Abstract
Background/Aims
The von Hippel-Lindau (pVHL) protein functions as an E3 ubiquitin ligase, controlling the stability of hypoxia inducible factor (HIF). Pre-induction of HIF-1α before pathological insult activates a self-defense mechanism and suppresses further aggravation of organ or cellular injury by ischemia. We investigated whether acute inactivation of the VHL gene might play a role in the response of mice to ischemic renal injury.
Methods
We generated tamoxifen-inducible conditional VHL knockout (VHL-KO) mice to inactivate the VHL gene in an acute manner during renal ischemia-reperfusion injury (IRI) induced by bilateral clamping of kidney arteries. Renal IRI is characterized by renal dysfunction and tubular damage.
Results
After the procedure of IRI, blood urea nitrogen (BUN) and creatinine (CRN) levels in control mice were significantly higher (BUN, 138.10±13.03 mg/dL; CRN, 0.72±0.16 mg/dL) than in VHL-KO mice (BUN, 52.12±6.61 mg/dL; CRN, 0.24±0.04 mg/dL; BUN: p<0.05; CRN: p<0.05). Histologically, tubular injury scores were higher in control mice than in VHL-KO mice (p<0.05).
Conclusion
We suggest that the acute inactivation of the VHL gene contributes to protective effects of ischemic preconditioning in renal tubules of the mouse.
Keywords: ischemia-reperfusion injury (IRI), von Hippel-Lindau (VHL) gene, hypoxia inducible factor (HIF), ischemic preconditioning
Introduction
Hypoxia in the tubulointerstitium has been thought to play pivotal roles in the pathophysiology of acute renal failure. Ischemic acute renal failure is traditionally referred to as acute tubular injury [1]. Proliferation of affected tubular cells seems to be essential for timely recovery of tubules after acute renal damage and for subsequent functional recovery of the injured kidney [2]. The identification of the mechanisms responsible for the ischemic tubular damage is important not only for an understanding of the pathophysiology of ischemic injury but also for considering therapeutic strategies. Although the pathogenesis of human acute tubular injury is not fully understood, a number of animal studies have shown that several kinds of mediators can be involved in ischemia-reperfusion injury (IRI) in renal tubules [2–8].
Many processes of adaptation to hypoxia are mediated by hypoxia-inducible factor (HIF), which is a master regulator of genes transactivated by low oxygen tension. The level of HIF-1α protein, an oxygen-regulated component of HIF-1, is tightly regulated by von Hippel-Lindau (pVHL) protein. pVHL forms a complex with HIF-1α during normoxic conditions but not in ischemic hypoxia, and then rapidly induces ubiquitin-mediated degradation of HIF-1α [9,10]. Several published studies indicate that pre-induction of HIF-1α before pathological insult activates a self-defense mechanism and suppresses further aggravation of organ or cellular injury by ischemia [11–14]. One previous report concluded that pre-treatment with cobalt, which inhibits HIF-1α degradation and elevates its protein level, protected against subsequent renal tubular damage in rats with glomerular injury [14]. Furthermore, these authors have recently demonstrated a pivotal role for HIF-2α in IRI using HIF-2α knockdown mice [15]. HIF-2α is known as another target of pVHL, but plays different roles and possesses contrasting properties compared with HIF-1α in terms of expression levels and localization [16–18]. Although the roles of HIF-1α and HIF-2α were extensively investigated in these previous studies, especially under hypoxic conditions, the role of pVHL remains unclear in the progression of renal tubular damage induced by IRI.
Given the potential importance of pVHL during ischemic conditions, we used VHL conditional knock out (VHL-KO) mice that expressed an inducible Cre recombinase transgene (CreERTM) [19] to delete the floxed VHL gene in an acute manner following tamoxifen induction. We then investigated whether pre-inactivation of VHL would afford protection of renal tubules from injury during IRI.
Materials and Methods
Generation of VHL conditional knockout (VHL-KO) mice
Mice carrying the VHL conditional (floxed) allele were generated by Ma et al. [20], using Cre/lox site-specific recombination technology. To generate VHL conditional knockout (VHL-KO) mice in which VHL was inactivated in multiple tissues in an inducible manner, we crossed VHLf/f mice with VHLd/+ mice carrying the tamoxifen-inducible Cre recombinase transgene (VHLd/+/ CreERTM) [19] and selected for VHLf/d/ CreERTM offspring by PCR-based genotyping [21]. As already reported, Cre recombinase expression in this system is under the control of a human β-actin promoter. Therefore, Cre recombinase was expressed throughout the kidney as demonstrated by using ROSA26 reporter mice (Figure 1A). Based on the data reported by Hong and colleagues, we determined the least toxic concentration of tamoxifen that was still effective in inducing Cre recombination [21]. For our experiments, VHLf/d/ CreERTM mice were injected i.p. with tamoxifen in corn oil (0.36mg/g body weight) to activate Cre recombinase one week prior to renal IRI. Eight to fifteen-week-old male and female VHLf/d/ CreERTM mice (n=10) and littermate VHLf/+/ CreERTM control mice (n=6) were subjected to tamoxifen induction for these experiments. Mice were housed in a specific pathogen-free facility and were confirmed to be negative for common murine viral pathogens by routine sera analysis.
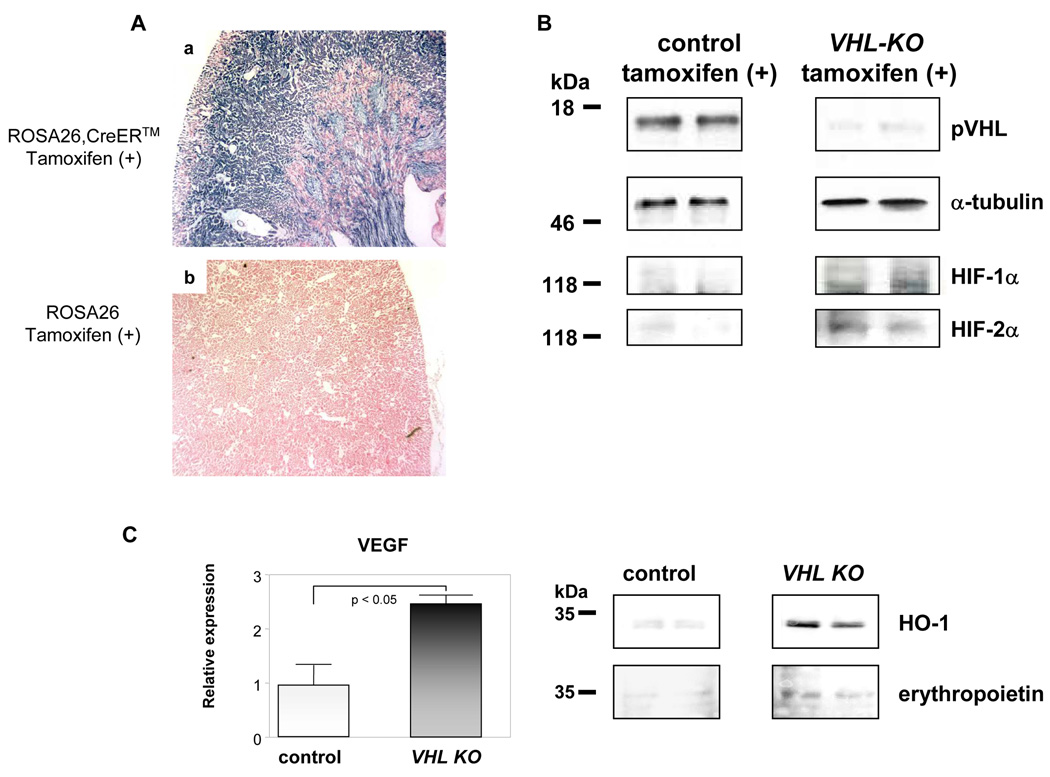
Figure 1. VHL protein level is decreased following tamoxifen induction in VHLf/d/CreERTM (VHL-KO) mice with reciprocal elevation of HIF-1α.
The distribution of Cre recombinase expression in the kidney was evaluated with ROSA26 reporter mice (A). After tamoxifen induction, Cre recombinase was expressed throughout the kidney in ROSA26 CreERTM mice (a), but not in ROSA26 mice lacking the CreERTM transgene (b). Each lane represents a protein extract from a tamoxifen-treated VHL-KO mouse kidney, in which Cre recombinase was induced, or a tamoxifen-treated control mouse kidney. α-tubulin is used as a loading control. VHL protein expression is decreased in tamoxifen-induced VHL-KO kidneys, with elevation of HIF-1α, HIF-2α (B), heme oxygenase (HO)-1, and erythropoietin (C) protein levels. Furthermore VEGF gene expression evaluated by RT-PCR is also increased (C). Representative data are shown (n=2 per group for Western blot analysis; n=3 per group for RT-PCR).
Western Blot Analysis
Protein from whole murine kidneys was prepared using Tissue Protein Extraction Reagent (T-PER) (Pierce Biotechnology, Rockford, Ill., USA). Western blot analysis was performed according to the methods in our previous study [22]. Kidney extracts were mixed with sample buffer, separated by electrophoresis on 15% SDS-PAGE gels and then transferred to PolyVinylidine DiFluoride (PVDF) membranes (Immobilon-P; Millipore, Bedford, Mass., USA). A rabbit polyclonal anti-VHL antibody (1:100; BD Biosciences, San Jose, CA, USA), a mouse monoclonal anti-HIF-1α antibody (1:500; Novus Biologicals, Inc., Littleton, CO, USA), a rabbit polyclonal anti-HIF-2α antibody (1:500; Novus Biologicals, Inc.), a rabbit polyclonal anti-erythropoietin antibody (1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), a mouse monoclonal anti-α-tubulin antibody (1:1000; Lab Vision, Fremont, CA, USA) and a mouse monoclonal anti-heme oxygenase (HO)-1 antibody (1:1000; Stressgen, Ann Arbor, MI, USA) were used with a horseradish peroxidase-conjugated secondary antibody (Promega, Madison, WI., USA). The ECL Plus Western blotting system (Amersham Bioscience, Piscataway, N.J., USA) was used for detection.
RT-PCR
Total RNAs were isolated from mouse kidney using Trizol reagent (Invitrogen) according to the manufacturer's instruction. To remove genomic DNA contamination, RNAs were digested with DNase I for 1 hr at 37°C followed by heat denaturation at 70°C for 20 min. Total RNAs (2.5 µg) were primed with 100 ng random primers and reverse-transcribed by Superscript II reverse transcriptase (Invitrogen) at 42°C for 1 hr. The same reactions were performed without reverse transcriptase to generate negative controls. The following PCR primers were generated by using Primer 3 software [21]:
VEGF forward, 5'- CAGGCTGCTGTAACGATGAA-3';
VEGF reverse, 5'-TATGTGCTGGCTTTGGTGAG-3';
β-actin forward, 5'-GACAGGATGCAGAAGGAGATTACTG-3';
β-actin reverse, 5'-GCTGATCCACATCTGCTGGAA -3'.
Quantitative RT-PCR was performed with SYBR-Green reagent (Applied Biosystems) with the ABI PRISM sequence detection system (Applied Biosystems) following the manufacturer's instruction. All reactions were run in triplicate using the β-actin gene as an internal control.
Antibody Microarray Analysis
In order to detect the proteins that were selectively expressed in the VHL-KO mouse kidney but not in control mouse kidney after tamoxifen induction, Panorama Cell Signaling Antibody Microarray kit (CSAA1, Sigma-Aldrich, St Louis, MO, USA) was used to detect a wide variety of proteins responsible for a broad range of biological functions, including apoptotic and cell cycle proteins. The antibody microarray was composed of 224 specific antibodies responsible for cell signaling, which were spotted in duplicate on nitrocellulose-coated glass slides. We prepared cell lysates from each kidney and the lysates were blotted according to manufacturer’s protocols. The complete list of arrayed antibodies can be found at the Sigma-Aldrich web site: http://www.sigmaaldrich.com/catalog/search/ProductDetail/SIGMA/CSAA1.
Induction of Renal Ischemia-Reperfusion Injury (IRI)
Mice to be analyzed were anesthetized by i.p. injection of pentobarbiturate (4 mg/kg). Following abdominal incisions, the bilateral renal arteries were occluded at the proximal ends of the abdominal aorta with arterial clips for 30 min. After removal of the clips, the kidneys were checked for reperfusion. Twenty-four hours later, mice were sacrificed for further sampling and blood was collected for evaluation of renal function.
Assessment of Renal Function
Blood urea nitrogen (BUN) and creatinine (CRN) levels were measured for assessment of the renal function. BUN and CRN levels were analyzed by automated analysis (Hitachi 7350, Hitachi, Ibaraki, Japan) in our laboratory center.
Histological Analysis
To evaluate the kidney damage induced by renal IRI, histological analysis was performed. Four-micrometer-paraffin sections were stained with hematoxylin and eosin (H&E). On the basis of the method described by Leemans et al. [6], we evaluated the morphological changes in renal tubules. Tubular injury was scored by estimating the percentage of injured tubules in the outer medulla and corticomedullary junction that showed tubular dilation, tubular epithelial injury, debris accumulation, and cast formation as follows: 0 normal: 1 mild: 2 moderate: 3 severe. Twenty viewing fields randomly selected from the outer medulla and corticomedullary junction on each slide section were examined at ×400 magnification. One slide per mouse kidney was evaluated.
Immunohistochemical Analysis
An immunohistochemical study was performed on paraffin sections of mouse kidneys using the Ventana automated immunohistochemistry system (Discovery TM, Ventana Medical system, Inc., Tucson, AZ, USA). VHL was identified with a polyclonal anti-VHL antibody, sc-5575 (1:150; Santa Cruz Biotechnology, Inc.). Antigen retrieval was performed for 60 min. in a preheated Dako Target Retrieval Solution (pH 6.0) using microwave treatment, followed by further steps including inhibition of intrinsic peroxidase, and blocking and reaction with a primary antibody.
TUNEL (Terminal Deoxynucleotide Transferase dUTP Nick-End Labeling) staining
TUNEL assay was performed to detect apoptosis in situ. Renal tissue sections were deparaffinized and rehydrated through three changes between xylene and graded alcohol, later washed in PBS for 5 min, and then incubated in 20µg/ml proteinase K for 15 min at room temperature. ApopTag Peroxidase In Situ Apoptosis Detection Kit (S7100, Chemicon International, Temecula, CA, USA) was used according to the manufacturer’s instructions. Endogenous peroxidase activity in the kidney sections was blocked by incubation for 5 min with 3% H2O2 in PBS, followed by incubation with equilibration buffer for 10 sec. The sections were then incubated for 60 min at 37°C with terminal deoxynucleotidyl transferase (TdT) enzyme in reaction buffer. The reaction was finished by incubation with stopping buffer at room temperature. Sections were incubated with peroxidase-conjugated anti-digoxigenin antibody for 30 min at room temperature, and the reaction was developed with diaminobenzidine (DAB) substrate for 6 min at room temperature. Sections were counterstained with methyl green stain, dehydrated through a graded series of alcohol, and mounted for microscopy viewing.
Statistical Analysis
Data are reported as means ± standard error of the mean (SEM). An unpaired t test was used for paired samples and Student’s t test was used to compare the two groups. A p value of < 0.05 was considered statistically significant.
Results
Expression level of VHL protein in VHL-KO mice
To examine the distribution of Cre recombinase expression in the kidney for evaluating regions of VHL-deletion, ROSA26 reporter mice were used. As shown in Figure 1A, Cre recombinase was expressed as purple staining throughout the kidneys of ROSA26 CreERTM mice treated with tamoxifen (a), but not in the kidneys from tamoxifen-treated ROSA26 mice without the CreERTM transgene (b).
To compare VHL protein expression in tamoxifen-treated VHLf/d/CreERTM (VHL-KO) mice and tamoxifen-treated VHLf/+/CreERTM (control) mice, we evaluated the levels of VHL protein in murine kidneys after tamoxifen induction by Western analysis (Fig. 1B). pVHL was detected in kidneys of control mice (left panel, Fig. 1B), but significantly reduced expression levels of VHL protein were detected in VHL-KO kidneys (right panel, Fig. 1B). In contrast to the VHL expression pattern, the protein levels of HIF-1α and HIF-2α were elevated in VHL-KO kidneys compared with control kidneys (Fig. 1B). These results demonstrated the effectiveness of the tamoxifen-inducible VHL conditional knockout mouse system, which causes VHL inactivation and stabilization of HIF-1α and HIF-2α protein, following tamoxifen injection.
To further evaluate HIFα-inducible target genes, the protein levels of heme oxygenase (HO)-1 and erythropoietin (Epo), and gene expression levels of VEGF were compared. As demonstrated in Figure 1C, they were elevated in VHL-KO kidneys compared with control kidneys.
BUN and CRN levels in VHL-KO and control mouse kidneys after IRI
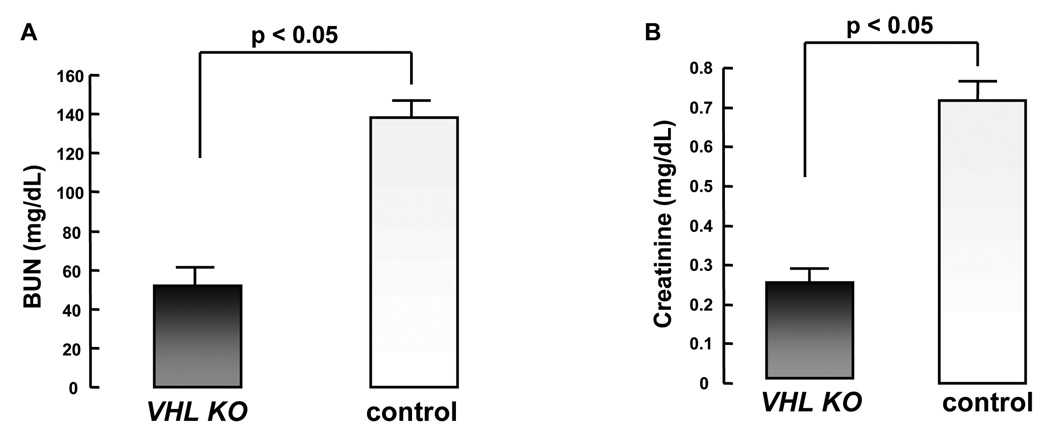
To assess whether VHL inactivation might play a role in renal protection during renal IRI, we subjected tamoxifen-injected VHLf/d/CreERTM (VHL-KO) and control mice to renal IRI by clamping the bilateral renal arteries. This procedure consisted of a 30 min ischemia followed by 24 h reperfusion. IRI significantly impaired renal function in control mice, as indicated by elevated serum BUN and CRN levels after 24 h reperfusion. The BUN and CRN levels in control mice (n=6) were 138.10 ± 13.03 mg/dL and 0.72 ± 0.16 mg/dl, respectively. In contrast, serum BUN and CRN levels in VHL-KO mice (n=10) were 52.12 ± 6.61 mg/dl and 0.24 ± 0.04 mg/dl, respectively. There were significant differences in the levels of these renal function markers in VHL-KO mice compared with control mice (Fig. 2A, 2B). Compared to control mice, VHL-KO mice demonstrated a dramatic reduction in BUN and CRN levels (p < 0.05 for each comparison), suggesting that VHL inactivation had contributed to protection of renal function following renal IRI.
Figure 2. VHL inactivation causes protection of kidney function during renal IRI.
BUN (A) and creatinine (B) levels in tamoxifen-injected VHL-KO mice (n=10) are significantly lower than those of control mice (n=6; p< 0.05) after renal IRI.
Histological analysis of the VHL-KO and control mouse kidneys after IRI
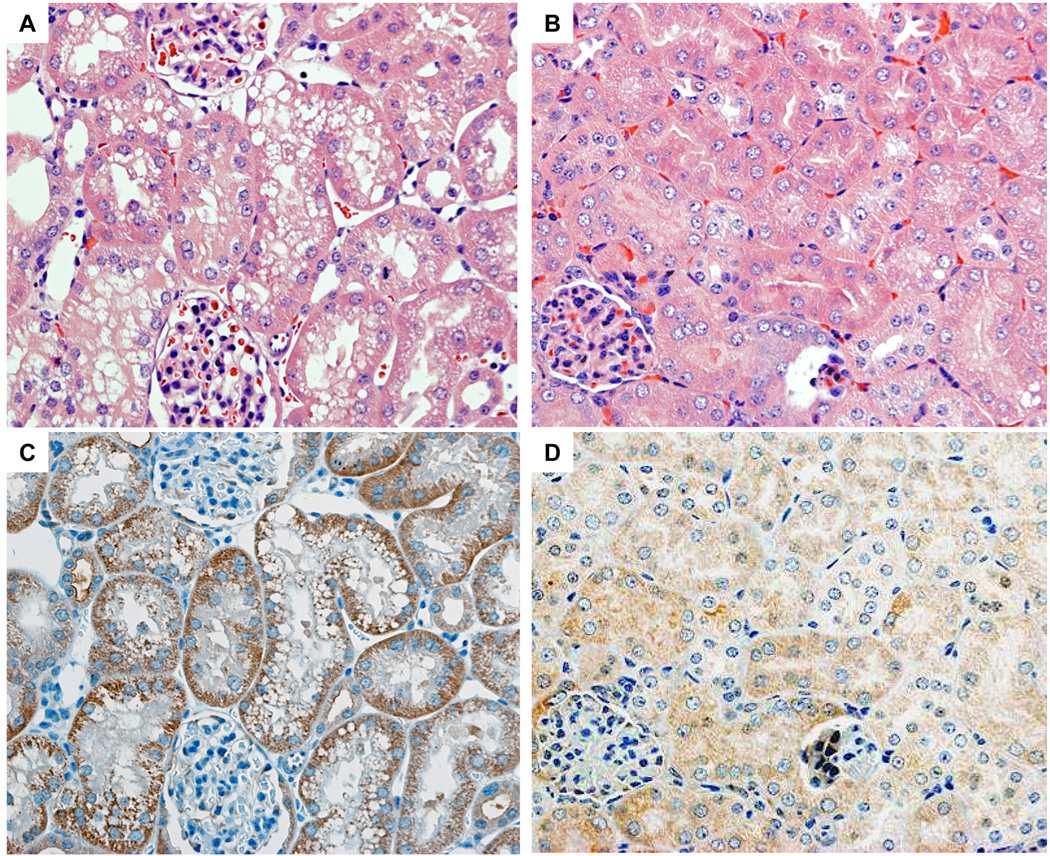
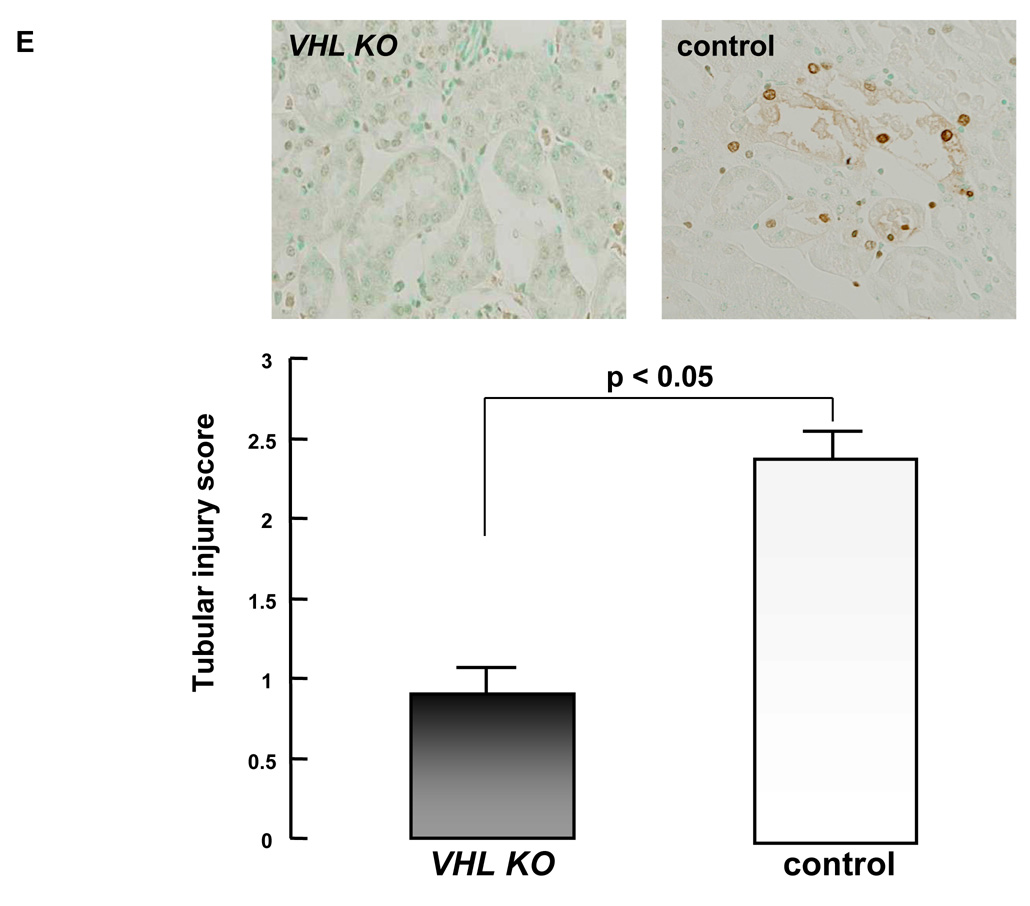
Impairment of renal function, as assessed by BUN and CRN levels, was corroborated by histological evidence. By H&E staining, more severe tubular damage was detected in tamoxifen-treated control kidney sections with extensive proximal tubular injury including tubular dilatation and tubular epithelial degeneration with necrosis (Fig. 3A). In contrast, tamoxifen-injected VHL-KO mice subjected to renal IRI showed less proximal tubular injury with even milder tubular epithelial degeneration in the kidney sections (Fig. 3B). Proximal tubular damage was mainly observed from the outer medulla to the medullary ray of the cortex in control mice. No regions of necrotic debris or cast in the damaged tubules were detected in the sections from either VHL-KO or control kidneys. Compatible with the pathological changes in the control kidney, positive cells in TUNEL staining were detected in those regions, however, no TUNEL positive cells were detected in the VHL-KO kidney (Fig. 3E). Semi-quantitative analysis of tubular damage demonstrated that the levels of tubular injury scores were significantly higher in control mice (n=6) than in VHL-KO mice (n=10) (Fig. 3E, p < 0.05). The scores in control mice and VHL-KO mice were 2.40 ± 0.08 and 0.90 ± 0.12, respectively. No pathological lesions of glomeruli and negligible numbers of infiltrated inflammatory cells were observed in VHL-KO and control kidney sections.
Figure 3. VHL inactivation reduces tubular damage caused by renal IRI.
H&E staining of kidney sections show renal tubules from control (A) and VHL-KO (B) mice, 24 h after reperfusion. Severe tubular damage is observed in control mice (A), compared with VHL-KO mice (B). Immunohistochemical staining shows less VHL protein expression in the renal tubules in VHL-KO mice (D) compared with control mice (C). The tubular injury score in VHL-KO mice (n=10) is lower than in control mice (n=6; p<0.05), compatible with fewer TUNEL positive cells in VHL-KO mice compared with control mice (E).
Immunohistochemical analysis of the tamoxifen-injected VHL-KO and control mouse kidneys after IRI
To further investigate the relationship between the localization of IRI-induced renal lesions and the distribution of VHL protein immunoreactivity in the kidneys, we performed immunohistochemical analysis of the VHL protein. The immunoreactivity of pVHL using anti-VHL antibody was mainly and strongly detected in the tubules of the tamoxifen-treated control mouse kidneys after renal IRI (Fig. 3C). In contrast, however, extremely weak immunoreactivity of pVHL was detected in the tubules of the tamoxifen-treated VHL-KO mice (Fig. 3D). We failed to detect pVHL immunoreactivity in glomeruli of either VHL-KO or control mice. TUNEL staining revealed positive apoptotic cells in the proximal tubules with the immunoreactivity of pVHL of the tamoxifen-treated control mouse kidneys after renal IRI (Fig. 3E), while there were almost no positive cells in VHL-KO mice.
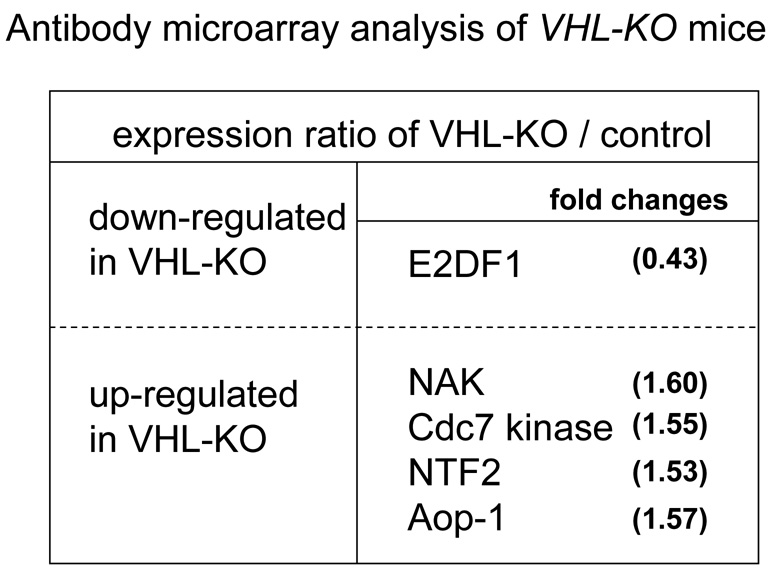
Antibody microarray analysis of VHL-KO and control mouse kidneys after IRI
In order to investigate downstream signaling molecules that may contribute to renal protection from IRI as a consequence of VHL inactivation, we used an antibody microarray containing 224 cell signaling proteins to compare the relative expression levels of these proteins in VHL-KO and control kidneys following tamoxifen induction. Proteins showing a >1.5-fold difference in expression were considered significantly differentially expressed. We observed an increase in protein expression levels of anti-oxidant-like protein (Aop-1), NF-κB activating kinase (NAK), and Cdc7 kinase in VHL-KO compared with control kidneys. In contrast, a decrease in the expression of E2F1 was seen in VHL-KO kidneys relative to control kidneys (Fig. 4).
Figure 4. Antibody microarray analysis of VHL-KO mice.
Representative data showing down-regulated and up-regulated protein expression levels in VHL-KO mice compared with control mice.
Discussion
To date, there have been no representative studies in the nephrology field that extensively investigate the role of VHL protein in renal injuries induced by ischemia. In this study, using murine genetic approaches, we have used a conditional VHL-KO mouse model based on the tamoxifen-inducible CreERTM system to inactivate the VHL gene in an acute manner during ischemia caused by bilateral renal IRI.
Here we demonstrated that VHL-KO mice subjected to renal IRI had extremely mild renal tubular injury without significant increases in BUN and CRN, while severe tubular damage and significantly higher levels of BUN and CRN were detected in control kidneys. Our histological analysis and evaluation of renal function showed that VHL inactivation could ameliorate ischemia-reperfusion injury of mouse renal tubules.
Matsumoto and colleagues reported that the most severe damage resulting from ischemic insult in a rat model extended from the outer medulla to the medullary ray of the cortex, suggesting that the proximal S3 segment and the thick ascending limb of the nephron are susceptible to ischemic injury [14]. Consistent with these findings, we frequently observed severe damage to the proximal tubules histologically in control mice. Furthermore, under normal conditions, VHL protein displays an expression pattern restricted to the proximal tubules of the kidney [23–25]. Our immunohistochemical analysis demonstrated that pVHL expression was detected strongly in the proximal tubules of the control kidneys, which coincided with the regions showing sustained moderate to severe injury after renal IRI. TUNEL staining of the tamoxifen-treated control mouse kidneys after renal IRI also revealed positive apoptotic cells in the proximal tubules with pVHL expression. In contrast, only weak immunoreactivity to VHL protein was found in the VHL-KO mouse kidney tubules that showed no damage.
Although the mechanisms are not fully elucidated in the present study, taken together these findings suggest that reduced expression of VHL protein in renal proximal tubules of VHL-KO mice could inhibit the transition to acute tubular injury. Our antibody microarray data indicate that inactivation of VHL leads to upregulation of several proteins reported to be responsible for activation of cell survival and inhibition of apoptosis (i.e., Aop-1, NAK, Cdc7 kinase) suggesting that renal protection resulting from VHL inactivation may involve signaling through these pathways. Nonetheless, the finding that VHL-KO mice were significantly less susceptible to renal IRI compared with control mice suggests that acute inactivation of the VHL gene contributes protective effects against ischemic damage to renal tubules of VHL-KO mice.
Using this CreERTM system, in vivo deletion of the VHL gene leads to increases in VHL target proteins HIF-1α, as well as HIF-2α. Recently, it has been reported that HIF-2α is also involved in adaptation to hypoxic conditions [15–18]. Unlike HIF-1α, HIF-2α is known to be restricted in expression to only a few types of cells including endothelial cells, and it was reported that loss of HIF-2α resulted in functional disruption of the endothelium [15]. In contrast to that study, which used HIF-2α knockdown mice, our VHL conditional knockout mice kidneys showed increased expression of both HIF-1α and HIF-2α proteins. Therefore, it is suggested that elevation of those HIFα subunits partly contributes to attenuation of IRI. In addition, other than the HIFα-dependent processes, HIFα-independent effects of VHL must also be taken into account. Several lines of evidence support a role for VHL in maintenance of epithelial tissue architecture and extracellular matrix deposition [26–28], suggesting that VHL may also be involved in inhibition of the cell cycle and, consequently, loss of pVHL might activate cell proliferation signals. Therefore, in our study both HIFα-dependent and -independent processes might contribute to the final phenotype in VHL-KO mice.
The present results also support our previous findings in another animal model, a rat model of glomerulonephritis, in which we demonstrated that VHL pre-induction by thrombin, a recently identified VHL inducer, aggravates glomerulonephritis [23]. Although the significance of VHL induction may vary in different animal models and cell types, our findings in the rat model suggest that VHL induction puts the kidney at a disadvantage, and may be reflection of the results presented here.
Several published reports have indicated that pre-induction of HIF-1α before pathological insult activates a self-defense mechanism and suppresses further aggravation of organ or cellular injury [11–13]. We have also previously shown that HIF-1α pre-induction by chemical hypoxia remarkably attenuated glomerulonephritis progression [24]. Furthermore, our recent studies demonstrated that HIF-1α was increased in VHL-null cells, even under normoxia, and directly enhanced apoptosis inhibitor (AI) gene expression, which suppresses mitochondrial function and reduces oxygen consumption [29]. Since the proximal tubules are rich in mitochondria for active transport and therefore oxygen demand is elevated, these regions with enhanced VHL expression levels are more susceptible to hypoxic events. Therefore, based on our recent study [29], it is suggested that VHL inactivation leads to HIFα stabilization, resulting in suppression of mitochondrial function and up-regulation of glucose metabolism. Our previous findings may suggest a possible mechanism, in the present study, by which VHL inactivation in the tamoxifen-inducible VHL-KO mice was protective against renal tubular injury. Bearing in mind that pVHL plays an important role in regulating the HIFα protein levels, reduction of VHL protein levels appears to be a positive modulator of survival signals in renal tubular cells. However, our data does not exclude the possibility that prolonged ischemia in VHL-KO mice may lead to the activation of the HIF-1α-regulated growth factors, such as EPO, PDGF, and TGFα, that could override these protective effects against renal tubular injuries, since other reports show that a broad variety of inflammatory mediators and cytokines can regulate HIF-1α, which in turn negatively controls inflammatory processes, and such a mechanism might be involved in protection from IRI [30,31]. Further studies in VHL-KO mice are needed to define further responsible signal transduction involved in renal tubular protection from the ischemic assault
In conclusion, we have shown that VHL-KO mice, in which acute VHL inactivation was induced by tamoxifen injection, were much less susceptible to renal IRI than control mice. This inducible VHL-inactivation system should prove useful as an in vivo model for evaluating drugs that can target HIF and HIF-regulated genes such as VEGF under ischemic conditions. Our present study has demonstrated a correlation between loss of VHL expression and protection against renal tubular injuries induced by ischemia. Further study is needed to elucidate the mechanisms by which VHL inactivation initiates cell signaling leading to renal tubule protective effects during renal IRI.
Acknowledgements
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-C0-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Heath and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. NCI-Frederick is accredited by AAALAC International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the “Guide for Care and Use of Laboratory Animals (National Research Council; 1996; National Academy Press; Washington DC).
References
- 1.Yamada K, Miwa T, Liu J, Nankoku M, Song W. Critical protection from renal ischemia reperfusion injury by CD55 and CD59. J Immunol. 2004;172:3869–3875. doi: 10.4049/jimmunol.172.6.3869. [DOI] [PubMed] [Google Scholar]
- 2.Fiaschi-Taesch NM, Santos S, Reddy V, Van Why SK, Philbrick WF, Ortega A, Esbrit P, Orloff J, Garcia-Ocana A. Prevention of acute ischemic renal failure by targeted delivery of growth factors to the proximal tubule in transgenic mice: The efficacy of parathyroid hormone-related protein and hepatocyte growth factor. J Am Soc Nephrol. 2004;15:112–125. doi: 10.1097/01.asn.0000102470.12285.c6. [DOI] [PubMed] [Google Scholar]
- 3.Park KM, Byun JY, Kramers C, Kim JI, Huang PL, Bonventre JV. Inducible nitric-oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J Biol Chem. 2003;278:27256–27266. doi: 10.1074/jbc.M301778200. [DOI] [PubMed] [Google Scholar]
- 4.Thakar CV, Zahedi K, Revelo MP, Wang Z, Burnham CE, Barone S, Bevans S, Lentsch AB, Rabb H, Soleimani M. Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J Clin Invest. 2005;115:3451–3459. doi: 10.1172/JCI25461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nath KA, Grande JP, Croatt AJ, Frank E, Caplice NM, Hebbel RP, Katusic ZS. Transgenic sickle mice are markedly sensitive to renal ischemia-reperfusion injury. Am J Pathol. 2005;166:963–972. doi: 10.1016/S0002-9440(10)62318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJD, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Woodward VK, Shelton JM, Richardson JA, Zhou XJ, Link D, Kielar ML, Jeyarajah DR, Lu CY. Ischemia-reperfusion induces G-CSF gene expression by renal medullary thick ascending limb cells in vivo and in vitro. Am J Physiol-Renal. 2004;286:1193–1201. doi: 10.1152/ajprenal.00379.2002. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Varghese TK, Zhang Z, Zhao LC, Thomas G, Hummel M, Abecassis M. Renal ischemia/reperfusion injury activates the enhancer domain of the human cytomegalovirus major immediate early promoter. Am J Transplant. 2005;5:1606–1613. doi: 10.1111/j.1600-6143.2005.00912.x. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 10.Min JH, Yang H, Ivan M, Gertler F, Kaelin WG, Jr, Pavletich NP. Structure of an HIF-1α–pVHL complex: hydroxyproline recognition in signaling. Science. 2002;296:1886–1889. doi: 10.1126/science.1073440. [DOI] [PubMed] [Google Scholar]
- 11.Jones NM, Bergeron M. Hypoxic preconditioning induces changes in HIF-1 α target genes in neonatal rat brain. J Cereb Blood Flow Metab. 2001;21:1105–1114. doi: 10.1097/00004647-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Yang YT, Ju TC, Yang DI. Induction of hypoxia inducible factor-1 attenuates metabolic insults induced by 3-nitropropionic acid in rat C6 glioma cells. Neurochem. 2005;93:513–525. doi: 10.1111/j.1471-4159.2005.03032.x. [DOI] [PubMed] [Google Scholar]
- 13.Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M, Reme CE. HIF-1 induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto M, Makino Y, Tanaka T, Tanaka H, Ishizaka N, Noiri E, Fujita T, Nangaku M. Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol. 2003;14:1825–1832. doi: 10.1097/01.asn.0000074239.22357.06. [DOI] [PubMed] [Google Scholar]
- 15.Kojima I, Tanaka T, Inagi R, Kato H, Yamashita T, Sakiyama A, Ohneda O, Takeda N, Sata M, Miyata T, Fujita T, Nangaku M. Protective role of hypoxia-inducible factor-2alpha against ischemic damage and oxidative stress in the kidney. J Am Soc Nephrol. 2007;18:1218–1226. doi: 10.1681/ASN.2006060639. [DOI] [PubMed] [Google Scholar]
- 16.Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 2006;66:6264–6270. doi: 10.1158/0008-5472.CAN-05-2519. [DOI] [PubMed] [Google Scholar]
- 17.Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006;291:F271–F281. doi: 10.1152/ajprenal.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo C, Yang W, Lobe CG. A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis. 2002;32:8–18. doi: 10.1002/gene.10021. [DOI] [PubMed] [Google Scholar]
- 20.Ma W, Tessarollo L, Hong S, Baba M, Southon E, Back T, Spence S, Lobe C, Sharma N, Maher G, Pack S, Vortmeyer A, Guo C, Zbar B, Schmidt L. Hepatic vascular tumors, angiectasis in multiple organs, and impaired spermatogenesis in mice with conditional inactivation of the VHL gene. Cancer Res. 2003;63:5320–5328. [PubMed] [Google Scholar]
- 21.Hong SB, Furihata M, Baba M, Zbar B, Schmidt LS. Vascular defects and liver damage by the acute inactivation of the VHL gene during mouse embryogenesis. Lab Invest. 2006;86:664–675. doi: 10.1038/labinvest.3700431. [DOI] [PubMed] [Google Scholar]
- 22.Kakinuma Y, Ando M, Kuwabara M, Katare RG, Okudera K, Kobayashi M, Sato T. Acetylcholine from vagal stimulation protects cardiomyocytes against ischemia and hypoxia involving non-hypoxic induction of HIF-1α. FEBS Lett. 2005;579:2111–2118. doi: 10.1016/j.febslet.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 23.Kudo Y, Kakinuma Y, Iguchi M, Sato T, Sugiura T, Furihata M, Shuin T. Modification in the von Hippel-Lindau protein is involved in the progression of experimentally induced rat glomerulonephritis. Nephron Exp Nephrol. 2007;106:e97–e106. doi: 10.1159/000103022. [DOI] [PubMed] [Google Scholar]
- 24.Kudo Y, Kakinuma Y, Mori Y, Morimoto N, Karashima T, Furihata M, Sato T, Shuin T, Sugiura T. Hypoxia-inducible factor-1α is involved in the attenuation of experimentally induced rat glomerulonephritis. Nephron Exp Nephrol. 2005;100:e95–e103. doi: 10.1159/000084575. [DOI] [PubMed] [Google Scholar]
- 25.Nagashima Y, Miyagi Y, Udagawa K, Taki A, Misugi K, Sakai N, Kondo K, Kaneko S, Yao M, Shuin T. Von Hippel-Lindau tumour suppressor gene. Localization of expression by in situ hybridization. J Pathol. 1996;180:271–274. doi: 10.1002/(SICI)1096-9896(199611)180:3<271::AID-PATH664>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Frew IJ, Krek W. Multitasking by pVHL in tumor suppression. Curr Opin Cell Biol. 2007;19:685–690. doi: 10.1016/j.ceb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Calzada MJ, Esteban MA, Feijoo-Cuaresma M, Castellanos MC, Naranjo-Suárez S, Temes E, Méndez F, Yánez-Mo M, Ohh M, Landázuri MO. von Hippel-Lindau tumor suppressor protein regulates the assembly of intercellular junctions in renal cancer cells through hypoxia-inducible factor-independent mechanisms. Cancer Res. 2006;66:1553–1560. doi: 10.1158/0008-5472.CAN-05-3236. [DOI] [PubMed] [Google Scholar]
- 28.Tang N, Mack F, Haase VH, Simon MC, Johnson RS. pVHL function is essential for endothelial extracellular matrix deposition. Mol Cell Biol. 2006;26:2519–2530. doi: 10.1128/MCB.26.7.2519-2530.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakinuma Y, Katare RG, Arikawa M, Muramoto K, Yamasaki F, Sato T. A HIF-1alpha-related gene involved in cell protection from hypoxia by suppression of mitochondrial function. FEBS Lett. 2008;582:332–340. doi: 10.1016/j.febslet.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frede S, Berchner-Pfannschmidt U, Fandrey J. Regulation of hypoxia-inducible factors during inflammation. Methods Enzymol. 2007;435:405–419. doi: 10.1016/S0076-6879(07)35021-0. [DOI] [PubMed] [Google Scholar]