Abstract
Transdermal nicotine is widely used for smoking cessation, but only ~20% of smokers quit successfully with this medication. Interindividual variability in nicotine metabolism rate may influence treatment response. This study sought to validate, and extend in a larger sample, our previous finding that the ratio of plasma nicotine metabolites 3′-hydroxycotinine (3-HC)/cotinine, a measure of nicotine metabolism rate, predicts response to nicotine patch. A sample of 568 smokers was enrolled in a study that provided counseling and 8-weeks of 21mg nicotine patch. Pretreatment 3-HC/cotinine ratio was examined as a predictor of 7-day point prevalence abstinence, verified with breath carbon monoxide (CO), 8 weeks after the quit date. Controlling for sex, race, age, and nicotine dependence, smokers in the upper 3 quartiles of 3-HC/cotinine ratio (faster metabolizers) were ~50% less likely to be abstinent vs. smokers in the first quartile (slow metabolizers; 28% vs. 42%; OR=.54 [95% CI: .36–.82], p=.003). Among abstainers, plasma nicotine levels (assessed 1 week after treatment began) decreased linearly across the 3-HC/cotinine ratio (β = −3.38, t[355]=−3.09, p<.05). These data support the value of the 3-HC/cotinine ratio as a biomarker to predict success with transdermal nicotine for smoking cessation.
Keywords: nicotine, metabolism, tobacco, smoking, addiction
1. Introduction
The transdermal nicotine patch is the most widely used form of nicotine replacement therapy (NRT) for smoking cessation (West et al., 2001), producing close to a two-fold increase in quit rates compared to placebo (Stead et al., 2008). Yet, only about 1 in 5 smokers who use this NRT quit smoking successfully (Stead et al., 2008). The identification of a pretreatment biomarker test to predict the effectiveness of transdermal nicotine for individual smokers may enable providers to optimize the choice and dose of pharmacotherapy and, in turn, to improve success rates for smoking cessation.
Nicotine, the addictive chemical in tobacco, is metabolized to cotinine and then subsequently to 3′-hydroxycotinine (3-HC) by the cytochrome P450 (CYP) 2A6 enzyme (Nakajima et al., 1996; Messina et al., 1997). The ratio of plasma 3-HC to its precursor cotinine provides a stable measure of individual differences in nicotine metabolism from cigarette smoking that is independent of time since last cigarette (Dempsey et al., 2004; Lea et al., 2006; Mooney et al., 2008). Previously, we reported that a faster nicotine metabolism rate, as indicated by a higher pre-treatment 3-HC/cotinine ratio derived from nicotine from cigarette smoking, predicts lower nicotine concentrations during treatment, more severe cravings to smoke among those abstinent, and lower quit rates with transdermal nicotine (Lerman et al., 2006). In addition, faster metabolizers based on the 3-HC/cotinine ratio also were less likely to quit with behavioral counseling and placebo in a bupropion randomized trial and with counseling provided along with placebo bupropion (Patterson et al., 2008).
The current study was designed to validate the predictive clinical validity of the nicotine metabolite ratio in an independent sample of smokers receiving 8 weeks of 21mg transdermal nicotine treatment. Such evidence could provide additional proof of concept for the application of this biomarker to personalized tailoring of pharmacotherapy for smoking cessation.
2. Methods
2.1. Participants
Subjects were recruited via advertisements for a free smoking cessation program at an academic setting. Subjects underwent a medical history and physical to confirm eligibility. To be eligible, subjects must have been age 18–65, report smoking ≥ 10 cigarettes/day, and interested in cessation treatment. Female subjects were excluded if they were pregnant, planning a pregnancy, or lactating. Subjects were also excluded if they had a current medical problem for which transdermal nicotine is contraindicated (e.g., uncontrolled hypertension, liver and/or kidney failure in the last 6 months), were currently receiving treatment for cancer or were diagnosed with cancer in the past 6 months, were currently diagnosed with a DSM-IV substance use disorder (i.e., alcohol, cocaine, marijuana, stimulants, benzodiazepines), were currently using NRT or concomitant medications (e.g., monoamine oxidase inhibitors within the past 14 days, antipsychotics, endogenous steroids, stimulants, antidepressants, including wellbutrin/bupropion), or were currently diagnosed with an Axis I psychiatric disorder as identified by a SCID interview (e.g., psychosis, major depression; Spitzer al. al., 1990). The study procedures and experimental protocol were approved by the University of Pennsylvania Institutional Review Committee for the use of human subjects. The flow of participants is shown in Figure 1. The final sample of 568 provided ≥ 80% power to detect a difference in quit rates between 3-HC/cotinine ratio groups of ≥ 14% (OR ~1.97; PASS software, NCSS, Orem, UT).
Figure 1.
Participant Flow.
Note. * A list of the reasons for participant ineligibility can be provided by the authors upon request; ** included in intent-to-treat analysis.
2.2. Study Procedures
All participants received 8 weeks of open-label 21mg transdermal NRT (Nicoderm CQ; GlaxoSmithKline, Research Triangle Park, NC). Participants received a pre-quit counseling session at week -2 (baseline), which focused on preparing for cessation, and set a quit date for week 0, at which time participants were to start NRT use. At weeks 0, 1, 4, and 8, participants received additional behavioral counseling. These counseling sessions, based on standard smoking cessation behavioral treatment (Fiore et al., 2008), focused on managing urges and triggers to smoking and developing strategies to avoid relapse, and was similar in content and duration to our previous study of 3-HC/cotinine ratio and quit rates following NRT (Lerman et al., 2006). Participants received 21mg nicotine patches for the full 8 weeks, consistent with prior studies reporting no differences in outcomes with tapered versus full dose therapy throughout the treatment phase (Stapleton et al., 1995). For the present study, we examined the effect of the rate of nicotine metabolism assessed at baseline on quit rates at 8 weeks. The study procedures, including assessment time-points, are illustrated in Figure 2.
Figure 2.
Study Design.
Note. R = Recruitment; BSL = Baseline; TN = transdermal nicotine; W = Week; * indicates CO-confirmed abstainers only; ** indicates inclusion of 63 participants who withdrew from the study and 136 participants who did not provide data at Week 8.
2.3. Assessments
Covariates
At baseline, participants completed self-report measures of demographics (e.g., age, race, sex) and smoking behavior (e.g., cigarettes per day, number of previous quit attempts, Fagerstrom Test for Nicotine Dependence [FTND]; Fagerstrom et al., 1996; Heatherton et al., 1991). CO, nicotine, and cotinine were measured at baseline as well.
Predictors
At baseline, participants provided a blood sample for analysis of plasma nicotine and metabolites and determination of 3-HC/cotinine ratio. The time of day for the blood draw was not standardized since the ratio of plasma cotinine and 3-HC concentrations is relatively stable throughout the day (Lea et al., 2006; Mooney et al., 2008). Plasma nicotine was measured using gas chromatography with nitrogen-phosphorus detection (Jacob et al., 1981). Cotinine and 3-HC were measured using liquid chromatography with tandem mass spectrometry (Dempsey et al, 2004). A breath sample was ascertained to assess carbon monoxide (CO) levels.
Intermediate Outcomes
At week 1 (after the first week of nicotine patch treatment), we measured plasma nicotine concentration, patch use, patch-related side effects, nicotine withdrawal, nicotine craving, and positive and negative affect to associate these measures with the baseline 3-HC/cotinine ratio among participants with confirmed abstinence (i.e., breath CO ≤ 10ppm; SRNT, 2002). A time-line follow-back measure assessed daily patch use from week 0–8. The average number of days the patch was used for week 1 was computed. A checklist of side effects from patch use was completed each week (e.g., rash, sleep problems). The severity of each side effect was graded (0–3), with higher scores indicating greater severity, and a total side effect score was computed by summing items. Withdrawal symptoms were assessed using an 18 item checklist (e.g., cravings, irritability; Hughes & Hatsukami, 1986). Two Likert-style items from this scale, shown to predict smoking relapse (Killen & Fortmann, 1997), represented craving for nicotine. The Positive and Negative Affect Schedule (PANAS), a 20-item Likert-format self-report measure, was used to assess positive (e.g., enthusiastic) and negative (e.g., distressed) affect (Watson et al., 1988).
Week 8 Outcome
Smoking cessation rate was assessed in-person at week 8 using a time-line follow-back measure (Brown et al., 1998). Participants who reported no smoking (not even a puff) for the 7 days prior to the week 8 assessment were asked to provide a breath sample for biochemical verification. Consistent with guidelines (SRNT, 2002), subjects who withdrew from the trial, failed to provide a sample, or provided a carbon monoxide (CO) sample >10ppm were considered smokers. All other subjects were considered abstinent (i.e., those with self-report cessation and CO ≤ 10 at week 8).
2.4. Statistical Analysis
First, the characteristics of the study sample were evaluated (e.g., demographics, smoking history, and metabolite ratio) using descriptive statistics. We created a log transformed measure for the 3-HC/cotinine ratio since: 1) assessment of the 3-HC/cotinine ratio values indicated a non-normal distribution (skewness = 2.3; kurtosis = 15.2), 2) nicotine clearance correlates best with the log metabolite ratio (Lerman et al., 2006; Levi et al., 2007); and 3) the log metabolite ratio would ease interpretation of the data and facilitate comparison of the present data to previous assessments of the relationship between 3-HC/cotinine ratio and response to NRT (Lerman et al., 2006). The relationship between covariates (e.g., age, sex, and education) and the baseline log 3-HC/cotinine ratio was examined with ANOVA, t-tests, or Pearson correlation. Participant retention to week 8 was assessed and differences between participants who remained in the study and those who failed to complete the week 8 assessment were evaluated in terms of the baseline log 3-HC/cotinine ratio and covariates using ANOVA.
Second, we assessed the relationship between the baseline log 3-HC/cotine ratio and baseline cigarettes/day, CO, plasma nicotine and cotinine concentrations, FTND, and number of previous quit attempts using Pearson correlation.
Third, we examined the relationship between the baseline log 3-HC/cotinine ratio and week 8 quit rates using logistic regression. We also assessed the relationship between baseline log 3-HC/cotinine ratio and week 1 plasma nicotine concentration and nicotine patch use, and differences in baseline to week 1 patch-related side effects, nicotine withdrawal, craving, and positive and negative affect among week 1 abstainers only, confirmed with CO (n = 386 of 568). Pearson correlation was used for these analyses, followed by linear regression for the relationship between week 1 plasma nicotine concentration and baseline log 3-HC/cotinine ratio.
Fourth, we conducted a Receiver Operator Characteristic (ROC) analysis, using the baseline log 3/HC-cotinine ratio values, to determine the optimal cut-point for distinguishing subjects who showed significantly improved response to transdermal nicotine (i.e., higher rate of week 8 abstinence) and examined this new measure of nicotine metabolism as a predictor of week 8 quit rates. Determining a cut-point in ROC analysis typically involves use of the point on the curve closest to (0,1) criterion (Coffin & Sukhatme, 1997; Sharir et al., 2001). The conceptual basis for this criterion is that this point on the curve represents the place in the distribution with the highest sensitivity and specificity, thus minimizing misclassification (Perkins & Schisterman, 2006). Lastly, we conducted a multivariate logistic regression, controlling for sex, race, and level of nicotine dependence, to assess the relationship between baseline 3-HC/cotinine ratio (using the cut-point from the ROC analysis) and week 8 quit rates.
3. Results
3.1. Sample Characteristics and Covariates
The average age of participants was 45 years (SD = 10.3), 55% of the sample was male, more than 90% of the sample had at least a high school diploma or additional education, and 84% of the sample was Caucasian. The average number of cigarettes smoked per day was 21.2 (SD = 9.2), the average number of previous quit attempts was 1.7 (SD = 3.8), and the average FTND score was 5.2 (SD = 2.1). Averages for nicotine metabolite measurements at baseline were as follows: cotinine (269 ng/mL, SD = 117), 3-HC (99 ng/mL, SD = 60), and 3-HC/cotinine ratio (0.38 ng/mL, SD = .20). The pre-log transformed 3-HC/cotinine quartile means, medians, upper and lower limits, and sample size were as follows: 1) 0.18, .20 (≤0.2591), 142, 2) 0.30, .30 (0.2592 – 0.3519), 142, 3) 0.40, .39 (0.352 – 0.466), 142, and 4) 0.63, .56 (> 0.466), 142.
There were some associations of the nicotine metabolite ratio, measured at baseline, with demographic characteristics. Older participants had higher baseline log 3-HC/cotinine ratio values (F[1,566] = 11.34, p < .05); women showed a higher baseline log 3-HC/cotinine ratio than men (t[566] = −3.46, p < .05); and Caucasians had higher baseline log 3-HC/cotinine ratio values than non-Caucasians (t[566] = 2.50, p < .05). In addition, participants lost to follow-up at week 8 were compared to participants who provided data at week 8 in terms of demographic characteristics, smoking behavior, and baseline metabolic ratio. Completers and non-completers had similar baseline log 3-HC/cotinine ratios (F[1,566] = 3.6, ns). However, compared to completers, non-completers reported higher FTND scores (F[1,567] = 19.44, p < .05) and smoked significantly more cigarettes per day (F[1,568] = 17.91, p < .05). In modeling analyses, sex, race, age, and FTND were treated as covariates (cigarettes per day was not included since FTND includes this variable and having both would violate co-linearity laws).
3.2. 3-HC/Cotinine Ratio, Smoking Behavior, Abstinence, and Intermediate Outcomes
3-HC/Cotinine Ratio and Baseline Smoking Variables
The baseline log 3-HC/cotinine ratio was correlated significantly with baseline cigarettes per day (r = .11, p = .01) and baseline plasma nicotine (r = −.21, p < .001). The baseline log 3-HC/cotinine ratio was not related to baseline FTND (r = .04, ns) or to number of previous quit attempts assessed at baseline (r = .04, ns). Further, the baseline log 3-HC/cotinine ratio was not related to baseline cotinine (r = −.01, ns). In part this may be a consequence of the log transformation, since the baseline 3-HC-cotinine ratio (non-transformed) was related to baseline cotinine (r = −.12, p < .05).
3-HC/Cotinine Ratio and Abstinence Rates
The baseline log 3-HC/cotinine ratio was a significant predictor of week 8 point prevalence quit rates (OR = .66 [95% CI: .48–.91]; p < .05), indicating that participants with lower baseline log 3-HC/cotinine ratios (i.e., slower nicotine metabolizers) showed higher quit rates at week 8, compared to participants with a higher baseline log 3-HC/cotinine ratios.
3-HC/Cotinine Ratio, Plasma Nicotine, Patch Use, Side Effects, Withdrawal, Craving, and Affect
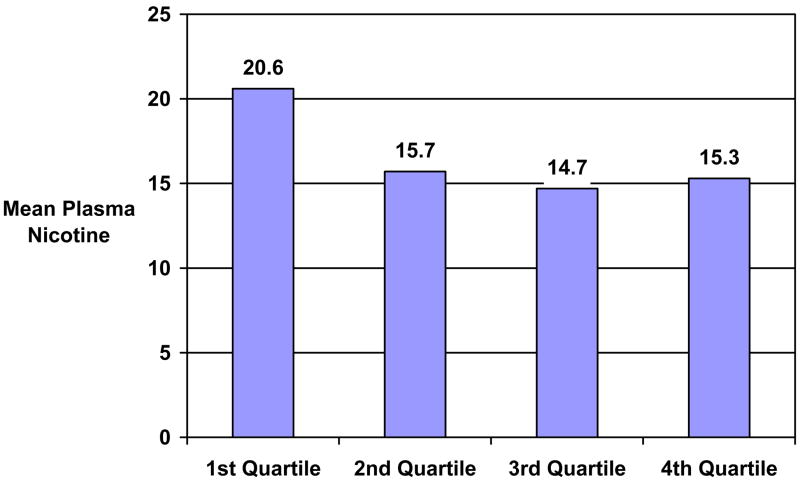
Among Week 1 abstainers only (n = 386), the log 3-HC/cotinine ratio measured at baseline was related to week 1 plasma nicotine levels (r = −.17, p < .01). The plasma nicotine levels at week 1, among abstainers at week 1, decreased linearly across the log 3-HC/cotinine ratio assessed at baseline (β = −3.48, t[385] = −3.33, p < .05). Week 1 plasma nicotine levels among week 1 abstainers are shown in Figure 3, across quartiles of the baseline log 3-HC/cotinine ratio. The baseline log 3-HC/cotinine ratio was not associated with patch use (r = .07, ns), patch-related side-effects (r = −.02, ns), nicotine withdrawal (r = −.02, ns), nicotine craving (r = −.02, ns), negative affect (r = .02, ns), or positive affect (r = −.03, ns) assessed after one week of treatment.
Figure 3.
Week 1 Plasma Nicotine Values Across 3-HC/Cotinine Ratio Quartiles (Abstainers Only, n = 386).
Note. Log 3-HC/cotinine ratio is significantly related to nicotine plasma levels one week after treatment began among confirmed abstainers (β = −3.48, t[385] = −3.33, p < .05).
ROC Analysis
The results of the ROC analysis indicated that the most appropriate cut-point, based on the closest-to-(0,1) criterion, was at the first quartile for the 3-HC/cotinine ratio (i.e., the lowest 25% of the 3-HC/cotinine ratio distribution). Thus, a binary 3-HC/cotinine ratio variable was created for subsequent analysis, with the lowest quartile compared to the remaining upper three quartiles of the 3-HC/cotinine ratio distribution.
3.3. Multivariate Modeling of Abstinence at Week 8
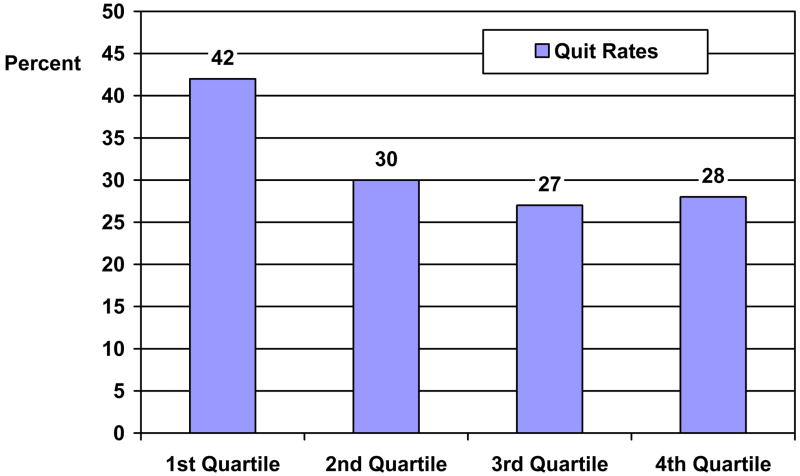
The final prediction model for week 8 abstinence rates is shown in Table 1. Controlling for sex, race, FTND, and age, the model revealed a significant effect for the binary (1ST vs. 2nd–4th quartiles) 3-HC/cotinine ratio variable assessed at baseline (OR = .54, 95% CI: .36 – .82, p = .003). Specifically, at week 8, the quit rates across the baseline 3-HC/cotinine quartiles were as follows: quartile 1 = 42%, quartile 2 = 30%, quartile 3 = 27%, and quartile 4 = 28% (see Figure 4). For the binary 3-HC/cotinine variable assessed at baseline, formed from the ROC analysis, the quit rates were: quartile 1 = 42% and quartiles 2–4 = 28%.
Table 1.
Multivariate Logistic Regression Analysis of Smoking Abstinence by 3-HC/cotinine Ratio Group, Controlling for Covariates (n = 568)
| Predictor | OR | 95% CI | p |
|---|---|---|---|
| Sex (Reference = Female) | 1.17 | 0.81 – 1.68 | 0.41 |
| Race (Reference = Non-Caucasian) | 0.87 | 0.69 – 1.09 | 0.23 |
| Age | 1.01 | 0.99 – 1.03 | 0.41 |
| FTND | 0.85 | 0.78 – 0.93 | 0.0001 |
| 3-HC/Cotinine Ratio (Reference = Slow) | 0.54 | 0.36 – 0.82 | 0.003 |
Note. FTND = Fagerström Test of Nicotine Dependence. Additional models that included the ability to quit on the target quit date and the number of previous quit attempts as covariates did not change the significance of the 3-HC/cotinine ratio for predicting abstinence at Week 8.
Figure 4.
Week 8 Quit Rates (7-day Point Prevalence, CO-confirmed) Across 3-HC/Cotinine Ratio Quartiles (n = 568).
Note. 3-HC/cotinine ratio is significantly related to 7-day point prevalence quit rates, CO-confirmed (OR = .66, 95% CI: .48–.91, p < .05). N’s for each quartile are 142.
4. Discussion
This study validates prior findings of the predictive validity of the 3-HC/cotinine ratio as a marker for successful quitting with 21mg transdermal NRT (Lerman et al., 2006). Across two independent studies we have demonstrated that the rate of nicotine metabolism, measured by the 3-HC/cotinine ratio, predicts cessation after treatment with the standard 21mg dose of nicotine patch. While smokers in the upper (faster) quartiles of nicotine metabolism show the expected approximately 30% 8-week response to transdermal NRT, smokers in the lowest quartile (i.e., the slow metabolizers) show quit rates comparable to varenicline (i.e., 47%), a recently marketed pharmacotherapy for nicotine dependence (Jorenby et al., 2006). While the binary 3-HC/cotinine ratio variable was not associated with baseline cigarettes per day, number of previous quit attempts, FTND, or gender, smokers in the lowest quartile of the 3-HC/cotinine ratio had significantly lower breath CO levels and plasma nicotine levels at baseline prior to treatment, vs. those in the highest three quartiles, and were more likely to be non-Caucasian (results not shown). Together with our initial study, the present data suggest the potential value of this phenotypic marker of nicotine metabolism to select a treatment modality for nicotine dependence.
There is, however, a difference in the pattern of associations between the present study and our previously published study. In the earlier study (Lerman et al., 2006), we found a relatively linear reduction in the probability of successful cessation from lowest to highest quartile, with the most robust effect detectable among those in the highest 3-HC/cotinine quartile (i.e., the fastest metabolizers), who showed the lowest end-of-treatment quit rates. In contrast, the present data show that the effect of the 3-HC/cotinine ratio on quit rates following transdermal NRT is largely attributable to a statistically significant and clinically meaningful increase in quit rate among those in the lowest 3-HC/cotinine ratio quartile (i.e., the slowest metabolizers), vs. other quartiles.
There are several potential explanations for this difference. First, the two studies differed in some aspects of methodology. The initial study used the step-down procedure for dosing transdermal nicotine, whereas the validation study maintained participants on 21mg patches for the full 8 weeks. Second, there were potentially meaningful differences in subject characteristics comparing the two samples. In the initial trial, participants had faster rates of nicotine metabolism as indicated by the 3-HC/cotinine ratio (mean = .44, SD = .9), versus the present study (mean = .38, SD = .20), and differences in the distributions of 3-HC/cotinine may influence relationships between nicotine metabolism and smoking behaviors. However, using the 3-HC/cotinine quartile cut-offs from our previous study to determine 3-HC/cotinine quartiles in the present sample did not change the results reported here (results not shown). The subject sample in the initial study was also more ethnically/racially diverse than the sample in the present study, and race affects rate of nicotine metabolism (Benowitz, 2008). Lastly, the sample in the initial study included a higher proportion of women than the present study, and women metabolize nicotine at a faster rate, especially if they are using oral contraceptives (Benowitz et al., 2006), and may respond less favorably to transdermal nicotine (Perkins and Scott, in press). Overall, these methodological and sample differences across the two studies may explain the disparity in the nature of the relationship between 3-HC/cotinine ratio groups and response to transdermal NRT observed across the studies.
The present findings also replicate our previous result showing that slow metabolizers have higher plasma nicotine levels during NRT treatment (Lerman et al., 2006). This finding provides additional validation of the important role played by 3-HC/cotinine ratio in determining therapeutic response to transdermal nicotine, since the slow metabolizers (with higher nicotine concentrations) were significantly less likely to relapse at 8 weeks, vs. intermediate or fast metabolizers (who showed lower levels of plasma nicotine). Thus, the 3-HC/cotinine ratio is related to both the extent of therapeutic nicotine replacement and quit rates.
The present results should, however, be considered in the context of methodological limitations. First, this study did not include a placebo to allow for assessment of whether or not the 3-HC/cotinine ratio effect on cessation is unique to treatment with transdermal nicotine. In a separate study, which included a placebo arm, among placebo-treated subjects, faster metabolizers of nicotine were more likely to relapse compared to slower metabolizers (Patterson et al., 2008), suggesting that the liability to relapse among fast metabolizers is likely a function of several mechanisms, not only nicotine replacement. Second, the present study did not include a long-term follow-up of cessation rates. We are currently following the present cohort to assess long-term outcomes and have demonstrated previously the effect of the 3-HC/cotinine ratio on long-term abstinence rates (Lerman et al., 2006; Patterson et al., 2008). Lastly, the present sample was comprised of treatment seeking smokers and it did not have substantial ethnic/racial variability. Therefore, the results are generalizable to treatment seeking smokers and should be extended to other racial/ethnic groups in future studies.
Nevertheless, this study underscores the potential value of assessing pretreatment nicotine metabolism rate from cigarette smoking when considering use of a standard dose of transdermal nicotine. Perhaps only slow metabolizers (i.e., the first quartile for 3-HC/cotinine ratio) should be considered good candidates for this dose of transdermal nicotine. Recent data suggest that faster metabolizers (those in the 4th quartile) achieve significant benefit from bupropion for smoking cessation, while slow metabolizers quit at similar rates on bupropion and placebo (Patterson et al., 2008). Since bupropion is not metabolized by CYP2A6, this finding suggests that the rate of nicotine metabolism influences the level of nicotine dependence and the need for additional pharmacotherapy. Additional randomized clinical trials are needed to determine whether other non-nicotine medications, such as varenicline, are also more efficacious for faster metabolizers, and to identify the optimal treatments for smokers with nicotine metabolism rates in the intermediate range. Further, additional studies are needed to determine if faster nicotine metabolizers are more effectively treated with high doses of transdermal nicotine. While the current and previous findings strongly suggest that physicians could use the nicotine metabolite ratio as a noninvasive biomarker of nicotine metabolism to select slow metabolizers for the transdermal patch, future studies are needed to determine the cost-effectiveness of such tailoring and the optimal cut-points for different populations. Once studies determine the appropriate treatments matched to level of nicotine metabolism, this phenotypic marker may be useful to screen individual smokers for a specific form of smoking cessation therapy, thereby increasing treatment response rates and reducing the overall rate of tobacco use.
Acknowledgments
The authors would like to thank the following individuals who participated in the implementation of this research project: Dr. Margaret Rukstalis, Dr. Daniel Heitjan, Angela Pinto, Susan Ware, and Lisa Yu.
Grant Support: This research was supported by a Transdisciplinary Tobacco Use Research Center Grant from the National Cancer Institute and National Institute on Drug Abuse P5084718 (CL), and National Institute on Drug Abuse grants DA02277 (NB) and DA20830 (NB, RFT and CL).
Footnotes
Conflicts of Interest: Dr. Lerman has served as a consultant to GlaxoSmithKline, the company that manufactures the nicotine patch used in this study. However, GSK did not provide medication or financial support for this study. Dr. Tyndale is a shareholder and CSO for Nicogen, a company focused on novel smoking cessation approaches. No funding was provided from Nicogen for this study. Dr Benowitz has been a paid consultant to several pharmaceutical companies that market and/or are developing medications for smoking cessation. He has also served as a paid expert witness in litigation against tobacco companies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benowitz NL. Clinical pharmacology of nicotine: Implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Thera. 2008;83:531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480–8. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Brown R, Burgess E, Sales S, Whiteley J. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12:101–12. [Google Scholar]
- Coffin M, Sukhatme S. Receiver operating characteristic studies and measurement errors. Biometrics. 1997;5:823–37. [PubMed] [Google Scholar]
- Dempsey D, Tutka P, Jacob P, 3rd, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Kunze M, Schoberberger R, et al. Nicotine dependence versus smoking prevalence: comparisons among countries and categories of smokers. Tob Cont. 1996;5:52–6. doi: 10.1136/tc.5.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. Treating tobacco use and dependence: 2008 Update. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatography. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP. Craving is associated with smoking relapse: findings from three prospective studies. Exp Clin Psychopharmacol. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Lea RA, Dickson S, Benowitz NL. Within-subject variation of the salivary 3HC/COT ratio in regular daily smokers: Prospects for estimating CYP2A6 enzyme activity in large-scale surveys of nicotine metabolic rate. J Anal Toxicol. 2006;30:386–389. doi: 10.1093/jat/30.6.386. [DOI] [PubMed] [Google Scholar]
- Lerman C, Tyndale R, Patterson F, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79:600–8. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Levi M, Dempsey DA, Benowitz NL, Sheiner LB. Prediction methods for nicotine clearance using cotinine and 3-hydroxy-cotinine spot saliva samples II. Model application. J Phamacokinetics Pharmacodynamics. 2007;34:23–24. doi: 10.1007/s10928-006-9026-0. [DOI] [PubMed] [Google Scholar]
- Messina ES, Tyndale RF, Sellers EM. A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J Pharmacol Exp Ther. 1997;282:1608–14. [PubMed] [Google Scholar]
- Mooney ME, Li ZZ, Murphy SE, et al. Stability of the nicotine metabolite ratio in ad libitum and reducing smokers. Cancer Epidemiol Biomarkers Prev. 2008;17:1396–00. doi: 10.1158/1055-9965.EPI-08-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Yamamoto T, Nunoya K, et al. Role of human cytochrome P4502A6 in C-oxidation of nicotine. Drug Metabol Disp. 1996;24:1212–17. [PubMed] [Google Scholar]
- Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein L, et al. Toward personalized therapy for smoking cessation: A randomized placebo-controlled trial of bupropion. Clin Pharmacol Thera. doi: 10.1038/clpt.2008.57. In press. [DOI] [PubMed] [Google Scholar]
- Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epi. 2006;163:670–5. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. doi: 10.1080/14622200802097506. In Press. [DOI] [PubMed] [Google Scholar]
- Sharir T, Berman DS, Waechter PB, et al. Quantitative analysis of regional motion and thickening by gated myocardial perfusion SPECT: normal heterogeneity and criteria for abnormality. J Nuc Med. 2001;42:1630–8. [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbon M. Structured Clinical Interview for DSM-III-R-Non-Patient Edition (SCID-NP, Version 1.0) American Psychiatric Press; 1990. [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stapleton J, Russel M, Feyerabend C. Dose effects and predictors of outcome in a randomized trial of transdermal nicotine patches in general practice. Addiction. 1995;90:31–42. doi: 10.1046/j.1360-0443.1995.901316.x. [DOI] [PubMed] [Google Scholar]
- Stead LR, Bullen P, Mant C, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Sys Rev. 2008;21:CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P, Nilsson F, et al. Individual differences in preferences for and responses to four nicotine replacement products. Psychopharmacology (Berl) 2001;153:225–30. doi: 10.1007/s002130000577. [DOI] [PubMed] [Google Scholar]