Abstract
Progressive human immunodeficiency virus (HIV)-1 infection and virus-induced neuroinflammatory responses effectuates monocyte-macrophage transmigration across the blood-brain barrier (BBB). A key factor in mediating these events is monocyte chemotactic protein-1 (MCP-1). Upregulated glial-derived MCP-1 in HIV-1 infected brain tissues generates a gradient for monocyte recruitment into the nervous system. We posit that the inter-relationships between MCP-1, voltage gated ion channels, cell shape and volume, and cell mobility underlie monocyte transmigration across the BBB. In this regard, MCP-1 serves both as a chemoattractant and an inducer of monocyte-macrophage ion flux affecting cell shape and mobility. To address this hypothesis, MCP-1 treated bone marrow derived macrophages (BMM) were analyzed for gene and protein expression, electrophysiology, and capacity to migrate across a laboratory constructed BBB. MCP-1 enhanced K+ channel gene (KCNA3) and channel protein expression. Electrophysiological studies revealed that MCP-1 increased outward K+ currents in a dose dependent manner. In vitro studies demonstrated that MCP-1 increased BMM migration across an artificial BBB and the MCP-1-induced BMM migration was blocked by tetraethylammonium, a voltage-gated K+ channel blocker. Together these data demonstrated that MCP-1 affects macrophage migratory movement through regulation of voltage-gated K+ channels and as such, provides a novel therapeutic strategy for neuroAIDS
Keywords: Monocyte, Monocyte Chemotactic Protein-1, K+ Channels, Blood-Brain Barrier
Introduction
Chemokines are structurally defined small proteins that serve as leukocyte chemoattractants for a variety of inflammatory diseases including those of the central nervous system (CNS) (Rebenko-Moll et al., 2006; Callewaere et al., 2007; Mines et al., 2007). They affect a variety of divergent biological activities that include cell migration to sites of disease and inflammation, leukocyte activation, leukocyte secretions, and microbial activities (infection and cellular clearance) (Callahan and Ransohoff, 2004; Banisadr et al., 2005; Rebenko-Moll et al., 2006; Ubogu et al., 2006; Li and Ransohoff, 2008). One of the most investigated and disease-associated chemokines is monocyte chemoattractant protein-1 (MCP-1), which plays a pivotal role in the recruitment of mononuclear phagocytes (MP; blood borne monocytes, dendritic cells, and macrophages) to sites of inflammation and malignancies (de la Rosa et al., 2003; Garlet et al., 2003; Yao et al., 2006; Buhling et al., 2007; Tsai et al., 2008). The mechanism of such recruitment is well studied. Indeed, migration of monocytes across the vascular endothelium is linked to cells tethering to the endothelium, rolling along the vascular surface, firm endothelial adhesion, and finally diapedesis between tight morphologically structured endothelial cells; processes that are explicatively linked to both chemokine and adhesion molecule expression (Strell et al., 2007; Vestweber, 2007; Crane JJ, 2008). After penetration of the endothelial basement membrane, monocytes migrate through the extracellular matrix of the tissues where they differentiate into tissue macrophages and migrate to sites of inflammation (Whatling et al., 2004; Fulcher et al., 2006; Reinhart et al., 2006; Cesar et al., 2008). Monocyte migration is mediated through selectins and their ligands, or integrins interacting with endothelial vascular cell adhesion molecule 1 (VCAM-1). VCAM-1 is expressed at low levels on the endothelium and up-regulated after immune activation (Lane et al., 1996; Nottet et al., 1996). On the apical endothelial surface, the chemokines MCP-1 and macrophage inflammatory proteins-alpha/beta, (MIP-1alpha/beta) activate beta(2) integrins to facilitate firm adhesion (Biernacki et al., 2004; Maslin et al., 2005). Diapedesis by monocytes occurs through interaction between integrins on both the monocyte and the endothelial cells, followed by homophilic adhesion (Muller and Randolph, 1999; Schenkel et al., 2002; Mamdouh et al., 2008). The intracellular mechanisms for these processes are linked to activation by tyrosine phosphorylation of cellular proteins and phosphorylation of the non-receptor tyrosine kinases Lyn, JAK2, the cytoskeletal binding protein paxillin, and downstream transcription factors STAT3 and STAT5 (Muller et al., 2005). In this manner, MCP-1-induces TNF-α and IL-1 production and promotes macrophage activation (Biswas and Sodhi, 2002).
Monocyte trafficking is of special interest in studies of the neuropathogenesis of HIV-infection, including establishment of viral reservoirs, and the development of HIV-associated neurocognitive disorders (HAND) (Venneti et al., 2004; Williams et al., 2005; Berman et al., 2006). However, the process of monocyte migration across the vascular endothelium and changes in migration that occur during HIV-infection are incompletely understood. We theorized that since MCP-1 can elicit activation responses for macrophages the actual influence of the chemokine on monocyte-macrophage physiology would be profound. In this manner, a linkage between MCP-1 and potassium (K+) channel expression would be apparent. Blockers of these channels altered the organization and structure of the cytoskeletal proteins actin, tubulin and vimentin; adherence-mediated macrophage activation; and maintenance of cytoskeletal integrity and cell shape. Such cytoskeletal changes were associated with cell morphology changes. We now demonstrate that MCP-1 activation of monocytes leads to morphological alterations that are directly linked to K+ channels. Chemokine mediated responses lead to a dosage escalation of channel responses and to alterations in size, shape, volume, and ingress across an artificial BBB. The observations, taken together, provide unique insights into how MCP-1 can affect movement of monocytes across barriers and most notably into the CNS.
Materials and Methods
Bone marrow derived-macrophage (BMM) cultivation
Bone marrow cells were obtained from femurs of 6-week-old C57/BL6 or BALB/c male mice (purchased from Jackson Laboratory, Bar Harbor, MN). Cells were cultured in Teflon flasks at 2×106 cells/ml in complete DMEM supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and 1% penicillin/streptomycin) in presence of mouse macrophage colony stimulating factor (M-CSF) 2 µg/ml (a gift from Wyeth, Cambridge, MA). After 5–6 days in culture, BMM were determined to be >95% CD11b+ by flow cytometry and utilized thereafter.
Whole-cell patch clamp recording
BMM were plated onto sterile glass cover slips at a density of 0.5×106 cells/slip. Whole cell patch-clamp recordings were made using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) interfaced with a Digidata 1322A digitizer (Molecular Devices) and controlled with pClamp Version 8.1 software (Molecular Devices). Whole cell currents were filtered at 1 kHz, digitized at 10 kHz and stored on a computer hard disk. Patch pipettes were fabricated from borosilicate glass capillaries using a Sutter P97 microelectrode puller, with tip resistances of 4–8 MΩ when filled with pipette solution containing (in mM) 130 K+ gluconate, 10 EGTA, 1 CaCl2, 1 MgCl2, and 10 HEPES (pH 7.2, 280 mOsm). The bathing solution contained (in mM) 135 Na+gluconate, 5 KCl, 1CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH 7.4, 290 mOsm). Values of access resistance ranging from 8–18 MΩ were compensated at 60–70% in most cells. Whole-cell voltage-dependent outward K+ currents were recorded in response to a series of voltage steps from −160 mV to +100 mV in 20 mV increments, starting from a holding potential of −60 mV. The steady-state current magnitudes were measured and normalized as a percentage of the magnitudes generated by a voltage step from −60 mV to +100 mV in control (perfused with normal bath solution). For statistical analyses, paired Student’s t tests were used with statistical significance at p<0.05.
Isolation and culture of primary human monocytes
Human monocytes were obtained from HIV-1, -2, and hepatitis B seronegative adults by leukophoresis and purified by countercurrent centrifugal elutriation. Monocytes were cultured in DMEM (Sigma, St. Louis) supplemented with 10% heat-inactivated human serum, 2mM L-glutamine, gentamicin (50 µg/ml), and 2 µg/ml M-CSF. After 7 days in culture, monocytes differentiated into macrophages and the monocyte-derived macrophages (MDM) were infected with HIV-1ADA at a multiplicity of infection (MOI) of 0.01 for 24 hr before being injected into the brain of severe combined immunodeficient (SCID) mice.
SCID mouse model of HIVE
SCID mice (male C.B-17, 4 weeks old) were purchased from the Charles River Laboratories (Wilmington, MA). Each animal was inoculated into the basal ganglia (caudate and putamen) with 5µl of either an HIV-1ADA-infected or uninfected human MDM suspension containing 3 × 105 cells. All animal procedures were approved by the Institutional Animal Care and Use Committee of University of Nebraska Medical Center.
BMM tracking by CT/SPECT
BMM were labeled with 111indium (111In) oxyquinoline (Indium oxine, GE Healthcare, Arlington Heights, IL) at a dose of 600 µCi per 30×106 cells in 1 ml RPMI 1640 supplemented with 10 mM HEPES for 45 min at 37°C. Cells were washed and resuspended in Hank’s buffered salt solution (HBSS). Labeling efficiency was determined by γ-scintillation spectrometry (Packard Instrument Co., Meriden, CT) and was > 75% of total input isotope. Each recipient mouse received 111In-labeled BMM by i.v. injection. Replicate groups of animals were simultaneously injected intraperitoneally (i.p.) with 4-aminopyridine daily (4-AP, 1 mg/kg) dissolved in phosphate-buffered saline (PBS). Mice were anesthetized with 0.5–1% isoflurane delivered in a 2:1 mixture of nitrous oxide and oxygen. Image acquisition was accomplished by a computed tomography/single photon emission computed tomography system (CT/SPECT) (FLEX Triumph, Gamma Medica-Ideas, Northridge, CA). CT images were acquired by an X-ray detector, while SPECT images were acquired with a γ-scintillation camera detector fitted with 5 pinhole collimator. Co-registration of anatomical CT images and functional SPECT were performed by 3D image visualization and analysis software VIVID Gamma Medica-Ideas). The specific tissue and region of interest (ROI) of active viral encephalitis were defined, and relative radioactivity for each area determined.
RNA Extraction and Real-Time RT-PCR
Total RNA was isolated from cells using TRIzol Reagent (Invitrogen, Carlsbad, CA) and was purified by RNeasy Mini Kit (QIAGEN, Inc., Valencia, CA). Real-time RT-PCR for mouse KCNA3, KCND1 and KCNN4 was performed using the one-step quantitative TaqMan real-time RT-PCR system (Applied Biosystems, Inc., Foster City, CA). Gene expression levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as internal control.
Western blots
Total proteins were prepared from BMM treated with MCP-1 or lipopolysaccharide (LPS) and equivalent amounts were electrophoretically separated on 4–15% sodium dodecyl sulfate (SDS) polyacrylamide gel then transferred to Trans-Blot nitrocellulose membrane (BioRad, Hercules, CA). The membranes were probed with rabbit polyclonal antibodies to Kv1.3 and Kv1.5 (Almonade Lab, Israel) and images visualized with a chemoilluminescent horseradish peroxidase substrate kit (Pierce Biotechnology, Rockford, IL).
Immunohistochemistry
BMM were fixed in 3.7% formaldehyde solution for 10 minutes at room temperature, permeabilized in 0.1% TRITON X-100 for 3 to 5 minutes. Nonspecific binding on BMM was blocked with 1% bovine serum albumin in phosphate buffered saline for 30 minutes, and stained for actin expression with rhodamine phalloidin. All samples were viewed on a confocal microscope.
Chemotaxis assay
BMM were labeled with 2.5 µM 5-chloromethylfluorescein diacetate (CMFDA) (Invitrogen) in serum free DMEM at 37°C for 15 minutes, washed, and adjusted to 3×106 cells/ml in complete DMEM. Tetraethylammonium chloride (TEA) was used to inhibit K+ channels. The CMFDA-labeled cells were loaded into chemotaxis µ-slides (ibidi, GmbH, Munich, Germany) in 10 µl per chamber and incubated at 37°C for 35 minutes to allow cell attachment. Fresh or conditioned media (CM) was loaded into adjacent reservoirs of central channel and the cells were imaged continuously for 2 hours using a Nikon swept-field laser confocal microscope (Nikon Instruments, New York, NY). Images were analyzed using different plugins interfaced with ImageJ 1.38X software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997–2007). Migration co-ordination data for each observed cell was acquired with the Manual Tracking plugin (Fabrice Cordelières, Institut Curie, Orsay, France). Chemotaxis plots and migration velocities of each cell were determined with the Chemotaxis and Migration Tool (ibidi).
Transendothelial migration
Transendothelial migration of BMM assay was measured using a quantitative fluorescence-based assay. First, mouse brain endothelial cells b.END3 (ATCC, Manassas, VA) were plated on rat-tail collagen type I coated FluoroBlok tinted tissue culture inserts (with 3 µm pores; BD Biosciences, San Diego, CA) at a density of 1.5×104 cells/insert (Ramirez et al., 2008; Yamamoto et al., 2008). Thereafter, endothelial cells and BMM were treated with TEA for 30 minutes and recombinant mouse MCP-1 (CCL2/MCP-1, 30 ng/ml; R&D Systems, Minneapolis, MN) was introduced into the lower chamber to create a chemokine gradient similar to that present in neuroinflammatory disorders. Labeled BMM (8×104 cells/insert loaded with calcein-AM at 5 µM/1×106 cells for 45 minutes) were placed in the upper chamber, and migration was allowed to continue up to 4 hour at 37°C. Migration was monitored by measuring the relative fluorescence of the labeled BMM found in the lower chamber by fluorescence spectrometry (Molecular Devices). The numbers of migrated BMM were derived from a standard curves of the relative fluorescence as a function of known numbers of labeled BMM.
Results
MCP-1 increased outward K+ currents in BMM
Voltage-gated K+ channels (Kv) play a key role in cell volume regulation and as such, cell movement. Under whole cell voltage clamp recording, outward currents were evoked on BMM by voltage steps (figure 1A). These voltage sensitive outward currents were blocked by TEA, a voltage-gated K+ channel blocker (figure 1B), suggesting the expression of Kv channels in BMM. Because MCP-1 is expressed in inflamed tissues serving as a pivotal chemoattractant for cell migration and Kv channels are involved in controlling macrophage migration and proliferation (Gallin, 1991), we investigated its influence on BMM Kv channels. As shown in figure 1A, bath perfusion of MCP-1 increased the outward K+ currents induced by a voltage step from −60mV to +100mV with the holding potential of −60mV in a dose-dependent manner. The average magnitude of steady-state outward K+ currents before application of MCP-1 was 100.0 ± 2.6% of control (M ± S.D., n=9). In contrast, bath application of MCP-1 at concentrations of 2, 20, and 200 ng/ml increased the steady-state K+ currents to 164.1±1.75%, 191.7±4.2%, and 344.0±24.0% of control, respectively (n=9, figure 1B). After washout of MCP-1, the outward K+ currents returned control levels (before application of MCP-1), with an average magnitude of 106.7±6.4% of control (n=9). Based on these observations, we next examined whether the Kv channel blocker, tetraethylammonium (TEA), could block K+ currents induced by MCP-1. In another set of BMM, bath application of MCP-1 (20 ng/ml) alone increased the steady-state outward currents to an average of 167.3±18.8% of control (n=5, figure 2). However, when MCP-1 was co-applied with TEA (20mM), the average steady-state outward K+ currents decreased (p<0.05) by 83.5±15.6% of control (n=5, figure 2) suggesting that MCP-1-mediated enhancement of outward K+ current is sensitive to Kv channel blockers. After washout of MCP-1 and TEA, the outward K+ currents returned to control levels, with an average of 96.6±9.7% of control (figure 2, n=4).
Figure 1. MCP-1 enhanced outward K+ currents in C57BL/6 mouse bone marrow-derived macrophages (BMM).
(A) Representative recordings from BMM before (control), during, and after (wash) bath perfusion with MCP-1 were assessed at different concentrations of 2 ng/ml, 20 ng/ml, and 200 ng/ml. Note that MCP-1 enhanced outward K+ currents in a concentration-dependent manner. (B) Concentration-dependent effects of MCP-1 were assessed on the steady-state outward K+ currents induced by a voltage step from −60 mV (holding potential) to +100 mV. Bath application of MCP-1 significantly increased K+ currents, as compared with control (n=9). (C) The voltage jump protocol employed in this study is shown. * p<0.05; ** p<0.01.
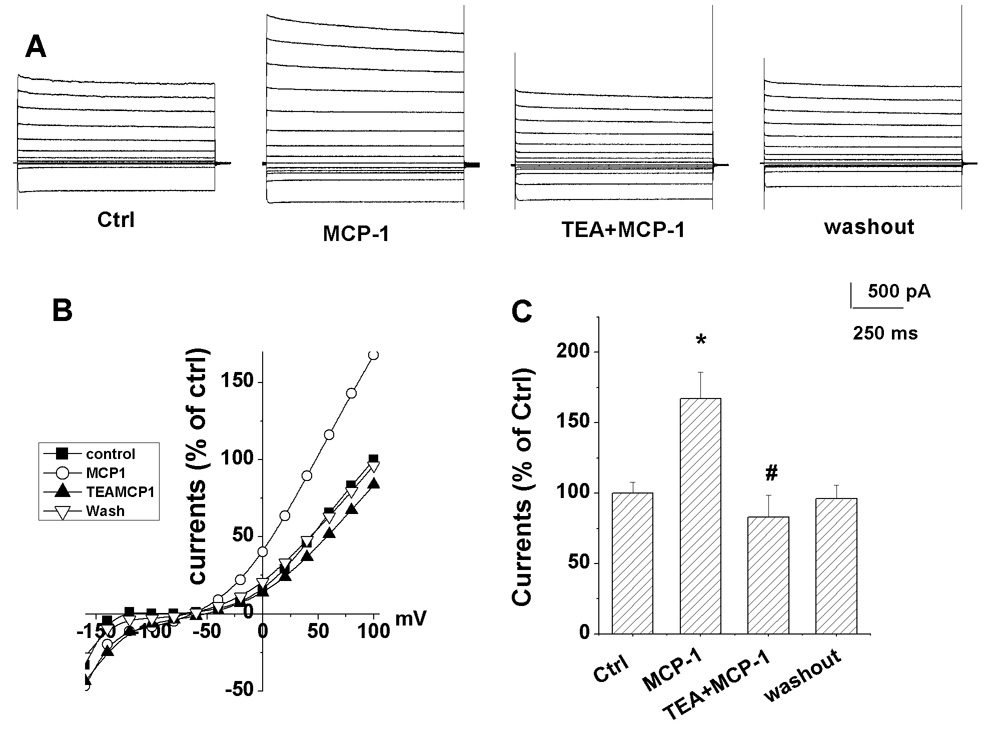
Figure 2. Blockade of MCP-1-induced enhancement of outward K+ current by TEA.
(A) Whole-cell outward K+ currents were recorded from a bone marrow derived macrophage before (control), during bath application of MCP-1 (MCP-1, 20 ng/ml) and TEA+MCP-1 (20 ng/ml of each) (TEA+MCP-1), and washout period (washout). Note the blockade of MCP-1-induced increase of outward K+ currents was significantly blocked by TEA (20 mM). (B) I-V curves were constructed from the results shown in panel A. (C) The steady-state K+ current induced by a voltage step from −60mV to +100mV was significantly increased by MCP-1(p<0.05, n=5). The MCP-1-induced increase of outward K+ currents was significantly blocked by bath application of TEA (p<0.01, n=5). Voltage jump protocol employed was the same as shown in figure 1C. *Compared with control group; # compared with MCP-1 alone group.
MCP-1 regulates K+ channel gene expression
To determine the responsiveness of K+ channel to MCP-1, we investigated the mRNA abundance and protein expression level of genes related to K+ channel. As shown in figure 3, we measured KCND1 (Kv4.1), KCNA3 (Kv1.3), and KCNN4 (KCa3.1) mRNA levels of BMM after treatment with MCP-1 and LPS. The results showed that KCNA3 gene expression is significantly increased 30 and 60 minutes after treatment with MCP-1 (figure 3C, P<0.01). Considering that Kv1.3 and Kv1.5 account for most of the voltage-gated K+ currents expressed by BMM, we tested the protein expression of Kv1.3 and Kv1.5. After treatment with MCP-1 for 30 and 60 minutes, Kv1.3 and Kv1.5 protein expression was readily increased by BMM (figure 3D). The results suggested that some K+ channel genes are regulated by MCP-1.
Figure 3. MCP-1 altered K+ channel gene and protein expression in mouse BMM cells.
BMM were stimulated with LPS or MCP-1 and mRNA was extracted at 0, 30, and 60 minutes, after stimulation, respectively. Abundance of mRNAs for individual samples was quantified using quantitative RT-PCR and normalized to GAPDH. Data were presented as fold-increase of specific mRNA species encoding for (A) KCND1, (B) KCNA3, and (C) KCNN4. (D) BMM cells were stimulated with LPS or MCP-1. Protein was extracted at 0, 30, and 60 minutes after stimulation, respectively for Western blot analysis. Western blot analysis results show that MCP-1 induced increases in Kv1.5 and Kv1.3 protein expression.
MCP-1 affects BMM F-actin reorganization
F-actin reorganization is essential for cell morphogenesis and migration. Based on morphology and F-actin distribution within BMM, macrophages can be categorized into four groups showing: (1) ramified macrophages with more than two protrusions; (2) elongated cells with two protrusions; (3) round cells with no protrusions; and (4) migratory cells with one or more rich lamellae and a tail (polarized F-actin). Migratory morphology was present in 25±2.9% of unstimulated control group, whereas after stimulation with 20 ng/ml MCP-1 for one hour, 48.56±1.7% of the cells exhibited a migratory morphology (p<0.05, figure 4A and B).
Figure 4. MCP-1 increased the migratory morphology of BMM.
(A) BMM cells were stimulated LPS or MCP-1 for 60 minutes, fixed with formaldehyde, and stained with rhodamine phalloidin. Examples of round, migratory, ramified, and elongated BMM cells are indicated by blue, yellow, green and white arrows, respectively. (B) BMM numbers were counted from slides based on different morphologies: round, migratory, ramified, and elongated for BMM cells treated with MCP-1 ( ), LPS (■) or media alone (control) (□). BMM numbers were normalized to percent of total number of BMM. *P<0.01 compared with control.
), LPS (■) or media alone (control) (□). BMM numbers were normalized to percent of total number of BMM. *P<0.01 compared with control.
MCP-1 facilitated BMM migration and is blocked by K+ channel antagonists
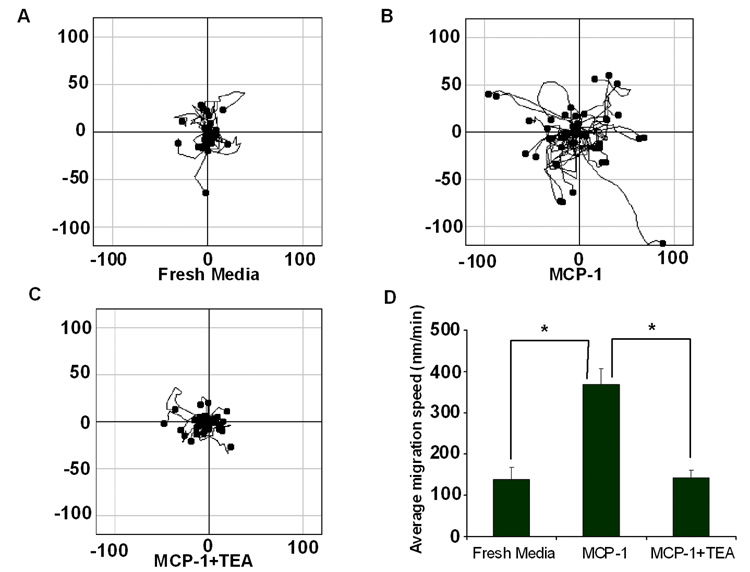
To investigate how MCP-1 influences BMM migration, we monitored the mobility of 5-chloromethylfluorescein diacetate (CMFDA)-labeled BMM in chemotaxis chambers by laser confocal microscopy live imaging (figure 5A–C). Minimal BMM migration was observed in fresh media (figure 5A). However, levels of BMM migration significantly increase when MCP-1 was introduced to the migratory system (figure 5B). This level of BMM migration was attenuated by TEA, a non-specific voltage-gated K+ channel blocker (figure 5C). These results were consistent with the measured mean migration speed. In control media, BMM migrated at 138.5±29.9 nm/min (mean ± SEM), which increased to 368.6±37.5 nm/min in MCP-1 conditioned media (figure 5D). However, after co-culture with TEA and MCP-1, BMM migration speed was reduced to 143.0±18.6 nm/min; levels comparable to those obtained in control media. Together, these observations demonstrated that MCP-1 significantly affects BMM mobility by activating K+ channels.
Figure 5. BMM mobility increased after treated with MCP-1 and blocked by TEA.
Chemotaxis Assay 2-Reservoir µ-slides (ibidi, LLC) was utilized to generate MCP-1 gradient and monitor BMM mobility by confocal microscopy live imaging. BMM migration over a defined area was determined for (A) both reservoirs filled with fresh media to generate a control environment; (B) MCP-1 loaded one reservoir and media and BMM in the other; and (C) MCP-1 applied to one reservoir, BMM introduced into the other, and TEA (20mM) applied to both reservoirs. BMM migration was assessed by live imaging over 2 h and analyzed by Image J software with chemotaxis tools plugins. (D) BMM migration in the presence of media alone, MCP-1, and MCP-1 in the presence of TEA was evaluated as a function of speed. *P<0.05 compared to MCP-1 treated BMM.
K+ channels regulate BMM BBB transmigration
Since MCP-1 increased BMM mobility and TEA attenuated mobility, we determined the biological importance of these observations by assessing whether MCP-1 induced BMM migration across an in vitro BBB was affected by a Kv channel antagonist. We used a mouse brain endothelial cell to construct the in vitro barrier system and evaluated BMM transendothelial migration in the absence or presence of MCP-1 and/or TEA. BMM cultured with MCP-1 increased BMM migration across the endothelium compared to migration in the absence of MCP-1 (figure 6A). In contrast, the ability of the BMM to migrate across the endothelium in the presence of TEA was significantly decreased. Similar results were obtained when both cell types were exposed to TEA (figure 6B). Taken together, these results suggested that K+ channels play a central role in MCP-1 induced BMM migration.
Figure 6. Effects of MCP-1 on BMM migration across a murine BBB.
BMM migration across mouse brain endothelial cells toward MCP-1 (20 ng/ml) was evaluated after 2 hours. (A) BMM were pretreated with TEA for 30 minutes and then removed before migration. (B) Both BMM and mouse brain endothelial cells were pretreated with TEA for 30 minutes. Data are represented as mean ± SEM for numbers of migrated BMM. *P<0.05 for comparisons of groups attached by indicated lines.
CT/SPECT analysis of 111In-labeled BMM transmigration in peripheral tissues
To assess real-time migration of macrophages, 1×107 111In-labeled BMM were adoptively transferred to normal recipients or recipients treated i.p. with 4-AP, and migration was assessed by CT/SPECT at 2 h, 24 h, 48 h and 72 h following adoptive transfer. At 2 h post-transfer time point, radiolabeled BMM was detected in lung tissue with reduced signal in spleen and liver (Fig. 7A), however by 24 h post-transfer the predominant signal was localized to spleen and liver. To quantify labeled BMM within each tissue, regions of interest were electronically mapped and total radioactivity counts determined. Relative number of counts/tissue volume confirmed that BMM levels in lungs were higher by 2 h then decreased quickly thereafter (figure 7B). Labeled BMM levels in liver and spleen was lower at the first 2 h, however by 24 h, higher levels were observed in liver and spleen and diminished thereafter, indicating that most BMM migrated to liver and spleen (figure 7C and D). Comparable levels of labeled BMM migration in animals were observed regardless of treatment with or without AP-4 K+ channel (p>0.05). These results suggest that K+ channel blocker had no effect on BMM migration into peripheral tissues under non-inflammatory conditions.
Figure 7. Peripheral BMM migration in non-HIVE mice is not affected by K+ channel blockade.
BMM were isolated from the femurs BALB/C mice and grown in MCSF containing media. After one week of cultivation, 1×107 BMM cells (purity by CD11b staining of 95%) were recovered, labeled with 111In oxine (indium), and transferred by i.v. injections to 2 groups of naïve mice (4 animals/group). Mice were then treated with either PBS (Control) or 1 mg/kg of the K+ channel blocker, AP-4 (Blocker), administered by daily i.p. injections. (A) A representative animal was imaged 2h (left panel) and 24 h (right panel) after injection of indium-labeled BMM and CT images were co-registered with SPECT images (Gamma Medica-Ideas Inc.). Intensities of radioactive signals from indium-labeled BMM are shown as pseudo-colored blue-green signals. Quantitative assessment of labeled BMM distributions in PBS (Control) and AP-4 (Blocker) treated mice were calculated for (B) lung, (C) liver, and (D) spleen by digital image software (VIVID, Gamma Medica-Ideas, Inc.). Relative radioactive counts as a function of pixel intensities were determined and presented as the means ± SEM for 4 mice/group.
CT/SPECT analysis of 111In-labeled BMM transmigration into brains of HIVE mice
We next assessed the effects of K+ channel blockade on BMM migration to the brain and across the BBB under inflammatory conditions exhibited in a murine model of HIVE. Here HIV-1ADA-infected MDMs were injected intracranially by stereotactic measures within the caudate and putamen. After 3 days, 1×107 111In-labeled BMM were adoptively transferred i.v. to control, HIVE and HIVE mice treated with AP-4 K+ blocker. BMM transmigration into the brain and the brain subregion with active encephalitis were tracked by CT/SPECT at 2 h, 24 h and 48 h post-transfer. Whole brain and injection site were electronically bit mapped in reconstructed 3D images and relative radioactive levels were determined for each recipient (figure 8A). of counts within the whole brain (Fig. 8B) and ROI area (Fig. 8C) are shown. Quantitative analyses of those bitmapped tissues demonstrated that radioactive counts of BMM from HIVE mice treated with AP-4 K+ blocker were significantly diminished within whole brains by 24 h post-transfer compared to control or HIVE mice (figure 8B). Upon closer examination of the area containing the injection site and thus the focal HIVE, relative counts from K+ blocker-treated animals significantly diminished by 2 h and times thereafter when compared to the HIVE animals (figure 8C). These results suggest that K+ channels clearly affect the migration of BMM to brain within the context of HIVE inflammatory conditions.
Figure 8.
Brain BMM migration for HIVE mice is affected by K+ channel blockade. HIVE was induced by the sterotactic injection of virus-infected human monocyte-derived macrophages (MDM). Mouse BMM were labeled with 111In oxine, washed and 1×107 labeled BMM were adoptive transferred to 4 animals/groups. One group of HIVE mice received PBS and the other group received 1 mg/kg of the K+ channel blocker, 4-AP administered by daily i.p. injections. BMM ingress into the brain was tracked by CT/SPECT imaging (Gamma Medica-Ideas Inc.) for each animal 2 h, 24 h and 72 h post transfer. Radioactivity in whole brain and in the area of MDM injection was assessed by digital image analysis software (VIVID, Gamma Medica-Ideas, Inc.). (A) CT/SPECT image from control mouse showing electronically mapped whole brain (pink) and the region of interest (ROI) (broken white line) that encompasses the injection site within the basal ganglia. Total counts as a function of pixel intensity were calculated by digital image analysis (VIVID software, Gamma Medica-Ideas, Inc.) from whole brain (B) and the ROI comprising the injection site and area of inflammation (C) for HIVE mice treated with PBS (Control) or 4-AP (Blocker). *Compared to the HIVE group, p<0.05.
Discussion
Several different subtypes of Kv channels are expressed in human and mouse monocytes and macrophages including, but not limited to, Kv1.3, Kv1.5 (Gallin, 1991; Mackenzie et al., 2003; Vicente et al., 2003). These channels play an important role in controlling macrophage functions such as proliferation, activation, migration, and cytokine production (Gallin, 1991; DeCoursey et al., 1996; Qiu et al., 2002). Studies have shown that Kv channels in macrophages can be regulated by many factors including pro-inflammatory cytokines. In the present study, we have demonstrated that MCP-1, a glial-derived cytokine, enhanced outward K+ currents and macrophage transmigration via regulation of Kv channel expression and channel activity. Our results herein fully support works by others demonstrating the expression of Kv channels in monocytes and macrophages (Mackenzie et al., 2003; Vicente et al., 2005; Vicente et al., 2006; Irvine et al., 2007; Villalonga et al., 2007). Voltage-gated K+ channels including Kv1.3 channels are operative in blood-borne macrophages, tissue macrophages, and microglia, and are prominently induced concomitant with M-CSF-dependent proliferation.
It is well known that differential channel regulation affects intracellular signals and is linked to macrophage activation and differentiation as well as to immune, morphological, and migratory responses of the cells (DeCoursey et al., 1996; Mackenzie et al., 2003; Fordyce et al., 2005; Rus et al., 2005; Vicente et al., 2005; Irvine et al., 2007). LPS-activation, for example, differentially regulates channel expression, as demonstrated by the induction of Kv1.3 channels and down-regulation of Kir2.1 channels. This modulation is dependent on the mode of macrophage immune responses (Gerth et al., 2005; Lei et al., 2005; Hoa et al., 2007; Villalonga et al., 2007). In this report, we have shown by pharmacological, mRNA and protein analyses that macrophage ion currents are operative through Kv1.3 and Kir2.1 channels. This provides further support for the physiological importance of potassium channels in macrophage immunity. For example, it is well known that Kv1.3 channels are those principally found in macrophages and in other blood leukocytes (Mackenzie et al., 2003; Vicente et al., 2003; Fordyce et al., 2005; Vicente et al., 2005). For T cells, these channels are important since they serve as a primary regulator and affector of cell activation, as well as in maintaining resting membrane potential and ion homeostasis (Estes et al., 2008; Poulopoulou et al., 2008). Importantly, less is known about the functional roles of channels in disease; although, Kv1.3 blockade has been shown to reduce proinflammatory cytokine secretion and cell proliferation (Irvine et al., 2007), as well as slow cell movement. Such findings serve as a foundation for investigations into whether channel modulation can be utilized as a therapeutic target for autoimmune disease such as multiple sclerosis as suggested from studies showing that selective Kv1.3 channel blockers diminish disease progression in experimental allergic encephalomyelitis (Gonsette, 2004; Vianna-Jorge and Suarez-Kurtz, 2004; Beeton and Chandy, 2005; Panyi, 2005; Rus et al., 2005; Beraud et al., 2006).
In hippocampal microglia, Kv channel toxins affect pathobiological responses and cell migration across tissue barriers (Bordey and Spencer, 2003; Fordyce et al., 2005; Thomas et al., 2007). Cytokines, toll receptor activation, and phorbol esters upregulate macrophage expression of Kv channels. Kv channel expression has been shown to be strongly upregulated in macrophage populations, and only recently has the functional role of these channels been shown. First, extracellular K+ can activate T cell β1 integrin and mediate adhesion and cell migration (Levite et al., 2000; Artym and Petty, 2002; Davis et al., 2002). The gating of leukocyte voltage-gated K+ channels (Kv1.3) affects physiological responses, including elevated extracellular K+ levels, integrin functions, and cell volume changes since they are linked to cell migration (Colden-Stanfield and Scanlon, 2000; Colden-Stanfield, 2002). Ion channels are known to affect macrophage function. For human macrophages, the major ionic currents are carried by an outwardly rectifying K+ channel activated by adhesion onto a substrate stretch applied to membrane patches. While K+ channels govern properties of cell movement, chloride and K+ channels contribute to the regulation of biological responses including nitric oxide. Indeed, nitric oxide synthase is inhibited by channel blockers. Thus, a range of ion channels expressed by macrophages and microglia is linked to cell biology (Gerth et al., 2005; Lei et al., 2005; Hoa et al., 2007; Villalonga et al., 2007).
The linkages shown in this report between MCP-1, K+ channels, and macrophage migration across the BBB are significant in regards to the pathogenesis of HAND and for a variety of reasons. HIV-1 transactivator protein Tat or progeny virus released from infected macrophages significantly increases astrocyte production and release of MCP-1 (Eugenin et al., 2005; El- Hage et al., 2006). MCP-1 is also expressed in the brains of patients at high levels with HIV-1 associated dementia (HAD), and MCP-1 levels within the cerebrospinal fluid correlate with the severity of neurological dysfunction (Sevigny et al., 2004; Maslin et al., 2005; Sevigny et al., 2007). Such observations are mirrored in simian immunodeficiency virus (SIV) infected macaques with pathological features of HIV-1 encephalitis (Zink and Clements, 2002; Mankowski et al., 2004; Harrington et al., 2007). Homozygosity for the MCP-1-2578G allele was linked to a 50% reduction in the risk of HIV-1 acquisition. However, following HIV-1 infection this MCP-1 genotype was associated with accelerated disease and a 4.5-fold increased risk of acquiring HAD. The mutant MCP-1-2578G allele in yields increased transcriptional activity, protein production, and serum MCP-1 levels concomitant with accelerated MP tissue migration (Gonzalez et al., 2002).
To evaluate the biological significance of potassium channel influence on BMM migration into the brain, we investigated BMM migration in a SCID mouse model with HIVE using CT/SPECT. HIVE was induced by i.c. injection of HIV-1ADA infected MDM into the caudate and putamen of SCID mice and is characterized by robust inflammation and the generation of an MCP-1 gradient for BMM attraction into sites of active disease. In this work, comparisons of BMM brain migration between uninfected and HIVE mice that were either untreated or treated with 4-aminopyridine (4-AP), a K+ channel blocker, showed that Kv channel blocker had no effects on BMM distribution in the peripheral organs, but significantly reduced BMM migration into the mouse brain. These results further reinforce the biological importance of potassium channels for macrophage migration and its links to MCP-1 and inflammatory responses.
The data, taken together, build on previous works demonstrating a link between MCP-1 induced biological responses and specific ion channels. MCP-1 certainly has a multifaceted role in HAND through its abilities to affect proinflammatory properties and contribute to disease progression and increased risk of dementia by affecting MP ingress into the brain. The importance of the current study rests in that these physiological responses to MCP-1 play a central role in HAND and as such opens up new therapeutic targets for disease.
Acknowledgements
The authors thank Mr. Anil Papugani for his help in BMM migration across murine blood-brain barrier experiments. The authors extend their thanks to Robin Taylor for her critical reading of the manuscript. The work was supported by the Frances and Louis Blumkin Foundation, the Community Neuroscience Pride of Nebraska Initiative, the Carol Swartz, M.D. Emerging Neuroscience Laboratory, and the Alan Baer Charitable Trust (to H.E.G.), NIH grants P01 NS31492 (to D.J.V.), R01 MH65151 (to Y.P.), R01 NS041862 (to H.X.), 2R37 NS36126, P01 NS043985, and P20 RR15635 (to H.E.G.).
References
- Artym VV, Petty HR. Molecular proximity of Kv1.3 voltage-gated potassium channels and beta(1)-integrins on the plasma membrane of melanoma cells: effects of cell adherence and channel blockers. J Gen Physiol. 2002;120:29–37. doi: 10.1085/jgp.20028607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Rostene W, Kitabgi P, Parsadaniantz SM. Chemokines and brain functions. Curr Drug Targets Inflamm Allergy. 2005;4:387–399. doi: 10.2174/1568010054022097. [DOI] [PubMed] [Google Scholar]
- Beeton C, Chandy KG. Potassium channels, memory T cells, and multiple sclerosis. Neuroscientist. 2005;11:550–562. doi: 10.1177/1073858405278016. [DOI] [PubMed] [Google Scholar]
- Beraud E, Viola A, Regaya I, Confort-Gouny S, Siaud P, Ibarrola D, Le Fur Y, Barbaria J, Pellissier JF, Sabatier JM, Medina I, Cozzone PJ. Block of neural Kv1.1 potassium channels for neuroinflammatory disease therapy. Ann Neurol. 2006;60:586–596. doi: 10.1002/ana.21007. [DOI] [PubMed] [Google Scholar]
- Berman JW, Carson MJ, Chang L, Cox BM, Fox HS, Gonzalez RG, Hanson GR, Hauser KF, Ho WZ, Hong JS, Major EO, Maragos WF, Masliah E, McArthur JC, Miller DB, Nath A, O'Callaghan JP, Persidsky Y, Power C, Rogers TJ, Royal W., 3rd NeuroAIDS, drug abuse, and inflammation: building collaborative research activities. J Neuroimmune Pharmacol. 2006;1:321–399. doi: 10.1007/s11481-006-9048-9. [DOI] [PubMed] [Google Scholar]
- Biernacki K, Prat A, Blain M, Antel JP. Regulation of cellular and molecular trafficking across human brain endothelial cells by Th1- and Th2-polarized lymphocytes. J Neuropathol Exp Neurol. 2004;63:223–232. doi: 10.1093/jnen/63.3.223. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Sodhi A. Tyrosine phosphorylation-mediated signal transduction in MCP-1-induced macrophage activation: role for receptor dimerization, focal adhesion protein complex and JAK/STAT pathway. Int Immunopharmacol. 2002;2:1095–1107. doi: 10.1016/s1567-5769(02)00055-3. [DOI] [PubMed] [Google Scholar]
- Bordey A, Spencer DD. Chemokine modulation of high-conductance Ca(2+)-sensitive K(+) currents in microglia from human hippocampi. Eur J Neurosci. 2003;18:2893–2898. doi: 10.1111/j.1460-9568.2003.03021.x. [DOI] [PubMed] [Google Scholar]
- Buhling F, Lieder N, Kuhlmann UC, Waldburg N, Welte T. Tiotropium suppresses acetylcholine-induced release of chemotactic mediators in vitro. Respir Med. 2007;101:2386–2394. doi: 10.1016/j.rmed.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Callahan MK, Ransohoff RM. Analysis of leukocyte extravasation across the blood-brain barrier: conceptual and technical aspects. Curr Allergy Asthma Rep. 2004;4:65–73. doi: 10.1007/s11882-004-0046-9. [DOI] [PubMed] [Google Scholar]
- Callewaere C, Banisadr G, Rostene W, Parsadaniantz SM. Chemokines and chemokine receptors in the brain: implication in neuroendocrine regulation. J Mol Endocrinol. 2007;38:355–363. doi: 10.1677/JME-06-0035. [DOI] [PubMed] [Google Scholar]
- Cesar B, Abud AP, de Oliveira CC, Cardoso F, Gremski W, Gabardo J, Buchi DD. Activation of mononuclear bone marrow cells treated in vitro with a complex homeopathic medication. Micron. 2008;39(4):461–470. doi: 10.1016/j.micron.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Colden-Stanfield M. Clustering of very late antigen-4 integrins modulates K(+) currents to alter Ca(2+)-mediated monocyte function. Am J Physiol Cell Physiol. 2002;283:C990–C1000. doi: 10.1152/ajpcell.00481.2001. [DOI] [PubMed] [Google Scholar]
- Colden-Stanfield M, Scanlon M. VCAM-1-induced inwardly rectifying K(+) current enhances Ca(2+) entry in human THP-1 monocytes. Am J Physiol Cell Physiol. 2000;279:C488–C494. doi: 10.1152/ajpcell.2000.279.2.C488. [DOI] [PubMed] [Google Scholar]
- Crane JJLJ. Mechanisms of leukocyte migration across the blood barrier. Semin Immunopathol. 2008;30:165–177. doi: 10.1007/s00281-008-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Gui P, Hill MA, Wilson E. Regulation of ion channels by integrins. Cell Biochem Biophys. 2002;36:41–66. doi: 10.1385/CBB:36:1:41. [DOI] [PubMed] [Google Scholar]
- de la Rosa G, Longo N, Rodriguez-Fernandez JL, Puig-Kroger A, Pineda A, Corbi AL, Sanchez-Mateos P. Migration of human blood dendritic cells across endothelial cell monolayers: adhesion molecules and chemokines involved in subset-specific transmigration. J Leukoc Biol. 2003;73:639–649. doi: 10.1189/jlb.1002516. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Kim SY, Silver MR, Quandt FN. Ion channel expression in PMA-differentiated human THP-1 macrophages. J Membr Biol. 1996;152:141–157. doi: 10.1007/s002329900093. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, Bruce-Keller AJ, Hauser KF. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53:132–146. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes DJ, Memarsadeghi S, Lundy SK, Marti F, Mikol DD, Fox DA, Mayer M. High-Throughput Profiling of Ion Channel Activity in Primary Human Lymphocytes. Anal Chem. 2008;80(10):3728–3735. doi: 10.1021/ac800164v. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Dyer G, Calderon TM, Berman JW. HIV-1 tat protein induces a migratory phenotype in human fetal microglia by a CCL2 (MCP-1)-dependent mechanism: possible role in NeuroAIDS. Glia. 2005;49:501–510. doi: 10.1002/glia.20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordyce CB CB, Jagasia R, Zhu X, Schlichter LC. Microglia Kv1.3 channels contribute to their ability to kill neurons. J Neurosci. 2005;25:7139–7149. doi: 10.1523/JNEUROSCI.1251-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher JA, Hashimi ST, Levroney EL, Pang M, Gurney KB, Baum LG, Lee B. Galectin-1-matured human monocyte-derived dendritic cells have enhanced migration through extracellular matrix. J Immunol. 2006;177:216–226. doi: 10.4049/jimmunol.177.1.216. [DOI] [PubMed] [Google Scholar]
- Gallin EK. Ion channels in leukocytes. Physiol Rev. 1991;71:775–811. doi: 10.1152/physrev.1991.71.3.775. [DOI] [PubMed] [Google Scholar]
- Garlet GP, Martins W, Jr, Ferreira BR, Milanezi CM, Silva JS. Patterns of chemokines and chemokine receptors expression in different forms of human periodontal disease. J Periodontal Res. 2003;38:210–217. doi: 10.1034/j.1600-0765.2003.02012.x. [DOI] [PubMed] [Google Scholar]
- Gerth A, Grosche J, Nieber K, Hauschildt S. Intracellular LPS inhibits the activity of potassium channels and fails to activate NFkappaB in human macrophages. J Cell Physiol. 2005;202:442–452. doi: 10.1002/jcp.20146. [DOI] [PubMed] [Google Scholar]
- Gonsette RE. New immunosuppressants with potential implication in multiple sclerosis. J Neurol Sci. 2004;223:87–93. doi: 10.1016/j.jns.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, Mummidi S, Kulkarni H, Bamshad MJ, Telles V, Anderson SA, Walter EA, Stephan KT, Deucher M, Mangano A, Bologna R, Ahuja SS, Dolan MJ, Ahuja SK. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci U S A. 2002;99:13795–13800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington PR, Connell MJ, Meeker RB, Johnson PR, Swanstrom R. Dynamics of simian immunodeficiency virus populations in blood and cerebrospinal fluid over the full course of infection. J Infect Dis. 2007;196:1058–1067. doi: 10.1086/520819. [DOI] [PubMed] [Google Scholar]
- Hoa NT, Zhang JG, Delgado CL, Myers MP, Callahan LL, Vandeusen G, Schiltz PM, Wepsic HT, Jadus MR. Human monocytes kill M-CSF-expressing glioma cells by BK channel activation. Lab Invest. 2007;87:115–129. doi: 10.1038/labinvest.3700506. [DOI] [PubMed] [Google Scholar]
- Irvine E, Keblesh J, Liu J, Xiong H. Voltage-gated potassium channel modulation of neurotoxic activity in human immunodeficiency virus type-1(HIV-1)-infected macrophages. J Neuroimmune Pharmacol. 2007;2:265–269. doi: 10.1007/s11481-007-9072-4. [DOI] [PubMed] [Google Scholar]
- Lane JH, Sasseville VG, Smith MO, Vogel P, Pauley DR, Heyes MP, Lackner AA. Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation. J Neurovirol. 1996;2:423–432. doi: 10.3109/13550289609146909. [DOI] [PubMed] [Google Scholar]
- Lei XJ, Ma AQ, Xi YT, Zhang W, Yao Y, Du Y. Expression of Kir2.1 channel during differentiation of human macrophages into foam cells. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:1461–1467. [PubMed] [Google Scholar]
- Levite M, Cahalon L, Peretz A, Hershkoviz R, Sobko A, Ariel A, Desai R, Attali B, Lider O. Extracellular K(+) and opening of voltage-gated potassium channels activate T cell integrin function: physical and functional association between Kv1.3 channels and beta1 integrins. J Exp Med. 2000;191:1167–1176. doi: 10.1084/jem.191.7.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol. 2008;84:116–131. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie AB, Chirakkal H, North RA. Kv1.3 potassium channels in human alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2003;285:L862–L868. doi: 10.1152/ajplung.00095.2003. [DOI] [PubMed] [Google Scholar]
- Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J Exp Med. 2008;205:951–966. doi: 10.1084/jem.20072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Clements JE, Zink MC. Cerebrospinal fluid markers that predict SIV CNS disease. J Neuroimmunol. 2004;157:66–70. doi: 10.1016/j.jneuroim.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Maslin CL, Kedzierska K, Webster NL, Muller WA, Crowe SM. Transendothelial migration of monocytes: the underlying molecular mechanisms and consequences of HIV-1 infection. Curr HIV Res. 2005;3:303–317. doi: 10.2174/157016205774370401. [DOI] [PubMed] [Google Scholar]
- Mines M, Ding Y, Fan GH. The many roles of chemokine receptors in neurodegenerative disorders: emerging new therapeutical strategies. Curr Med Chem. 2007;14:2456–2470. doi: 10.2174/092986707782023686. [DOI] [PubMed] [Google Scholar]
- Muller MM, Singer BB, Klaile E, Obrink B, Lucka L. Transmembrane CEACAM1 affects integrin-dependent signaling and regulates extracellular matrix protein-specific morphology and migration of endothelial cells. Blood. 2005;105:3925–3934. doi: 10.1182/blood-2004-09-3618. [DOI] [PubMed] [Google Scholar]
- Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999;66:698–704. doi: 10.1002/jlb.66.5.698. [DOI] [PubMed] [Google Scholar]
- Nottet HS, Persidsky Y, Sasseville VG, Nukuna AN, Bock P, Zhai QH, Sharer LR, McComb RD, Swindells S, Soderland C, Gendelman HE. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156:1284–1295. [PubMed] [Google Scholar]
- Panyi G. Biophysical and pharmacological aspects of K+ channels in T lymphocytes. Eur Biophys J. 2005;34:515–529. doi: 10.1007/s00249-005-0499-3. [DOI] [PubMed] [Google Scholar]
- Poulopoulou C, Papadopoulou-Daifoti Z, Hatzimanolis A, Fragiadaki K, Polissidis A, Anderzanova E, Davaki P, Katsiari CG, Sfikakis PP. Glutamate levels and activity of the T cell voltage-gated potassium Kv1.3 channel in patients with systemic lupus erythematosus. Arthritis Rheum. 2008;58:1445–1450. doi: 10.1002/art.23446. [DOI] [PubMed] [Google Scholar]
- Qiu MR, Campbell TJ, Breit SN. A potassium ion channel is involved in cytokine production by activated human macrophages. Clin Exp Immunol. 2002;130:67–74. doi: 10.1046/j.1365-2249.2002.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez SH, Heilman D, Morsey B, Potula R, Haorah J, Persidsky Y. Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) suppresses Rho GTPases in human brain microvascular endothelial cells and inhibits adhesion and transendothelial migration of HIV-1 infected monocytes. J Immunol. 2008;180:1854–1865. doi: 10.4049/jimmunol.180.3.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebenko-Moll NM, Liu L, Cardona A, Ransohoff RM. Chemokines, mononuclear cells and the nervous system: heaven (or hell) is in the details. Curr Opin Immunol. 2006;18:683–689. doi: 10.1016/j.coi.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Reinhart B, DeWitte-Orr SJ, Van Es SJ, Bols NC, Lee LE. Cell adhesion characteristics of a monocytic cell line derived from rainbow trout, Oncorhynchus mykiss. Comp Biochem Physiol A Mol Integr Physiol. 2006;144:437–443. doi: 10.1016/j.cbpa.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Rus H, Pardo CA, Hu L, Darrah E, Cudrici C, Niculescu T, Niculescu F, Mullen KM, Allie R, Guo L, Wulff H, Beeton C, Judge SI, Kerr DA, Knaus HG, Chandy KG, Calabresi PA. The voltage-gated potassium channel Kv1.3 is highly expressed on inflammatory infiltrates in multiple sclerosis brain. Proc Natl Acad Sci U S A. 2005;102:11094–11099. doi: 10.1073/pnas.0501770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, Schifitto G, Selnes OA, Stern Y, McClernon DR, Palumbo D, Kieburtz K, Riggs G, Cohen B, Epstein LG, Marder K. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63:2084–2090. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- Sevigny JJ, Albert SM, McDermott MP, Schifitto G, McArthur JC, Sacktor N, Conant K, Selnes OA, Stern Y, McClernon DR, Palumbo D, Kieburtz K, Riggs G, Cohen B, Marder K, Epstein LG. An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. Arch Neurol. 2007;64:97–102. doi: 10.1001/archneur.64.1.97. [DOI] [PubMed] [Google Scholar]
- Strell C, Lang K, Niggemann B, Zaenker KS, Entschladen F. Surface molecules regulating rolling and adhesion to endothelium of neutrophil granulocytes and MDA-MB-468 breast carcinoma cells and their interaction. Cell Mol Life Sci. 2007;64:3306–3316. doi: 10.1007/s00018-007-7402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MP, Chartrand K, Reynolds A, Vitvitsky V, Banerjee R, Gendelman HE. Ion channel blockade attenuates aggregated alpha synuclein induction of microglial reactive oxygen species: relevance for the pathogenesis of Parkinson's disease. J Neurochem. 2007;100:503–519. doi: 10.1111/j.1471-4159.2006.04315.x. [DOI] [PubMed] [Google Scholar]
- Tsai WH, Shih CH, Lin CC, Ho CK, Hsu FC, Hsu HC. Monocyte chemotactic protein-1 in the migration of differentiated leukaemic cells toward alveolar epithelial cells. Eur Respir J. 2008;31:957–962. doi: 10.1183/09031936.00135707. [DOI] [PubMed] [Google Scholar]
- Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wang G, Bissel SJ, Mathis CA, Meltzer CC, Boada F, Capuano S, 3rd, Kress GJ, Davis DK, Ruszkiewicz J, Reynolds IJ, Murphey-Corb M, Trichel AM, Wisniewski SR, Wiley CA. PET imaging of brain macrophages using the peripheral benzodiazepine receptor in a macaque model of neuroAIDS. J Clin Invest. 2004;113:981–989. doi: 10.1172/JCI20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev. 2007;218:178–196. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- Vianna-Jorge R, Suarez-Kurtz G. Potassium channels in T lymphocytes: therapeutic targets for autoimmune disorders? BioDrugs. 2004;18:329–341. doi: 10.2165/00063030-200418050-00005. [DOI] [PubMed] [Google Scholar]
- Vicente R, Escalada A, Soler C, Grande M, Celada A, Tamkun MM, Solsona C, Felipe A. Pattern of Kv beta subunit expression in macrophages depends upon proliferation and the mode of activation. J Immunol. 2005;174:4736–4744. doi: 10.4049/jimmunol.174.8.4736. [DOI] [PubMed] [Google Scholar]
- Vicente R, Escalada A, Coma M, Fuster G, Sanchez-Tillo E, Lopez-Iglesias C, Soler C, Solsona C, Celada A, Felipe A. Differential voltage-dependent K+ channel responses during proliferation and activation in macrophages. J Biol Chem. 2003;278:46307–46320. doi: 10.1074/jbc.M304388200. [DOI] [PubMed] [Google Scholar]
- Vicente R, Escalada A, Villalonga N, Texido L, Roura-Ferrer M, Martin-Satue M, Lopez-Iglesias C, Soler C, Solsona C, Tamkun MM, Felipe A. Association of Kv1.5 and Kv1.3 contributes to the major voltage-dependent K+ channel in macrophages. J Biol Chem. 2006;281:37675–37685. doi: 10.1074/jbc.M605617200. [DOI] [PubMed] [Google Scholar]
- Villalonga N, Escalada A, Vicente R, Sanchez-Tillo E, Celada A, Solsona C, Felipe A. Kv1.3/Kv1.5 heteromeric channels compromise pharmacological responses in macrophages. Biochem Biophys Res Commun. 2007;352:913–918. doi: 10.1016/j.bbrc.2006.11.120. [DOI] [PubMed] [Google Scholar]
- Whatling C, Bjork H, Gredmark S, Hamsten A, Eriksson P. Effect of macrophage differentiation and exposure to mildly oxidized LDL on the proteolytic repertoire of THP-1 monocytes. J Lipid Res. 2004;45:1768–1776. doi: 10.1194/jlr.M400195-JLR200. [DOI] [PubMed] [Google Scholar]
- Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol. 2008;172:521–533. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao TC, Kuo ML, See LC, Ou LS, Lee WI, Chan CK, Huang JL. RANTES and monocyte chemoattractant protein 1 as sensitive markers of disease activity in patients with juvenile rheumatoid arthritis: a six-year longitudinal study. Arthritis Rheum. 2006;54:2585–2593. doi: 10.1002/art.21962. [DOI] [PubMed] [Google Scholar]
- Zink MC, Clements JE. A novel simian immunodeficiency virus model that provides insight into mechanisms of human immunodeficiency virus central nervous system disease. J Neurovirol. 2002;8 Suppl 2:42–48. doi: 10.1080/13550280290101076. [DOI] [PubMed] [Google Scholar]