Abstract
The clinical phenotype of Huntington’s disease (HD) is far more complex and variable than depictions of it as a progressive movement disorder dominated by neostriatal pathology represent. The availability of novel neuro-imaging methods has enabled us to evaluate cerebral cortical changes in HD, which we have found to occur early and to be topographically selective. What is less clear, however, is how these changes influence the clinical expression of the disease. In this study, we used a high-resolution surface based analysis of in vivo MRI data to measure cortical thickness in 33 individuals with HD, spanning the spectrum of disease and 22 age- and sex-matched controls. We found close relationships between specific functional and cognitive measures and topologically specific cortical regions. We also found that distinct motor phenotypes were associated with discrete patterns of cortical thinning. The selective topographical associations of cortical thinning with clinical features of HD suggest that we are not simply correlating global worsening with global cortical degeneration. Our results indicate that cortical involvement contributes to important symptoms, including those that have been ascribed primarily to the striatum, and that topologically selective changes in the cortex might explain much of the clinical heterogeneity found in HD. Additionally, a significant association between regional cortical thinning and total functional capacity, currently the leading primary outcome measure used in neuroprotection trials for HD, establishes cortical MRI morphometry as a potential biomarker of disease progression.
Keywords: Huntington’s disease, cortex, phenotypic variability, biomarker
Introduction
Huntington’s disease (HD) is a progressive lethal neurodegenerative disorder that is inherited in an autosomal dominant fashion. The hallmark of neuropathology in HD is selective neurodegeneration in which vulnerable populations of neurons degenerate while less vulnerable populations are spared. The earliest and most striking neuropathological changes are found in the neostriatum (Hersch et al., 2004) but neuronal loss has been identified in many other regions of the brain, including the cerebral cortex (Hedreen et al., 1991; Braak and Braak, 1992; Heinsen et al., 1994; Halliday et al., 1998;). Projection neurons (pyramidal cells) from cortical layers III, V and VI are known to degenerate in HD (Hedreen et al., 1991; Heinsen et al., 1994; Jackson et al., 1995; Gutekunst et al., 1999; Sieradzan and Mann, 2001). Morphological changes also occur in cortical pyramidal cells prior to degeneration, including dendritic remodelling and altered size and number of dendritic spines (Sapp et al., 1997). This has suggested that these neurons undergo a prolonged period of stress and injury prior to succumbing.
Much of the difficulty of understanding the involvement of the cerebral cortex in HD lies in its heterogeneous and convoluted anatomy. As a result, investigators using neuropathology approaches have only been able to study it fractionally. Recently, computational MRI methods have enabled whole-brain analyses that can identify regional grey matter loss in the cerebral cortex of HD patients. Using these methods, changes in the cortex have been identified by several groups in both symptomatic HD and in pre-manifest individuals known to carry the genetic mutation but still unaffected by motor symptoms (Rosas et al., 2002, 2005; Kassubek et al., 2004; Douaud et al., 2006).
The clinical phenotype is also far more complex and variable than depictions of it as a progressive movement disorder represent. Symptoms encompass multiple clinical domains including motor, cognitive and psychiatric. Chorea is the clinical hallmark of the disease, however, akinaesia, slowed initiation of movement, bradykinaesia, slowness in the execution of movement and dystonia are also recognized motor features of HD and may be the prevalent clinical features in some patients. Cognitive difficulties encompass multiple domains, including executive dysfunction, as well as disturbances in memory, visuospatial attention and praxis. Psychiatric symptoms can be disabling and include depression, apathy, obsessive compulsive symptoms, delusions and psychosis. As of yet, no satisfactory explanation has been offered as to why the clinical symptoms can be so complex and variable in a disorder that has traditionally been conceived principally as disabling the basal ganglia circuitry in which other pathologies such as strokes and surgical or functional lesions can have few ill effects.
In the present study, we have more fully characterized the selectivity, progression and heterogeneity of cortical atrophy in HD and demonstrate relationships between regional cortical thinning, progressive functional decline and prominent clinical features. We show that topologically selective involvement of the cortex explains some of the phenotype and its heterogeneity. Moreover, our finding of correspondence between progressive functional decline, as measured by the total functional capacity (TFC) and regional cortical thinning, as well as striatal atrophy is novel and clinically important as it establishes cortical MRI morphometry as a potential biomarker of disease progression.
Methods
Subjects
A total of 55 subjects were recruited for this study, 33 with HD and 22 age- and sex-matched controls. Control subjects had no history of neurological or psychiatric symptoms. A diagnosis of HD was based on the presence of an unequivocal movement disorder as well as a positive family history and known trinucleotide repeat expansion. Duration of the illness ranged from 6 months to more than 7 years in order to study a spectrum of disease severity, and included patients from Stage I to IV, as defined by the TFC measure from the Unified Huntington’s Disease Rating Scale (UHDRS). Subjects’ consent was obtained according to the Declaration of Helsinski (Br Med J 1991; 302: 1194). All study protocols were approved by the Massachusetts General Hospital Internal Review Board. Basic demographic information was acquired. Twenty-nine HD subjects underwent the functional and cognitive portions of the UHDRS, including Stroop Color Word, Symbol Digit and Verbal Fluency. All subjects were assessed by a neurologist with expertise in HD (HDR). Characteristics of the study groups are given in Table 1. Blood was collected for genetic analysis.
Table 1.
Subject demographics
| Controls | HD | Stage I | Stage II | Stage III and IV | |
|---|---|---|---|---|---|
| Demographics | N = 22 | N = 33 | N = 8 | N = 14 | N = 9 |
| Age | 42.1 ± 8.2 (30–57) | 45.3 ± 8.4 (30–57) | 48.7 ± 5.9 (42–57) | 42.4 ± 9.0 (30–55) | 44.3 ± 7.7 (33–57) |
| Gender | 13F, 9M | 19F, 14M | 4F, 4M | 9F, 5M | 4F, 5M |
| Education (years)a | 16.7 (12–21) | 14.4 (11–18) | 15.7 (11.5–18) | 13.2 (11–18) | 14.8 (12–18) |
| CAG Repeat Lengthb | N/A | 45.4 (41–56) | 43.4 (42–47) | 46.6 (43–56) | 46.7 (45–50) |
| TFCc | N/A | 8.5 ± 3.0 | 11.5 ± 0.8 | 9.1 ± 1.0 | 4.3 ± 1.7 |
| UHDRS Cognitive Scores† | |||||
| Verbal Fluency | 43.4 ± 11.5 | 22.0 ± 12.9 | 40.3 ± 7.7 | 19.0 ± 7.3 | 12.3 ± 8.5 |
| Symbol Digit | 58.7 ± 9.7 | 24.9 ± 12.1 | 37.5 ± 11.5 | 23.5 ± 6.9 | 13.1 ± 7.0 |
| Stroop Color Word | 44.5 ± 7.8 | 26.4 ± 11.2 | 40.4 ± 11.7 | 24.5 ± 7.3 | 18.3 ± 5.0 |
Data presented as mean ± SD, where appropriate. UHDRS = Unified Huntington’s Disease Rating Scale; TFC = total functional capacity; N/A= not available.
Education available for N = 26 HD, N = 22 controls.
CAG available for N = 30 HD.
UHDRS cognitive scores available for 29 HD subjects, N = 21 controls.
Image acquisition and processing
Scan acquisition
Two T1-weighted images [TE = 3.31 ms, TR = 2730 ms, flip angle 7°, FOV 256 mm, matrix 256 × 171, 1.33 mm sagittal acquisition, Siemens Avanto System (Erlangen, Germany)] were acquired for each subject with 1.3 × 1 × 1.3 mm resolution at the MGH Athinoula A. Martinos Center for Biomedical Imaging.
Automated surface reconstruction and cortical thickness determination
The image processing methods used have been previously described in detail (Salat et al., 2004). Briefly, the two MP-RAGE scans for each subject were motion corrected, averaged to create a single image with high contrast-to-noise and subsequently registered to standard space. The resulting averaged volume was used to segment and classify brain volumes into three major brain tissues: grey matter, white matter and CSF. The grey/white boundary was used as the starting point for a deformable surface algorithm designed to find the pial surface with sub-millimetre precision. Cortical thickness measurements were obtained by calculating the shortest distance between the pial and white matter segmentations (Fischl et al., 2001).
The surface representing the grey–white border was subsequently inflated, morphed and registered to an average spherical surface representation that optimally aligns gyral patterns. A surface-based Gaussian smoothing kernel of full-width half-maximum, equivalent to 485 iterations in an iterative nearestneighbour averaging procedure was used, as described previously (Salat et al., 2004). The thickness values were calculated in the native MRI scanner space of an individual subject’s brain rather than in Talairach transformed space and computed as the shortest distance between the pial surface and the grey/white surface. These methods have been previously shown to be reliable (Han et al., 2006) and comparable to manual measurements (Salat et al., 2004). This procedure provides accurate matching of morphologically homologous cortical locations, minimizing geometric distortion and resulting in a mean measure of thickness at each vertex on the reconstructed surface.
Automated segmentation for caudate volume determination
Whole-brain and striatal volumes were determined using an automated segmentation algorithm previously described (Fischl et al., 2002). Striatal volumes were adjusted for intra-cranial volume.
Statistical analysis
Areas of significant thinning in HD as compared to healthy controls
A vertex-by-vertex analysis was carried out using a multivariate general linear model in which the main effects of group (thickness difference) are shown, co-varying for age. Thickness across subjects was modelled as [offset + (slope × age + slope × group) + an error term]. Thickness difference maps were constructed using a t-statistic; differences between groups at each vertex were calculated by comparing their offsets. Multiple comparisons were taken into account appropriately for all analyses, using a false discovery rate correction at a 0.05 level of significance (Genovese et al., 2002).
Correlation of thickness to clinical measures
In order to more precisely determine the relationship between clinical scores and regional thinning, thickness was regressed on a vertex-by-vertex basis against the score for TFC, Verbal Fluency, Symbol Digit and Stroop Color Word. The TFC represents a 13-point measure of functional capacity derived from the UHDRS. The Verbal Fluency, Symbol Digit and Stroop Color word from the cognitive battery that is part of the UHDRS (Group, 1996), a tool that has undergone extensive reliability and validity testing and which has provided many of the outcome measures for controlled clinical trials in HD.
Each score was modelled independently, using a model of the thickness for each subtest: [offset + (slope × subtest score) + (slope × age) + an error term]. The offset and slope are subject-independent regression coefficients estimated separately for each vertex using a general linear model. T-statistics at each vertex were used to test the hypothesis that the slope coefficient was equal to zero. Regions of interest (ROIs) were derived for the thickness/TFC and thickness/UHDRS cognitive score correlations, respectively.
A conjunction analysis was performed for the purpose of identifying regions commonly correlated amongst the various cognitive UHDRS subscores. We computed an analysis that tested the null hypothesis that the relationship between cortical thinning and worse performance in each sub-test did not differ. The criterion of significant conjunction was defined using a joint probability threshold of P < 0.05 uncorrected for multiple comparisons. We created a single P-value map: [−log10(P-value) map]. Voxels that survive this threshold are locations where both individual maps were significant at P < 0.05 (and so a conjunction). In the disjunction analysis, unique areas were determined by masking one contrast exclusively with the other. Additional regressions were created for thickness versus CAG repeat length, which represents the CAG trinucleotide expansion within the HD gene.
Results
Sample characteristics
Characteristics of the study groups are given in Table 1. The groups did not differ significantly with respect to age or gender distribution.
Topology of regional cortical thinning in early symptomatic HD
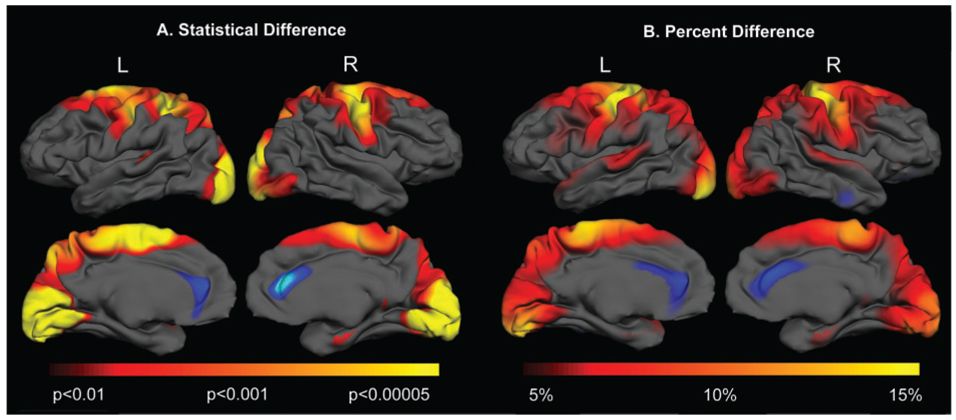
We sought to confirm that cortical thinning was not uniform in HD and to characterize the topology and magnitude of regional cortical changes in early HD. Stage I and Stage II have been operationally defined as ‘early’ HD, and the target population of therapeutic interventional studies (Phase II and Phase III clinical trials). The vertex-by-vertex statistical and mean thickness maps comparing early HD subjects (n = 22) versus age- and sex-matched controls are shown in Fig. 1A and B.
Fig. 1.
The topology of cortical thinning in early HD. Surface maps of cortical thinning were generated by using a general linear model at each vertex across the entire cortical mantle, shown in (A). In Stage I and II HD subjects, significant cortical thinning was present over sensori-motor cortex, portions of parietal cortex and occipital cortex, with relative sparing of anterior frontal and temporal regions. Maps are presented on a semi-inflated cortical surface of an average brain. The colour scale at the bottom represents the significance of the thickness change, transitioning from red (P < 0.01) to yellow (P < 0.00005). The magnitude of cortical thinning in HD is shown in (B). The colour scale at the bottom represents the magnitude of the thickness change, transitioning from red (5% loss) to yellow (>15% loss).
In HD subjects, significant thinning was present in sensori-motor cortex (BA 4, 3,2,1), superior parietal cortex (BA 5), occipital cortex (BA 17, 18, 19), portions of superior temporal cortex (BA 41,42), precuneus (BA 7), parahippocampal gyrus and posterior portions of superior and middle frontal regions (Fig. 1A). The pattern of cortical thinning was decidedly non-uniform across the brain. In contrast, there appeared to be regions of thicker cortex within the anterior cingulate. The magnitude of cortical thinning was also non-uniform, on the order of more than 15% over sensori-motor and primary visual cortex and ~<5% over posterior superior frontal cortical regions, as shown in Fig. 1B. Even within gyri, cortical thinning was heterogeneous. For example, the dorsal portions of the motor cortex, corresponding topographically to cortical areas of the lower limbs, were more significantly thinned than more ventral portions, suggesting that the involvement of the cortex in HD has a highly selective topography. Parenthetically, the gradient of striatal degeneration proposed by Vonsattel would suggest that striato-cortical projections to the lower limbs would be affected earliest in HD (Vonsattel et al., 1985). In contrast, there was no significant thinning over more anterior portions of frontal or temporal cortex.
Modelling progressive cortical thinning
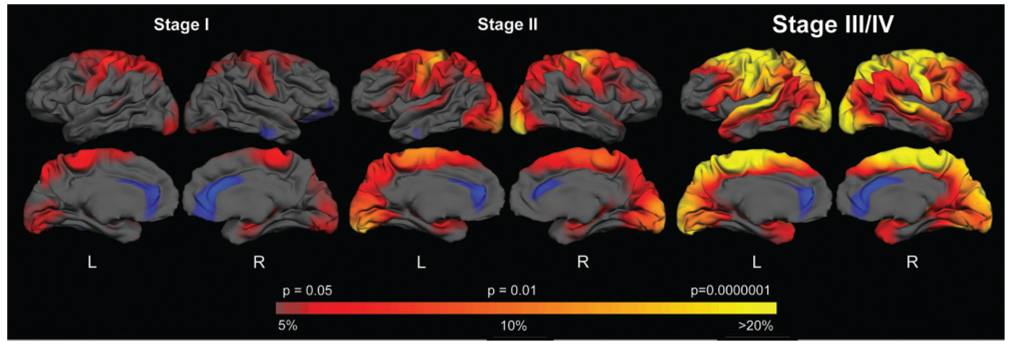
In a second analysis, subjects from each stage of disease were grouped together and compared to the same control group. We proposed a model of disease progression using cross-sectional information from individuals in the earliest to more advanced stages of disease. Given the range of TFC from 3 to 13, this would give us a ‘window’ of approximately one decade of disease progression, assuming that each decrement in TFC corresponds roughly to ~1 year (Marder et al., 2000). Importantly, there was no significant difference in age across groups (age: controls 42.1 ± 8.22, Stage I 48.7 ± 5.9; Stage II 42.37 ± 9.0; Stage III/IV 44.2 ± 7.6).
Results of this analysis are shown in Fig. 2. In Stage I, primary motor (BA4), sensory (superior portions of BA 3,2,1) portions of visual cortical areas, including BA17 and 18) and portions of the precuneus were the most significantly thinned. This corresponded to loss of thickness of ~5 to 15%. In Stage II, cortical thinning extended to include portions of pre-motor cortex (BA 6), more anterior frontal cortex (BA6), parieto-occipital areas (BA 40, BA 19), superior temporal (BA 41, 42) and portions of entorhinal cortex. The thinning in these areas was more significant and the magnitude of the thinning corresponded to ~15–20% as compared to age- and gender-matched controls. By Stage III, most of the cortex was thinned, with the relative preservation of the most anterior frontal and inferior temporal cortical regions. In the most severely thinned regions, thinning exceeded 20% (in some areas, 30%; data not shown).
Fig. 2.
A model of disease progression. HD subjects were grouped according to Stage. The colour scale at the bottom represents the significance of the thickness difference, with red to yellow indicating regions of more significant thinning in HD compared to matched controls, P < 0.05 to P < 0.0000001. The magnitude of the thickness change is displayed as well, transitioning from red (5% loss) to yellow (>20% loss).
To more accurately quantify thickness at each stage, particularly in areas affected early in the disease, an ROI was generated over the entire pre-central gyrus and the mean thickness across this gyrus was calculated (Desikan et al., 2006). In Stage I subjects, the mean thickness was reduced by ~10% (P < .05), by Stage II the thickness was reduced by ~15% (P < 0.01) and by Stage III, the thickness of the pre-central gyrus was reduced by ~25% (P < 0.000001). In contrast, the average cortical thickness across the entire brain was reduced by only 3% in Stage I (P = .20), by 6% (P < 0.05) in Stage II and by 11% in Stage III/IV (P < 0.00001).
The relationship between regional thinning and UHDRS cognitive scores
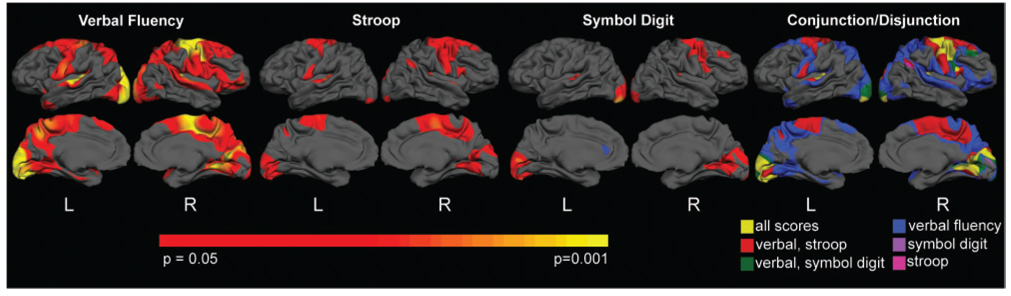
We also sought to characterize the relationship between regional thinning and the cognitive measures from the UHDRS. We found a distinct relationship for each of the cognitive measures. Performance on the Verbal Fluency correlated inversely with thickness measures in both hemispheres including the pre-central gyrus, superior temporal, portions of posterior superior frontal cortex, lingual gyrus, precuneus and cuneus. Performance on the Stroop Color Word correlated inversely with the thickness of portions of R pre-central gyrus, bilateral paracentral lobule and occipital cortex, including R lingual regions. Performance on the Symbol Digit correlated inversely with the thickness of occipital cortex, including the cuneus and with R pre-central gyrus. In each case, poorer performance correlated with thinner cortex. In the conjunction analysis, voxels that survived a threshold P-value of 0.05 in both analyses were identified. Areas of conjunction for the three cognitive tests were primarily in portions of pre-central, lingual and occipital cortex. In the disjunction analysis, unique areas were determined by masking one contrast exclusively with the other. The results of these analyses are shown in Fig. 3.
Fig. 3.
Relationship between cortical thinning and UHDRS cognitive scores. Performance on the Verbal Fluency, Stroop Color Word and Symbol Digit was inversely correlated with thinning in a number of distinct cortical regions. These findings suggest that greater clinical impairment is associated with a greater magnitude of thinning. Importantly, the topological relationship is unique for each cognitive measure, supporting clinical deficits reflect regional, and not global pathology, and also suggest that regional changes in the cortex may underlie clinical heterogeneity. The conjunction/disjunction analysis of the right more clearly shows correlations that overlap and areas that are unique to each.
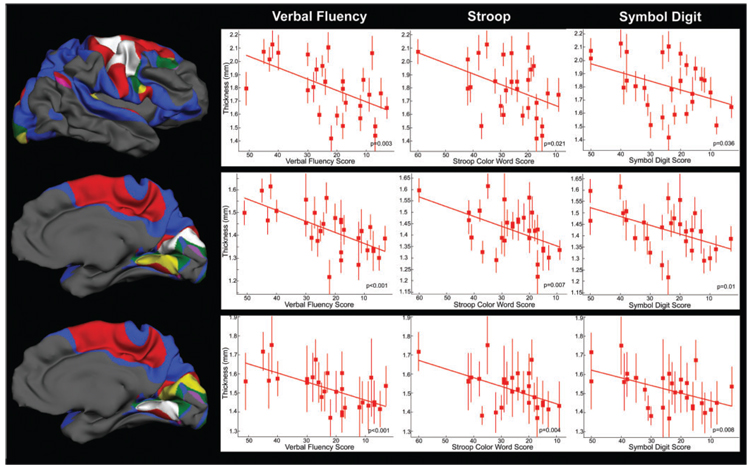
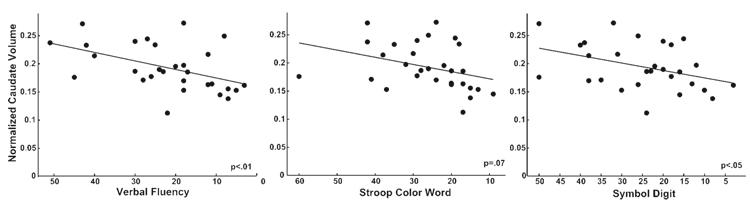
Scatter plots illustrating select relationships between cortical thickness and performance are shown in Fig. 4.
Fig. 4.
Scatter plots detailing the inverse relationship between performance on Verbal Fluency, Stroop and Symbol Digit and cortical thickness in areas of conjunction.
We also sought to investigate the relationship between cognitive performance and caudate volume, as the caudate has been implicated in various cognitive functions. As expected, there was a relationship between caudate volume and performance on the Verbal Fluency and the Symbol Digit; however, there was no relationship between caudate volume and performance on the Stroop Color Word. Scatter plots illustrating these relationships are shown in Fig. 5. This further supports that important influence of the cortex in cognitive dysfunction in HD.
Fig. 5.
Scatter plots detailing the relationship between performance on Verbal Fluency, Stroop and Symbol Digit and normalized caudate volume. There was a significant relationship between caudate volume and performance on the Verbal Fluency and Symbol Digit, but not with Stroop Color Word.
The relationship between regional cortical atrophy and clinical phenotype
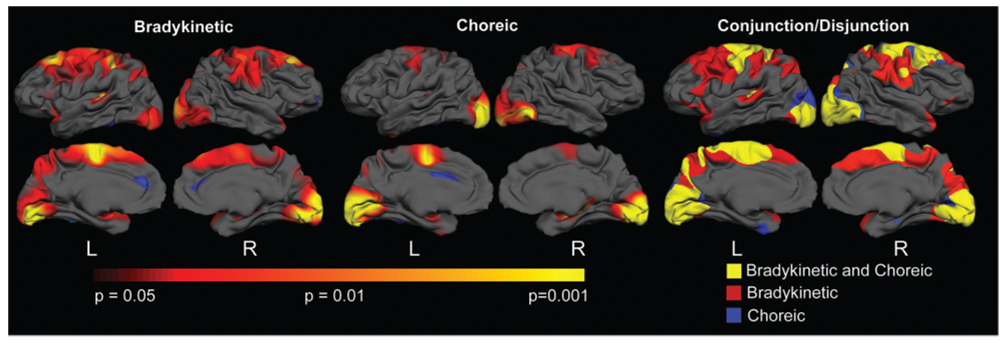
It is known that some patients with HD present with more prominent bradykinaesia and dystonia, while others have more prominent chorea. We hypothesized that some of the clinical heterogeneity in motor phenotype could be explained by differences in the regional distribution of cortical changes. Six individuals with more prominent bradykinaesia, rigidity and dystonia (TFC 8.3, 3F, 3M) and six individuals with more prominent chorea (TFC 8.6, 3F, 3M) were compared to age- and gender-matched controls. A formal conjunction disjunction analysis was also done to determine areas of overlap and discordance between the two. As shown in Fig. 6, both groups demonstrated significant thinning over sensori-motor, portions of superior frontal and occipital cortical areas. In contrast, subjects with more prominent bradykinaesia and dystonia, demonstrated more significant cortical thinning over anterior frontal cortical regions, including the pre-motor and supplementary motor areas. In contrast, striatal volumes did not differ between the two phenotypes (P = 0.08).
Fig. 6.
Differential cortical involvement in distinct motor phenotypes in HD. This suggests that cortical involvement superimposes its own distinctive contributions to the phenotype of HD. In the subjects with more prominent bradykinaesia and dystonia, more significant thinning was present over pre-motor and supplementary motor areas, as more clearly shown in the conjunction/disjunction analyses.
The relationship between regional thinning and TFC
The TFC is an integrated clinical measure of functional disability that has been accepted by clinical investigators and the FDA as the leading primary outcome measure used in clinical trials assessing whether a treatment can slow the progression of HD. In symptomatic patients with HD at mild to moderate stages of disease, it has been shown to progress in a predictable manner. We hypothesized that if cortical thinning were related to functional impairment, we would find an association between thinner cortex and greater functional disability, as measured by the TFC.
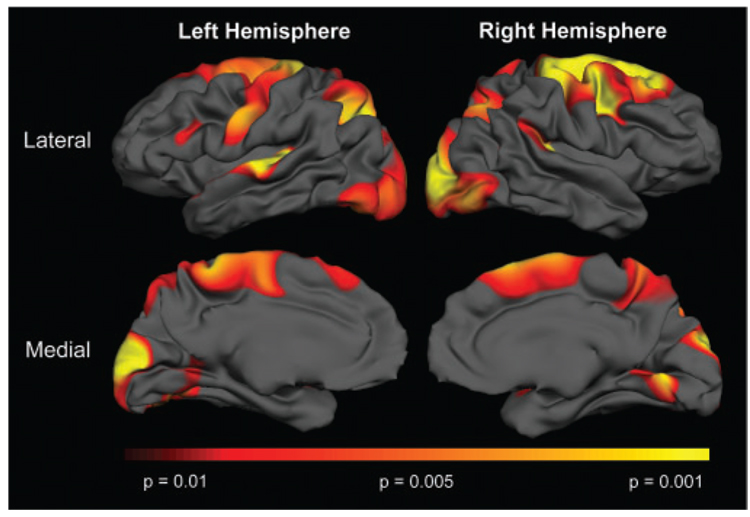
We used a linear regression model to determine the relationship between TFC and regional cortical thinning in HD subjects (TFC range 1:13). The results of this analysis are shown in Fig. 7.
Fig. 7.
Relationship between thinning and TFC. More severe impairment was associated with more thinning; red to yellow indicating regions of more significant correlations P < 0.01 to P < 0.001. This implicates the cortex as a major contributor to the progressive symptoms of HD.
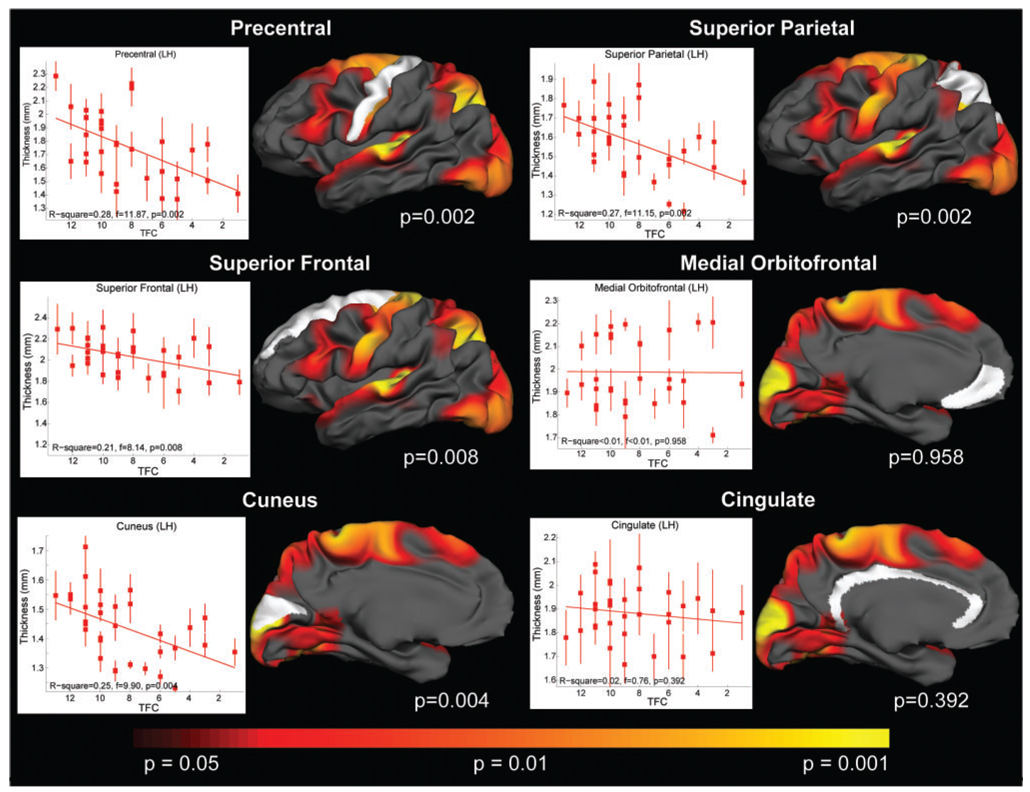
We found a significant relationship between lower TFC scores and cortical thinning in several regions. The most significant associations were found between TFC and motor cortex (P < 0.002), superior parietal (P < 0.002) and cuneus (P < 0.004), cortical regions that we had identified as the most significantly thinned in HD. In contrast, no association was found between TFC and cortical areas in which we did not find significant thinning such as cingulate (P = 0.4) or orbito-frontal (P = 1.0) cortex. This is illustrated in the scatter plots for select cortical ROIs shown in Fig. 8.
Fig. 8.
Scatter plots detailing the relationship between TFC and specific regions.
Discussion
The work presented here demonstrates that neurodegeneration of the cortex in HD occurs in a topographically predictable, selective and progressive manner that contributes importantly to the clinical expression of the disease. The distribution and magnitude of the changes reported here are strikingly comparable to those reported previously in a smaller pilot study (Rosas et al., 2002), where we found heterogeneous involvement of the sensori-motor cortex, portions of superior parietal, occipital and superior, middle and inferior temporal cortical regions. The current study examines the clinical impact of regional cortical thinning and indicates that cortical involvement contributes to important symptoms in HD, including many of those that have been ascribed primarily to the striatum. Furthermore, our findings suggest that topographically selective changes in the cortex might explain much of the clinical heterogeneity found in HD. As a potential biomarker of HD, morphometric neuroimaging that includes the cerebral cortex may more accurately represent the disease and its progression than the striatum alone.
Cortical thinning in HD is topographically selective
Using the TFC, we have developed a temporal model of disease that corresponds roughly to a decade of progression. While we believe this is representative of the natural course of HD, it must be validated in a longitudinal study. Nevertheless, it provides a basis from which to better understand important pathophysiological processes that take place and to understand clinical progression. Using this model we found that the earliest (Stage 1 HD) and most severely affected cortical regions are the primary sensory, motor and visual cortical regions (as well as superior parietal and superior frontal cortex). The finding of significant early changes in the motor cortex (pre-central gyrus) is understandable clinically in a disease known to affect both involuntary and voluntary motor systems, although poor voluntary motor control in HD has historically been attributed to degeneration of the striatum and associated dysfunction of striatal outflow pathways. Altered motor cortex excitability has also been recently reported in early HD (Nardone et al., 2007). Several post-mortem studies primarily using late-stage tissue have demonstrated degeneration of the primary motor cortex (de la Monte et al., 1988; Mann et al., 1993a; Macdonald and Halliday, 2002; DiProspero et al., 2004). Our data demonstrate that this degeneration occurs early and is especially pronounced.
Profound neuronal loss has been reported in primary somatosensory cortex (Lange et al., 1976; Mann et al., 1993b; Heinsen et al., 1994) and alterations in sensory processing have also been reported (Boecker et al., 1999). Apraxia is seen in HD (Hamilton et al., 2003), and while it has been postulated to be due to basal ganglia dysfunction, it may actually be due to changes in superior parietal cortex (Heilman et al., 1986). The occipital cortex also appeared thinned early in our study. In one post-mortem study, the occipital lobe was found to demonstrate the most significant atrophy among cortical regions (Lange et al., 1976). While primary visual deficits have not been reported in HD, deficits in spatial cognition and spatial memory have been (Lawrence et al., 2000; Brandt et al., 2005). Changes in the metabolism of the occipital cortex (Feigin et al., 2001; Jenkins et al., 2005) have also been reported. The anterior cingulate has received little attention in HD, but it may play an important role in cognitive impairment (Georgiou-Karistianis et al., 2007; Thiruvady et al., 2007). We have found that the anterior cingulate cortical ribbon was thicker, perhaps due to gliosis. Indeed, a recent publication reported increased microglial activation in anterior cingulate cortex in HD (Pavese et al., 2006).
In Stage II subjects, primary auditory cortex and cortical areas adjacent to those seen in Stage I subjects were also significantly thinned. There was more extensive involvement of the occipital cortex and of additional parietal regions, including precuneus, and more anterior frontal cortical regions, corresponding to pre-frontal and supplementary motor area. Thinning was also present within entorhinal cortex, consistent with a previous finding of allocortical involvement in HD (Braak and Braak, 1992). By Stage III/IV, most of the cortex was thinned, including entorhinal cortex and higher order cortical regions including Areas 49 and 9, as shown in previous neuropathological studies (Selemon et al., 2004). However, even in advanced subjects, portions of the most anterior frontal and inferior temporal regions were relatively preserved.
In the striatum, medium spiny neurons are the most vulnerable and they also receive most of their glutamatergic projections from the cortex. Since the cortex provides glutamatergic stress to the striatum as well as necessary trophic support (e.g. BDNF) and loss of striatal neurons could potentially promote retrograde degeneration of corticostriatal pyramidal cells, the inter-relationships of cortical and striatal pathology are likely to be quite complicated. Interestingly, in HD the indirect striatal efferent pathway has been hypothesized to be affected earliest and the sensori-motor cortex primarily projects to the striatal projection neurons that give rise to the indirect pathway (Parthasarathy and Graybiel, 1997). However, in HD transgenic mice, cortical lesions are actually protective of the striatum (Stack et al., 2007), which would implicate the cortex as having a strong role in promoting striatal degeneration.
It is interesting that, with the exception of the precuneus, thinning first appears to involve primary cortical idiotypic regions and progresses to involve unimodal, or homotypical, cortical areas by Stage II. By Stage III/IV, heteromodal areas are also thinned. This suggests that the cortex undergoes a topologically selective degeneration. The mechanisms underlying this regional selectivity and the apparent heterogeneous topographical distribution of cortical changes in HD are unknown, but there are several possible explanations.
Huntington aggregation has been shown to be non-uniform throughout the cortex (Gutekunst et al., 1999), and it is possible that there is a correlation between heightened physiological stress regionally, aggregates, and neurodegeneration, reflected in the cortical pathology. However, it is unlikely that aggregate formation underlies the regional selectivity of the cortical pathology in HD. The distribution of aggregates that has been reported does not overlap with the distribution of cortical thinning (van Dellen et al., 2001). Alterations in transcription, which likely contribute to the pathogenesis of HD (Cha, 2007), have also been recently shown to have a distinct regional pattern, with greater abnormalities in mRNA expression in the motor cortex than in pre-frontal association cortex (Hodges et al., 2006), suggesting that changes in transcription differ regionally and perhaps correspond to the regional severity of cortical involvement.
Primary motor, sensory and visual areas and the precuneus are areas of high metabolic activity (Fox et al., 1988; Raichle, 1998; Cavanna and Trimble, 2006). Alterations in various neurotransmitter concentrations, including glutamate, have been found to be most profound in primary visual and motor cortex (Storey et al., 1992; Browne and Beal, 2006). Kynurenic acid concentrations, which correlate with neuronal stress (Giorgini et al., 2005) have been found to be significantly higher in motor cortex (Connick et al., 1989). Together, these data suggest that the regional selectivity of the cortical changes may be in part due to underlying metabolic differences, exacerbated by the dysregulation of excitatory neurotransmitters.
One other hypothesis is that functional networks are affected in parallel in HD. The motor network is a complex one that includes not only primary motor and sensory cortex, but also pre-motor, parietal, precuneal and dorsal lateral pre-frontal cortical regions as well as the basal ganglia and cerebellum. The cortical regions that receive input from basal ganglia structures through the thalamus are regions that are thinned by Stage I. However, early thinning also occurs in parietal and occipital cortical regions, which do not receive striatal outflow. These data suggest that the pattern of regional cortical degeneration is independent of the striatal connectivity.
Topographical changes are correlated to clinical measures
Relationship to UHDRS cognitive measures
The regions of cortical thinning associated with the cognitive subtests of the UHDRS, showed some overlap and some marked differences. The Symbol Digit task assesses visual-motor dexterity, speed of performance and ability to learn a new task. Performance on this test begins to decline before subjects with HD have motor symptoms and it declines progressively in parallel with the TFC. Performance was inversely correlated with thinning in lingual, fusiform, cuneus, occipital, superior temporal, pre-motor and insular areas.
The Verbal Fluency task is a standard measure of language production that is included in the UHDRS. Performance in this task was inversely correlated with thinning in pre-motor, inferior frontal, superior parietal, cuneus, lingual/occipital and superior temporal areas. Functional neuro-imaging studies have revealed activation in the inferior frontal cortex, pre-motor cortex, lateral temporal gyrus, occipital cortex and precuneus (Gaillard et al., 2000; Billingsley et al., 2004; Costafreda et al., 2006; Halari et al., 2006) on similar tasks.
The Stroop Color Word task and Symbol Digit modality examine selective attention. In our HD subjects, performance on this task was inversely correlated with thinning of pre-central cortex, portions of pre-motor, dorsolateral pre-frontal, precuneus, insular cortex and fusiform gyrus. Functional neuro-imaging studies have reported increased activation in the precuneus, fusiform gyrus, portions of inferior frontal, superior and inferior parietal, parietooccipital and insular cortical regions (Banich et al., 2000; Gaillard, 2000; Leung et al., 2000; Adleman et al., 2002; Gurd, 2002). Performance on the Stroop Color Word was inversely correlated with thinning of portions of pre-frontal, occipital, lingual, superior temporal and pre-central regions.
The conjunction analysis demonstrated that performance on all three tasks correlated inversely with thinning over motor and pre-motor, lingual and occipital regions. The inverse correlation between performance and cortical thickness was the most discordant for Verbal Fluency, where a unique relationship was present in several cortical regions, including superior temporal, superior parietal, L insular and entorhinal cortex.
The conjunction/disjunction analyses suggest that topologically selective degeneration of the cortex contributes to specific cognitive deficits in HD. Interestingly, caudate volumes correlated with performance on both Verbal Fluency and Symbol Digit, but not with the Stroop Color Word. Clearly, the relationships between cortical pathology, subcortical pathology and cognitive measures are complex and require further investigation. However, our data does suggest that the cortex contributes significantly to cognitive symptoms in HD.
Relationship to motor phenotype
The motor phenotype in HD can be quite variable. In some patients, dystonia, bradykinaesia and rigidity are more common whereas in others, chorea is more prominent. We found that while there was overlap in regions of thinning in both phenotypes, including over sensori-motor cortex, subjects with more prominent bradykinaesia demonstrated more extensive cortical involvement including more anterior portions of the frontal cortex, including BA6 (pre-motor and supplementary motor areas), BA 8 (pre-frontal) and BA9. It is important to note that the groups were matched for disease severity as assessed by the TFC and for age. The same areas are relatively under-activated in patients with Parkinson’s disease (Playford et al., 1992; Haslinger et al., 2001). Although the supplementary motor, pre-motor and pre-frontal regions are highly interconnected and essential for the successful execution of voluntary movements, they have different roles in motor control. The role of the supplementary motor area in the planning and coordination of complex movements is well established, and it is understandable that its involvement could contribute to bradykinaesia. The pre-motor cortex is important in the temporal organization of sequential movements (Halsband et al., 1993, 1994), selection of movements (Deiber et al., 1991) and in the generation of motor sequences from memory that fit into a precise plan (Grafton et al., 1992). In contrast, caudate volumes did not differ between the two clinical phenotypes. These findings together suggest that while striatal degeneration clearly plays a major role in the movement disorders of HD, dysfunctional motor cortices do so as well.
Relationship to TFC
We have demonstrated a remarkable correspondence between regional cortical thinning and the global progressive functional and cognitive decline that occurs in HD, as measured by the TFC. The TFC score declines progressively through the early and middle stages of HD (Marder et al., 2000), after which there is a floor effect. Because of this relationship with disease progression and because it reflects clinically significant disability, the TFC has been accepted by the FDA as the primary end-point in clinical trials testing neuroprotective therapies in symptomatic HD. The most significant relationship between the TFC and cortical thinning was in regions of sensori-motor and occipital cortex, areas that we identified to be the earliest affected in HD. In contrast, regions that demonstrated relative preservation of thickness, such as orbito-frontal cortex, did not correlate with TFC. As expected, caudate atrophy also correlated with TFC decline. This implicates the cortex, along with the striatum, as a major contributor to the symptoms of HD. Our interpretation is that neurodegeneration affecting the striatum and cerebral cortex both contribute to the progressive functional decline that occurs in HD. We would suggest that while the clinical consequences of striatal involvement may play out by the early to middle stages of HD, the clinical consequences of cortical involvement also begin early but play an ever-increasing role in the expression of the disease phenotype as the disease progresses involving more and more of the cortex. The correspondence between regional cortical thinning and the TFC also suggests that cortical MRI morphometry could serve as a biomarker of disease progression and possibly a surrogate end-point in clinical trials. By comparison to the 30 or so HD subjects in our study, 100s of HD subjects must be followed for at least a few years to give the TFC power to monitor progression. Moreover, this is a potential advance over monitoring the striatum alone in symptomatic HD because the striatum is already severely atrophic by the time individuals are diagnosed clinically. Therefore, a biomarker of disease progression encompassing the striatum and cortex will likely have enhanced power and range. In pre-manifest HD, the TFC and other clinical outcome measures may not be very useful for examining disease progression since these patients are not yet symptomatic. Neuro-imaging techniques are able to detect changes during this HD prodrome (Aylward et al., 1994; Thieben et al., 2002; Reading et al., 2004; Rosas et al., 2005, 2006) and have potential utility for monitoring progression and responses to disease-modifying therapy.
Summary
Our growing understanding of HD underscores the complexity of the disease. A single, well-defined genetic mutation causes a cascade of events, whose final result is an aggregate insult of homoeostatic processes. Our cross-sectional study demonstrates that the cortex is both selectively thinned and that cortical thinning is progressive. We believe our results represent a paradigm shift in how HD is conceived in that it is a systems disorder in which many brain regions contribute in patterns that unfold temporally and heterogeneously depending on the differential involvement of specific regions and circuits. Finally, because cortical thinning correlates with important clinical outcome measures, especially the TFC, its measurement is a potential biomarker for assessing neuroprotective therapies.
Acknowledgements
Special thanks to our patients who so generously contributed their time and energy to this work and without whom it would not have been possible. Thanks also to Lindsay Muir and Talia Landau for their contribution. This work was supported by National Institutes of Health, National Institute for Neurological Disorders and Stroke (NS042861 HDR NS045242 SMH PO1NS058793 SMH and HDR), National Center for Complementary and Alternative Medicine (AT000613 (SMH and HDR), the National Institute on Aging (NIAAG024898 DHS), the National Center for Research Resources (RR4075 and R16594 BF), the Mental Illness and Neuroscience Discovery (MIND) Institute, the Center for Functional Neuroimaging Technologies (P01RR4075 BF) and the High Q Foundation (HDR).
Abbreviations
- HD
Huntington’s disease
- ROI
region of interest
- TFC
total functional capacity
- UHDRS
Unified Huntington’s Disease Rating Scale
References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, et al. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Brandt J, Codori AM, Mangus RS, Barta PE, Harris GJ. Reduced basal ganglia volume associated with the gene for Huntington’s disease in asymptomatic at-risk persons. Neurology. 1994;44:823–828. doi: 10.1212/wnl.44.5.823. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, et al. fMri studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J Cogn Neurosci. 2000;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Billingsley RL, Simos PG, Castillo EM, Sarkari S, Breier JI, Pataraia E, et al. Spatio-temporal cortical dynamics of phonemic and semantic fluency. J Clin Exp Neuropsychol. 2004;26:1031–1043. doi: 10.1080/13803390490515333. [DOI] [PubMed] [Google Scholar]
- Boecker H, Ceballos-Baumann A, Bartenstein P, Weindl A, Siebner HR, Fassbender T, et al. Sensory processing in Parkinson’s and Huntington’s disease: investigations with 3D H(2)(15)O- PET. Brain. 1999;122(Pt 9):1651–1665. doi: 10.1093/brain/122.9.1651. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Allocortical involvement in Huntington’s disease. Neuropathol Appl Neurobiol. 1992;18:539–547. doi: 10.1111/j.1365-2990.1992.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Brandt J, Shpritz B, Munro CA, Marsh L, Rosenblatt A. Differential impairment of spatial location memory in Huntington’s disease. J Neurol Neurosurg Psychiatry. 2005;76:1516–1519. doi: 10.1136/jnnp.2004.059253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SE, Beal MF. Oxidative damage in Huntington’s disease pathogenesis. Antioxid Redox Signal. 2006;8:2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cha J-HJ. Transcriptional signatures in Huntingtons disease. Progress in Neurobiology. 2007 doi: 10.1016/j.pneurobio.2007.03.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connick JH, Carla V, Moroni F, Stone TW. Increase in kynurenic acid in Huntington’s disease motor cortex. J Neurochem. 1989;52:985–987. doi: 10.1111/j.1471-4159.1989.tb02552.x. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Vonsattel JP, Richardson EP., Jr Morphometric demonstration of atrophic changes in the cerebral cortex, white matter, and neostriatum in Huntington’s disease. J Neuropathol Exp Neurol. 1988;47:516–525. doi: 10.1097/00005072-198809000-00003. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84:393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- DiProspero NA, Chen EY, Charles V, Plomann M, Kordower JH, Tagle DA. Early changes in Huntington’s disease patient brains involve alterations in cytoskeletal and synaptic elements. J Neurocytol. 2004;33:517–533. doi: 10.1007/s11068-004-0514-8. [DOI] [PubMed] [Google Scholar]
- Douaud G, Gaura V, Ribeiro MJ, Lethimonnier F, Maroy R, Verny C, et al. Distribution of grey matter atrophy in Huntington’s disease patients: a combined ROI-based and voxel-based morphometric study. Neuroimage. 2006;32:1562–1575. doi: 10.1016/j.neuroimage.2006.05.057. [DOI] [PubMed] [Google Scholar]
- Feigin A, Leenders KL, Moeller JR, Missimer J, Kuenig G, Spetsieris P, et al. Metabolic network abnormalities in early Huntington’s disease: an [(18)F]FDG PET study. J Nucl Med. 2001;42:1591–1595. [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintum MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988:463–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Georgiou-Karistianis N, Sritharan A, Farrow M, Cunnington R, Stout J, Bradshaw J, et al. Increased cortical recruitment in Huntington’s disease using a Simon task. Neuropsychologia. 2007;45:1791–1800. doi: 10.1016/j.neuropsychologia.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Giorgini F, Guidetti P, Nguyen Q, Bennett SC, Muchowski PJ. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nat Genet. 2005;37:526–531. doi: 10.1038/ng1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Woods RP, Phelps ME. Human functional anatomy of visually guided finger movements. Brain. 1992;115(Pt 2):565–587. doi: 10.1093/brain/115.2.565. [DOI] [PubMed] [Google Scholar]
- Group HS. Unified Huntington’s Disease Rating Scale: reliability and consistency. Huntington Study Group. Mov Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- Gutekunst CA, Li SH, Yi H, Mulroy JS, Kuemmerle S, Jones R, et al. Nuclear and neuropil aggregates in Huntington’s disease: relationship to neuropathology. J Neurosci. 1999;19:2522–2534. doi: 10.1523/JNEUROSCI.19-07-02522.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halari R, Sharma T, Hines M, Andrew C, Simmons A, Kumari V. Comparable fMRI activity with differential behavioural performance on mental rotation and overt verbal fluency tasks in healthy men and women. Exp Brain Res. 2006;169:1–14. doi: 10.1007/s00221-005-0118-7. [DOI] [PubMed] [Google Scholar]
- Halliday GM, McRitchie DA, Macdonald V, Double KL, Trent RJ, McCusker E. Regional specificity of brain atrophy in Huntington’s disease. Exp Neurol. 1998;154:663–672. doi: 10.1006/exnr.1998.6919. [DOI] [PubMed] [Google Scholar]
- Halsband U, Ito N, Tanji J, Freund HJ. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain. 1993;116(Pt 1):243–266. doi: 10.1093/brain/116.1.243. [DOI] [PubMed] [Google Scholar]
- Halsband U, Matsuzaka Y, Tanji J. Neuronal activity in the primate supplementary, pre-supplementary and premotor cortex during externally and internally instructed sequential movements. Neurosci Res. 1994;20:149–155. doi: 10.1016/0168-0102(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Hamilton JM, Haaland KY, Adair JC, Brandt J. Ideomotor limb apraxia in Huntington’s disease: implications for corticostriate involvement. Neuropsychologia. 2003;41:614–621. doi: 10.1016/s0028-3932(02)00218-x. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat DH, van der Kouwe A, Quinn BT, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, et al. Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain. 2001;124:558–570. doi: 10.1093/brain/124.3.558. [DOI] [PubMed] [Google Scholar]
- Hedreen JC, Peyser CE, Folstein SE, Ross CA. Neuronal loss in layers V and VI of cerebral cortex in Huntington’s disease. Neurosci Lett. 1991;133:257–261. doi: 10.1016/0304-3940(91)90583-f. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Rothi LG, Mack L, Feinberg T, Watson RT. Apraxia after a superior parietal lesion. Cortex. 1986;22:141–150. doi: 10.1016/s0010-9452(86)80038-7. [DOI] [PubMed] [Google Scholar]
- Heinsen H, Strik M, Bauer M, Luther K, Ulmar G, Gangnus D, et al. Cortical and striatal neurone number in Huntington’s disease. Acta Neuropathol (Berl) 1994;88:320–333. doi: 10.1007/BF00310376. [DOI] [PubMed] [Google Scholar]
- Hersch S, Rosas HD, Ferrante RJ. Neuropathology and pathophysiology of Huntington’s disease. New York: McGraw-Hill; 2004. [Google Scholar]
- Hodges A, Strand AD, Aragaki AK, Kuhn A, Sengstag T, Hughes G, et al. Regional and cellular gene expression changes in human Huntington’s disease brain. Hum Mol Genet. 2006;15:965–977. doi: 10.1093/hmg/ddl013. [DOI] [PubMed] [Google Scholar]
- Jackson M, Gentleman S, Lennox G, Ward L, Gray T, Randall K, et al. The cortical neuritic pathology of Huntington’s disease. Neuropathol Appl Neurobiol. 1995;21:18–26. doi: 10.1111/j.1365-2990.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, Andreassen OA, Dedeoglu A, Leavitt B, Hayden M, Borchelt D, et al. Effects of CAG repeat length, HTT protein length and protein context on cerebral metabolism measured using magnetic resonance spectroscopy in transgenic mouse models of Huntington’s disease. J Neurochem. 2005;95:553–562. doi: 10.1111/j.1471-4159.2005.03411.x. [DOI] [PubMed] [Google Scholar]
- Kassubek J, Bernhard Landwehrmeyer G, Ecker D, Juengling FD, Muche R, Schuller S, et al. Global cerebral atrophy in early stages of Huntington’s disease: quantitative MRI study. Neuroreport. 2004;15:363–365. doi: 10.1097/00001756-200402090-00030. [DOI] [PubMed] [Google Scholar]
- Lange H, Thorner G, Hopf A, Schroder KF. Morphometric studies of the neuropathological changes in choreatic diseases. J Neurol Sci. 1976;28:401–425. doi: 10.1016/0022-510x(76)90114-3. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Watkins LH, Sahakian BJ, Hodges JR, Robbins TW. Visual object and visuospatial cognition in Huntington’s disease: implications for information processing in corticostriatal circuits. Brain. 2000;123(Pt 7):1349–1364. doi: 10.1093/brain/123.7.1349. [DOI] [PubMed] [Google Scholar]
- Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An event-related functional MRI study of the stroop color word interference task. Cereb Cortex. 2000;10:552–560. doi: 10.1093/cercor/10.6.552. [DOI] [PubMed] [Google Scholar]
- Macdonald V, Halliday G. Pyramidal cell loss in motor cortices in Huntington’s disease. Neurobiol Dis. 2002;10:378–386. doi: 10.1006/nbdi.2002.0528. [DOI] [PubMed] [Google Scholar]
- Mann DM, Oliver R, Snowden JS. The topographic distribution of brain atrophy in Huntington’s disease and progressive supranuclear palsy. Acta Neuropathol (Berl) 1993a;85:553–559. doi: 10.1007/BF00230496. [DOI] [PubMed] [Google Scholar]
- Mann DMA, Oliver R, Snowden JS. The topographic distribution of brain atrophy in Huntington’s disease and progressive supranuclear palsy. Acta Neuropathol (Berl) 1993b;85:553–559. doi: 10.1007/BF00230496. [DOI] [PubMed] [Google Scholar]
- Marder K, Zhao H, Myers RH, Cudkowicz M, Kayson E, Kieburtz K, et al. Rate of functional decline in Huntington’s disease. Huntington Study Group. Neurology. 2000;54:452–458. doi: 10.1212/wnl.54.2.452. [DOI] [PubMed] [Google Scholar]
- Nardone R, Lochner P, Marth R, Ausserer H, Bratti A, Tezzon F. Abnormal intracortical facilitation in early-stage Huntington’s disease. Clin Neurophysiol. 2007;118:1149–1154. doi: 10.1016/j.clinph.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J Neurosci. 1997;17:2477–2491. doi: 10.1523/JNEUROSCI.17-07-02477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavese N, Gerhard A, Tai YF, Ho AK, Turkheimer F, Barker RA, et al. Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology. 2006;66:1638–1643. doi: 10.1212/01.wnl.0000222734.56412.17. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Ann Neurol. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci USA. 1998;95:765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading SA, Dziorny AC, Peroutka LA, Schreiber M, Gourley LM, Yallapragada V, et al. Functional brain changes in presymptomatic Huntington’s disease. Ann Neurol. 2004;55:879–883. doi: 10.1002/ana.20121. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65:745–747. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, et al. Diffusion tensor imaging in presymptomatic and early Huntington’s disease: selective white matter pathology and its relationship to clinical measures. Mov Disord. 2006;21:1317–1325. doi: 10.1002/mds.20979. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sapp E, Schwarz C, Chase K, Bhide PG, Young AB, Penney J, et al. Huntingtin localization in brains of normal and Huntington’s disease patients. Ann Neurol. 1997;42:604–612. doi: 10.1002/ana.410420411. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Evidence for progression in frontal cortical pathology in late-stage Huntington’s disease. J Comp Neurol. 2004;468:190–204. doi: 10.1002/cne.10938. [DOI] [PubMed] [Google Scholar]
- Sieradzan KA, Mann DM. The selective vulnerability of nerve cells in Huntington’s disease. Neuropathol Appl Neurobiol. 2001;27:1–21. doi: 10.1046/j.0305-1846.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- Stack EC, Dedeoglu A, Smith KM, Cormier K, Kubilus JK, Bogdanov M, et al. Neuroprotective effects of synaptic modulation in Huntington’s disease R6/2 mice. J Neurosci. 2007;27:12908–12915. doi: 10.1523/JNEUROSCI.4318-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey E, Kowall NW, Finn SF, Mazurek MF, Beal MF. The cortical lesion of Huntington’s disease: further neurochemical characterization, and reproduction of some of the histological and neurochemical features by N-methyl-D-aspartate lesions of rat cortex. Ann Neurol. 1992;32:526–534. doi: 10.1002/ana.410320408. [DOI] [PubMed] [Google Scholar]
- Thieben MJ, Duggins AJ, Good CD, Gomes L, Mahant N, Richards F, et al. The distribution of structural neuropathology in pre-clinical Huntington’s disease. Brain. 2002;125:1815–1828. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
- Thiruvady DR, Georgiou-Karistianis N, Egan GF, Ray S, Sritharan A, Farrow M, et al. Functional connectivity of the prefrontal cortex in Huntington’s disease. J Neurol Neurosurg Psychiatry. 2007;78:127–133. doi: 10.1136/jnnp.2006.098368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dellen A, Deacon R, York D, Blakemore C, Hannan AJ. Anterior cingulate cortical transplantation in transgenic Huntington’s disease mice. Brain Res Bull. 2001;56:313–318. doi: 10.1016/s0361-9230(01)00655-4. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]