Abstract
Conditionally replicative adenoviruses (CRAds) are often evaluated in mice; however, normal and cancerous mouse tissues are poorly permissive for human CRAds. As the cotton rat (CR) is a semipermissive animal and the Syrian hamster (SH) is a fully permissive model for adenoviral replication, we compared them in a single study following intracranial (i.c.) injection of a novel glioma-targeting CRAd. Viral genomic copies were quantified by real-time PCR in brain, blood, liver and lung. The studies were corroborated by immunohistochemical, serological and immunological assays. CR had a multiple log higher susceptibility for adenoviral infection than SH. A similar amount of genomic copies of CRAd-Survivin-pk7 and human adenovirus serotype 5 (AdWT) was found in the brain of CR and in all organs from SH. In blood and lung of CR, AdWT had more genomic copies than CRAd-Survivin-pk7 in some of the time points studied. Viral antigens were confirmed in brain slices, an elevation of serum transaminases was observed in both models, and an increase in anti-adenoviral antibodies was detected in SH sera. In conclusion, CR represents a sensitive model for studying biodistribution of CRAds after i.c. delivery, allowing for the detection of differences in the replication of CRAd-Survivin-pk7 and AdWT that were not evident in SH.
Keywords: oncolytic adenovirus, Syrian hamster, cotton rat, biodistribution, replication, glioma
Introduction
Oncolytic adenoviral therapy is a novel modality of anticancer treatment that has led to promising results in the preclinical setting as well as in recent clinical trials. This therapy consists of the use of conditionally replicative adenoviruses (CRAds) to kill neoplastic cells.1,2 To do so, these vectors are modified to preferentially replicate in tumor cells, and subsequently, the adenoviral replicative process culminates with the destruction of its host cell. The specificity of adenoviral replication is achieved by different strategies such as capsid modifications to bind proteins found on tumor cell membranes,3–6 incorporation of tumor promoter sequences to control the expression of viral genes,7–9 and the deletion of viral genomic sequences to limit the replication to cells with particular pathway alterations that are characteristic of cancer cells.10 To date, a series of CRAds are currently undergoing various stages of preclinical and clinical testing, and in fact one of these vectors was recently approved for the treatment of patients with head and neck cancer in China.11,12
Our group has explored the application of the oncolytic vector CRAd-Survivin-pk7 (CRAd-S-pk7) for the treatment of malignant glioma.13 For transcriptional targeting toward gliomas, CRAd-S-pk7 contains a survivin promoter incorporated upstream from viral gene E1A. The rationale for the use of this promoter is that it is highly active in gliomas, whereas it remains relatively silent in the surrounding brain parenchyma.8,9 To enhance viral transduction into glioma cells, the capsid of this vector has been modified to bind heparan sulfate proteoglycans expressed in these tumors.13,14 In preclinical studies, CRAd-S-pk7 exhibited a potent antitumoral activity in mice bearing intracranial (i.c.) human glioma xenografts. In addition, this virus has recently been shown to elicit a synergistic therapeutic effect when combined with low-dose radiation15 and with the chemotherapeutic agent temozolomide (submitted for publication), two therapies that constitute part of the standard of care for patients bearing these tumors.
To proceed with the besting of CRAd-S-pk7 in the clinical scenario, the assessment of replication kinetics and toxicity of this novel viral vector is imperative. To study the pharmacokinetics of a CRAd, various factors need to be considered. In the first place, human adenoviruses do not undergo their classic replication cycle in murine tissues,16–18 which is inconvenient, as mice are commonly used as models for testing novel therapies against gliomas. This issue has been classically addressed by testing the efficacy of CRAds in human tumor xenografts established in immunodeficient mice. Such models have limitations; given the fact that mice are poorly permissive for replication of human adenoviruses, the murine models do not recapitulate the replication profile of these vectors in different tissues of an organism that is susceptible to human adenoviral infection. In addition, the lack of a host's competent immune system in xenograft's host does not resemble the replication kinetics, the toxicity and the efficacy of these viruses in the clinical setting where the human organism is capable of eliciting an antiviral immunity.
In the search for a model to study CRAd in vivo, different rodent species have been explored.19–22 Toth et al.19,21 reported on the semipermissive nature of the cotton rat whereas most recently, Thomas et al.,20,22 described the use of Syrian hamster (SH) as a fully permissive species that is susceptible to the replication of human adenoviruses. Thus, these species have been proposed as models to study CRAd kinetics, toxicity and biodistribution. Nevertheless, it is unclear which of these two organisms constitutes a better model to study CRAds, as the susceptibility to human adenoviral infection in cotton rats and SHs has not yet been compared in a single study.
To assess the replication kinetics, the antiviral immune response and the toxicity elicited by CRAd-S-pk7, we studied the biodistribution of this vector in SHs and cotton rats. We administered this vector intracranially to resemble the clinical scenario in which patients bearing malignant brain tumors could be injected with the virus directly into the brain. We then examined vector biodistribution in target and nontarget tissues using quantitative PCR to analyze E1A expression with a demonstrated limit of quantitation of ≤50 copies of vector per 1 μg genomic DNA.
To the best of our knowledge, this is the first study to compare these two models directly following CNS administration, and therefore represents an important tool for other researchers in the field as well as the food and drug administration (FDA), as it evaluates the safety and toxicity of CRAds, which are being developed for brain tumor applications.
Materials and methods
Animals and i.c. injection
Four- to five-week-old male cotton rats (Sigmodon hispidus) and SHs (Mesocricetus auratus) were purchased from Harlan (Indianapolis, IN). Animals (n = 4 per group per time point) were injected intracranially with 4.5 × 109 viral particles in a final volume of 5 μl suspended in PBS. The i.c. injection was performed in the right parietal lobe as previously described by our group.23–25 All animals were housed and maintained under pathogen-free condition in accordance with a protocol approved by the Institutional Animal Care and Use Committee at the University of Chicago.
Viruses
Human adenovirus serotype 5 (AdWT) and CRAd-S-pk7 have been described previously.13,26 CRAd-S-pk7 contains the human survivin promoter driving E1A expression. In this construct, the survivin-controlled E1A expression cassette replaced the original viral E1A region as described before.8 A poly(A) signal was inserted between the inverted terminal repeat and the survivin promoter to enhance the transcriptional specificity. CRAd-S-pk7 was obtained by homologous recombination in 911 cells between a shuttle vector pScs/PA/S, which carries a human survivin promoter, and a pVK700-based wild-type adenoviral 5 backbone containing a polylysine modification of the fiber knob.27 Viruses were selected from single plaques on 911 cells, expanded in A549 cells and purified by double CsCl gradient ultracentrifugation.28 Before i.c. injection, viral stock solutions were dialyzed and viral particles were quantified by spectrophotometry. To correlate viral particles with infective particles, viral stock was titrated by TCID50. For AdWT, 4.5 × 109 viral particles containing 1.8 × 109 plaque-forming units were injected per animal, whereas in the case of CRAd-S-pk7, 4.5 × 109 viral particles containing 7.35 × 108 plaque-forming units were injected per animal.
Organ harvest, DNA isolation and PCR
Organs were harvested at the time of killing. For each organ, the set of instruments used was cleaned previously with chloride. Organs were stored at −80 °C. The brain tissue samples were obtained as follows: after dissecting the brain from the cranium, a coronal cut was performed in a plane that crossed the injection site. The anterior portion of the brain was fixed in formalin and the posterior was further divided into two symmetrical pieces through midline. These two pieces were processed separately and PCR was performed independently for each right and left sample.
Total DNA was extracted from frozen tissue samples and frozen anticoagulated blood using DNeasy Tissue kit (Qiagen) according to the manufacturer's instructions. DNA was quantified by spectrophotometry and was subject to quantitative PCR (qPCR) using primers targeting E1A. The sequences of E1A primers used were the following: forward: 5′-AACCAGTTGCCGTGAGAGTTG-3′; and reverse: 5′-CTCGTTAAGCAAGTCCTCGATACAT-3′. Reactions ran in triplicate under the following conditions: 2 min at 95 °C, 20 s at 94 °C, 20 s at 56 °C and 20 s at 72 °C for 40 cycles. Quantification using SYBR GREEN PCR Master Mix (Bio-Rad, Hercules, CA) was performed according to the vendor. For each qPCR, a no-template reaction was included as the negative control. For reference, a standard curve was prepared by mixing different dilutions of viral DNA with DNA from untreated SHs or cotton rats to obtain a final concentration ranging from 5 (lowest) to 5 × 107 (highest) viral genomic copies per 100 ng of host DNA. SH and cotton rat samples were analyzed with their respective standard curves. Nevertheless, the results are comparable, as the same viral dilutions were utilized for preparation of SH as well as cotton rat standard curves. Results were converted to viral genomic copies per μg of total DNA and each reaction was run in triplicate.
Histological staining and immunohistochemistry
After fixation in formalin, brain samples were embedded in paraffin and 5-μm coronal slices were obtained. Tissue was stained with hematoxylin and eosin, and for detection of viral antigens, an immunohistochemistry against viral protein E1A was performed with an anti-E1A M58 antibody (MS587-P1, LabVision, Fremont, CA) and a secondary antibody conjugated to horseradish peroxidase (sc2314, Santa Cruz Biotechnology, Santa Cruz, CA).
ELISA
For detection of anti-adenoviral antibodies in hamster serum, an ELISA was performed by coating 96-well plates with 1 × 108 viral particles per 100 μl overnight at 4 °C. Blocking was done with a solution containing 2% albumin. After incubation with hamster serum (1:10 and 1:100), hamster antibodies were detected with a goat anti-hamster Ig (6061, Southern Biotech, Birmingham, AL), and for developing the plate, an horseradish peroxidase-conjugated donkey anti-goat Ig antibody and TMB substrate were used. After incubation, the plates were read at 450 nm.
Neutralizing antibody assay
In a 96-well plate, 1 × 104 of HEK293 cells were cultured in complete medium (modified Eagle's medium with 10% FBS, 0.09 g NaHCO3, 2 mm l-glutamine, 200 U ml−1 penicillin, 100 μg ml−1 streptomycin and Fungizone (1.0 μg ml−1) for 24 h at 37 °C in 5% CO2. Type-specific immune serum was heat-inactivated at 56 °C for 30 min. Equal volumes of type-specific sera diluted serially from 1:2 to 1:64 in FBS-free medium were mixed with 200 MOI of AdWT and incubated for 1 h at 37 °C in 5% CO2. As control, hyperimmune rabbit sera raised against Ad2 adenovirus was used. The mixture of diluted serum with virus dose was added to the appropriate wells. The plates were further incubated for 7 days. The titer of adenoviral antibodies was determined by inhibition of the cytopathic effect. Hyperimmune rabbit serum is expected to show inhibition at a ratio of 1:64. Samples pretreated with pre-immune serum without antibodies are expected to show inhibition at 1:2. Viral neutralization was considered confirmed when no infection was observed at 1:16.
Determination of liver enzymes
Hamster and cotton rat aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were performed by RADIL MU Research Animal Diagnostic Laboratory (Columbia, MO).
Statistical analysis
Before statistical analysis, a log transformation was applied to the viral genomic copy number data that had a large range and a right skewed distribution. ANOVA was used to analyze the viral genomic copy number and liver enzyme data, with animal type (SHs or cotton rats), virus type (CRAd-S-pk7 or AdWT), organ type and time point (day after i.c. injection) and their two-way and three-way interaction as factors. In the notation F(X,Y) = Z, F is a typical statistical procedure to test whether a factor is sufficiently related to the outcome when utilizing ANOVA. The first number (X) is the degree of freedom (df) of the factor, the second number (Y) is the degree of freedom of the residuals and Z is the F-value from ANOVA, which has an F distribution with df1 = X and df2 = Y. Regression analysis was used to correlate antibody to gene expression after controlling for time point. A P-value less than 0.05 was considered statistically significant.
Results
Comparison of viral distribution between SHs and cotton rats
To characterize the replication of the adenoviral vectors in immunocompetent and permissive organisms, we have quantified viral genomic copies in the brain, blood, lung and liver at days 1, 7, 14 and 30 after i.c. injection of CRAd-S-pk7 or AdWT. To do so, we have amplified viral gene E1A by real-time PCR (≤50 copies of vector per 1 μg genomic DNA). Following injection of the same amount of viral particles (4.5 × 109 virus particles per 5 μl) in both models, cotton rats exhibited multiple log higher number of viral genomic copies than that found on SHs. The difference between the two animal models remained significant (animal type: F(1,301) = 146.6, P<0.001) after controlling virus type, organ and time point (Figure 1). Moreover, in cotton rats, but not in SHs, there was a significant interaction between organ and day, meaning that the relation between viral copies over time was different among organs (animal type × organ × time interaction: F(12, 301) = 2.20, P = 0.01). In SHs as well as cotton rats, the number of viral copies among different organs varied significantly, with higher number of viral copies in the brain and blood than that seen in the liver and lung for both CRAd-S-pk7 and AdWT (virus type × organ interaction: F(4,301) = 2.67, P = 0.03) (Figure 1).
Figure 1.
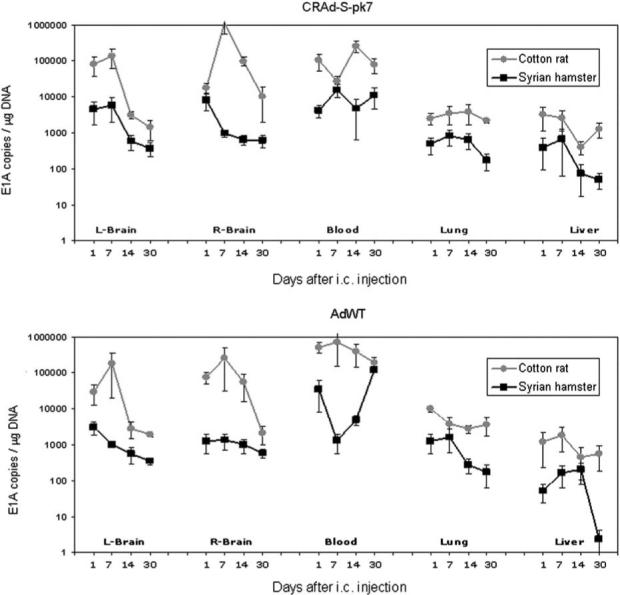
Viral genomic copies were determined by amplification of E1A viral gene by qPCR after i.c. injection of 4.5 × 109 virus particles of CRAd-S-pk7 (top) or AdWT (bottom) into the right parietal lobe of Syrian hamsters (SHs) (black lines) and cotton rats (gray lines). Animals were killed 1, 7, 14 and 30 days after i.c. injection. Cotton rats exhibited a multiple log higher number of viral genomic copies than SHs in all organs and time points studied (P<0.001). In SHs as well as cotton rats, the number of viral copies among different organs varied significantly, with higher number of viral copies in the brain and blood than that seen on the liver and lung for both CRAd-S-pk7 and AdWT (P = 0.03).
Viral distribution and kinetics in the brain
To investigate the presence of the viruses in the brain, we performed immunohistochemical analysis for viral antigen E1A. We confirmed that i.c. injection of both vectors led to a positive staining for viral antigen E1A in SH as well as cotton rat brain slices at day 7 after i.c. injection (Figure 2). Interestingly, in the samples analyzed, E1A staining was observed in brain parenchyma as well as in ependymal cells in the case of animals injected with AdWT, whereas those injected with CRAd-S-pk7 did not exhibit E1A staining in ependymal cells (Figure 2).
Figure 2.
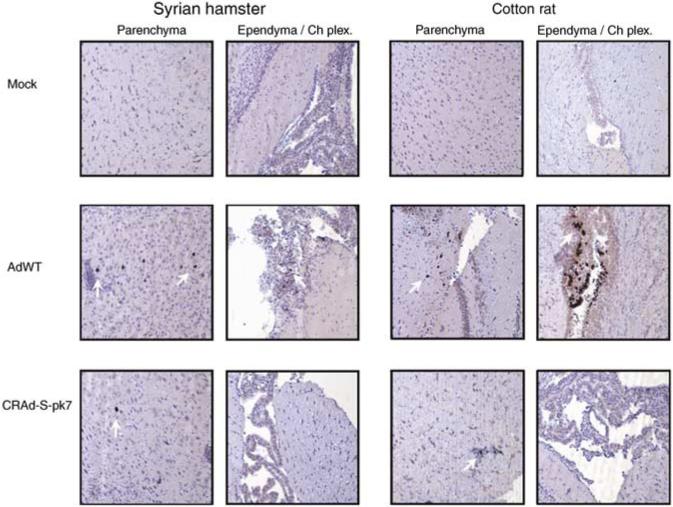
Immunohistochemical staining for viral antigen E1A in Syrian hamster (SH) and cotton rat brain slices. At day 7 after i.c. injection of CRAd-S-pk7 or AdWT, SHs and cotton rats were killed and brain samples were fixed, sliced and analyzed for the presence of viral antigen E1A by immunohistochemistry. White arrows point to cells that stained positive for E1A antigen. Representative × 10 power fields are presented from brain parenchyma and ependyma/choroid plexus from SHs (left) and cotton rats (right). Mock-treated animals (top) did not exhibit any staining, whereas animals injected with AdWT (middle) or CRAd-S-pk7 (bottom) stained positively for E1A in some cells.
To obtain a quantitative assessment of the presence of CRAd-S-pk7 and AdWT in the brain of SHs and cotton rats, we compared the genomic copies of these two vectors in the injected (right brain) and noninjected (left brain) hemispheres (Figure 3a). Interestingly, there is no statistically significant difference in the number of viral genomic copies for CRAd-S-pk7 in comparison with AdWT in the case of the injected hemisphere (right brain) or noninjected hemisphere (left brain) in any of the time points studied in the brain of SHs or cotton rats (Figure 3a). Moreover, in the case of SH brains, a post hoc analysis failed to show a significant increase in viral genomic copies subsequent to day 1 after i.c. injection, which is the first time point studied (Figure 3a). In the case of cotton rat brain, we observed only an increase of CRAd-S-pk7 genomic copies on day 7 (1.14 × 106 ± 5.82 × 105 E1A copies per μg DNA) in comparison with day 1 (1.8 × 104 ± 6.04 × 103 E1A copies per μg DNA) following i.c. injection on the injected hemisphere (right brain) (Figure 3a) (P = 0.0126).
Figure 3.
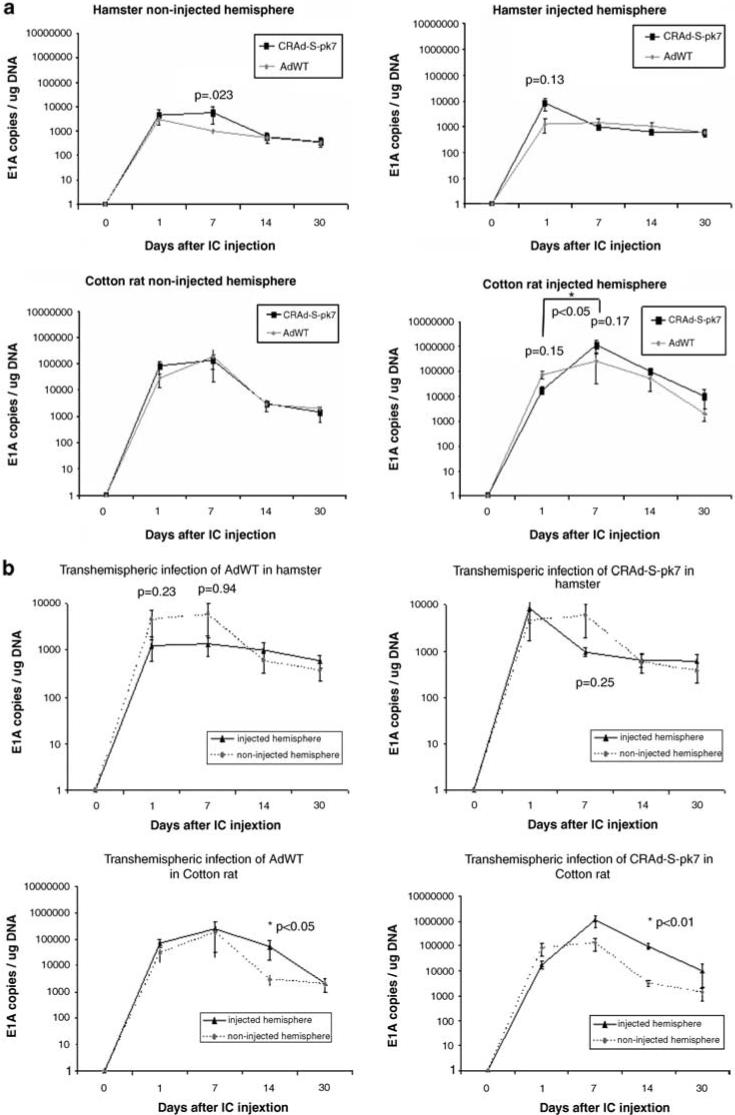
Viral genomic copies in the brain of Syrian hamsters (SHs) and cotton rats were quantified by qPCR for viral gene E1A following i.c. injection of CRAd-S-pk7 or AdWT. (a) A comparison of viral genomic copies of CRAd-S-pk7 (black lines) and AdWT (gray lines) in the injected (right) and noninjected (left) hemispheres. In the case of SHs (top) as well as cotton rats (bottom), there is no statistically significant difference in the number of viral genomic copies of CRAd-S-pk7 and AdWT (P>0.05). An increase in the number of viral genomic copies over time was noted in the injected hemisphere of cotton rats at day 7 following i.c. injection of CRAd-S-pk7 in comparison with day 1 (P<0.05). (b) A comparison of the viral genomic copies of CRAd-S-pk7 (right) and AdWT (left) was carried out between the injected (black line) and noninjected (intermittent gray line) hemispheres. In the case of SHs (top), there was no difference in the number of viral genomic copies between hemispheres for any of the vectors (P>0.05). In contrast, in the case of cotton rats, at day 14 after i.c. injection, the injected hemisphere had a significantly higher number of viral genomic copies in comparison with the noninjected hemisphere for AdWT (P<0.05) and CRAd-S-pk7 (P<0.01). For graphic representation on a logarithmic scale, 0 was substituted for 1.
To understand the distribution of our vectors throughout the brain, we have compared the genomic copies of CRAd-S-pk7 and AdWT in the ipsilateral (right brain) and contralateral (left brain) hemispheres to the site of injection (Figure 3b). In the brain of SHs, the number of genomic copies was similar in both hemispheres for CRAd-S-pk7 and AdWT, suggesting an even distribution of the virus throughout the brain with a similar amount of viral particles independent of the distance from the injection site (Figure 3b). In contrast, in the case of cotton rat brain, a difference in the number of genomic copies of both vectors was noted between the injected and the noninjected hemispheres. There was a lower number of CRAd-S-pk7 genomic copies in the hemisphere contralateral to the site of injection (left brain) (3.12 × 103 ± 7.02 × 102 E1A copies per μg DNA) in comparison with the injected hemisphere (right brain) (9.79 × 104 ± 2.64 × 104 E1A copies per μg DNA) at day 14 after i.c. injection (P<0.01). Similarly, cotton rats injected with AdWT had a significantly lower number of viral genomic copies in the hemisphere contralateral to the site of injection (left brain) (2.89 × 103 ± 1.37 × 103 E1A copies per μg DNA) in comparison with the injected hemisphere (right brain) (5.48 × 104 ± 3.84 × 104 E1A copies per μg DNA) at day 14 after i.c. injection (P<0.05) (Figure 3b).
Irrespective of the amount of viral particles encountered in the brain of SHs or cotton rats, all animals were reactive and appeared to have maintained a normal behavior and movements until the time of their killing, dismissing the possibility of obvious neurotoxicity. In addition, no histological signs of neurotoxicity were noted on brain slices stained by hematoxylin and eosin (data not shown).
Viral biodistribution after i.c. injection
The modifications that have been made to target CRAd-S-pk7 toward gliomas might have an effect on its pharmacokinetics in comparison with AdWT in the setting of permissive and immunocompetent organisms. To explore this possibility, we compared the distribution of CRAd-S-pk7 and AdWT in the blood, lung and liver of SHs (Figure 4a) and cotton rats (Figure 4b) after i.c. injection.
Figure 4.
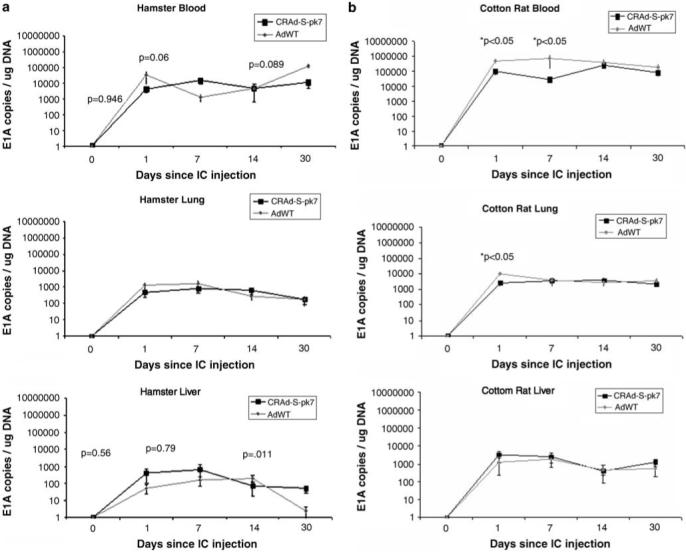
Viral biodistribution following i.c. injection of CRAd-S-pk7 and AdWT was investigated in the blood, lung and liver by determination of viral genomic copies using qPCR for viral gene E1A. (a) Comparison of the number of viral genomic copies of AdWT (gray line) and CRAd-S-pk7 (black line) in the blood (left), lung (middle) and liver (right) of Syrian hamsters showed no difference between these two vectors (P>0.05). (b) Comparison of the number of viral genomic copies of AdWT (gray line) and CRAd-S-pk7 (black line) in the blood (left), lung (middle) and liver (right) of cotton rats showed a significantly lower number of genomic copies of CRAd-S-pk7 in comparison with AdWT in blood (days 1 and 7 following i.c. injection) and in the lung (day 1 following i.c. injection) (P<0.05). For graphic representation on a logarithmic scale, 0 was substituted for 1.
In the case of SHs, there was no statistically significant difference between CRAd-S-pk7 and AdWT in the blood, lung or liver in any of the time points studied (Figure 4a). In the case of cotton rats, there was a significantly higher number of viral genomic copies in the blood for AdWT compared with CRAd-S-pk7 on days 1 and 7 after i.c. injection (P<0.05), and in lung on day 1 after i.c. injection (P<0.05) (Figure 4b).
Intracranial injection of adenoviral vectors leads to transaminitis
Within clinical trials employing adenoviral vectors, mild hepatotoxicity characterized by hyperbilirubinemia and/or mild transaminitis is frequently encountered.29–31 Given the fact that we have encountered viral genomic copies in the liver of animals injected with AdWT or CRAd-S-pk7 in the brain (Figure 4), we assessed the serum ALT and AST levels at the time of killing. Intracranial injection of the adenoviral vectors led to an increase of ALT and AST in both SHs (Figure 5a) and cotton rats (Figure 5b) in comparison with mock-treated animals (P<0.05). Even though such difference appears to be mainly on day 1 after i.c. injection, a post hoc analysis showed no difference in the level of liver enzymes between days 1, 7, 14 and 30 after i.c. injection in SHs or cotton rats.
Figure 5.
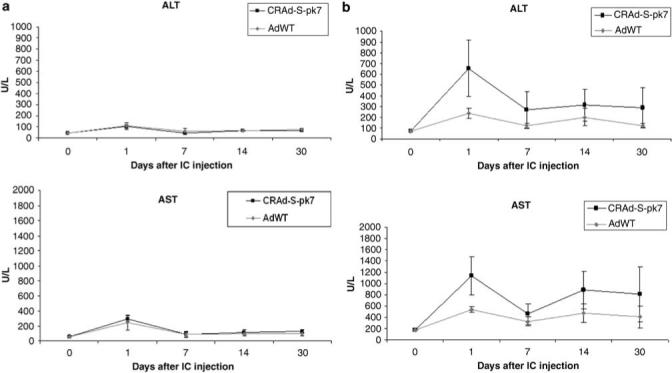
Serum ALT and AST levels following i.c. injection of AdWT and CRAd-S-pk7. (a) Comparison of serum levels of ALT (left) and AST (right) following i.c. injection of CRAd-S-pk7 (black line) and AdWT (gray line) in Syrian hamsters shows a similar transaminitis for both vectors (P>0.05). (b) Comparison of serum levels of ALT (left) and AST (right) following i.c. injection of CRAd-S-pk7 (black line) and AdWT (gray line) in cotton rats shows a lower ALT level for AdWT than that seen for CRAd-S-pk7 (P<0.05).
The interaction between virus and animal type is significant for ALT (P<0.05) and marginally significant for AST (P = 0.056). In the case of SHs, there was no difference between the two viruses tested, whereas in the case of cotton rats, i.c. injection of AdWT led to a lower ALT level than that of CRAd-S-pk7 (Figure 5). Regression analysis failed to find a correlation of ALT or AST levels and viral genomic copy number in SHs or cotton rats.
Humoral immune response against adenovirus
The administration of oncolytic adenoviral vectors in patients has often led to a humoral immune response characterized by the generation of neutralizing anti-adenoviral antibodies.29,32–34 To investigate whether i.c. injection of CRAd-S-pk7 leads to a humoral immune response, we have performed an ELISA to detect anti-adenoviral antibodies in the serum of SHs. We could not perform a similar experiment in cotton rats due to the lack of commercially available antibodies; however, the results of a viral neutralization assay are shown in Table 1. We have detected a significant increase in absorbance (optical density (OD) 450 nm) in the assays corresponding to serum samples from SHs 14 and 30 days after i.c. injection of CRAd-S-pk7 or AdWT, reflecting an elevation of anti-adenoviral antibodies in 1:10 and 1:100 dilutions in these animals (P<0.05) (Figure 6a). Interestingly, the elevation of anti-adenoviral antibodies was associated with a decrease in viral genomic copies in the hamster brain. A regression analysis controlled for time points and virus type showed a significant correlation between OD 450 nm from the anti-adenoviral antibody ELISA and the number of viral genomic copies found in the noninjected hemisphere (left brain) (P = 0.013). Moreover, a marginally significant correlation of anti-adenoviral antibodies and viral genomic copies was found in the injected hemisphere (right brain) (P = 0.053) (Figure 6b).
Table 1.
Viral neutralization assay
| Day of killing |
Hamster seraa,b,c |
Rat seraa,b,c |
||
|---|---|---|---|---|
| AdWT | CRAd-S-pk7 | AdWT | CRAd-S-pk7 | |
| Day 0 | ND | ND | ND | ND |
| Day 1 | ND | ND | ND | ND |
| Day 7 | ND | ND | ND | ND |
| Day 14 | ND | ND | ND | ND |
| Day 30 | 4−8 | 4−8 | 4−8 | 8−16 |
Abbreviation: ND, none detected.
Inverse dilution giving 50% neutralization.
Animal anti-AdWT and anti-CRAd-S-pk7 sera derived from intracranially injected hamster and cotton rats was heated at 56 °C for 30 min before assay.
Viral neutralization assay was performed against 200 multiplicity of infection of AdWT virus.
Figure 6.
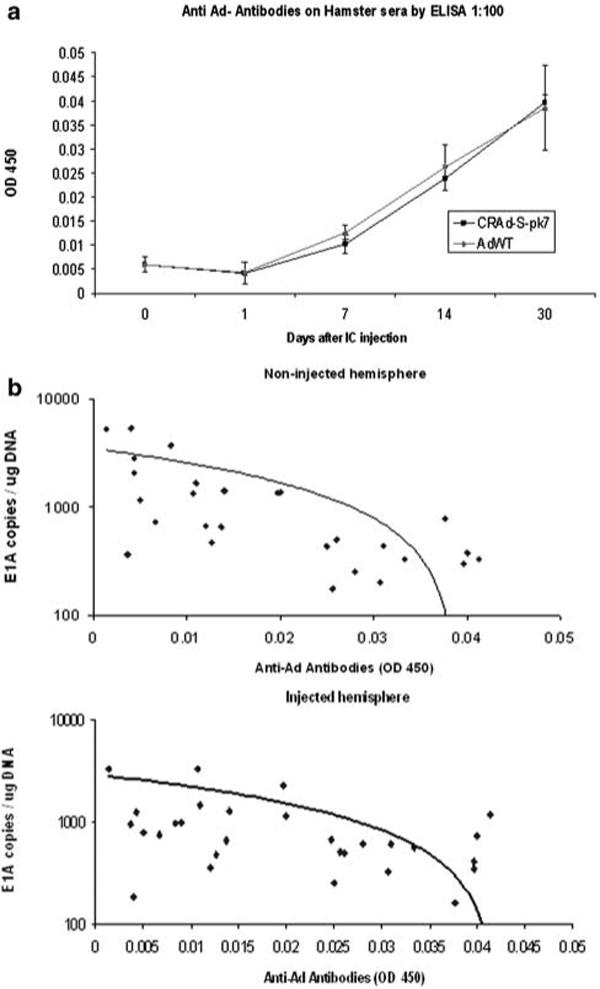
Determination of anti-adenoviral antibodies following i.c. injection of adenoviral vectors in Syrian hamsters (SHs). (a) Anti-adenoviral antibodies were detected by ELISA in sera from SHs at days 14 and 30 following i.c. injection of AdWT and CRAd-S-pk7 at a 1:10 and 1:100 serum dilutions(P<0.05). (b) A regression analysis showed a significant negative correlation in the number of viral genomic copies found in the noninjected hemisphere and the anti-adenoviral antibody signal (ELISA, OD 450 nm) (P<0.05) and a marginally significant negative correlation of viral genomic copies found in the injected hemisphere and the anti-adenoviral antibody signal (ELISA, OD 450 nm) (P = 0.053).
Discussion
In this study, we evaluated SHs and cotton rats as putative immunocompetent models that are permissive for replication of human adenoviruses. We have found that following i.c. injection of the same amount of viral particles, the susceptibility of cotton rats for infection by these vectors is considerably higher than that exhibited by SHs. The increased sensibility of cotton rats as a model allowed us to find differences between AdWT and CRAd-S-pk7 in blood and the lung, and differences among injected and noninjected cerebral hemispheres that would otherwise not have been noticed in the SH model. Moreover, in cotton rats, there was a difference in ALT levels between CRAd-S-pk7 and AdWT that was not present in SHs. The superior susceptibility of cotton rats for human adenoviral infection was consistent for CRAd-S-pk7 and AdWT. Thus, in our study, the difference in permissiveness among these two models proved to be meaningful.
Separate studies in cotton rats and SHs have assessed their permissiveness for human adenoviral infection. Toth et al.19,21 compared the infectivity of oncolytic adenoviruses between the human lung carcinoma cell line A549 and LCRT, a cotton rat fibrosarcoma cell line. In this experiment, the human cell line led to multiple fold higher progeny release than the cotton rat counterpart. Moreover, in an in vivo experiment, intratumoral injection of an oncolytic adenovirus led to no viral yield in the blood, lung or liver 2 days after injection. Later, Thomas et al.,20,22 compared the infectivity of the same oncolytic adenovirus between SH kidney cell line HaK and A549, showing a multiple fold higher progeny release by the human cell line in comparison with the SH cell line. In the case of the SH experiment, the difference between rodent and human cell line's susceptibility toward adenoviral infection seems to be relatively smaller than that seen in the case of the cotton rat experiment. Moreover, in an in vivo experiment, intratumoral injection of the same oncolytic adenovirus in SHs led to viral progeny in the blood, lung and liver that varied from 4 to up to 7 days following injection,20,22 in comparison with the absence of infective viruses at day 2 seen in the cotton rat study.19,21
In contrast to our findings, the results from the above-mentioned studies suggest that SHs might be more permissive than cotton rats for human adenoviral infection. Nevertheless, various considerations should be kept in mind. In the first place, the studies by Toth et al. and Thomas et al. have not made a direct comparison between SHs and cotton rats. Moreover, viral yield was assessed by TCID50, whereas we have quantified viral genomic copies by qPCR. We believe that the fact that we encountered the presence of viral DNA up to 30 days following i.c. injection in contrast to the fast adenoviral clearance from the organs described previously might be explained by the high sensitivity of qPCR. Finally, it is important to stress that our study has made a comparison of SHs and cotton rats in the setting of i.c. injection of oncolytic adenoviruses. The previous in vivo studies that are discussed described the biodistribution following intratumoral injection into subcutaneous tumors.19–22 We therefore propose that different organs may show different susceptibilities to adenoviral replication in the two models and that the cotton rat model might be advantageous over the SH model for the study of biodistribution of human adenoviruses in the setting of i.c. injection.
With regard to the biodistribution of CRAd-S-pk7 and AdWT, a difference in the genomic copy number between these two vectors was encountered only in cotton rat organs. In all cases where there was a difference between these two viruses, AdWT had significantly higher number of copies than CRAd-S-pk7. Thus, we believe that the incorporation of the polylysine motif and the presence of the survivin promoter that characterize CRAd-S-pk7 do not increase the length of the clearance and do not increase the tropism toward the studied organs in comparison with AdWT. These results suggest that transcriptional/transductional modifications of Ad5 are associated with decreased toxicity. Whereas our group has previously found a multiple-fold higher replication of CRAd-S-pk7 in comparison with AdWT in human glioma cells Kings, U118, No.10 and A172 in vitro,13 in the case of brain parenchyma of SHs and cotton rats, there is no difference in the number of genomic copies for these two vectors. In comparison with the case of an ex vivo experiment for viral replication in human brain where AdWT had significantly higher replication than that of CRAd-S-pk7 13, in our in vivo study, there was no significant difference in the viral genomic copies of these two vectors (Figure 3). This is most likely due to the presence of an immune response; however, further investigation will be needed to fully characterize such difference in the clinical setting.
Similar to some clinical trials utilizing CRAds, we have encountered transaminitis following administration of adenoviruses. Interestingly, a regression analysis failed to correlate the serum liver enzyme level with the number of genomic copies found in the liver. We hypothesize that the observed transaminitis might not be secondary to viral replication in the liver but to the presence of viral proteins, but further studies are needed to explore this possibility.
The most significant adverse effect ever described to be caused by an adenoviral vector was a systemic inflammatory response that led to the death of a patient in a safety trial using a replication-deficient adenovirus.35 Nevertheless, in subsequent studies, other adenoviral vectors have been proven safe for human use.29–31 In our study, in spite of the fact that we have used immunocompetent organisms, no animals were subject to any lethal toxicity. We have found that i.c. injection of oncolytic adenoviruses leads to a humoral response in SHs. Moreover, a regression analysis suggests a negative correlation between the level of anti-adenoviral antibodies and the number of viral genomic copies in the brain. This finding favors the possibility that viral neutralization by anti-adenovirus antibodies might limit the replication of viruses in the brain following i.c. injection. Whether the humoral response could limit the toxicity or the efficacy of CRAd therapy for brain tumors is not yet known; therefore this possibility should be further explored.
In summary, we have shown that CRAd-S-pk7 has a comparable safety and biodistribution profile to AdWT following i.c. injection into permissive and immunocompetent organisms. By comparing SHs and cotton rats, we present evidence that might be useful to other investigators who pursue a better understanding of the systemic behavior of human adenoviruses in the preclinical setting.
Acknowledgements
We are grateful to the animal facility at the University of Chicago for their advice and technical support. In addition, we acknowledge the support of Ms Terri Li from the immunohistochemistry facility at the University of Chicago. This work was supported by the National Cancer Institute (R01-CA122930, MSL), the National Institute of Neurological Disorders and Stroke (K08-NS046430, MSL), The Alliance for Cancer Gene Therapy Young Investigator Award (MSL) and the American Cancer Society (RSG-07-276-01-MGO, MSL).
References
- 1.Jiang H, McCormick F, Lang FF, Gomez-Manzano C, Fueyo J. Oncolytic adenoviruses as antiglioma agents. Expert Rev Anticancer Ther. 2006;6:697–708. doi: 10.1586/14737140.6.5.697. [DOI] [PubMed] [Google Scholar]
- 2.Sonabend AM, Ulasov IV, Han Y, Lesniak MS. Oncolytic adenoviral therapy for glioblastoma multiforme. Neurosurg Focus. 2006;20:E19. [PubMed] [Google Scholar]
- 3.Ulasov IV, Tyler MA, Han Y, Glasgow JN, Lesniak MS. Novel recombinant adenoviral vector that targets the interleukin-13 receptor alpha2 chain permits effective gene transfer to malignant glioma. Hum Gene Ther. 2007;18:118–129. doi: 10.1089/hum.2006.146. [DOI] [PubMed] [Google Scholar]
- 4.Tyler MA, Ulasov IV, Borovjagin A, Sonabend AM, Khramtsov A, Han Y, et al. Enhanced transduction of malignant glioma with a double targeted Ad5/3-RGD fiber-modified adenovirus. Mol Cancer Ther. 2006;5:2408–2416. doi: 10.1158/1535-7163.MCT-06-0187. [DOI] [PubMed] [Google Scholar]
- 5.Sebestyen Z, de Vrij J, Magnusson M, Debets R, Willemsen R. An oncolytic adenovirus redirected with a tumor-specific T-cell receptor. Cancer Res. 2007;67:11309–11316. doi: 10.1158/0008-5472.CAN-07-0739. [DOI] [PubMed] [Google Scholar]
- 6.Wohlfahrt ME, Beard BC, Lieber A, Kiem HP. A capsid-modified, conditionally replicating oncolytic adenovirus vector expressing TRAIL Leads to enhanced cancer cell killing in human glioblastoma models. Cancer Res. 2007;67:8783–8790. doi: 10.1158/0008-5472.CAN-07-0357. [DOI] [PubMed] [Google Scholar]
- 7.Ulasov IV, Rivera AA, Nettelbeck DM, Rivera LB, Mathis JM, Sonabend AM, et al. An oncolytic adenoviral vector carrying the tyrosinase promoter for glioma gene therapy. Int J Oncol. 2007;31:1177–1185. [PubMed] [Google Scholar]
- 8.Van Houdt WJ, Haviv YS, Lu B, Wang M, Rivera AA, Ulasov IV, et al. The human survivin promoter: a novel transcriptional targeting strategy for treatment of glioma. J Neurosurg. 2006;104:583–592. doi: 10.3171/jns.2006.104.4.583. [DOI] [PubMed] [Google Scholar]
- 9.Ulasov IV, Rivera AA, Sonabend AM, Rivera LB, Wang M, Zhu ZB, et al. Comparative evaluation of survivin, midkine and CXCR4 promoters for transcriptional targeting of glioma gene therapy. Cancer Biol Ther. 2007;6:679–685. doi: 10.4161/cbt.6.5.3957. [DOI] [PubMed] [Google Scholar]
- 10.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 11.Xia ZJ, Chang JH, Zhang L, Jiang WQ, Guan ZZ, Liu JW, et al. [Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus.]. Ai Zheng. 2004;23:1666–1670. [PubMed] [Google Scholar]
- 12.Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 13.Ulasov IV, Zhu ZB, Tyler MA, Han Y, Rivera AA, Khramtsov A, et al. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther. 2007;18:589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- 14.Zheng S, Ulasov IV, Han Y, Tyler MA, Zhu ZB, Lesniak MS. Fiber-knob modifications enhance adenoviral tropism and gene transfer in malignant glioma. J Gene Med. 2007;9:151–160. doi: 10.1002/jgm.1008. [DOI] [PubMed] [Google Scholar]
- 15.Nandi S, Ulasov IV, Tyler M, Sugihara AQ, Molinero L, Han Y, et al. Low-dose radiation enhances survivin-mediated virotherapy against malignant glioma stem cells. Cancer Res. 2008;68:5778–5784. doi: 10.1158/0008-5472.CAN-07-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginsberg HS, Moldawer LL, Sehgal PB, Redington M, Kilian PL, Chanock RM, et al. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci USA. 1991;88:1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hjorth RN, Bonde GM, Pierzchala WA, Vernon SK, Wiener FP, Levner MH, et al. A new hamster model for adenoviral vaccination. Arch Virol. 1988;100:279–283. doi: 10.1007/BF01487691. [DOI] [PubMed] [Google Scholar]
- 18.Duncan SJ, Gordon FC, Gregory DW, McPhie JL, Postlethwaite R, White R, et al. Infection of mouse liver by human adenovirus type 5. J Gen Virol. 1978;40:45–61. doi: 10.1099/0022-1317-40-1-45. [DOI] [PubMed] [Google Scholar]
- 19.Toth K, Spencer JF, Tollefson AE, Kuppuswamy M, Doronin K, Lichtenstein DL, et al. Cotton rat tumor model for the evaluation of oncolytic adenoviruses. Hum Gene Ther. 2005;16:139–146. doi: 10.1089/hum.2005.16.139. [DOI] [PubMed] [Google Scholar]
- 20.Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K, et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- 21.Toth K, Spencer JF, Wold WS. Immunocompetent, semi-permissive cotton rat tumor model for the evaluation of oncolytic adenoviruses. Methods Mol Med. 2007;130:157–168. doi: 10.1385/1-59745-166-5:157. [DOI] [PubMed] [Google Scholar]
- 22.Thomas MA, Spencer JF, Wold WS. Use of the Syrian hamster as an animal model for oncolytic adenovirus vectors. Methods Mol Med. 2007;130:169–183. doi: 10.1385/1-59745-166-5:169. [DOI] [PubMed] [Google Scholar]
- 23.Hsu W, Lesniak MS, Tyler B, Brem H. Local delivery of interleukin-2 and adriamycin is synergistic in the treatment of experimental malignant glioma. J Neurooncol. 2005;74:135–140. doi: 10.1007/s11060-004-6597-8. [DOI] [PubMed] [Google Scholar]
- 24.Lesniak MS, Gabikian P, Tyler BM, Pardoll DM, Brem H. Dexamethasone mediated inhibition of local IL-2 immuno-therapy is dose dependent in experimental brain tumors. J Neurooncol. 2004;70:23–28. doi: 10.1023/b:neon.0000040821.50347.c5. [DOI] [PubMed] [Google Scholar]
- 25.Lesniak MS, Upadhyay U, Goodwin R, Tyler B, Brem H. Local delivery of doxorubicin for the treatment of malignant brain tumors in rats. Anticancer Res. 2005;25:3825–3831. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu ZB, Chen Y, Makhija SK, Lu B, Wang M, Rivera AA, et al. Survivin promoter-based conditionally replicative adenoviruses target cholangiocarcinoma. Int J Oncol. 2006;29:1319–1329. [PubMed] [Google Scholar]
- 27.Wu H, Han T, Lam JT, Leath CA, Dmitriev I, Kashentseva E, et al. Preclinical evaluation of a class of infectivity-enhanced adenoviral vectors in ovarian cancer gene therapy. Gene Ther. 2004;11:874–878. doi: 10.1038/sj.gt.3302249. [DOI] [PubMed] [Google Scholar]
- 28.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 29.Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62:6070–6079. [PubMed] [Google Scholar]
- 30.Reid T, Galanis E, Abbruzzese J, Sze D, Andrews J, Romel L, et al. Intra-arterial administration of a replication-selective adenovirus (dl1520) in patients with colorectal carcinoma metastatic to the liver: a phase I trial. Gene Ther. 2001;8:1618–1626. doi: 10.1038/sj.gt.3301512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin X, Huang H, Li S, Li H, Li Y, Cao Y, et al. A phase I clinical trial of an adenovirus-mediated endostatin gene (E10A) in patients with solid tumors. Cancer Biol Ther. 2007;6:648–653. doi: 10.4161/cbt.6.5.4004. [DOI] [PubMed] [Google Scholar]
- 32.Galanis E, Okuno SH, Nascimento AG, Lewis BD, Lee RA, Oliveira AM, et al. Phase I-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Ther. 2005;12:437–445. doi: 10.1038/sj.gt.3302436. [DOI] [PubMed] [Google Scholar]
- 33.Nemunaitis J, Senzer N, Sarmiento S, Zhang YA, Arzaga R, Sands B, et al. A phase I trial of intravenous infusion of ONYX-015 and enbrel in solid tumor patients. Cancer Gene Ther. 2007;14:885–893. doi: 10.1038/sj.cgt.7701080. [DOI] [PubMed] [Google Scholar]
- 34.Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L, et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14:107–117. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Miller HI. Gene therapy on trial. Science. 2000;287:591–592. doi: 10.1126/science.287.5453.591c. [DOI] [PubMed] [Google Scholar]


