Abstract
Gliogenesis in the mammalian CNS continues after birth, with astrocytes being generated well into the first two postnatal weeks. In this study we have isolated an A2B5+ astrocyte precursor (APC) from the postnatal rat forebrain that is capable of differentiating into mature astrocytes in serum-free medium without further trophic support. Exposure to bFGF selectively induces the APCs to proliferate, forming clusters of vimentin+ cells, which, within two weeks, differentiate into GFAP+ astrocytes. While bFGF functions as a potent mitogen, it is not necessary to induce or maintain astrocyte differentiation, nor is it capable of maintaining the precursors in an immature, proliferative state. APCs exit the cell cycle and differentiate, even in the continued presence of FGF alone or in combination with other mitogenic factors such as PDGF. Under the culture conditions employed, it was not possible to cause the astrocytes to re-enter cell cycle. After transplantation into the neonatal forebrain, APCs differentiated exclusively into astrocytes, regardless of brain region. Initially distributed widely within the forebrain, the precursors are most greatly concentrated within the SVZ and subcortical white matter, where they are maintained throughout postnatal development. APCs can be isolated from the SVZ and white matter of animals as late as four weeks after birth.
Keywords: glia, growth factor, SVZ
INTRODUCTION
Astrocytogenesis spans prenatal and postnatal periods in the mammalian brain. The origins of astrocytes and the sequence of developmental stages through which astrocyte precursors (APCs) travel are only partially understood. A number of laboratories have isolated immature cells from the developing CNS that are able to differentiate into astrocytes. These cells vary in their fate restrictions and in their growth factor requirements for promoting differentiation (Behar et al. 1988; Levi et al. 1987; Noble et al. 1988; Raff et al. 1983; Herrera et al. 2001; Mi and Barres 1999; Rao and Mayer-Proschel 1997, Liu et al. 2004)). In most of these experiments, astrocyte differentiation is achieved either through the withdrawal of mitogens or via the addition of factors such as CNTF and BMP family members.
Nearly all known APCs require basic fibroblast growth factor (bFGF), or FGF-2, as a survival factor. However, bFGF influences a wide range of cellular processes, playing a role in the regulation of the proliferation, migration, and differentiation of astrocytes as well as many other cell types (Bikfalvi et al. 1997; Ornitz and Itoh 2001; Reuss and von Bohlen und Halbach 2003). Basic FGF is expressed throughout the brain, though the level of expression varies according to region and cell type throughout development. Basic FGF can be detected in the embryonic rat brain as early as E13, with levels increasing throughout the first month before reaching adult levels (Gomez-Pinilla et al. 1994). Early in development, bFGF appears to be expressed mainly by neurons; localization in astrocytes begins during the first two postnatal days and increases until high levels of astrocytic bFGF can be detected in the adult brain (Gomez-Pinilla et al. 1994; Gonzalez et al. 1995). The FGF family is large, consisting of 23 members. Mature astrocytes produce high levels of bFGF and express FGF receptors 1, 2, and 3 (Miyake et al. 1996; Reilly et al. 1998; Takami et al. 1998; Yazaki et al. 1994). Several studies have reported mitogenic effects of bFGF on astrocytes in vitro and in vivo. Senescent cultured astrocytes can be driven back into cycle by exposure to bFGF (Kniss and Burry 1988). Infusion of bFGF into both neonatal and adult rat brain increases the proliferation of astrocytes as well (Goddard et al. 2002; Gomez-Pinilla et al. 1995). Similar effects were observed in experiments in which avian viruses were used to overexpress bFGF in astrocytes via the human GFAP promoter (Holland and Varmus 1998). In addition to an increase in their proliferation, infected cells and/or their progeny were detected far from the injection site, indicating migration of those cells that produced increased levels of bFGF. Finally, bFGF may have an indirect effect on the differentiation of astrocytes, since studies have suggested that bFGF increases astrocyte process length and may influence the expression of GFAP (Gomes et al. 1999; Reilly et al. 1998).
In this study we describe a bFGF-responsive APC that can be isolated from the postnatal forebrain that is capable of differentiating into mature astrocytes in serum-free media without further trophic support. These precursors cannot be maintained in a cycling state indefinitely in the presence of mitogenic factors and do not require additional factors for astrocyte differentiation. In addition, after transplantation into the neonatal forebrain, the precursors differentiate exclusively into astrocytes.
MATERIALS AND METHODS
Media
Unless otherwise stated, all media reagents were purchased from Sigma (St. Louis, MO). CDMEM: DMEM (Invitrogen, Carlsbad, CA) with 10% FBS (Invitrogen, Carlsbad, CA), 1mM sodium pyruvate (Invitrogen), and 100 μg/ml penicillin/streptomycin (Invitrogen). Chemically Defined Media (CDM): A modified version of the N2 media described in (Bottenstein and Sato 1979), N2B3, that is composed of DMEM/F12 (Invitrogen), 1 mg/ml BSA, 10 ng/ml d-biotin, 5 μg/ml insulin, 20 nM progesterone, 100 μM putrescine, 1.2 g/L sodium bicarbonate, 5 ng/ml selenium, 50 μg/ml transferrin, 15 mM HEPES, 30 nM triiodothyronine (T3) and 100 μg/ml penicillin/streptomycin. Sorting Media: CDM made with phenol-red free DMEM/F12 (Invitrogen) and without T3. Isolation Media: 0.9M sucrose in 1× MEM (Invitrogen) supplemented with 20mM HEPES, pH 7.2. Wash Media: Composed of 1:1 mix of calcium-magnesium-free-PBS (CMF-PBS) and phenol red-free DMEM supplemented with 15mM HEPES, 5 μg/ml insulin, 50 μg/ml transferrin, 5 ng/ml selenium, 100 μg/ml penicillin/streptomycin, and 1% FBS. Sorting Wash: Composed of a 1:1 mix of Isolation Media and Wash Media.
Cell Culture
Forebrains of P2 Sprague-Dawley rat pups were removed, stripped of meninges, and mechanically and enzymatically dissected in a modification of a procedure described elsewhere (Gensert and Goldman 2001). Briefly, tissue was shredded using forceps and digested in a solution containing 0.125% trypsin (Invitrogen), 20U/ml papain (Roche Applied Science, Indianapolis, IN), and 285U/ml DNase (Sigma, St. Louis, MO) at 37°C for 35 min. in a shaking water bath. Undigested tissue was triturated with a Pasteur pipet, filtered through 70 μm Nitex mesh and the trypsin neutralized with an equal volume of CDMEM. The single cell solution was centrifuged at 1000×g for 10 minutes and the cell pellet resuspended in sorting media supplemented with 10 ng/ml bFGF (Sigma) and A2B5 hybridoma supernate (1:5; American Type Culture Collection (ATCC), Manassas, VA) and incubated for 20 min at RT. Cells were washed with isolation media (1000×g, 10 min.) and resuspended in sorting media containing Alexa Fluor 647 conjugated goat anti-mouse IgM antibody (1:2,000; Invitrogen) and incubated for 10 min. Cells were then washed with wash media and resuspended in sorting media containing Hoescht 33342 (1:10,000; Molecular Probes, Eugene, OR). Cells were sorted into CDM using a BD FACSAria flow cytometer (BD Biosciences, San Jose, CA). Sorting media and the collection media (CDM) contained 10 ng/ml bFGF for all experiments except those in which bFGF addition was delayed or the FGF concentration altered. Collected cells were pelleted and resuspended in CDM at 2×105 cells/ml and plated onto poly-l-lysine coated 60 mm tissue culture dishes or 8 or 16-well glass or plastic chamber slides (Nunc, Rochester, NY) in droplets and allowed to settle for 10 min before the addition of CDM to the appropriate volume. The final density was 2×104 cells/cm2. Regional dissections were performed from P2, P7, P14, and P28 Sprague-Dawley rats. The forebrains were removed, stripped of meninges, and coronally sliced to isolate tissue from the SVZ, striatum, subcortical WM, and neocortex. The tissue was dissociated, processed for flow cytometry, and plated as indicated above with the following exceptions. After primary antibody incubation, cells isolated from the SVZ, WM, and cortex were washed with a 1:1 mixture of isolation media and wash media. Cells from the striatum were washed with wash media only. Media was changed every two to three days. When necessary, cells were passaged after five days in culture. Briefly, media was aspirated, 3ml of TrypLE (Invitrogen) was added and cells were incubated at 36°C for 1 min. Cells were then collected, centrifuged at 1000×g for 10 min and replated at a density of 2×104 cells/cm2. All animal experiments were performed under the guidelines of the Columbia University Institutional Animal Care and Use Committee.
Immunofluorescence Staining
Cultured cells were fixed in 4% paraformaldehyde (PFA) for 20 min at RT stored in PBS at 4°C until use. Cells stained with intracellular markers were permeabilized with ice-cold acetone for 7 minutes. Slides were blocked with 20% goat serum (Sigma) for 20 min and all primary antibodies were applied overnight at 4°C. Cultures stained with surface markers were incubated in the surface marker antibody overnight, washed 3X with PBS, and fixed with 4% PFA before permeabilization with ice-cold acetone for 8 min. Cultures were then blocked and incubated in primary antibodies to intracellular markers. Surface markers include: A2B5 hybridoma supernatant (1:5), O4 hybridoma supernatant (1:5; ATCC), and guinea pig anti-GLT-1 (1:1000; Chemicon, Temecula, CA). Intracellular markers include: mouse anti-vimentin (1:500; DAKO Cytomation, Carpinteria, CA), rabbit anti-GFAP (1:500; DAKO Cytomation), rabbit anti-S100β (1:500; DAKO Cytomation), mouse anti-TUJ1 (1:500; Covance, Berkeley, CA), mouse anti-nestin (1:100; Developmental Studies Hybridoma Bank, Iowa City, IA), CC1 (1:50; Calbiochem; San Diego, CA), rabbit anti-GLAST (1:100; Alpha Diagnostic, San Antonio, TX), mouse anti-NeuN (1:500; Chemicon), mouse anti-smooth muscle actin (1:200; DAKO Cytomation), mouse anti-Zebrin II IgG1(1:100; provided by R. Hawkes, University of Calgary, Alberta, Canada), guinea pig anti-Olig2 (1:20,000; provided by T. Jessel, Columbia University, New York, NY), mouse anti-NG2 (1:400; provided by W. Stallcup, The Burnham Institute, La Jolla, CA), rabbit-anti Ki67 (1:1250; NovoCastra, New Castle, UK), and mouse anti-Ki67 (1:250; NovoCastra). Antigen retrieval was utilized for Zebrin II staining as described in (Marshall and Goldman 2002). Cells were washed 3X with PBS and incubated in Alexa-Fluor conjugated secondary antibodies (1:2000, Invitrogen, Carlsbad, CA) or FITC conjugated goat-anti rabbit secondary antibody (Southern Biotech, Birmingham, AL) for one hour at room temperature and then counter-stained with Hoescht 33342 nuclear stain. Images were captured using a Nikon Eclipse E800 microscope and Spot imaging software. Immunohistochemistry was performed following the same procedure with the following exceptions. Sections were stained floating and blocked in 20% goat serum with 1% Triton X-100 (Sigma). After secondary antibody treatment, sections were mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and images were captured with a Zeiss laser scanning microscope (LSM) 510 confocal microscope and associated imaging software.
Retroviral injections
GFP retrovirus was produced using HEK 293 cells stably transfected with the pNIT vector (provided by T. Palmer and S. Suhr, The Salk Institute, La Jolla, CA), a bicistronic replication incompetent retrovirus that uses a 5′-LTR to drive expression of the tetracycline activator and the tetracycline operon enhancer/promoter to drive the expression of eGFP. pNIT virus was prepared as described previously (Kakita and Goldman, 1999). P0 Sprague Dawley rat pups at were deeply anesthetized by immersion in ice water for 8 min, positioned in a stereotactic apparatus (Stoelting, Wood Dale, IL), and 1μl of retrovirus solution was injected bilaterally into the SVZ at a rate of 0.2 μl/min using a 10μl Hamilton syringe (Fisher Scientific). Coordinates of the injection site are 1 mm anterior and 2.0 mm lateral to bregma at a depth of 2.0 mm. Animals were sacrificed after two days and astrocyte precursors were isolated from the SVZ and proximal striatum and WM.
Transplantation
A2B5+ APCs were isolated from P2 Sprague Dawley rat pups and plated onto 60mm tissue culture dishes in CDM supplemented with 10 ng/ml bFGF. APCs were labeled with GFP by adding pNIT-GFP retrovirus to the medium after 1 and 3 days in culture. After 5 days in culture, cells were harvested and GFP+ cells collected using flow cytometry. Approximately 2000 GFP+ cells in 1μl of CDM were injected into P0 Sprague Dawley rat pups as described above. At P14, rats were anesthetized by intraperitoneal injection of ketamine (75 mg/kg; Aveco, Fort Dodge, IA) and xylazine (5 mg/kg; Mobay, Shawnee, KS) and were transcardially perfused with 4% paraformaldehyde. Brains were postfixed overnight, and cryoprotected with 30% sucrose in PBS. 50μm coronal sections were cut using a cryostat and stored in PBS at 4°C until use.
RESULTS
An A2B5+ Astrocyte Precursor in the Neonatal Forebrain
To determine if an A2B5+ APC can be isolated from postnatal forebrain, P2 rat forebrains were dissociated into single cell suspensions and A2B5+ cells were purified by immunolabeling followed by flow cytometry (Figure 1A). Cells were plated onto eight-well chamber slides at 200 cells/μl to a density of 2×104 cells/cm2 in a serum-free medium supplemented with the growth factors, platelet-derived growth factor-AA (PDGF-AA) (10ng/ml), basic fibroblast growth factor (bFGF) (10ng/ml), and insulin-like growth factor-1 (IGF-1) (10ng/ml), individually and in combinations. The initial composition of the A2B5+ cell population was examined after 2–3 hours to allow the cells time to settle. Cells were immunostained with TuJ1 to label neurons, O4 to label oligodendrocyte precursors, vimentin to label immature astrocytes, and GFAP to label mature astrocytes. At this time approximately 75% of the A2B5+ cells were TuJ1+, 7% were O4+, and 7% were vimentin+. No GFAP+ cells were present. Approximately 10% of the cells did not label with any of these markers and were not further identified. After four days, TuJ1+ neurons accounted for about 40% of the cells, regardless of the culture conditions. There was a steady decrease in the total number and, consequently, the proportion of immature neurons over time, so that after twelve days in culture, less than 1% of the cells were TuJ1+. The proportion of O4+ cells increased to 12% after 4 days in vitro, with no significant growth factor contribution over medium alone. However, over the next eight days, the proportion of O4+ cells decreased to 3%.
Figure 1.
A2B5+ Cells Isolated from P2 Rat Forebrain.
A: A histogram of the FACS sorted A2B5+ cells. The A2B5+ population was approximately 25% of the parent sample. Mid+: A population with weaker A2B5 immunoreactivity; Neg: A2B5− population.
B: A2B5+ cells cultured for 5 days in CDM supplemented with bFGF (10ng/ml). Vimentin+ (Vim) clusters are marked: 1. large cluster >60 cells (grey arrows); 2. medium cluster 15–60 cells (white arrows); 3. small cluster <15 cells (arrowheads) scale bar- 200μm. Representative clusters: scale bar- 25μm.
C: Histogram depicting the distribution of the size of vimentin+ clusters in 5day cultures of A2B5+ cells grown in CDM with bFGF (10ng/ml) (n=370).
In the presence of FGF, by 4 days in vitro all cultures displayed clusters of bipolar, vimentin+ cells, ranging in size from four cells to over a hundred (Figure 1B and 1C). With PDGF-AA by itself, clusters never exceeded more than eight cells. However, PDGF-AA potentiated the effects of bFGF, increasing the percent of vimentin+ cells to approximately 65% in comparison to bFGF alone, in which about 55% of the cells were positive (Figure 2). In contrast, IGF-1 appeared to inhibit the effects of bFGF moderately.
Figure 2. Percentage of Vimentin+ Cells in A2B5+ Cultures Grown in the Presence of PDGF-AA, bFGF, and IGF-1.
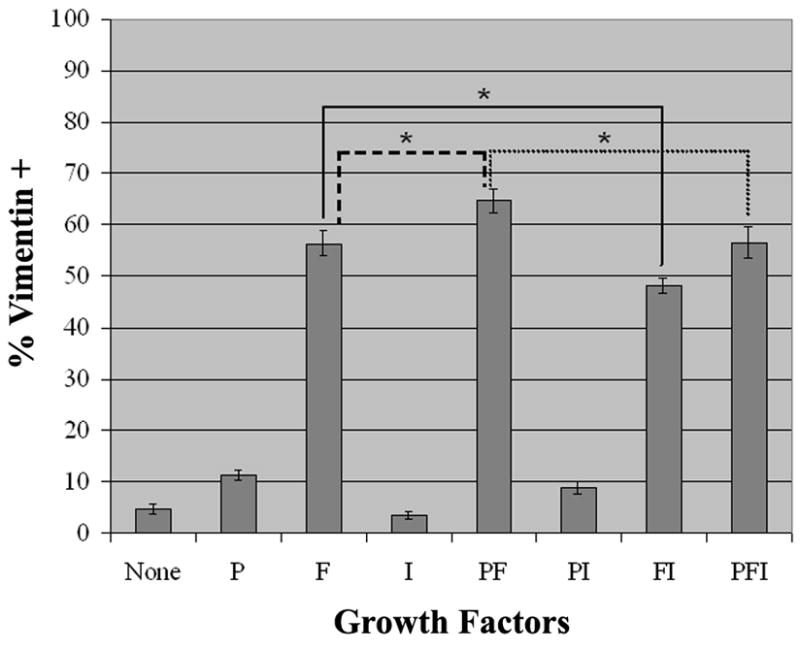
Forebrain A2B5+ cells were grown in CDM (C) supplemented with the following growth factors (10ng/ml): PDGF-AA (P), bFGF (F), and IGF-1 (I) alone and in combination. After four days in culture, cells were fixed and stained for vimentin and Hoescht 33342 (m ± SEM n=4). The presence of bFGF resulted in large increase in vimentin+ cells. This effect was further amplified by the addition of PDGF. The presence of IGF in addition to bFGF and/or PDGF decreased the number of vimentin+ cells (*P<0.05 as determined by paired Student’s t-Test).
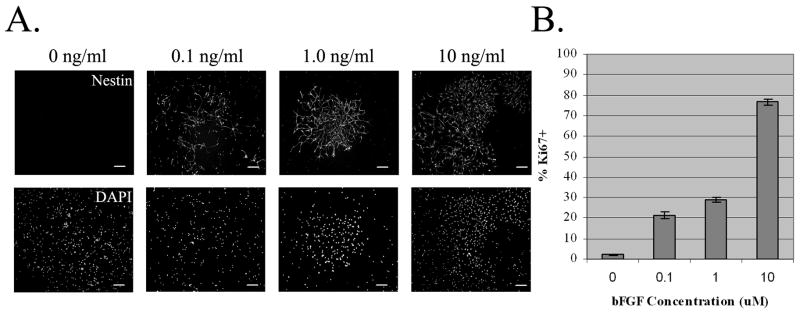
A bFGF concentration as low as 0.1ng/ml was sufficient to drive proliferation (Figure 3A). However, clusters grown in the 0.1ng/ml of bFGF were smaller, suggesting that the rate of proliferation at this lower concentration was less than that at higher levels. The proliferative index of cultures exposed to 0, 0.1, 1.0, and 10 ng/ml of bFGF increased, as judged by the proportion of Ki67+ cells (Figure 3B). In the absence of bFGF, only a fraction of the Ki67+ cells were also nestin+ or vimentin+. However, in the presence of 0.1 ng/ml of bFGF, 95% of the Ki67+ cells co-expressed nestin and vimentin. This percentage increased with the addition of higher levels of bFGF, such that at a concentration of 10 ng/ml, all Ki67+ cells were nestin+ and vimentin+, demonstrating that bFGF selectively amplifies the astrocyte precursors.
Figure 3. Effect of Lower bFGF Concentrations on APCs.
A2B5+ SVZ APCs were sorted into media without bFGF and plated in CDM containing 0, 0.1, 1.0, and 10 ng/ml bFGF. After five days, cells were fixed and stained for nestin and/or vimentin, Hoescht 33342 (DAPI), and Ki67. A. Higher levels of bFGF produced larger clusters of nestin+ precursors. Scale bar- 50μm.
B. Bar graph depicting the percentage of Ki67+ precursors out of the total number of cells present under each condition (m ± SEM n=3). Increasing concentrations of bFGF correspond to increasing mitotic indices.
The degree of Ki67 labeling increased with increasing cluster size. Large clusters were composed of tightly-spaced small cells, nearly all of which were Ki67+. In contrast, small clusters consisted of larger cells with longer processes, many of which had little or no Ki67 staining. This suggests that cluster size correlates with the proliferative capacity of individual precursors and introduces the possibility that precursors generating small clusters may be a later stage of the highly proliferative cells, less able to respond to the FGF.
Antigen Expression Profile of Astrocyte Precursors
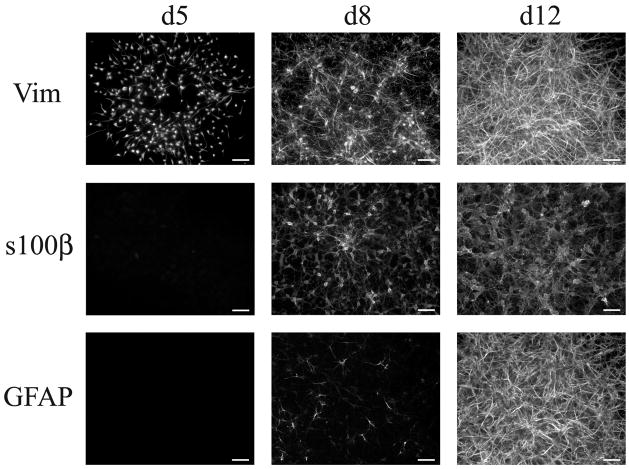
We examined the marker profile of these cells at 5, 8, and 12 days in culture (Table 1). By 5 days, in addition to being A2B5+ and vimentin+, the cells also expressed zebrinII/aldolase, the glutamate transporter GLT-1, nestin (Supplemental Figure 1), and the transcription factor, olig2 (not shown). The cells did not express S100β, or GFAP (Figure 4). While some NG2+ cells were also present in the cultures, they were not vimentin+ and did not constitute part of the vimentin+ clusters. By 8 days in vitro, along with substantial process growth, the cells had acquired S100β and some had begun to express GFAP (Figure 4). Over the next few days, the majority of the cells developed into GFAP+ astrocytes. The developmental expression of the glutamate transporter, GLAST, was similar to that of S100β. Although these cells continued to express vimentin, S100β, and GLT-1, some had apparently downregulated expression of nuclear Olig2, diffusely expressing Olig2 within the cell body and at times within the processes (data not shown).
Table 1.
Antigen Expression of A2B5+ Precursors on d5, d8, and d12. Cultures of A2B5+ precursors were fixed and immunostained for the above antigens on d5, d8 and d12.
| Antigen | d5 | d8 | d12 |
|---|---|---|---|
| Vimentin | + | + | + |
| Zebrin | + | + | + |
| Olig2 | + | + | −/+ |
| Nestin | + | + | + |
| Glt-1 | + | + | + |
| GLAST | − | + | + |
| S100b | − | + | + |
| GFAP | − | −/+ | + |
Figure 4. Expression of Astrocyte Markers on APCs.
Cultures of A2B5+ APCs were fixed and immunostained on d5, d8 and d12 to determine expression of the following markers: vimentin, S100β, and GFAP. Regions of comparable cellular density are depicted for each time point. Scale bar- 25μm.
Few Astrocytes Arise from the A2B5− Population
To determine if these APCs exist only within the A2B5+ population, we also collected the A2B5− population from flow cytometry, and placed these in culture under the same conditions as above (Figure 1A – Neg). Occasional (no more than 1 per 103 cells plated, 20-fold less than the A2B5+ population) clusters of vimentin+, A2B5+, spindly cells resembling the clusters in the A2B5+ sorted population appeared over the 5-day culture period. It is possible that the A2B5+ clusters arising from the A2B5− cell sorted population are due to minor contamination with cells expressing low levels of the A2B5 antigen. We also examined these intermediately A2B5+ cells, encompassing a wide range of A2B5 expression levels between the A2B5+ population and the A2B5− population (Figure 1A – Mid+). Cultures of these A2B5+ cells that fell into the intermediate category also gave rise to occasional clusters, raising the possibility that some contamination occurred between the cells expressing low levels of A2B5 and the negative population. Another explanation for the presence of a few A2B5+ APCs in the A2B5− population is that the precursors are derived from an A2B5−predecessor that later acquires A2B5 expression. In either case, all clusters formed from the A2B5− population expressed A2B5 and appeared identical to those isolated from the A2B5+ population, indicating that the APCs are indeed A2B5+.
While few astrocytes were detected in the A2B5− population when cultured in CDM supplemented with bFGF, the addition of 10% FBS resulted in the production of GFAP+ astrocytes within 4 days. Similarly, the addition of serum to the culture medium of A2B5+ APCs also accelerated the acquisition of GFAP by 4 days in culture. Far more astrocytes were detected in the A2B5+ population, however, most likely due to the proliferation of the astrocyte precursors. Therefore, while astrocyte can be generated from both A2B5+ and A2B5− cells, the astrocyte precursors exist primarily in the A2B5+ population.
More A2B5+ Astrocyte Precursors can be Isolated from SVZ than Cerebral Cortex
To delineate further the origin of these precursors, different regions of the brain were microdissected from coronal slices of P2 rat pup forebrains, dissociated, and sorted via flow cytometry. A2B5+ cells from the subventricular zone (SVZ), striatum, subcortical white matter (WM), and cortex were cultured in eight-well chamber slides with serum-free medium supplemented with bFGF. After 5 days in vitro, the slides were stained and analyzed to determine the quantity and size of clusters from each region. The majority of the precursors that gave rise to large clusters were localized in the SVZ and WM (Table 2). Even though the striatum also contained proliferative precursors, the clusters formed were smaller than those from the SVZ and WM, suggesting a lower proliferative potential. In contrast, the cortex yielded precursors that formed small clusters no greater than 4 to 6 cells. Thus, the highly proliferative precursors that generated large clusters in the whole forebrain cultures appeared to have originated from SVZ and WM.
Table 2. Spatiotemporal Distribution and Proliferative Capacity of A2B5+ Astrocyte Precursors.
A2B5+ precursors isolated from the SVZ, striatum, subcortical white matter, and cortex of P2, P7, P14, and P28/P30 rats were cultured in CDM supplemented with bFGF (10ng/ml) for five days before fixation and analysis. The number of astrocyte precursors present was determined by vimentin immunostaining. Proliferative capacity was determined by the total number of precursors located within each cluster as well as by cellular morphology. Representative clusters are depicted in Figure 1B: 1. large cluster (“++++” and “+++”); 2. medium cluster (“++”); 3. small cluster (“+”). The data presented was gathered from a minimum of three experiments.
| SVZ | Striatum | WM | Cortex | ||
|---|---|---|---|---|---|
| Age |
|

|
|
|
|
| P2 | # of precursors | ++++ | +++ | ++++ | ++ |
| Proliferation | ++++ | +++ | ++++ | + | |
| P7 | # of precursors | ++++ | ++ | +++ | + |
| Proliferation | ++++ | + | +++ | + | |
| P14 | # of precursors | +++ | + | +++ | + |
| Proliferation | +++ | + | ++− | − | |
| P28 | # of precursors | +++ | + | +++ | + |
| Proliferation | +++ | − | ++ | − |
Some A2B5+ Astrocyte Precursors are Derived from Cells Cycling in vivo
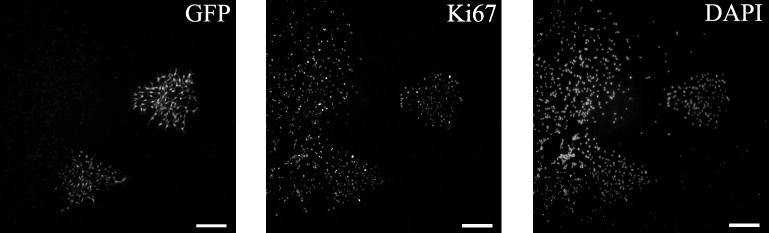
The release of FGF as a result of injury to the brain is believed to induce the proliferation of normally quiescent astrocytes. The same effect has been demonstrated in vitro where quiescent cultured astrocytes are induced to proliferate upon exposure to bFGF. Therefore, it is possible that the mitogenic effects of bFGF observed in the isolated A2B5+ cells are being exerted upon quiescent GFAP− astrocytes rather than on actively dividing precursors. The most common method of identifying cycling cells in vivo is BrdU labeling. However, the rapid division of the A2B5+ cells could dilute the BrdU. As an alternative, retroviral delivery of an inheritable marker was used to label cycling cells. P0 rat pups were stereotactically injected bilaterally with a GFP-expressing retrovirus targeted into the SVZ. The pups were then sacrificed two days later and their forebrains were dissociated. The isolated A2B5+ cells were plated in chamber slides at a density of 2×104 cells/cm2 with serum-free medium supplemented with bFGF. After 5 days the cultures contained GFP+ clusters, which were found interspersed among unlabeled clusters, indicating that at least some of the astrocyte precursors were derived from cells that were cycling in vivo (Figure 5). Each vimentin+ cluster was composed entirely of either GFP+ cells or GFP− cells. This observation bolsters our view that the clusters represent clones. Since a minority of cycling cells are labeled with the retrovirus, and if clusters were formed from multiple precursors, those clusters that contained GFP+ cells would also contain GFP− cells. Similar to previous experiments, in vivo labeled precursors also gave rise to a wide range of cluster sizes, suggesting that the variable proliferative capacity of the precursors reflects intrinsic cellular potential rather than culture conditions that are driving otherwise quiescent cells to proliferate.
Figure 5. A2B5+ APCs labeled with GFP in vivo.
A2B5+ APCs were isolated from P2 rat pups that had been injected with a GFP-expressing retrovirus targeted to the SVZ at P0. Cells were cultured in CDM containing 10 ng/ml bFGF for five days, fixed and immunostained for Ki67 and Hoescht 33342 (DAPI). The clusters of Ki67+ cells correspond to the vimentin+ APC clusters. Scale bar- 100μm.
A2B5+ Astrocyte Precursors Differentiate into Astrocytes in vivo
To determine the in vivo differentiation capabilities of these cells, A2B5+ cells were isolated from P2 rat SVZ. The cells were plated onto 60mm tissue culture dishes and grown in serum-free medium supplemented with bFGF. On the first and third days in vitro, the cells were infected with a low-titer (104 virus particles/ml) GFP-expressing retrovirus, labeling a large majority of the clusters. Only vimentin+ APCs were labeled; no TUJ1+ neurons or O4+ oligodendrocytes were infected. After 5 days the cells were harvested and unilaterally injected into the SVZ or the lateral ventricle of P0 rat pups. Approximately 2,000 cells were transplanted into each of 4 pups per experiment, which was repeated 4 times. The animals were sacrificed at P14 and their brains cryosectioned and stained for GFAP, NeuN, CC1 and NG2 to identify oligodendrocytes, and pan-endothelium (RECA-1) to detect blood vessels. Precursors injected into the lateral ventricle were dispersed throughout the brain, engrafting into the striatum and hippocampus. Transplants into the SVZ resulted in cells integrated into subcortical white matter tracts. All transplanted precursors displayed characteristic astrocyte morphology, displaying thick, highly branched processes (Figure 6). Approximately 70% of the GFP-labeled cells expressed GFAP. Those that did not appear to express GFAP extended endfeet that wrapped around nearby blood vessels, again suggesting an astrocytic fate. None of the cells expressed either NeuN or CC1, indicating that the precursors had not acquired neuronal or oligodendrocytic cell fates. The remaining 10% of cells possessed short bipolar morphologies and appeared to be immature, precluding further classification.
Figure 6. GFP-infected A2B5+ APC Transplants.
SVZ APCs were retrovirally labeled with GFP and transplanted into P0 pups. Animals were sacrificed at P14, and sections immunostained for GFAP and RECA (A–H) or GFAP and CC1 (I–K). Precursors transplanted into striatum (A–D). B and D show only GFAP and RECA to visualize GFP+/GFAP+ cells in A and C, respectively (arrows indicate GFP-labeled APCs). Precursors transplanted into SVZ (E–H). E shows only GFP to display the morphology of transplanted precursors shown in F. Precursors transplanted into WM (I–K) I displays the morphology, J shows GFP+/GFAP+ cells and K demonstrating the absence of co-labeling with CC1. Scale bar- 40μm.
Spatiotemporal Distribution of A2B5+ Astrocyte Precursors Through Postnatal Development
Since astrocytes continue to be generated during the first two weeks of postnatal rat forebrain development, it is possible that the A2B5+ APCs isolated at P2 are retained as the animals age, representing the source of these late developing astrocytes in vivo. To determine the persistence of the astrocyte precursors, P7, P14, and P28/30 rats were sacrificed and the SVZ, striatum, WM, and cortex were dissected from coronal slices. After dissociation, A2B5+ cells were purified using flow cytometry. The sorted cells were plated on eight-well chamber slides with serum-free medium supplemented with bFGF. After culturing for 5 days, the cells were fixed and stained.
At P2, highly proliferative APCs resided in the SVZ, striatum, and WM, forming large clusters consisting of dozens of cells. Precursors from the cortex possessed a lower proliferative capability, forming small clusters of no more than 8 cells. As development progressed, fewer A2B5+ cells could be isolated from all regions of the forebrain. However, highly proliferative precursors were retained in the SVZ through P30. WM also retained these highly proliferative cells through P30 (Table 2). In comparison, the number and proliferative capacity of the precursors in the striatum and cortex decreased between P7 and P30. At P7, virtually no bFGF-responsive, proliferative precursors could be isolated from the cortex, while those in the striatum proliferated less, forming small clusters. By P14, the proliferative APCs could no longer be isolated from the striatum. P30 brains yielded only occasional immature astrocytes from both the striatum and cortex. In addition, A2B5− cells were collected from all regions and ages. No clusters were detected from any of the samples from the P7 and older animals. This suggests that the disappearance of the astrocyte precursors from the gray matter with age is not due to a loss of A2B5 expression by the astrocyte precursors, but rather to the loss of the precursors themselves.
Astrocytes Differentiate and do not Continue to Proliferate in the Presence of FGF
While bFGF is a potent mitogen early in culture, APCs do not proliferate indefinitely. To determine the proliferative index of the precursors, A2B5+ cells were isolated from P2 rat SVZ and grown in chamber slides at a density of 2×104 cells/cm2 with serum-free medium supplemented with bFGF (10ng/ml). Some cells were plated into 6cm tissue culture dishes in the same medium. After 5 days in vitro, precursors grown on the tissue culture dishes were passaged and replated into chamber slides at a density of 2×104 cells/cm2. Slides from both passaged and non-passaged cells were cultured from 5 to 12 days and stained for vimentin and Ki67. At 5 days, over 70% of the cells were Ki67+. By day 8, approximately 20% of the cells were Ki67+. The level of proliferation decreased still further by day 12. Passage of the cells appeared to maintain a higher level of proliferation at day 6 (65% vs. 50%), compared to non-passaged cells. However, by the next day the proliferative index of both cultures was equivalent (Figure 7). Thus, the continued presence of bFGF is insufficient to keep the astrocyte precursors from exiting cell cycle. The addition of PDGF-AA (10ng/ml) to the medium in combination with bFGF allows the cells to proliferate for slightly longer, but not indefinitely. Similarly, EGF (10ng/ml), alone or in combination with bFGF, supported proliferation for a slightly longer period of time, but was unable to promote continued self-renewal. The astrocyte precursors will still stop cycling. In addition, cell density does not appear to play a role in the cessation of proliferation.
Figure 7. Proliferation Levels of Passaged and Non-Passaged APCs.
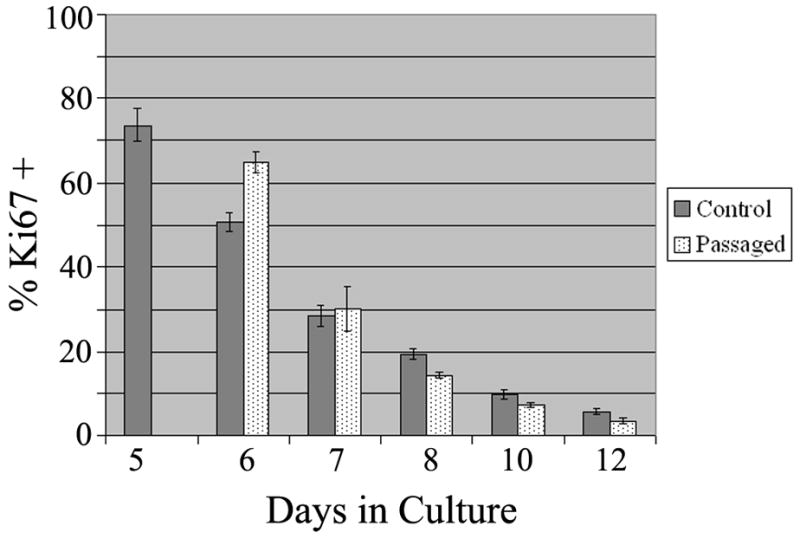
A2B5+ APCs were cultured in CDM with bFGF and fixed on d5, d6, d7, d8, d10, and d12. The cells were immunostained for Ki67 and counted. Control: un-passaged cells. Passaged: cells passaged on d5 and re-plated at 2×104 cells/cm2 (m ± SEM n=4).
To determine if the continuous presence of bFGF had caused the cells to become desensitized, cells, both passaged and non-passaged, were grown in the presence of bFGF. After 7 and 14 days, bFGF was withdrawn and the cells cultured in serum-free medium only. After one week, bFGF was added back to the medium. In all cases there was no increase in proliferation. Therefore, the absence of bFGF is insufficient to return the astrocyte precursors to their previous proliferative stage upon reintroduction of the growth factor. In addition, PDGF-AA, alone and in combination with bFGF, and EGF were added after bFGF withdrawal to induce the astrocytes to enter cell cycle. These other factors were incapable of promoting proliferation. Therefore, once the astrocyte precursors had ceased to proliferate, it was not possible to induce them to reenter cell cycle under the culture conditions employed.
DISCUSSION
We have characterized an APC isolated from the postnatal rat forebrain using the A2B5 monoclonal antibody. Clearly, A2B5-sorted cells from this area at this time are a heterogeneous mix. The culture conditions apparently select strongly for the proliferation and survival of APCs and against neurons and oligodendrocytes. We do not think that the A2B5+ neuroblasts or immature oligodendrocytes transdifferentiate into APCs. We have never observed cells co-labeled with vimentin, GLT-1, or zebrinII and TuJ1 or O4. Furthermore, all of the vimentin+ and eventually GFAP+ clonal clusters are homogenous, not containing any cells with either neuronal or oligodendrocyte markers.
What do these cells correspond to in vivo? Vimentin and zebrinII are both expressed in the perinatal period by radial glia and by SVZ cells that have migrated into gray and white matter (Staugaitis et al. 2001; Zerlin et al. 1995), both of which populations generate astrocytes (Levison and Goldman 1993; Ventura and Goldman 2007). The fact that we can label some of these cells in vivo with retrovirus indicates that those labeled cells belong to the migratory SVZ population, since we do not label radial glia with retroviral injections into the SVZ (Levison et al. 1993; Levison and Goldman 1993). Nevertheless, we cannot rule out that some of the postnatal forebrain APCs originate directly from radial glia. It would eventually be useful to label dorsal radial glia specifically (Ventura and Goldman 2007) to ask if these generate APCs and if so, whether those APCs are different from those generated by dividing SVZ cells.
Alternatively, these postnatal APCs may originate from neural stem cells (NSCs). Most NSCs readily differentiate into astrocytes upon the removal of the factors, bFGF or EGF, which maintain the cells in an immature proliferative state. Indeed, it has been suggested that the default cell fate of NSCs is that of an astrocyte (Nakayama et al. 2006). However, the two hallmarks of an NSC are the potential for extensive self-renewal and multipotency. Unlike NSCs, the postnatal APCs possess a limited ability to self-renew. Despite constant exposure to bFGF and/or EGF, the majority of APCs cease to proliferate after 2 weeks in culture. APCs also appear to lack the ability to acquire multiple cell fates, differentiating solely into astrocytes both in vitro and in vivo. However, it is possible that even a few days exposure to culture conditions in vitro was sufficient to drive the prospective NSCs to a lineage-restricted stage. Therefore, while it is unlikely that the cultured APCs isolated from the postnatal forebrain are NSCs, they may be the progeny of NSCs whose potential for self-renewal and multipotency have been restricted in culture.
Comparison with other APCs
While other APCs have been identified in the CNS, the majority has been isolated from embryonic spinal cord and optic nerve. The A2B5+ APCs of the embryonic rat optic nerve (Mi and Barres 1999) require CNTF or LIF to induce differentiation. In comparison, the A2B5+ APCs of the postnatal forebrain do not require further trophic support to differentiate into mature astrocytes. One consideration is that some optic nerve APCs were GFAP+ after one day in culture, whereas forebrain APCs are initially GFAP−, with GFAP expression beginning around 7 days in culture and increasing thereafter. Given the development of the optic nerve, it is likely that the embryonic optic nerve APCs are derived from radial glial that initially populated the nascent optic nerve outpouching. SVZ cells that migrate into the optic nerve in the postnatal period differentiate into oligodendrocytes.
CD44+ APCs of the embryonic spinal cord differentiated exclusively into astrocytes in vivo after transplantation (Liu et al. 2004), like the forebrain APCs. These CD44+ APCs arise from A2B5 progenitors. However, whereas CD44+ APCs retain CD44 expression upon differentiation, only rare CD44+ cells can be detected in our early cultures (< 1%, unpublished data), and once GFAP expression is achieved, CD44 immunoreactivity is no longer distinguishable. The expression of CD44 may be specific to precursors of the spinal cord.
The O2A glial progenitor, also an A2B5+ cell, has been isolated throughout the CNS. There are several key differences between these and the A2B5+ APCs we have characterized in the forebrain. First, O2A cells are able to cycle indefinitely in the presence of bFGF and PDGF-AA (Bogler et al. 1990). In contrast, the A2B5+ APCs initially proliferate in response to bFGF, but eventually exit cell cycle. The addition of PDGF-AA to bFGF extends the period of proliferation beyond that of bFGF alone but is unable to induce the cells to divide continuously. Next, the production of astrocytes in vitro requires the presence of serum in the medium, since without serum, O2A cells develop into oligodendrocytes (Raff et al. 1983). The cell fate of the forebrain A2B5+ APCs is astrocytic under all the culture conditions we examined. Finally, in contrast to the A2B5+ APCs, O2A cells give rise to oligodendrocytes when transplanted into the brain (Espinosa de los Monteros et al. 1993).
A bipotential A2B5+ precursor cell, isolated from the postnatal rat spinal cord (Fok-Seang and Miller 1992), gives rise to astrocytes in culture. However, like the optic nerve O2A cells, these precursors proliferate in response to PDGF and bFGF and are highly migratory. In the absence of serum, instead of differentiating into oligodendrocytes, most precursors retain an immature phenotype. Exposure to serum is necessary to induce the cells to differentiate into astrocytes and also induces proliferation.
Astrocytes Stop Proliferating in the Continued Presence of bFGF
bFGF, which initially functions as a potent mitogen, is unable to keep the cells proliferating indefinitely. It is possible that they become desensitized to bFGF. Withdrawal of bFGF for one week is not enough to return cells to a responsive state, although it is possible that the period of withdrawal is insufficient. In previous studies the mitogenic response of astrocytes to bFGF is density dependent. Thus, confluent layers of cultured astrocytes do not proliferate in response to the presence of bFGF (Nakatsuji and Miller 1998), but when the astrocytes are passaged to a lower density, they begin to proliferate when exposed to bFGF. It is possible that contact inhibition in a dense culture can override the mitogenic effects of bFGF, and replating relieves this inhibition. However, cell density does not appear to play a role in regulating the cell cycle arrest of the APCs in the present study. Passage of the cells to a lower density produces a slightly higher proliferation for one day, but soon matches that of un-passaged cells.
We do not yet know the full reason for this cessation of proliferation in the presence of mitogen. It is possible that a change in their expression of FGF receptors may render them insensitive to extracellular bFGF. However, semi-quantitative RT-PCR analysis of the transcript levels of FGF receptors 1, 2, and 3 in APCs cultured for 5 and 12 days suggest the continued expression of the receptors, and Western blot analysis of FGF receptors 1 and 2 indicate that the APCs maintain expression of these receptors as they differentiate (G. Lin, unpublished observations). We have found that as APCs differentiate they begin to produce and secrete TGFβ1, and increase intracellular concentrations of such cell cycle regulators as p27 and p53 (G. Lin, unpublished observations). Further study of the control of the FGF-induced proliferation and subsequent cessation of proliferation will shed light on molecular mechanisms underlying the regulation of astrocyte proliferation. Furthermore, since FGF is present during development of the CNS, the regulation of FGF-induced proliferation of APCs may be an important means of controlling astrocyte numbers during gliogenesis.
The inability of the APCs to continue to proliferate indefinitely in the presence of bFGF in vitro may be analogous to the limits on proliferative capacity in vivo. A previous study utilizing retrovirus to label neonatal SVZ progenitors investigated the size and number of clonal clusters generated by glial precursors over a period of several months (Levison et al. 1999). Astrocyte clusters were consistently small at one month, containing only a few cells, and continued to remain small over the next seven months, whereas oligodendrocyte clusters increased in size, indicating continued proliferation or better survival of the oligodendrocytes. The constitutive expression of bFGF in (GFAP+) astrocytes does not generate brain tumors, but does lead to an initial, transient, period of proliferation and migration (Holland and Varmus 1998). All of these observations suggest that the proliferative capacity of astrocytes is tightly regulated.
Astrocytes May Produce Their Own Differentiation Factors
bFGF is initially a potent mitogen for APCs. Removal of bFGF from the culture medium after one day in culture resulted in smaller clusters in comparison to those that formed from precursors exposed to bFGF for longer periods. However, after only a 24 hour exposure to bFGF, APCs were still able to differentiate into GFAP+ astrocytes (data not shown). Therefore, continuous exposure to bFGF is not necessary to promote or maintain astrocyte differentiation. We should consider the evidence that APCs themselves produce trophic factors that induce differentiation, negating the need for exogenous application of differentiation factors. Indeed, mature astrocytes produce and contain CNTF (Stockli et al. 1991), LIF (Aloisi et al. 1994; Wesselingh et al. 1990), and BMP’s (Xin et al. 2006).
To examine the ability of the astrocytes to regulate their own differentiation, we collected conditioned medium from A2B5+ APCs that had been cultured for over a month. Newly isolated A2B5+ cells cultured in a 1:1 ratio of serum-free FGF-supplemented medium and conditioned medium began to express GFAP after only 5 days in culture (G. Lin, unpublished observations). The increased levels of maturation factors may also negate the mitogenic effects of bFGF, preventing excessive numbers of astrocytes from being generated when sufficient cells are already present. However, further experiments using various antibodies and inhibitors to block the effects of trophic factors would be required to determine exactly what factors are responsible for inducing the precursors to differentiate.
Potential in vivo Roles of Postnatal APCs
The APCs presumably represent a source of precursors utilized during development to populate the brain during astrocytogenesis. The most highly proliferative A2B5+ astrocyte precursors appear to reside within the SVZ and WM. The less proliferative precursors in the striatum and cortex may represent cells that have migrated out of the SVZ and WM into the surrounding parenchyma to establish regional astrocyte populations. As astrocytogenesis completes in each region, the A2B5+ APCs differentiate, lose A2B5 antigens, and therefore can no longer be isolated by this FACS technique. Another possible explanation for the loss of A2B5+ precursors in older animals may be due to changes in the efficiency of recovery. Older tissue is more difficult to dissociate and more cells may be lost in the process, yielding fewer precursors.
By P14, an age at which astrocytogenesis has primarily concluded, A2B5+, isolatable, precursors have disappeared from gray matter regions, but are retained within the SVZ and WM. Do these residual APCs play a role in replenishing astrocytes due to turnover? Early 3H-thymidine experiments in aged mice demonstrated a slow turnover of the labeled astrocytes in the corpus callosum over a six-month period (McCarthy and Leblond 1988; Paterson 1983). In addition, BrdU-labeled cells isolated from the brains of adult rats differentiated into astrocytes in vitro (Gensert and Goldman 2001). Those precursors retained within the SVZ and WM may provide a constant source of precursors to reconstitute astrocytes lost as a result of injury or cellular turnover. It has been difficult, however, to identify these APCs in situ.
Supplementary Material
Antigen Expression of APCs
A2B5+ APCs were fixed and immunostained on d5 and d12 to determine expression of the following markers:
A. A2B5 and vimentin (Vim). B. GLT-1 and nestin (Nes) C. ZebrinII (ZII). Areas of low density were chosen for all d12 images to visualize antigen expression patterns more clearly. All cells were counterstained for Hoescht 33342 (DAPI). Scale Bar- 25μm.
Acknowledgments
This work was supported by NIH Grant NS17125. We thank the members of the lab, as well as Peter Canoll, Carol Mason, Steve Levison, and Lloyd Greene, for constructive criticisms and comments. In addition, we would also like to thank T. Jessel and W. Stallcup for their generous gifts of antibodies.
References
- Aloisi F, Rosa S, Testa U, Bonsi P, Russo G, Peschle C, Levi G. Regulation of leukemia inhibitory factor synthesis in cultured human astrocytes. J Immunol. 1994;152(10):5022–31. [PubMed] [Google Scholar]
- Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26(25):6781–90. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar T, McMorris FA, Novotny EA, Barker JL, Dubois-Dalcq M. Growth and differentiation properties of O-2A progenitors purified from rat cerebral hemispheres. J Neurosci Res. 1988;21(2–4):168–80. doi: 10.1002/jnr.490210209. [DOI] [PubMed] [Google Scholar]
- Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18(1):26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- Bogler O, Wren D, Barnett SC, Land H, Noble M. Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc Natl Acad Sci U S A. 1990;87(16):6368–72. doi: 10.1073/pnas.87.16.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979;76(1):514–7. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth GS, Walsh FS, Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979;76(10):4913–7. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engele J, Bohn MC. Effects of acidic and basic fibroblast growth factors (aFGF, bFGF) on glial precursor cell proliferation: age dependency and brain region specificity. Dev Biol. 1992;152(2):363–72. doi: 10.1016/0012-1606(92)90143-5. [DOI] [PubMed] [Google Scholar]
- Espinosa de los Monteros A, Zhang M, De Vellis J. O2A progenitor cells transplanted into the neonatal rat brain develop into oligodendrocytes but not astrocytes. Proc Natl Acad Sci U S A. 1993;90(1):50–4. doi: 10.1073/pnas.90.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. J Neurobiol. 2001;48(2):75–86. [PubMed] [Google Scholar]
- Goddard DR, Berry M, Kirvell SL, Butt AM. Fibroblast growth factor-2 induces astroglial and microglial reactivity in vivo. J Anat. 2002;200(Pt 1):57–67. doi: 10.1046/j.0021-8782.2001.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FC, Paulin D, Moura Neto V. Glial fibrillary acidic protein (GFAP): modulation by growth factors and its implication in astrocyte differentiation. Braz J Med Biol Res. 1999;32(5):619–31. doi: 10.1590/s0100-879x1999000500016. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Lee JW, Cotman CW. Distribution of basic fibroblast growth factor in the developing rat brain. Neuroscience. 1994;61(4):911–23. doi: 10.1016/0306-4522(94)90412-x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vu L, Cotman CW. Regulation of astrocyte proliferation by FGF-2 and heparan sulfate in vivo. J Neurosci. 1995;15(3 Pt 1):2021–9. doi: 10.1523/JNEUROSCI.15-03-02021.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain Res. 1995;701(1–2):201–26. doi: 10.1016/0006-8993(95)01002-x. [DOI] [PubMed] [Google Scholar]
- Herrera J, Yang H, Zhang SC, Proschel C, Tresco P, Duncan ID, Luskin M, Mayer-Proschel M. Embryonic-derived glial-restricted precursor cells (GRP cells) can differentiate into astrocytes and oligodendrocytes in vivo. Exp Neurol. 2001;171(1):11–21. doi: 10.1006/exnr.2001.7729. [DOI] [PubMed] [Google Scholar]
- Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci U S A. 1998;95(3):1218–23. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniss DA, Burry RW. Serum and fibroblast growth factor stimulate quiescent astrocytes to re-enter the cell cycle. Brain Res. 1988;439(1–2):281–8. doi: 10.1016/0006-8993(88)91485-0. [DOI] [PubMed] [Google Scholar]
- Levi G, Aloisi F, Wilkin GP. Differentiation of cerebellar bipotential glial precursors into oligodendrocytes in primary culture: developmental profile of surface antigens and mitotic activity. J Neurosci Res. 1987;18(3):407–17. doi: 10.1002/jnr.490180305. [DOI] [PubMed] [Google Scholar]
- Levison SW, Chuang C, Abramson BJ, Goldman JE. The migrational patterns and developmental fates of glial precursors in the rat subventricular zone are temporally regulated. Development. 1993;119(3):611–22. doi: 10.1242/dev.119.3.611. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10(2):201–12. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Levison SW, Young GM, Goldman JE. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res. 1999;57(4):435–46. [PubMed] [Google Scholar]
- Liu Y, Han SS, Wu Y, Tuohy TM, Xue H, Cai J, Back SA, Sherman LS, Fischer I, Rao MS. CD44 expression identifies astrocyte-restricted precursor cells. Dev Biol. 2004;276(1):31–46. doi: 10.1016/j.ydbio.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Marshall CA, Goldman JE. Subpallial dlx2-expressing cells give rise to astrocytes and oligodendrocytes in the cerebral cortex and white matter. J Neurosci. 2002;22(22):9821–30. doi: 10.1523/JNEUROSCI.22-22-09821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy GF, Leblond CP. Radioautographic evidence for slow astrocyte turnover and modest oligodendrocyte production in the corpus callosum of adult mice infused with 3H-thymidine. J Comp Neurol. 1988;271(4):589–603. doi: 10.1002/cne.902710409. [DOI] [PubMed] [Google Scholar]
- Mi H, Barres BA. Purification and characterization of astrocyte precursor cells in the developing rat optic nerve. J Neurosci. 1999;19(3):1049–61. doi: 10.1523/JNEUROSCI.19-03-01049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Hattori Y, Ohta M, Itoh N. Rat oligodendrocytes and astrocytes preferentially express fibroblast growth factor receptor-2 and -3 mRNAs. J Neurosci Res. 1996;45(5):534–41. doi: 10.1002/(SICI)1097-4547(19960901)45:5<534::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Nakatsuji Y, Miller RH. Homotypic cell contact-dependent inhibition of astrocyte proliferation. Glia. 1998;22(4):379–89. doi: 10.1002/(sici)1098-1136(199804)22:4<379::aid-glia7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333(6173):560–2. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2(3):REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson JA. Dividing and newly produced cells in the corpus callosum of adult mouse cerebrum as detected by light microscopic radioautography. Anat Anz. 1983;153(2):149–68. [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303(5916):390–6. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Rao MS. Multipotent and restricted precursors in the central nervous system. Anat Rec. 1999;257(4):137–48. doi: 10.1002/(SICI)1097-0185(19990815)257:4<137::AID-AR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Rao MS, Mayer-Proschel M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Dev Biol. 1997;188(1):48–63. doi: 10.1006/dbio.1997.8597. [DOI] [PubMed] [Google Scholar]
- Reilly JF, Maher PA, Kumari VG. Regulation of astrocyte GFAP expression by TGF-beta1 and FGF-2. Glia. 1998;22(2):202–10. doi: 10.1002/(sici)1098-1136(199802)22:2<202::aid-glia11>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Reuss B, von Bohlen und Halbach O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313(2):139–57. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- Sauvageot CM, Stiles CD. Molecular mechanisms controlling cortical gliogenesis. Curr Opin Neurobiol. 2002;12(3):244–9. doi: 10.1016/s0959-4388(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Staugaitis SM, Zerlin M, Hawkes R, Levine JM, Goldman JE. Aldolase C/zebrin II expression in the neonatal rat forebrain reveals cellular heterogeneity within the subventricular zone and early astrocyte differentiation. J Neurosci. 2001;21(16):6195–205. doi: 10.1523/JNEUROSCI.21-16-06195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockli KA, Lillien LE, Naher-Noe M, Breitfeld G, Hughes RA, Raff MC, Thoenen H, Sendtner M. Regional distribution, developmental changes, and cellular localization of CNTF-mRNA and protein in the rat brain. J Cell Biol. 1991;115(2):447–59. doi: 10.1083/jcb.115.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami K, Matsuo A, Terai K, Walker DG, McGeer EG, McGeer PL. Fibroblast growth factor receptor-1 expression in the cortex and hippocampus in Alzheimer’s disease. Brain Res. 1998;802(1–2):89–97. doi: 10.1016/s0006-8993(98)00552-6. [DOI] [PubMed] [Google Scholar]
- Ventura RE, Goldman JE. Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J Neurosci. 2007;27(16):4297–302. doi: 10.1523/JNEUROSCI.0399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselingh SL, Gough NM, Finlay-Jones JJ, McDonald PJ. Detection of cytokine mRNA in astrocyte cultures using the polymerase chain reaction. Lymphokine Res. 1990;9(2):177–85. [PubMed] [Google Scholar]
- Xin H, Li Y, Chen X, Chopp M. Bone marrow stromal cells induce BMP2/4 production in oxygen-glucose-deprived astrocytes, which promotes an astrocytic phenotype in adult subventricular progenitor cells. J Neurosci Res. 2006;83(8):1485–93. doi: 10.1002/jnr.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki N, Hosoi Y, Kawabata K, Miyake A, Minami M, Satoh M, Ohta M, Kawasaki T, Itoh N. Differential expression patterns of mRNAs for members of the fibroblast growth factor receptor family, FGFR-1-FGFR-4, in rat brain. J Neurosci Res. 1994;37(4):445–52. doi: 10.1002/jnr.490370403. [DOI] [PubMed] [Google Scholar]
- Zerlin M, Levison SW, Goldman JE. Early patterns of migration, morphogenesis, and intermediate filament expression of subventricular zone cells in the postnatal rat forebrain. J Neurosci. 1995;15(11):7238–49. doi: 10.1523/JNEUROSCI.15-11-07238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antigen Expression of APCs
A2B5+ APCs were fixed and immunostained on d5 and d12 to determine expression of the following markers:
A. A2B5 and vimentin (Vim). B. GLT-1 and nestin (Nes) C. ZebrinII (ZII). Areas of low density were chosen for all d12 images to visualize antigen expression patterns more clearly. All cells were counterstained for Hoescht 33342 (DAPI). Scale Bar- 25μm.