Abstract
Rationale
Benzodiazepines (BZs) are effective anxiolytics and hypnotics, but their use is limited by unwanted side effects, such as motor impairment.
Objectives
To assess the contribution of α1 subunit-containing GABAA receptor subtypes to the motor-impairing effects of BZs, the present study evaluated two observable measures of motor coordination (balance on a pole, resistance to hind-limb flexion) engendered by non-selective and selective BZ-site agonists in squirrel monkeys.
Methods
Multiple doses of non-selective BZs (triazolam, alprazolam, diazepam, chlordiazepoxide) and α1 subunit-preferring agonists (zolpidem and zaleplon) were administered to adult male squirrel monkeys (N=4–6), and experimenters rated the monkey’s ability to balance on a horizontal pole (“ataxic-like effects”), as well as the degree of resistance to hind-limb flexion (“myorelaxant-like effects”).
Results
Administration of all BZ-type drugs resulted in ataxic-like and myorelaxant-like effects. Pretreatment with the α1 subunit-preferring antagonist βCCT (β-carboline-3-carboxylate-tbutyl ester) attenuated the ataxic-like effects engendered by both types of drugs. However, βCCT was largely ineffective at blocking the ability of both BZs and non-BZs to induce myorelaxant-like effects.
Conclusions
These experiments demonstrate dose-dependent motor impairment in squirrel monkeys using quantitative behavioral observation techniques. Altogether, these findings suggest a lack of a prominent role for α1 subunit-containing receptors in the alteration of hind-limb flexion, a putative measure of myorelaxation, induced by BZ-type drugs in monkeys.
Keywords: Ataxia, Myorelaxation, Motor impairment, Benzodiazepine, GABAA receptor, Monkey
Benzodiazepines (BZs) are among the most widely prescribed drugs worldwide. These drugs produce their pharmacological effects by allosterically modulating the inhibitory action of γ-aminobutyric acid (GABA) at the GABAA receptor (for review, see Möhler 2006; Whiting 2006). Although the GABAA receptor is a pentameric structure formed by the assembly of subunits from at least five different families (α 1–6, β 1–3, γ 1–3, ρ1–2, and δ1), only the α, β, and γ subunits are necessary to confer BZ sensitivity (McKernan and Whiting 1996; Rudolph et al. 2001). Moreover, the behavioral effects of BZs appear to depend upon the presence of a particular α subunit. In this respect, GABAA receptors containing α1 subunits (α1GABAA receptors) have been proposed to mediate the sedative-like effects of BZs (Rudolph et al. 1999; McKernan et al. 2000). In contrast, the α2 and/or α3-containing GABAA receptors (α2GABAA, α3GABAA receptors) may be the substrates for the anxiolytic and myorelaxant effects of BZs (Rudolph et al. 1999; Löw et al. 2000; McKernan et al. 2000; Collins et al. 2002; Dias et al. 2005), while α5-containing receptors (α5GABAA receptors) may be involved in processes associated with memory (Collinson et al. 2002; Crestani et al. 2002).
In general, the motor-impairing effects of BZs limit their clinical utility as anxiolytics (for review, see Korpi et al. 1997), although some effects, e.g., myorelaxation, can be desirable from a therapeutic perspective. Therefore, understanding the GABAA receptor mechanisms underlying specific motor-impairing effects such as ataxia and myorelaxation would clearly benefit drug discovery efforts geared toward anxiolytic development. In rodents, BZ-induced ataxia is often measured using the rotarod test (Gallaher et al. 1991; Griebel et al. 1999b), while the myorelaxant effects of BZs are typically assessed using a test of grip strength (Griebel et al. 1999a; Crestani et al. 2001; Bach-Rojecky and Samarzija 2005). In non-human primates, observational procedures have been used to evaluate the effects of BZs on these measures. For example, observers were trained to interpret slow and uncertain movements as ataxia in baboons, while lip droop was interpreted as myorelaxation (Weerts et al. 1998; Ator et al. 2000).
Platt et al. (2002) first described a quantitative procedure to assess motor effects in squirrel monkeys in which trained handlers evaluated the ability of monkeys to maintain normal balance on a transport pole following administration of BZ agonists. Subsequent studies employing this procedure have suggested that α1GABAA receptors may play a critical role in mediating this putative measure of ataxia, a term defined medically as a lack of muscle coordination when performing voluntary movements (Licata et al. 2005; Rowlett et al. 2005b). Preliminary descriptions of methods for evaluating myorelaxation have been described in later studies from our laboratory (Licata et al. 2005; Rowlett et al. 2005b). This procedure involves evaluation of the degree to which monkeys resist the extension of a hind limb. Results from studies using this procedure have suggested that in contrast to the effects of BZs on balancing on a horizontal pole, the ability of BZ-type drugs to decrease resistance to hind-limb flexion may involve α2 and/or α3GABAA subtypes (Licata et al. 2005; Rowlett et al. 2005b).
The present study evaluated hands-on measures of motor behavior in squirrel monkeys, with the overall aim of examining the contribution of specific GABAA receptor subtypes to the ataxic vs. myorelaxant effects of BZs and BZ-site agonists. For these experiments, we chose representative 1,4-benzodiazepines and triazolobenzodiazepines with high intrinsic efficacy and no selectivity for GABAA receptor subtypes (triazolam, alprazolam, diazepam, and chlordiazepoxide), as well as two α1GABAA receptor-selective agonists with high intrinsic efficacy (zolpidem and zaleplon). To delineate further the extent to which α1GABAA receptors contribute to BZ-induced ataxia and myorelaxation, studies were conducted with the α1GABAA–preferring antagonist β-carboline-3-carboxylate-t-butyl ester (βCCT; Cox et al. 1995; for review see Rowlett et al. 2005a). In squirrel monkeys, βCCT has been shown to block motor behaviors involving α1GABAA receptors. For example, βCCT antagonized both zolpidem-induced decreases in operant responding, as well as zolpidem- and triazolam-induced increases in ataxia (Paronis et al. 2001; Platt et al. 2002). While other studies conducted by our group have found that βCCT did not attenuate the ability of BZ-site compounds to decrease hind-limb flexion at a dose that did reduce ataxic-like effects (Licata et al. 2005; Rowlett et al. 2005b), those experiments were conducted with single doses of a relatively limited range of BZ agonists. Thus, another goal of the present study was to compare βCCT’s ability to shift full dose-response functions of conventional and receptor-preferring BZ-site ligands.
Methods
Subjects
Adult male squirrel monkeys (Saimiri sciureus, N= 4–6), weighing 750–1100g, were studied in daily experimental sessions (Monday through Friday). Between experimental sessions all monkeys were housed individually and maintained under a 12 hr light/dark cycle in a temperature- and humidity-controlled room where they had unrestricted access to food (Teklad Monkey Diet supplemented with fresh fruit) and water. All procedures were conducted with the approval and under the supervision of the Harvard University Institutional Animal Care and Use Committees. Animals in this study were maintained in accordance with the guidelines of the Committee on Animals of the Harvard Medical School and the “Guide for Care and Use of Laboratory Animals” National Research Council, Department of Health, Education and Welfare Publication No. (NIH) 85-23, revised 1996.
Apparatus
Studies were conducted in a ventilated, transparent Plexiglas arena (114 cm × 122 cm × 213 cm) located in an isolated room. This arena was equipped with perches, suspended plastic chains, and a wood chip foraging substrate to allow the monkeys to express a range of species-typical behaviors. Monkeys were transferred to and from the observation arena using a pole and leash system. The stainless steel chain leash was attached to a collar around the neck of the monkey, and during transfer the leash was passed through a ring at the end of a 1 cm diameter stainless steel transport pole. This technique restricted the monkey’s movement to the region of the transport pole farthest away from the handler and required the monkey to balance atop the transport pole.
Procedure
Monkeys were habituated to the observation arena and the experimental procedures described below for a period of approximately one month. Following habituation, 30-min behavioral assessment sessions were conducted daily. During the 6th, 18th, and 30th minute of each 30-min session, the monkeys were removed briefly from the observation arena by a trained handler following the technique described above for transferring the monkeys, and evaluated for ataxic-like effects and myorelaxant-like effects. Ataxic-like effects were evaluated according to the criteria described by Platt et al. (2002), and were defined as the inability to balance on the transport pole held in the horizontal plane. A score of 0 indicated that the monkey was able to balance normally on the pole, a score of (+1) indicated that the monkey was able to hold onto the pole, but unable to maintain balance (e.g. suspended by limbs below the pole), and a score of (+2) indicated that the monkey could neither balance on nor hold onto the pole. Myorelaxant-like effects were defined as decreased resistance to extension of a hind limb. A score of 0 indicated normal resistance to extension, a score of (−1) indicated a decreased resistance to extension, and a score of (−2) indicated no resistance to extension (i.e., the monkey was flaccid and completely relaxed). Handlers were trained for at least 1 month prior to data collection, and were unaware of the drug being tested (i.e., “blinded” to the drug condition). Inter-rater reliability scores were determined prior to the study to be ≥ 0.90.
Drug testing occurred once or twice per week, with control sessions preceded by saline injections on intervening days. Doses of diazepam (0.3–10 mg/kg; 30 min pretreatment), chlordiazepoxide (3.0–56 mg/kg; 30 min pretreatment), triazolam (0.01–0.3 mg/kg; 30 min pretreatment), alprazolam (0.03–1.0 mg/kg; 30 min pretreatment), zolpidem (1.0–17.8 mg/kg; 10 min pretreatment), or zaleplon (1.0–17.8 mg/kg; 10 min pretreatment) were evaluated in separate test sessions. Pretreatment intervals were chosen on the basis of preliminary studies which determined the approximate time to peak effect, or were obtained from published reports of these drugs administered i.m. to squirrel monkeys (cf. Platt et al., 2002; Licata et al., 2005). In a subset of four monkeys, antagonism studies were conducted in which selected doses of βCCT (3.0 mg/kg; 10 min pretreatment) were studied in combination with a range of doses of the agonists. All drugs, as well as saline controls, were administered via intramuscular injections in a calf or thigh muscle.
Analysis of drug effects
For each subject, the median scores for each measure were obtained across the three evaluation time points of a session, and median scores were obtained across subjects for each treatment. To determine statistical reliability of treatment effects, the effect of dose was determined for each drug by separate Friedman repeated measures analysis of variance (ANOVA) on ranks. Treatment effects were assessed further using Dunn’s Q statistic, in which the effects of different doses of each drug were compared to data from saline control sessions. Data are presented graphically as medians with variability presented as the interquartile range.
The potency of compounds to engender ataxia and muscle relaxation was estimated by calculating the ED50 for each drug. ED50 values correspond to the doses that produced 50% of the maximum effect observed for a particular drug or drug combination. Linear regression analysis was used in cases where the portion of the log dose-response function was defined by three or more data points or by linear interpolation in cases where the log dose-response function was defined best by two points.
Drugs
Triazolam, alprazolam, diazepam (Sigma/RBI; St. Louis, MO), zolpidem (Merck Sharp Dohme; Harlow, UK; gift from G. Dawson), βCCT (Department of Chemistry, University of Wisconsin-Milwaukee, Milwaukee, WI), and zaleplon (Wyeth-Ayerst; Princeton, NJ) were prepared in a vehicle of 50% propylene glycol (Fisher Scientific; Suwanee, GA), 40% saline, and 10% ethanol. Chlordiazepoxide (Research Biochemicals; Natick, MA) was dissolved in 0.9% saline. All doses are expressed in the base form of the compound. Injection volumes were 0.1–0.4 ml/kg body weight. All drugs were prepared the day of a test session.
Results
Ataxic-Like and Myorelaxant-Like Effects of Non-selective BZs
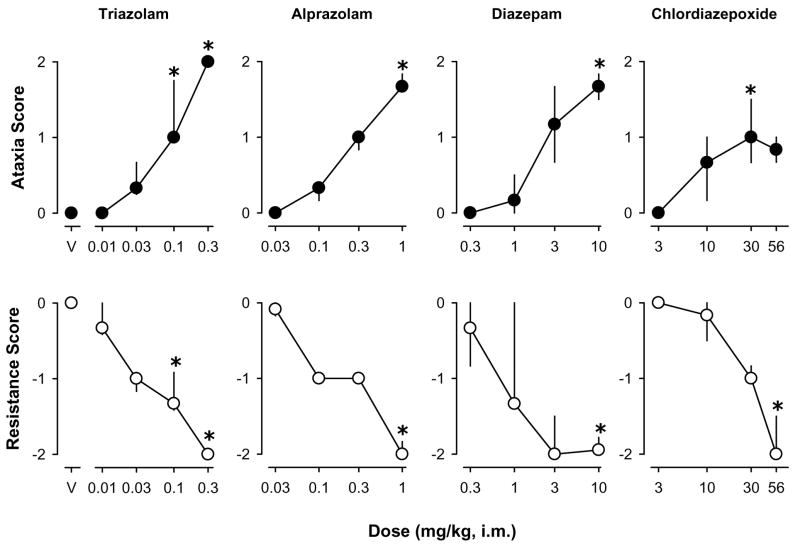
Following saline administration, no animal exhibited ataxic-like or myorelaxant-like effects (i.e., ataxic-like effects score=0, resistance score=0). Dose-related increases in ataxic-like effects were observed following administration of the non-selective BZs triazolam, alprazolam, diazepam, and chlordiazepoxide (Fig. 1, top panels). Triazolam engendered increases in ataxic-like effects such that doses of 0.1 or 0.3 mg/kg produced ataxic-like effects scores that were reliably greater than those obtained following saline administration [χ2(4)= 19.30, p<0.05, Dunn’s Q statistic, p<0.05]. Similarly, the highest dose of alprazolam (1.0 mg/kg) [χ2(4)= 15.51, p<0.05, Dunn’s Q statistic, p<0.05], diazepam (10.0 mg/kg) [χ2(4)= 14.51, p<0.05, Dunn’s Q statistic, p<0.05], and the 30 mg/kg dose of chlordiazepoxide [χ2(4)= 12.97, p<0.05, Dunn’s Q statistic, p<0.05] engendered reliable increases in the scores for ataxic-like effects compared to those obtained following saline.
Figure 1.
Ataxic-like and myorelaxant-like effects of the non-selective benzodiazepines triazolam, alprazolam, diazepam, and chlordiazepoxide. Data are median ± interquartile range from N=4–5 monkeys. Asterisks represent significant differences relative to saline control sessions (p<0.05).
Dose-related decreases in hind limb resistance also were observed following administration of triazolam [χ2(4)= 19.50, p<0.05], alprazolam [χ2(4)= 15.78, p<0.05], diazepam [χ2(4)= 13.13, p<0.05], and chlordiazepoxide [χ2(4)= 15.13, p<0.05,] (Fig. 1, bottom panels). The highest doses of alprazolam, diazepam, and chlordiazepoxide induced reliable decreases in resistance scores, with the two highest doses reliable for triazolam (Dunn’s Q statistic, p<0.05).
Ataxic-Like and Myorelaxant-Like Effects of the α1GABAA-Preferring Agonists
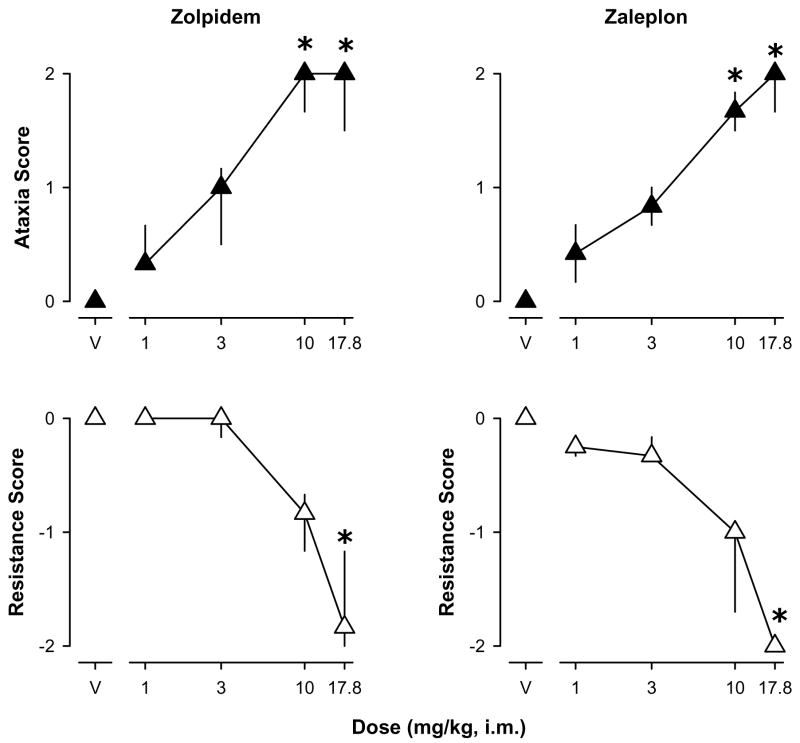
Similar to the non-selective BZs, the α1GABAA-preferring BZ-site agonists zolpidem and zaleplon engendered dose-dependent increases in ataxic-like effects above saline levels (Fig. 2, top panels). Zolpidem engendered a significant increase in the ataxic-like effects score at doses of 10 and 17.8 mg/kg [χ2(4)= 13.11, p<0.05, Dunn’s Q statistic, p<0.05]. The effects of zaleplon were similar such that doses of 10 and 17.8 mg/kg engendered a significant ataxic-like effects [χ2(4)= 15.64, p<0.05, Dunn’s Q statistic, p<0.05].
Figure 2.
Ataxic-like and myorelaxant-like effects of the α1 GABAA receptor-preferring agonists zolpidem and zaleplon. Data are median ± interquartile range from N=4 monkeys. Asterisks represent significant differences relative to saline control sessions (p<0.05).
Zolpidem and zaleplon also engendered dose-related decreases in the hind limb resistance scores (Fig. 2, bottom panels). Unlike the scores for ataxic-like effects, decreases in resistance (i.e. increases in myorelaxant-like effects) only were induced by the highest dose of both zolpidem [χ2(4)= 14.75, p<0.05, Dunn’s Q statistic, p<0.05] and zaleplon [χ2(4)= 14.86, p<0.05, Dunn’s Q statistic, p<0.05].
Effects of the α1GABAA-Preferring Antagonist βCCT
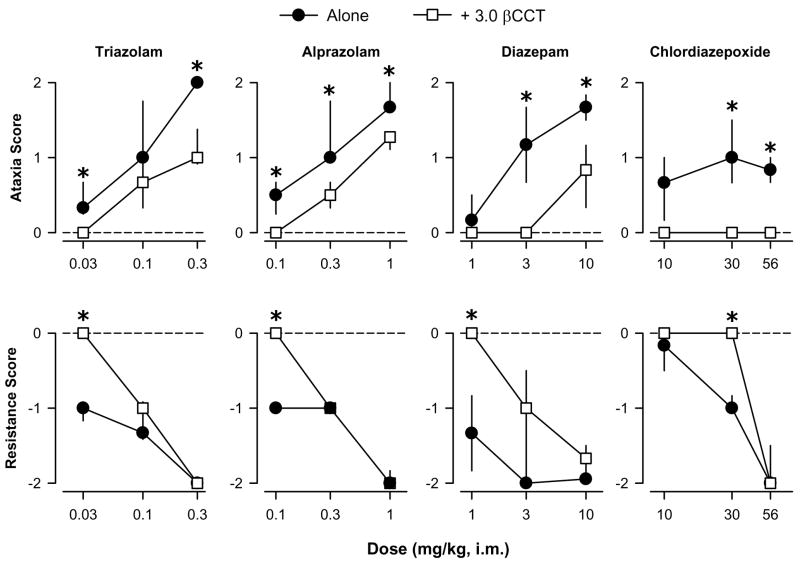
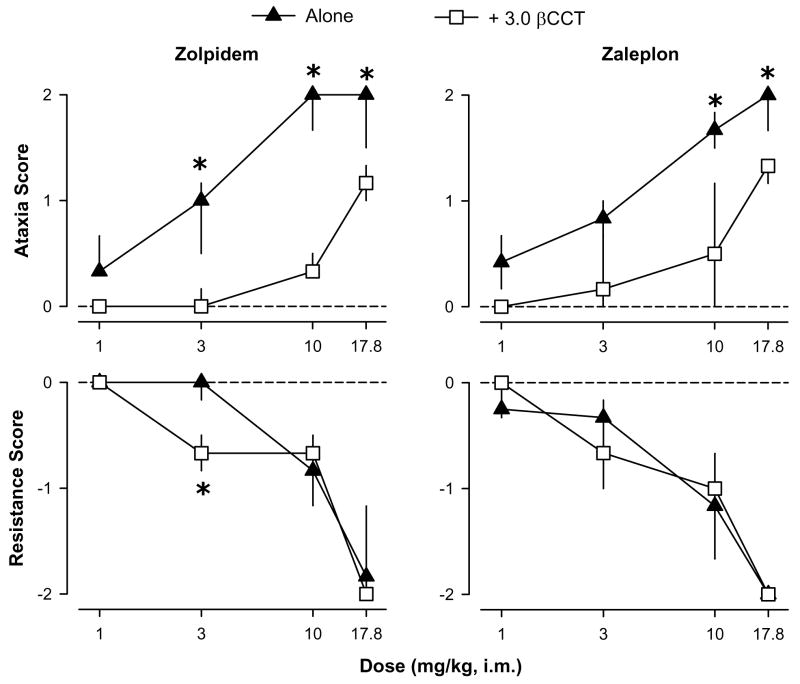
Previous results have shown that βCCT (3.0 mg/kg, i.m.) does not affect motor behavior when administered alone, and it antagonizes some of the behavioral effects of zolpidem and non-selective BZs (Platt et al. 2002). When administered as a pretreatment, βCCT attenuated the ataxic-like effects of low-to-high doses of both the non-selective BZs and the α1GABAA-preferring agonists (Figs. 3 and 4, top panels). With the exception of chlordiazepoxide, this attenuation of ataxic-like effects by βCCT was surmountable (higher doses of chlordiazepoxide were not tested due to solubility limitations leading to relatively high injection volumes). Overall, βCCT pretreatment resulted in rightward shifts in the dose-response functions for ataxic-like effects for all compounds except chlordiazepoxide, and this antagonism is evident in the 2- to 4-fold greater ED50 values computed for the combination of each agonist plus βCCT compared to agonist alone (Table 1).
Figure 3.
Ataxic-like and myorelaxant-like effects of the non-selective benzodiazepines triazolam, alprazolam, diazepam, and chlodiazepoxide, in the presence and absence of the α1 GABAA receptor-preferring antagonist βCCT (3.0 mg/kg). Data are median ± interquartile range from N=4 monkeys. Asterisks represent significant differences relative to agonist alone (p<0.05). Horizontal dashed lines represent median values for vehicle and 3.0 mg/kg βCCT alone. βCCT had no effects when tested alone under any condition (all scores = 0).
Figure 4.
Ataxic-like and myorelaxant-like effects of the α1 GABAA receptor-preferring agonists zolpidem and zaleplon in the presence and absence of the α1 GABAA receptor-preferring antagonist βCCT (3.0 mg/kg). Data are median ± interquartile range from N=4 monkeys. Asterisks represent significant differences relative to agonist alone (p<0.05). Horizontal dashed lines represent median values for vehicle and 3.0 mg/kg βCCT alone. βCCT had no effects when tested alone under any condition (all scores = 0).
Table 1.
Potencies and antagonism of the ataxic and myorelaxant effects of benzodiazepine-type drugs by βCCT in squirrel monkeys
| ED50 mean mg/kg ± SEM)
|
||||
|---|---|---|---|---|
| Drug | Alone | + βCCT (3.0 mg/kg) | Dose Ratioa (mean ± SEM) | P<0.05?b |
| Ataxia | ||||
| lam | 0.07 ± 0.01 | 0.3 ± 0.05 | 4.0 ± 0.50 | Yes |
| Alprazolam | 0.3 ± 0.03 | 0.8 ± 0.08 | 2.7 ± 0.27 | Yes |
| Diazepam | 2.6 ± 0.8 | 8.8 ± 0.5 | 3.6 ± 0.73 | Yes |
| Chlordiazepoxide | 11± 7.1 | >56 | > 3.0 | |
| Zolpidem | 3.0 ± 0.9 | 16 ± 1.2 | 6.0 ± 1.1 | Yes |
| Zaleplon | 3.3 ± 0.3 | 15 ± 1.6 | 4.5 ± 0.8 | Yes |
| Muscle Resistance | ||||
| Triazolam | 0.09 ± 0.01 | 0.1 ± 0.01 | 1.1 ± 0.1 | No |
| Alprazolam | 0.2 ± 0.02 | 0.3 ± 0.00 | 1.5 ± 0.3 | No |
| Diazepam | 1.7 ± 0.2 | 4.4 ± 1.7 | 2.3 ± 0.8 | No |
| Chlordiazepoxide | 27 ± 2.8 | 41 ± 0.00 | 1.5 ± 0.2 | No |
| Zolpidem | 8.8 ± 0.8 | 7.6 ± 0.9 | 0.9 ± 0.2 | No |
| Zaleplon | 9.0 ± 0.7 | 9.2 ± 0.8 | 1.0 ± 0.4 | No |
ED50 is the dose of drug resulting in 50% of the maximum effect.
Dose ratio = agonist + βCCT ED50 divided by agonist alone ED50.
Difference between agonist and agonist + βCCT, Student’s t-tests.
βCCT also attenuated myorelaxant-like effects induced by the non-selective BZs but only at the lowest dose of agonist (or “middle” dose for chlordiazepoxide, 30 mg/kg); and for the α1GABAA-selective agonists no antagonism was apparent (Figs. 3 and 4, bottom panels). In fact, the myorelaxant-like effects of one dose of zolpidem (3.0 mg/kg) were enhanced by administration of βCCT (Bonferroni t-test, p<0.05). For both the non-selective BZs and the α1GABAA-preferring agonists, βCCT did not significantly alter the ED50 values (Table 1).
Discussion
These studies further established observational methods for evaluating potential measures of ataxia and myorelaxation induced by BZ-type drugs in a non-human primate species (squirrel monkey). We also investigated the potential role of α1GABAA receptor mechanisms underlying these effects. Although previous reports from our laboratory have provided an analysis of the ability of BZ-type compounds to engender motor effects (Platt et al. 2002; Licata et al. 2005; Rowlett et al. 2005b), the present studies were designed to compare more fully the ability of both conventional BZs and α1GABAA receptor-preferring agonists to disrupt balance on a pole (ataxic-like effects) and decrease resistance to hind-limb flexion (myorelaxant-like effects). The primary advantage of these techniques, in addition to the use of quantitative observation methods to improve reliability and reproducibility across laboratories, is the ease of use and non-invasive nature of the methodology (i.e., no implants are required, as with electromyography). However, it should be noted that as with all indirect measures of motor coordination and function, validation by direct measures is required before firm conclusions can be made regarding the actual phenomena of interest (e.g., myorelaxation is most precisely measured via electromyography), hence the use of the phrases “ataxic-like” and “myorelaxant-like” in this report. Finally, our data provide support for the hypothesis that α1 subunit-containing GABAA receptors likely do not play a key role in the myorelaxant-like effects of BZ-type drugs in monkeys.
Of the compounds tested, nearly all induced maximum median scores for both ataxic-like and myorelaxant-like effects. The primary exception to this pattern of effects was chlordiazepoxide, which consistently engendered lower-than-maximal median scores for ataxic-like effects. These observations are consistent with reports of chlordiazepoxide’s relatively distinctive profile in behavioral studies. For example, drug discrimination studies have suggested that chlordiazepoxide may interact more selectively with α2, α3, and α5GABAA receptors than α1GABAA receptors (Sanger and Benavides 1993; Lelas et al. 2001a). However, the complete abolition of chlordiazepoxide’s ataxic effects by pretreatment with βCCT in the present study suggests that chlordiazepoxide’s effects may be influenced by α1GABAA receptors as well, consistent with it’s profile as a non-selective agonist. Alternatively, the variable behavioral effects induced by chlordiazepoxide could result from its complex metabolic pathway since it is transformed into four active metabolites, none of which have been characterized in the present behavioral methods or with respect to their interactions with the GABAA receptor (Greenblatt et al. 1978). However, diazepam shares many of these same metabolites and did not differ appreciably from the triazolobenzodiazepines with respect to ataxic-like and myorelaxant-like profles.
Our findings are consistent with a growing body of literature suggesting that BZ-induced ataxia may be an α1GABAA receptor-mediated phenomenon. In support of this idea, the experiments in which monkeys were pretreated with the α1GABAA-preferring antagonist βCCT showed a clear attenuation of the ataxic-like effects produced by both the α1GABAA-preferring and non-selective BZ agonists. Rightward shifts were observed for those dose-response functions. These findings are consistent with an earlier study from our laboratory (Platt et al. 2002), and more recently, we have shown that L-838,417, a compound lacking efficacy at α1GABAA receptors, failed to engender ataxia (Rowlett et al. 2005b), and SL651498 (6-fluoro-9-methyl-2-phenyl-4-(pyrrolidin-1-yl-carbonyl)-2,9-dihydro-1H-pyridol[3,4-b]indol-1-one), a compound with only partial agonist efficacy at α1 receptors, demonstrated a relatively weak ataxic effect at the highest dose tested (Licata et al. 2005). Nonetheless, it is important to note that our studies cannot rule out a role for other receptor subtypes in mediating ataxic-like effects induced by BZ-type drugs—this type of information awaits the availability of selective antagonists for each of the subtypes.
Our findings with monkeys are very similar to data obtained in studies using rodent models. For example, reports with several compounds that have weak or no activity at α1GABAA receptors compared with α2GABAA, α3GABAA, and α5GABAA receptors have shown a reduced propensity to engender ataxia evaluated by the rotarod test and measures of spontaneous locomotor activity (e.g., Savic et al. 2007; but see also Popik et al. 2006). Finally, studies performed in genetically altered mice in which the α1 subunit is rendered insensitive to the effects of BZs have shown that diazepam engendered less ataxia in these mice compared to their wild-type counterparts (McKernan et al. 2000; Rudolph et al. 2001). Together, these findings provide support for the idea that stimulation of α1GABAA receptors plays a role in BZ-induced ataxia, and extend these observations to a monkey species.
In addition to BZ-induced ataxic-like effects, all six agonists engendered robust myorelaxant-like effects. Unlike ataxic-like effects, studies using functionally selective compounds in monkeys (Licata et al. 2005; Rowlett et al. 2005b) or genetically altered mice (Crestani et al. 2001; 2002) have suggested a more prominent role for α2 and/or α3GABAA receptors in the myorelaxant effects of BZs and BZ-type compounds. At first glance, this hypothesis does not appear to be supported by the data presented here, since both zolpidem and zaleplon induced significant myorelaxant-like effects. Despite their binding preference for α1GABAA receptors, however, zolpidem and zaleplon bind to and have intrinsic efficacy at α2GABAA and α3GABAA receptors (see Sanna et al. 2002). Potential mediation of zolpidem- and zaleplon-induced myorelaxation by these subtypes of the GABAA receptor may be implied in our studies by the need for somewhat higher (and presumably less selective) doses of both drugs to induce myorelaxant-like effects.
Although βCCT attenuated the myorelaxant-like effects engendered by triazolam, alprazolam, diazepam, and chlordiazepoxide, this antagonism occurred only at relatively low doses of the agonists and did not result in an overall change in potency (measured via ED50 values). Consistent with this finding, we previously demonstrated that βCCT was generally ineffective in blocking myorelaxation, but not ataxia, induced by single doses of alprazolam, triazolam, chlordiazepoxide, and SL651498 (Licata et al. 2005; Rowlett et al. 2005a). Most strikingly, βCCT clearly did not antagonize the myorelaxant-like effect engendered by zolpidem and zaleplon, raising the possibility that myorelaxant effects induced by these agonists primarily involve the α2GABAA and/or α3GABAA receptor subtypes. Overall, this hypothesis is supported by previous results in which myorelaxant-like effects in monkeys were engendered by compounds functionally-selective for α2GABAA and/or α3GABAA receptor subtypes (Rowlett et al. 2005b; Licata et al. 2005).
In summary, these findings suggest that BZ agonist-induced ataxic-like and myorelaxant-like effects in squirrel monkeys can be dissociated by antagonism studies with βCCT, implying that these two observable effects may require activation of different GABAA receptor subtypes. These studies particularly raise the possibility that α1GABAA receptors contribute to a lesser extent to the myorelaxant effects of these drugs. These studies further suggest that compounds with limited efficacy and/or potency at α1GABAA receptors may be better poised to have fewer motor side effects than either non-selective or α1GABAA-selective compounds.
Acknowledgments
This work was supported by U.S. Public Health Services Grants DA11792, AA16179, RR00168, and MH46851. The experiments reported here comply with the current laws of the United States of America. A preliminary report of these data was made at the 2004 Annual Meeting of the Society for Neuroscience.
References
- Ator NA, Weerts EM, Kaminski BJ, Kautz MA, Griffiths RR. Zaleplon and triazolam physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. Drug Alcohol Depend. 2000;61:69–84. doi: 10.1016/s0376-8716(00)00122-8. [DOI] [PubMed] [Google Scholar]
- Bach-Rojecky L, Samarzija I. Influence of ethanol on the myorelaxant effect of diazepam in rats. Acta Pharm. 2005;55:115–122. [PubMed] [Google Scholar]
- Collins I, Moyes C, Davey WB, Rowley M, Bromidge FA, Quirk K, Atack JR, McKernan RM, Thompson S-A, Wafford K, Dawson GR, Pike A, Sohal B, Tsou NN, Ball RG, Castro JL. 3-Heteroaryl-2-pyridones: Benzodiazepine site ligands with functional selectivity for α2/α3-subtypes of human GABAA receptor-ion channels. J Med Chem. 2002;45:1887–1900. doi: 10.1021/jm0110789. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Out FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox ED, Hagen TJ, McKernan RM, Cook JM. BZ1 receptor subtype specific ligands. Synthesis and biological properties of beta-CCt, a BZ1 receptor subtype specific antagonist. Med Chem Res. 1995;5:710–718. [Google Scholar]
- Crestani F, Keist R, Fritschy J-M, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Löw K, Keist R, Mandelli MJ, Mohler H, Rudolph U. Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol. 2001;59:442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- Dias R, Sheppard WF, Fradley RL, Garrett EM, Stanley JL, Tye SJ, Goodacre S, Lincoln RJ, Cook SM, Conley R, Hallett D, Humphries AC, Thompson SA, Wafford KA, Street LJ, Castro JL, Whiting PJ, Rosahl TW, Atack JR, McKernan RM, Dawson GR, Reynolds DS. Evidence for a significant role of {alpha}3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci. 2005;46:10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher EJ, Gionet SE, Feller DJ. Behavioral and neurochemical studies in diazepam-sensitive and–resistant mice. J Addict Dis. 1991;10:45–60. doi: 10.1300/J069v10n01_04. [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ, Shader RI, MacLeod SM, Sellers EM. Clinical pharmacokinetics of chlordiazepoxide. Clin Pharmacokinet. 1978;3:381–394. doi: 10.2165/00003088-197803050-00004. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Letang V, Granger P, Avenet P, Schoemaker H, Sanger DJ. New evidence that the pharmacological effects of benzodiazepine receptor ligands can be associated with activities at different BZ (ω) receptor subtypes. Psychopharmacology. 1999a;146:205–213. doi: 10.1007/s002130051108. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Tan S, Schoemaker H, Sanger DJ. Comparison of the pharmacological properties of classical and novel BZ-omega receptor ligands. Behav Pharmacol. 1999b;10:483–495. doi: 10.1097/00008877-199909000-00007. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Mattila MJ, Wisden W, Lüddens H. GABAA-receptor subtypes: clinical efficacy and selectivity of benzodiazepine site ligands. Ann Med. 1997;29:275–282. doi: 10.3109/07853899708999348. [DOI] [PubMed] [Google Scholar]
- Lelas S, Rowlett JK, Spealman RD. Isobolographic analysis of chlordiazepoxide and triazolam combinations in squirrel monkeys discriminating triazolam. Psychopharmacology. 2001a;158:181–189. doi: 10.1007/s002130100868. [DOI] [PubMed] [Google Scholar]
- Licata SC, Platt DM, Cook JM, Sarma PVVS, Griebel G, Rowlett JK. Contribution of GABAA receptor subtypes to the anxiolytic-like, motor, and discriminative stimulus effects of benzodiazepines: Studies with the functionally-selective ligand SL 651498 [6-fluoro-9-methyl-2-phenyl-4-(pyrrolidin-1-yl-carbonyl)-2,9-dihydro-1H-pyridol[3,4-b]indol-1-one] J Pharmacol Exp Ther. 2005;313:1118–1125. doi: 10.1124/jpet.104.081612. [DOI] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Pharmacol Sci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor alpha1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Möhler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Cox ED, Cook JM, Bergman J. Different types of GABAA receptors may mediate the anticonflict and response-rate decreasing effects of zaleplon, zolpidem, and midazolam in squirrel monkeys. Psychopharmacology. 2001;156:461–468. doi: 10.1007/s002130100754. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD, Cook JM, Ma C. Selective antagonism of the ataxic effects of zolpidem and triazolam by the GABAA/α1-preferring antagonist β–CCt in squirrel monkeys. Psychopharmacology. 2002;164:151–159. doi: 10.1007/s00213-002-1189-9. [DOI] [PubMed] [Google Scholar]
- Popik P, Kostakis E, Krawczyk M, Nowak G, Szewczyk B, Krieter P, Chen Z, Russek SJ, Gibbs TT, Farb DH, Skolnick P, Lippa AS, Basile AS. The anxioselective agent 7-(2-chloropyridin-4-yl)pyrazolo-[1,5-a]-pyrimidin-3-yl](pyridin-2-yl)methanone (DOV 51892) is more efficacious than diazepam at enhancing GABA-gated currents at alpha1 subunit-containing GABAA receptors. J Pharmacol Exp Ther. 2006;319:1244–1252. doi: 10.1124/jpet.106.107201. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Cook JM, Duke AN, Platt DM. Selective antagonism of GABAA receptor subtypes: An in vivo approach to exploring the therapeutic and side effects of benzodiazepine-type drugs. CNS Spectr. 2005a;10:40–48. doi: 10.1017/s1092852900009895. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci. 2005b;102:915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid (A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Mohler H. GABAA receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Benavides J. Discriminative stimulus effects of omega (BZ) receptor ligands: correlation with in vivo inhibition of [3H]-flumazenil binding in different regions of the rat central nervous system. Psychopharmacology. 1993;111:315–322. doi: 10.1007/BF02244947. [DOI] [PubMed] [Google Scholar]
- Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, Maciocco E, Biggio G. Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol. 2002;451:103–110. doi: 10.1016/s0014-2999(02)02191-x. [DOI] [PubMed] [Google Scholar]
- Savic MM, Huang S, Furtmuller R, Clayton T, Huck S, Obradovic DI, Ugresic ND, Sieghart W, Bokonjic DR, Cook JM. Are GABA(A) receptors containing alpha5 subunits contributing to the sedative properties of benzodiazepine site agonists? Neuropsychopharmacology. 2007 Mar 28; doi: 10.1038/sj.npp.1301403. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Weerts EM, Ator NA, Grech DM, Griffiths RR. Zolpidem physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. J Pharmacol Exp Ther. 1998;285:41–59. [PubMed] [Google Scholar]
- Whiting PJ. GABA-A receptors: a viable target for novel anxiolytics? Curr Opin Pharmacol. 2006;6:24–29. doi: 10.1016/j.coph.2005.08.005. [DOI] [PubMed] [Google Scholar]