Abstract
Methicillin-resistant Staphylococcus aureus is increasingly responsible for staphylococcal infections in the community. A large percentage of the community-acquired methicillin-resistant (CA-MRSA) strains in the USA produce Panton–Valentine leukocidin (PVL), which is associated with severe infections. The virulence of the clinical CA-MRSA strain USA300 was compared to that of its isogenic pvl-deleted mutant, and it was shown that PVL contributes to lung and muscle tissue destruction, respectively, in murine necrotizing pneumonia and skin infection models. Mice infected with the USA300 strain developed a dominant anti-PVL response. The PVL subunits were therefore tested as vaccinogens against this isolate, and their vaccine efficacy correlated with both the route of vaccination and infection. These data suggest that PVL is a virulence factor in murine CA-MRSA infections.
Keywords: CA-MRSA, Panton–Valentine leukocidin, Staphylococcus aureus
INTRODUCTION
Staphylococcus aureus is an opportunistic pathogen that colonizes the skin of approximately 20–30% of the population and can cause a diverse spectrum of disease in humans [1]. In the last decade, steadily growing numbers of community-acquired methicillin-resistant S. aureus (CA-MRSA) infections have been detected globally [2–5]. Although the most frequent manifestations of CA-MRSA are skin and soft tissue infections, these strains can cause highly invasive, rapidly progressing fatal diseases such as necrotizing pneumonia and necrotizing fasciitis [6–11].
Three major genetic markers distinguish CA-MRSA strains from other S. aureus strains: their genetic lineage, the composition of their methicillin resistance element, and the presence of the genes encoding Panton–Valentine leukocidin (PVL). PVL is a bi-component, pore-forming toxin with the ability to lyse leukocytes [12]. Epidemiological data, as well as our own experimental pneumonia animal infection models, demonstrated that PVL contributes to the severity of necrotizing pneumonia [13].
In contrast to recent reports, it was demonstrated, using the clinical LAC strain and the isogenic LACΔpvl mutant, that PVL is a virulence determinant in both lung and skin murine infection models [14,15].
The importance of PVL in the infection process was further highlighted by immunization studies (either intranasally or intradermally) that demonstrated that LukS-PV conferred protection against CA-MRSA challenge (unpublished observations).
MATERIALS AND METHODS
Bacterial strains and culture
S. aureus strains LAC (USA300) and LACΔpvl were generously provided by F. DeLeo. Isolates were cultured aerobically on blood agar at 37°C overnight, and then in 5 mL of casein hydrolysate and yeast extract medium (CCY) or tryptic soy broth (TSB) at 37°C as previously described [13].
Murine lung and skin infections
Animal experiments were carried out with 6-week-old female Balb/c mice (Harlan, Indianapolis, IN, USA), in accordance with the National Institute of Health guidelines, and were approved by the Institutional Animal Care Use Committee at the Texas A&M HSC Institute of Biosciences and Technology. Lung infections were induced as previously described [13]. For the skin infections, the lower back of the animals was shaved and cleaned with 70% ethanol; they were then anaesthetized [13], and injected intradermally with S. aureus (1–3 × 107 bacteria in 50 µL). Survival data were analysed with a two-sided Fisher’s exact test with the Yates continuity correction; weight changes were analysed with an unpaired t-test with Welch correction.
Tissue histology and serological analysis
Mouse lungs were perfused with formalin as previously described [13]. For the skin infection model, the skin and muscle regions surrounding the infected site were processed as previously described [13]. IgG-specific serum antibody responses to S. aureus proteins were determined by an ELISA, as previously described [16]. S. aureus proteins (Efb27–166 [17], Map1950–237 [18], FnbpA620–881 [19],CNA3530–331 [20], SdrC182–496, SdrD50–600, SdrE51–606, ClfA229–545 [21], ClfB201–542 [22], LukS-PV29–312 [13] and LukF-PV35–325 [13]) were expressed as His-tagged recombinant fragments, with the exception of FnbpA, which was expressed using the pGEX-2T vector.
Immunizations
Balb/c mice were immunized with either recombinant LukF-PV, LukS-PV, DbpA (a Borrelia burgdorferi adhesin used as a control antigen), toxoided alpha toxin or adjuvant alone. Purified alpha toxin (List Biological Laboratories, Inc., Campbell, CA, USA) was toxoided with formaldehyde (0.2%, w/v) as previously described [23]. The recombinant forms of LukF-PV, LukS-PV and DbpA were expressed as previously described [13,24].
Intranasally vaccinated mice were immunized once per week prior to infection on day 35. The first immunization was performed with 10 µg of antigen mixed with 3 µg of cholera toxin (CT) (List Biological Laboratories), and subsequent vaccinations were performed with 5 µg of antigen mixed with 1 µg of CT (10 µL).
Subcutaneously vaccinated mice were immunized twice prior to infection on day 35 [16]. The first immunization in complete Freund’s adjuvant (Sigma, St. Louis, MO, USA) was done with either 20 µg of a single antigen or 10 µg of LukF and 10 µg of LukS administered ventrally on the right and left sides, respectively, and boosted at day 14 in incomplete Freund’s adjuvant (IFA) using the same amounts of antigen (100 µL/flank) [16].
Antibody profile analysis
Isotype-specific biotin-conjugated rat anti-IgG1 (clone A85-1), anti-IgG2a (clone R19-15), anti-IgG2b (clone R12-3), anti-IgG3 (clone R40–82), anti-IgE (clone R35–72), anti-IgM (clone II/41) (BD Biosciences Pharmingen, San Jose, CA, USA) or biotinylated polyclonal goat anti-mouse IgA (SBA104004) (Accurate Chemical, Westbury, NY, USA) were used to identify isotype-specific serum antibody responses to either recombinant LukF-PV or LukS-PV by ELISA as previously described [16]. Serum from each mouse was taken at 0 and 28 days post-immunization, diluted 1 : 100, and added (100 µL/well) to the respective wells [16]. Differences in absorbance values were analysed using the unpaired t-test [16].
Delayed-type hypersensitivity (DTH) analysis
Prior to infection with S. aureus, mice were challenged with 2.5 µg of either LukF (right footpad) or LukS (left footpad) diluted in 50 µL of phosphate-buffered saline (PBS). Footpads were measured at 0 and 24 h post-challenge as previously described [18]. Differences in footpad swelling were analysed using the unpaired t-test with Welch correction [16].
RESULTS
The expression of PVL is linked to disease severity
Mice were infected with 1 × 108 CFUs of LAC or LACΔpvl and examined for 8 days. Seventy-two per cent of mice (n = 57) infected with the LAC pulse-field type isolate died 2 days post-infection, as compared to 19% of mice (n = 32) infected with the LACΔpvl isogenic mutant (p <0.0001). Mortality rates did not change after day 2 post-infection (Fig. 1).
Fig. 1. Pneumonia survival curve.
Balb/c mice were intranasally inoculated with a suspension of 1–3 × 108 CFUs of either Staphylococcus aureus USA300 LAC (●) or S. aureus LACΔpvl (■). Mortality was recorded at 24 and 48 h post-infection, and animals were monitored for up to 7 days.
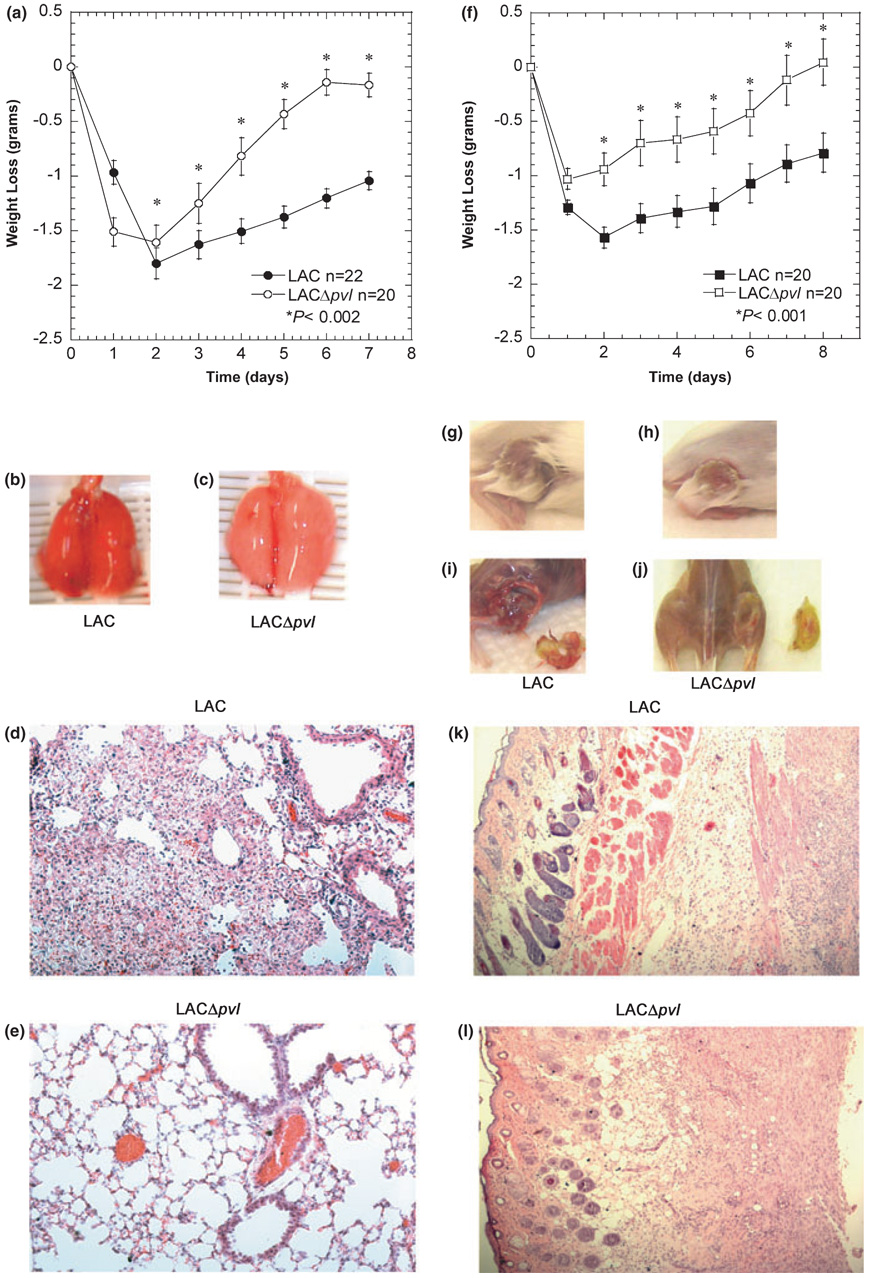
To evaluate disease progression, 5 × 107 CFUs were used in subsequent experiments. LAC-infected mice were lethargic, had hunched posture and laboured breathing, and lost 14% of their body weight during the first 48–72 h as compared to 8.5% weight loss in LACΔpvl-infected mice (Fig. 2a). As mice were most severely ill 48 h post-infection, this time-point was chosen for histological and gross analysis of lung tissue. Lung damage was greater in LAC-infected mice than in LACΔpvl-infected controls (Fig. 2b,c). To assess tissue damage, a histological grading system was used where a score of 0 represents no inflammation and a score of 5 represents severely damaged and inflamed tissue [13]. Histological scores were 3.42 ± 0.23 (n = 7) and 2.83 ± 0.09 (n = 6) for LAC-infected (Fig. 2d) and LACΔpvl-infected (Fig. 2e) mice, respectively (p <0.024, Student’s t-test).
Fig. 2. Panton–Valentine leukocidin-deleted mutants cause less severe infections.
(a–e) Mice were infected intranasally with 3–5 × 107 CFUs of each Staphylococcus aureus strain, LAC (●) or LACΔpvl (○). (a) Weight loss in grams over time (days). (b, c) Gross lung morphology 2 days post-infection (b, LAC; c, LACΔpvl). (d, e) Five-micrometre lung sections were stained with haematoxylin and eosin (H&E), ×20 magnification. (f–l) Mice were infected intradermally with 1 × 107 CFUs of each S. aureus strain, LAC (■) or LACΔpvl (□). (f) Weight loss in grams over time (days). (g, h) Gross superficial skin morphology. Intradermally infected animals were killed and photographed 7 days post-inoculation (g, LAC; h, LACΔpvl). (i, j) Gross morphology of tissues underlying the infection site (i, LAC; j, LACΔpvl). (k, l) Five-micrometre tissue sections stained with H&E, 10× magnification.
In parallel experiments, mice were infected intradermally (1 × 107 CFUs) with LAC or LACΔpvl [14]. Symptoms of disease, including ruffled coat, lethargy and weight loss, were observed as early as 24 h post-infection in the LAC-infected mice. This group lost an average of 9% of their total body weight during the first 48–72 h, as compared to a 5% loss in LACΔpvl-infected mice (Fig. 2f). Regardless of the infecting strain, mice developed an initial blister at the site of inoculation (Fig. 2g,h); however, mice infected with LAC (but not with LACΔpvl) had developed severe myositis beneath the infection site by day 7 post-infection (Fig. 2i,j, respectively). Histological analysis of the skin and muscle tissues harvested from the infection site of LAC-infected mice showed a strong inflammatory cell infiltrate visualized as dark blue aggregates throughout the tissue. Necrosis of the skin and underlying muscle layers was also evident (Fig. 2k). Staphylococcal aggregates were also visible in the fascia, dermis, epidermis, and particularly at the base of the hair follicles (Fig. 2k). In contrast, tissues harvested from LACΔpvl-infected mice had reduced inflammatory infiltrates (Fig. 2l), which correlated with the CFUs in these tissues (Fig. S1).
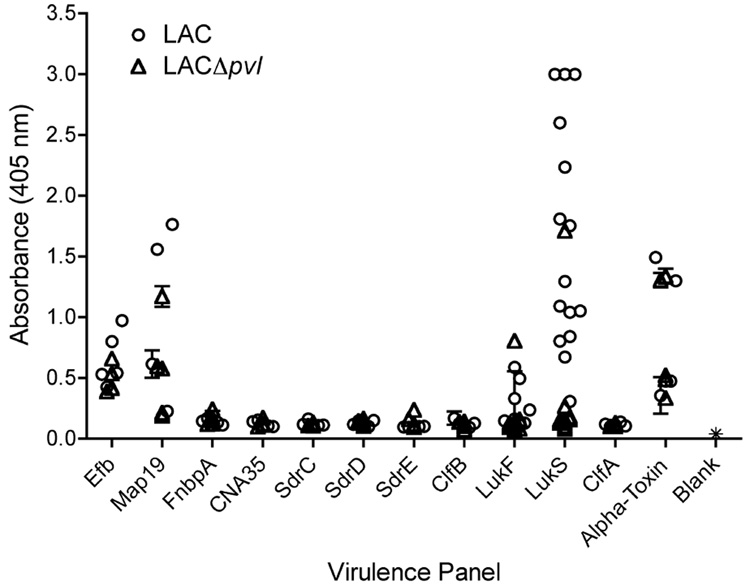
To understand the nature of the immune response during the course of a persistent skin infection, antibody reactivity to a panel of S. aureus proteins was measured 28 days after skin inoculation with LAC or LACΔpvl (Fig. 3). Representative cell wall-anchored and secreted proteins known to be involved in staphylococcal adherence, toxicity or immune evasion were examined, including the collagen adhesin CNA as a negative control, as this protein is not encoded in the LAC genome. This analysis demonstrated that mice intradermally infected with LAC developed significant and dominant responses to the LukS-PV subunit of PVL. Detectable IgG reactivity was also observed against LukF-PV, the immunomodulator Map, alpha toxin and the complement-binding protein Efb. LACΔpvl elicited a similar response except that there was no response to PVL.
Fig. 3. Antibody response to virulence factors.
Mice were infected intradermally with 1 × 107 CFUs of either Staphylococcus aureus LAC or S. aureus LACΔpvl. Twenty-eight days later, sera from five mice were collected, and the reactivity of antibodies towards selected recombinant S. aureus virulence factors was tested. CNA was used as a negative control, as it is not encoded in the S. aureus USA300 genome. The presence of antibodies from groups of five animals infected with LAC (●), infected with LACΔpvl (▽) or uninfected (*), that were reactive to the S. aureus virulence factors was tested using ELISA as previously described. Each symbol represents the response of each animal. The data are expressed as the mean ± SD of three wells.
Subcutaneous vaccinations
Mice were immunized subcutaneously (Fig. 4) with recombinant PVL subunits (delivered individually or together) or with inactivated alpha toxin. The non-staphylococcal His-tagged negative control protein DbpA and mice treated with Freund’s adjuvant alone or non-immunized were used as controls. The humoral immune responses to recombinant LukF-PV or LukS-PV were measured in mice immunized with the different antigen formulations. On day 28 post-immunization, mice from all vaccine groups were bled and the sera were analysed by ELISA to detect different isotypes reactive to either LukF-PV or LukS-PV (Fig. 4a). In addition, six mice from each vaccination group were challenged in contralateral footpads with either LukF-PV or LukS-PV for DTH analysis (Fig. 4b).
Fig. 4. Subcutaneous immunizations.
(a) Isotype profile analysis following vaccination. Twenty-eight days following a subcutaneous immunization regimen with either LukF or LukS (or LukF and LukS in one group of subcutaneously vaccinated mice), mice were bled and their sera were analysed for isotype-specific (IgG1, IgG2a, IgG2b, IgG3, IgE, IgA, or IgM) anti-LukF or anti-LukS responses. Only IgG1-, IgG2a-, IgG2b- and IgA-specific antibodies were detected. The IgG3 response is representative of negative responses, i.e. IgE and IgM. The number of mice used in each treatment group is indicated in parentheses. Each symbol represents the mean ± SE response within each group. †p <0.05, IgG2a anti-LukS response as compared to the IgG2a anti-LukF response in subcutaneously vaccinated mice; unpaired t-test with Welch correction. (b) Delayed-type hypersensitivity response to LukF and LukS following vaccination. Thirty-four days post-vaccination, mice from all groups were challenged on the right and left footpads with 2.5 µg of LukF and LukS, respectively. Footpads were measured at 0 and 24 h after challenge, and the response to LukS (black) and to LukF (white) is expressed as the mean ± SE of six mice/group (*p <0.0001 as compared to the contralateral footpad or to the challenge-only control; unpaired t-test with Welch correction). (c) Percent survival following intranasal inoculation with Staphylococcus aureus USA300. Balb/c mice (total numbers used/group indicated in parentheses) were vaccinated subcutaneously with recombinant LukF, LukS, control proteins or adjuvant alone prior to infection with 5 × 107 CFUs of S. aureus in a volume of 20 µL. Mouse survival was monitored for up to 7 days post-infection. (d) Weight change in vaccinated mice following intradermal infection. Vaccinated mice were intradermally infected with S. aureus USA300 and weighed before and after infection. Symbols represent the weight loss in mice vaccinated with LukS (●), LukF (■), CFA only (▼), or unvaccinated (○). †p <0.01 LukF- and LukS-vaccinated mice as compared to CFA-only-vaccinated groups of mice; unpaired t-test with Welch correction.
Isotype profile analysis revealed a dominant and specific IgG1 anti-LukF-PV and anti-LukS-PV response following subcutaneous vaccination with the respective recombinant PVL components (Fig. 4a). Mice immunized with a single component had an IgG1 response similar to that seen in mice immunized subcutaneously with both components (data not shown and Fig. 4a). The IgG2a and IgG2b responses were not as robust as the IgG1 response; IgG2b responses were significantly greater to LukS-PV than to LukF-PV, and in the group immunized with both subunits, the IgG2a response to LukS-PV was greater than that to LukF-PV (Fig. 4a). IgG3, IgM or IgE reactivity was not observed (Fig. 4a and data not shown).
The DTH response to LukF-PV or LukS-PV demonstrated that immunization with either LukF-PV or LukS-PV resulted in a specific response to each respective component (Fig. 4b). The DTH response to the PVL components in the subcutaneously immunized mice paralleled the IgG2b antibody response described above, and mice that received equal amounts of both components developed a dominant anti-LukS-PV response. Mice immunized with control proteins (alpha toxin or DbpA) or adjuvant alone did not develop a significant response when compared to the challenge-only (untreated) group, demonstrating that neither the control proteins nor adjuvant alone elicited a cross-reactive LukF-PV or LukS-PV response.
The route of vaccination correlated with vaccine efficacy in the context of the type of infection studied. Immunized animals were inoculated with bacterial suspensions intranasally or intradermally to determine the efficacy of the vaccine formulations against either lung or skin infections (Fig. 4c,d). For each immunization group, mortality and weight loss were assessed 8 and 14 days post-infection, respectively. Subcutaneously administered LukF-PV and LukS-PV (LukFS) may have resulted in a more effective immune response, as mice receiving either a multicomponent formulation of LukFS or alpha toxin were better protected; however, the differences in the pneumonia challenge were not statistically significant over the complete Freud’s adjuvant CFA-only control (Fig. 4c).
Although 60–55% of mice vaccinated with LukF-PV-CFA or LukS-PV-CFA survived 8 days post-infection, as compared to 13.3% of the infection-only group (p 0.01 and p 0.03), when these animals were compared to the DbpA-only or CFA-only groups, the differences were not significant (Fig. 4c).
In addition to the pneumonia challenge, the effectiveness of the different vaccination strategies against a non-lethal, intradermal infection was examined, which allowed the monitoring of weight loss as a measure of chronic disease over a 2-week period (Fig. 4d).
The protective effect of subcutaneously administered LukF-PV-CFA or LukS-PV-CFA, as compared to the infection-only or the CFA-only control groups, was apparent as early as day 3 post-infection (p <0.0003, unpaired t-test) and remained statistically different through day 14 post-infection (Fig. 4d).
Mucosal vaccinations
Mice were intranasally immunized with either LukF-PV, LukS-PV or alpha toxin with CT as an adjuvant. Administration of both LukF-PV and LukS-PV intranasally resulted in significant inflammation of the respiratory epithelium even if they were administered 2 days apart (data not shown); therefore, a multicomponent approach was not viable for this vaccination route. However, each subunit administered independently was well tolerated. Control animals received DbpA or PBS mixed with CT. As observed for subcutaneously vaccinated animals, intranasally immunized mice developed a dominant IgG1 anti-LukF-PV and anti-LukS-PV response (Fig. 5a). Both LukF-PV and LukS-PV intranasally vaccinated mice had elevated IgG2a responses; IgG2b responses were significantly greater to LukS-PV than to LukF-PV (Fig. 5a). IgG3, IgM or IgE reactivity was not detected in the sera obtained from any of the vaccination groups (Fig. 5a and data not shown). The only isotype detected in the sera of intranasally vaccinated groups (LukS-PV-vaccinated mice only) that was not observed in subcutaneously vaccinated groups was IgA (Fig. 5a).
Fig. 5. Mucosal immunizations.
(a) Isotype profile analysis following vaccination. Twenty-eight days following a intranasal immunization regimen with either LukF or LukS (or control proteins), mice were bled and their sera were analysed for isotype-specific (IgG1, IgG2a, IgG2b, IgG3, IgE, IgA, or IgM) anti-LukF or anti-LukS responses. Only IgG1-, IgG2a-, IgG2b- and IgA-specific antibodies were detected. The IgG3 response is representative of negative responses, i.e. IgE and IgM. The number of mice used in each treatment group is indicated in parentheses. Each symbol represents the mean ± SE response within each group. ‡p <0.005, IgG2b and IgA anti-LukS response as compared to the IgG2b and IgA anti-LukF response in intranasally vaccinated mice; unpaired t-test with Welch correction. (b) Delayed-type hypersensitivity response to LukF and LukS following vaccination. Thirty-four days post-vaccination, mice from all groups were challenged on the right and left footpads with 2.5 µg of LukF and LukS, respectively. Footpads were measured at 0 and 24 h after challenge, and the response to LukS (black) and to LukF (white) is expressed as the mean ± SE of six mice/group (*p <0.0001 as compared to the contralateral footpad or to the challenge-only control; unpaired t-test with Welch correction). (c) Percent survival following intranasal inoculation with Staphylococcus aureus USA300. Balb/c mice (total numbers used/group indicated in parentheses) were vaccinated subcutaneously with recombinant LukF, LukS, control proteins or adjuvant alone prior to infection with 5 × 107 CFUs of S. aureus in a volume of 20 µL. Mouse survival was monitored for up to 7 days post-infection. The percent survival of mice immunized intranasally with LukS was significantly different when compared to LukF-, DbpA- and alpha toxin-immunized mice (‡p <0.05) or to non-immunized mice (†p <0.0001; Fisher’s exact test). (d) Weight change in vaccinated mice following intradermal infection. Vaccinated mice were intradermally infected with S. aureus USA300 and weighed before and after infection. Symbols represent the weight loss in mice vaccinated with LukS (●), LukF (■), cholera toxin (CT) only (▲), or unvaccinated (○).
The cellular response to LukF-PV or LukS-PV demonstrated that intranasal immunization with either component resulted in a specific, respectively, response (Fig. 5b) as described for intradermally immunized mice.
The intranasal vaccination was very effective in protecting mice against pneumonia; mice vaccinated with LukS-PV were significantly protected against an intranasal challenge (75% survival at day 8 post-infection; p <0.0001) when compared to the infection-only, DbpA or adjuvant-only controls (13.3%, 26.6% and 6.9% survival, respectively, at day 8 post-infection) and they were significantly protected (p <0.05) when compared to LukF-PV-vaccinated mice or mice intranasally vaccinated with alpha toxin (48.3% and 43.7% survival, respectively, at day 8 post-infection) (Fig. 5c). The animals vaccinated with LukF-PV and alpha toxin were also significantly protected as compared to the control groups (p <0.005 and p <0.02) (Fig. 5c), although these components were less effective than LukS-PV.
In contrast, intranasally vaccinated mice were not well protected against intradermal challenge, as weight differences in LukF-PV- or LukS-PV-vaccinated mice were only apparent at days 14–15 post-infection (p <0.001 and p <0.04) (Fig 5d).
DISCUSSION
Because the severity of CA-MRSA infections has been linked to strains expressing the pvl determinant, disease presentation was examined in mice infected either intranasally or intradermally with either LAC or LACΔpvl, and the hypothesis that vaccination with the PVL components could protect against LAC infections was tested [4,25,28]. Before this was done, the functionality of LAC PVL was checked by comparing the kinetics of propidium iodide incorporation into polymorphonuclear neutrophils treated with supernatants from LAC vs. LACΔpvl cultures (Fig. S2). The importance of anti-PVL immunity in protection against LAC infections (of lung or skin) was strengthened by the observations that mice infected with LAC presented with more severe disease manifestations than mice infected with LACΔpvl. These data appear to contradict recent experiments, carried out in a superficially similar infection model, which led to the conclusion that PVL is not a virulence determinant in murine infections [15,29,30]. However, significant technical differences exist between the models that may explain the different conclusions reached by Wardenburg et al. [15]. First, in the skin model, assessing the role of PVL by measuring the lesion size following an intradermal inoculation is insufficient. Intradermal infection in the present model also revealed no differences in superficial wound size; however, mice infected with LAC developed severe, deep myositis beneath the infection site that was nearly absent in LACΔpvl-infected mice. These differences can only be appreciated by observing the depth of the lesions by analysis of gross morphology and histology. Second, the pneumonia model results are questionable, as the number of CFUs obtained 2 days after infection are much lower than expected, based on similar experiments carried out in the present model. These data suggest that the mice may not have been properly infected. In addition, when mice are inoculated via the left nostril, most of the inoculum enters into the left lung; that is, the inoculum is not equally distributed to both lobes. Therefore, drawing conclusions regarding the infectivity of LAC inoculated via the left nostril by determining the CFUs from the right lobe [15] does not accurately reflect infection of the entire lung.
Third, the intranasal administration of almost any bacterium results in some degree of lung inflammation; it is therefore not surprising that inflammation was observed in mice infected with either LAC or LACΔpvl. However, to clearly discern degrees of inflammation resulting from LAC or LACΔpvl infections, the lungs must be fixed by perfusing and inflating them with formaldehyde to preserve tissue structures normally filled with air (such as alveolar spaces). This is not a minor point, as the procedure facilitates the discernment of differences in tissue damage, oedema and inflammation resulting from infection by the different strains. As shown by Wardenburg et al. [15], the morphology of a collapsed lung pictured at low magnification is difficult to interpret. Fourth, when evaluating LAC-derived strains, weight loss data over a 2-day period are not sufficient to allow weight differences to manifest [15], as opposed to a 7–14-day period post-infection, monitored for both lung- and skin-infected mice, as reported in this study. Fifth, the age and weight of the mice used in the infection studies also play significant roles in disease presentation. The data shown in this article were obtained from 6-week-old infected mice. Finally, although we have performed experiments in which LAC cultures were grown in both TSB and CCY media with similar results, the strains used in this study were cultured in CCY medium as opposed to the TSB medium used by other investigators [15,29]. This may be significant, as the production of PVL is greatly enhanced when strains are grown in CCY rather than TSB medium.
The data presented in this article demonstrate that PVL is linked to disease severity in murine models of pneumonia and cutaneous infection, and that the LukS-PV subunit administered intranasally protects mice against LAC-induced pneumonia but not against intradermal infection.
In contrast, mice subcutaneously vaccinated with LukF-PV and LukS-PV were protected against an intradermal infection but not against a pulmonary infection. The primary difference between the mice vaccinated intranasally with LukS-PV and the groups that received other vaccines was the generation of anti-LukS-PV IgA, which may explain the significant protective effects of this formulation against lung but not skin challenge.
The antigenic dominance of LukS-PV was also observed in the subcutaneously vaccinated mice, as a dominant anti-LukS-PV IgG2a, IgG2b and DTH response in mice immunized with both components suggested that the immune response to LukS-PV was most likely associated with protection.
In summary, these data demonstrate the importance of the immunization route with regard to diminishing specific disease manifestations caused by the same pathogen and that humoral responses resulting from intranasal or subcutaneous vaccination directed at the LukS-PV component could protect mice accordingly against either a pulmonary or an intradermal infection.
Finally, these data demonstrate that the murine model is a valid tool for evaluating the role of PVL in disease when performed carefully.
Supplementary Material
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Proliferation of S. aureus in infected tissues.
Fig. S2. Kinetics of PMN propidium iodide incorporation.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
ACKNOWLEDGEMENT
We are grateful to F. DeLeo for providing the S. aureus strains, and to E. M. Barbu, E. Smeds, S. Prabhakaran and X. Liang for purified proteins.
TRANSPARENCY DECLARATION
This work was supported by funds from the University of Texas to E. L. Brown, The Texas A&M Health Science Center and the Hamill Foundation to M. G. Bowden and the French Ministry of research to F. Vandenesch. The authors declare no relationship (commercial or otherwise) that may constitute a dual or conflicting interest.
REFERENCES
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 4.Davis SL, Perri MB, Donabedian SM, et al. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol. 2007;45:1705–1711. doi: 10.1128/JCM.02311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez BE, Hulten KG, Dishop MK, et al. Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus infection. Clin Infect Dis. 2005;41:583–590. doi: 10.1086/432475. [DOI] [PubMed] [Google Scholar]
- 6.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–1453. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 7.Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton–Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 8.Garnier F, Tristan A, Francois B, et al. Pneumonia and new methicillin-resistant Staphylococcus aureus clone. Emerg Infect Dis. 2006;12:498–500. doi: 10.3201/eid1203.051040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis JS, Doherty MC, Lopatin U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton–Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–107. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 10.Monaco M, Antonucci R, Palange P, Venditti M, Pantosti A. Methicillin-resistant Staphylococcus aureus necrotizing pneumonia. Emerg Infect Dis. 2005;11:1647–1648. doi: 10.3201/eid1110.050776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vayalumkal JV, Whittingham H, Vanderkooi O, et al. Necrotizing pneumonia and septic shock: suspecting CA-MRSA in patients presenting to Canadian emergency departments. CJEM. 2007;9:300–303. doi: 10.1017/s1481803500015219. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko J, Kamio Y. Bacterial two-component and heteroheptameric pore-forming cytolytic toxins: structures, poreforming mechanism, and organization of the genes. Biosci Biotechnol Biochem. 2004;68:981–1003. doi: 10.1271/bbb.68.981. [DOI] [PubMed] [Google Scholar]
- 13.Labandeira-Rey M, Couzon F, Boisset S, et al. Staphylococcus aureus Panton–Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1082–1085. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 14.Voyich JM, Otto M, Mathema B, et al. Is Panton–Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–1770. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 15.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton–Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198:1166–1170. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown EL, Reisenbichler ES, Kim J-H, Höök M. Multicomponent Lyme vaccine: three is not a crowd. Vaccine. 2005;23:3687–3696. doi: 10.1016/j.vaccine.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Lee LY, Höök M, Haviland D, et al. A secreted Staphylococcus aureus protein inhibits complement activation. J Infect Dis. 2004;190:571–579. doi: 10.1086/422259. [DOI] [PubMed] [Google Scholar]
- 18.Lee LY, Miyamoto YJ, McIntyre BW, et al. The Staphylococcus aureus Map protein is an immunomodulator that interferes with T cell-mediated responses. J Clin Invest. 2002;110:1461–1471. doi: 10.1172/JCI16318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto YJ, Wann ER, Fowler T, Duffield E, Höök M, McIntyre BW. Fibronectin binding protein A of Staphylococcus aureus can mediate human T lymphocyte adhesion and coactivation. J Immunol. 2001;166:5129–5138. doi: 10.4049/jimmunol.166.8.5129. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Rivas JM, Brown EL, Liang X, Höök M. Virulence potential of the Staphylococcal adhesin CNA in experimental arthritis is determined by its affinity for collagen. J Infect Dis. 2004;189:2323–2333. doi: 10.1086/420851. [DOI] [PubMed] [Google Scholar]
- 21.Hartford OM, Wann ER, Höök M, Foster TJ. Identification of residues in the Staphylococcus aureus fibrinogen-binding MSCRAMM clumping factor A (C1fA) that are important for ligand binding. J Biol Chem. 2001;276:2466–2473. doi: 10.1074/jbc.M007979200. [DOI] [PubMed] [Google Scholar]
- 22.Perkins S, Walsh EJ, Deivanayagam CC, Narayana SV, Foster TJ, Hook M. Structural organization of the fibrinogen-binding region of the clumping factor B MSCRAMM of Staphylococcuss aureus. J Biot Chem. 2001;276:44721–44728. doi: 10.1074/jbc.M106741200. [DOI] [PubMed] [Google Scholar]
- 23.Bernheimer AW, Freer JH, Lominski I, Sessa G. Some properties of staphylococcal alpha- toxoid. J Bacteriol. 1968;96:1429–1430. doi: 10.1128/jb.96.4.1429-1430.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo UP, Brown EL, Dorward DW, Rosenberg LC, Höök M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- 25.Kourbatova EV, Halvosa JS, King MD, Ray SM, White N, Blumberg HM. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA 300 clone as a cause of health care-associated infections among patients with prosthetic joint infections. Am J Infect Control. 2005;33:385–391. doi: 10.1016/j.ajic.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006;42:647–656. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 27.Hulten KG, Kaplan SL, Gonzalez BE, et al. Three-year surveillance of community onset health care-associated Staphylococcus aureus infections in children. Pediatr Infect Dis J. 2006;25:349–353. doi: 10.1097/01.inf.0000207404.50143.1e. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez BE, Martinez-Aguilar G, Hulten KG, et al. Severe Staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics. 2005;115:642–648. doi: 10.1542/peds.2004-2300. [DOI] [PubMed] [Google Scholar]
- 29.Wardenburg JB, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bubeck Wardenburg J, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun. 2007;75:1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Proliferation of S. aureus in infected tissues.
Fig. S2. Kinetics of PMN propidium iodide incorporation.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.