Abstract
Although current treatments for opioid detoxification are not always effective, medical detoxification remains a required step before long-term interventions. The use of opioid antagonist medications to improve detoxification has produced inconsistent results. Very low dose naltrexone (VLNTX) was recently found to reduce opioid tolerance and dependence in animal and clinical studies. We decided to evaluate safety and efficacy of VLNTX adjunct to methadone in reducing withdrawal during detoxification. In a multi-center, double-blind, randomized study at community treatment programs, where most detoxifications are performed, 174 opioid-dependent subjects received NTX 0.125 mg, 0.250 mg or placebo daily for 6 days, together with methadone in tapering doses. VLNTX-treated individuals reported attenuated withdrawal symptoms [F = 7.24 (2,170); P = 0.001] and reduced craving [F = 3.73 (2,107); P = 0.03]. Treatment effects were more pronounced at discharge and were not accompanied by a significantly higher retention rate. There were no group differences in use of adjuvant medications and no treatment-related adverse events. Further studies should explore the use of VLNTX, combined with full and partial opioid agonist medications, in detoxification and long-term treatment of opioid dependence.
Keywords: Community treatment programs, craving, opioid agonist/antagonist interaction, opioid antagonist, opioid dependence, opioid withdrawal
INTRODUCTION
Opioid dependence is a substantial public health problem and a major treatment challenge. About one-half of the estimated 2.4 million opioid-dependent individuals in the United States are not in treatment (Lucas 2004; Substance Abuse and Mental Health Services Administration 2007a; Huang et al. 2006). Among those who are in treatment, medication-based opioid detoxification is the main approach to bring patients from a drug-dependent to a drug-free state. From 1995 to 2005, annual treatment admissions for opioid dependence showed a steady increase, from 244 110 to 322 232, and almost 40% of the patients received detoxification (Substance Abuse and Mental Health Services Administration 2007b). In particular, the proportion of injection heroin abusers admitted to detoxification rose from 20% to 37%, as opposed to a decrease from 55% to 31% of those receiving opioid agonist substitution treatments (DASIS Report 2007). The results are not encouraging: as many as 50% of patients leave facilities before the detoxification is complete and less than 10% of those who stay seek further treatment (Gossop et al. 1987; Broers et al. 2000; Chutuape et al. 2001). Thus, there is a substantial need for improved detoxification treatments.
Detoxification without subsequent effective relapse prevention treatment is usually not successful intervention for opioid dependence (National Consensus Development Panel on Effective Medical Treatment of Opioid Addiction 1998). The most effective relapse prevention strategies include agonist substitution and maintenance treatment (reviewed by Mattick et al. 2003). For patients who enter maintenance treatment, there is no rationale to first undergo detoxification. However, even in an optimal treatment system, detoxification will continue to be indicated for some patients, e.g. those who do not want or for whom maintenance treatment is not indicated, or those who must discontinue maintenance treatment. Thus, efforts to improve the effectiveness of this intervention through safe and simple treatment paradigms remain clinically important. In particular, an adequate control of opioid withdrawal discomfort may be instrumental in getting patients into addiction treatment and improving long-term retention and completion rates (see Gowing & Ali 2006, for a review on matching detoxification options with long-term treatment entry).
Several studies have used opioid antagonist medications to ease the detoxification process, including rapid, intensive interventions (reviewed by Gowing, Ali & White 2006). Recent clinical trials have confirmed that antagonist-based, anesthesia-assisted, ultra-rapid opioid detoxification is neither safe nor effective (Collins et al. 2005; De Jong, Laheij & Krabbe 2005). A variety of other detoxification protocols have employed the opioid antagonist naltrexone (NTX) to facilitate treatment after methadone discontinuation (Riordan & Kleber 1980; Charney et al. 1982; Kleber et al. 1987). In some cases, high levels of medical supervision and monitoring were needed, while methods and results were heterogeneous or inconsistent. Thus, translation to clinical practice has been difficult (see O’Connor & Kosten 1998 for a review of clinical trials).
In recent pre-clinical investigations, administration of very low doses of NTX (VLNTX) and opioid agonist medications was associated with reduced development of tolerance and dependence (for a review, see Burns 2005). Morphine-dependent rodents receiving VLNTX have shown decreased withdrawal intensity (Shen & Crain 1997). Attenuation of withdrawal symptoms was accompanied by reduced activity in brainstem noradrenergic nuclei following daily administration of VLNTX in rats (Mannelli et al. 2004; Van Bockstaele et al. 2006). Clinical trials have confirmed that chronic VLNTX administration reduces opioid tolerance, manifest as increased opioid analgesia in chronic pain patients (Webster et al. 2006). VLNTX treatment of opioid dependence has been studied only in open pilot investigations (Mannelli et al. 2003). A controlled trial found that VLNTX alone is ineffective in preventing relapse following detoxification (Rea et al. 2004).
Pre-clinical research offers potentially useful information on the clinical use of VLNTX. In animal studies where opiate agonist agents were previously administered, withdrawal severity was reduced only with repeated VLNTX doses (Powell et al. 2002; Mannelli et al. 2004); single doses had little or no effect (Gracy, Dankiewicz & Koob 2001). On the other hand, previous experience casts doubt on the utility of NTX in actual clinical practice (Jaffe 2006). With this in mind, it was decided to evaluate the effectiveness of repeated, daily VLNTX administration among opioid-dependent individuals undergoing methadone-based detoxification at community treatment programs. This is the ‘real world’ setting where the majority of opioid withdrawal treatments are performed (Amato et al. 2005).
The objective of this study was to evaluate in a double-blind, randomized fashion whether the addition of VLNTX to tapering doses of methadone would be safe and effective in attenuating the severity of withdrawal during inpatient opioid detoxification.
MATERIALS AND METHODS
Study design
This was a 6-day, double-blind, randomized, multi-site clinical trial of two different adjunct oral NTX regimens (0.125 mg/day and 0.250 mg/day) for the treatment of withdrawal during inpatient detoxification in opioid-dependent subjects receiving daily tapering doses of methadone.
Subjects
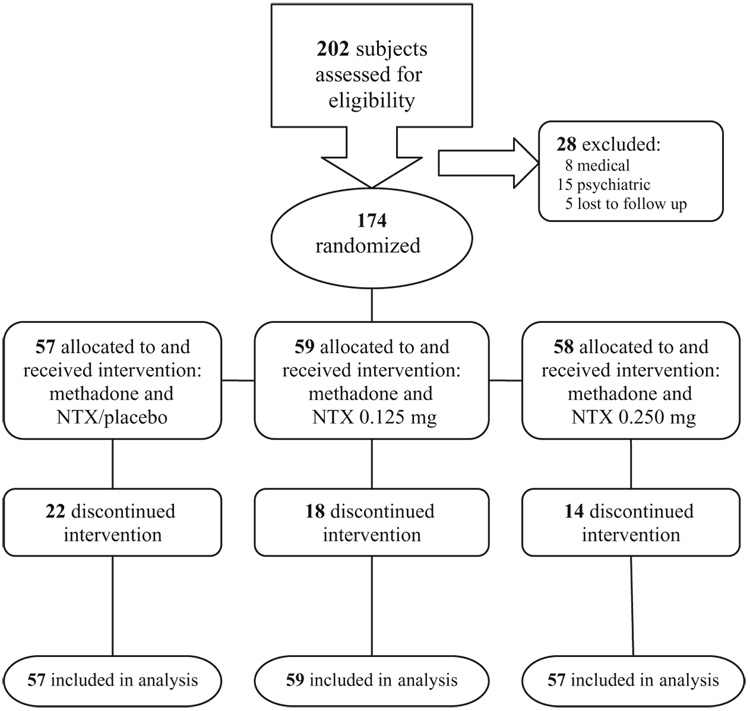
Participants were recruited between March 2004 and June 2006 from among opioid-dependent subjects 18 years of age or older seeking detoxification at two community-based treatment programs in Chapel Hill, NC (site I) and Philadelphia, PA (site II). The diagnosis of opioid dependence with physiological dependence was confirmed by the DSM IV checklist for that disorder (American Psychiatric Association 2000) and by urine drug testing. Potential subjects were excluded for any of the following criteria: inability to give informed consent, history of hypersensitivity to NTX, pregnancy or medical conditions that would make participation hazardous (e.g. acute hepatitis, unstable cardiovascular status, liver disease, renal disease), suicide risk, DSM IV diagnosis of psychotic disorder, major depression, bipolar disorder, or current dependence on substances other than opioids. The investigation was carried out in accordance with the Declaration of Helsinki of 1975. The institutional review boards of Duke University and Thomas Jefferson University approved the study. All subjects provided voluntary oral and written informed consent. Figure 1 shows the patient flow for the 202 individuals assessed for eligibility. In total, 174 participants were randomly assigned.
Figure 1.
Flow chart of clinical trial participants.
Procedures
Screening and intake
Screening assessments included a medical history, physical examination, routine clinical laboratory tests, including pregnancy test and urine testing for opioids, cocaine, amphetamine, tetrahydrocannabinol and benzodiazepines. The Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, was used to obtain a complete psychiatric history and to determine any possible psychiatric exclusion. The Addiction Severity Index (McLellan et al. 1992) provides severity profiles in seven domains (medical, employment, alcohol, drugs, family/social, legal and psychiatric), with composite score ranging from 0 (no problem) to 1 (extreme severity). Severity of opioid withdrawal at baseline was assessed with vital signs and two rating scales. The Subjective Opiate Withdrawal Scale (SOWS, Handelsman et al. 1987) is a self-rating scale which evaluates 16 symptoms whose intensity the patient rates on a 5-point Likert scale. One specific item ‘I feel like shooting up/taking the drug right now’ is also a reliable index of opioid craving (Kanof et al. 1992). The Objective Opiate Withdrawal Scale (OOWS, Handelsman et al. 1987) contains 13 observable physical signs, rated present or absent by a trained staff observer during the time the subject was filling out the SOWS.
Subjects were randomly assigned to one of the three medication groups by a computerized random numbers generator, maintained by an offsite researcher. All subjects, research and medical staff remained blind to the randomization sequence throughout the study. The groups were as follows: (1) NTX placebo; (2) NTX 0.125 mg/day; and (3) NTX 0.250 mg/day. Subjects in all three groups received a methadone taper.
Medications and treatments
Methadone hydrochloride USP (United States Pharmacopeia) 5 mg oral tablets were administered every morning at 10 am consistent with protocols in use at the participating treatment programs. Subjects received a single 30 mg dose on day 1 upon baseline assessment, after which methadone was tapered by 5 mg/day, with treatment completion and discharge on day 6.
Naltrexone hydrochloride USP powder (Medisca Inc. New York, USA) and matching lactose-free placebo filling powder were compounded into identical appearing 0.125 mg and 0.250 mg capsules. Offsite research staff assembled subject medication packs labeled with the subject’s ID number. Each medication pack contained six capsules of the same formulation. One capsule/day was administered with methadone. NTX doses were chosen based on previous animal and clinical studies (Shen & Crain 1997; Mannelli et al. 2003, 2004).
Ancillary medications available for symptomatic treatment included: ibuprofen 200–400 mg and acetaminophen 325 mg orally (po) every 4–6 hours as needed for muscular/bone pain (q4-6h PRN); chlordiazepoxide 25–75 mg po q6h PRN anxiety; hydroxyzine 25–50 mg po q6h PRN nausea, vomiting, or anxiety; prochlorperazine 25 mg po q6h PRN nausea; loperamide 2–4 mg po q4-6h PRN diarrhea; and cyclobenzaprine 5 mg po q6h PRN myalgias or muscle spasm. Clonidine, 0.2 mg po q6-8h PRN, and olanzapine 2.5 mg po q6h PRN anxiety, agitation, were available at site I. Need for and use of PRN medications was determined in routine clinical fashion by the nursing and medical staff.
All subjects received psychosocial support: 1 hour a day of abstinence-focused group activity led by a drug dependence counselor and one individual session to discuss outpatient treatment placement following discharge.
Assessments
Daily evaluations were conducted between 9 and 10 am, prior to administration of methadone and NTX. Withdrawal severity and craving were assessed using SOWS and OOWS scales. Subjects’ conditions were rated using a 4-point Clinical Global Improvement Scale (CGI-S, 0 = very much improved, 1 = much improved, 2 = minimally improved, 3 = no change or worse) by a physician blind to the treatments. Requests for ancillary drugs by subjects were rated on a 3-point PRN scale (PRN-S): 0 = no request made, 1 = request made, 2 = medication ordered. Vital signs were taken four times daily after 30 minutes in a sitting position. Adverse events were noted as reported by patients and observed by medical staff during inpatient treatment.
Compensation
Participants received $30 in gift certificates for completing screening and detoxification. All medications and other treatments were provided at no cost to subjects.
Outcome measures
The primary outcome measure for this study was opioid withdrawal severity during the 6-day inpatient detoxification, assessed using SOWS and OOWS scales. Secondary measures were: (1) craving, self-rated using one SOWS item; (2) global improvement assessed by CGI-S scores; (3) retention in treatment, based on the number of days from first dose to the last dose of study medication and measured by proportion of patients completing detoxification and by number of detoxification days; and (4) need for ancillary medications, based on number of subjects who received additional medications, PRN-S scores and quantity of medications administered.
Statistical analysis
All analyses were carried out on the intent-to-treat population with last observation carried forward and included all subjects started on study medication. All tests were two-tailed, with the alpha significance level set at 0.05. Baseline demographic and clinical characteristics were compared among groups using ANOVA for continuous variables and χ2 test for categorical variables. Analyses of change of withdrawal severity and craving over time (measured by SOWS and OOWS) were performed using repeated measure two-factor (time × treatment) ANOVA, or ANCOVA to control for differences between groups (baseline scores study site and recent alcohol use as covariates for OOWS, baseline scores as covariate for craving). Post hoc Bonferroni and Tukey’s tests were used to control experiment-wise error. SOWS total scores were not normally distributed and were analyzed as change from baseline. Completion rates, CGI-S and PRN-S scores were compared using χ2 test. Average daily quantity of ancillary medications, number of subjects receiving them and number of detoxification days were compared using ANOVA. The number of subject discontinuing treatment over time was examined using Kaplan–Meier survival analysis. Power calculations suggested that enrolling 50 patients in each group would give a power of about 0.80 to observe a difference in withdrawal intensity score between groups equal to one-half standard deviation. This is considered amoderate treatment effect size (Cohen 1988).
RESULTS
Subjects
Demographic and clinical characteristics of the 174 participants are summarized in Table 1. Fifty-seven subjects were randomized to placebo, 59 to NTX 0.125 mg/day, and 58 to NTX 0.250 mg/day. Sixty-three percent of the subjects were white and 57.6% were male, with a mean (SD) age of 32.3 years (9.3), 11.7 (2.4) years of education and 7.5 (8.7) years of opioid use. At least, one additional illicit substance was found in the urine of 52.5% of subjects, in the majority of cases being cocaine or marijuana. There were significant site differences in admission OOWS scores and medication group differences in the recent frequency, lifetime duration and severity of alcohol use, which were higher among subjects randomized to VLNTX (Table 1).
Table 1.
Sociodemographic and substance use characteristics of 174 opioid-dependent inpatients undergoing 6-day methadone detoxification.
| Placebo (n = 57) | NTX 0.125 mg (n = 59) | NTX 0.250 mg (n = 58) | |
|---|---|---|---|
| Demographics | |||
| Age | 30.83 (9.9) | 31.38 (9.0) | 34.56 (8.6) |
| Male | 62.3% | 66.7% | 70.4% |
| African American | 19.2% | 31.3% | 29.6% |
| Years of education | 11.7 (2.3) | 11.8 (2.9) | 11.8 (2.4) |
| Married or cohabitant | 18.2% | 11.9% | 22.2% |
| Unemployed | 64% | 61% | 64% |
| Substance use | |||
| Substance use last 30 days | |||
| Opioids | 21.6 (2.9) | 18.4 (4.4) | 20.9 (2.5) |
| Alcohola | 2.86 (6.5) | 7.05 (9.9) | 10.62 (11.8) |
| Marijuana | 7.91 (11.93) | 9.07 (12.9) | 3.29 (5.9) |
| Cocaine | 10.54 (12.5) | 7.18 (9.8) | 12.67 (13.2) |
| Lifetime substance use (years) | |||
| Opioids | 7.5 (9.2) | 6.4 (7.7) | 8.7 (9.1) |
| Alcoholb | 4.3 (6.0) | 7.7 (9.2) | 10.3 (10.9) |
| Marijuana | 6.6 (6.5) | 7.3 (6.8) | 11.3 (8.9) |
| Cocaine | 5.4 (6.2) | 7.1 (8.5) | 7.2 (7.2) |
| N previous detoxifications | 1.93 (2.5) | 1.16 (1.64) | 1.85 (2.6) |
| N other previous treatment | 3.49 (5.0) | 2.16 (2.24) | 2.64 (3.35) |
| ASI Drug Comp Score | 0.281 (0.18) | 0.269 (0.17) | 0.281 (0.16) |
| ASI Alcohol Comp Scorec | 0.032 (0.16) | 0.127 (0.23) | 0.136 (0.24) |
| ASI Psychiatric Comp Score | 0.252 (0.22) | 0.264 (0.26) | 0.267 (0.23) |
| Baseline measures | |||
| Positive urine cocaine | 58.2% | 48.1% | 50.9% |
| Positive urine THC | 48.4% | 36.7% | 50% |
| Positive urine amphetamine | 6.3% | 3.7% | 6.1% |
| Withdrawal scores | |||
| SOWS (0–64) | 35.49 (12.7) | 33.51 (15.1) | 37.66 (15.4) |
| OOWS (0–13)d | 4.42 (2.8) | 4.70 (3.1) | 5.26 (2.5) |
Difference between placebo and 0.250 group by Bonferroni test; F = 5.515 (2,171); P = 0.004.
Difference between placebo and 0.250 mg group by Bonferroni test; F = 3.846 (2, 172); P = 0.024.
Difference between placebo and 0.125 mg as wells as 0.250 mg groups by Bonferroni test; F = 5.319 (2, 169); P = 0.006.
Difference between sites, F = 67.0 (1, 174); P = 0.001. ASI = Addiction Severity Index; Comp = composite; NTX = naltrexone; OOWS = Objective Opiate Withdrawal Scales; SOWS = Subjective Opiate Withdrawal Scales; THC = tetrahydrocannabinol.
Opioid withdrawal
Both NTX groups reported significantly reduced SOWS withdrawal scores than did the placebo group [F = 7.24 (2,170); P = 0.001; 0.125 mg/day vs. placebo, P = 0.04; 0.250 mg/day vs. placebo, P = 0.001]. No differences between sites were identified by covariate analysis (not shown). There was a significant treatment × time interaction [F = 5.64 (10, 200); P = 0.001], with the most relevant changes in withdrawal scores on the last day of treatment (Tukey’s test = 0.001). OOWS ratings were also different, after adjusting for baseline scores and site effect [F = 13.28 (2, 168); P = 0.001]. In particular, objective withdrawal scores were significantly lower than placebo in the 0.125 mg NTX group (P = 0.001) and did not differ between NTX conditions. A group × time interaction was detected also for the OOWS scores [F = 7.12 (10, 202); P = 0.001], with a significant reduction of the objective withdrawal symptoms at discharge (Tukey’s test = 0.001).
Naltrexone is a common treatment for alcohol dependence (Srisurapanont & Jarusuraisin 2005) and subjects receiving VLNTX entered detoxification with a more severe pattern of alcohol use. To investigate if this could have influenced response to treatment, we repeated the analysis, controlling for alcohol consumption in the last month. Subjective withdrawal scores remained lower among VLNTX-treated subjects compared with the placebo group (SOWS, F = 5.39 (2,171); P = 0.006). If use of alcohol in the last 30 days was included among the covariates, the difference in observed withdrawal became not significant among groups (OOWS, F = 1.44 (2,155); P = 0.14). However, recent alcohol use did not have a direct influence on OOWS scores [F = 0.37 (2,155); P = 0.44] or show a time × effect interaction [F = 0.56 (5, 101); P = 0.72].
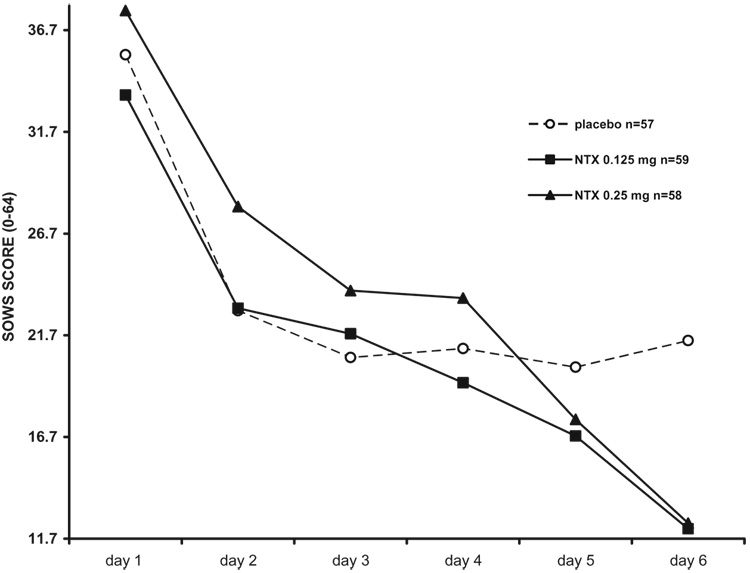
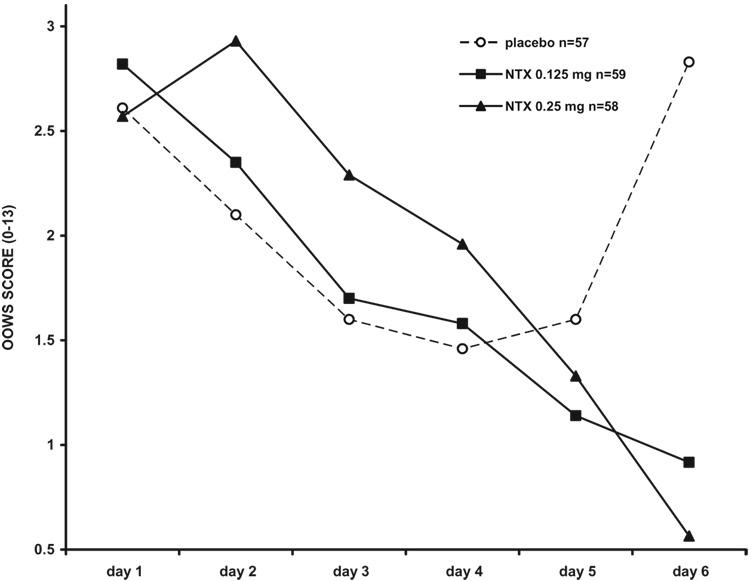
Intensity and time course of withdrawal during detoxification are shown in Fig 2 and Fig 3, and summarized in Table 2.
Figure 2.
Subjective opioid withdrawal scores (SOWS) in 174 opioid-dependent inpatients undergoing 6-day methadone detoxification. VLNTX treatment was associated with significantly reduced withdrawal [repeated measures ANOVA, change of scores, F = 7.24 (2,170); P = 0.001] and a significant time × treatment interaction [F = 5.64 (10, 200); P = 0.001; day 6 Tukey’s test = 0.001]. Withdrawal reduction was still significant after controlling for recent alcohol use [F = 5.39 (1,171); P = 0.006]. VLNTX, very low dose naltrexone.
Figure 3.
Objective opioid withdrawal scores (OOWS) in 174 opioid-dependent inpatients undergoing 6-day methadone detoxification. VLNTX treatment was associated with significantly reduced withdrawal [repeated measures ANCOVA, F = 13.28 (2,170); P = 0.001] and a significant time × treatment interaction [F = 7.12 (10, 202); P = 0.001; day 6 Tukey’s test = 0.001]. Withdrawal reduction became non-significant after controlling for recent alcohol use [F = 1.44 (2, 155); P = 0.14].VLNTX, very low dose naltrexone.
Table 2.
Detoxification outcome in 174 opioid-dependent inpatients undergoing 6-day methadone detoxification.
| Placebo (n = 57) | NTX 0.125 mg (n = 59) | NTX 0.250 mg (n = 58) | ||
|---|---|---|---|---|
| Outcome measures | Mean score days 2–6 | Mean score days 2–6 | Mean score days 2–6 | F/χ2 |
| SOWS (0–64) | 22.28 (11.9) | 18.63 (12.4) | 19.1 (10.1) | 7.24* |
| OOWS (0–13) | 2.47 (1.7) | 1.71 (1.2) | 1.80 (1.5) | 13.28*a |
| Craving (0–4) | 1.5 (1.2) | 1.1 (1.2) | 1 (1.1) | 3.73** |
| CGI-S vast/decided improvement at discharge | 69.2% | 89.4% | 89.1% | 29.49* |
| Completers | 63.2% | 69.5% | 75.9% | 2.19 |
| Days in treatment (1–6) | 5.12 (1.4) | 5.30 (1.3) | 5.57 (0.9) | 2.04 |
| PRN-S request of medication | 63% | 51% | 62.5% | 1.56 |
P = 0.001
P = 0.03.
Not significant after controlling for alcohol use (F = 1.44). CGI-S = Clinical Global Improvement Scale; NTX = naltrexone; OOWS = Objective Opiate Withdrawal Scales; PRN-S = PRN scale; SOWS = Subjective Opiate Withdrawal Scales.
Craving
There was an overall 37% decrease in opiate craving scores across treatment condition between days 1 and 6 (data not shown). Patients taking NTX 0.125 mg or 0.250 mg had less craving (both P = 0.02) than those taking placebo, adjusting for baseline scores [F = 3.73 (2,107); P = 0.03, Table 2]. There was a time × group interaction [F = 11.82 (10, 180); P = 0.001], with significantly lower craving on days 4, 5 and 6 among subjects receiving VLNTX (Tukey’s test, all Ps = 0.001).
Global improvement
At the end of treatment, there were more subjects rated ‘much’ or ‘very much’ improved compared with baseline in the NTX groups (χ2 = 29.49 (2); P = 0.001; Table 2). In particular, 30.8% of patients receiving methadone and placebo NTX either were doing worse than at admission, or showed minimal or no improvement by discharge, compared with <11% in each VLNTX group (Table 2).
Treatment retention
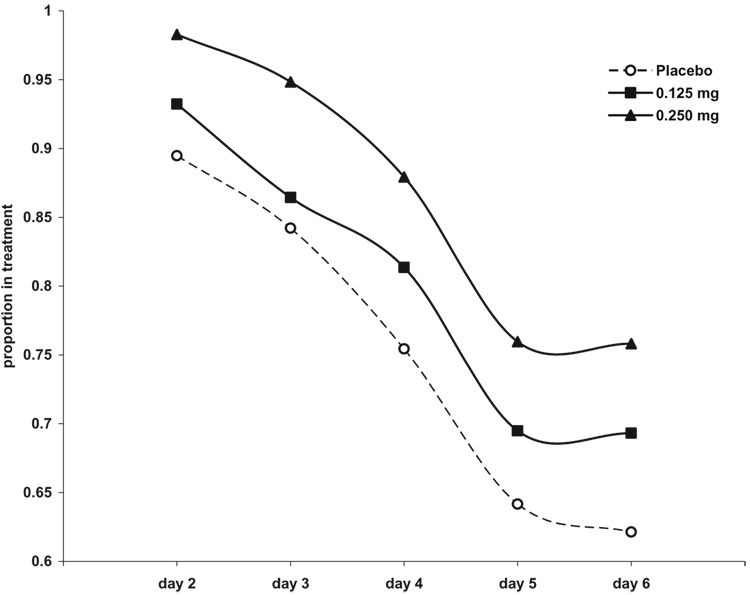
A total of 120 subjects (69%) completed treatment. The proportion of completing subjects did not differ significantly by medication group [χ2 = 2.19 (2); P = 0.33, Table 2]. The number of dropouts did not differ across treatment groups (Fig. 4, Kaplan–Meier survival analysis. χ2 [Breslow] = 2.93, d.f. = 2, P = 0.23). There were no significant differences in treatment retention among the three medication groups [F = 2.04 (2,173); P = 0.13].
Figure 4.
Time until discontinuation from treatment in 174 opioid-dependent inpatients undergoing 6-day methadone detoxification. Kaplan–Meier survival analysis: χ2 [Breslow] = 2.93, d.f. = 2, P = 0.23.
Other medications
All subjects received ancillary medications and in 58.8% of cases the medication was administered following a request. There were no significant differences in number or requests of adjuvant medications (Table 2) and PRN-S score [χ2 for days 1–6 range = 0.62–3.62 (2); P = 0.16– 0.73], or in daily amount of ancillary medications administered (data not shown). The mean (SD) dose of medication received per subject per day was: 743.5 mg (200.1) ibuprofen, 613.7 mg (252.1) acetaminophen, 8 mg (2.2) loperamide, 50.8 mg (5.0) hydroxyzine, 19.8 mg (1.2) prochlorperazine, 31.1 mg (4.62) chlordiazepoxide and 10 mg (2.1) cyclobenzaprine. Clonidine [0.45 mg (0.3)] and olanzapine [5.17 mg (2.7)] were administered only at site I and also showed comparable daily doses across medication groups [F = 0.15 (2,94); P = 0.86 and F = 0.27 (2,81); P = 0.78, respectively].
Adverse events
There were no serious adverse events. One subject in the placebo group presented with seizure-like symptoms that required intervention and was discontinued from the study. One subject receiving NTX 0.250 mg/day group was found to have high fasting blood glucose during treatment, which returned to normal without specific intervention. This subject recalled later a history of high blood glucose prior to this study. The only other adverse events reported were occasional mild complaints of restlessness, sweating, nausea and anxiety, considered indistinguishable from ongoing signs of opiate withdrawal that were documented by scores on the withdrawal scales. There were no episodes of medication-precipitated withdrawal. There were no significant medication group differences in peak heart rate [F = 0.24 (2); P = 0.79; total mean (SD) = 69.7 (5.3)], or peak systolic [F = 0.64 (2); P = 0.21; total mean (SD) = 116.2 (13.1)] or diastolic BP [F = 0.14 (2); P = 0.12; total mean (SD) = 68.9 (6.8)].
DISCUSSION
This clinical trial showed VLNTX to be safe and well-tolerated when administered with methadone. Opiate withdrawal symptoms and craving were less severe at discharge among subjects who received VLNTX during detoxification, with no significant differences between NTX regimens. Although potential symptom modifiers (ancillary medications) were allowed by the ‘real world’ design of the study, there were no significant medication group differences in proportion of subjects requesting ancillary medications, mean daily PRN-S score, or mean daily doses of ancillary medications. Thus, it is very unlikely that ancillary medications biased the observed VLNTX findings. Objective opioid withdrawal scores were not significantly reduced in VLNTX-treated subjects when alcohol use in the last month was included as a covariate. This finding requires further investigation. VLNTX may differentially affect some aspects of withdrawal in opioid-dependent subjects who use alcohol. On the other hand, the results do not suggest that recent alcohol use ‘per se’ influenced directly and significantly the manifestation of withdrawal in this sample. Subjects did not differ significantly in their use of other drugs, such as cocaine and marijuana (Table 1), so it is unlikely that non-opioid drug withdrawal influenced the findings.
The addition of VLNTX was superior to methadone taper alone in reducing withdrawal, with an effect size of 0.71. One out of every two to three subjects receiving NTX = 0.250 mg/day showed significantly less withdrawal discomfort than those treated with only methadone (NNT (number needed to treat) analysis: SOWS = 3, 95% CI 2–5; OOWS = 2, 95% CI 2–3). The methadone detoxification that was considered ‘treatment-as-usual’ in this study has been demonstrated to be more effective than non-opiate medications in controlling withdrawal severity (Amato et al. 2004), and displays comparable efficacy to newer opiate agents such as buprenorphine (Gowing, Ali, White 2004).
This is the first randomized, controlled trial to evaluate the use of VLNTX for the treatment of opioid withdrawal. The mechanisms of symptom reduction remain to be elucidated. Pre-clinical investigations suggest that VLNTX attenuates chronic opioid excitatory signaling at the mu-opioid receptor, blocking intracellular cyclic adenosine monophosphate (cAMP) up-regulation (Wang et al. 2005). Up-regulation of the cAMP pathway is associated with development of dependence and with the expression of withdrawal (Nestler, Alreja & Aghajanian 1994). Reduced opioid withdrawal severity by VLNTX was accompanied in opioid-dependent animal models by lower intracellular levels of enzymes of the cAMP system in brainstem noradrenergic areas that are activated during withdrawal (Mannelli et al. 2004). The administration in this study of ancillary drugs that influence noradrenergic activity prevents direct interpretation of putative noradrenergic mechanisms of VLNTX.
Subjects in the VLNTX groups showed more pronounced clinical differences from the methadone-only group in the last few days of detoxification, a time-related treatment effect that may be reinforced by a medication dose–effect mechanism. The reduction of dependence is not immediate with VLNTX treatment. Multiple doses are required to restore opioid agonist action in animals, suggesting a slow reversal of the mechanisms that contribute to tolerance and dependence (Powell et al. 2002). Animal studies also show that VLNTX enhances opioid analgesia and reward to a greater extent when the quantity of opioid drug is reduced, as it is at the end of methadone tapering and before discontinuation (Crain & Shen 2001; Powell et al. 2002).
The effects observed with VLNTX add-on were not associated with an increased proportion of patients completing detoxification. It is a rather common finding that a more comfortable detoxification is insufficient to encourage retention in treatment (Buydens-Branchey, Branchey & Reel-Brander 2005; Herman et al. 2005; Oreskovich et al. 2005; Reed et al. 2007). Among other factors that can influence treatment completion, the use of nonopioid drugs immediately prior to detoxification, individual expectations, lack of health insurance and the weekday of admission to treatment have received attention (Perez de los Cobos et al. 1997; Armenian, Chutuape & Stitzer 1999; Gossling et al. 2001; Blondell et al. 2006). In our study, the relatively late onset of the main clinical differences between NTX and placebo-treated subjects may have played a role, preventing individuals from receiving full benefit from the treatment.
The main intention of our research was to study the effects of VLNTX within an opioid agonist/antagonist administration paradigm and not to identify the best detoxification protocol for this treatment. The investigation was conducted in community hospital programs, to evaluate the feasibility of the approach in a ‘real world’ setting. Although this can be considered one strong point of the study, it is at the same time a weakness, in that major limitations to the design were necessitated to minimize the conventional requirements of a controlled clinical trial and by adopting without modifications protocols in use at the community programs. In particular, (1) considering the methadone tapering schedule, it is unclear if subjects were actually ‘detoxified’ at discharge; (2) no specific, rigid protocol existed for the administration of ancillary medications and for consistency between the two study sites. As for the first limitation, a follow-up of individuals who received VLNTX would provide useful information following methadone discontinuation. With regard to the use of ancillary drugs, subjects did not receive significantly different amounts across medications groups, nor did site-related differences in ancillary drugs influence response to treatment. Together, these potential limitations seem unlikely to bias our main findings.
Conclusions
This two-site study provides evidence that the administration of VLNTX is a safe and effective method to reduce withdrawal severity and treatment discomfort in opioid-dependent participants undergoing methadone detoxification.
Opioid dependence is a chronic disorder, with relapse to drug use a frequent occurrence even after multiple detoxification episodes (McLellan et al. 1996). VLNTX adjunct showed improvement over a standard treatment for opioid-dependent patients attempting detoxification, although its utility in reducing relapse after discharge and facilitating access to long-term treatment remains to be determined. The use of VLNTX may help improve detoxification, potentially resulting in improved NTX maintenance induction or long-term, agonist substitution treatment. In particular, treatment combinations of methadone and buprenorphine with VLNTX should be tested. Finally, a number of research questions could be explored using existing animal models that have proven to be a reliable translational tool to test the properties of VLNTX in opioid dependence (Mannelli, Gottheil & Van Bockstaele 2006).
Acknowledgements
This research was supported by grant DA15469 from the National Institute on Drug Abuse to P. Mannelli. Dr. Gorelick is supported by the Intramural Research Program, NIH, National Institute on Drug Abuse. The authors wish to thank Stephen P. Weinstein for helping with recruitment, Michelle Kay Anderberg for recruitment and data collection, and Neena Ajwani for recruitment, data collection and manuscript editing. They also thank the personnel at Freedom House, Chapel Hill, NC and Kensington Hospital, Philadelphia, PA and all the individuals who participated in the clinical trial. Portions of this study were presented at the 160th annual meeting of the American Psychiatric Association, San Diego, CA, 19–24 May 2007.
Footnotes
This work was carried out at Duke University, Durham, NC, and Thomas Jefferson University, Philadelphia, PA.
References
- Amato L, Davoli M, Ferri M, Gowing L, Perucci CA. Effectiveness of interventions on opiate withdrawal treatment: an overview of systematic reviews. Drug Alcohol Depend. 2004;73:219–226. doi: 10.1016/j.drugalcdep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Amato L, Davoli M, Minozzi S, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev. 2005;20:CD003409. doi: 10.1002/14651858.CD003409.pub3. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic criteria from DSM-IV-TR / American Psychiatric Association. Washington, D.C.: 2000. [Google Scholar]
- Armenian SH, Chutuape MA, Stitzer ML. Predictors of discharges against medical advice from a short-term hospital detoxification unit. Drug Alcohol Depend. 1999;56:1–8. doi: 10.1016/s0376-8716(99)00027-7. [DOI] [PubMed] [Google Scholar]
- Blondell RD, Amadasu A, Servoss TJ, Smith SJ. Differences among those who complete and fail to complete inpatient detoxification. J Addict Dis. 2006;25:95–104. doi: 10.1300/J069v25n01_12. [DOI] [PubMed] [Google Scholar]
- Broers B, Giner F, Dumont P, Mino A. Inpatient opiate detoxification in Geneva: follow-up at 1 and 6 months. Drug Alcohol Depend. 2000;58:85–92. doi: 10.1016/s0376-8716(99)00063-0. [DOI] [PubMed] [Google Scholar]
- Burns LH. Ultra-low-dose opioid antagonists enhance opioid analgesia while reducing tolerance, dependence and addictive properties. In: Capasso A, editor. Recent developments in Pain Research. Kerala: Trivandrum: 2005. pp. 115–136. [Google Scholar]
- Buydens-Branchey L, Branchey M, Reel-Brander C. Efficacy of buspirone in the treatment of opioid withdrawal. J Clin Psychopharmacol. 2005;25:230–236. doi: 10.1097/01.jcp.0000162804.38829.97. [DOI] [PubMed] [Google Scholar]
- Charney DS, Riordan CE, Kleber HD, Murburg M, Braverman P, Sternberg DE, Heninger GR, Redmond DE. Clonidine and naltrexone: a safe, effective, and rapid treatment of abrupt withdrawal from methadone therapy. Arch Gen Psychiatry. 1982;39:1327–1332. doi: 10.1001/archpsyc.1982.04290110077013. [DOI] [PubMed] [Google Scholar]
- Chutuape MA, Jasinski DR, Fingerhood MI, Stitzer ML. One-, three-, and six-month outcomes after brief inpatient opioid detoxification. Am J Drug Alcohol Abuse. 2001;27:19–44. doi: 10.1081/ada-100103117. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analyses for the Social Science. New York: Lawrence, Erlbaum & Associates; 1988. [Google Scholar]
- Collins ED, Kleber HD, Whittington RA, Heitler NE. Anesthesia-assisted vs. buprenorphine- or clonidine-assisted heroin detoxification and naltrexone induction: a randomized trial. JAMA. 2005;294:903–913. doi: 10.1001/jama.294.8.903. [DOI] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Acute thermal hyperalgesia elicited by low-dose morphine in normal mice is blocked by ultra-low-dose naltrexone, unmasking potent opioid analgesia. Brain Res. 2001;888:75–82. doi: 10.1016/s0006-8993(00)03010-9. [DOI] [PubMed] [Google Scholar]
- De Jong CA, Laheij RJ, Krabbe PF. General anaesthesia does not improve outcome in opioid antagonist detoxification treatment: a randomized controlled trial. Addiction. 2005;100:206–215. doi: 10.1111/j.1360-0443.2004.00959.x. [DOI] [PubMed] [Google Scholar]
- Gossling HW, Gunkel S, Schneider U, Melles W. Frequency and causes of premature termination (drop-out) during in-patient opiate detoxification. Fortschr Neurol Psychiatr. 2001;69:474–481. doi: 10.1055/s-2001-17565. [DOI] [PubMed] [Google Scholar]
- Gossop M, Green L, Phillips G, Bradley B. What happens to opiate addicts immediately after treatment: a prospective follow-up study. BMJ. 1987;294:1377–1380. doi: 10.1136/bmj.294.6584.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing L, Ali R. The place of detoxification in treatment of opioid dependence. Curr Opin Psychiatry. 2006;19:3266–3270. doi: 10.1097/01.yco.0000218596.54054.a1. [DOI] [PubMed] [Google Scholar]
- Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2004;18:CD002025. doi: 10.1002/14651858.CD002025.pub2. [DOI] [PubMed] [Google Scholar]
- Gowing L, Ali R, White J. Opioid antagonists under heavy sedation or anaesthesia for opioid withdrawal. Cochrane Database Syst Rev. 2006;19:CD002022. doi: 10.1002/14651858.CD002022.pub2. [DOI] [PubMed] [Google Scholar]
- Gracy KN, Dankiewicz LA, Koob GF. Opiate withdrawal-induced fos immunoreactivity in the rat extended amygdala parallels the development of conditioned place aversion. Neuropsychopharmacol. 2001;24:152–160. doi: 10.1016/S0893-133X(00)00186-X. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Herman I, Shamir D, Bar-Hamburger R, Pick CG, Schreiber S. The effect of mianserin add-on, on the intensity of opioid withdrawal symptoms during detoxification program —a randomized, double blind, placebo controlled, prospective study. Addict Behav. 2005;30:1154–1167. doi: 10.1016/j.addbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Huang B, Dawson DA, Stinson FS, Hasin DS, Ruan WJ, Saha TD, Smith SM, Goldstein RB, Grant BF. Prevalence, correlates, and comorbidity of nonmedical prescription drug use and drug use disorders in the United States: results of the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2006;67:1062–1073. doi: 10.4088/jcp.v67n0708. [DOI] [PubMed] [Google Scholar]
- Jaffe JH. Do antagonists have a role in the treatment of opioid dependence? Addiction. 2006;101:468–469. doi: 10.1111/j.1360-0443.2006.01402.x. [DOI] [PubMed] [Google Scholar]
- Kanof PD, Handelsman L, Aronson MJ, Ness R, Cochrane KJ, Rubinstein KJ. Clinical characteristics of naloxone-precipitated withdrawal in human opioid-dependent subjects. J Pharmacol Exp Ther. 1992;260:355–363. [PubMed] [Google Scholar]
- Kleber HD, Topazian M, Gaspari J, Riordan CE, Kosten T. Clonidine and naltrexone in the outpatient treatment of heroin withdrawal. Am J Drug Alcohol Abuse. 1987;13:1–17. doi: 10.3109/00952998709001497. [DOI] [PubMed] [Google Scholar]
- Lucas GM. Buprenorphine in primary HIV care clinics: a big pill to swallow. Hopkins HIV Rep. 2004;16:5–7. [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Woody GE, Metzger D, McKay J, Durrell J, Alterman AI, O’Brien CP. Evaluating the effectiveness of addiction treatments: reasonable expectations, appropriate comparisons. Milbank Q. 1996;74:51–85. [PubMed] [Google Scholar]
- Mannelli P, Gottheil E, Buonanno A, De Risio S. Use of very low-dose naltrexone during opiate detoxification. J Addict Dis. 2003;22:63–70. doi: 10.1300/J069v22n02_05. [DOI] [PubMed] [Google Scholar]
- Mannelli P, Gottheil E, Peoples JF, Oropeza VC, Van Bockstaele EJ. Chronic very low dose naltrexone administration attenuates opioid withdrawal expression. Biol Psychiatry. 2004;56:261–268. doi: 10.1016/j.biopsych.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Mannelli P, Gottheil E, Van Bockstaele EJ. Antagonist treatment of opioid withdrawal translational low dose approach. J Addict Dis. 2006;25:1–8. doi: 10.1300/J069v25n02_01. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2003;2:CD002209. doi: 10.1002/14651858.CD002209. [DOI] [PubMed] [Google Scholar]
- National Consensus Development Panel on Effective Medical Treatment of Opioid Addiction. Effective medical treatment of opiate addiction. JAMA. 1998;280:1936–1943. [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular and cellular mechanisms of opiate action: studies in the rat locus coeruleus. Brain Res Bull. 1994;35:521–528. doi: 10.1016/0361-9230(94)90166-x. [DOI] [PubMed] [Google Scholar]
- O’Connor PG, Kosten TR. Rapid and ultrarapid opioid detoxification techniques. JAMA. 1998;279:229–234. doi: 10.1001/jama.279.3.229. [DOI] [PubMed] [Google Scholar]
- Oreskovich MR, Saxon AJ, Ellis ML, Malte CA, Reoux JP, Knox PC. A double-blind, double-dummy, randomized, prospective pilot study of the partial mu opiate agonist, buprenorphine, for acute detoxification from heroin. Drug Alcohol Depend. 2005;77:71–79. doi: 10.1016/j.drugalcdep.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Perez de los Cobos J, Trujols J, Ribalta E, Casas M. Cocaine use immediately prior to entry in an inpatient heroin detoxification unit as a predictor of discharges against medical advice. Am J Drug Alcohol Abuse. 1997;23:267–279. doi: 10.3109/00952999709040946. [DOI] [PubMed] [Google Scholar]
- Powell KJ, Abul-Husn NS, Jhamandas A, Olmstead MC, Beninger RJ, Jhamandas K. Paradoxical effects of the opioid antagonist naltrexone on morphine analgesia, tolerance, and reward in rats. J Pharmacol Exp Ther. 2002;300:588–596. doi: 10.1124/jpet.300.2.588. [DOI] [PubMed] [Google Scholar]
- Rea F, Bell JR, Young MR, Mattick RP. A randomised, controlled trial of low dose naltrexone for the treatment of opioid dependence. Drug Alcohol Depend. 2004;75:79–88. doi: 10.1016/j.drugalcdep.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Glasper A, de Wet CJ, Bearn J, Gossop M. Comparison of buprenorphine and methadone in the treatment of opiate withdrawal: possible advantages of buprenorphine for the treatment of opiate-benzodiazepine codependent patients? J Clin Psychopharmacol. 2007;27:188–192. doi: 10.1097/JCP.0b013e318032ec2a. [DOI] [PubMed] [Google Scholar]
- Riordan CE, Kleber HD. Rapid opiate detoxification with clonidine and naloxone. Lancet. 1980;1:1079–1080. doi: 10.1016/s0140-6736(80)91516-0. [DOI] [PubMed] [Google Scholar]
- Shen KF, Crain SM. Ultra-low doses of naltrexone or etorphine increase morphine’s antinociceptive potency and attenuate tolerance/dependence in mice. Brain Res. 1997;757:176–190. doi: 10.1016/s0006-8993(97)00197-2. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005;25:CD001867. doi: 10.1002/14651858.CD001867.pub2. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Treatment Episode and Data Set Highlights-2005. Rockville, MD: Office of Applied Studies; 2007a. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. DASIS Report: Heroin Trends-Changes in How it is Used 1995–2005. Rockville, MD: Office of Applied Studies; 2007b. [Google Scholar]
- Van Bockstaele EJ, Rudoy C, Mannelli P, Oropeza V, Qian Y. Elevated mu-opioid receptor expression in the nucleus of the solitary tract accompanies attenuated withdrawal signs after chronic low dose naltrexone in opiate-dependent rats. J Neurosci Res. 2006;83:508–514. doi: 10.1002/jnr.20738. [DOI] [PubMed] [Google Scholar]
- Wang HY, Friedman E, Olmstead MC, Burns LH. Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and Gbetagamma signaling. Neuroscience. 2005;135:247–261. doi: 10.1016/j.neuroscience.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Webster LR, Butera PG, Moran LV, Wu N, Burns LH, Friedmann N. Oxytrex minimizes physical dependence while providing effective analgesia: a randomized controlled trial in low back pain. J Pain. 2006;7:937–946. doi: 10.1016/j.jpain.2006.05.005. [DOI] [PubMed] [Google Scholar]