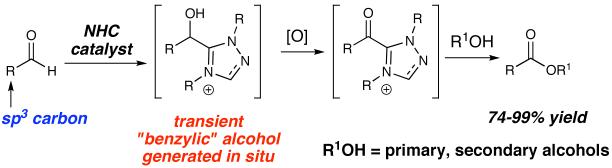
Abstract
N-Heterocyclic carbenes catalyze the oxidation of unactivated aldehydes to esters with manganese(IV) oxide in excellent yield. The reaction proceeds through a transient activated alcohol, which, when generated in situ allows for the selective oxidation of the aldehyde under mild conditions. These conditions successfully oxidize potentially epimerizable aldehydes and alcohols while preserving stereochemical integrity. A variety of ester derivatives can be synthesized with variation of the acylated alcohol as well as the unactivated aldehyde.
Efficient oxidations carried out under mild conditions are important organic transformations.1 Of particular interest are processes that achieve multiple oxidations and/or functionalization in a single procedural step.2 Transformations of this type have several attractive attributes including their potential to generate and/or transform challenging products in situ. These advantages have inspired numerous investigations into the direct oxidation of aldehydes to esters.3 Recent methods have employed Oxone,4 pyridinium hydrobromide perbromide,5 or peroxides6 as oxidants, but these approaches can suffer from toxic or expensive reagents or competing oxidation of the alcohol nucleophile. The less efficient two-step process involving the oxidation of the aldehyde to a carboxylic acid and subsequent alkylation can be significantly complicated by over-oxidation of electron-rich aromatic rings and chemoselectivity issues during the alkylation step. Lastly, the use of saturated aldehydes in either approach can be problematic due to enolization/aldol issues.
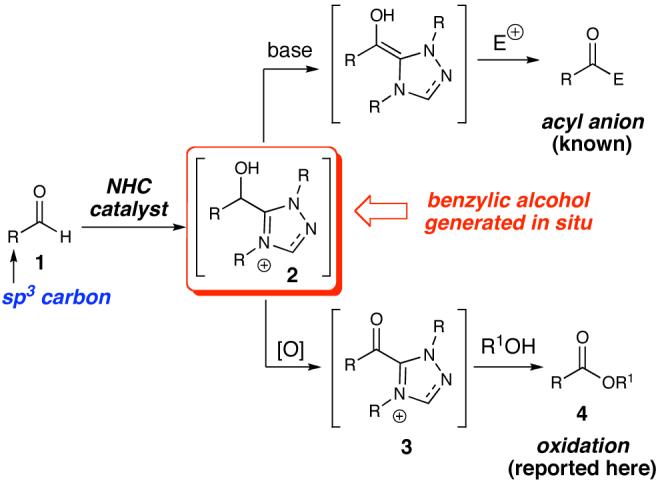
While N-heterocyclic carbenes (NHCs) are known to catalyze interesting redox processes,7 the potential and synthetic utility of these reactions are far from being fully realized.8 During our recent investigations of carbene-catalyzed redox reactions9 and related process,10 we recognized that a transient benzylic alcohol intermediate should be accessible through addition of an NHC to any aldehyde (Figure 1). This process fundamentally diverges from previous reports since the aromatic nature of the NHC catalyst imparts unique reactivity in the presence of a selective oxidant upon addition to a saturated (unactivated) aldehyde. Connon and coworkers have very recently reported an extension of our 2007 report9 using a thiazolium catalyst.11 However, their method was low yielding when attempts were made with saturated aldehydes as substrates.12 In our 2007 paper, we reported a single example of the oxidation of a saturated aldehyde9 (91% yield for the oxidation of hydrocinnamaldehyde). Given the potential of this mild oxidative transformation, we engaged in a full exploration of this process which has taken us well beyond our recent NHC-catalyzed tandem oxidations of allylic or benzylic alcohols. Herein, we report the general oxidation of saturated aldehydes (1) to esters (4) via activated alcohol intermediates (2) using carbene catalysis.
Figure 1.
Oxidation of Unactivated Aldehydes
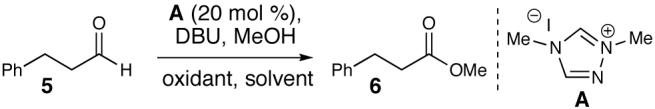
Initial efforts were directed toward finding the optimal oxidizing agent for the activated alcohol intermediate. Metal-free oxidation conditions13 were investigated for the conversion of aldehyde 5 to the methyl ester (6, Table 1, entries 1-4). A screen of azolium salts14 (not shown) indicated that the simple dimethyl triazolium iodide (A) was the optimal pre-catalyst in terms of conversion. These preliminary reactions showed promise, but decomposition was evident before complete consumption of the aldehyde starting material. Fortunately, manganese(IV) oxide proved to be an ideal oxidant for this process, efficiently providing the methyl ester in less than three hours in excellent yields with 10 mol % of azolium A. The reaction can be carried out either in methanol (entry 5) or with 5 equivalents of the alcohol in dichloromethane (entry 6). On a multi-gram scale, the optimized reaction provides nearly quantitative conversion to the ester (entry 7).15
Table 1.
Oxidation Optimization
 | |||
|---|---|---|---|
| entry | oxidant | solvent | yieldb |
| 1 | o-chloranil (2 equiv) | THF | (<10) |
| 2 | TEMPO (1 mol %), NaOCl (aq.) (2 equiv) | CH2Cl2/H2O | 0 |
| 3 | TEMPO (1 mol %), Oxone (4 equiv) | CH2Cl2 | (52) |
| 4 | TEMPO (1 mol %), n-Bu4NSO5c (2 equiv) | CH2Cl2 | (59) |
| 5 | MnO2 (5 equiv) | MeOH | 99 |
| 6 | MnO2 | CH2Cl2 | 98d |
| 7 | MnO2 19 mmol scale | CH2Cl2 | 98d |
20 mol% A, 1.2 equiv DBU, 2-5 equiv methanol, 0.2 M in solvent.
Isolated yields (yields in parentheses calculated by GC with dodecane as an internal standard).
Prepared from commercial Oxone (see Supporting Information).
10 mol% A, 1.1 equiv DBU, 5 equiv methanol, 5 equiv MnO2, 0.2 M in CH2Cl2.
This new carbene-catalyzed transformation is remarkable when compared to the cyanide-promoted Corey-Gilman oxidation of allylic alcohols.16 In distinct contrast to the transformation in Table 1, a full equivalent of NaCN with MnO2 and aldehyde 5 does not provide any saturated ester.17 Furthermore, no oxidation is observed when A is omitted, highlighting the unique ability of the NHC catalyst to generate activated alcohols in situ.
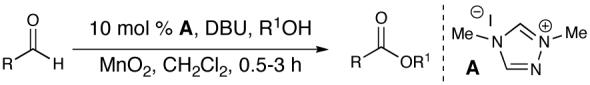
A key feature of this process is that a wide variety of saturated aldehydes and alcohols can be employed (Table 2). For example, methanol as the nucleophile affords the fastest reactions, but other primary (entries 2, 4, 5), and secondary alcohols (Table 2, entries 3, 6) afford the desired esters. With this process, direct access to protected carboxylate derivatives (TMSE and trichloroethyl) from the corresponding aldehydes is high yielding.18
Table 2.
NHC-Catalyzed Oxidation Scope
 | |||
|---|---|---|---|
| entry | substrate | product | yielda |
| 1 | R1OH = MeOH | 98 (6) | |
| 2 | = n-PrOH | 82 (7) | |
| 3 | = CyOH | 88 (8) | |
| 4 | 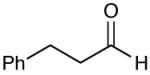 |
85 (9) | |
| 5 | 87 (10) | ||
| 6 | 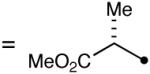 |
74b (11) | |
| 7 | 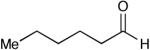 |
91 (12) | |
| 8 | 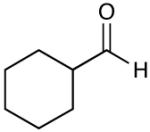 |
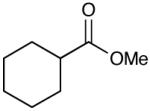 |
96 (13) |
| 9 | 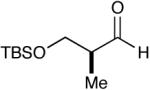 |
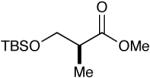 |
91c (14) |
| 10 | 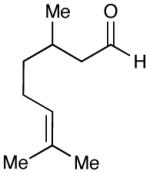 |
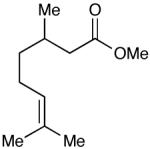 |
93 (15) |
| 11 | 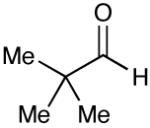 |
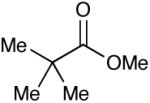 |
56 (16) |
| 12 | 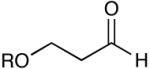 |
94 (17) | |
| 13 | 95 (18) | ||
| 14 | 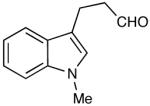 |
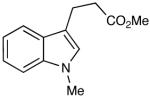 |
90 (19) |
| 15 | 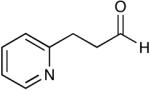 |
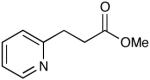 |
88 (20) |
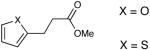
| 16 | 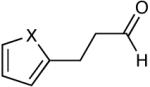 |
 |
92 (21) |
| 17 | 96 (22) | ||
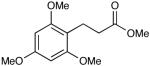
| 18 | 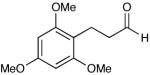 |
 |
99 (23) |
Isolated yield. See Table 1 for general procedure.
Methyl (S)-(-)-lactate (97% ee) yields the ester in 93% ee.
25 mol % triazolium salt A yields the methyl ester in 92% ee from enantiopure aldehyde.
Most importantly, this new oxidation is successful with electron-rich aromatic rings present (entries 16, 17, 18). These results are distinct from established chlorine-based oxidation conditions which can promote significant electrophilic aromatic chlorination.19 Substitution at the α-(entries 8, 9) or β-position (entry 10) has little effect on the yield, although moderate yields are observed with more congested aldehydes such as pivalaldehyde (entry 11). This decrease in efficiency is most likely due to the slow formation of the activated alcohol resulting from the difficult addition of the carbene catalyst to the hindered aldehyde.
The oxidation is compatible with a range of functional groups, including potentially epimerizable substrates. The acylation of methyl lactate occurs with minimal loss of stereochemical information (entry 6). An aldehyde with a stereogenic center at the α-position undergoes a minor amount of epimerization (from 99% ee to 92% ee), with 25 mol% catalyst to increase the rate of oxidation (entry 9) so that the reaction was complete in 30 to 45 minutes. Despite previous reports of silicon activation by NHCs,20 aldehydes containing silyl ethers are stable to these conditions (entries 9, 12, 13) and smoothly undergo oxidation. Multiple nitrogen-containing heterocycles are compatible, yielding the indolyl-substituted ester and the pyridyl-substituted ester in high yields (entries 14, 15). Electron-rich oxygen and sulfur-containing heterocycles (entries 16, 17) give similarly excellent results.
In summary, saturated esters are produced in a single flask from a wide variety of aldehydes by a carbene-catalyzed oxidation. In this process, the N-heterocyclic carbene catalyst formed in situ undergoes addition to a saturated aldehyde and the aromatic framework of the catalyst activates the carbinol intermediate generated in situ for a mild manganese(IV) oxidation. The scope of the reaction includes electron-rich heterocyclic aldehydes and substrates containing potentially sensitive stereochemistry. Further investigations capitalizing on the unique aspects of carbene catalysis for oxidations and reductions are underway and will be reported in due course.
Supplementary Material
Acknowledgment
Research support was generously provided by NIH/NIGMS (GM73072), Abbott Laboratories, Amgen, AstraZeneca, GlaxoSmithKline, the Sloan Foundation, and Boehringer-Ingelheim. B. E. M. was supported by a GAANN Fellowship.
References
- 1.(a) Tojo G, Fernández M. Oxidation of Alcohols to Aldehydes and Ketones. Springer; New York: 2006. [Google Scholar]; (b) Sheldon RA, Arends I, Ten Brink GJ, Dijksman A. Acc. Chem. Res. 2002;35:774–781. doi: 10.1021/ar010075n. [DOI] [PubMed] [Google Scholar]; (c) Caron P, Dugger RW, Ruggeri SG, Ragan JA, Ripin DHB. Chem. Rev. 2006;106:2943–2989. doi: 10.1021/cr040679f. [DOI] [PubMed] [Google Scholar]; (d) Zhan BZ, Thompson A. Tetrahedron. 2004;60:2917–2935. [Google Scholar]
- 2.For a review of tandem oxidation processes, see:Taylor RJK, Reid M, Foot J, Raw SA. Acc. Chem. Res. 2005;38:851–869. doi: 10.1021/ar050113t.
- 3.For examples, see:Ekoue-Kovi K, Wolf C. Chem.-Eur. J. 2008;14:6302–6315. doi: 10.1002/chem.200800353.Marko IE, Mekhalfia A, Ollis WD. Synlett. 1990:347–348.Grigg R, Mitchell TRB, Sutthivaiyakit S. Tetrahedron. 1981;37:4313–4319.Williams DR, Klingler FD, Allen EE, Lichtenthaler FW. Tetrahedron Lett. 1988;29:5087–5090.McDonald C, Holcomb H, Kennedy K, Kirkpatrick E, Leathers T, Vanemon P. J. Org. Chem. 1989;54:1213–1215.
- 4.Travis BR, Sivakumar M, Hollist GO, Borhan B. Org. Lett. 2003;5:1031–1034. doi: 10.1021/ol0340078. [DOI] [PubMed] [Google Scholar]
- 5.Sayama S, Onami T. Synlett. 2004:2739–2745. [Google Scholar]
- 6.(a) Gopinath R, Barkakaty B, Talukdar B, Patel BK. J. Org. Chem. 2003;68:2944–2947. doi: 10.1021/jo0266902. [DOI] [PubMed] [Google Scholar]; (b) Yoo WJ, Li CJ. Tetrahedron Lett. 2007;48:1033–1035. [Google Scholar]
- 7.(a) Enders D, Niemeier O, Henseler A. Chem. Rev. 2007;107:5606–5655. doi: 10.1021/cr068372z. [DOI] [PubMed] [Google Scholar]; (b) Marion N, Diez-Gonzalez S, Nolan SP. Angew. Chem., Int. Ed. 2007;46:2988–3000. doi: 10.1002/anie.200603380. [DOI] [PubMed] [Google Scholar]; (c) Zeitler K. Angew. Chem., Int. Ed. 2005;44:7506–7510. doi: 10.1002/anie.200502617. [DOI] [PubMed] [Google Scholar]
- 8.For relevant examples, see:Castells J, Llitjos H, Morenomanas M. Tetrahedron Lett. 1977:205–206.Inoue H, Higashiura K. Chem. Commun. 1980:549–550.Miyashita A, Suzuki Y, Nagasaki I, Ishiguro C, Iwamoto K, Higashino T. Chem. Pharm. Bull. 1997;45:1254–1258.Tam SW, Jimenez L, Diederich F. J. Am. Chem. Soc. 1992;114:1503–1505.Chan A, Scheidt KA. J. Am. Chem. Soc. 2006;128:4558–4559. doi: 10.1021/ja060833a.
- 9.Maki BE, Chan A, Phillips EM, Scheidt KA. Org. Lett. 2007;9:371–374. doi: 10.1021/ol062940f. [DOI] [PubMed] [Google Scholar]
- 10.(a) Chan A, Scheidt KA. Org. Lett. 2005;7:905–908. doi: 10.1021/ol050100f. [DOI] [PubMed] [Google Scholar]; (b) Chan A, Scheidt KA. J. Am. Chem. Soc. 2007;129:5334–5335. doi: 10.1021/ja0709167. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wadamoto M, Philllips EM, Reynolds TE, Scheidt KA. J. Am. Chem. Soc. 2007;129:10098–10099. doi: 10.1021/ja073987e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Phillips EM, Wadamoto M, Chan A, Scheidt KA. Angew. Chem. Int. Ed. 2007;46:3107–3110. doi: 10.1002/anie.200605235. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Phillips EM, Reynolds TE, Scheidt KA. J. Am. Chem. Soc. 2008;130:2416–2417. doi: 10.1021/ja710521m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chan A, Scheidt KA. J. Am. Chem. Soc. 2008;130:2740–2741. doi: 10.1021/ja711130p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noonan C, Baragwanath L, Connon SJ. Tetrahedron Lett. 2008;49:4003–4006. [Google Scholar]
- 12.A 16% yield for the oxidation of hexanal to methyl hexanoate was reported in reference 10. A 91% yield is achieved using triazolium salt A and MnO2 (see Table 2, entry 7).
- 13.Adam W, Saha-Moller CR, Ganeshpure PA. Chem. Rev. 2001;101:3499–3548. doi: 10.1021/cr000019k. [DOI] [PubMed] [Google Scholar]
- 14.Substitution of A with 1,3-bis(2,4,6-trimethylphenyl)imidazolium chloride resulted in only partial conversion (∼25%) of the aldehyde, with significant decomposition under the standard reaction conditions.
- 15.The use of water in these reactions currently does not provide high yields of the corresponding carboxylic acid. Investigations to understand this observation and apply this new oxidation for the synthesis of carboxylic acids are underway.
- 16.(a) Corey EJ, Gilman NW, Ganem BE. J. Am. Chem. Soc. 1968;90:5616–5617. [Google Scholar]; (b) Corey EJ, Katzenellenbogen JA, Gilman NW, Roman SA, Erickson BW. J. Am. Chem. Soc. 1968;90:5618–5620. [Google Scholar]; (c) Gilman NW. Chem. Commun. 1971:733–734. [Google Scholar]
-
17.Following the procedure ofFoot JS, Kanno H, Giblin GMP, Taylor RJK. Synthesis. 2003:1055–1064.
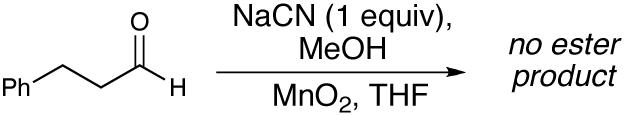
- 18.Wuts PGM, Greene TW. Protective Groups in Organic Synthesis. 4th ed. Wiley & Sons; Hoboken, NJ: 2007. [Google Scholar]
-
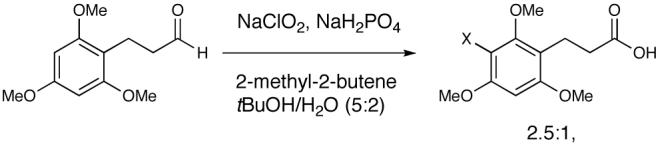
19.Bal BS, Childers WE, Pinnick HW. Tetrahedron. 1981;37:2091–2096.Wilson SR, Tofigh S, Misra RN. J. Org. Chem. 1982;47:1360–1361.Zhao MZ, Li J, Mano E, Song ZG, Tschaen DM, Grabowski EJJ, Reider PJ. J. Org. Chem. 1999;64:2564–2566.Subjecting 3-(2,4,6-trimethoxyphenyl)propanal to Pinnick oxidation conditions resulted in significant chlorinations of the aromatic ring. See Supporting Information for details.
- 20.(a) Reynolds TE, Stern CA, Scheidt KA. Org. Lett. 2007;9:2581–2584. doi: 10.1021/ol0710515. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Song JJ, Tan ZL, Reeves JT, Yee NK, Senanayake CH. Org. Lett. 2007;9:1013–1016. doi: 10.1021/ol0630494. [DOI] [PubMed] [Google Scholar]; (c) Fukuda Y, Maeda Y, Kondo K, Aoyama T. Synthesis. 2006:1937–1939. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.