Abstract
This article discusses the current techniques and future directions of infection imaging with particular attention to respiratory, CNS, abdominal, and postoperative infections. The agents currently in use localize to areas of infection and inflammation. An infection specific imaging agent would greatly improve the utility of scintigraphy in imaging occult infections. The superior spatial resolution of 18F-FDG PET and its lack of reliance on a functional immune system, gives this agent certain advantages over the other radiopharmaceuticals.
In respiratory infection imaging, an important advancement would be the ability to quantitatively delineate lung inflammation, allowing one to monitor the therapeutic response in a variety of conditions. Current studies suggest PET should be considered the most accurate quantitative method.
Scintigraphy has much to offer in localizing abdominal infection as well as inflammation. We may begin to see a gradual increase in the usage of FDG PET in detecting occult abdominal infections. Commonly used modalities for imaging inflammatory bowel disease are scintigraphy with 111In-oxine/99mTc-HMPAO labeled autologous white blood cells.
The literature on CNS infection imaging is relatively scarce. Few clinical studies have been performed and numerous new agents have been developed for this use with varying results. Further studies are needed to more clearly delineate the future direction of this field.
In evaluating the post-operative spine, 99mTc-ciprofloxacin SPECT was reported to be >80% sensitive in patients more than 6 months post-surgery. FDG PET has also been suggested for this purpose and may play a larger role than originally thought.
It appears PET/CT is gaining support, especially in imaging those with fever of unknown origin or nonfunctional immune systems. While an infection specific agent is lacking, the development of one would greatly advance our ability to detect, localize, and quantify infections. Overall, imaging such an agent via SPECT/CT or PET/CT will pave the way for greater clinical reliability in the localization of infection.
The Development of Infection Specific Imaging Agents
Since the advent of 67Ga citrate for routine infection imaging, a variety of agents have been developed and evaluated to better localize and detect areas of infection within the body. There has certainly been some advancement since the introduction of 67Ga citrate in 1971, but a true infection-specific imaging agent has yet to be developed. Almost all of the commonly used imaging agents localize to areas of inflammation rather than specifically those of infection, which makes clinical interpretation difficult and at times unreliable, particularly when the infection requires aggressive therapeutic intervention.
Inflammation and infection are different processes. Inflammation is merely a nonspecific immune response - one which does not require the presence of micro-organisms to occur. Inflammation can occur from trauma, ischemia, neoplasm, autoimmune attack, or invasion by micro-organisms. Conversely, the presence of a locus of micro-organisms may not lead to inflammation in the immunocompromised patient, but still constitutes a site of infection. It should be recognized that all radiopharmaceuticals accumulate to some extent in this quality due to inflammation at the site of infection (1, 2).
Granulocytes play an important role in the pathophysiology of infections and the development of imaging agents concerning infections. There are 3 physiological compartments that are involved in the granulocyte kinetics: the circulating and marginating granulocytes which constitute the total blood pool, the granulocyte pool in the bone marrow responsible for the development and release of granulocytes and the pools within which the blood granulocytes are physiologically destroyed. The average granulocyte residence time is 10 days and is replaced at a rate of 10 h. The hallmark of an infective process is enhanced vascular permeability, leading to the leakage of fluid and small molecules at the affected site and associated transudation or diapedesis of leukocytes leading to local accumulation of these cells. The process of migration of granulocytes from the second compartment towards the sites of infection is considered to be an important factor for targeting foci of infection (3). Radiopharmaceuticals utilize these properties to localize the lesion. It is for these reasons that the goal of developing an infection-specific imaging agent is a topic of much ongoing research. In this article we will review the current progress of non-osseous infection imaging and discuss those agents that hold promise for further research and future clinical utility.
An advancement over the original 67Ga citrate infection imaging was the development of in vitro radiolabeled white blood cells (WBCs) using 111In-oxine (4) or 99mTc-hexamethylpropylene amine oxime (99mTc-HMPAO). The chemotactic properties of the activated leukocytes form the basis of labeled leukocyte imaging. The various problems encountered in leukocyte labeling are discussed in the next section. One disadvantage worth mentioning is the inability to differentiate infection related to urinary and gastrointestinal systems. Continued research in this field led to the development of labeled antibiotics. 99mTc-labeled ciprofloxacin was reported to bind to the DNA gyrase of living bacteria, thereby distinguishing bacterial infection from inflammation (5). The other agents which have found their place in infection imaging are ubiquicidin analogs which are chemotactic peptide fragments, and nonpeptide molecules, such as leukotriene antagonists which bind to the leukocytes (6, 7). A newer technique that has recently gained favor in clinical use is that of Positron Emission Tomography – Computerized Tomography (PET-CT) imaging for the rapid detection and localization of occult infection. 18Fluoro-deoxy-glucose (18F-FDG) has been shown to target sites of infection via increased glucose utilization by infiltrated granulocytes and macrophages due to metabolic requirements. However this is nonspecific since 18F-FDG is also taken up at non-specific sites of inflammation as well as sites of tumor (8). The sensitivity of 18F-FDG PET (FDG PET) in patients suspected with focal infection is reported to be similar to that of 111In-labeled leukocytes; however false-positive changes are seen in patients with malignancy and in the postoperative period leading to low specificity (9).
It is clear that as of now, an ideal infection imaging agent does not exist. Table 1 lists the current available strategies for developing these agents. The generally accepted characteristics that constitute an ideal imaging agent are listed below. The goal in development is to strive for an agent that comes as close as possible to meeting the following criteria:
Table 1.
Strategies in infection imaging with specific tracers
Label leukocytes migrating to foci of infection
|
Target inflammatory mediators
|
Target locally present micro-organisms
|
Target the increased metabolic activity of infected areas
|
Accumulation only in those areas of active infection. Easily distinguishable from background uptake or low uptake in non-infected tissues.
Absence of physiological accumulation of the tracer in the blood and bone marrow, liver, spleen and rapid washout of background activity.
No adverse toxic effects and free of immune-reactions.
Safe, quick, and cost-effective preparation.
The ability of uptake at sites of infection in vivo, without requiring outside preparation and handling of blood products.
Low radiation burden to the patient.
Differentiation between infection and non-specific inflammation.
Rapid blood clearance and rapid accumulation at the site of infection.
Agents for Infection/Inflammation Imaging Currently in Clinical Use
67 Ga Citrate
Since 1971, 67Ga citrate has been used to detect infection and inflammation. Certain studies have suggested that following injection, the 67Ga citrate forms a complex with transferrin, and undergoes extravasation at the site of inflammation/infection. The exact mechanism of retention in the infectious lesion is not entirely clarified, but data from various models support the role of bacterial siderophores and lactoferrin in leukocytes (10).
Discrimination of infection is difficult using 67Ga citrate due to the normal uptake that is found in the bowel, liver, spleen, bone and genitourinary system and its slow blood clearance. Laxative administration can clear unwanted colonic activity, but is cumbersome and undesirable for the patient. In addition, 67Ga citrate has unfavorable imaging characteristics (long physical half-life and a low abundance of high-energy gamma radiations), causing high-radiation absorbed doses and relatively poor image quality. Furthermore, optimal imaging often requires delayed imaging for up to 72 hours post-injection (10).
Figure 1 demonstrates a positive 67Ga citrate scan in a patient with Pneumocystis carinii (PCP) pneumonia. Note the background uptake that occurs in the bowel, spleen, bone, and genitourinary tract. This does not interfere with making a clear diagnosis of a respiratory infection in this case; however in other locations, it can make interpretation difficult or impossible. In addition, lactating women can have high background uptake in breast tissue, potentially interfering with respiratory tract imaging.
Figure 1.

Radiolabeled WBCs
Radiolabeled WBCs are the most commonly used method to detect and localize infection today. The WBCs can be labeled using either 111In or 99mTc-hexamethylpropylene amine oxime HMPAO. McAfee and Thakur developed a technique to label autologous leukocytes with 111In by using oxinate as a chelator to transfer the tracer into the cell (10, 11). Peters et al developed HMPAO, a lipophilic chelator that allows the efficient labeling of white blood cells with 99mTc. Because of its more favorable radiation characteristics for imaging, 99mTc-HMPAO is more commonly used over 111In (2). One drawback of 99m Tc-HMPAO, however, is that of in vivo tracer elution from labeled cells. Also, the technique of WBC labeling is not without its own set of drawbacks. First, there is the risk to health-care workers involved with the handling of blood products. Secondly, there is a cost and time concern involved with the labeling of the WBCs. Indeed, it typically can take up to 3–4 hours from phlebotomy to completion of injection. In patients suffering from neutropenia there is difficulty associated with obtaining a sufficient amount of WBCs to label. In addition to this, the functional status of the WBCs must also be taken into account. Another concern involves patients receiving chemotherapy or other immune modulating agents that can affect WBC harvesting and/or function when injected back into the patient. Therefore, patients undergoing chemotherapy, receiving glucocorticoids, or infected with HIV may be difficult or impossible to image. The reported sensitivity and specificity for radiolabeled WBCs ranges from 86–90%, with a slightly higher sensitivity for acute infections likely due to the increased granulocytic response (12). This discovery was a significant advancement over the traditional 67Ga-citrate agent. Yet, WBCs are also able to localize to non-specific areas of inflammation and thus lack specificity for infection. Nonetheless, ex vivo labeling of leukocytes to 18F-FDG for 18F-FDG -WBC hybrid PET/CT imaging was found to detect foci of infection with high precision.
Nonspecific Immunoglobulins
Nonspecific polyclonal human immunoglobulins have been shown to localize to sites of infection/inflammation by extravasation from the bloodstream due to the induced local hyperemia (13) and this agent is thus, nonspecific for infection. One noteworthy benefit of this agent is that since it is derived from human antibodies, it negates the possibility of a Human Anti Murine Antibody (HAMA) response. This agent can be labeled with either 111In or 99mTc and thus suffers from some of the limitations of biodistribution of these agents. Studies have shown that detection of infection is reliable, but drawbacks include long-imaging time, and delayed blood-pool clearance. It is uncertain if this agent will play a promising role in clinical utility, especially given the potentially harmful side effects of other previously studied labeled antibodies, and the promising results of other newer agents.
PET Agents
18F-FDG is known to accumulate at sites of infection, inflammation and in autoimmune and granulomatous diseases. The inflammatory cells produce an excess of glycolytic enzymes and also overexpress glucose transporter (GLUT) isotypes (mainly GLUT-1 and GLUT-3) (14).
FDG PET can diagnose a variety of infections with a fairly high degree of certainty. Just a few examples include large-vessel vasculitis, abdominal infections such as inflammatory bowel disease, thoracic and soft-tissue infection. It is also useful in tumor induced fever, secondary to Hodgkin’s disease, aggressive non-Hodgkin’s lymphoma, colorectal cancer and sarcoma. In patients with fever of unknown origin (FUO), in vitro or in vivo labeled WBC methods are of limited value because of the rather low prevalence of granulocytic processes in this clinical setting (15).
Various in vitro studies of 18F-FDG labeling of human leukocytes using mixed/pure preparations of neutrophils and mononuclear cells have been reported. Granulocyte uptake accounted for 78%–87% of the activity in mixed preparations. Labeling of WBCs with 18F-FDG was not stable and the labeling yield ranged from 40% to 80% when pure preparations of WBC were used. Neutrophils when stimulated for 60 min by N-formyl-methionyl-leucyl-phenylalanine, a chemotactic peptide, revealed a significant increase in 18 F-FDG uptake (16–19).
In vivo labeling of WBCs using antibodies or antibody fragments
After the development of the above described techniques for labeling WBCs in vitro, the focus on discovery of an agent with the ability to label WBCs in vivo became an increasingly popular area of research. This would eliminate many of the drawbacks to the techniques of in vitro labeling that are still in common practice today. This would include a shorter preparation time, as well as elimination of the possible degradation in WBC function as a result of removal and re-injection into the body. The first reported case of in vivo WBC labeling was performed by Locher et al. using radiolabeled monoclonal antibody (MoAb) in 1986.
To date, at least three antigranulocyte antibodies have been tested for infection imaging: anti-NCA-95 immunoglobulin G (IgG) (BW250/183), anti-NCA-90 Fab’ (Immu-MN3, Leukoscan: anti-CD66 [Immunomedics Inc, Morris Plains, NJ]), and anti-CD15 (LeuTech [Palatin Technologies, Princeton, NJ]) (20). Each of these antigranulocyte antibodies labeled with 99mTc were determined to allow the accurate delineation of infection and inflammation. It was reasoned that since neutrophils increase the expression of CD15 antigens when they become activated, these compounds should increase the amount of specific binding around sites of infection and, as a consequence, enhance the efficacy of the radiopharmaceutical as a diagnostic test (21). None of these compounds, however, were specific for infection only as was original hypothesized. After further investigation it appeared that the behavior of these antigranulocyte antibodies differed from that of radiolabeled WBCs. In short, it appears that they did not accumulate in the lungs post-injection and their circulatory clearance differed from that of their in vitro WBC labeled counterparts.
Mozley et al. later showed that a transient leukopenia typically occurred 15–20 min post-injection of the anti-CD15 antibody and recovered with a mild overcompensation in approximately one hour (21). This transient leukopenia is not uncommon after injections of any compound and it has never been shown to be harmful or increase susceptibility to infections. Since that time, it has become widely accepted that the behavior of these MoAbs is largely due to a localized hyperemia and an increased vascular permeability which is non-specifically associated with both infection and inflammation.
The future of radiolabeling WBCs using antibodies
The future of these compounds is uncertain due to the results of the few clinical trials that have been performed. Also there is the theoretic and real possibility of a HAMA to any antibody that is made of nonhumanized protein. Thus, the investigation of partial antibody fragments was undertaken to minimize these adverse reactions and to potentially avoid the transient pancytopenia that had been documented to occur with use of these agents (21).
Respiratory Inflammation/Infection imaging
The literature and research surrounding respiratory infection imaging in nuclear medicine is relatively scarce. This is not to say that the imaging of this organ system does not hold promise and the studies to date have not been encouraging. As of now an important advance would be the development and general acceptance of an imaging modality that could quantitatively delineate lung inflammation, leading to the ability to monitor the therapeutic response in such diverse conditions as pneumonia, acute respiratory distress syndrome, cystic fibrosis, and Chronic Obstructive Pulmonary Disease (COPD).
Chen et al. have suggested that PET should be considered the most accurate quantitative radionuclide imaging method currently available for imaging pulmonary inflammation (22). They were also able to show a positive correlation between the rate of 18F-FDG uptake in lung field and the amount of neutrophils present in broncho-alveolar lavage fluid in patients with cystic fibrosis, suggesting this may be a useful adjunct to monitoring inflammatory burden (23). Another study conducted by Chen et al., performed in healthy volunteers, also showed that the inflammatory response to low-dose endotoxin in a single lung segment can be visualized and quantified by imaging with FDG PET (24). FDG PET has also shown promise in delineating inflammation resulting from bleomycin induced pneumonitis. Bleomycin is commonly used in the treatment of Hodgkin’s lymphoma and bleomycin induced pneumonitis is a common cause of morbidity and even mortality resulting from treatment. A case report of a 47 year old woman receiving treatment utilized FDG PET to distinguish resolution of disease activity, even in the presence of residual pulmonary scarring (25).
In patients with lobar pneumonia, an increase in 18F-FDG uptake was evident which was not so in the case of patients having brochiectasis. In addition, neutrophil accumulation at the site of the lesion was evident with 111In-leukocytes (26). In a study published in 2005, Mahfouz et al. examined the value of FDG PET in diagnosing infections in a population affected with multiple myeloma. Ninety-nine of the one-hundred and sixty-five infections identified were in the respiratory tract. The results of this study determined that FDG PET imaging (whether for cancer staging or infection work-up) contributed to the overall patient management by 24–28% depending on the indication (infection vs. staging) (27). While PET seems promising for respiratory infection imaging, this study was performed on a unique patient population that made any focal lung uptake unlikely to be due to malignancy. Therefore, perhaps the clinical utility of FDG PET in diagnosing respiratory infections lies in certain specific sub-groups of patients where infection is suspected and malignancy highly unlikely.
The diagnosis of a pulmonary lesion seen on plain film or CT typically raises the question of infection (acute or chronic) which can be a difficult one to answer. However, the lack of clinical trials in respiratory infection imaging makes CT scanning and biopsy a necessity in a symptomatic patient in order to answer this question. With the increasing awareness of radiation dosages and discomfort associated with biopsy, it would be extremely beneficial to continue research in this area, especially with some of the newer, postulated infection-specific agents. This could negate biopsy or at the very least reduce patient anxiety and possibly decrease the necessity of high-resolution CT scanning.
Abdominal Inflammation/Infection Imaging
Inflammatory Disorders
The area of abdominal inflammation imaging centers on the imaging of Inflammatory Bowel Disease (IBD). IBD comes in two well described forms. The first, ulcerative colitis (UC) typically is limited to the colon, sparing the small bowel and anus. Lesions are confined to the bowel mucosa, and they typically extend from a primary site rather than patches of inflammation. The other entity, Crohn’s Disease (CD), can affect the entire length of the bowel, primarily displays skip lesions, and shows transmural inflammation on histopathology. More importantly, distinguishing the diagnosis between CD and UC is crucial, since therapeutic options differ greatly.
In the past, there were no general guidelines as to which of the many available tests was the best to use in a given clinical situation. In 2005, Annovazzi et al. conducted a meta-analysis of peer reviewed articles published between 1984 and 2004 describing the use of nuclear medicine for the study of inflammatory bowel disorders, appendicitis and vascular graft infections (28).
The main modalities used for IBD imaging are scintigraphy with autologous white blood cells, labeled with 111In-oxine or 99mTc-HMPAO. These should be considered as the studies of choice in acute phases of disease, since endoscopic and barium studies are contraindicated. For this indication, Annovazzi et al. analyzed a total of 4388 patients from 49 different studies (Table 2). Their results revealed that for imaging IBD, alternative nuclear medicine techniques (scintigraphy with MoAbs and human polyclonal immunoglobulins) should be used only when a 99mTc-HMPAO WBC scan was not available, because of their lower overall sensitivity. However, these scans should be used to address specific questions, as ultrasound and barium studies have their clinical role as well. That is, a labeled WBC scan should only be considered when radiological and/or endoscopic techniques are inconclusive (28). Another specific indication is for determining recurrences on pre-anastomotic loops. In this case, WBC uptake can be classified semi-quantitatively comparing the activity to that of iliac crest, liver and or spleen (28).
Table 2.
Imaging modalities for imaging inflammatory bowel diseases. Results of meta-analysis
| Imaging Modality | Patients | Sensitivity (%) | Specificity (%) | Patients | Accuracy (%) | Patients | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| 111In-WBCs | 682 | 87.97 | 93.43 | 651 | 91.71 | 374 | 93.27 | 80.06 |
| 99mTc-WBCs | 1427 | 88.36 | 91.54 | 1195 | 85.45 | 889 | 93.12 | 83.39 |
| 99mTc-WBCs > 3h | 1459 | 90.69 | 84.94 | 1071 | 89.12 | 808 | 89.45 | 82.02 |
| 99mTc/111In-HIG | 110 | 75.73 | 83.93 | 110 | 76.28 | 110 | 92.38 | 62.67 |
| Endoscopy | 58 | 91.33 | 72.30 | 58 | 86.22 | 58 | 91.03 | 75.52 |
| Computed | 110 | 80.32 | 88.93 | 71 | 83.68 | 0 | NA | NA |
| Tomography |
WBCs-white blood cells; HIG – Human Immunoglobulin; PPV – Positive Predictive Values; NPV - Negative Predictive Value; NA – Not Available
Infectious Abdominal Disorders
Appendicitis is a very common disorder accounting for more than 1 out of 100 emergency room visits and affecting nearly all age groups. However, it has been shown that 30% of appendicitis presentations are misdiagnosed leading to the patient being discharged from the hospital with an erroneous diagnosis (29). Moreover, the percentage of appendectomy where the appendix is normal is around 16%. This is particularly true for atypical clinical manifestations, or in certain populations, such as young children, pregnant women and elderly patients (28).
Annovazzi et al. reviewed data from a total of 1548 patients from 24 different papers. The results of their analysis revealed that many nuclear medicine techniques show a high diagnostic accuracy: 99mTc-HMPAO, 111In-oxine WBCs, 99mTc-HIG, 99mTc-MoAbs and can be indistinctly used (Table 3). All agents showed good sensitivity and specificity. However, this paper was published before the question arose as to certain complications resulting from the usage of these MoAbs, as these agents are no longer available for use in the US. This leaves only WBC labeling as a viable option, with the caveat of having a significant delay in time to diagnosis, again highlighting the need for the development of more effective in vivo WBC labeling techniques. It appears that at this time the role of nuclear medicine in appendiceal infection imaging (at least in the US) is not substantial.
Table 3.
Nuclear medicine modalities in the diagnosis of appendicitis: results of meta-analysis
| Imaging Modality | Number of scans | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV | NPV |
|---|---|---|---|---|---|---|
| 111In-WBCs | 128 | 89.53 | 95.01 | 92.97 | 90.97 | 93.65 |
| 99mTc-WBCs < 2h | 224 | 93.83 | 92.06 | 92.41 | 85.60 | 96.51 |
| 99mTc-WBCs < 4h | 572 | 92.86 | 88.00 | 89.29 | 88.69 | 92.20 |
| 99mTc-MoAb | 629 | 90.81 | 87.26 | 89.04 | 79.55 | 94.15 |
WBCs-white blood cells; PPV – Positive Predictive Values; NPV - Negative Predictive Value; NA – Not Available
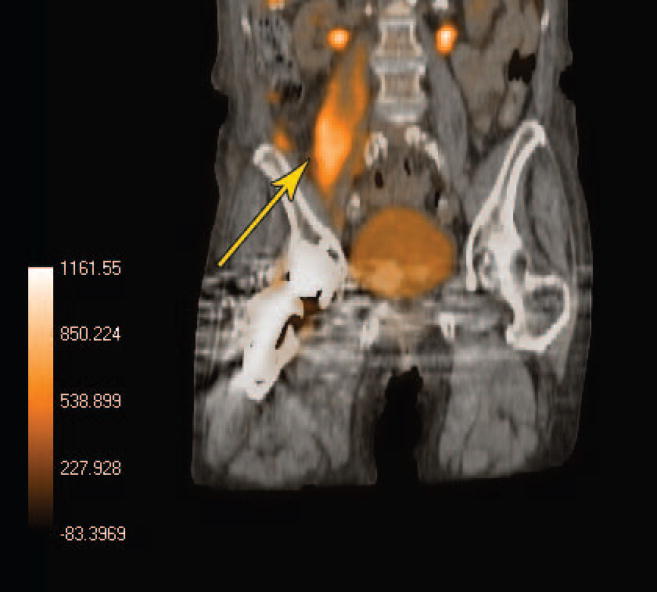
The advent of PET/CT also shows promise for the future use of 18F-FDG to detect abdominal infections and allow for greater anatomic localization, leading to better diagnostic confidence. Figure 2 demonstrates a right psoas muscle abscess found on FDG PET imaging. The greater confidence achieved in localizing the infection played an important role in determining the management of this particular patient. FDG PET has been shown to have an overall sensitivity of 90% in detecting abdominal and pelvic abscesses, bacterial colitis, diverticulitis, and infected vascular grafts (27, 30–32). Unfortunately, large scale studies in this area are lacking, and only time will tell if this agent can outperform 111In or 99mTc labeled WBCs.
Figure 2.
Interestingly, a study conducted by Ingui et al. in 2007, suggests that the use of software fusing Single Photon Emission Computerized Tomography (SPECT) with CT is superior over traditional side-by-side SPECT and CT for 111In-labeled-WBCs or 67Ga Citrate scans. The authors reported that a greater confidence was achieved in 71% of cases and the fused images altered interpretations in almost half of the studies examined (33). In a comparison of SPECT/CT vs. traditional SPECT in general, Roach et al. found that final reports were significantly different in 26% patients compared to the reporting with planar/SPECT alone (34).
It is clear that we have only begun to scratch the surface of the many uses of PET/CT in delineating abdominal infections. Given PET’s superior spatial resolution and anatomic localization when fused with CT, we may begin to see a gradual increase in the usage of FDG PET in detecting both known and suspected abdominal infections. Appendicitis is only one common illness that stands to benefit from FDG PET. There are numerous other abdominal infections such as gastroenteritis, colitis, cholecystitis, pyogenic abscesses, prostatitis, cystitis, pyelonephritis, and gynecologic infections that may stand to benefit from this imaging modality. With further research and clinical trials we can begin to better delineate the true extent of FDG PET or other positron emitting infection agents’ clinical utility.
CNS Inflammation/Infection Imaging
The literature on CNS inflammation and infection imaging is relatively scarce at this point in time. There have been very few clinical studies performed and most articles are case reports or involve only a small cohort of patients. One such study evaluated the use of dynamic SPECT with 99mTc-ethyl cysteinate dimer (99mTc-ECD) in 9 patients suspected of having herpes simplex viral encephalitis (HSVE) or other types of viral encephalitis. It is thought that 99mTc-ECD SPECT is able to more accurately detect subtle focal changes in uptake because of a superior contrast to background ratio of activity in white matter. The authors’ results showed that dynamic SPECT findings were positive in patients with HSVE and negative in those with non-HSVE. It was reasoned that HSV primarily damages endothelial cells of the capillaries of the brain, leading to the higher focal uptake whereas other viruses may damage neurons more directly, with less vascular involvement (35).
An increasing amount of research has been performed on PET imaging techniques and their usefulness in CNS infection imaging. One article looked at the imaging characteristics of PET in a patient with a known tuberculous brain abscess. The possibility of a tuberculous brain abscess should be considered when 18F-FDG accumulates at the periphery of a ring-enhancing lesion in a chronically ill or immunocompromised patient (36). This paper, however, only looked at a single patient and it has been previously suggested that any type of neoplastic or non-neoplastic lesion could produce this pattern of enhancement in the immunocompromised patients (37).
The development of the tracer O-(2-[18F]fluoroethyl)-]L-tyrosine (18F-FET) has been reported to allow tumor recurrence to be distinguished from radiation-induced changes in cases of suspected recurrent high and low grade gliomas, and it was also postulated that it would better able to differentiate neoplastic vs. non-neoplastic ring-enhancing lesions. A recent study however, concluded that 18F-FET PET has limited specificity in distinguishing between neoplastic and non-neoplastic ring-enhancing intracerebral lesions thus making biopsy and histologic confirmation necessary (37).
A study performed in 2006 by Falagas et al., looked at the utility of 99mTc-ciprofloxacin in diagnosing suspected spinal infections due to other tracers’ lack of sensitivity in this area. The results of that preliminary study suggested that scintigraphy with 99mTc-ciprofloxacin may be useful in the diagnosis of active spinal infections (38). However, the authors’ also suggest that its usefulness in this particular area stems from this agents’ ability to distinguish between bacterial and sterile inflammation. It should be noted that no controls were used in this study and since the time of publication it has been shown that 99mTc-ciprofloxacin is more likely a nonspecific indicator of inflammation than an infection specific agent.
In the inflammatory imaging spectrum of CNS imaging is the possibility of FDG PET usage to aid in diagnosing paraneoplastic neurological syndrome (PNS). In the patient with PNS, brain FDG PET and brain Magnetic Resonance Imaging (MRI) are both complementary to each other, and frequently, abnormalities noted on FDG PET images can provide additional information which is clinically significant (39). In a review of four different published articles, the authors also found that in the setting of paraneoplastic cerebellar degeneration, FDG PET generally reveals cerebellar hypometabolism analogous to the cerebellar atrophy on MRI, but it also tends to show the abnormality more often than MRI (39). These findings suggest that FDG PET may be a future tool for the initial workup of a patient with PNS having either a known or unknown malignancy.
Postoperative Infections
The diagnosis of a postoperative infection is often a difficult one to make clinically. Imaging can play a crucial role in helping to rule in or out the presence of infection. Often times the decision still becomes a difficult one due to post operative inflammation and healing. This difficulty increases even more so in the presence of orthopedic devices. Consequently, bone, WBC and 67Ga scanning, as well as CT and MRI, are of limited use in this setting. In aging adults, the problem of delay in diagnosing postoperative infections is even worse since the clinical manifestations in this population are frequently atypical.
A study performed in 2003 evaluated the utility of 99mTc-ciprofloxacin in diagnosing infections in postoperative spinal surgery patients. In the case of postoperative spinal patients, WBC scanning is of limited use, but the low normal bone marrow uptake of 99mTc-ciprofloxacin along with its postulated bacterial specificity made it an ideal candidate to be evaluated in this setting (40). In 2003, a study published by De Winter et al., found that of 49 postoperative spinal surgery patients, 99mTc-ciprofloxacin SPECT is up to 81% sensitive in evaluating infections in the postoperative spine, when operations performed less than 6 months prior were excluded. Not surprisingly, sensitivity was much higher for SPECT than for planar imaging (40).
FDG PET has also been suggested as a means for evaluating postoperative infections. A 2005 study evaluated the utility of FDG PET in assessing postoperative infections in a rabbit model. Comparisons were made between noninfected rabbits and rabbits in which infection with S. aureas was initiated at the time of surgery. The results of this study found that SUV comparisons from the surgical site could not be used to distinguish between the infected and uninfected groups until day 15. However, visual analysis of scans revealed a significant difference between the infected and uninfected groups as early as day 8 (41). These findings suggest that FDG PET may have more utility in postoperative infection imaging than was originally thought.
Another study from 2002, evaluated the sensitivity and specificity of various tests in diagnosing postoperative abdominal infections. Thirty-three patients undergoing colorectal surgery and aged over 60 years were enrolled in the study. All patients underwent a gallium scan and C-Reactive Protein, WBC and endoscopic examinations. Of the four diagnostic modalities used, 67Ga scan had the highest overall diagnostic accuracy (90.9%) while the WBC test had the lowest sensitivity (60.6%) (42). This study just reiterates the importance of establishing a better means of postoperative infection imaging.
The advent of PET and SPECT imaging along with CT hybridization allows for much greater accuracy and confidence in diagnosis. These modalities combined with newer infection imaging agents suggest that the future of postoperative infection imaging is bright. Unfortunately, to date, only limited clinical trials have been done on a small number of agents. If we are to properly advance the field of postoperative infection imaging, much larger and more numerous trials are needed to best compare each agents pros and cons, and develop a widely accepted, working algorithm.
Modern Uses for 18F-FDG PET
Fever of Unknown Origin
Of all the newer agents in development, none has received as much attention as the use of FDG PET in detecting infection. The widespread availability of this agent has made it a convenient choice for many different clinical situations, and case reports regarding its usage in certain specific situations are abundant. One situation that has received particular attention is the usage of FDG PET in patients with fever of unknown origin (FUO). Fever of unknown origin in adults is defined as a temperature higher than 38.3°C that lasts for greater than three weeks with no obvious source despite an appropriate investigation. The majority of cases of FUO are due to infections, malignancy, collagen vascular diseases, and autoimmune disorders. Among these causes, infections account for the most cases of FUO, followed by malignancy and other non-infectious inflammatory diseases.
An early prospective study performed by Meller et al. involving 20 consecutive patients compared the role of FDG PET imaging with that of 67Ga-SPECT in patients with FUO. The authors reported a sensitivity of 81% and a specificity of 86% for FDG PET vs. a sensitivity and specificity of 67% and 78%, respectively, for 67Ga SPECT (30). At this time, however, there is no general consensus as to the role of FDG PET in cases of FUO. Although FDG PET is certainly useful in the setting of FUO, well-designed prospective studies remain necessary to quantify the efficacy of FDG PET for the evaluation of FUO, as it may potentially become a first-line imaging technique for assessments of these patients (43).
A prospective multi-center study analyzing the value of FDG PET in 70 patients with FUO by Bleeker-Rovers and colleagues found that FDG PET contributed to determining the etiology in 30% of patients. Interestingly, they also found that FDG PET was not helpful in any patient with a normal erythrocyte sedimentation rate (ESR) and CRP. These findings suggest that FDG PET should be strongly considered as an early diagnostic study in patients presenting with FUO with an elevated ESR or CRP (43, 44).
A more recent article from Bleeker-Rovers et al. nicely summarized the results of seven recent studies of FDG PET in patients with FUO. However, comparing one study to another is difficult due to the difference in study design, PET technique, time of imaging, and varying definitions of FUO (45, 46).
FDG PET seems to be increasingly recognized as having utility in FUO and infection imaging as a whole. While the search for an infection-specific agent continues, in patients with FUO, 18F-FDG can provide useful benefits. Its ability to show areas of increased glucose utilization, allows for visualization of more than one etiology such as: infection, inflammatory disorders, malignancy, etc.
Immunocompromised Infection Imaging
FDG PET can be a useful tool for diagnosing and managing infections even in the setting of severe neutropenia or lymphopenia (27). In addition to this, FDG PET uptake has also been shown to correlate with the degree of HIV infection and increasing viral load (47). We have already discussed the uses of PET in distinguishing between CNS infection and malignancy in patients with AIDS. But in addition to this, there seems to be utility in all cases of inherited or acquired neutropenia.
For example, Gungor et al. sampled 43 patients born immunocompromised with chronic granulomatous disease (CGD) and compared FDG PET vs. CT in 22 and 19 patients, respectively. Their results showed that PET was able to exclude 59 lesions suspicious for active infection on CT and also reveal 49 infective lesions not seen on CT. These findings showed that whole body FDG PET can be used to screen for active infections in patients with CGD (48). Another article from Depas et al. reported the clinical impact of PET/CT usage in immunocompromised neonates. The authors suggest that, based on their findings, FDG PET should be considered in difficult cases of neonatal infection or in challenging diagnoses. They also felt that PET/CT allows for more accurate staging and treatment monitoring of the in invasive infections of immunocompromised children (49).
Promising Agents
99mTc-labeled Interleukin-8
Interleukin 8 (IL-8) is a well known chemotactic cytokine that binds with a high affinity to receptors expressed on activated neutrophils. A 99mTc-labeled IL-8 preparation was developed with hydrazinonicotinamide (HYNIC) as a chelating agent was shown to have some promising characteristics in initial animal model experiments (49). This led to the development of the first clinical trial in humans performed by Bleeker-Rovers et al., on 20 patients suspected of having localized infections. In 10 of 12 patients with infections, 99mTc-IL-8 localized the infection at 4 h after injection. In 1 patient with vertebral osteomyelitis and in 1 patient with an infected knee prosthesis, 99mTc-IL-8 scintigraphy results were false (50). Of the 8 patients determined to have noninfectious disorders, no focal accumulation of 99mTc-IL-8 was noted. In addition, focal accumulation to sites of infection occurred as early as 4 hours after injection. No significant side effects were noted (50).
Due to the positive results and lack of significant side effects, this appears to be a promising new agent. However, it is not yet reasonable to assume this agent is infection specific. Larger scale trials must be performed to determine what role, if any, this agent will play in the future of infection imaging.
fMLP
N-formyl products, such as N-formyl-methionyl-leucyl-phenylalanine (fMLF or fMLP) are formed via the cleavage of bacterial and mitochondrial proteins. These products are among the first identified potent chemoattractants for activated leukocytes The first studies on these compounds were carried out in the early 1980’s (51,52).
Early studies using these agents showed localization of infection within 1 hour post-injection. However, even a dose as low as 10 ng/kg of the agent induced a transient leukopenia. One study using 99mTc-HYNIC-fMLFK in rabbits with intramuscular abscesses showed visualization of the infected foci at 2 hours post-injection. The authors’ demonstrated a moderate abscess-to-normal-tissue ratio at 4 hours post-injection. This study again noted a transient leukopenia (65% reduction) that occurred 30 minutes after injection (53,54).
Future Outlook
Overall, the area of infection imaging in nuclear medicine is one rich with ongoing research. While an infection specific imaging agent is still lacking, the development of one would greatly advance our ability to detect, localize, and quantify respiratory, abdominal, CNS, and post-operative infections. It appears that the superior spatial resolution of PET combined with CT for better anatomic localization is gaining support as a more effective means of infection imaging, especially in those with FUO or nonfunctional immune systems. Therefore, the hybridization of an infection specific radiopharmaceutical, imaged via SPECT or PET and combined with CT will pave the way for greater reliability and clinical utilization of these technologies in the evaluation of difficult patients.
Acknowledgments
Research was supported by: NIH CA109231, C027175, EB001809, 1S10RR23709 and PA ME-03-184
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rennen HJ, Boerman OC, Oyen WJ, et al. Imaging infection/inflammation in the new millennium. Eur J Nucl Med. 2001;28:241–252. doi: 10.1007/s002590000447. [DOI] [PubMed] [Google Scholar]
- 2.Boerman OC, Rennen H, Oyen WJ, et al. Radiopharmaceuticals to image infection and inflammation. Semin Nucl Med. 2001;31:286–295. doi: 10.1053/snuc.2001.26189. [DOI] [PubMed] [Google Scholar]
- 3.Tulchinsky M, Peters AM. Leukocyte receptor-binding radiopharmaceuticals for infection and inflammation scintigraphy. J Nucl Med. 2005;46:718–721. [PubMed] [Google Scholar]
- 4.Thakur ML, Lavender JP, Arnot RN, Silvester DJ, Segal AW. Indium-111-labeled autologous leukocytes in man. J Nucl Med. 1977;18(10):1014–21. [PubMed] [Google Scholar]
- 5.Vinjamuri S, Hall AV, Solanki KK, et al. Comparison of 99mTc infecton imaging with radiolabelled white-cell imaging in the evaluation of bacterial infection. Lancet. 1996;347:233–235. doi: 10.1016/s0140-6736(96)90407-9. [DOI] [PubMed] [Google Scholar]
- 6.van Eerd JE, Oyen WJ, Harris TD, et al. A bivalent leukotriene B(4) antagonist for scintigraphic imaging of infectious foci. J Nucl Med. 2003;44:1087–1091. [PubMed] [Google Scholar]
- 7.Fischman AJ, Babich JW, Strauss HW. A ticket to ride: Peptide radiopharmaceuticals. J Nucl Med. 1993;34:2253–2263. [PubMed] [Google Scholar]
- 8.Rennen HJ, Laverman P, van Eerd JE, et al. PET imaging of infection with a HYNIC-conjugated LTB4 antagonist labeled with F-18 via hydrazone formation. Nucl Med Biol. 2007;34:691–695. doi: 10.1016/j.nucmedbio.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Kjaer A, Lebech AM, Eigtved A, et al. Fever of unknown origin: Prospective comparison of diagnostic value of 18F-FDG PET and 111In-granulocyte scintigraphy. Eur J Nucl Med Mol Imaging. 2004;31:622–626. doi: 10.1007/s00259-003-1425-5. [DOI] [PubMed] [Google Scholar]
- 10.Tsan MF. Mechanism of gallium-67 accumulation in inflammatory lesions. J Nucl Med. 1985;26:88–92. [PubMed] [Google Scholar]
- 11.McAfee JG, Thakur ML. Survey of radioactive agents for in vitro labeling of phagocytic leukocytes. II. particles. J Nucl Med. 1976;17:488–492. [PubMed] [Google Scholar]
- 12.Datz FL. Abdominal abscess detection: Gallium, 111In-, and 99mTc-labeled leukocytes, and polyclonal and monoclonal antibodies. Semin Nucl Med. 1996;26:51–64. doi: 10.1016/s0001-2998(96)80016-x. [DOI] [PubMed] [Google Scholar]
- 13.Fischman AJ, Fucello AJ, Pellegrino-Gensey JL, et al. Effect of carbohydrate modification on the localization of human polyclonal IgG at focal sites of bacterial infection. J Nucl Med. 1992;33:1378–1382. [PubMed] [Google Scholar]
- 14.Shepherd PR, Kahn BB. Glucose transporters and insulin action--implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341:248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 15.Meller J, Altenvoerde G, Munzel U, et al. Fever of unknown origin: Prospective comparison of [18F]FDG imaging with a double-head coincidence camera and gallium-67 citrate SPET. Eur J Nucl Med. 2000;27:1617–1625. doi: 10.1007/s002590000341. [DOI] [PubMed] [Google Scholar]
- 16.Osman S, Danpure HJ. The use of 2-[18F]fluoro-2-deoxy-D-glucose as a potential in vitro agent for labelling human granulocytes for clinical studies by positron emission tomography. Int J Rad Appl Instrum B. 1992;19:183–190. doi: 10.1016/0883-2897(92)90006-k. [DOI] [PubMed] [Google Scholar]
- 17.Forstrom LA, Dunn WL, Mullan BP, et al. Biodistribution and dosimetry of [(18)F]fluorodeoxyglucose labelled leukocytes in normal human subjects. Nucl Med Commun. 2002;23:721–725. doi: 10.1097/00006231-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Forstrom LA, Mullan BP, Hung JC, et al. 18F-FDG labelling of human leukocytes. Nucl Med Commun. 2000;21:691–694. doi: 10.1097/00006231-200007000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Dumarey N, Egrise D, Blocklet D, et al. Imaging infection with 18F-FDG-labeled leukocyte PET/CT: Initial experience in 21 patients. J Nucl Med. 2006;47:625–632. [PubMed] [Google Scholar]
- 20.Thakur ML, Thiagarajan P, White F, et al. Monoclonal antibodies for specific cell labeling: considerations, preparations and preliminary evaluation. Nucl Med and Biol. 1987;14:51–58. doi: 10.1016/0883-2897(87)90161-9. [DOI] [PubMed] [Google Scholar]
- 21.Mozley PD, Thakur ML, Alavi A, et al. Effects of a 99mTc-labeled murine immunoglobulin M antibody to CD15 antigens on human granulocyte membranes in healthy volunteers. J Nucl Med. 1999;40:2107–2114. [PubMed] [Google Scholar]
- 22.Chen DL, Ferkol TW, Mintun MA, et al. Quantifying pulmonary inflammation in cystic fibrosis with positron emission tomography. Am J Respir Crit Care Med. 2006;173:1363–1369. doi: 10.1164/rccm.200506-934OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen DL, Schuster DP. Imaging pulmonary inflammation with positron emission tomography: A biomarker for drug development. Mol Pharm. 2006;3:488–495. doi: 10.1021/mp060050w. [DOI] [PubMed] [Google Scholar]
- 24.Chen DL, Rosenbluth DB, Mintun MA, et al. FDG PET imaging of pulmonary inflammation in healthy volunteers after airway instillation of endotoxin. J Appl Physiol. 2006;100:1602–1609. doi: 10.1152/japplphysiol.01429.2005. [DOI] [PubMed] [Google Scholar]
- 25.Buchler T, Bomanji J, Lee SM. FDG PET in bleomycin-induced pneumonitis following ABVD chemotherapy for hodgkin’s disease--a useful tool for monitoring pulmonary toxicity and disease activity. Haematologica. 2007;92:e120–1. doi: 10.3324/haematol.11856. [DOI] [PubMed] [Google Scholar]
- 26.Jones HA, Sriskandan S, Peters AM, et al. Dissociation of neutrophil emigration and metabolic activity in lobar pneumonia and bronchiectasis. Eur Respir J. 1997;10:795–803. [PubMed] [Google Scholar]
- 27.Mahfouz T, Miceli MH, Saghafifar F, et al. 18F-fluorodeoxyglucose positron emission tomography contributes to the diagnosis and management of infections in patients with multiple myeloma: A study of 165 infectious episodes. J Clin Oncol. 2005;23:7857–7863. doi: 10.1200/JCO.2004.00.8581. [DOI] [PubMed] [Google Scholar]
- 28.Annovazzi A, Bagni B, Burroni L, et al. Nuclear medicine imaging of inflammatory/infective disorders of the abdomen. Nucl Med Commun. 2005;26:657–664. doi: 10.1097/01.mnm.0000169202.68011.47. [DOI] [PubMed] [Google Scholar]
- 29.Calder JD, Gajraj H. Recent advances in the diagnosis and treatment of acute appendicitis. Br J Hosp Med. 1995;54:129–133. [PubMed] [Google Scholar]
- 30.Meller J, Sahlmann CO, Scheel AK. 18F-FDG PET and PET/CT in fever of unknown origin. J Nucl Med. 2007;48:35–45. [PubMed] [Google Scholar]
- 31.Stumpe KD, Dazzi H, Schaffner A, et al. Infection imaging using whole-body FDG PET. Eur J Nucl Med. 2000;27:822–832. doi: 10.1007/s002590000277. [DOI] [PubMed] [Google Scholar]
- 32.Bleeker-Rovers CP, de Kleijn EM, Corstens FH, et al. Clinical value of FDG PET in patients with fever of unknown origin and patients suspected of focal infection or inflammation. Eur J Nucl Med Mol Imaging. 2004;31:29–37. doi: 10.1007/s00259-003-1338-3. [DOI] [PubMed] [Google Scholar]
- 33.Ingui CJ, Shah NP, Oates ME. Infection scintigraphy: Added value of single-photon emission computed tomography/computed tomography fusion compared with traditional analysis. J Comput Assist Tomogr. 2007;31:375–380. doi: 10.1097/01.rct.0000237815.11054.d2. [DOI] [PubMed] [Google Scholar]
- 34.Roach PJ, Schembri GP, Ho Shon IA, et al. SPECT/CT imaging using a spiral CT scanner for anatomical localization: Impact on diagnostic accuracy and reporter confidence in clinical practice. Nucl Med Commun. 2006;27:977–987. doi: 10.1097/01.mnm.0000243372.26507.e7. [DOI] [PubMed] [Google Scholar]
- 35.Kataoka H, Inoue M, Shinkai T, et al. Early dynamic SPECT imaging in acute viral encephalitis. J Neuroimaging. 2007;17:304–310. doi: 10.1111/j.1552-6569.2007.00154.x. [DOI] [PubMed] [Google Scholar]
- 36.Kang K, Lim I, Roh JK. Positron emission tomographic findings in a tuberculous brain abscess. Ann Nucl Med. 2007;21:303–306. doi: 10.1007/s12149-007-0023-1. [DOI] [PubMed] [Google Scholar]
- 37.Floeth FW, Pauleit D, Sabel M, et al. 18F-FET PET differentiation of ring-enhancing brain lesions. J Nucl Med. 2006;47:776–782. [PubMed] [Google Scholar]
- 38.Falagas ME, Valotassiou VJ, Papadouli D, et al. 99mTechnetium-ciprofloxacin scintigraphy for the evaluation of spinal infections: A preliminary report. Clin Orthop Relat Res. 2006;444:34–37. doi: 10.1097/01.blo.0000201173.02883.6e. [DOI] [PubMed] [Google Scholar]
- 39.Basu S, Alavi A. Role of FDG PET in the clinical management of paraneoplastic neurological syndrome: Detection of the underlying malignancy and the brain PET-MRI correlates. Mol Imaging Biol. 2008;10:131–137. doi: 10.1007/s11307-008-0134-7. [DOI] [PubMed] [Google Scholar]
- 40.De Winter F, Gemmel F, Van Laere K, et al. 99mTc-ciprofloxacin planar and tomographic imaging for the diagnosis of infection in the postoperative spine: Experience in 48 patients. Eur J Nucl Med Mol Imaging. 2004;31:233–239. doi: 10.1007/s00259-003-1349-0. [DOI] [PubMed] [Google Scholar]
- 41.Jones-Jackson L, Walker R, Purnell G, et al. Early detection of bone infection and differentiation from post-surgical inflammation using 2-deoxy-2-[18F]-fluoro-D-glucose positron emission tomography (FDG PET) in an animal model. J Orthop Res. 2005;23:1484–1489. doi: 10.1016/j.orthres.2005.03.010.1100230635. [DOI] [PubMed] [Google Scholar]
- 42.Lin WY, Chao TH, Wang SJ. Clinical features and gallium scan in the detection of post-surgical infection in the elderly. Eur J Nucl Med Mol Imaging. 2002;29:371–375. doi: 10.1007/s00259-001-0727-8. [DOI] [PubMed] [Google Scholar]
- 43.Kumar R, Basu S, Torigian D, et al. Role of modern imaging techniques for diagnosis of infection in the era of 18F-fluorodeoxyglucose positron emission tomography. Clin Microbiol Rev. 2008;21:209–224. doi: 10.1128/CMR.00025-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bleeker-Rovers CP, Vos FJ, de Kleijn EM, et al. A prospective multicenter study on fever of unknown origin: The yield of a structured diagnostic protocol. Medicine (Baltimore) 2007;86:26–38. doi: 10.1097/MD.0b013e31802fe858. [DOI] [PubMed] [Google Scholar]
- 45.Bleeker-Rovers CP, Vos FJ, Mudde AH, et al. A prospective multi-centre study of the value of FDG PET as part of a structured diagnostic protocol in patients with fever of unknown origin. Eur J Nucl Med Mol Imaging. 2007;34:694–703. doi: 10.1007/s00259-006-0295-z. [DOI] [PubMed] [Google Scholar]
- 46.Bleeker-Rovers CP, Vos FJ, Corstens FH, et al. Imaging of infectious diseases using [18F] fluorodeoxyglucose PET. Q J Nucl Med Mol Imaging. 2008;52:17–29. [PubMed] [Google Scholar]
- 47.Brust D, Polis M, Davey R, et al. Fluorodeoxyglucose imaging in healthy subjects with HIV infection: Impact of disease stage and therapy on pattern of nodal activation. AIDS. 2006;20:985–993. doi: 10.1097/01.aids.0000222070.52996.76. [DOI] [PubMed] [Google Scholar]
- 48.Gungor T, Engel-Bicik I, Eich G, et al. Diagnostic and therapeutic impact of whole body positron emission tomography using fluorine-18-fluoro-2-deoxy-D-glucose in children with chronic granulomatous disease. Arch Dis Child. 2001;85:341–345. doi: 10.1136/adc.85.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Depas G, Decortis T, Francotte N, et al. F-18 FDG PET in infectious diseases in children. Clin Nucl Med. 2007;32:593–598. doi: 10.1097/RLU.0b013e3180a1abe8. [DOI] [PubMed] [Google Scholar]
- 50.Bleeker-Rovers CP, Rennen HJ, Boerman OC, et al. 99mTc-labeled interleukin 8 for the scintigraphic detection of infection and inflammation: First clinical evaluation. J Nucl Med. 2007;48:337–343. [PubMed] [Google Scholar]
- 51.McAfee JG, Subramanian G, Gagne G. Technique of leukocyte harvesting and labeling: Problems and perspectives. Semin Nucl Med. 1984;14:83–106. doi: 10.1016/s0001-2998(84)80023-9. [DOI] [PubMed] [Google Scholar]
- 52.Thakur ML. New radionuclides in the diagnosis of obscure infections. Conn Med. 1981;45:302–304. [PubMed] [Google Scholar]
- 53.van Eerd JE, Boerman OC, Corstens FH, et al. Radiolabeled chemotactic cytokines: New agents for scintigraphic imaging of infection and inflammation. Q J Nucl Med. 2003;47:246–255. [PubMed] [Google Scholar]
- 54.Edwards DS, Liu S, Ziegler MC, et al. RP463: A stabilized technetium-99m complex of a hydrazino nicotinamide derivatized chemotactic peptide for infection imaging. Bioconjug Chem. 1999;10:884–891. doi: 10.1021/bc990049y. [DOI] [PubMed] [Google Scholar]