Abstract
The upper interventricular septum may be prominent in elderly individuals, a finding referred to as discrete upper septal thickening (DUST). We examined the prevalence, clinical and echocardiographic correlates, and prognostic significance of DUST in a community-based sample. We evaluated Framingham Study participants who underwent routine echocardiography. In 3562 Framingham Study participants (mean age 58 years, 57% women), DUST was observed in 52 participants. The clinical correlates of DUST were: increasing age (odds ratio [OR] per 10 year increment 2.59, 95% confidence intervals [CI] 1.64-4.08), and systolic blood pressure (OR per SD increment 1.55, 95% CI 1.15-2.09). DUST was positively associated with left ventricular (LV) fractional shortening and mitral annular calcification but inversely with LV diastolic dimensions (p<0.02 for all). On follow-up (mean 15 years), 732 individuals died (33 with DUST) and 560 experienced a cardiovascular disease event (18 with DUST). Adjusting for cardiovascular risk factors, DUST was not associated with CVD or mortality risk (p>0.30 for both). The follow-up component of our study suggests that DUST is not independently associated with adverse prognosis.
Keywords: Echocardiography, ventricular septum, epidemiology, prognosis
Introduction
Asymmetric or disproportionate septal hypertrophy is a hallmark of hypertrophic cardiomyopathy,1 but it may be found also in older hypertensive individuals1-4 and in a variety of congenital and acquired lesions in the absence of hypertrophic cardiomyopathy.5 Indeed, disproportionate septal hypertrophy appears to be a normal finding in the fetus and disappears by the age of 1 or 2 years.6 In older children and adults, discrete upper septal thickening (DUST) may be seen with lesions producing long-standing right ventricular hypertension (such as pulmonary stenosis and primary pulmonary hypertension, as well as other congenital heart diseases).5
The thickening of the ventricular septum and its changing shape in some older adults has been the subject of several prior reports.7-12 Investigators have reported that interventricular septum may become “S” shaped and may bulge into the left ventricular outflow tract.7-12 Several terms have been used to describe this phenomenon, including DUST, sigmoid septum; and discrete upper septal knuckle. Although several investigators have evaluated the functional significance of age-related changes in septal shape in hospital-based studies,7,9-12 the prevalence and clinical correlates of DUST in the general population has not been investigated. Furthermore, the prognostic significance of DUST in the community is unknown. Accordingly, we investigated the prevalence, clinical correlates and echocardiographic features, and prognostic significance of DUST in a large community-based sample.
Methods
Study Sample
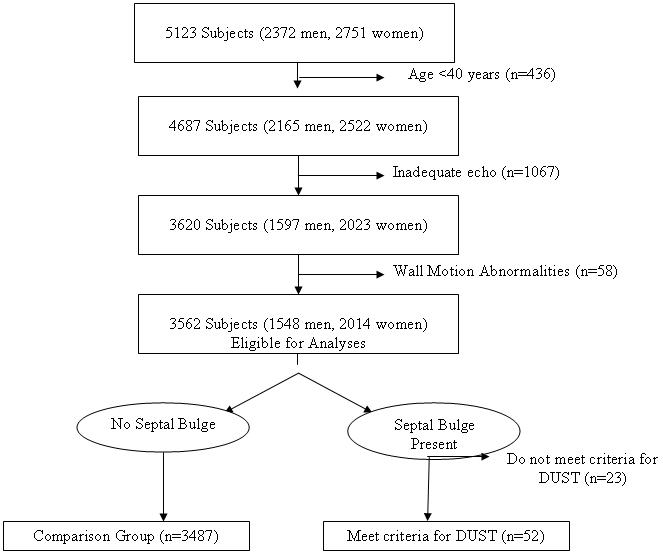
The selection criteria and study design of the Framingham Heart Study and Framingham Offspring study have been detailed extensively.13,14 Between 1987 and 1990, 5723 participants underwent routine echocardiography at the 20th examination cycle of the original cohort (n=1200) and the 4th examination of the Offspring cohort (n=3923). We excluded 1561 participants from the present investigation for the reasons displayed in Figure 1. After these exclusions, 3562 participants remained eligible. The Institutional Review Board at Boston University Medical Center approved the study, and all participants gave written informed consent.
Figure 1.
Exclusion criteria and derivation of study sample.
Echocardiographic Methods
At the index examinations, all attendees underwent routine M-mode and two-dimensional echocardiography with Doppler color flow imaging (model 77020AC; Hewlett- Packard Co; Andover, Mass.). M-mode measurements of: LV internal dimension in diastole (LVDD) and systole (LVDS), and end-diastolic posterior wall (PW) and interventricular septum (IVS) thicknesses, and aortic root diameter, and end-systolic left atrium (LA) were obtained using a ‘leading edge’ technique, averaging measurements in 3 cardiac cycles according to the American Society of Echocardiography guidelines.15 LV mass was calculated by using the formula 0.8[1.04(LVDD+IVS+PW)3 - (LVDD)3 ] +0.6.16 LV wall thickness was defined as the sum of the end-diastolic thicknesses of the PW and IVS. LV fractional shortening was calculated as (LVDD-LVDS)/LVDD. Mitral annular calcification was considered present if an echo-density was visualized throughout systole and diastole, was distinguishable from the posterior mitral-valve leaflet, and was located anterior and parallel to the posterior left ventricular wall. Valve disease was defined as mild or greater degree of stenosis or regurgitation of the aortic or mitral valves on Doppler echocardiography.
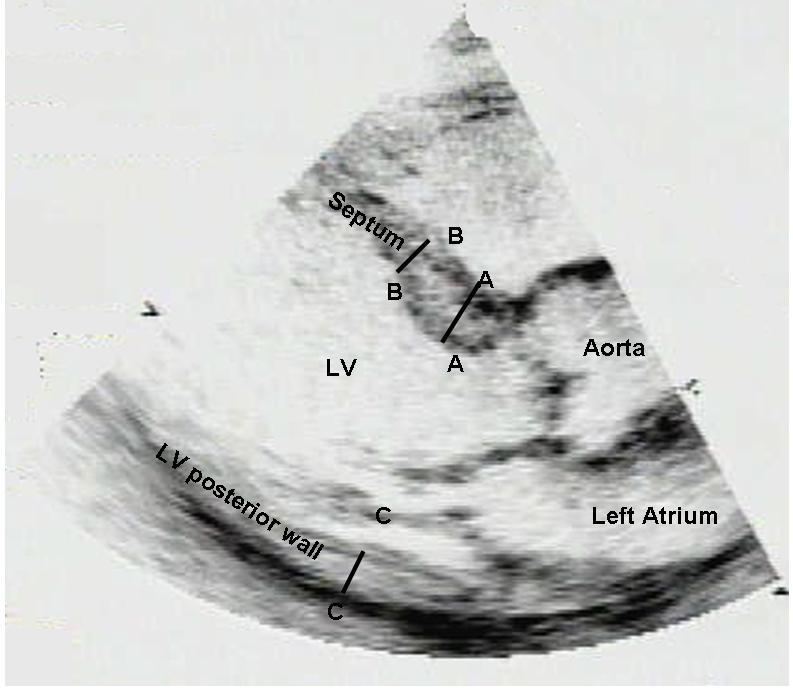
Two-dimensional echocardiographic studies of the 3562 eligible participants were evaluated routinely for the presence of a discrete upper septal “knuckle” upon visual assessment by a cardiologist or a sonographer. The presence of a septal knuckle served as a screening test for further evaluation for the presence of DUST. Seventy-five participants had a prominent upper septal knuckle on visual assessment of the two-dimensional echocardiogram. These 75 studies were then reviewed by a sonographer (DF) to determine which participants fulfilled criteria for DUST. For this purpose, two-dimensional measurements of the upper septal, mid septal and posterior wall thicknesses were made at end-diastole (from the parasternal long-axis view) using a ‘leading edge to leading edge’ technique (Figure 2). We defined DUST based on presence of all 4 of the following criteria: 1. Upper septal knuckle by visual assessment; 2. Upper interventricular septum thickness ≥1.4 cm; 3. Upper septum thickness/mid septum thickness ≥1.3; 4. No wall motion abnormalities or scar in the mid septum that could result in isolated septal thickening. We chose these criteria to emphasize specificity of a diagnosis of DUST.
Figure 2.
Schematic representation of criteria for DUST: discrete upper septal ‘knuckle’ on visual assessment, and upper septal diastolic thickness ≥1.4 cm (AA in Figure), and upper (AA)/mid-septum (BB) diastolic thickness ≥1.3 in the absence of septal wall motion abnormalities. CC represents the posterior wall.
Follow-up
All Framingham Heart Study participants are under continuous surveillance for the occurrence of CVD events and death. A committee of three investigators reviewed all suspected cardiovascular events by examining hospitalization records, physician office notes, and pathology reports. Investigators adjudicating endpoints had no knowledge of the echocardiographic measurements. A Framingham Heart Study neurologist evaluated participants with suspected cerebrovascular events, and a separate review committee that included a neurologist adjudicated these events. Incident CVD events during follow-up were defined as: recognized or unrecognized myocardial infarction, angina pectoris, coronary insufficiency, coronary heart disease death, stroke, transient ischemic attack, congestive heart failure, or intermittent claudication. Criteria for the diagnoses of cardiovascular events have been described elsewhere.17
Statistical analysis
We evaluated the prevalence of DUST in our sample within 5-year age groups. We used multiple logistic regression analyses to examine the association of select clinical variables and echocardiographic findings with the presence of DUST. Clinical variables considered included age, sex, body mass index, systolic blood pressure, diastolic blood pressure, prevalent CVD, atrial fibrillation and syncope. Echocardiographic variable evaluated included LV mass, LV end-diastolic internal dimensions, wall thickness, left atrium, aortic root, LV fractional shortening, valvular or stenosis, and mitral annular calcification. Multivariable analyses used a forward stepwise selection procedure with p<0.05 criterion for retention in the model.
We used sex-pooled multivariable-adjusted Cox proportional-hazards regression analysis18 to examine the association of DUST with the incidence of first CVD event and death (separate analyses for each outcome), after confirming that the assumption of proportionality of hazards was met. Individuals with prevalent CVD at baseline were excluded from the analyses of incident CVD. Multivariable models adjusted for sex and the following covariates defined at baseline: age, current smoking, systolic blood pressure, diastolic blood pressure, total/HDL cholesterol, antihypertensive treatment, and diabetes. Prevalent CVD was included as an additional covariate in analyses with death as the outcome.
Results
Study Sample Characteristics
The mean age of the 3562 Framingham Study participants was 58 years and 57% were women. Fifty-seven of the 75 participants with a visual septal knuckle met the criteria for DUST and constituted our “cases”; the 3487 individuals without a visual knuckle served as the referent groups. Twenty-three participants with a septal knuckle but who did not meet the criteria for DUST were included in the analyses for estimating the prevalence of DUST (included in the denominator), but were excluded from the analyses evaluating the clinical and echocardiographic correlates of DUST (Figure 1). Considering the 52 participants (15 men, 37 women) that met our criteria for DUST, the overall prevalence was 1.5 percent. Figure 2 displays the prevalence of DUST in relation to study participant age. The prevalence of DUST was markedly higher (17.8%) in the eighth decade of life.
The clinical & echocardiographic characteristics of DUST participants and referents are compared in Table 1. Participants with DUST were on average 17 years older, more likely to be women, and had a higher prevalence of hypertension, diabetes and CVD compared to participants without DUST (unadjusted comparisons). After adjustment for age, the association of DUST with female sex became statistically non-significant. Participants with DUST had also a higher prevalence of mitral annular calcification, smaller end-diastolic LV dimensions, a higher mean value of fractional shortening and a higher prevalence of aortic or mitral valvular regurgitation (age and sex-adjusted comparisons).
Compared to individuals with DUST, participants with a septal bulge but who did not meet the criteria for DUST were more likely to be male, but had a similar clinical profile in terms of risk factor burden (Appendix Table 1); they had larger mean LV end-diastolic dimensions but lower mean fractional shortening in comparison.
Clinical and Echocardiographic Correlates of DUST: Multivariable Analyses
After adjustment for age and sex, only hypertension treatment was associated with DUST (Table 2); syncope, CVD and atrial fibrillation were not associated with DUST. Of the echocardiographic variables evaluated, after adjustment for age and sex, LV end-diastolic dimensions (inverse), and fractional shortening and mitral annular calcification (both positive) were associated with DUST.
Incidence of CVD events and death
During a mean follow-up of 15 years (range 0.3-18 years), 732 participants died (including 33 individuals with DUST), 560 experienced a first CVD event (including 18 with DUST). In multivariable analyses, presence of DUST was not associated with CVD risk (hazards ratio [HR] 1.29, 95% CI 0.77-2.16, p=0.32), or with mortality (HR 1.05, 95% CI 0.71-1.55, p=0.82).
Discussion
Our observations suggest that DUST occurs predominantly in elderly individuals with higher systolic blood pressure. Although 69% of our participants with DUST were women, female sex was not associated with prevalent DUST after accounting for age. Echocardiographic findings associated with presence of DUST include increasing LV systolic function, mitral annular calcification, and smaller LV end-diastolic dimensions. On prospective follow-up, DUST was not associated with risk of CVD or death in multivariable analyses. Although DUST is not independently associated with an adverse prognosis, its presence may serve to direct the attention of clinicians to the concomitant presence of systolic hypertension, and thereby promote recognition and treatment of high blood pressure in the elderly.
Comparison with Published Literature
Prior investigations of clinical correlates of DUST have been hospital-based, utilized a small number of patients, and did not include a comparison referent group.7-12 Our observation that DUST is associated with higher systolic pressure is consistent with that of other investigators who have suggested that the hypertrophy in the basal part of the IVS in the elderly may represent a pattern of cardiac hypertrophy caused by hypertension.2-4,19 Whereas development of concentric left ventricular hypertrophy is more typical, some hypertensive individuals may exhibit isolated upper septal hypertrophy. However, our findings differ from those of Toth et al. who reported that men had consistently greater sigmoidicity of the septum compared with women.12 It is noteworthy that a septal bulge in the absence of criteria for DUST was observed more often in men.
Strengths and Limitations
To our knowledge, the present investigation is the first attempt to evaluate the prevalence, correlates and outcome of DUST in the community. Several limitations of our study must be acknowledged, however. Several studies have reported technical artifacts in imaging the ventricular septum by two-dimensional echocardiography.20 We used stringent criteria for DUST to obviate the labeling of septal bulge due to technical artifacts as DUST. An additional limitation is the overwhelmingly white study sample; the generalizability to other racial groups is unknown. Our clinical correlates study was cross-sectional in design; the predictors of DUST are unknown. To definitively describe predictors of DUST would require a longitudinal study, examining the factors associated with development of DUST.
Clinical Implications
The increased prevalence of DUST in the elderly and the association with higher systolic blood pressure in the present study would suggest that cardiac adaptation/responses to elevated blood pressure may vary. Some individuals develop preferential thickening of the upper interventricular septum. The presence of DUST on a routine echocardiogram is not associated with an adverse prognosis.
Supplementary Material
Figure 3.
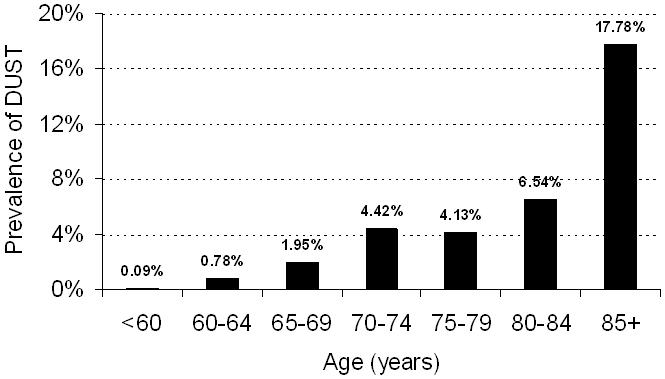
Prevalence of DUST with age. No. of individuals with DUST by number of individuals in age groups (years): 55-<60: 2/2113; 60-<65: 3/386; 65-<70: 6/308; 70-<75: 17/385; 75-<80: 9/218; 80-<85: 7/107; 85 or more: 8/45.
Acknowledgments
Sources of Support: This work was supported by NIH/NHLBI NO1-HC-25195, HL080124 (RSV), and K24-HL-04334 (Dr. Vasan).
Reference List
- (1).Klues HG, Schiffers A, Maron BJ. Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: morphologic observations and significance as assessed by two-dimensional echocardiography in 600 patients. J Am Coll Cardiol. 1995;26:1699–1708. doi: 10.1016/0735-1097(95)00390-8. [DOI] [PubMed] [Google Scholar]
- (2).Lewis JF, Maron BJ. Diversity of patterns of hypertrophy in patients with systemic hypertension and marked left ventricular wall thickening. Am J Cardiol. 1990;65:874–881. doi: 10.1016/0002-9149(90)91429-a. [DOI] [PubMed] [Google Scholar]
- (3).Maron BJ, Edwards JE, Epstein SE. Disproportionate ventricular thickening in patients with systemic hypertension. Chest. 1978;73:466–470. doi: 10.1378/chest.73.4.466. [DOI] [PubMed] [Google Scholar]
- (4).Shapiro LM, Howat AP, Crean PA, Westgate CJ. An echocardiographic study of localized subaortic hypertrophy. Eur Heart J. 1986;7:127–132. doi: 10.1093/oxfordjournals.eurheartj.a062034. [DOI] [PubMed] [Google Scholar]
- (5).Maron BJ, Clark CE, Henry WL, Fukuda T, Edwards JE, Mathews EC, Jr., Redwood DR, Epstein SE. Prevalence and characteristics of disproportionate ventricular septal thickening in patients with acquired or congenital heart diseases: echocardiographic and morphologic findings. Circulation. 1977;55:489–496. doi: 10.1161/01.cir.55.3.489. [DOI] [PubMed] [Google Scholar]
- (6).Maron BJ, Verter J. Disproportionate ventricular septal thickening in the developing normal human heart. Circulation. 1978;57:520–526. doi: 10.1161/01.cir.57.3.520. [DOI] [PubMed] [Google Scholar]
- (7).Azechi N, Morita Y, Inoue M, Kuzuyama R, Imataka K. Age-associated morphological change in interventricular septum. Rinsho Byori. 1993;41:285–288. [PubMed] [Google Scholar]
- (8).Goor D, Lillehei CW, Edwards JE. The “sigmoid septum”. Variation in the contour of the left ventricular outt. Am J Roentgenol Radium Ther Nucl Med. 1969;107:366–376. [PubMed] [Google Scholar]
- (9).Krasnow N. Subaortic septal bulge simulates hypertrophic cardiomyopathy by angulation of the septum with age, independent of focal hypertrophy. An echocardiographic study. J Am Soc Echocardiogr. 1997;10:545–555. doi: 10.1016/s0894-7317(97)70009-9. [DOI] [PubMed] [Google Scholar]
- (10).Oki T, Yamada H, Fukuda N, Minagoes S. Sigmoid septum. Ryoikibetsu Shokogun Shirizu. 1996:519–523. [PubMed] [Google Scholar]
- (11).Swinne CJ, Shapiro EP, Jamart J, Fleg JL. Age-associated changes in left ventricular outflow tract geometry in normal subjects. Am J Cardiol. 1996;78:1070–1073. doi: 10.1016/s0002-9149(96)00542-5. [DOI] [PubMed] [Google Scholar]
- (12).Toth AB, Engel JA, McManus AM, McManus BM. Sigmoidity of the ventricular septum revisited: progression in early adulthood, predominance in men, and independence from cardiac mass. Am J Cardiovasc Pathol. 1988;2:211–223. [PubMed] [Google Scholar]
- (13).Dawber TR, Meadors GF, Moore FE. Epidemiologic approaches to heart disease: the Framingham Study. Am J Public Health. 1951;41:279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- (15).Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- (16).Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- (17).Kannel WB, Wolf PA, Garrison RJ, editors. Framingham Heart Study, 30 year follow-up. US Department of Health and Human Services; Bethesda, MD: 1987. Section 34: Some risk factors related to the annual incidence of cardiovascular disease and death in pooled repeated biennial measurements. [Google Scholar]
- (18).Cox DR. Regression models and life tables (with discussion) (Series B).J Royal Stat Soc. 1972;34:187–220. [Google Scholar]
- (19).Ieki K, Imataka K, Sakurai S, Okamoto E, Ashida T, Fujii J. Differentiation of hypertrophic cardiomyopathy and hypertensive cardiac hypertrophy using the patterns of interventricular septum hypertrophy. J Cardiol. 1996;27:309–314. [PubMed] [Google Scholar]
- (20).Bernstein RF, Tei C, Child JS, Shah PM. Angled interventricular septum on echocardiography: anatomic anomaly or technical artifact? J Am Coll Cardiol. 1983;2:297–304. doi: 10.1016/s0735-1097(83)80166-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


