ability to produce detectable levels of thyroid hormones yet somehow is able to maintain increased VO2 and heat per gram body weight compared with normal controls.
The influence of GH signaling on the metabolic parameters investigated here is confounding in light of the putative roles of this hormone. GH is an anabolic hormone known to be calorigenic and has been shown to accelerate fat metabolism, prevent triglyceride accumulation, and facilitate lipid mobilization (31). This is exemplified by the lean body composition of bGH TG mice (32) and conversely, markedly increased adiposity of GHRKO mice (1,6,25). However, bGH TG mice that overexpress GH have a higher daily RQ, pointing to increased carbohydrate oxidation relative to lipid oxidation in these animals, whereas the GHRKO and Ames dwarf mice both have decreased GH signaling and yet maintain a lower daily RQ, indicating increased fat oxidation. Because about 90% of mammalian VO2 is mitochondrial (27), increased VO2 levels expressed per gram seen in long-lived mice are a direct reflection of increased mitochondrial oxidative metabolism. The RQ and VO2 data from this experiment show that fat is more relied upon as mitochondrial fuel substrate in long-lived animals than in their littermate controls and less in short-lived animals relative to their littermate controls, in both the fed and the fasted state. This is interesting when considering the increased adiposity of the GHRKO mice and the lean body composition of bGH TG mice.
The increased metabolism per gram body weight in long-lived mutant mice may be explained by several aspects of the physiology of these mice. First, because adiponectin is known to activate 5-AMP-activated protein kinase (AMPK) in the liver and skeletal muscle, the effects of elevated adiponectin in GHRKO (33) and Ames dwarf (34) mice may mask or override the effects of reduced GH, insulin, and T3 (in the Ames dwarf) and increase catabolism. Once activated, AMPK in turn stimulates multiple processes: phosphorylation (deactivation) of acetyl CoA carboxylase (ACC), fatty acid oxidation, glucose uptake, and lactate production in myocytes; phosphorylation of ACC and reduction of molecules involved in gluconeogenesis in the liver; and a reduction in glucose levels in mice (35). In addition, decreased plasma adiponectin and AMPK in bGH TG mice (36) may relate to the decreased catabolism per body weight seen in these mice. Also, genes related to the hydrolysis of triglycerides and the transport of fatty acids into mitochondria as well as mitochondrial biogenesis and oxidation, such as PGC-1 alpha, are upregulated in the examined long-lived mice with reduced somatotrophic signaling (33,34), and downregulated in short-lived bGH TG mice (36).
Another possible explanation for increased VO2 and heat per gram in long-lived mice may be the decreased mass –to –surface area ratio seen in these mice. The size and weight reduction of the long-lived Ames d warf, GHRKO, and normal mice subjected to CR would cause them to lose body heat more rapidly than normal animals. In fact, lower core body temperature is found in GHRKO mice (3), Ames dwarf mice (37), and mice on CR (38). Therefore, one would expect thermogenic mechanisms to be more active in these mice to maintain homeothermy. Because there is a significant energy cost attached to maintenance of body temperature (21), this may partially account for the increased metabolism seen in long-lived mice. GHRKO mice are known to have enlarged brown adipose tissue stores and increased uncoupling protein (UCP) 1-messenger-RNA (mRNA) in these stores, indicating increased nonshivering thermogenesis (39). In addition, T3 actively stimulates UCP1, UCP2, and UCP3 mRNA in several tissues including brown fat (40,41), and the activity of some or all of these UCPs may increase overall metabolism. This fact may relate to the increased metabolism in GHRKO mice.
Elevated metabolism has long been associated with premature mortality. The rate of living theory proposed by Pearl (14) and the “free-radical” theory proposed by Harman (42) have provided a potential mechanism to link the rate of aging to metabolic rate: Increased mitochondrial activity leads to increased ROS generation, which leads to increased rate of aging. However, evidence is emerging that suggests that the relationship between metabolic rate and free-radical production is not directly proportional and may be inversely proportional. For instance, it has been known for decades that when VO2 is acutely increased in isolated mitochondria, the production of ROS is sharply decreased (43). VO2 was increased in calorie-restricted yeast cells whose replicative life span was also increased (44). Mice on CR have significantly higher mass-specific metabolic rates than mice fed more calories after 6 months on their respective diets but exhibit decreased rates of ROS production (10). Additionally, CR has been shown to increase mitochondrial proliferation and density, while decreasing mitochondrial membrane potential, ROS production, and oxygen consumption of individual mitochondria and yet maintaining ATP production in vitro (45). CR has also been shown to increase mitochondrial biogenesis, ATP production, and oxygen consumption in vivo (46). These examples show that a high rate of metabolism does not necessarily cause an increased production of ROS; in fact, the opposite is often seen. This unexpected phenomenon may be explained by the “uncoupling to survive” hypothesis proposed by Brand (47). Simply, this hypothesis states that in addition to thermogenesis, UCPs play a protective role in the mitochondria by allowing the dissipation of the electrochemical potential built up by the pumping of protons across the mitochondrial inner membrane. This dissipation is facilitated by UCPs, which allow the flow of ions back across the mitochondrial inner membrane without activating ATP synthases and creating ATP. In addition to creating heat, this “wasteful” release of electrical potential may decrease the production of superoxide and other ROS. This could be important in helping to minimize oxidative damage to DNA and, ultimately, in slowing aging. Alternatively, an increased number of efficient mitochondria with electron transport tightly coupled to ATP production and low membrane potential could also provide an explanation for the decreased ROS seen in CR and long-lived animals. Future experiments will elucidate which phenomenon is responsible for the decreased oxidative damage seen in the long-lived Ames dwarf and GHRKO mice, and increased oxidative damage seen in bGH TG mice (48,49). Recently, in Caenorhabditis elegans, systemic lipolysis occurring by induction of a fat-specific lipase via germline stem cell arrest or constitutive lipase expression was shown to increase fat mobilization and prolong life span in these animals (50). This provides further evidence of the important role of increased fat metabolism in promoting longevity.
In summary, this study shows that both long-lived Ames dwarf and GHRKO mice have increased VO2 and heat expressed per gram, and decreased RQ compared with normal controls, whereas short-lived bGH TG mice have reduced VO2 and heat expressed per gram body weight, and increased RQ compared with their corresponding controls. In addition, we have shown that male GHRKO mice have increased T3 levels and T3/T4 ratio, whereas male bGH TG mice have no significant change in thyroid hormone levels compared with normal controls. These data show that alterations in GH signaling cause marked changes in energy metabolism. Decreased GH signaling causes increased T3 (GHRKO), increased metabolism per body weight, increased fatty acid oxidation, and may have a beneficial effect on mitochondrial flexibility by increasing the ability to utilize fat as a fuel substrate, whereas GH excess has generally opposite effects in mice. These results indicate that increased metabolism is associated with increased life span in these mice.
Supplementary Material
Supplementary material can be found at: http://biomed.gerontologyjournals.org/.
Supplementary Material
Acknowledgments
Contributions of numerous colleagues, students, and lab members to the progress of our work are gratefully acknowledged. This work was supported by grants from the National Institute on Aging (National Institutes of Health) AG019899 and U19AG023122 (Longevity Consortium), as well as by the Ellison Medical Foundation. The authors thank Oge Arum for critical comments on the manuscript and Steve Sandstrom for editorial assistance.
References
- 1.Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- 2.Benedict FG, Lee RC. La production de chaleur de la souris. Etude de plusieurs races de souris. Ann Physiol Physicochim Biol. 1936;12:983–1064. [Google Scholar]
- 3.Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp Biol Med. 2001;226:552–558. doi: 10.1177/153537020122600607. [DOI] [PubMed] [Google Scholar]
- 4.Hagen TM. Oxidative stress, redox imbalance, and the aging process. Antioxid Redox Signal. 2003;5:503–506. doi: 10.1089/152308603770310149. [DOI] [PubMed] [Google Scholar]
- 5.Harman D. Free radical theory of aging. Mutat Res. 1992;275:257–266. doi: 10.1016/0921-8734(92)90030-s. [DOI] [PubMed] [Google Scholar]
- 6.Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology. 2006;147:2801–2808. doi: 10.1210/en.2006-0086. [DOI] [PubMed] [Google Scholar]
- 7.Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–321. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- 8.Weindruch R. The retardation of aging by caloric restriction: studies in rodents and primates. Toxicol Pathol. 1996;24:742–745. doi: 10.1177/019262339602400618. [DOI] [PubMed] [Google Scholar]
- 9.Mobbs CV, Bray GA, Atkinson RL, et al. Neuroendocrine and pharmacological manipulations to assess how caloric restriction increases life span. J Gerontol A Biol Sci Med Sci. 2001;56A:34–44. doi: 10.1093/gerona/56.suppl_1.34. [DOI] [PubMed] [Google Scholar]
- 10.Faulks SC, Turner N, Else PL, Hulbert AJ. Calorie restriction in mice: effects on body composition, daily activity, metabolic rate, mitochondrial reactive oxygen species production, and membrane fatty acid composition. J Gerontol A Biol Sci Med Sci. 2006;61:781–794. doi: 10.1093/gerona/61.8.781. [DOI] [PubMed] [Google Scholar]
- 11.Lee DW, Yu BP. Modulation of free radicals and superoxide dismutases by age and dietary restriction. Aging (Milano) 1990;2:357–362. doi: 10.1007/BF03323951. [DOI] [PubMed] [Google Scholar]
- 12.McCarter R, Masoro EJ, Yu BP. Does food restriction retard aging by reducing the metabolic rate? Am J Physiol. 1985;248:E488–E490. doi: 10.1152/ajpendo.1985.248.4.E488. [DOI] [PubMed] [Google Scholar]
- 13.Liu RK, Walford RL. Mid-life temperature-transfer effects on life-span of annual fish. J Gerontol. 1975;30:129–131. doi: 10.1093/geronj/30.2.129. [DOI] [PubMed] [Google Scholar]
- 14.Pearl R. The Rate of Living. London, UK: University of London Press; 1928. [Google Scholar]
- 15.Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGrane MM, DeVente J, Yun JS, et al. Tissue-specific expression and dietary regulation of a chimeric phosphoenolpyruvate carboxykinase/bovine growth hormone gene in transgenic mice. J Biol Chem. 1988;263:11443–11451. [PubMed] [Google Scholar]
- 17.Zurlo F, Lillioja S, Esposito-Del Puente A, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259:E650–E657. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 18.Seidell JC, Muller DC, Sorkin JD, Andres R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. Int J Obes Relat Metab Disord. 1992;16:667–674. [PubMed] [Google Scholar]
- 19.Snitker S, Tataranni PA, Ravussin E. Respiratory quotient is inversely associated with muscle sympathetic nerve activity. J Clin Endocrinol Metab. 1998;83:3977–3979. doi: 10.1210/jcem.83.11.5265. [DOI] [PubMed] [Google Scholar]
- 20.Ukropcova B, Sereda O, de Jonge L, et al. Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes. 2007;56:720–727. doi: 10.2337/db06-0521. [DOI] [PubMed] [Google Scholar]
- 21.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 22.Cecim M, Bartke A, Yun JS, Wagner TE. Expression of human, but not bovine growth hormone genes promotes development of mammary tumors in transgenic mice. Transgenics. 1994;1:431–437. [Google Scholar]
- 23.Rubner M. Ueber den Einfluss der Körpergrösse auf Stoff-und Kraftwechsel. Zeitschrift fur Biologie. 1883;19:536–562. [Google Scholar]
- 24.Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315–353. [Google Scholar]
- 25.Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, Bartke A. Long-lived growth hormone receptor knockout mice show a delay in age-related changes of body composition and bone characteristics. J Gerontol A Biol Sci Med Sci. 2006;61:562–567. doi: 10.1093/gerona/61.6.562. [DOI] [PubMed] [Google Scholar]
- 26.Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14:309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 28.Heiman M, Tinsley F, Mattison J, Hauck S, Bartke A. Body composition of prolactin-, growth hormone-, and thyrotropin-deficient Ames dwarf mice. Endocrine. 2003;20:149–154. doi: 10.1385/ENDO:20:1-2:149. [DOI] [PubMed] [Google Scholar]
- 29.Bartke A. Genetic Variation in Hormone Systems. Boca Raton, FL: CRC Press; 1979. Genetic models in the study of anterior pituitary hormones; pp. 113–126. ShireJGM. [Google Scholar]
- 30.Bartke A, Chandrashekar V, Turyn D, et al. Effects of growth hormone overexpression and growth hormone resistance on neuroendocrine and reproductive functions in transgenic and knock-out mice. Proc Soc Exp Biol Med. 1999;22:113–123. doi: 10.1046/j.1525-1373.1999.d01-121.x. [DOI] [PubMed] [Google Scholar]
- 31.Goodman HM. The Endocrinology of Growth, Development, and Metabolism in Vertebrates. San Diego, CA: Academic Press; 1993. Growth hormone and metabolism; pp. 93–115. Schreibman MPScanes CGPang PKT, [Google Scholar]
- 32.Frick F, Bohlooly YM, Linden D, et al. Long-term growth hormone excess induces marked alterations in lipoprotein metabolism in mice. Am J Physiol Endocrinol Metab. 2001;281:E1230–E1239. doi: 10.1152/ajpendo.2001.281.6.E1230. [DOI] [PubMed] [Google Scholar]
- 33.Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor 1/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and caloric restricted mice. J Gerontol A Biol Sci Med Sci. 2006;61A:323–331. doi: 10.1093/gerona/61.4.323. [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Masternak MM, Al-Regaiey KA, Bartke A. Adipocytokines and the regulation of lipid metabolism in growth hormone transgenic and calorie-restricted mice. Endocrinology. 2007;148:2845–2853. doi: 10.1210/en.2006-1313. [DOI] [PubMed] [Google Scholar]
- 37.Hunter WS, Croson WB, Bartke A, Gentry MV, Meliska CJ. Low body temperature in long-lived Ames dwarf mice at rest and during stress. Physiol Behav. 1999;67:433–437. doi: 10.1016/s0031-9384(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson M, Sohal BH, Forster MJ, Sohal RS. Effect of long-term caloric restriction on oxygen consumption and body temperature in two different strains of mice. Mech Ageing Dev. 2007;128:539–545. doi: 10.1016/j.mad.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Knapp J, Kopchick JJ. Enlargement of interscapular brown adipose tissue in growth hormone antagonist transgenic and in growth hormone receptor gene-disrupted dwarf mice. Exp Biol Med. 2003;228:207–215. doi: 10.1177/153537020322800212. [DOI] [PubMed] [Google Scholar]
- 40.Branco M, Ribeiro M, Negrao N, Bianco AC. 3,5,3′-Triiodothyronine actively stimulates UCP in brown fat under minimal sympathetic activity. Am J Physiol. 1999;276:E179–E187. doi: 10.1152/ajpendo.1999.276.1.E179. [DOI] [PubMed] [Google Scholar]
- 41.Jekabsons MB, Gregoire FM, Schonfeld-Warden NA, Warden CH, Horwitz BA. T(3) stimulates resting metabolism and UCP-2 and UCP-3 mRNA but not nonphosphorylating mitochondrial respiration in mice. Am J Physiol. 1999;277:E380–E389. doi: 10.1152/ajpendo.1999.277.2.E380. [DOI] [PubMed] [Google Scholar]
- 42.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 43.Loschen G, Flohe L, Chance B. Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett. 1971;18:261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- 44.Lin SJ, Kaeberlein M, Andalis AA, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Lluch G, Hunt N, Jones B, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nisoli E, Tonello C, Cardile A, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 47.Brand MD. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol. 2000;35:811–820. doi: 10.1016/s0531-5565(00)00135-2. [DOI] [PubMed] [Google Scholar]
- 48.Brown-Borg H, Johnson W, Rakoczy S, Romanick M. Mitochondrial oxidant generation and oxidative damage in Ames dwarf and GH transgenic mice. J Amer Aging Assoc. 2001;24:85–96. doi: 10.1007/s11357-001-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hauck SJ, Aaron JM, Wright C, Kopchick JJ, Bartke A. Antioxidant enzymes, free-radical damage, and response to paraquat in liver and kidney of long-living growth hormone receptor/binding protein gene-disrupted mice. Horm Metab Res. 2002:481–486. doi: 10.1055/s-2002-34787. [DOI] [PubMed] [Google Scholar]
- 50.Wang MC, O’Rourke EJ, Ruvkun G. Fat metabolism links germline stem cells and longevity in C. elegans. Science. 2008;322:957–960. doi: 10.1126/science.1162011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.