Abstract
Increased expression of heme oxygenase-1 (HO-1) in response to physiological stress is considered to be a protective response, which may be altered with aging. In this study, HO-1 expression was assessed following heat stress by immunoblotting of liver homogenates and isolated hepatocytes from young (6 months) and old (24 months) Fischer 344 rats and by immunohistochemistry. Livers of old rats showed higher baseline levels of HO-1, which was predominately localized to Kupffer cells. After heat stress, young animals showed a greater relative increase in hepatic HO-1, part of which was caused by increased numbers of nonparenchymal cells that were immunoreactive to HO-1. Consistent with these data, HO-1 was significantly upregulated after hyperthermia in vitro only in hepatocytes from young rats. Hence, aging alters stress-induced expression of HO-1 in a cell-specific manner, which may contribute to the diminished stress tolerance observed in older organisms.
Keywords: Oxidative stress, Kupffer cells, Inflammation, Hyperthermia
THE progressive biological alterations that accompany the aging process reduce an organism's ability to cope with physiological and pathological challenges. Aging alters the expression pattern of genes involved in metabolism, cellular signaling, and stress defense in multiple organs (1, 2), which likely contributes to an enhanced susceptibility to various insults. Indeed, previous studies from our laboratory have shown marked effects of both aging and hyperthermia on gene expression profiles within the liver (2).
Heme oxygenase-1 (HO-1) is a potentially informative protein to study in the context of aging and physiological stress due to its exquisite sensitivity to stressors and to its protective role in cellular homeostasis (3–7). HO-1 catalyzes the degradation of heme into ferrous iron, biliverdin, and carbon monoxide. Because bilirubin [the reduced form of biliverdin (8)] and carbon monoxide (9) in low concentrations have antioxidant and anti-inflammatory properties, respectively, HO-1 is considered cytoprotective (10). However, because HO-1 produces iron, it may also catalyze deleterious cellular reactions (11). Thus, HO-1 can serve both pro- and antioxidative roles and it is of interest to study the regulation of HO-1 with aging, a condition in which stress defense is compromised.
With aging, there is a disruption of homeostasis as cellular redox balance is shifted toward a more pro-oxidative state. In addition to this basal increase in oxidative status, when challenged with environmental heat stress, old organisms show further elevations in reactive oxygen species as well as oxidative liver injury (12). HO-1 is quite sensitive to changes in both temperature (6) and redox state (7); hence, both aging and heat stress likely affect hepatic HO-1 expression due to enhanced oxidative stress. Interestingly, investigators have observed both an upregulation (13) and a downregulation (14) of hepatic HO-1 messenger RNA with advanced age in rats. Studies have also shown that both HO-1 activity (15) and protein levels (16) increase in the liver with age; yet, the regulation of HO-1 protein after a physiologically relevant challenge has not been examined. We have shown that aging is associated with hepatic dysfunction after environmental heat stress that is manifested at tissue, cellular, and subcellular levels (17–19). Because hepatic HO-1 may protect against hyperthermia-induced alterations in homeostasis, and its expression is altered with aging, our goal was to compare the expression of HO-1 protein in young and old rats after heat stress.
When evaluating HO-1 protein expression in the liver, the heterogeneity of hepatic cells should be taken into consideration. Hepatic macrophages (Kupffer cells) constitutively express HO-1 under basal conditions (20,21), which reflects their prominent role in heme degradation from senescent erythrocytes. Although various stressors have been shown to induce HO-1 expression in hepatocytes, Kupffer cells are the sole hepatic cell type that demonstrates immunoreactivity for HO-1 in normal, unstressed rat liver (20). Previous studies have reported an increase in HO-1 expression with aging (13,15,16), but whether this increase reflects alterations in the cell types that express HO-1 has not previously been addressed. Hence, the first aim of this study was to characterize age-related changes in steady-state levels and immunolocalization of HO-1 protein in the liver. Although a difference in HO-1 protein induction between young and old animals has been observed with X-irradiation (22), the effects of a physiologically relevant environmental stressor such as hyperthermia have not been investigated. Therefore, the second goal of this study was to characterize the pattern of hepatic HO-1 protein expression after environmental heat stress. Based on observations from our laboratory and others, we hypothesized that old rats would show higher steady-state levels of HO-1 protein than their younger counterparts. Because old rats typically show an altered response to stressors (2,23), we postulated that they would not upregulate HO-1 protein to the same extent as young rats after heat stress.
METHODS
Animal Protocols
Young (6 months, 300–400 g) and old (24 months, 325–425 g) rats were obtained from the National Institute on Aging. Rats were housed in the University of Iowa animal care facility and maintained on a 12:12 hour light/dark cycle. All animal protocols were approved by the institutional animal care and use committee.
Heat Stress Experiments
All rats were handled daily and familiarized with a colonic temperature probe during the week preceding the heat stress protocol. This two–heat stress protocol has been described in detail previously and is used as a physiologically relevant stress model that mimics conditions that elderly humans experience during repeated exposures to heat stress (eg, a heat wave) (17,18). Briefly, core temperature was increased from approximately 37°C to 41.0°C for 60 minutes and maintained at 41.0°C for an additional 30 minutes. After this period, rats were allowed to passively cool at room temperature. Twenty-four hours after the first heat stress, animals underwent a second identical heat stress. Control animals underwent sham heatings and were euthanized at similar times as the heat stress groups. At designated times after the second heat stress, animals were euthanized with an overdose of pentobarbital sodium (80 mg/kg i.p.). Livers were collected and rinsed in phosphate-buffered saline (PBS) and then immediately frozen in liquid nitrogen or fixed overnight in 10% neutral buffered formalin for subsequent analysis.
Primary Hepatocyte Experiments
In a second set of studies, primary hepatocytes were isolated from young and old rats according to Seglen's method as described previously (24). Typical cell yields from both young and old rats were 5 × 106 cells per isolation and cell viability exceeded 90%. Cells were plated on cell culture dishes (106 cells per 100-mm dish or 1.2 × 105 cells per 35-mm dish) that were coated with type 1 collagen (BD Biosciences, San Jose, CA) and incubated in Hepatostim cell culture media (BD Biosciences). At these densities, cells were approximately 90% confluent and formed a monolayer. Hepatocytes in culture were uniformly immunoreactive for albumin, and freshly isolated hepatocytes were negative for HO-1 as assessed by immunoblotting. These data indicate that the isolated cells were essentially pure hepatocytes. The medium was changed 3 hours after plating to remove nonadherent cells. On the morning after isolation (16–20 hours after isolation), the medium was again changed and cells were incubated for 1 hour in a separate incubator maintained at 42°C. Cells were harvested at the indicated times after heat stress. The medium was removed and cells were rinsed with PBS, pelleted, and then frozen for immunoblot analysis. Separate dishes of cells (for each time point examined) from the same animal served as controls and were maintained at 37°C. This ex vivo system was used as a complementary approach to examine the effect of an acute stressor on HO-1 regulation in hepatocytes. To avoid the potential confounding effect of multiple heat stresses on cell viability, a single heat stress was used, and experiments were performed within a shorter time frame than the in vivo studies.
Immunoblotting
Portions of liver and primary hepatocytes were homogenized in lysis buffer (1% sodium dodecyl sulfate [SDS], 1.0 mM sodium orthovanadate, 10 mM tris, pH 7.4). Homogenates were centrifuged at 15,000 g, and aliquots of the supernatant were used for protein determination and SDS polyacrylamide gel electrophoresis. For whole-liver homogenates, 30 μg of protein per sample was loaded in each lane and 5 μg of protein was loaded for primary hepatocytes. Immunoblotting was performed as previously described (25). To increase statistical power in the in vivo studies, samples from 2 hours after heat stress were pooled with additional samples from a previous study that used the 1.5-hour time point (25); HO-1 expression did not differ significantly between these time points. The primary antibodies used were as follows: HO-1 (Assay Designs/Stressgen, Ann Arbor, MI) 1:1,000, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (AbCam, Cambridge, MA) 1:10,000, and β-actin (Sigma Aldrich, St Louis, MO) 1:10,000. Membranes were probed simultaneously for HO-1 and a loading control. GAPDH was a reliable loading control for in vivo samples, but we found it difficult to resolve GAPDH and HO-1 protein bands on ex vivo samples. It is likely that the high magnitude of HO-1 expression ex vivo contributed to this observation. Therefore, we used β-actin as a loading control in ex vivo samples. Immunoblotting was performed in duplicate for all samples, and one sample that was positive for HO-1 was used in each determination to allow for comparison between gels. Protein bands were quantified with Gel Pro Analyzer software (Media Cybernetics Inc., Bethesda, MD). Expression of HO-1 protein was normalized to GAPDH in the in vivo studies and to β-actin in the ex vivo experiments.
Immunohistochemistry
After fixation, liver sections were dehydrated through increasing grades of ethanol and xylenes, and then embedded in paraffin. Sections (4 μm) were cut from paraffin blocks and affixed to glass slides. Slides were deparaffinized with xylene and rehydrated through a graded series of ethanol solutions and rinsed in water. Endogenous peroxidase activity was blocked with a 15-minute incubation in 3% hydrogen peroxide. Slides were washed with water, then placed in citrate buffer (1.8 mM citric acid monohydrate, 8.2 mM trisodium citrate dihydrate, pH 6.0), and subjected to antigen retrieval in a pressure cooker. Slides were rinsed and then nonspecific protein binding was blocked by a 40-minute incubation in blocking buffer (1% bovine serum albumin, 10% normal horse serum, and 0.1% tween in PBS). Sections were incubated with a primary antibody against HO-1 (1:200) in blocking solution overnight at 4°C, followed by washing in PBS and incubation for a second time in blocking solution for 20 minutes. Sections were then incubated for 30 minutes at room temperature with a biotin-conjugated, horse anti-mouse antibody at a 1:250 dilution in blocking buffer, then rinsed with PBS, and incubated with avidin-biotin complex (Vector Laboratories, Burlingame, CA) for 30 minutes. Sections were then incubated with diaminobenzidine (Vector Laboratories) to develop the signal. A negative control was carried out on each slide by omitting the primary antibody.
Microscopy
Cell counts were performed on micrographs obtained in two discrete regions of the liver lobule: the area around the portal triad, which consists of the portal vein, hepatic artery, and bile duct (periportal region), and the area surrounding the terminal hepatic venule (perivenous region). The number of nonparenchymal cells positive for HO-1 (herein designated HO-1+ cells) was counted by a blinded observer in three 40× fields (88, 400-μm2 area) per animal in each liver region. Results are expressed as the number of HO-1+ cells per field.
Statistics
Results were analyzed with analysis of variance (SPSS software; SPSS Inc, Chicago, IL), and where appropriate, a Bonferroni adjustment was performed on individual p values to adjust for multiple comparisons. An adjusted p value less than .05 was considered significant for the pair-wise comparisons.
RESULTS
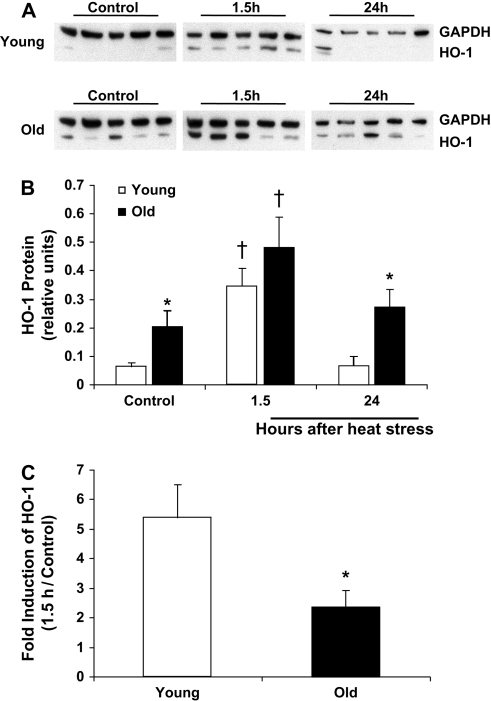
Under nonstressed conditions, hepatic HO-1 protein expression was barely detectable by immunoblot in young rat livers. Compared with young rats, levels of HO-1 in the livers of nonstressed old rats were elevated twofold (Figure 1). Both young and old rats demonstrated a robust increase in HO-1 protein at an early time point after hyperthermia (Figure 1A and B). However, when compared with the basal level of expression in their respective age groups, young rats showed a greater than fivefold increase in HO-1 protein after heat stress, whereas old animals showed only a twofold increase (Figure 1C). By 24 hours after heat stress, HO-1 protein had returned to nonstressed levels in both age groups and remained higher in the old animals compared with the young animals.
Figure 1.
Hepatic HO-1 protein levels are elevated with aging and in response to heat stress. Liver homogenates from young and old rats under control conditions and at the indicated times after heat stress were analyzed for HO-1 protein via immunoblotting. (A) Representative immunoblots from young and old rats. (B) Quantitation of relative HO-1 protein levels via densitometry: optical densities of HO-1 bands were normalized to those of the loading control, GAPDH. (C) Fold increase of HO-1 protein at the 1.5-hour time point in young and old rats. Results are presented as mean + SE; n = 5–6 animals per group. *Significant difference between young and old animals within a time point. †Significant effect of heat stress within an age group. HO-1, heme oxygenase-1. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
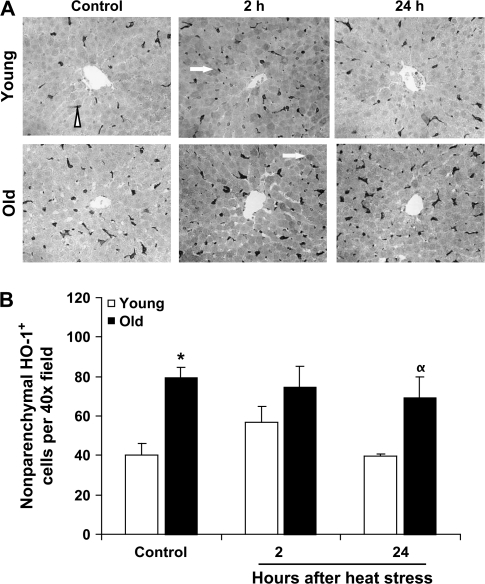
Under basal conditions in both young and old rat livers, HO-1 immunoreactivity was confined to nonparenchymal cells located along the hepatic sinusoids whose morphological appearance was consistent with Kupffer cells (Figures 2 and 3). In both young and old rats, HO-1+ cells were more abundant in the periportal zones than in the perivenous regions (Figures 2 and 3). In agreement with the immunoblotting results showing increased HO-1 protein levels in old rat livers, aging was associated with a large and significant increase in HO-1+ Kupffer cells under control conditions in both liver zones (Figures 2 and 3). There were approximately 60% more HO-1+ Kupffer cells in the periportal regions and nearly 100% more in the perivenous regions in the livers of old rats compared with young rats.
Figure 2.
Increases in the number of nonparenchymal HO-1+ cells in the periportal liver region contribute to the elevations in HO-1 protein with aging and heat stress. (A) Representative micrographs of HO-1 immunostaining in liver samples from young and old rats under control conditions and at the indicated times after heat stress. HO-1+ nonparenchymal cells are indicated by an arrowhead and HO-1+ hepatocytes are indicated by arrows. (B) Quantitation of the number of HO-1+ nonparenchymal cells per microscopic field. Results are presented as mean + SE; n = 3–4 animals per group (except in the old 2-hour group, where n = 2). *Significant difference between young and old animals within a time point. †Significant effect of heat stress within an age group. HO-1, heme oxygenase-1.
Figure 3.
HO-1+ cells are increased with aging in the perivenous liver region. (A) Representative micrographs of HO-1 immunostaining in liver samples from young and old rats under control conditions and at 2 and 24 hours after heat stress. HO-1+ nonparenchymal cells are indicated by an arrowhead and HO-1+ hepatocytes are indicated by arrows. (B) Quantitation of the number of nonparenchymal HO-1+ cells per microscopic field. Results are presented as mean + SE; n = 3–4 animals per group (except in the old 2-hour group, where n = 2). *Significant difference between young and old animals within a time point. α: p = .063 young versus old. HO-1, heme oxygenase-1.
At 2 hours after heating, young rats showed a modest increase (approximately 30%) in HO-1+ Kupffer cells in the periportal regions (Figure 2). A similar trend was observed in the perivenous regions in young rats but did not reach statistical significance (p = .15; Figure 3). At 24 hours after heat stress, numbers of HO-1+ Kupffer cells were not statistically different than in the control condition. In old rats, heat stress did not affect the number of HO-1+ nonparenchymal cells at any time point.
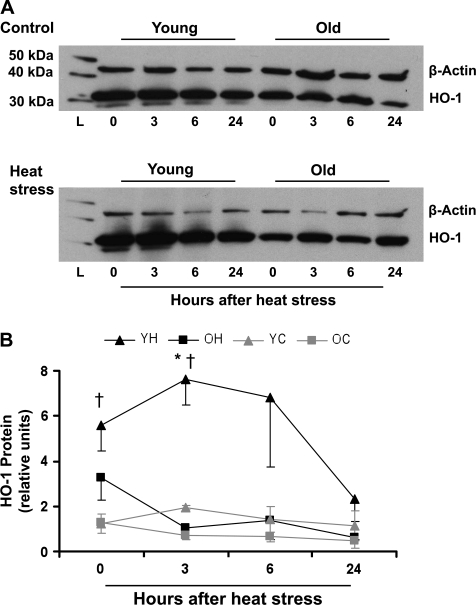
In contrast to the nonstressed livers, HO-1+ hepatocytes were observed following heat stress in both age groups. For this reason and because age-related and hyperthermia-induced changes in hepatic HO-1 protein levels could not be accounted for solely by an increase in the number of HO-1+ nonparenchymal cells in either the young or old animals, we assessed the effects of heat stress on HO-1 expression in isolated primary hepatocytes (Figure 4). To preserve the viability of hepatocytes, experiments were performed during a shorter time period and only one heat stress was used. High basal levels of HO-1 protein expression were observed in cells from both young and old rats, likely resulting from the stress of the isolation procedure (26). Nonetheless, hyperthermia further elevated HO-1 protein in hepatocytes from young rats. This increase was evident immediately after heating and was sustained for at least 3 hours. At 24 hours after heat stress, HO-1 protein levels did not differ from those in the nonstressed condition. In contrast, although hepatocytes from old rats showed a small increase in HO-1 immediately after heat stress, this was of lesser magnitude than that in the cells from young rats and did not reach statistical significance.
Figure 4.
Primary hepatocytes from young but not from old animals upregulate HO-1 protein after heat stress. Homogenates from primary hepatocytes were assayed for HO-1 protein expression via immunoblotting. (A) Representative immunoblots from young and old cells under control conditions (top panel) and at the indicated time points after heat stress (bottom panel). (B) Quantitation of relative HO-1 protein levels via densitometry: optical densities of HO-1 bands were normalized to those of the loading control, β-actin. YH, young heated; OH, old heated; YC, young control; OC, old control. Results are presented as mean ± SE. *Significant difference between YH and OH within a time point. †Significant difference between YH and YC within a time point. “L” designates the protein ladder. HO-1, heme oxygenase-1.
DISCUSSION
The crucial role of HO-1 in cellular protection (3–5) emphasizes the importance of understanding its regulation in models of physiological stress in vivo. In this study, we show novel effects of age and physiological stress on hepatic HO-1 protein expression and localization. Specifically, we show that although hepatic HO-1 protein is significantly elevated with age, upregulation of HO-1 expression by hepatocytes in response to stress is attenuated in old rats. Furthermore, we found that the basal increase in hepatic HO-1 in old animals is largely attributable to greater numbers of HO-1+ Kupffer cells in those livers. In contrast, the robust increase in HO-1 protein following heat stress in young rats was accompanied by only a modest increment in the number of HO-1+ Kupffer cells, suggesting a proportionately greater enhancement of HO-1 expression by hepatocytes. This interpretation is supported by the finding that isolated hepatocytes from young rats demonstrated a much greater increase in HO-1 expression following heat stress ex vivo. Taken together, our data point to the potential importance of differential expression of HO-1 by specific cell populations in response to physiological stressors.
Increased expression of HO-1 following exposure to a stress is usually considered to serve a protective function. Thus, the differences in HO-1 expression between young and old rats observed in the current study are intriguing. Previous work from our laboratory has demonstrated that young rats cope with hyperthermia better than old rats and that old rats show higher levels of oxidative damage following heat stress, particularly in hepatocytes (18,19). Thus, it is tempting to speculate that the baseline increase in HO-1 in the old rat livers, which is confined to Kupffer cells, does not contribute substantially to protection of the liver against heat-related damage. This situation differs from that of young rat livers, in which heat stress efficiently upregulates HO-1 in hepatocytes, where it can protect this critical cellular component. A more thorough time-course evaluation of HO-1 protein expression after heat stress will be important to fully characterize HO-1 kinetics and how this response might affect hepatic redox balance.
Results from the present study clearly show that the increase in HO-1 expression with aging can be explained by age-associated changes in the cellular composition of the liver. As reported in other laboratories, hepatic HO-1 protein is restricted to Kupffer cells under nonstressed conditions (20,21). Here we show that HO-1+ nonparenchymal cells increase with age, suggesting an increase in Kupffer cell numbers in livers from old rats. These data are consistent with the recent report by Hilmer and colleagues (27), which found a threefold increase in Kupffer cells in 24-month-old rats compared with 6-month-old rats. This increase in Kupffer cell number may be due to gradual infiltration of monocytes or in situ division of resident macrophages (28). To the extent that the greater abundance of HO-1 in old rat livers under basal conditions primarily reflects changes in Kupffer cell numbers, the increase in HO-1 may paradoxically indicate a greater susceptibility to liver injury because Kupffer cells play prominent roles in inflammation and oxidative stress (29).
Numbers of HO-1+ periportal Kupffer cells increased in the young animals following heat stress, which is a unique observation that merits further investigation. It is noteworthy that HO-1-positive cells tended to increase in the perivenous region as well. Although the change in nonparenchymal cell number appears to be a small proportion of the total increase in HO-1, it highlights a novel physiological response to heat stress. Possible explanations for this observation include either in situ cell division after stress or monocyte infiltration. The possibility of monocyte infiltration is particularly intriguing and has experimental support in other models (30,31). After liver transplant, there is an increase in cells that are both are HO-1 and ED1 positive in the isograft (31). The ED1 antigen identifies cells of the monocyte lineage (32); thus, an increase in this cell type likely reflects monocyte infiltration. It is also known that monocytes express HO-1 (33) and that they home to sites of tissue injury (31). Because branches of the hepatic artery make up the periportal zone, monocytes would be expected to take up residence in this region. Indeed, Baier and colleagues (30) have shown that ED2 (a marker of mature tissue macrophages) increases in the periportal region within 2 hours after the induction of sepsis. In that study, the authors speculated that the increase in ED2+ cells was due to infiltration rather than cellular proliferation because Ki-67+ (a marker of proliferation) cells increased at later time points (30). Because we assessed cell numbers approximately 50 hours after the first heat stress, we cannot rule out cellular division; therefore, future studies should examine time points after the first heat stress to determine whether the increase in cell numbers is due to infiltration or cellular division. Studies that more thoroughly evaluate the regulation of monocytes after heat stress using specific markers of this cell type will be important approaches for future investigations. Hence, young animals show a robust response to heat stress that may involve infiltration of monocytes.
In addition to heat stress, the process of hepatocyte isolation and culture itself can be viewed as a stressor because it resulted in a striking upregulation of HO-1 protein. This observation might be explained by the findings that hepatocyte isolation results in the activation of the immediate early transcription factor, AP-1 (26), and the HO-1 promoter region contains an AP-1-binding site (34). In the ex vivo studies, we observed that hepatocyte HO-1 is highly sensitive not only to changes in ambient temperature but also to other perturbations that challenge cellular homeostasis. Despite the higher baseline levels of HO-1 ex vivo, HO-1 was upregulated in hepatocytes from young rats at early time points after heat stress and declined to nonstressed levels after 24 hours. Notably, this pattern of HO-1 expression over time parallels the trends that were observed in vivo. In hepatocytes from old rats, levels of HO-1 tended to be higher immediately after heat stress and declined at later time points. Although they are different experimental systems, our ex vivo results support the in vivo observations showing that young animals have a more robust response to stressors than their older counterparts.
Delineating the role of HO-1 in this model will be an important area for future investigation and will require manipulation of HO-1 protein to determine under what circumstances and in which cell type its upregulation is protective after heat stress. In this study, we show clear differences in HO-1 protein expression that occur with the aging process and after heat stress. The altered regulation of HO-1 between young and old rats after heat stress observed in the present study suggest the possibility that HO-1 has different roles after heat stress in each age group. Overall, our results highlight age-associated modifications in the hepatic response to heat stress and suggest that changes in the cellular composition of the liver with aging contributes to the alteration in HO-1 protein expression.
FUNDING
This research was supported by National Institutes of Health Grant AG-12350. K.E.B. was supported by a Merit Review grant from the Veterans Administration.
Acknowledgments
The authors thank Gail Kurriger, Rong Fan, Megan Conner, Stephanie Shaw, and Mindy Nowak for expert technical assistance and Joan Seye for assistance with manuscript preparation.
References
- 1.Lee C-K, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 2.Zhang HJ, Drake VJ, Morrison JP, Oberley LW, Kregel KC. Molecular biology of thermoregulation: selected contribution: differential expression of stress-related genes with aging and hyperthermia. J Appl Physiol. 2002;92:1762–1769. doi: 10.1152/japplphysiol.00733.2001. [DOI] [PubMed] [Google Scholar]
- 3.Gong P, Cederbaum AI, Nieto N. Heme oxygenase-1 protects HepG2 cells against cytochrome P450 2E1-dependent toxicity. Free Radic Biol Med. 2004;36:307–318. doi: 10.1016/j.freeradbiomed.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Hori R, Kashiba M, Toma T, et al. Gene transfection of H25A mutant heme oxygenase-1 protects cells against hydroperoxide-induced cytotoxicity. J Biol Chem. 2002;277:10712–10718. doi: 10.1074/jbc.M107749200. [DOI] [PubMed] [Google Scholar]
- 5.Lee PJ, Alam J, Wiegand GW, Choi AM. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci U S A. 1996;93:10393–10398. doi: 10.1073/pnas.93.19.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibahara S, Muller RM, Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem. 1987;262:12889–12892. [PubMed] [Google Scholar]
- 7.Vile G, Basu-Modak S, Waltner C, Tyrrell R. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci U S A. 1994;91:2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 9.Otterbein LE, Bach FH, Alam J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 10.Bauer M, Bauer I. Heme oxygenase-1: redox regulation and role in the hepatic response to oxidative stress. Antioxid Redox Signal. 2002;4:749–758. doi: 10.1089/152308602760598891. [DOI] [PubMed] [Google Scholar]
- 11.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999;13:1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
- 12.Zhang HJ, Xu L, Drake VJ, Xie L, Oberley LW, Kregel KC. Heat-induced liver injury in old rats is associated with exaggerated oxidative stress and altered transcription factor activation. FASEB J. 2003;17:2293–2295. doi: 10.1096/fj.03-0139fje. [DOI] [PubMed] [Google Scholar]
- 13.Lavrovsky Y, Song CS, Chatterjee B, Roy AK. Age-dependent increase of heme oxygenase-1 gene expression in the liver mediated by NF-ΚB. Mech Ageing Dev. 2000;114:49–60. doi: 10.1016/s0047-6374(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 14.Patriarca S, Furfaro AL, Cosso L, et al. Heme oxygenase 1 expression in rat liver during ageing and ethanol intoxication. Biogerontology. 2007;8:365–372. doi: 10.1007/s10522-006-9079-x. [DOI] [PubMed] [Google Scholar]
- 15.Abraham NG, Levere RD, Freedman ML. Effect of age on rat liver heme and drug metabolism. Exp Gerontol. 1985;20:277–284. doi: 10.1016/0531-5565(85)90053-1. [DOI] [PubMed] [Google Scholar]
- 16.Kang MJ, Kim HJ, Kim HK, et al. The effect of age and calorie restriction on HIF-1-responsive genes in aged liver. Biogerontology. 2005;6:27–37. doi: 10.1007/s10522-004-7381-z. [DOI] [PubMed] [Google Scholar]
- 17.Bloomer SA, Brown KE, Buettner GR, Kregel KC. Dysregulation of hepatic iron with aging: implications for heat stress-induced oxidative liver injury. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1165–R1174. doi: 10.1152/ajpregu.00719.2007. [DOI] [PubMed] [Google Scholar]
- 18.Oberley TD, Swanlund JM, Zhang HJ, Kregel KC. Aging results in increased autophagy of mitochondria and protein nitration in rat hepatocytes following heat stress. J Histochem Cytochem. 2008;56:615–627. doi: 10.1369/jhc.2008.950873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang HJ, Doctrow SR, Xu L, et al. Redox modulation of the liver with chronic antioxidant enzyme mimetic treatment prevents age-related oxidative damage associated with environmental stress. FASEB J. 2004;18:1547–1549. doi: 10.1096/fj.04-1629fje. [DOI] [PubMed] [Google Scholar]
- 20.Bauer I, Wanner GA, Rensing H, et al. Expression pattern of heme oxygenase isoenzymes 1 and 2 in normal and stress-exposed rat liver. Hepatology. 1998;27:829–838. doi: 10.1002/hep.510270327. [DOI] [PubMed] [Google Scholar]
- 21.Goda N, Suzuki K, Naito M, et al. Distribution of heme oxygenase isoforms in rat liver. Topographic basis for carbon monoxide-mediated microvascular relaxation. J Clin Invest. 1998;101:604–612. doi: 10.1172/JCI1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oztezcan S, Kirgiz B, Unlucerci Y, et al. Heme oxygenase induction protects liver against oxidative stress in x-irradiated aged rats. Biogerontology. 2004;5:99–105. doi: 10.1023/B:BGEN.0000025073.46100.01. [DOI] [PubMed] [Google Scholar]
- 23.Hall DM, Xu L, Drake VJ, et al. Aging reduces adaptive capacity and stress protein expression in the liver after heat stress. J Appl Physiol. 2000;89:749–759. doi: 10.1152/jappl.2000.89.2.749. [DOI] [PubMed] [Google Scholar]
- 24.Hall DM, Sattler GL, Sattler CA, et al. Aging lowers steady-state antioxidant enzyme and stress protein expression in primary hepatocytes. J Gerontol A Biol Sci Med Sci. 2001;56:B259–B267. doi: 10.1093/gerona/56.6.b259. [DOI] [PubMed] [Google Scholar]
- 25.Zhang HJ, Doctrow SR, Oberley LW, Kregel KC. Chronic antioxidant enzyme mimetic treatment differentially modulates hyperthermia-induced liver HSP70 expression with aging. J Appl Physiol. 2006;100:1385–1391. doi: 10.1152/japplphysiol.01046.2005. [DOI] [PubMed] [Google Scholar]
- 26.Rana B, Mischoulon D, Xie Y, Bucher NL, Farmer SR. Cell-extracellular matrix interactions can regulate the switch between growth and differentiation in rat hepatocytes: reciprocal expression of C/EBP alpha and immediate-early growth response transcription factors. Moll Cell Biol. 1994;14:5858–5869. doi: 10.1128/mcb.14.9.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilmer SN, Cogger VC, Le Couteur DG. Basal activity of Kupffer cells increases with old age. J Gerontol A Biol Sci Med Sci. 2007;62:973–978. doi: 10.1093/gerona/62.9.973. [DOI] [PubMed] [Google Scholar]
- 28.Widmann JJ, Fahimi HD. Proliferation of mononuclear phagocytes (Kupffer cells) and endothelial cells in regenerating rat liver. A light and electron microscopic cytochemical study. Am J Pathol. 1975;80:349–366. [PMC free article] [PubMed] [Google Scholar]
- 29.Videla LA, Tapia G, Fernandez V. Influence of aging on Kupffer cell respiratory activity in relation to particle phagocytosis and oxidative stress parameters in mouse liver. Redox Rep. 2001;6:155–159. doi: 10.1179/135100001101536265. [DOI] [PubMed] [Google Scholar]
- 30.Baier PK, Baumgartner U, Hempel S, Wolff-Vorbeck G, von Dobschuetz E, Hopt UT. Kupffer cells infiltrate liver tissue early after ischemia-reperfusion and partial hepatectomy. Eur Surg Res. 2005;37:290–297. doi: 10.1159/000089239. [DOI] [PubMed] [Google Scholar]
- 31.Kato H, Amersi F, Buelow R, et al. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant. 2001;1:121–128. [PubMed] [Google Scholar]
- 32.Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2, and ED3. Immunology. 1985;54:589–599. [PMC free article] [PubMed] [Google Scholar]
- 33.Philippidis P, Mason JC, Evans BJ, et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94:119–126. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- 34.Alam J, Den Z. Distal AP-1 binding sites mediate basal level enhancement and TPA induction of the mouse heme oxygenase-1 gene. J Biol Chem. 1992;267:21894–21900. [PubMed] [Google Scholar]