Abstract
Cellular differentiation, organization, proliferation and apoptosis are determined by a combination of an intrinsic genetic program, matrix/substrate interactions, and extracellular cues received from the local microenvironment. These molecular cues come in the form of soluble (e.g. cytokines) and insoluble (e.g. ECM proteins) factors, as well as signals from surrounding cells that can promote specific cellular processes leading to tissue formation or regeneration. Recent developments in the field of tissue engineering have employed biomaterials to present these cues, providing powerful tools to investigate the cellular processes involved in tissue development, or to devise therapeutic strategies based on cell replacement or tissue regeneration. These inductive scaffolds utilize natural and/or synthetic biomaterials fabricated into three-dimensional structures. This review summarizes the use of scaffolds in the dual role of structural support for cell growth and vehicle for controlled release of tissue inductive factors, or DNA encoding for these factors. The confluence of molecular and cell biology, materials science and engineering provides the tools to create controllable microenvironments that mimic natural developmental processes and direct tissue formation for experimental and therapeutic applications.
Introduction
Many experimental and clinical applications of tissue engineering rely on the ability of stem or progenitor cells to self-organize into functional tissue.1–3 These cells can be either transplanted into an injured or diseased site, or recruited from the surrounding tissue.4 The intrinsic genetic potential of these progenitor cells to regenerate the desired tissue acts in concert with the molecular cues present in the extracellular microenvironment to navigate the multiple differentiation pathways and produce the appropriate cell types. These progenitor cells respond to the molecular cues, which are biochemical and biophysical signals transmitted via cell surface receptors and integrated via a complex array of intracellular signaling pathways. Stimulation of specific signaling pathways by these molecular cues affects cellular gene expression, resulting in protein production and secretion. The secreted proteins provide feedback to the microenvironment and can subsequently affect gene expression. This dynamic process is essential to normal tissue development and function. Inappropriate environmental signals, or cells lacking proliferation constraints, however, may lead to pathologic states exemplified by cancer.5 Thus, the controlled presentation of specific molecular cues, at the appropriate time and location, is an underlying objective of many tissue engineering approaches.
The molecular cues that define the microenvironment consist of soluble macromolecules (e.g., growth factors, chemokines, cytokines), insoluble factors (e.g., ECM proteins, glycoproteins, and proteoglycans), and proteins presented on the surface of adjacent cells (e.g., integrins, cadherins).6 Environments created by isolating ECM with entrapped growth factors have been valuable tools to examine the interplay between these various molecular cues and the cellular responses they influence. Matrigel, a multicomponent matrix composed of basement membrane preparations that is highly enriched in laminin and type IV collagen (reviewed in ref. 7), can induce breast epithelial cells to form ducts capable of milk production,8 and vascular endothelial cells to form capillaries with a central lumen.9 Alternatively, small intestinal submucosa (SIS), an ECM containing a variety of immobilized growth factors, promotes healing with minimal scar formation.10 These natural systems have enabled numerous studies, yet their lack of characterization and complexity complicates the identification of individual factors and the role they play in tissue formation. Synthetic systems, though lacking the intrinsic functionality of naturally-derived ECMs, can be more precisely controlled to specifically investigate isolated factors or combinations of defined constituents. These synthetic systems provide flexibility to control the concentration and physical placement of the molecular signals, which will be required for their translation to the engineering of tissue structures, such as the intricate tracks of the nervous system or complex vascular networks.11,12
This review focuses on the design of synthetic extracellular matrices for providing growth factors within a three-dimensional microenvironment. Growth factors regulate many cellular processes involved in tissue formation, and expression of growth factors during tissue formation varies temporally, spatially, or occurs in gradients that pattern tissue structures. Tissue inductive scaffolds can serve as experimental tools to investigate the function of individual molecular cues on cellular and tissue-specific processes, and may ultimately be translated to therapeutic applications for repair of tissues that are damaged due to trauma or disease. Growth factors are a primary factor for stimulating tissue formation, which can be delivered directly from the scaffold, or their expression may be induced through gene delivery. A brief introduction of natural and synthetic materials, and protein and DNA delivery systems, provides the foundation to examine the advantages and limitations with the different delivery modalities and mechanisms.
Tissue engineering scaffolds
Natural and synthetic biomaterials serve as fundamental research and therapeutic tools to investigate and facilitate the repair of damaged or dysfunctional tissues, both in cell-based and acellular therapies.13–15 Fabrication of these materials into three-dimensional (3-D) structures, referred to as scaffolds, attempts to mimic key features of the native extracellular microenvironment. The 3-D environment defined by the scaffold can dramatically affect cellular behavior relative to 2-D systems, and may be more representative of the in vivo physiological environment.16 Relative to 2-D systems, 3-D cultures more effectively recreate the complex interplay between mechanical and biochemical signals that affect matrix adhesions and integrin usage,5,17 and alter proliferation rates, cell morphology and migration.18–20 Scaffolds engineered with the appropriate concentration and types of molecular cues can be fabricated into a defined structure to direct cell growth and differentiation. In addition to presenting the molecular cues driving tissue formation, scaffolds must be both macroscopically stable and microscopically dynamic; two opposing yet complimentary characteristics that natural ECMs possess. Macroscopic stability indicates that scaffolds must create a space for new tissue formation, and provide a substrate for cell adhesion and migration. The microscopic dynamics of tissue formation, however, require that scaffolds must also be degradable to enable cellular remodeling of the microenvironment. Finally, scaffolds must support cellular infiltration from the surrounding tissue to allow for vascular ingrowth that provides both nutrient delivery and waste removal. Common materials used in scaffold fabrication are briefly described below, though a more complete description of design strategies and applications can be found in several excellent reviews.6,14,21,22
Hydrogels
Hydrogels are composed of hydrophilic polymers, either natural (e.g., collagen, hyaluronic acid) or synthetic (e.g., polyethylene glycol (PEG)), that can self-assemble or be crosslinked into three-dimensional structures. Typically, cells can be suspended within the hydrophilic solution and are entrapped upon gelation. Self assembling gels, such as PuraMatrix23,24 or peptide amphiphiles25 spontaneously form hydrogels and provide for gentle encapsulation, while providing sites for cell adhesion and promotion of differentiated cell processes. Many of these polymers contain reactive sites or groups that enable the attachment of functional chemical moieties. Numerous cell adhesion proteins or peptides have been attached at controlled densities for fundamental studies of cell–ECM interactions.26–28
The stability of the hydrogel can be regulated through its mechanical properties and degradation rate, which is typically controlled by varying the extent of crosslinking or self-assembly.29–31 Degradation of hydrogels formed from natural polymers occurs by cell-secreted enzymes, which enables cellular infiltration and vascular ingrowth. Synthetic polymers, however, which are not sensitive to degradation by cell-secreted enzymes, can form degradable hydrogels using hydrolysable crosslinkers or incorporating matrix metalloproteinase (MMP)-sensitive cleavage sites that are targets for invading fibroblasts.32 The role of degradation is exemplified by PEG gels delivering bone morphogenetic protein-2 (BMP-2), which induced intramembranous bone formation that was directly related to the rate of gel degradation via cell-derived MMPs.32
Microporous scaffolds
Porous scaffolds composed of natural (e.g., collagen, chitosan) and synthetic polymers (e.g., poly(lactide-co-glycolide) (PLG)), ethylene vinyl co-acetate (EVAc)) are fabricated using processes such as gas foaming, electrospinning, solvent casting, and extrusion. Microporous scaffolds can have porosities ranging from as low as 30% to more than 95%, and pore sizes ranging from ten to hundreds of microns. An interconnected pore structure ensures that infiltrating cells and nascent vascular networks can penetrate throughout the entire scaffold. In some applications, however, it may be desirable to create scaffolds, or portions of scaffolds that are non-porous to allow selective repopulation of cells within and around the site of implantation.33 Porous scaffolds have a high surface area/volume ratio and provide ample area for cellular attachment and migration. Many synthetic polymers are coated with extracellular matrix molecules, such as collagen, laminin, fibronectin and vitronectin.34 These factors markedly increase cell adhesion and influence cellular response by promoting specific receptor–ligand interactions. Synthetic polymers have been used in the engineering of multiple tissues, including skin,35,36 cartilage,37,38 bone39,40 and liver.41 A variety of biodegradable synthetic polymers have been explored for use in tissue engineering scaffolds and have been reviewed elsewhere.6,21,42
Protein and DNA delivery systems
Growth factors initiate and control a variety of cellular processes involved in tissue formation (Table 1). Their use in the clinic, however, has been facilitated following advances in recombinant protein technology. Growth factors, growth factor receptors and monoclonal antibodies are currently being employed to treat clinical conditions such as obesity,43,44 cancer,45,46 and idiopathic short stature,47,48 with the potential for use in wound healing and tissue regeneration.39,49 Localized delivery of tissue inductive factors from scaffolds can function to direct progenitor cell differentiation toward the desired cell fate. Although in vitro studies of tissue formation on scaffolds can be performed simply by adding growth factors to cell culture media, translation of these studies to applications with in vivo tissue formation requires the use of delivery systems that can make these factors available at the appropriate concentration and duration. These delivery systems may also impact in vitro studies by providing the ability to create concentration gradients in one or more factors, or to spatially pattern substrates.11 Alternatively, gene therapy approaches can be employed to induce the expression of tissue inductive factors.
Table 1.
Growth factors delivered to promote tissue formation
| Growth factor | MW/kDa | Functions | Reference |
|---|---|---|---|
| NGF | 27 | Promotes neuron survival and extension in CNS and PNS; modulates differentiation of various neuron types in vivo and in vitro; role in tissue repair and fibrosis | 73,77,131–133 |
| IGF-1 | 27.9 | Mediates actions of GH; increases proteoglycans and type II collagen synthesis | 134–137 |
| IGF-2 | 35.1 | Promotes myogenic differentiation of ES cells | 138 |
| EGF | 6.2 | Wound healing | 139 |
| FGF-1 | 17.5 | Would healing, vascular repair; fibroblast mitogen | 140,141 |
| FGF-2 | 17.3 | Chondrogenesis; angiogenesis; neuronal and endothelial cell proliferation | 103,142–145 |
| PDGF | 22–25 | Maturation of blood vessels, recruitment of SMCs to developing vasculature; wound healing; neural regeneration | 83,146–148 |
| BMP-2 | 44.7 | Osteogenesis; angiogenesis | 149–152 |
| TGF-β1 | 25 | Promotes chondrogenic differentiation; increases cartilage matrix synthesis and chondrocyte proliferation | 153–156 |
| VEGF | 19–22 | Angiogenesis; vasculogenesis; osteogenesis; neurotrophic factor for motor neurons; cartilage remodeling | 86,149,150,157–160 |
Delivery systems for protein and DNA from tissue engineering scaffolds can generally be categorized as controlled release or substrate immobilization. Sustained delivery from polymer substrates not only protects the protein or DNA from degradation but also helps to maintain elevated levels within the extracellular environment by continual replacement of factors that are cleared or degraded.50,51 Thus, the effectiveness of polymeric delivery at maintaining therapeutic levels of the delivered factor can be far greater than that achieved by bolus delivery. Since these factors are transported via diffusion following release, concentration gradients may be established through appropriate manipulation of the release kinetics. Alternatively, substrate immobilization is based on the association or tethering of factors to the scaffold. This places the factor directly in the cell microenvironment and can avoid mass transfer limitations. Immobilization maintains the factor locally, which limits potentially undesirable diffusion to distant sites. Immobilization is also a means to regulate the distribution of factors and create gradients.52–55
Physical properties of delivered factors
Growth factors and other proteins are amphiphilic molecules that vary widely in their physical properties (e.g., disulfide links, multiple subunits, isoelectric points). While most growth factors have molecular weights in the range of 15 kDa to 45 kDa, many proteins with potential therapeutic applications have MW less than 10 kDa (e.g., hormones) or as high as 160 kDa (e.g., antibodies). Growth factors often have short half-lives (on the order of minutes to hours) and are rapidly degraded or cleared, thereby minimizing their biological effect. In order to be effective, delivery systems must maintain the three-dimensional conformation of the protein in order to maximize bioactivity. The processing strategies for incorporating and releasing proteins from scaffolds must also avoid aggregation or chemical inactivation (e.g., deamidation, oxidation), which could reduce activity, alter the half-life, or expose potentially immunogenic residues.56
Vectors for gene delivery consist of DNA that may be packaged with proteins, polymers or lipids to create particles that can effectively overcome the extracellular and intracellular barriers to gene transfer.57 The extracellular obstacles include mass transport to the desired cell populations, DNA degradation, and clearance from the delivery site, whereas the intracellular barriers include cellular internalization, endosomal escape, vector unpacking, and transport into the nucleus. DNA can be delivered alone (i.e., plasmid), or can be packaged using viral or non-viral vectors. Viral vectors provide greater efficiency than either naked plasmid or non-viral vectors, yet provoke an immune response that can lead to clearance of the vector, and cells expressing the transgene. Viruses utilized for gene delivery vectors include retrovirus, adenovirus, and adeno-associated virus (AAV).58 Naked plasmid and non-viral vectors initiate inflammatory responses that are milder than viral vectors, yet lack the intrinsic efficiency of viral-based gene delivery. Plasmid alone is able to transfect cells in vivo, but generally has a low efficiency in vitro. Non-viral vectors composed of cationic polymers or lipids complexed with DNA can provide for enhanced gene transfer in vitro and in vivo of plasmid. A thorough description of viral and non-viral vectors can be found in several recent reviews.58–60
Controlled release
Some of the initial approaches to control delivery from tissue engineering scaffolds involved the addition of protein or DNA to solutions prior to gelation. Gels, such as collagen, Matrigel, and agarose form into hydrogel upon a change in temperature. Fibrin and other polymer scaffolds can be chemically crosslinked with minimal effects on the protein stability. Entrapped factors are soluble within the hydrated gel and can diffuse through the pores and into the surrounding tissue.61 The diffusivity through the gel may be controlled by the extent of crosslinking and the degradation rate of the hydrogel, which determines the average pore size. Typical times for release by diffusion from these hydrogels can range from days to weeks.62 Alternatively, mechanical stimulation of the hydrogel can enhance growth factor release.63
To prolong release from hydrogels, microspheres with encapsulated proteins can be embedded within synthetic polymers. Materials that have traditionally been used for the formation of microspheres or microparticles include PLG, chitosan, polyanhydride, and EVAc. EVAc was one of the first materials used for long-term protein release.64,65 Microspheres are fabricated using a variety of emulsion techniques, and may be modified to control the release rate of embedded proteins and DNA.66 Microspheres can release encapsulated growth factors,50 or viral and non-viral gene therapy vectors.51 When molded into 3-D constructs,67–70 the drug delivery capacity of microspheres is coupled with the structural support afforded by a scaffold (Fig. 1A). Although the release profile of factors from the microsphere is determined by its composition, entrapment within a hydrogel or formation into a scaffold also influences release.71,72
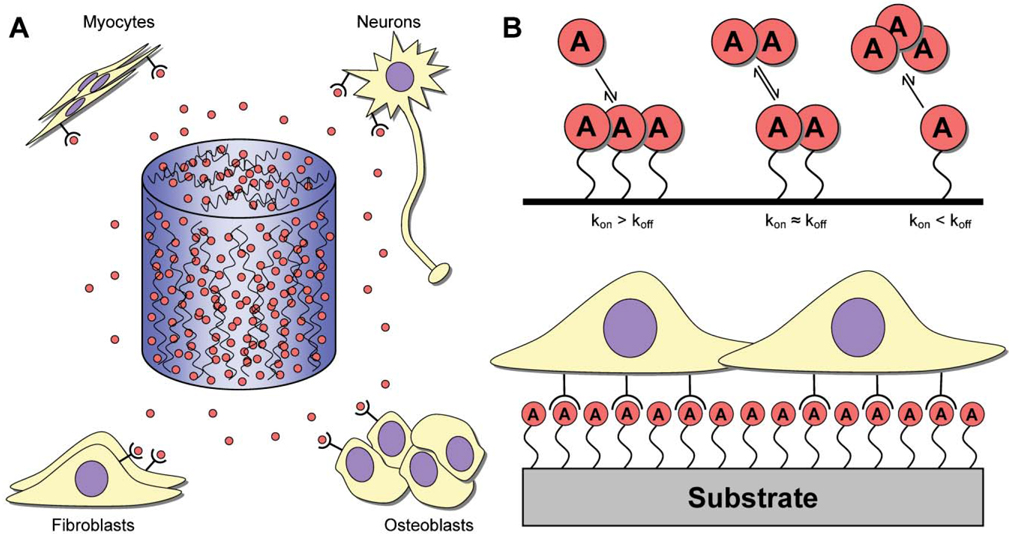
Fig. 1. Release mechanisms for protein and DNA.
(A) Inductive factors (red circles) may be embedded or encapsulated within hydrogels or microspheres, which may, in turn, be used to form 3-D scaffolds that are capable of releasing factors at the site of implantation. The highest factor concentration exists within the scaffold, with lower concentrations found within the surrounding tissue. Delivered factors target a variety of different cell populations (e.g., myocytes, neurons, fibroblasts or osteoblasts) for various applications (e.g., muscle, nerve, bone, wound healing). (B) Substrate immobilization is characterized by factors being bound to a biomaterial. The interaction between the factor and biomaterial can be (top): (1) kon ≫ koff, such that the factor is effectively bound irreversibly; (2) kon ≈ koff, where the factor associates and dissociates from the surface at roughly equal rates; and (3) kon ≪ koff, such that the factor is loaded onto the biomaterial and dissociates to serve as a delivery vehicle. Cells interact with the immobilized factors upon interaction with the biomaterial (bottom).
Scaffolds capable of protein and DNA release have also been fabricated directly from the same materials used for microsphere fabrication. EVAc loaded with neurotrophic factors has been fabricated into tubes for nerve regeneration.65,73 EVAc, however, is not biodegradable, and thus requires a second surgical procedure for removal. Alternatively, PLG has been widely used to produce tissue engineering scaffolds. A gas foaming process has been developed that allows encapsulation and release of a variety of factors.74,75 This process is relatively mild, avoiding the high temperature and organic solvents typical of many polymer processing approaches. Recently, the ability to fabricate more complex architectures using a gas foaming process has been demonstrated, while retaining the ability to control release.76
Substrate immobilization
Polymer scaffolds can be modified to interact with either protein or DNA, thereby slowing or preventing release from the matrix (Fig. 1B). The repeated binding of factors to the matrix hinders their diffusion, thereby prolonging release.77 The number of binding sites, the affinity of growth factor for these sites, and the degradation rate of the hydrogel are key determinants of the amount of bound growth factor, as well as the release profile.78 For protein delivery, a common approach involves the incorporation of heparin to support the interaction with heparin-binding growth factors.79 This affinity-based drug delivery system has been employed with fibrin, in which a synthetic linker peptide is covalently attached to fibrin that is capable of binding heparin. Immobilized heparin is then able to bind many growth factors, thereby slowing their diffusion. NGF, BDNF, and Neurotrophin-3 (NT-3) bind heparin with low affinities (estimated KD on the order of 10−6 M) via a basic sequence at their C-terminus. NT-3 was released within 3 days in the absence of heparin, whereas release occurred over 14 days in the presence of optimal heparin.78
Protein and DNA delivery: efficiency and efficacy
Protein
Growth factors have strong tissue inductive properties,39 but their short half-lives combined with high clearance rates have motivated the development of delivery systems capable of maintaining elevated concentrations to achieve therapeutic efficacy. Bone morphogenic proteins (BMPs), for example, are currently being used in clinical trials for the treatment of open fractures.80–82 Tissue engineering scaffolds releasing proteins mimic the natural reservoir capacity of the extracellular matrix. Coupling delivery of protein with a degradable carrier capable of organizing tissue formation is a powerful approach for tissue regeneration (Table 2). Controlling the release kinetics of inductive agents allows for regulation over the type, dose and duration of exposure, which is sure to impact therapeutic success. Protein delivery systems have demonstrated the ability to release multiple proteins, or create concentration gradients of inductive factors.83–85 A critical challenge to developing delivery systems for multiple proteins involves identifying processing conditions that maintain the bioactivity of all constituents.84,86
Table 2.
Scaffolds delivering protein for tissue regeneration
| Material | Proteins | Applications | Reference |
|---|---|---|---|
| Natural: | |||
| Collagen | FGF-2 | Tissue development and remodeling, cellular proliferation, angiogenesis | 139,161 |
| PDGF-BB | Blood vessel maturation | 139 | |
| VEGF | Angiogenesis | 139 | |
| HB-EGF | Wound healing | 139 | |
| TGF-β | Chondrogenesis | 110 | |
| Fibrin | NGF | Promote neurite extension and axonal guidance in CNS and PNS injuries | 77,101 |
| bFGF | Angiogenesis; wound healing | 79,109 | |
| Synthetic: | |||
| PLGA | BMP-4/VEGF | Osteoinduction and angiogenesis | 86 |
| NGF | Neural regeneration | 64,73,76,131,132,162–165 | |
| VEGF | Angiogenesis | 166,167 | |
| EVAc | bFGF | Neural regeneration; angiogenesis | 145,168 |
| PEG | TGF-β | Smooth muscle matrix deposition | 169 |
| Hybrid: | |||
| PLGA/Fibrin | FGF-1 | Promote wound healing while inducing angiogenesis | 140 |
| PCL/TCP/Fibrin | BMP-2 | Bone regeneration | 170 |
| HAC-PLA | BMP | Osteogenesis, osteoinduction | 171 |
| PLGA/PEG/PPF | Osteogenic peptide TP508 | Osteogenesis | 172 |
DNA
The delivery of DNA encoding for tissue inductive factors represents a versatile alternative to direct protein delivery. Such a strategy has been used to successfully induce synthesis and secretion of various proteins (e.g., growth factors), with transgene expressing cells serving as bioreactors for localized protein production (Table 3).74,87,88 In this way, targeted gene delivery can stimulate local protein production capable of activating autocrine and paracrine loops that may play important roles in tissue development and physiology.89 Genes can be delivered in vivo that (i) encode for the desired protein(s), or (ii) encode for transcription factors that induce expression of the desired protein(s). Relative to protein delivery, gene delivery can potentially provide protein expression for a longer period of time and at higher concentrations, target more cellular processes, and is not restricted to proteins that interact with cell-surface receptors. DNA delivery can also be used to express genes encoding for intracellular proteins, which could be used to control the fate of pluripotent cells. In general, the therapeutic efficacy of gene delivery for tissue engineering and other clinical applications is dependent upon the development of safe and efficient delivery systems.90
Table 3.
Scaffolds delivering DNA for tissue regeneration
| Material | Vectors/gene | Applications | Reference |
|---|---|---|---|
| Collagen | siRNA for TGFβR | Therapeutic inhibitory vector to block renal interstitial fibrosis | 173 |
| pPDGF | Wound healing | 174 | |
| hPTH | Bone regeneration | 88 | |
| pPDGF-A, -B | Wound healing | 174 | |
| Fibrin | hEGF | Wound healing | 175,176 |
| Chitosan-gelatin | pTGF-β1 | Cartilage regeneration | 177 |
| PLG | BMP-4 | Bone regeneration | 104,178 |
| VEGF | Angiogenesis | 87 | |
| PDGF | Granulation tissue formation and vascularization | 74 | |
| Ca2+-alginate microcapsules | TGF-β1 | Tissue engineered bioartificial cartilage growth | 179 |
From a pharmaceutical perspective, DNA may be preferred over protein delivery as the essential information is encoded in the linear sequence of bases, and not its three dimensional conformation. Since DNA has similar physical properties, independent of its linear sequence, multiple plasmids can easily be incorporated into a single delivery system. Finally, the quantity and duration of protein production can be manipulated using inducible promoters, or restricted to a specific tissue through tissue-specific promoters. As listed, delivery of DNA has several potential advantages over protein delivery that make it attractive for tissue engineering. As discussed in the previous section, however, these advantages are dependent upon finding delivery systems that provide efficient gene transfer and transgene expression.
Immobilization vs. release
The efficacy of protein and DNA delivery systems depends substantially on the mechanism of delivery. The traditional approach has been to introduce growth factors or DNA as soluble factors into culture media in vitro, or by injection in vivo. Increasingly, however, the material and/or the factor are being engineered to provide specific interactions that mediate their release or retention in the scaffold, which can affect their function within the biological system. The following sections address the issue of the factors interacting with the scaffold and their biological consequences for controlled release and substrate immobilization of protein and DNA, respectively.
Protein
Receptor binding of growth factors that are free in solution, as opposed to immobilized to the matrix, may induce significantly different biological responses. Growth factors are routinely added to cultures in vitro, and have been incorporated and released from polymeric systems with retention of bioactivity, which is exemplified by neurotrophins,49 BMPs,84,91 and VEGF.92 In vivo, these soluble factors can be transported from the delivery site, where the design parameter for the delivery system is the duration over which therapeutic concentrations can be maintained. Alternatively, growth factor immobilization can occur through reversible association with the scaffold, irreversible binding to the polymer, or immobilized with release dependent upon degradation of a linking tether or the matrix itself. For growth factor immobilization to fibrin, cell migration results in cell-activated plasmin degradation that can catalyze release of the factor. These scaffolds have been termed “cell-responsive” due to release of the factor upon cellular demand. Once released, these soluble factors can bind their receptors and initiate a signaling cascade. Alternatively, immobilized growth factors can ligate their receptors directly from the material surface; however, this type of interaction may not exactly replicate signaling through the soluble factor, as growth factor internalization can stimulate signaling pathways separate from those activated at the surface.93,94 For example, NGF induces neurite outgrowth by signaling at the plasma membrane, yet promotes neuron survival when internalized.95–97 Surface immobilization has been successfully used to attach growth factors such as EGF,98 BMP-7,99 BMP-2,100 VEGF,92 NGF,77,79,101 and NT-378 to a variety of natural and synthetic biomaterials. Signaling by these immobilized or locally released growth factors may be more potent than signaling by soluble versions added directly to culture media.102 These studies also demonstrate that the immobilization strategy must consider protein structure and active region topology when designing suitable delivery systems in order to maximize growth factor bioactivity. Ultimately, some factors may be best delivered in a sustained manner, while others benefit from direct attachment to the biomaterial substrate.103
DNA
Gene delivery by polymeric release or substrate immobilization can result in substantially different transfection profiles, suggesting unique opportunities for each in various tissue engineering applications. Early attempts at using scaffolds as gene delivery devices focused on the polymeric release approach, while attempts at substrate immobilization have developed more recently. Relatively high concentrations of naked plasmid are required to induce transgene expression at levels capable of inducing new tissue formation.87,88 Polymeric release systems have the capacity to deliver large quantities of vector (mg quantities) with transgene expression correlating to the dose of DNA delivered and release occurring over a period of weeks to months.87,88 Non-viral vectors can also be used to deliver DNA, and may potentially reduce the amount of plasmid required to achieve appreciable transfection and tissue inductive effects.104 Relative to release approaches, surface immobilization prevents aggregation of DNA complexes and places the vector directly onto the substrate to which cells are adhered, mimicking the natural process used by some viruses.105 This approach has been used to efficiently transfect cells with significantly less vector than more conventional methods.106 One explanation for this increased efficiency is that by maintaining elevated concentrations of DNA directly in the cell microenvironment, significantly less DNA is required to drive transgene expression at levels comparable to release approaches. Likely owing to the low amount of DNA used, this expression is more transient, occurring for relatively short times (days to weeks). In fact, short-lived expression may be advantageous in some applications, such as initiating a cascade of events (e.g., osteoinduction in bone repair), or when prolonged expression may lead to undesirable effects. To date, substrate immobilization of DNA has not been extensively investigated in vivo, although continued development of this delivery strategy may increase the duration of transgene expression through manipulation of the DNA/biomaterial interaction.
Delivery systems: key issues and requirements
Exploiting the therapeutic potential of proteins requires knowledge of the most efficacious mode of delivery—an area of increasing activity. Delivery systems for tissue inductive factors must consider both the specific application and the requirements for efficacy. For example, systems developed for nerve regeneration in spinal cord injury will likely have a vastly different design compared to systems that promote angiogenesis in ischemic cardiac tissue. The following sections address specific issues associated with the design of delivery systems for tissue regeneration, though the design criteria will likely be further refined with ongoing studies.
Concentration and duration
The concentration and duration of function for tissue inductive factors at the regenerating tissue site are critical parameters involved in promoting developmental processes and the formation of mature tissues (Fig. 2A). Therapeutic angiogenesis and anti-angiogenesis illustrates these concepts, as immature vessels or vessels that regress over time can lead to unsuccessful or abnormal tissue formation.107 Gene therapy approaches have been employed to investigate the relationship between protein concentration and blood vessel formation. Sustained expression of low to medium levels of VEGF was required to promote the growth of blood vessels displaying normal morphological and functional characteristics.108 Cells transplanted that expressed low levels of VEGF avoided the formation of aberrant vessels and hemangiomas observed with cells expressing high levels of VEGF. The prolonged release of fibroblast growth factor-2 (FGF-2) from fibrin gels significantly increased the micro-vessel density relative to free injection of FGF-2.109 Similar concentration and dose effects have been observed with other tissues. For example, elevated concentration and prolonged release of TGF-β1 has been shown to affect chondrocyte function.110 Chondrocytes seeded onto polymeric matrices composed of microspheres with encapsulated TGF-β1 have enhanced glycosaminoglycan production and exhibit higher proliferation rates relative to control scaffolds. These results demonstrate that the concentration of tissue inductive factors must fall within a therapeutic range—lacking efficacy at one extreme while producing abnormal tissues at the other.
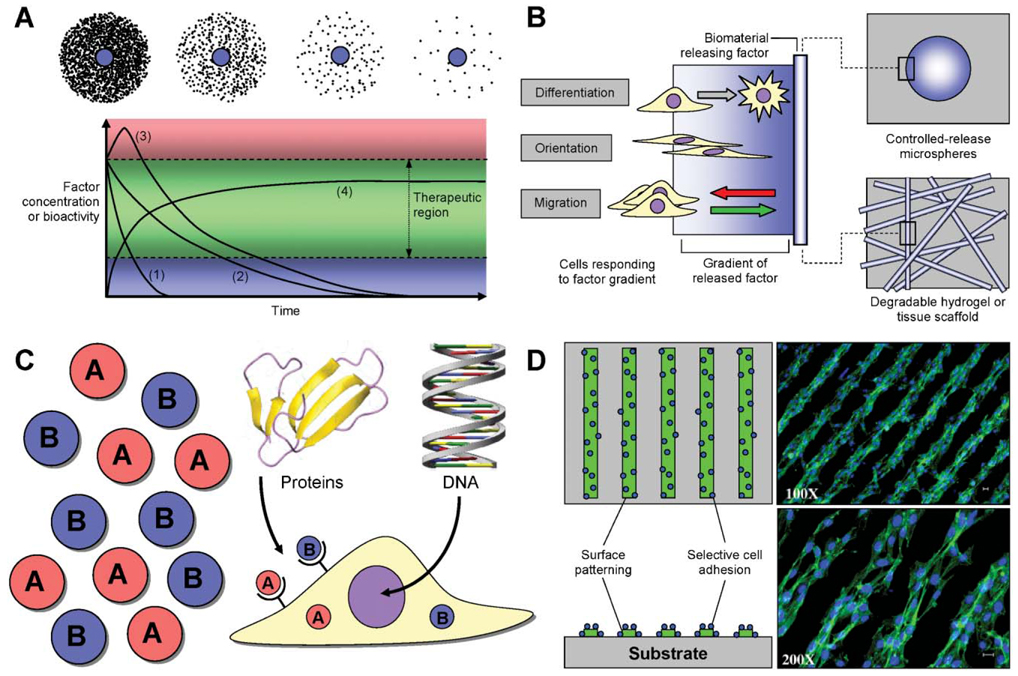
Fig. 2. Design parameters for controlled release systems.
(A) Local factor concentration (top) can be customized to provide for high to low levels of factor released in the vicinity of the delivery device (represented by blue circles). Release profiles (bottom) can be designed to allow for short- (1) or long-term (2) release that slowly decays over time, or for a burst release (3) that then decays. Alternatively, DNA delivery can result in long-term protein production (4). Tailoring the release profile based on the degradation and clearance rates of the local environment can sustain therapeutic protein levels. Importantly, excess factor may produce undesirable side effects (e.g., toxicity), whereas insufficient protein will not produce the desired effect. (B) Concentration gradients of soluble factors can induce a variety of cellular processes, including cellular differentiation, orientation and migration. Parts of (B) adapted from ref. 11. (C) Multiple factors may be delivered simultaneously with variable kinetics to take advantage of their synergistic effects. In the case of proteins (e.g., growth factors), factor effect may be mediated by receptor binding and subsequent cellular internalization. Alternatively, DNA must be internalized and successfully transported to the nucleus for expression. Protein graphic prepared using MOLMOL.180 DNA graphic used with permission. (D) Spatial patterning of factors on biomaterial substrates can lead to selective cell adhesion or orientation. Photomicrographs reprinted from ref. 30, Copyright 2005, with permission from Elsevier.
Concentration gradients
Diffusible factors exert their action not only by binding to cell surface receptors but also through the gradients established by their release (Fig. 2B). Adhesion peptides immobilized in a gradient can induce cellular alignment and greater cellular elongation than on homogeneous surfaces.111 Gradients in the concentration of growth factors can similarly direct cell migration, and may also function to create patterns of cellular differentiation among a progenitor cell population.11,112–114 Controlling the location of release can create concentration gradients by diffusion of the factors from the release site. Concentration gradients established in an agarose gel oriented neurite outgrowth by PC12 and dorsal root ganglion along the direction of the gradient.85 Immobilized gradients of NGF and FGF-2 have similarly been shown to direct neurite outgrowth and cell alignment.53,115 A heparin-modified fibrin gel capable of immobilizing NGF has been used to direct axonal extension in a sciatic nerve injury model.101 The ability to effectively create, control, and maintain concentration gradients in vitro and in vivo is still under development, and could provide a valuable tool to guide progenitor cell differentiation and organize tissue formation.
Multiple factor delivery
Tissue morphogenesis and regeneration are typically driven by the concomitant action of multiple factors, which can work synergistically on the same process, or can target different barriers to regeneration (Fig. 2C). The synergistic effect of growth factors has been reported for many developmental processes including angiogenesis,116 where mature blood vessels form by the combined action of VEGF and platelet-derived growth factor (PDGF) to form stable vessels. Although VEGF is able to initiate angiogenesis, PDGF promotes vessel maturation via recruitment of smooth muscle cells to the developing endothelium.117 PLG scaffolds releasing both VEGF and PDGF formed a mature vascular network within and around the scaffolds.83 Similarly, delivery of multiple growth factors to sites of bone injury was shown to dramatically enhance bone regeneration. Dual delivery of BMP-2 and TGF-β3 from a hydrogel promoted significant bone formation by co-transplanted BMSCs within 6 weeks of implantation.84 Interestingly, the synergistic activity of these two factors allowed them to be provided at a low dose, whereas supraphysiological concentrations of the individual factors resulted in negligible bone tissue formation. In addition to dual protein delivery, DNA and protein delivery can be combined to provide the necessary factors for tissue formation. Combined delivery of plasmid DNA encoding for BMP-4, VEGF protein, and human bone marrow stromal cells (hBMSCs) significantly enhanced bone formation relative to delivery of any single factor.86
Alternatively, multiple factor delivery can be employed to block or degrade inhibitory molecules, or to supply factors that actively stimulate cellular processes leading to regeneration. Spinal cord regeneration exemplifies this concept with the use of bridges that span the area of trauma, creating an environment in which glial scar formation does not inhibit axonal outgrowth, yet one in which neuron survival and axonal outgrowth are stimulated.118 Indeed, delivery of neurotrophic factors to promote neuron survival and axon outgrowth functions synergistically with delivery of chondroitinase, which degrades the chondroitin sulfate proteoglycans that comprise the glial scar.119 Taken together, these studies demonstrate that multi-factorial presentation of growth factors can be more effective at stimulating natural developmental processes leading to tissue formation. These examples represent only a few of the many opportunities to deliver multiple factors that function synergistically to promote regeneration.
Spatial patterning
Natural tissues develop as a result of complex temporal-spatial patterns in the expression of various cytokines, growth factors, and matrix molecules in the cellular microenvironment.120 Both ligands and receptors exhibit distinct expression profiles that correlate with a diverse range of developmental functions. Engineering functional structures such as nerves and blood vessels will depend on the ability to direct cells to organize into spatially complex arrangements on length scales ranging from micrometers to centimeters. While the technology is developing in this area of tissue engineering, a proposed set of design criteria could direct system development to guide the dynamic organization, maturation, and remodeling of cells, leading to the formation of mature and functional tissues.121
Until recently, the development of spatially patterned surfaces has focused primarily on the attachment of proteins to specifically promote or prevent cell adhesion. Surfaces patterned with cell adhesion peptides or extracellular matrix molecules can direct cell attachment, for applications such as nerve regeneration.55,122,123 New microfabrication techniques, however, such as three-dimensional printing, laser ablation and similar procedures are being developed and provide the means to create scaffolds with controllable feature size and pattern topography (Fig. 2D).21,22,124,125 These patterning strategies can be merged with various drug delivery technologies to specifically promote or prevent the adhesion of cells to defined areas of a substrate. In one study, DNA polyplexes were immobilized to a micropatterned hyaluronic acid-collagen hydrogel.105 Cells cultured on the hydrogel aligned along the ridges of the pattern, and were transfected. Thus, cellular transfection was spatially patterned on the scaffold through controlled cell adhesion.105,106 Spatial patterning combined with controlled protein and DNA delivery systems can be employed to create complex tissue structures, or to regenerate across tissue interfaces, such as the bone–cartilage interface. In addition to impacting tissue engineering, these technologies are also being applied to the design and fabrication of biosensors, cell-based biochips and neural networks.126–129
Conclusions
Clinical implementation of tissue engineering based therapies has produced a handful of FDA approved products, with others making their way down the pipeline.35 Successes with tissues such as skin, cartilage, and bladder35,130 have illustrated the potential for inducing cells to form into functional tissues using cell-seeded biomaterials combined with the appropriate combination of molecular cues. Protein and DNA delivery systems, using either controlled release or substrate immobilization, combined with existing and developing scaffold technologies, offers unique opportunities for promoting regeneration and/or repair of damaged or defective tissues. Importantly, the impact of inductive scaffolds on functional tissue engineering will also depend upon an improved understanding of the molecular cues necessary for directing essential cellular processes in tissue development.
Scaffolds capable of controlled protein delivery can also serve as model systems to molecularly dissect tissue formation. Knockout models have commonly been employed to examine the role of specific gene products in tissue development; however, tissue engineering scaffolds can readily manipulate multiple molecular cues and require significantly less time to produce than transgenic animals. Importantly, the presence of tissue inductive factors must be considered within the context that they are presented. Thus, properties such as the ECM composition, degradation, and scaffold mechanics must also be investigated. The ability to regulate the spatial and temporal presentation of these molecular cues within a three-dimensional context will be essential to recreate the environmental complexity that governs cellular organization in natural tissues. Tissue engineering scaffolds capable of protein and DNA delivery will serve as indispensable tools in investigating the biology underlying tissue formation, which may ultimately be translated into clinical therapies.
Acknowledgements
We apologize to all the scientists whose work we could not cite due to space restrictions. Support for this manuscript was provided by NIH (RO1 EB003806) and the Juvenile Diabetes Research Foundation (JDRF).
Biographies

David M. Salvay
David M. Salvay was born in 1978 in Tel-Aviv, Israel. He studied at Washington University in St. Louis, where he received a BA in Biochemistry and Molecular Biology, and a BS and MS in Biomedical Engineering in 2001. In addition to conducting undergraduate research at Washington University, he has trained in the Controlled Biological Systems Division of NASA and at the National Cancer Institute (NCI/NIH). He is currently in his fourth year of the Medical Scientist Training Program (MD/PhD) at Northwestern University in Chicago where he is pursuing a PhD in the Department of Chemical and Biological Engineering. His research interests include the use of tissue engineering scaffolds as inductive platforms for directing cell and tissue development.

Lonnie D. Shea
Lonnie D. Shea was born in 1969 in Michigan. He received a BS and MS degree in Chemical Engineering at Case Western Reserve University, and his PhD in Chemical Engineering and Scientific Computing at the University of Michigan in 1997. Postdoctoral research was also performed at the University of Michigan in the Department of Biologic and Materials Science in the Dental School. After his postdoctoral research, he joined the faculty at Northwestern University and is currently an Associate Professor in the Department of Chemical and Biological Engineering. His research interests are focused on combining gene and drug delivery with biomaterials to create controllable microenvironments for tissue engineering applications, and his laboratory is applying them to areas such as islet transplantation, ovarian follicle maturation, and nerve regeneration.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol. Rev. 2005;85(2):635–678. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- 3.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19(10):1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 4.Murphy WL, Mooney DJ. Controlled delivery of inductive proteins, plasmid DNA and cells from tissue engineering matrices. J. Periodontal Res. 1999;34(7):413–419. doi: 10.1111/j.1600-0765.1999.tb02275.x. [DOI] [PubMed] [Google Scholar]
- 5.Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J. Cell Sci. 2003;116(Pt 12):2377–2388. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 7.Kleinman HK, Martin GR. Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 2005;15(5):378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 1992;89(19):9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant DS, Tashiro K, Segui-Real B, Yamada Y, Martin GR, Kleinman HK. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- 10.Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transplant Immunol. 2004;12(3–4):367–377. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Saltzman WM, Olbricht WL. Building drug delivery into tissue engineering. Nat. Rev. Drug Discov. 2002;1(3):177–186. doi: 10.1038/nrd744. [DOI] [PubMed] [Google Scholar]
- 12.Lauffenburger DA, Griffith LG. Who’s got pull around here? Cell organization in development and tissue engineering. Proc. Natl. Acad. Sci. U. S. A. 2001;98(8):4282–4284. doi: 10.1073/pnas.081083698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hubbell JA. Materials as morphogenetic guides in tissue engineering. Curr. Opin. Biotechnol. 2003;14(5):551–558. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428(6982):487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 15.Rahaman MN, Mao JJ. Stem cell-based composite tissue constructs for regenerative medicine. Biotechnol. Bioeng. 2005;91(3):261–284. doi: 10.1002/bit.20292. [DOI] [PubMed] [Google Scholar]
- 16.Abbott A. Cell culture: biology’s new dimension. Nature. 2003;424(6951):870–872. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- 17.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Chen K, Yang ST. Effects of three-dimensional culturing on osteosarcoma cells grown in a fibrous matrix: analyses of cell morphology, cell cycle, and apoptosis. Biotechnol. Prog. 2003;19(5):1574–1582. doi: 10.1021/bp034024w. [DOI] [PubMed] [Google Scholar]
- 19.Edelman DB, Keefer EW. A cultural renaissance: in vitro cell biology embraces three-dimensional context. Exp. Neurol. 2005;192(1):1–6. doi: 10.1016/j.expneurol.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Lao J, Chen BP, Li YS, Zhao Y, Chu J, Chen KD, Tsou TC, Peck K, Chien S. Genomic analysis of smooth muscle cells in 3-dimensional collagen matrix. FASEB J. 2003;17(1):97–99. doi: 10.1096/fj.02-0256fje. [DOI] [PubMed] [Google Scholar]
- 21.Hollister SJ. Porous scaffold design for tissue engineering. Nat. Mater. 2005;4(7):518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 22.Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues—state of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001;12(1):107–124. doi: 10.1163/156856201744489. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003;21(10):1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S, Holmes TC, DiPersio CM, Hynes RO, Su X, Rich A. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials. 1995;16(18):1385–1393. doi: 10.1016/0142-9612(95)96874-y. [DOI] [PubMed] [Google Scholar]
- 25.Arnold MS, Guler MO, Hersam MC, Stupp SI. Encapsulation of carbon nanotubes by self-assembling peptide amphiphiles. Langmuir. 2005;21(10):4705–4709. doi: 10.1021/la0469452. [DOI] [PubMed] [Google Scholar]
- 26.Ruoslahti E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez AL, Gobin AS, West JL, McIntire LV, Smith CW. Integrin interactions with immobilized peptides in polyethylene glycol diacrylate hydrogels. Tissue Eng. 2004;10(11–12):1775–1786. doi: 10.1089/ten.2004.10.1775. [DOI] [PubMed] [Google Scholar]
- 28.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24(24):4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 29.Anseth KS, Bowman CN, Brannon-Peppas L. Mechanical properties of hydrogels and their experimental determination. Biomaterials. 1996;17(17):1647–1657. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- 30.Segura T, Anderson BC, Chung PH, Webber RE, Shull KR, Shea LD. Crosslinked hyaluronic acid hydrogels: a strategy to functionalize and pattern. Biomaterials. 2005;26(4):359–371. doi: 10.1016/j.biomaterials.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 31.Hartgerink JD, Beniash E, Stupp SI. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc. Natl. Acad. Sci. U. S. A. 2002;99(8):5133–5138. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. U. S. A. 2003;100(9):5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binzen E, Lendlein A, Kelch S, Rickert D, Franke RP. Biomaterial-microvasculature interaction on polymers after implantation in mice. Clin. Hemorheol. Microcirc. 2004;30(3–4):283–288. [PubMed] [Google Scholar]
- 34.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11(1–2):1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 35.Lysaght MJ, Hazlehurst AL. Tissue engineering: the end of the beginning. Tissue Eng. 2004;10(1–2):309–320. doi: 10.1089/107632704322791943. [DOI] [PubMed] [Google Scholar]
- 36.Zacchi V, Soranzo C, Cortivo R, Radice M, Brun P, Abatangelo G. In vitro engineering of human skin-like tissue. J. Biomed. Mater. Res. 1998;40(2):187–194. doi: 10.1002/(sici)1097-4636(199805)40:2<187::aid-jbm3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 37.Ma PX, Langer R. Morphology and mechanical function of long-term in vitro engineered cartilage. J. Biomed. Mater. Res. 1999;44(2):217–221. doi: 10.1002/(sici)1097-4636(199902)44:2<217::aid-jbm12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Elisseeff J. Injectable cartilage tissue engineering. Expert Opin. Biol. Ther. 2004;4(12):1849–1859. doi: 10.1517/14712598.4.12.1849. [DOI] [PubMed] [Google Scholar]
- 39.Boontheekul T, Mooney DJ. Protein-based signaling systems in tissue engineering. Curr. Opin. Biotechnol. 2003;14(5):559–565. doi: 10.1016/j.copbio.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004;32(3):477–486. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 41.Kaufmann PM, Heimrath S, Kim BS, Mooney DJ. Highly porous polymer matrices as a three-dimensional culture system for hepatocytes. Cell Transplant. 1997;6(5):463–468. doi: 10.1177/096368979700600505. [DOI] [PubMed] [Google Scholar]
- 42.Ratner BD, Hoffman AS, Schoen FJ, Lemons JE. Biomaterials science : An introduction to materials in medicine. 2nd edn. Amsterdam, Boston: Elsevier Academic Press; 2004. [Google Scholar]
- 43.Albert SG, Mooradian AD. Low-dose recombinant human growth hormone as adjuvant therapy to lifestyle modifications in the management of obesity. J. Clin. Endocrinol. Metab. 2004;89(2):695–701. doi: 10.1210/jc.2003-031264. [DOI] [PubMed] [Google Scholar]
- 44.Franco C, Brandberg J, Lonn L, Andersson B, Bengtsson BA, Johannsson G. Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J. Clin. Endocrinol. Metab. 2005;90(3):1466–1474. doi: 10.1210/jc.2004-1657. [DOI] [PubMed] [Google Scholar]
- 45.Wong SF. Cetuximab: an epidermal growth factor receptor monoclonal antibody for the treatment of colorectal cancer. Clin. Ther. 2005;27(6):684–694. doi: 10.1016/j.clinthera.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Jaquet P, Gunz G, Saveanu A, Dufour H, Taylor J, Dong J, Kim S, Moreau JP, Enjalbert A, Culler MD. Efficacy of chimeric molecules directed towards multiple somatostatin and dopamine receptors on inhibition of GH and prolactin secretion from GH-secreting pituitary adenomas classified as partially responsive to somatostatin analog therapy. Eur. J. Endocrinol. 2005;153(1):135–141. doi: 10.1530/eje.1.01950. [DOI] [PubMed] [Google Scholar]
- 47.Lanes R. Long-term outcome of growth hormone therapy in children and adolescents. Treat. Endocrinol. 2004;3(1):53–66. doi: 10.2165/00024677-200403010-00006. [DOI] [PubMed] [Google Scholar]
- 48.Ranke MB. Insulin-like growth factor-I treatment of growth disorders, diabetes mellitus and insulin resistance. Trends Endocrinol. Metab. 2005;16(4):190–197. doi: 10.1016/j.tem.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Whittlesey KJ, Shea LD. Delivery systems for small molecule drugs, proteins, and DNA: the neuroscience/biomaterial interface. Exp. Neurol. 2004;190(1):1–16. doi: 10.1016/j.expneurol.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 50.Langer R. Drug delivery and targeting. Nature. 1998;392(6679 Suppl):5–10. [PubMed] [Google Scholar]
- 51.Pannier AK, Shea LD. Controlled release systems for DNA delivery. Mol. Ther. 2004;10(1):19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 52.Bryant SJ, Anseth KS. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J. Biomed. Mater. Res. A. 2003;64(1):70–79. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 53.Kapur TA, Shoichet MS. Immobilized concentration gradients of nerve growth factor guide neurite outgrowth. J. Biomed. Mater. Res. A. 2004;68(2):235–243. doi: 10.1002/jbm.a.10168. [DOI] [PubMed] [Google Scholar]
- 54.Ito Y, Hayashi M, Imanishi Y. Gradient micropattern immobilization of heparin and its interaction with cells. J. Biomater. Sci. Polym. Ed. 2001;12(4):367–378. doi: 10.1163/156856201750195270. [DOI] [PubMed] [Google Scholar]
- 55.Schmalenberg KE, Uhrich KE. Micropatterned polymer substrates control alignment of proliferating Schwann cells to direct neuronal regeneration. Biomaterials. 2005;26(12):1423–1430. doi: 10.1016/j.biomaterials.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 56.Putney SD, Burke PA. Improving protein therapeutics with sustained-release formulations. Nat. Biotechnol. 1998;16(2):153–157. doi: 10.1038/nbt0298-153. [DOI] [PubMed] [Google Scholar]
- 57.De Laporte L, Cruz Rea J, Shea LD. Design of modular non-viral gene therapy vectors. Biomaterials. 2005 doi: 10.1016/j.biomaterials.2005.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kootstra NA, Verma IM. Gene therapy with viral vectors. Annu. Rev. Pharmacol. Toxicol. 2003;43:413–439. doi: 10.1146/annurev.pharmtox.43.100901.140257. [DOI] [PubMed] [Google Scholar]
- 59.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 60.Roth CM, Sundaram S. Engineering synthetic vectors for improved DNA delivery: insights from intracellular pathways. Annu. Rev. Biomed. Eng. 2004;6:397–426. doi: 10.1146/annurev.bioeng.6.040803.140203. [DOI] [PubMed] [Google Scholar]
- 61.Davis KA, Anseth KS. Controlled release from crosslinked degradable networks. Crit. Rev. Ther. Drug Carrier Syst. 2002;19(4–5):385–423. doi: 10.1615/critrevtherdrugcarriersyst.v19.i45.30. [DOI] [PubMed] [Google Scholar]
- 62.Burdick JA, Mason MN, Hinman AD, Thorne K, Anseth KS. Delivery of osteoinductive growth factors from degradable PEG hydrogels influences osteoblast differentiation and mineralization. J. Controlled Release. 2002;83(1):53–63. doi: 10.1016/s0168-3659(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 63.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408(6815):998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 64.Saltzman WM, Mak MW, Mahoney MJ, Duenas ET, Cleland JL. Intracranial delivery of recombinant nerve growth factor: release kinetics and protein distribution for three delivery systems. Pharm. Res. 1999;16(2):232–240. doi: 10.1023/a:1018824324275. [DOI] [PubMed] [Google Scholar]
- 65.Fine EG, Decosterd I, Papaloizos M, Zurn AD, Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur. J. Neurosci. 2002;15(4):589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 66.Varde NK, Pack DW. Microspheres for controlled release drug delivery. Expert Opin. Biol. Ther. 2004;4(1):35–51. doi: 10.1517/14712598.4.1.35. [DOI] [PubMed] [Google Scholar]
- 67.Hu Y, Hollinger JO, Marra KG. Controlled release from coated polymer microparticles embedded in tissue-engineered scaffolds. J. Drug Target. 2001;9(6):431–438. doi: 10.3109/10611860108998777. [DOI] [PubMed] [Google Scholar]
- 68.Jang JH, Shea LD. Controllable delivery of non-viral DNA from porous scaffolds. J. Controlled Release. 2003;86(1):157–168. doi: 10.1016/s0168-3659(02)00369-3. [DOI] [PubMed] [Google Scholar]
- 69.Mercier NR, Costantino HR, Tracy MA, Bonassar LJ. Poly(lactide-co-glycolide) microspheres as a moldable scaffold for cartilage tissue engineering. Biomaterials. 2005;26(14):1945–1952. doi: 10.1016/j.biomaterials.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 70.Meilander NJ, Pasumarthy MK, Kowalczyk TH, Cooper MJ, Bellamkonda RV. Sustained release of plasmid DNA using lipid microtubules and agarose hydrogel. J. Controlled Release. 2003;88(2):321–331. doi: 10.1016/s0168-3659(03)00007-5. [DOI] [PubMed] [Google Scholar]
- 71.Xu X, Yu H, Gao S, Ma HQ, Leong KW, Wang S. Polyphosphoester microspheres for sustained release of biologically active nerve growth factor. Biomaterials. 2002;23(17):3765–3772. doi: 10.1016/s0142-9612(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 72.Leach JB, Schmidt CE. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials. 2005;26(2):125–135. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 73.Bloch J, Fine EG, Bouche N, Zurn AD, Aebischer P. Nerve growth factor- and neurotrophin-3-releasing guidance channels promote regeneration of the transected rat dorsal root. Exp. Neurol. 2001;172(2):425–432. doi: 10.1006/exnr.2001.7778. [DOI] [PubMed] [Google Scholar]
- 74.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat. Biotechnol. 1999;17(6):551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 75.Sheridan MH, Shea LD, Peters MC, Mooney DJ. Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J. Controlled Release. 2000;64(1–3):91–102. doi: 10.1016/s0168-3659(99)00138-8. [DOI] [PubMed] [Google Scholar]
- 76.Yang Y, De Laporte L, Rives CB, Jang JH, Lin WC, Shull KR, Shea LD. Neurotrophin releasing single and multiple lumen nerve conduits. J. Controlled Release. 2005;104(3):433–446. doi: 10.1016/j.jconrel.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J. Controlled Release. 2000;69(1):149–158. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 78.Taylor SJ, McDonald JW. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J. Controlled Release. 2004;98(2):281–294. doi: 10.1016/j.jconrel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 79.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J. Controlled Release. 2000;65(3):389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 80.Riedel GE, Valentin-Opran A. Clinical evaluation of rhBMP-2/ACS in orthopedic trauma: a progress report. Orthopedics. 1999;22(7):663–665. [PubMed] [Google Scholar]
- 81.Starr AJ. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures. J. Bone Joint Surg. Am. 2003;85-A(10) doi: 10.2106/00004623-200310000-00027. 2049 author replies 2049–2050. [DOI] [PubMed] [Google Scholar]
- 82.Groeneveld EH, Burger EH. Bone morphogenetic proteins in human bone regeneration. Eur. J. Endocrinol. 2000;142(1):9–21. doi: 10.1530/eje.0.1420009. [DOI] [PubMed] [Google Scholar]
- 83.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 84.Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35(2):562–569. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 85.Cao X, Shoichet MS. Defining the concentration gradient of nerve growth factor for guided neurite outgrowth. Neuroscience. 2001;103(3):831–840. doi: 10.1016/s0306-4522(01)00029-x. [DOI] [PubMed] [Google Scholar]
- 86.Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J. Bone Miner. Res. 2005;20(5):848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 87.Jang JH, Rives CB, Shea LD. Plasmid Delivery in Vivo from Porous Tissue-Engineering Scaffolds: Transgene Expression and Cellular Transfection. Mol. Ther. 2005 doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat. Med. 1999;5(7):753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 89.Jang JH, Houchin TL, Shea LD. Gene delivery from polymer scaffolds for tissue engineering. Exp. Rev. Med. Dev. 2004;1(1):127–138. doi: 10.1586/17434440.1.1.127. [DOI] [PubMed] [Google Scholar]
- 90.Wolff JA, Budker V. The mechanism of naked DNA uptake and expression. Adv. Genet. 2005;54:3–20. doi: 10.1016/S0065-2660(05)54001-X. [DOI] [PubMed] [Google Scholar]
- 91.Kroese-Deutman HC, Ruhe PQ, Spauwen PH, Jansen JA. Bone inductive properties of rhBMP-2 loaded porous calcium phosphate cement implants inserted at an ectopic site in rabbits. Biomaterials. 2005;26(10):1131–1138. doi: 10.1016/j.biomaterials.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 92.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmokel H, Bezuidenhout D, Djonov V, Zilla P, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17(15):2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 93.Haugh JM, Schooler K, Wells A, Wiley HS, Lauffenburger DA. Effect of epidermal growth factor receptor internalization on regulation of the phospholipase C-gamma1 signaling pathway. J. Biol. Chem. 1999;274(13):8958–8965. doi: 10.1074/jbc.274.13.8958. [DOI] [PubMed] [Google Scholar]
- 94.Wiedlocha A, Sorensen V. Signaling, internalization, and intracellular activity of fibroblast growth factor. Curr. Top. Microbiol. Immunol. 2004;286:45–79. doi: 10.1007/978-3-540-69494-6_3. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Moheban DB, Conway BR, Bhattacharyya A, Segal RA. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J. Neurosci. 2000;20(15):5671–5678. doi: 10.1523/JNEUROSCI.20-15-05671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ye H, Kuruvilla R, Zweifel LS, Ginty DD. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron. 2003;39(1):57–68. doi: 10.1016/s0896-6273(03)00266-6. [DOI] [PubMed] [Google Scholar]
- 97.Bronfman FC, Tcherpakov M, Jovin TM, Fainzilber M. Ligand-induced internalization of the p75 neurotrophin receptor: a slow route to the signaling endosome. J. Neurosci. 2003;23(8):3209–3220. doi: 10.1523/JNEUROSCI.23-08-03209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuhl PR, Griffith-Cima LG. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nat. Med. 1996;2(9):1022–1027. doi: 10.1038/nm0996-1022. [DOI] [PubMed] [Google Scholar]
- 99.Kirkwood K, Rheude B, Kim YJ, White K, Dee KKC. In vitro mineralization studies with substrate-immobilized bone morphogenetic protein peptides. J. Oral Implantol. 2003;29(2):57–65. doi: 10.1563/1548-1336(2003)029<0057:IVMSWS>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 100.Karageorgiou V, Meinel L, Hofmann S, Malhotra A, Volloch V, Kaplan D. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J. Biomed. Mater. Res. A. 2004;71(3):528–537. doi: 10.1002/jbm.a.30186. [DOI] [PubMed] [Google Scholar]
- 101.Lee AC, Yu VM, Lowe JB. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp. Neurol. 2003;184(1):295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 102.Seliktar D, Zisch AH, Lutolf MP, Wrana JL, Hubbell JA. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J. Biomed. Mater. Res. A. 2004;68(4):704–716. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- 103.Dinbergs ID, Brown L, Edelman ER. Cellular response to transforming growth factor-beta1 and basic fibroblast growth factor depends on release kinetics and extracellular matrix interactions. J. Biol. Chem. 1996;271(47):29822–29829. doi: 10.1074/jbc.271.47.29822. [DOI] [PubMed] [Google Scholar]
- 104.Huang YC, Riddle K, Rice KG, Mooney DJ. Long-term in vivo gene expression via delivery of PEI-DNA condensates from porous polymer scaffolds. Hum. Gene Ther. 2005;16(5):609–617. doi: 10.1089/hum.2005.16.609. [DOI] [PubMed] [Google Scholar]
- 105.Segura T, Chung PH, Shea LD. DNA delivery from hyaluronic acid-collagen hydrogels via a substrate-mediated approach. Biomaterials. 2005;26(13):1575–1584. doi: 10.1016/j.biomaterials.2004.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bengali Z, Pannier AK, Segura T, Anderson BC, Jang JH, Mustoe TA, Shea LD. Gene delivery through cell culture substrate adsorbed DNA complexes. Biotechnol. Bioeng. 2005;90(3):290–302. doi: 10.1002/bit.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 108.Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, McDonald DM, Blau HM. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J. Clin. Invest. 2004;113(4):516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jeon O, Ryu SH, Chung JH, Kim BS. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J. Controlled Release. 2005;105(3):249–259. doi: 10.1016/j.jconrel.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 110.Lee JE, Kim KE, Kwon IC, Ahn HJ, Lee SH, Cho H, Kim HJ, Seong SC, Lee MC. Effects of the controlled-released TGF-beta 1 from chitosan microspheres on chondrocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold. Biomaterials. 2004;25(18):4163–4173. doi: 10.1016/j.biomaterials.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 111.Kang CE, Gemeinhart EJ, Gemeinhart RA. Cellular alignment by grafted adhesion peptide surface density gradients. J. Biomed. Mater. Res. A. 2004;71(3):403–411. doi: 10.1002/jbm.a.30137. [DOI] [PubMed] [Google Scholar]
- 112.Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87(4):661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- 113.Gurdon JB, Harger P, Mitchell PA, Lemaire P. Activin signalling and response to a morphogen gradient. Nature. 1994;371(6497):487–492. doi: 10.1038/371487a0. [DOI] [PubMed] [Google Scholar]
- 114.Tanabe Y, Jessell TM. Diversity and pattern in the developing spinal cord. Science. 1996;274(5290):1115–1123. doi: 10.1126/science.274.5290.1115. [DOI] [PubMed] [Google Scholar]
- 115.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26(16):3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 116.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat. Med. 2003;9(5):604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 117.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta(+) perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat. Cell Biol. 2005 doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Geller HM, Fawcett JW. Building a bridge: engineering spinal cord repair. Exp. Neurol. 2002;174(2):125–136. doi: 10.1006/exnr.2002.7865. [DOI] [PubMed] [Google Scholar]
- 119.Tropea D, Caleo M, Maffei L. Synergistic effects of brain-derived neurotrophic factor and chondroitinase ABC on retinal fiber sprouting after denervation of the superior colliculus in adult rats. J. Neurosci. 2003;23(18):7034–7044. doi: 10.1523/JNEUROSCI.23-18-07034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carlson BM. Human embryology & developmental biology. 2nd edn. St. Louis: Mosby; 1999. [Google Scholar]
- 121.Nishimura I, Garrell RL, Hedrick M, Iida K, Osher S, Wu B. Precursor tissue analogs as a tissue-engineering strategy. Tissue Eng. 2003;9:S77–S89. doi: 10.1089/10763270360696996. [DOI] [PubMed] [Google Scholar]
- 122.Ranieri JP, Bellamkonda R, Bekos EJ, Gardella JA, Jr, Mathieu HJ, Ruiz L, Aebischer P. Spatial control of neuronal cell attachment and differentiation on covalently patterned laminin oligopeptide substrates. Int. J. Dev. Neurosci. 1994;12(8):725–735. doi: 10.1016/0736-5748(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 123.Barrett EW, Phelps MV, Silva RJ, Gaumond RP, Allcock HR. Patterning poly(organophosphazenes) for selective cell adhesion applications. Biomacromolecules. 2005;6(3):1689–1697. doi: 10.1021/bm049193z. [DOI] [PubMed] [Google Scholar]
- 124.Iwanaga S, Akiyama Y, Kikuchi A, Yamato M, Sakai K, Okano T. Fabrication of a cell array on ultrathin hydrophilic polymer gels utilising electron beam irradiation and UV excimer laser ablation. Biomaterials. 2005;26(26):5395–5404. doi: 10.1016/j.biomaterials.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 125.Rosoff WJ, McAllister R, Esrick MA, Goodhill GJ, Urbach JS. Generating controlled molecular gradients in 3D gels. Biotechnol. Bioeng. 2005 doi: 10.1002/bit.20564. [DOI] [PubMed] [Google Scholar]
- 126.Folch A, Jo BH, Hurtado O, Beebe DJ, Toner M. Microfabricated elastomeric stencils for micropatterning cell cultures. J. Biomed. Mater. Res. 2000;52(2):346–353. doi: 10.1002/1097-4636(200011)52:2<346::aid-jbm14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 127.Kim C, Burrows PE, Forrest SR. Micropatterning of organic electronic devices by cold-welding. Science. 2000;288(5467):831–833. doi: 10.1126/science.288.5467.831. [DOI] [PubMed] [Google Scholar]
- 128.Liu QY, Coulombe M, Dumm J, Shaffer KM, Schaffner AE, Barker JL, Pancrazio JJ, Stenger DA, Ma W. Synaptic connectivity in hippocampal neuronal networks cultured on micropatterned surfaces. Brain Res. Dev. Brain Res. 2000;120(2):223–231. doi: 10.1016/s0165-3806(00)00014-6. [DOI] [PubMed] [Google Scholar]
- 129.Liu XH, Wang HK, Herron JN, Prestwich GD. Photopatterning of antibodies on biosensors. Bioconjugate Chem. 2000;11(6):755–761. doi: 10.1021/bc000006d. [DOI] [PubMed] [Google Scholar]
- 130.Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat. Biotechnol. 1999;17(2):149–155. doi: 10.1038/6146. [DOI] [PubMed] [Google Scholar]
- 131.Camarata PJ, Suryanarayanan R, Turner DA, Parker RG, Ebner TJ. Sustained release of nerve growth factor from biodegradable polymer microspheres. Neurosurgery. 1992;30(3):313–319. doi: 10.1227/00006123-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 132.Cao X, Schoichet MS. Delivering neuroactive molecules from biodegradable microspheres for application in central nervous system disorders. Biomaterials. 1999;20(4):329–339. doi: 10.1016/s0142-9612(98)00172-0. [DOI] [PubMed] [Google Scholar]
- 133.Hoffman D, Wahlberg L, Aebischer P. NGF released from a polymer matrix prevents loss of ChAT expression in basal forebrain neurons following a fimbria-fornix lesion. Exp. Neurol. 1990;110(1):39–44. doi: 10.1016/0014-4886(90)90049-x. [DOI] [PubMed] [Google Scholar]
- 134.Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA, O’Driscoll SW. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage. 2003;11(1):55–64. doi: 10.1053/joca.2002.0869. [DOI] [PubMed] [Google Scholar]
- 135.LeBaron RG, Athanasiou KA. Ex vivo synthesis of articular cartilage. Biomaterials. 2000;21(24):2575–2587. doi: 10.1016/s0142-9612(00)00125-3. [DOI] [PubMed] [Google Scholar]
- 136.Morales TI. The role and content of endogenous insulin-like growth factor-binding proteins in bovine articular cartilage. Arch. Biochem. Biophys. 1997;343(2):164–172. doi: 10.1006/abbi.1997.0166. [DOI] [PubMed] [Google Scholar]
- 137.Silver FH, Glasgold AI. Cartilage wound healing. An overview. Otolaryngol. Clin. North Am. 1995;28(5):847–864. [PubMed] [Google Scholar]
- 138.Prelle K, Wobus AM, Krebs O, Blum WF, Wolf E. Overexpression of insulin-like growth factor-II in mouse embryonic stem cells promotes myogenic differentiation. Biochem. Biophys. Res. Commun. 2000;277(3):631–638. doi: 10.1006/bbrc.2000.3737. [DOI] [PubMed] [Google Scholar]
- 139.Kanematsu A, Yamamoto S, Ozeki M, Noguchi T, Kanatani I, Ogawa O, Tabata Y. Collagenous matrices as release carriers of exogenous growth factors. Biomaterials. 2004;25(18):4513–4520. doi: 10.1016/j.biomaterials.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 140.Royce SM, Askari M, Marra KG. Incorporation of polymer microspheres within fibrin scaffolds for the controlled delivery of FGF-1. J. Biomater. Sci. Polym. Ed. 2004;15(10):1327–1336. doi: 10.1163/1568562041960016. [DOI] [PubMed] [Google Scholar]
- 141.Xue L, Tassiopoulos AK, Woloson SK, Stanton DL, Jr, Ms CS, Hampton B, Burgess WH, Greisler HP. Construction and biological characterization of an HB-GAM/FGF-1 chimera for vascular tissue engineering. J. Vasc. Surg. 2001;33(3):554–560. doi: 10.1067/mva.2001.112229. [DOI] [PubMed] [Google Scholar]
- 142.Isogai N, Morotomi T, Hayakawa S, Munakata H, Tabata Y, Ikada Y, Kamiishi H. Combined chondrocyte-copolymer implantation with slow release of basic fibroblast growth factor for tissue engineering an auricular cartilage construct. J. Biomed. Mater. Res. A. 2005;74(3):408–418. doi: 10.1002/jbm.a.30343. [DOI] [PubMed] [Google Scholar]
- 143.Shen B, Duan H, Chen J, Yang J, Pei F. Preparation of controlled release microsphere incorporating bFGF and its effect on Schwann cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2005;22(4):719–724. [PubMed] [Google Scholar]
- 144.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc. Pathol. 2003;12(6):295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 145.Perets A, Baruch Y, Weisbuch F, Shoshany G, Neufeld G, Cohen S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J. Biomed. Mater. Res. A. 2003;65(4):489–497. doi: 10.1002/jbm.a.10542. [DOI] [PubMed] [Google Scholar]
- 146.Fressinaud C. Repeated injuries dramatically affect cells of the oligodendrocyte lineage: effects of PDGF and NT-3 in vitro. Glia. 2005;49(4):555–566. doi: 10.1002/glia.20136. [DOI] [PubMed] [Google Scholar]
- 147.Lee JA, Conejero JA, Mason JM, Parrett BM, Wear-Maggitti KD, Grant RT, Breitbart AS. Lentiviral transfection with the PDGF-B gene improves diabetic wound healing. Plast. Reconstr. Surg. 2005;116(2):532–538. doi: 10.1097/01.prs.0000172892.78964.49. [DOI] [PubMed] [Google Scholar]
- 148.Fiedler J, Etzel N, Brenner RE. To go or not to go: Migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. J. Cell Biochem. 2004;93(5):990–998. doi: 10.1002/jcb.20219. [DOI] [PubMed] [Google Scholar]
- 149.Vartanian KB, Chen HY, Kennedy J, Beck SK, Ryaby JT, Wang H, Hoying JB. The non-proteolytically active thrombin peptide TP508 stimulates angiogenic sprouting. J. Cell Physiol. 2005 doi: 10.1002/jcp.20442. [DOI] [PubMed] [Google Scholar]
- 150.Wang H, Li X, Tomin E, Doty SB, Lane JM, Carney DH, Ryaby JT. Thrombin peptide (TP508) promotes fracture repair by up-regulating inflammatory mediators, early growth factors, and increasing angiogenesis. J. Orthop. Res. 2005;23(3):671–679. doi: 10.1016/j.orthres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 151.Szpalski M, Gunzburg R. Recombinant human bone morphogenetic protein-2: a novel osteoinductive alternative to autogenous bone graft? Acta Orthop. Belg. 2005;71(2):133–148. [PubMed] [Google Scholar]
- 152.Chen F, Wu Z, Wang Q, Wu H, Zhang Y, Nie X, Jin Y. Preparation and Biological Characteristics of Recombinant Human Bone Morphogenetic Protein-2-Loaded Dextran-co-Gelatin Hydrogel Microspheres, in vitro and in vivo Studies. Pharmacology. 2005;75(3):133–144. doi: 10.1159/000088212. [DOI] [PubMed] [Google Scholar]
- 153.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J. Controlled Release. 2005;101(1–3):111–125. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 154.Morales TI. Transforming growth factor-beta 1 stimulates synthesis of proteoglycan aggregates in calf articular cartilage organ cultures. Arch. Biochem. Biophys. 1991;286(1):99–106. doi: 10.1016/0003-9861(91)90013-9. [DOI] [PubMed] [Google Scholar]
- 155.Rosier RN, O’Keefe RJ, Crabb ID, Puzas JE. Transforming growth factor beta: an autocrine regulator of chondrocytes. Connect. Tissue Res. 1989;20(1–4):295–301. doi: 10.3109/03008208909023900. [DOI] [PubMed] [Google Scholar]
- 156.Seyedin SM, Rosen DM, Segarini PR. Modulation of chondroblast phenotype by transforming growth factor-beta. Pathol Immunopathol Res. 1988;7(1–2):38–42. doi: 10.1159/000157090. [DOI] [PubMed] [Google Scholar]
- 157.Widenfalk J, Lipson A, Jubran M, Hofstetter C, Ebendal T, Cao Y, Olson L. Vascular endothelial growth factor improves functional outcome and decreases secondary degeneration in experimental spinal cord contusion injury. Neuroscience. 2003;120(4):951–960. doi: 10.1016/s0306-4522(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 158.Eckardt H, Bundgaard KG, Christensen KS, Lind M, Hansen ES, Hvid I. Effects of locally applied vascular endothelial growth factor (VEGF) and VEGF-inhibitor to the rabbit tibia during distraction osteogenesis. J. Orthop. Res. 2003;21(2):335–340. doi: 10.1016/S0736-0266(02)00159-6. [DOI] [PubMed] [Google Scholar]
- 159.Elcin YM, Dixit V, Gitnick G. Extensive in vivo angiogenesis following controlled release of human vascular endothelial cell growth factor: implications for tissue engineering and wound healing. Artif. Organs. 2001;25(7):558–565. doi: 10.1046/j.1525-1594.2001.025007558.x. [DOI] [PubMed] [Google Scholar]
- 160.Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, Van Damme P, Rutten B, Man WY, De Mol M, Wyns S, Manka D, Vermeulen K, Van Den Bosch L, Mertens N, Schmitz C, Robbererecht W, Conway EM, Collen D, Moons L, Carmeliet P. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat. Neurosci. 2005;8(1):85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 161.Cote MF, Laroche G, Gagnon E, Chevallier P, Doillon CJ. Denatured collagen as support for a FGF-2 delivery system: physicochemical characterizations and in vitro release kinetics and bioactivity. Biomaterials. 2004;25(17):3761–3772. doi: 10.1016/j.biomaterials.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 162.Hadlock T, Sundback C, Koka R, Hunter D, Cheney M, Vacanti J. A novel, biodegradable polymer conduit delivers neurotrophins and promotes nerve regeneration. Laryngoscope. 1999;109(9):1412–1416. doi: 10.1097/00005537-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 163.Menei P, Daniel V, Montero-Menei C, Brouillard M, Pouplard-Barthelaix A, Benoit JP. Biodegradation and brain tissue reaction to poly(D,L-lactide-co-glycolide) microspheres. Biomaterials. 1993;14(6):470–478. doi: 10.1016/0142-9612(93)90151-q. [DOI] [PubMed] [Google Scholar]
- 164.Pean JM, Boury F, Venier-Julienne MC, Menei P, Proust JE, Benoit JP. Why does PEG 400 co-encapsulation improve NGF stability and release from PLGA biodegradable microspheres? Pharm. Res. 1999;16(8):1294–1299. doi: 10.1023/a:1014818118224. [DOI] [PubMed] [Google Scholar]
- 165.Pean JM, Venier-Julienne MC, Boury F, Menei P, Denizot B, Benoit JP. NGF release from poly(D,L-lactide-co-glycolide) microspheres. Effect of some formulation parameters on encapsulated NGF stability. J. Controlled Release. 1998;56(1–3):175–187. doi: 10.1016/s0168-3659(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 166.Cleland JL, Duenas ET, Park A, Daugherty A, Kahn J, Kowalski J, Cuthbertson A. Development of poly-(D,L-lactide-co-glycolide) microsphere formulations containing recombinant human vascular endothelial growth factor to promote local angiogenesis. J. Controlled Release. 2001;72(1–3):13–24. doi: 10.1016/s0168-3659(01)00258-9. [DOI] [PubMed] [Google Scholar]
- 167.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21(24):2521–2527. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 168.Aebischer P, Salessiotis AN, Winn SR. Basic fibroblast growth factor released from synthetic guidance channels facilitates peripheral nerve regeneration across long nerve gaps. J. Neurosci. Res. 1989;23(3):282–289. doi: 10.1002/jnr.490230306. [DOI] [PubMed] [Google Scholar]
- 169.Mann BK, Schmedlen RH, West JL. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22(5):439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 170.Rai B, Teoh SH, Hutmacher DW, Cao T, Ho KH. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials. 2005;26(17):3739–3748. doi: 10.1016/j.biomaterials.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 171.Hu Y, Zhang C, Zhang S, Xiong Z, Xu J. Development of a porous poly(L-lactic acid)/hydroxyapatite/collagen scaffold as a BMP delivery system and its use in healing canine segmental bone defect. J. Biomed. Mater. Res. A. 2003;67(2):591–598. doi: 10.1002/jbm.a.10070. [DOI] [PubMed] [Google Scholar]
- 172.Hedberg EL, Tang A, Crowther RS, Carney DH, Mikos AG. Controlled release of an osteogenic peptide from injectable biodegradable polymeric composites. J. Controlled Release. 2002;84(3):137–150. doi: 10.1016/s0168-3659(02)00261-4. [DOI] [PubMed] [Google Scholar]
- 173.Kushibiki T, Nagata-Nakajima N, Sugai M, Shimizu A, Tabata Y. Delivery of plasmid DNA expressing small interference RNA for TGF-beta type II receptor by cationized gelatin to prevent interstitial renal fibrosis. J. Controlled Release. 2005;105(3):318–331. doi: 10.1016/j.jconrel.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 174.Tyrone JW, Mogford JE, Chandler LA, Ma C, Xia Y, Pierce GF, Mustoe TA. Collagen-embedded platelet-derived growth factor DNA plasmid promotes wound healing in a dermal ulcer model. J. Surg. Res. 2000;93(2):230–236. doi: 10.1006/jsre.2000.5912. [DOI] [PubMed] [Google Scholar]
- 175.Trentin D, Hubbell J, Hall H. Non-viral gene delivery for local and controlled DNA release. J. Controlled Release. 2005;102(1):263–275. doi: 10.1016/j.jconrel.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 176.Andree C, Voigt M, Wenger A, Erichsen T, Bittner K, Schaefer D, Walgenbach KJ, Borges J, Horch RE, Eriksson E, Stark GB. Plasmid gene delivery to human keratinocytes through a fibrin-mediated transfection system. Tissue Eng. 2001;7(6):757–766. doi: 10.1089/107632701753337708. [DOI] [PubMed] [Google Scholar]
- 177.Guo T, Zhao J, Chang J, Ding Z, Hong H, Chen J, Zhang J. Porous chitosan-gelatin scaffold containing plasmid DNA encoding transforming growth factor-beta1 for chondrocytes proliferation. Biomaterials. 2005 doi: 10.1016/j.biomaterials.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 178.Huang YC, Simmons C, Kaigler D, Rice KG, Mooney DJ. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene Ther. 2005;12(5):418–426. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- 179.Paek HJ, Campaner AB, Kim JL, Aaron RK, Ciombor DM, Morgan JR, Lysaght MJ. In vitro characterization of TGF-beta1 release from genetically modified fibroblasts in Ca(2+)-alginate microcapsules. ASAIO J. 2005;51(4):379–384. doi: 10.1097/01.mat.0000169116.84336.c3. [DOI] [PubMed] [Google Scholar]
- 180.Koradi R, Billeter M, Wuthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graphics. 1996;14(1) doi: 10.1016/0263-7855(96)00009-4. 51–5529–32. [DOI] [PubMed] [Google Scholar]