Abstract
hrR3 is an oncolytic Herpes simplex virus 1 (HSV-1) mutant that replicates preferentially in tumors compared to normal tissues. Portal venous administration of hrR3 in mice bearing diffuse colorectal carcinoma liver metastases significantly reduces tumor burden and prolongs animal survival. In the current study, we compared survival benefit and biodistribution of hrR3 following intravenous (IV) administration versus intraperitoneal (IP) administration in immune-competent mice bearing colon carcinoma peritoneal metastases.
Mice bearing peritoneal metastases received 1×108 pfu hrR3 or mock-infected media every other day for 3 doses, and were randomized to have the viruses administered by either a IP or IV route. Biodistribution was assessed by PCR amplification of HSV-1 specific sequences from tumor and normal tissue including small bowel, liver, spleen, kidney, lung, heart and brain. LD50 for IP administration was compared to the LD50 for IV administration. In subsequent experiments, animals were monitored for survival.
The frequency of HSV-1 detection in peritoneal tumors was similar in mice randomized to either IP or IV administration. However, IP administration resulted in a more restricted systemic biodistribution, with a reduced frequency of virus detected in the kidney, lung, and heart. The LD50 associated with IP administration was higher than with IV administration. Tumor burden was more effectively reduced with IP compared to IV administration. Median survival following IP administration was approximately twice that observed with IV administration.
IP administration of an HSV-1 oncolytic mutant is associated with more restricted biodistribution, less toxicity, and greater efficacy against peritoneal metastases compared to IV administration.
INTRODUCTION
Peritoneal implants are detected in approximately 10% of the patients at the time of primary colorectal cancer resection (1) (2). Furthermore, the peritoneal cavity is the only site of metastatic disease in 25% of patients with colorectal cancer recurrence (2-4). The two proposed mechanisms for intraperitoneal spread of intraabdominal tumors (5) are natural tumor progression and surgical manipulation. During tumor progression, intraperitoneal spread may occur as a result of full thickness invasion of the bowel wall. Peritoneal seeding may also occur as a result of tumor rupture or organ rupture, such as with mucus-producing cystadenocarcinoma of the appendix. Iatrogenic intraperitoneal spread may occur during surgery from tumor cells spilled from dissected lymph vessels or from transected viscera. Tumor cells can also theoretically reach the peritoneal cavity through hemorrhage into the surgical field. Peritoneal colorectal carcinomatosis is associated with high morbidity and mortality. Regional delivery of therapeutic agents (e.g. IP administration) remains an active area of clinical investigation (6), (7).
hrR3 is a Herpes simplex virus 1 (HSV-1) mutant defective in viral ribonucleotide reductase (8). In previous work, we demonstrated that hrR3 replicates preferentially in liver metastases rather than in normal liver (9, 10). A single intravascular administration of hrR3 significantly reduces tumor burden and enhances survival in mice bearing diffuse colorectal liver metastases (11). Pre-existing immunity to HSV-1 does not reduce anti-tumor efficacy (12, 13). Here we investigate in a murine model of syngeneic peritoneal colon carcinoma metastases any differences in biodistribution and efficacy of hrR3 as a function of route of administration. We demonstrate that intraperitoneal (IP) administration of hrR3 leads to more restricted systemic biodistribution of virus than intravenous (IV) treatment. The more restricted biodistribution observed following IP delivery compared to IV delivery was also associated with a higher LD50. Furthermore, overall survival of animals is significantly prolonged in IP treated mice as compared to IV treated animals.
MATERIALS AND METHODS
Cell Lines and Viruses
The human colon carcinoma cell lines HT29 and SW480, as well as the African Green Monkey kidney cell line Vero, were obtained from the American Type Culture Collection (Rockville, MD). The MC26 mouse colon carcinoma cell line was obtained from the National Cancer Institute Tumor Repository (Frederick, MD). All cell lines were maintained in Dulbecco-modified Eagle's medium, 10% fetal bovine serum, 100 U/mL penicillin, and 100 g/mL streptomycin. The HSV-1 vector, hrR3 (8) (kindly provided by Sandra Weller, University of Connecticut, New Haven, CT), is comprised of an insertion of the lacZ gene into the UL39 locus of the parent virus, KOS. F strain Herpes simplex virus was obtained from Antonia Chiocca (Ohio State University, Columbus, OH). The viruses were propagated and titered on Vero cells.
Viral Cytotoxicity Assays
Viral cytotoxicity assays were performed as previously described (10). Briefly, cells were plated onto 96-well plates at 5000 cells per well for 36 hours. Virus was added at MOI values ranging from 0.001 to 10 and incubated for 6 days. The number of surviving cells was quantitated using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Experiments were performed in triplicate.
Animal Studies
Balb/c and A/J mice were obtained from Charles River Labs (Wilmington, MA). Animal studies were performed in accordance with policies of the Massachusetts General Hospital Subcommittee on Research Animal Care. To examine the biodistribution of IP or IV hrR3, peritoneal metastases of colorectal cancer were induced by IP injection of a single-cell suspension consisting of 5 × 105 MC26 cells in 500 μl PBS (n = 5 per group). Beginning on day 4, mice were treated IP or IV with 1×108 pfu hrR3 every other day for a total of 3 doses. Mice were sacrificed 48 hours after the last dose, and presence of virus was assessed by polymerase chain reaction (PCR) amplification of HSV-1 specific sequences from tissue samples including tumor and normal bowel, liver, spleen, kidney, lung, heart and brain.
LD50 was assessed by a single inoculation of a variety of doses of F strain into A/J mice. The mice (n = 5 per group) were followed 10 days for signs of morbidity or mortality. Mice were randomized to either IV or IP administration. As per the institutionally approved protocol, doses above the LD50 were not tested.
To assess macroscopic differences in tumor progression, peritoneal metastases were induced by IP injection of a single-cell suspension consisting of 1 × 105 MC26 cells in 500 μl PBS (n = 3 per group). Beginning on day 4, mice were treated with three doses of 1×108 pfu hrR3 either IP or IV every other day. Control animals received mock-infected media. Twenty days after tumor induction, all mice were euthanized and their tumor burden was examined. All abdominal organs (liver, stomach, small and large bowel, mesentery, kidneys, spleen, omentum, and all associated tumor) were removed en bloc. Abdominal organ specimens from mice naive to virus and tumor were removed en bloc in a similar fashion and a baseline weight was established. Tumor weight was determined by subtracting the average naive animals' (baseline) abdominal organ weight from the weights of abdominal organ specimens.
To examine the effect of route of administration on survival of mice bearing peritoneal colorectal metastases, mice were randomized to receive hrR3 by either IP or IV administration. Control animals received IP mock-infected media. On day 0, Balb/c mice were injected IP with a single-cell suspension consisting of 1 × 105 MC26 cells in 500 μl PBS. On days 4, 6 and 8, IP or IV treatments of 1 × 108 pfu hrR3 (in a volume of 500 μl for IP injections and 100 μl for IV injections) were performed (n = 10 per group). Survival times were monitored and distribution was displayed by the method of Kaplan and Meier. Experiments were repeated once to ensure reproducibility.
PCR-Assay
PCR amplification of HSV-1-specific sequences to investigate the biodistribution of HSV-1 in mice was performed as described (13). Forward oligonucleotide primer 5′-GGAGGCGCCCAAGCGTCCGGCCG-3′ and reverse oligonucleotide primer 5′-TGGGGTACAGGCTGGCAAAGT-3′ were used to amplify a 229-bp fragment of HSV-1 DNA polymerase gene. DNA was extracted from BALB/c mouse tissues using the MasterPure Purification kit (Epicentre, Madison, WI) following the manufacturer's instructions. PCR amplifications were performed on 100 ng of DNA in a 25-μl volume using 2X PCR Master Mix (Promega Corporation, Madison, WI) according to the manufacturer's instructions in a DNA Thermal Cycler 480 (Perkin-Elmer Applied Biosystems). Cycling conditions were for 35 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 1 min. Negative controls were used for all PCR reactions, and no contamination of reagents was detected.
Statistical Methods
Two nonparametric statistical analyses, the log-rank test and Peto-Wilcoxon test, were used to compare survival between groups (Intercooled Stata 6.0, Stata Corporation, College Station, TX). Quantitative data are reported as the mean ± standard deviation. Student's t test was used for statistical comparisons.
RESULTS
IP administration of hrR3 results in a restricted biodistribution and higher LD50 than systemic delivery
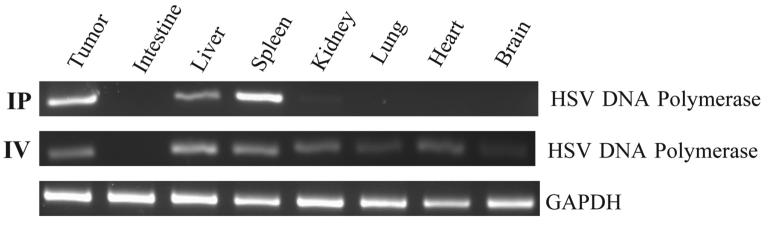
For these studies we used mice bearing MC26 colorectal carcinoma peritoneal metastases, as we have previously characterized susceptibility of this particular tumor to HSV-1 mutants (11, 14-16). Mice were randomized to treatment with 1×108 pfu hrR3 by IV or IP delivery every other day for a total of 3 doses. Forty-eight hours after the last dose, biodistribution was assessed by PCR amplification of HSV-1 specific sequences from tissue samples including tumor and normal gut, liver, spleen, kidney, lung, heart and brain (Fig. 1). The frequency of detection by PCR of hrR3 in tumors was essentially similar in IP- and IV-treated mice, although the “effective” multiplicity of infection (MOI) was higher in the IP group relative to the IV group. The location of lacZ staining detected histochemically in the tumor nodules did not differ between IV and IP administration mice. Importantly, IP administration resulted in a reduced frequency of hrR3 detection in normal tissues such as kidney, lung, heart, and brain (Table 1).
Figure 1. Biodistribution of hrR3 after IP or IV administration.
The presence of HVS-1 and GAPDH DNA was identified by the PCR amplification of specific sequences from DNA extracted from tissue samples 48 hours after IP (n=5) or IV (n=5) administration. Representative gels are shown, and data for all mice are summarized in Table 1.
Table 1.
Biodistribution of hrR3 to mice organs
| Site of Herpes simplex-1 infection as assessed by PCR | ||||||||
|---|---|---|---|---|---|---|---|---|
| Positive / tested (n) | ||||||||
| Tumor | Intestine | Liver | Spleen | Kidney | Lung | Heart | Brain | |
| IP | 3/5 | 0/5 | 4/5 | 3/5 | 1/5 | 1/5 | 0/5 | 0/5 |
| IV | 2/5 | 3/5 | 4/5 | 4/5 | 4/5 | 5/5 | 5/5 | 3/5 |
To determine if reduced toxicity is also observed with this more restricted biodistribution, LD50 was assessed following IP or IV administration of F strain in mice. Initial experiments revealed no balb/c mouse mortality following administration of even the highest achievable titers of hrR3, KOS, and F strain, and we therefore instead tested AJ mice, which are more sensitive to HSV-1 mortality. While the LD50 of IV administration of F strain was determined to be approximately 1 × 107 pfu, the LD50 of IP administration could not be reached because of stock viral titer limitations, but it is demonstrated to be higher than 1 × 108 pfu (Table 2). Thus, the more restricted biodistribution observed following IP delivery compared to IV delivery was also associated with reduced toxicity and a higher LD50.
Table 2.
Survival after inoculation of Herpes simplex virus-1
| Survival (%) | ||||
|---|---|---|---|---|
| 1 × 105 | 1 × 106 | 1 × 107 | 1 × 108 | |
| IP | ND | 0/5 | 0/5 | 1/5 |
| IV | 0/5 | 1/5 | 3/5 | ND |
In mice bearing peritoneal colorectal metastases, IP administration of hrR3 reduces tumor burden and enhances survival
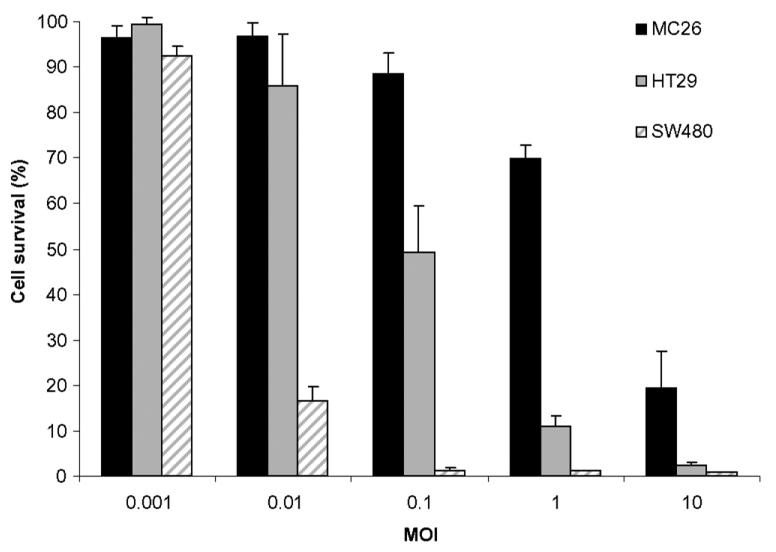
hrR3 is cytotoxic to MC26 murine colon carcinoma cells and the human colon carcinoma cell lines HT29 and SW480 at low multiplicities of infection (MOI) (Fig. 2). Because herpes viruses have a greater tropism for human cell lines, it is not surprising that the murine MC26 cell line was less susceptible to lytic oncolysis than the human colon cancer cell linces. We measured in vivo antineoplastic efficacy against MC26 peritoneal metastases following either IP or IV administration. Mice bearing MC26 colon carcinoma peritoneal metastases were established as above, and randomized to receive IP or IV injections of 1 × 108 pfu hrR3 (n = 4 for each group) on days 4, 6 and 8. Control animals received mock-infected media IP. Twenty days after tumor induction, all mice were euthanized and their tumor burden was examined (Fig. 3A). IP administered hrR3 reduced tumor burden from 280 ± 98 mg in controls to 72 ± 48 mg (P<0.01). Systemic administration of hrR3 had no effect on tumor burden. These animals had comparable tumor burden (290 ± 141 mg) as the control animals (Fig. 3B).
Figure 2. Cytotoxicity of hrR3 against human and murine colorectal carcinoma cell lines in vitro.
MC26 cells and two human colon carcinoma cell lines were infected with hrR3 and mock-infected media at several MOI values, and surviving cells were quantitated 6 days later.
Figure 3. Effect of IP and IV hrR3 administration on tumor growth.
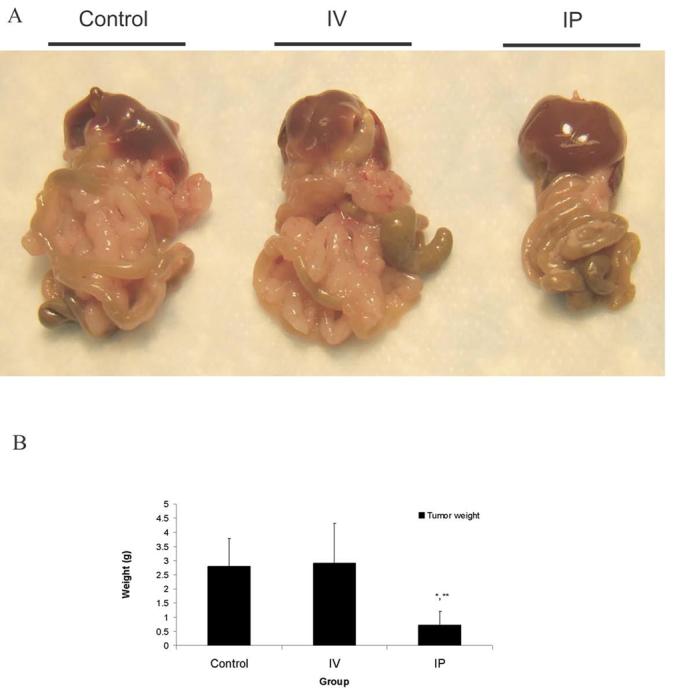
Mice with MC26 peritoneal metastases were randomized to treatment with IP or IV injections of 1 × 108 pfu hrR3 on day 4, 6 and 8. Control animals received mock-infected media. Mice were sacrificed and examined on day 20. A, representative specimen from each group. B, tumor weight in control animals, compared to after IV or IP treatment. *, P < 0.01 for IP compared to Control. **, P < 0.05 for IP compared to IV.
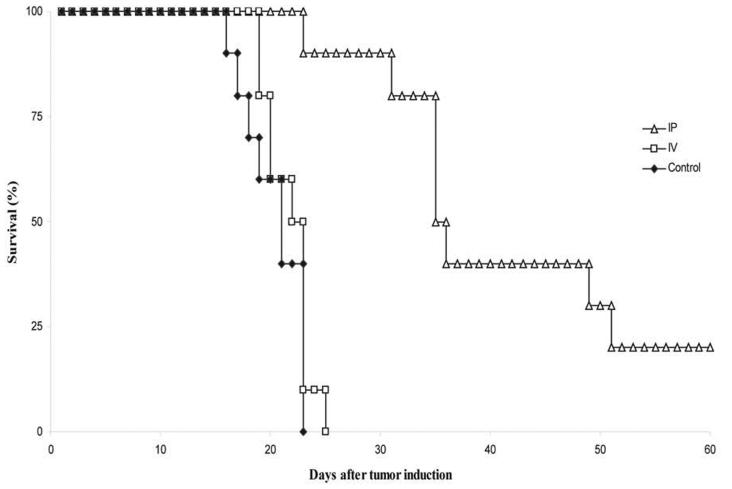
To examine the effect of route of administration on survival of mice bearing peritoneal colorectal metastases, mice were randomized to receive hrR3 by either IP or IV administration. Control animals received mock-infected media IP. On day 0, Balb/C mice were injected IP with a single-cell suspension consisting of 1 × 105 MC26 cells in 500 μl PBS, followed by IP or IV injections of 1 × 108 pfu hrR3 on days 4, 6 and 8. Mice were monitored for survival (Fig. 4). The IP group had two long term survivors, which were euthanized on day 78. Necropsy revealed minimal tumor burden in these animals, and necropsy of other mice demonstrated that they died of overwhelming tumor burden. Median survival of animals in the control, IV and IP groups were 22, 24 and 37 days, respectively. The difference in survival between the IP group and the IV group was statistically significant (P < 0.01). There was no significant difference between IV-treated and control groups. These results indicate that in mice bearing peritoneal metastases, regional delivery of the hrR3 oncolytic virus has anti-neoplastic effects, whereas systemic delivery does not.
Figure 4. Survival after IP or IV inoculation of hrR3 in mice bearing MC26 peritoneal metastases.
BALB/c mice bearing peritoneal metastases were treated with a total of 3 doses of 1 × 108 pfu hrR3 IP or IV. Control animals received mock-infected media IP (P < 0.01 for IP compared with IV).
DISCUSSION
Viral oncolysis represents a therapeutic approach that exploits the natural process of lytic viral replication as a means to destroy tumors (17). Because hrR3 is deficient in the large subunit of ribonucleotide reductase it replicates preferentially in cells with high intracellular nucleotide pools (18). For example, hrR3 preferentially replicates in colon carcinoma rather than in normal hepatocytes (9, 10), and a single intravascular (intrasplenic) injection of hrR3, significantly reduces liver tumor burden (11).
Several strategies have been investigated to restrict viral replication to tumor cells (rather than normal cells) including modulation of surface receptors (19), deletion of critical viral genes that are complemented preferentially by tumor cells (9), use of promoters for tumor associated antigen to drive critical genes critical to viral replication (20), and regional virus administration (21). Hepatic arterial administration has been favored as a delivery route to achieve preferential distribution to liver tumors rather than normal liver (22). Similarly, intraperitoneal administration has been examined as a delivery route to achieve preferential distribution to small peritoneal nodules (6),(7).
Previous preclinical studies have evaluated the therapeutic efficacy of IP injected HSV-1 mutants against peritoneal implants, but all these studies were conducted in nude mice bearing human peritoneal cancer xenografts. For example, in a peritoneal disseminated pancreatic cancer xenograft, IP hrR3 was shown to be effective (23). And Coukos and colleagues investigated the effect of another HSV-1 mutant (G207) in a human epithelial ovarian cancer mouse xenograft model (24). Benett et al. treated peritoneally disseminated gastric cancer cells in a mouse xenograft model using two different HSV-1 mutants, and demonstrated a significant reduction in tumor burden and prolongation of survival (25). But as noted above, all of these studies were conducted in immunodeficient mice bearing human tumors, which precludes a reliable and accurate comparison of biodistribution to tumor versus normal tissues, since human cells are much more susceptible to Herpes infection than murine cells.
Here we compared the impact of route of administration (IP versus IV) on therapeutic efficacy and biodistribution in immune-competent mice with syngeneic murine colon carcinoma peritoneal metastases. Inoculation of hrR3 via the IP route of administration leads to a more restricted biodistribution and reduced toxicity (higher LD50). The IP route was also more efficacious in reducing tumor burden and prolonging survival relative to the IV route. Additional theoretical advantages of IP administration are that the vascular drainage of the peritoneal surface is directed to the liver via the portal vein, providing exposure of potential hepatic microscopic metastases to IP administered virus. The liver also provides first pass clearance of most virus.
The dose that was selected for comparing the two routes of delivery proved to be ineffective against the peritoneal metastases following IV administration. However, hrR3 was detected in peritoneal tumor samples. The lack of antineoplastic efficacy despite the presence of hrR3 in peritoneal tumors after IV treatment was likely secondary to an “effective MOI” that was too low. IP administration increases direct contact between tumor cells and viral particles. We and others have shown than induction of immunity against HSV-1 prior to HSV-1 administration does not reduce oncolytic efficacy (12, 13); however, natural immunity and other defenses in the vascular compartment may reduce the effective MOI (26). The innate immune system has been demonstrated to sequester infected cells as well as free viral particles, thereby reducing oncolytic efficacy (27). Virus delivered IV is more susceptible to the neutralizing effects of innate immunity than virus administered IP or directly into a tumor. In addition, the blood supply to peritoneal metastases has been shown to be quite limited, leading to reduced bioavailability of systemically administered virus (28). Our results provide support to IP administration of oncolytic agents as a means to both increase efficacy against peritoneal metastases and limit toxicity.
Intraperitoneal HSV-1 oncolysis could theoretically be combined with other IP therapies. For example, improved survival in patients with peritoneal dissemination of colorectal cancer after treatment with surgical cytoreduction followed by hyperthermic IP chemotherapy has been reported (29). And in selected gastric cancer patients, IP chemotherapy compared to surgery (30), or hyperthermic IP chemotherapy compared to either surgery alone or normothermic IP chemotherapy resulted in higher survival rates (31). Hyperthermia has been shown to improve drug potency as well as tissue penetration (32). Unfortunately, hyperthermia is inhibitory to lytic HSV-1 replication (33, 34), including replication of ICP6-defective HSV-1 mutants such as hrR3 (35). Cytotoxic chemotherapy also appears to be inhibitory to HSV-1 replication (unpublished data). Thus, additional studies examining chemotherapy and hyperthermia induced modulation of cellular pathways that are intimately linked to viral replication are required to gain a better understanding how to involve oncolytic HSV-1 as a part of multimodality approach to treatment.
Acknowledgments
Grant support: 2R01CA76183 [KKT]; 5T32CA009535 [JDD]; Massachusetts General Hospital Tucker Gosnell Gastrointestinal Cancer Center [KKT, BCF], and Fund for Medical Discovery Fellowship [BCF]; Deutsche Forschungsgemeinschaft [YK]
Abbreviations
- HSV-1
Herpes Simplex Virus 1
- IP
intraperitoneal
- IV
intravenous
- MOI
multiplicity of infection
REFERENCES
- 1.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89(12):1545–50. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 2.Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63(2):364–7. doi: 10.1002/1097-0142(19890115)63:2<364::aid-cncr2820630228>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243(2):212–22. doi: 10.1097/01.sla.0000197702.46394.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugarbaker PH, Cunliffe WJ, Belliveau J, et al. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol. 1989;16(4 Suppl 6):83–97. [PubMed] [Google Scholar]
- 5.Sugarbaker PH. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. Cancer Treat Res. 1996;82:79–100. doi: 10.1007/978-1-4613-1247-5_6. [DOI] [PubMed] [Google Scholar]
- 6.Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–92. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Rothenberg ML, Liu PY, Braly PS, et al. Combined intraperitoneal and intravenous chemotherapy for women with optimally debulked ovarian cancer: results from an intergroup phase II trial. J Clin Oncol. 2003;21(7):1313–9. doi: 10.1200/JCO.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein DJ, Weller SK. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988;62(1):196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon SS, Carroll NM, Chiocca EA, Tanabe KK. Cancer gene therapy using a replication-competent herpes simplex virus type 1 vector. Ann Surg. 1998;228(3):366–74. doi: 10.1097/00000658-199809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll NM, Chiocca EA, Takahashi K, Tanabe KK. Enhancement of gene therapy specificity for diffuse colon carcinoma liver metastases with recombinant herpes simplex virus. Ann Surg. 1996;224(3):323–9. doi: 10.1097/00000658-199609000-00008. discussion 9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon SS, Nakamura H, Carroll NM, Bode BP, Chiocca EA, Tanabe KK. An oncolytic herpes simplex virus type 1 selectively destroys diffuse liver metastases from colon carcinoma. Faseb J. 2000;14(2):301–11. [PubMed] [Google Scholar]
- 12.Delman KA, Bennett JJ, Zager JS, et al. Effects of preexisting immunity on the response to herpes simplex-based oncolytic viral therapy. Hum Gene Ther. 2000;11(18):2465–72. doi: 10.1089/10430340050207957. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura H, Kasuya H, Mullen JT, et al. Regulation of herpes simplex virus gamma(1)34.5 expression and oncolysis of diffuse liver metastases by Myb34.5. J Clin Invest. 2002;109(7):871–82. doi: 10.1172/JCI10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuruppu D, Brownell AL, Zhu A, et al. Positron emission tomography of herpes simplex virus 1 oncolysis. Cancer Res. 2007;67(7):3295–300. doi: 10.1158/0008-5472.CAN-06-4062. [DOI] [PubMed] [Google Scholar]
- 15.Mullen JT, Donahue JM, Chandrasekhar S, et al. Oncolysis by viral replication and inhibition of angiogenesis by a replication-conditional herpes simplex virus that expresses mouse endostatin. Cancer. 2004;101(4):869–77. doi: 10.1002/cncr.20434. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura H, Mullen JT, Chandrasekhar S, Pawlik TM, Yoon SS, Tanabe KK. Multimodality therapy with a replication-conditional herpes simplex virus 1 mutant that expresses yeast cytosine deaminase for intratumoral conversion of 5-fluorocytosine to 5-fluorouracil. Cancer Res. 2001;61(14):5447–52. [PubMed] [Google Scholar]
- 17.Mullen JT, Tanabe KK. Viral oncolysis. Oncologist. 2002;7(2):106–19. doi: 10.1634/theoncologist.7-2-106. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein DJ, Weller SK. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988;166(1):41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds P, Dmitriev I, Curiel D. Insertion of an RGD motif into the HI loop of adenovirus fiber protein alters the distribution of transgene expression of the systemically administered vector. Gene Therapy. 1999;6(7):1336–9. doi: 10.1038/sj.gt.3300941. [DOI] [PubMed] [Google Scholar]
- 20.Mullen JT, Kasuya H, Yoon SS, et al. Regulation of herpes simplex virus 1 replication using tumor-associated promoters. Ann Surg. 2002;236(4):502–12. doi: 10.1097/00000658-200210000-00013. discussion 12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasuya H, Kuruppu DK, Donahue JM, Choi EW, Kawasaki H, Tanabe KK. Mouse models of subcutaneous spleen reservoir for multiple portal venous injections to treat liver malignancies. Cancer Res. 2005;65(9):3823–7. doi: 10.1158/0008-5472.CAN-04-2631. [DOI] [PubMed] [Google Scholar]
- 22.Kemeny N, Daly J, Oderman P, et al. Hepatic artery pump infusion: toxicity and results in patients with metastatic colorectal carcinoma. JClin Oncol. 1984;2(6):595–600. doi: 10.1200/JCO.1984.2.6.595. [DOI] [PubMed] [Google Scholar]
- 23.Kasuya H, Nishiyama Y, Nomoto S, Hosono J, Takeda S, Nakao A. Intraperitoneal delivery of hrR3 and ganciclovir prolongs survival in mice with disseminated pancreatic cancer. J Surg Oncol. 1999;72(3):136–41. doi: 10.1002/(sici)1096-9098(199911)72:3<136::aid-jso5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Coukos G, Makrigiannakis A, Montas S, et al. Multi-attenuated herpes simplex virus-1 mutant G207 exerts cytotoxicity against epithelial ovarian cancer but not normal mesothelium and is suitable for intraperitoneal oncolytic therapy. Cancer Gene Ther. 2000;7(2):275–83. doi: 10.1038/sj.cgt.7700130. [DOI] [PubMed] [Google Scholar]
- 25.Bennett JJ, Delman KA, Burt BM, et al. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene Ther. 2002;9(11):935–45. doi: 10.1038/sj.cgt.7700510. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda K, Ichikawa T, Wakimoto H, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5(8):881–7. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 27.Wakimoto H, Johnson PR, Knipe DM, Chiocca EA. Effects of innate immunity on herpes simplex virus and its ability to kill tumor cells. Gene Ther. 2003;10(11):983–90. doi: 10.1038/sj.gt.3302038. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki T, Yanagi K, Ookawa K, Hatakeyama K, Ohshima N. Blood flow and leukocyte adhesiveness are reduced in the microcirculation of a peritoneal disseminated colon carcinoma. Ann Biomed Eng. 1998;26(5):803–11. doi: 10.1114/1.67. [DOI] [PubMed] [Google Scholar]
- 29.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–43. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 30.Yu W, Whang I, Chung HY, Averbach A, Sugarbaker PH. Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: results of a prospective randomized trial. World J Surg. 2001;25(8):985–90. doi: 10.1007/s00268-001-0067-7. [see comment] [DOI] [PubMed] [Google Scholar]
- 31.Yonemura Y, de Aretxabala X, Fujimura T, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepato-Gastroenterology. 2001;48(42):1776–82. [PubMed] [Google Scholar]
- 32.Teicher BA, Kowal CD, Kennedy KA, Sartorelli AC. Enhancement by hyperthermia of the in vitro cytotoxicity of mitomycin C toward hypoxic tumor cells. Cancer Res. 1981;41(3):1096–9. [PubMed] [Google Scholar]
- 33.Panasiak W, Oraczewska A, Luczak M. Influence of hyperthermia on experimental viral infections in vitro. Advances in Experimental Medicine & Biology. 1990;267:471–5. doi: 10.1007/978-1-4684-5766-7_50. [DOI] [PubMed] [Google Scholar]
- 34.Szmigielski S, Luczak M, Janiak M, et al. In vitro and in vivo inhibition of virus multiplicaton by microwave hyperthemia. Arch Virol. 1977;53(12):71–7. doi: 10.1007/BF01314848. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson JG, Leib DA, Goldstein DJ, et al. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology. 1989;173(1):276–83. doi: 10.1016/0042-6822(89)90244-4. [DOI] [PubMed] [Google Scholar]