Abstract
Gonorrhea remains an important clinical and public health problem throughout the world. Gonococcal infections have historically been diagnosed by Gram stain and culture, but are increasingly diagnosed through nucleic acid tests thereby eliminating the opportunity for antimicrobial susceptibility testing. Gonococcal infections are typically treated with single-dose therapy with an agent found to cure >95% of cases. Unfortunately, the gonococcus has repeatedly developed resistance to antimicrobials including sulfonamides, penicillin, tetracyclines, and fluoroquinolones. This has left third-generation cephalosporins as the lone class of antimicrobials currently recommended as first line therapy for gonorrhea in some regions. However, resistance to oral third-generation cephalosporins has emerged and spread in Asia, Australia and elsewhere. The mechanism of this resistance seems to be associated with a mosaic penicillin binding protein (penA) in addition to other chromosomal mutations previously found to confer resistance to beta-lactam antimicrobials (ponA, mtrR, penB, pilQ). Few good options exist or are in development for treating cephalosporin resistant isolates as most have had multidrug resistance. Preventing the spread of resistant isolates will depend on ambitious antimicrobial management programs, strengthening and expanding surveillance networks, and through effective sexually transmitted disease control and prevention.
Keywords: Neisseria gonorrhoeae, cephalosporin resistance
1. Introduction
Urethritis from gonorrhea has probably been affecting humans for thousands of years. Gonorrhea was recognized by ancient physicians such as Galen, and scholars believe that it was mentioned in the bible.1 The gonococcus was first discovered by Albert Neisser in 1879 and was the second pathogenic bacterium to be isolated in history.2 Though infections historically were treated with various local and systemic preparations of questionable effectiveness, the first curative treatment came with the introduction of sulfanilamide in 19373 and was followed by the use of penicillin for gonorrhea in 1943.2 Resistance to sulfonamides,4 penicillin, and each subsequent antimicrobial used to treat gonorrhea has inevitably developed over time.5 Most recently, the gonococcus has developed resistance to fluoroquinolones.6, 7 As a result, currently in some regions only third-generation cephalosporins are recommended as first line therapy for gonococcal infections.7, 8 However, consistent with the history of the gonococcus, resistance to this class of antimicrobials is now emerging and will almost certainly present significant future challenges to the treatment and control of gonococcal infections and their complications.
1.1 Morbidity of gonococcal infections
Gonococcal infections in males cause predominantly symptomatic urethritis that can be complicated by epididymitis and urethral strictures. In women, gonococcal infections cause cervicitis —only approximately half of which occur with symptoms— and which can go on to cause pelvic inflammatory disease, ectopic pregnancies, and infertility.1 In addition, in both men and women exposed orally or anally, gonococcal infections can cause a predominantly asymptomatic pharyngitis or proctitis. Especially among gay men and other men who have sex with men (MSM), these non-urethral sites can be the predominant site of infection.9 Less commonly, N. gonorrhoeae can cause conjunctivitis, endocarditis, tenosynovitis, arthritis, meningitis, inflammation of the liver capsule (Fitzhugh-Curtis syndrome) and disseminated blood stream infections.1 N. gonorrhoeae can also cause ophthalmic infections among newborns.10, 11
Like other sexually transmitted infections (STIs), gonococcal infections of the cervix, urethra, and rectum have been shown to substantially increase the risk of acquiring and transmitting human immunodeficiency virus (HIV) infection,12, 13 making gonorrhea control an important part of HIV prevention.
1.2 Diagnosis of gonococcal infections
Diagnosis of gonococcal infection has historically been a combination of clinical signs and symptoms of cervicitis/urethritis, a Gram stain of urethral or cervical discharge revealing the characteristic Gram-negative intracellular diplococci, and the use of culture on selective media, usually Thayer-Martin media.14, 15 However, over the last 20 years new molecular methods for diagnosing gonococcal infections have been developed and have entered widespread use, mostly in resource rich settings. These assays are generally much more sensitive than culture and are highly specific for urogenital infections,14, 16, 17 however, depending on the assay used (e.g. PCR) some concerns have arisen about the specificity of these tests from other anatomic sites.18, 19 Because these assays can be performed on easily collected specimens such as urine or self-collected vaginal or rectal swabs, in resource rich settings, especially the United States, they have supplanted culture in many clinical settings and have expanded screening to many non-clinical settings.20-23 This move away from culture has made routine clinical antimicrobial susceptibility testing impossible in many cases so that nearly all information regarding susceptibility now comes from relatively small surveillance systems set up specifically for this purpose.
In resource limited settings where diagnostic testing for gonococcal infections is difficult or impossible, persons are typically treated for gonococcal and chlamydial infections using syndrome-based algorithms for urethritis, vaginitis, or pelvic inflammatory disease (PID).24, 25 In these settings the etiologic agent (and the antimicrobial susceptibility) is not known.
1.3 Epidemiology of gonococcal infections
Gonococcal infections are among the most common reportable infections around the world. In the United States, gonorrhea is consistently the second-most frequently reported notifiable infection with more than 350,000 infections reported in 2006.26 Many more infections likely go unreported and the actual annual cumulative incidence of gonococcal infections in the United States during 2000 was estimated to be >700,000.27 In the United Kingdom during 2007, there were 18,710 uncomplicated gonococcal infections diagnosed in STD (Genito-Urinary Medicine) clinics.28
In other regions of the world, gonococcal infections are much more common. According to World Health Organization (WHO) estimates for 1999 (updated global estimates are forthcoming), approximately 62.4 million gonococcal infections occur each year worldwide, nearly half (27.2 million) of which occur in South and Southeast Asia, with another 17 million in Sub-Saharan Africa.10
Gonococcal infection is more common among young persons, particularly those aged 15–24 years.26, 28 Rates of disease are also higher among persons with lower socio-economic status, MSM, illicit drug users, commercial sex workers, persons held in correctional facilities, and racial/ethnic minority groups.1, 26, 29 In the United States the disparity in rates between whites and blacks is the highest for gonorrhea than for any other reportable disease with the rate among blacks more than 24 times the rate among whites in 2002.30 In 2006, gonorrhea cases among blacks accounted for 69% of all gonorrhea in the United States while blacks make up approximately 12% of the population.26
2. The use of antimicrobials against Neisseria gonorrhoeae and the history of development of antimicrobial resistance
2.1 General Principles of Therapy
Several general principles of the treatment of gonococcal infections are important. Single dose, directly observed therapy has become the norm in most areas of the world. Single dose therapy has been effective and assures adequate treatment. WHO recommendations for selecting treatments have stated that cure rates should be >95%.31 In the United States, recommendations have further stated that the lower bound of the 95% confidence interval around the estimated treatment efficacy should also be higher than 95%.32 Additionally, candidate medications should achieve and sustain serum levels of at least 4 times the MIC90 for 10 hours.32 Recently, as a consequence of limited treatment options and few studies, it has been proposed that a slightly less stringent criteria of >95% cure rate with the lower bound of the 95% confidence interval >90% be used for alternative regimens in the US Centers for Disease Control and Prevention (CDC) STD Treatment Guidelines.33
Treatment of sex partners is important to prevent reinfection. Efforts to improve partner treatment have been ongoing in the United States and elsewhere, often through the use of expedited partner therapy which involves the patient delivering medications or a prescription for medication along with instructions for use to his or her sex partners. This has been shown to lower gonococcal reinfection rates in randomized trials,34-36 but depends on the efficacy and availability of an easily deliverable oral treatment.
Following treatment, in the absence of recurrent symptoms, generally no test of cure is needed for uncomplicated gonorrhea and this is not recommended routinely by the CDC or WHO.8, 25 Retesting 3 months following treatment is recommended because of the high rate of reinfection,8 but this recommendation is difficult to implement in many settings.
Last, because gonococcal and chlamydial coinfection rates are high, persons treated for gonococcal infections are also treated for chlamydia unless chlamydia has already been ruled out. This means that many persons will also receive a macrolide or a tetracycline in addition to treatment for gonorrhea.
2.2 Penicillin
Though sulfonamides were the first antimicrobials used to treat gonococcal infections, resistance quickly developed.3, 4 Alexander Fleming documented the ability of penicillin to inhibit growth of the gonococcus in his 1929 paper describing his monumental discovery,37 and penicillin became the gonorrhea treatment of choice in 1943.38-40 Penicillin served as the mainstay of treatment for several decades. However, soon after introduction, N. gonorrhoeae began developing low-level resistance to penicillin. Nearly all isolates collected in the pre-penicillin era had MICs of <0.0125 mg/L (0.02 IU/mL).5, 41 This gradually climbed so that 22% of isolates had MIC ≥0.125 mg/L by 1956,5, 42 and by 1974 11–23% of isolates in some US cities were resistant (MIC ≥0.5 mg/L).43 This MIC rise required numerous escalations in the recommended effective dose of penicillin from 50,000 units in 1945 to 4.8 Million units by the 1970s.5, 44, 45 Increasing low-level penicillin resistance was the additive effect of multiple chromosomal mutations resulting in altered penicillin binding proteins, increased antibiotic efflux, and decreased antimicrobial penetration of the outer membrane.46
The emergence of N. gonorrhoeae with plasmid-mediated β-lactamase (penicillinase) production, which confers high-level penicillin resistance, was first identified in N. gonorrhoeae in 1976.5, 47, 48 In Africa and Asia especially, the rates of penicillinase-producing strains rose rapidly whereas in regions such as North America, Europe, and Australia spread was slower and was likely imported from Africa and Asia.5, 49, 50 However, by 1989 penicillin was no longer an effective treatment option and penicillin is not currently recommended in the United States.8 Penicillin regimens (amoxicillin/probenicid) are recommended in European guidelines for known susceptible isolates though resistance rates are high (21.3%).51
2.3 Tetracyclines
Chromosomally-mediated tetracycline resistance emerged in the 1970s along with, and via some of the same mechanisms as, chromosomally-mediated penicillin resistance.5 Plasmid-mediated tetracycline resistance emerged independently in 1985 in the United States and the Netherlands and was the result of the acquisition on a plasmid of a streptococcal tetM determinant that restored ribosomal protein synthesis in the presence of tetracycline.46, 52
2.4 Fluoroquinolones
Fluoroquinolones became widely available in the mid-1980s. They were highly effective against N. gonorrhoeae infections at all anatomic sites, had few side effects in adults, and required only one oral dose of medication.6, 53, 54 Ciprofloxacin became the mainstay of treatment for uncomplicated gonococcal infections with CDC recommending it as an alternative regimen in 198955 and as a first line therapy in 1993.56 However, resistance was already developing with the first fluoroquinolone-resistant isolates described in the mid 1980s.6, 57 This resistance, through alteration of DNA gyrase (gyrA) or topoisomerase IV (parC), first became prevalent in Asia; by 1992 ciprofloxacin resistant isolates made up >40% of isolates in Japan. As had been seen with penicillinase-producing N. gonorrhoeae, resistant strains quickly spread from Asia to Australia, Hawaii, North America, and Europe,6, 58-61 likely via travelers.61, 62 Prevalence of resistant isolates continued to increase in the United States especially in California, Hawaii, and among MSM such that fluoroquinolones were no longer recommended in those populations by the early 2000s.63, 64 Finally, in 2007, the US CDC recommended that no gonococcal infections in the United States be treated with ciprofloxacin as first-line therapy.7 In Europe, though the last published guideline lists fluoroquinolones as recommended for the treatment of gonococcal infections, recent surveillance shows that quinolone resistance is high (30.9%) and several European countries have removed fluoroquinolones from lists of recommended therapies.51, 65 Other antimicrobials that remain options for the treatment of gonococcal infections, including spectinomycin, are discussed below in section 7.
3. Cephalosporins for the treatment of gonococcal infections
3.1 History and General Characteristics of Cephalosporins
Cephalosporins were discovered in 1945 by Guiseppe Brotzu when he isolated a mold from sewage effluvium in Sardinia, Italy that had broad spectrum antibacterial activity.66 Modern cephalosporins are variations on the prototypic molecule produced by Cephalosporin acremonium. These variations are achieved by side chain substitutions at R1 (C7) and R2 (C3) of the cephalosporin nucleus with R1 alterations generally being responsible for stability against β-lactamases and R2 substitutions affecting elimination half-life (Figure 1).67 Cephalosporins are classified into “generations” on the basis of their spectrum of activity. First-generation agents are most active against aerobic Gram-positive cocci including Staphylococcus aureus (methicillin sensitive), whereas second-generation agents have more activity against Gram-negatives and less activity against S. aureus. Third-generation agents have broader activity against Gram-negatives than second-generation agents. Fourth-generation agents, such as cefipime, have broad activity against both Gram-negative and Gram-positive organisms.
Figure 1.
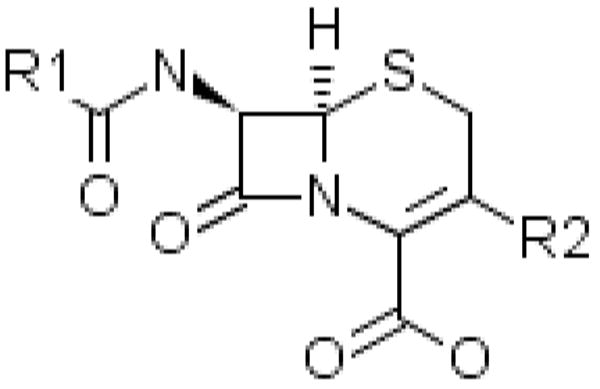
Basic Cephalosporin Nucleus
In general, third generation cephalosporins and cephamycins (i.e. cefoxitin) are active against N. gonorrhoeae. Some second-generation agents have also been studied, however ceftriaxone and several oral third-generation agents are the most frequently used for treating gonococcal infections.
Like other β-lactam antimicrobials, cephalosporins work by inhibiting cell wall synthesis through binding and inhibiting enzymes responsible for inserting peptidoglycan cross-linkage structures into the cell wall. These enzymes, including transpeptidases, carboxypeptidases, and endopeptidases, are also termed penicillin binding proteins (PBPs).66 Cephalosporins are considered bactericidal drugs with time-dependent killing and maximal bacterial killing occurring at 4 times the MIC.67, 68 These characteristics make the peak serum drug level and rate of elimination particularly important in selection of agents for one time dosing.
3.1.1 Oral Cephalosporins for Gonorrhea
Oral cephalosporins with activity against N. gonorrhoeae include cefuroxime axetil,69, 70 cefaclor,71 cefixime,72-75 cefpodoxime proxetil,76, 77 ceftibuten,78 cefdinir,79 and cefoperozone (see Table 1).80, 81 The WHO recommends cefixime 400mg and in the United States, cefixime 400mg is the only oral regimen recommended as first line therapy. This is because it is the only oral option to date which has met the criterion of the lower bound of the 95% confidence interval of the cure rate >95% (97.5% cure; 95% confidence interval, 95.4–98.8%).33 Cefixime is also recommended in the UK.65 Cefixime was not available in the United States from 2002 until 2008,82 and at that time cefpodoxime 400mg became more widely used.83 Other countries have used options including ceftibuten in Hong Kong84 and cefditoren and cefdinir in Japan.
Table 1.
Chemical, pharmacologic, and microbiologic characteristics of selected oral cephalosporins used to treat infections caused by Neisseria gonorrhoeae.
| Cephalosporin Usual Dose IM (Alternate Dose IM) | Chemical Structure* | Peak Serum Level** (mg/L) | Half Life** (hrs) | Serum Level 10 hours after peak (mg/L)***** | Hypothetical MIC90 limit*** (10hr conc/4) (mg/L) | Breakpoints (CLSI unless indicated otherwise) |
|---|---|---|---|---|---|---|
| Cefixime 400mg | 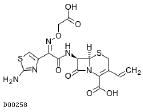 |
4.5 | 3–4 | 0.446–0.795 | 0.112–0.199 | S: ≤0.25 I: ND R: ND |
| Cefpodoxime (proxetil)**** 400mg | 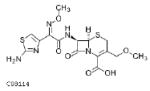 |
4.5 | 2–3 | 0.141–0.446 | 0.035–0.112 | S: ≤0.5 I: ND R: ND |
| Cefdinir 600mg | 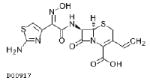 |
2.87 | 1.7 | 0.049 | 0.012 | |
| Cefuroxime (axetil)**** 1000mg | 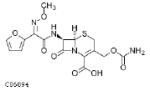 |
13.6 | 1.3 | 0.066 | 0.016 | (IV formulation) S: ≤1 I: 2 R: ≥4 |
| Ceftibuten 400mg | 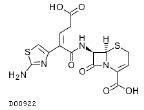 |
15 | 1.5–2.5 | 0.148–0.938 | 0.037–0.234 |
S= sensitive; I=Intermediate; R=resistant; ND=Not determined
Source of chemical structures is the Kyoto Encyclopedia of Genes and Genomes Drug database available at: http://www.genome.jp/kegg/drug/ (Accessed August 23, 2008)
Source of elimination half life and peak concentration is Micromedex DRUGDEX® Evaluations, Thomson Healthcare. http://www.micromedex.com (Accessed September 30, 2008)
Moran JS, Levine WC. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin Infect Dis 1995 Apr;20 Suppl 1:S47-65.32
Cefuroxime axetil and cefpodoxime proxetil are administered as a prodrug ester and are passively absorbed and hydrolyzed by intestinal epithelial cells to the active cephalosporin form which is then transferred into the bloodstream.
serum concentration at time
Table 1 lists the properties of selected oral cephalosporins including the calculated serum level 10 hours after peak level. Using this information to apply the theoretical guideline of Moran and Levine that medications used in one-time doses for treatment of gonorrhea should stay 4 times above the MIC90 for 10 hours, one can see that there might not be much excess pharmacologic capacity in many of these agents to accommodate increases in the MIC.
3.1.2 Parenteral Cephalosporins for Gonorrhea
Among the parenteral cephalosporins, ceftriaxone has been extensively studied and is the parenteral treatment of choice for gonorrhea.85-90 It is the recommended first line antimicrobial for treatment of gonorrhea in the United States and the United Kingdom and is recommended by the WHO.7, 8, 31, 65 However, the dose of ceftriaxone is the subject of debate with 125mg recommended in the United States and by WHO, but many countries recommend 250mg.8, 31, 65 In Japan, 1000mg IV is recommended.91 The chemical structure of ceftriaxone, particularly the heterocyclic thiomethyl group at the R2 (C3) position greatly prolongs the elimination half-life because of extended protein binding.66 Other parenteral cephalosporins have been studied and recommended as alternative regimens.8 These include ceftizoxime 500 mg IM,92-94 cefoxitin 2 gm IM with 1gm of probenecid,95-97 and cefotaxime 500mg IM.98-100 Cefuroxime 1.5gm IM is occasionally used in the United Kingdom. 70 Cefodizime has also been studied and used in Japan and has shown activity against recent multidrug resistant Japanese isolates.33, 101-103 However, these agents do not provide any advantage over ceftriaxone (See Table 2) and so are not routinely recommended.
Table 2.
Chemical, pharmacologic, and microbiologic characteristics of selected parenteral cephalosporins used to treat infections caused by Neisseria gonorrhoeae.
| Cephalosporin Usual Dose (Alternate Dose) | Chemical Structure* | Peak Serum Level** (mg/L) | Half Life** (hrs) | Serum Level 10 hours after peak (mg/L)***** | Hypothetical MIC90 limit*** (10hr conc/4) (mg/L) | Breakpoints (CLSI unless indicated otherwise) |
|---|---|---|---|---|---|---|
| Ceftriaxone 125mg or 250mg (pk data for 125mg) | 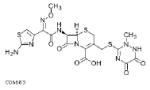 |
13.5 | 5.8–8.7 | 4.086–6.086 | 1.022–1.521 | S: ≤0.25**** I: ND R: ND |
| Cefuroxime 1500mg | 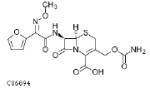 |
13.6 | 1.3 | 0.066 | 0.016 | (IV formulation) S: ≤1 I: 2 R: ≥4 |
| Ceftizoxime 500mg | 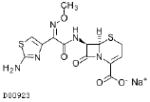 |
13 | 1.1–2.3 | 0.024–0.638 | 0.006–0.160 | S: ≤0.5 1:ND R:ND |
| Cefodizime 1000mg | 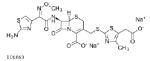 |
75 | 2.5–4 | 0.141–0.446 | 0.035–0.112 | |
| Cefotaxime 1000mg | 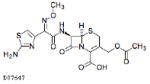 |
20.5 | 0.8 | 0.001–0.054 | 0–0.013 |
S= sensitive; I=Intermediate; R=resistant; ND=Not determined
Source of chemical structures is the Kyoto Encyclopedia of Genes and Genomes Drug database available at: http://www.genome.jp/kegg/drug/ (Accessed August 23, 2008)
Source of elimination half life and peak concentration is Micromedex DRUGDEX® Evaluations, Thomson Healthcare. http://www.micromedex.com (Accessed September 30, 2008)
Moran JS, Levine WC. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin Infect Dis 1995 Apr;20 Suppl 1:S47-65.32
Other authors and organizations have picked lower cutpoints to define isolates that are “less susceptible.” For example, the European Surveillance of Sexually Transmitted Infections (ESSTI) network uses 0.125 as the upper limit of sensitivity.51
serum concentration at time
4. Epidemiology of cephalosporin resistance
Despite their historic reliability for treating gonococcal infections, resistance to cephalosporins has begun to develop and spread in Asia with possible importation into Australia and Europe.
4.1 Japan
Case reports of treatment failures with the use of third-generation cephalosporins were reported in Japan as early as 2000,104 though a published report including isolates collected in Japan during 1991–1996 also documented elevated MICs to cephalosporins including cefpodoxime and cefdinir105(See Table 3). Several subsequent reports from various regions in Japan documented the rapid spread and increase of resistance to oral third-generation cephalosporins during the late 1990s and early 2000s.103-112 As a result of cephalosporin resistance in Japan, beginning in 2006, cefixime was no longer recommended as first line therapy for gonorrhea in Japan with only the parenteral agents ceftriaxone and spectinomycin remaining first line treatment options.91, 110, 111
Table 3.
Reports from Japan of Neisseria gonorrhoeae isolates with elevated MICs to third-generation cephalosporins.
| Author, publication year | Location | Year of Specimen Collection | Criteria and Number of isolates assessed | Cephalosporin MICs mg/L (range) | Comment |
|---|---|---|---|---|---|
| Japan | |||||
| Yamaguchi 1998105 | Several Areas of Japan | 1991 and 1996 | All isolates: 27 |
Cefpodoxime MIC90=4 Cefditoren MIC90=4 |
In vitro study of investigational antimicrobial. |
| Akasaka 2001104 | Kitakyushu | 1999 | Cefdinir Treatment Failure: 2 |
Cefpodoxime MIC = 4 Cefdinir MIC = 1 Ceftriaxone MIC=0.125 |
Case report. |
| Muratani 2001106 | Kitakyushu | 1999 | Cefozopran ≥ 8: 17 of 54 |
For 17 isolates: Ceftriaxone MIC90=0.125 (0.03–0.25) Cefpodoxime MIC90=4 (0.5–4) Cefixime MIC90=0.5 (0.125–0.5) |
|
| Ito 2004107 | Central Japan | 1999–2000 2001 2002 |
Cefixime ≥0.5: 0 of 91 39 of 150 67 of 221 |
1999: Cefixime MIC90 = 0.06 (≤0.004–0.125) Ceftriaxone MIC90 = 0.03 (≤0.004–0.06) 2002: Cefixime MIC90 = 0.5 (≤0.004–2) Ceftriaxone MIC90 = 0.06 (≤0.004–0.5) |
Emerging cefixime resistance. |
| Ameyama 2002108 | Tokyo | 2000 2001 |
Cefixime ≥ 0.25: 9 of 53 4 of 24 |
2000 and 2001: Cefixime MIC90 = 0.25 Ceftriaxone MIC90 = 0.06 |
Report also described a mosaic penA gene among isolates with cefixime MIC ≥0.25. |
| Tanaka 2002103 | Fukuoka City | 1995 2000 |
Cefixime ≥ 0.5 0 of 55 5 of 100 |
1995: Cefixime MIC90 = 0.015 (0.002–0.06) Ceftriaxone MIC90 = 0.015 (0.001–0.03) 2000: Cefixime MIC90 = 0.25 (0.002–0.5) Ceftriaxone MIC90 = 0.06 (0.002–0.5) |
Emerging cefixime resistance. |
| Tanaka 2006109 | Fukuoka City | 2000–2001 | Ceftriaxone = 0.5: 1 of 398 |
Cefixime MIC = 0.5 | Analysis of 1 ceftriaxone resistant isolate with mosaic penA and mtrR, ponA, penB mutations. |
| Yokoi 2007110 | Toyota | 2002–2003 | Cefixime Treatment Failure: 4 |
Cefixime (0.5–1) Ceftriaxone (0.125–0.5) |
Case report. |
| Osaka 2006111 | Tokyo | 2006 | Cefixime ≥0.125: 17 of 47 |
Cefixime MIC90 = 0.125 (0.004–0.25) Ceftriaxone MIC90 = 0.06 (0.002–0.125) |
MIC values compared to those of Ameyama in 2001. |
| Takahata 2006112 | Tokyo | 2006 | Cefixime ≥ 0.125 28 of 58 |
For 28 isolates with mosaic penA gene: Cefixime MIC90 = 0.5 (0.12–0.5) Ceftriaxone MIC90 = 0.12 (0.016–0.12) |
Cefixime MICs correlated with presence of mosaic penA. |
| Europe | |||||
| Olsen 2008125 | Sweden | 2002–2005 | Cefixime MIC >0.06–≤0.5: 6 of 679 |
All had ceftriaxone MIC < 0.125 | |
| Hoffmann 2005126 | Denmark | 2004 | Ceftriaxone MIC >0.023 <0.094 81 of 434 |
No PenA sequencing performed. Serotype, NG-MAST sequence type were related within group of ceftriaxone MIC >0.023 and <0.094 | |
| Martin 200651 | Europe (ESSTI) | 2004 | Ceftriaxone > 0.125: 3 of 965 |
Ceftriaxone MIC = 0.25 | Surveillance report. Resistant isolates included 2 from Italy and 1 from Sweden. |
| Vazquez 2007127 | Spain | 2004–2005 | 204 isolates | Ceftriaxone MIC90=0.007 (≤0.007–0.12) Cefditoren MIC90=0.12 (≤0.007–0.25) |
|
| Tzelepi 2008128 | Greece | Dec 2006–Jan 2008 | Cefotaxime MIC 0.25–1: 17 of 195 |
Ceftriaxone MIC90= 0.125 (0.064–0.125) Cefixime MIC90=0.25 (0.125–0.25) |
Isolates were part of a cluster with related serotypes and PFGE patterns in Northern Greece. Isolates were multidrug resistant including penicillin, tetracycline, and fluoroquinolones. |
| Gonococcal Resistance to Antimicrobials Surveillance Programme 200828 | United Kingdom | 2007 | Cefixime MIC = 0.25: 2 of 1113 |
Ceftriaxone MIC = 0.015 | Surveillance Report. |
| Australia | |||||
| Tapsall 2008113 | Australia | 1997–2006 | Ceftriaxone MIC 0.06–0.5: 134 of ~15,000 |
Isolates with elevated ceftriaxone MIC mostly from travelers or contacts. | |
| Australian Gonococcal Surveillance Program 2008114 | Australia | 2007 | Ceftriaxone MIC 0.06–0.25: 25 of 3,042 |
Surveillance report. | |
| Elsewhere in Asia | |||||
| Ray 2005123 | Chennai, India Hyderabad, India Nagpur, India Pune, India Kolkata, India Bangladesh |
2001 2001 2001 2001 2001 1999–2000 |
Ceftriaxone LS (disk diffusion): 4 of 80 9 of 46 10 of 74 4 of 37 4 of 58 2 of 110 |
Resistant isolates not confirmed at regional reference laboratory. | |
| Bala 2007124 | New Delhi, India | 2002–2006 | Ceftriaxone MIC≥0.06: 9 of 382 |
Ceftriaxone MIC 0.064–0.094 | No treatment failures reported. |
| Ye 2002116 | China (various) | 1993–1998 | Ceftriaxone R (not defined): 16 of 2801 |
Results not confirmed at national reference laboratory. | |
| Guoming 2000117 | Zhanjiang, China | 1998–1999 | Ceftriaxone MIC ≥1: 15 of 98 Ceftriaxone MIC ≥0.06: 34 of 98 |
Ceftriaxone MIC90=2 (0.016–2) | |
| Wong 2008118 | Taipei, Taiwan | Apr 2006–Aug 2007 | Cefixime R (disk diffusion): 24 of 146 Cefpodoxime R (disk diffusion): 31 of 146 |
All sensitive to ceftriaxone by disk diffusion. | NGMAST ST 835 and 2180 associated with cephalosporin resistance. |
| Lo 200884 | Hong Kong | Oct 2006–Aug 2007 | Ceftibuten Treatment Failure: 42 of 1228 |
Ceftibuten MIC90=1 (0.06–8) Ceftriaxone MIC90=0.06 (<0.016–0.125) Cefixime MIC90=0.125 (<0.016–0.25) |
NG MAST ST 835 and 2469 associated with cephalosporin resistance. |
| Clendennen 1992120 | Philippines | September 1989 | Ceftriaxone ≥0.5: 8 of 134 Cefpodoxime ≥4: 4 of 134 |
||
| Clendennen 1992121 | Thailand | May 1990 | Ceftriaxone ≥0.5: 3 of 333 Cefixime ≥4: 1 of 328 Cefpodoxime ≥4: 2 of 331 |
||
| Cao 2008119 | Ho Chi Minh Ville, Vietnam |
Mar 2004–Jun 2006 | Ceftriaxone MIC=0.5: 1 of 121 |
No other cephalosporins were evaluated. | |
| United States | |||||
| Wang 2003130 | Hawaii | 2001 | Multidrug resistance: 4 isolates |
Cefixime MIC 0.25–0.5 Ceftriaxone MIC 0.125 |
Case report. All 3 patients with links to Asia. |
| Gonococcal Isolate Surveillance Project 200883 | 2006 | 1992–2006 1988–2006 |
Cefixime MIC 0.5–2: 48 isolates Ceftriaxone MIC=0.5 4 isolates |
Surveillance Report. | |
R= Resistant; LS= Less Sensitive; NG MAST= Neisseria gonorrhoeae multiple antigen sequence typing
4.2 Australia
The Australian Gonococcal Surveillance Programme began to identify isolates with ceftriaxone MIC 0.06–0.5 mg/L (termed “less susceptible”) in 2001.113, 114 Isolates were predominately from urban centers and isolated from international travelers and their sex partners, though some domestic transmission was suspected as well.113
4.3 China, Hong Kong, and Taiwan
Cephalosporin resistance might also be emerging in China. The 2006 report of the WHO Western Pacific Region mentions that resistance was “particularly prominent” in China though no more information is reported.115 Other reports from China have reported elevated ceftriaxone MICs among isolates collected from different regions of China during the 1990s, however some of these results were not confirmed at the national reference laboratory.116, 117
Recently, investigators in Hong Kong reported a rate of ceftibuten (400mg PO once) treatment failure of 3.7% during October 2006–August 2007 (n=1228). Among the 42 persons with clinical ceftibuten failure, 7 had MIC ≥1 mg/L. A total of 23 isolates had ceftriaxone MIC of 0.06 or 0.125 mg/L.84 Other investigators in Taiwan recently reported oral cephalosporin resistance there as well.118
4.4 Elsewhere in Asia
Reports from Vietnam, Thailand, and the Philippines documented sporadic isolates with ceftriaxone MIC ≥0.5,119-121 though further testing on these isolates were not performed and clinical outcomes were not reported. Plans for a more extensive survey of gonococcal antimicrobial resistance patterns in the WHO Western Pacific Regions are underway.122 A surveillance report from India, Bangladesh, Nepal, and Sri Lanka reported significant rates of ceftriaxone less susceptible/intermediate isolates (1.5–20%) among 767 total isolates collected and tested in local laboratories during 1999–2001, however these results were not able to be confirmed in the regional reference laboratory.123 In India, Bala et al recently reported 9 isolates with ceftriaxone MIC of 0.064 or 0.094 mg/L among 382 isolates collected in New Delhi during 2002–2006. All cases were treated with ceftriaxone 250mg or cefixime 400mg and there were no treatment failures.124
4.5 Europe
Recently a Europe-wide surveillance system, European Surveillance of Sexually Transmitted Infections (ESSTI), has been implemented to monitor antimicrobial resistance patterns in N. gonorrhoeae. This system identified 3 isolates with ceftriaxone MIC=0.25 mg/L from Italy and Sweden (ESSTI defined reduced susceptibility to ceftriaxone as ≥ 0.125 mg/L).51 The UK gonococcal surveillance system reported their first two isolates with decreased cefixime susceptibility in 2007 (MIC ≥0.25 mg/L).28 Other reports from Denmark, Spain, Sweden, and Greece have documented isolates with increased cephalosporin MICs. 125-128
4.6 United States
Since the start of a national surveillance system in 1986 for gonococcal resistance in the United States (Gonococcal Isolate Surveillance Program; GISP) there have been four sporadic isolates with a ceftriaxone MIC of 0.5 mg/L in San Diego (1987), Cincinnati (1992 and 1993), and Philadelphia (1997).83, 129 GISP incorporated testing for cefixime in 1992 and through 2006 there have been 48 isolates with cefixime MIC of 0.5–2.0 mg/L.83 However, the percent of isolates with elevated MIC to cefixime has decreased over time.83 In 2001, three patients were identified in Hawaii with multidrug resistant N. gonorrhoeae including isolates with cefixime MIC of 0.25–0.5 mg/L and ceftriaxone MIC of 0.125 mg/L. Those 3 persons had epidemiologic links to Asia.130
4.7 Other global regions including Africa and Latin America
Very limited recent data exist from other parts of the world, but there have not been isolates with documented elevated MICs to cephalosporins among recent published reports. These have included reports from Africa (South Africa, Madagascar, Cameroon, Central African Republic),119, 131-133 and Latin America (Argentina, Uruguay, Colombia, Peru, and Venezuela).134
5. Neisseria gonorrhoeae mechanism of resistance to cephalosporins
5.1 Neisseria Biology Review
Gonococci have several features that might be important in the development of antimicrobial resistance. These include surface structures such as a porin protein, Por, encoded by the porB gene, and pilQ, another porin coded by the pilQ (formerly penC) gene through which pili are thought to project.135 Gonococci are unusual in that they are constitutively competent for exogenous DNA transformation. The gonococcus is able to take up exogenous DNA that has a specific 10 base pair uptake sequence frequently found in the genome of many Neisseria species. There are approximately 1900 copies of this uptake sequence in Neisseria genomes compared with 4 copies in H. influenzae.136-138 Gonococci frequently release DNA. This DNA can be taken up and integrated into the recipient gonococcal genome. Some gonococci also do contain a 36-kb conjugal plasmid but are not thought to transfer chromosomal genes via plasmids. There is evidence that gonococci take up genetic information much more efficiently through transformation than through plasmids.138
5.2 Definitions of Resistance
Defining resistance to cephalosporins is difficult because up to now documented clinical treatment failures have been rare. As a result, the Clinical and Laboratory Standards Institute (CLSI) does not define resistance breakpoints for most cephalosporins, including ceftriaxone, but only defines sensitive isolates.139 This has made terminology and surveillance difficult with programs and authors using varying definitions and terms. Complicating this are inherent differences in laboratory techniques that might render MICs not directly comparable.115, 140, 141 Most definitions of cephalosporin resistance are based on ceftriaxone, though there might be important differences in the susceptibility of isolates to ceftriaxone and other oral cephalosporins.106, 107, 112 Some authors define N. gonorrhoeae with increased ceftriaxone MIC as ≥0.06 mg/L,113, 124, 142 other authors and UK Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP), have used ≥0.125 mg/L28, 143 while the ESSTI has chosen >0.125 mg/L,51 and the CLSI defines isolates ≤ 0.25 mg/L as susceptible, making ≥0.5 “non-susceptible.”139 In this review, we attempt to report actual MICs and the criteria used for determination of non-susceptibility.
5.3 Resistance Mechanisms
5.3.1 Altered PBPs
Neisseria gonorrhoeae has three penicillin-binding proteins (PBPs), designated 1, 2, and 3. PBP2 has a 10-fold higher affinity for penicillin G than PBP1144 and is thought to be the major binding site for β-lactam antimicrobials like the cephalosporins. Alterations in PBP2, coded for by the penA gene, have been demonstrated to cause decreased binding of penicillin through a single amino acid insertion (Asp-345a).145, 146 Several additional PBP alterations have been documented to be associated with resistance to β-lactam antimicrobials including cephalosporins (See Table 4). However, much is still not known regarding the importance of specific mutations in PBPs, their interactions with each other, and with alterations in other genes.
Table 4.
Genetic alterations linked to Neisseria gonorrhoeae reduced susceptibility to β-lactam antimicrobials.
| Gene (Amino Acid alteration) | Gene Product | Phenotype | Source |
|---|---|---|---|
| ponA (L421P) | PBP1 | Altered PBP1. Requires penC for high level resistance. Role in cephalosporin resistance questioned. | 150 |
| penA (Asp-345a) | PBP2 | Insertion PBP2 resulting in penicillin resistance | 146 |
| penA (mosaic PBP2) | PBP2 | Oral cephalosporin resistance Possibly increased MIC for parenteral cephalosporins |
108, 154 |
| penA (A501V) | PBP2 | Possibly similar effect to mosaic; 2-4 fold increase in cephalosporin MIC | 112, 142 |
| penB (porB1b) | PorB1b | Altered porin and membrane permeability to hydrophobic antibiotics and tetracycline | 143, 152 |
| pilQ (penC) | PilQ Major outer membrane protein through which pilus projects also is a porin 135 | Increases resistance to penicillin when penA, mtrR, and penB mutations are present; thought to form outer membrane pore through which antimicrobials diffuse into periplasm | 143, 185 |
| MtrR | Transcription repressor | Results in MtrC-D-E efflux pump upregulation decreased susceptibility to hydrophobic agents such as azithromycin, rifampin. Possible increased in vivo fitness | 186, 187 |
The most frequently cited PBP alteration related to cephalosporin resistance is the altered PBP2 linked to cefixime resistance in Japanese male urethritis isolates by Ameyama et al in 2002.108 In this group of isolates, 13 of 77 (17%) had cefixime MIC ≥0.25 mg/L. Sequencing of penA revealed a mosaic genotype.108 This genotype consists of multiple genetic changes in the penA transpeptidase domain forming a mosaic penA with segments that are nearly identical to the homologous regions of the penA genes of related Neisseria commensal species such as N. flavescens, N. perflava, N. subflava, N. cinerea, and N. meningiditis.108, 109 Presence of these multiple penA alterations are thought to have occurred through transformation of N. gonorrhoeae penA genes with genetic sequences from commensal Neisseria organisms.108, 109 This has previously been shown to occur in the development of chromosomally-mediated penicillin resistance in both N. gonorrhoeae and N. meningiditis.147, 148
In order to define the role of this mosaic penA, Ameyama et al attempted to genetically transform a cefixime-sensitive isolate with cloned copies of a mosaic penA gene amplified from an isolate with cefixime MIC of 0.5. The resulting transformant had increased MIC from the initial sensitive transformee isolate, but did not completely replicate the susceptibility profile of the penA donor isolate: cefixime MIC increased from 0.001 to 0.06 mg/L; ceftriaxone 0.00025 to 0.002 mg/L.108 In a recent similar experiment, other investigators showed that the introduction of the mosaic penA into a penicillin and cephalosporin susceptible isolate increased the cefixime MIC by 100-fold (to 0.12 mg/L) and the ceftriaxone MIC 20-fold to 0.012 mg/L. When the mosaic penA was introduced into a chromosomally-mediated penicillin resistant isolate possessing several other mutations (ponA, mtrR, penB) the ceftriaxone MIC increased to 0.25 mg/L and cefixime increased to 0.5 mg/L.149 Data from Lindberg also suggest that multiple mutations in addition to PBP2 are needed to attain MICs to cephalosporins equivalent to that seen in vivo.143
Within the mosaic penA, which specific substitutions are important is not yet clear, but the amino acid substitutions G545S, I312M, V316T, and possibly A501V were demonstrated to be responsible for most of the observed reduced susceptibility to cefixime.112 Of these substitutions, I312M and V316T occur in the PBP2 of N. perflava/sicca and N. flavescens, reinforcing the hypothesis that these mosaic sequences might be the result of transformation with commensal Neisseria species.
Osaka et al did comparative penA sequencing and homology modeling of isolates from Japan with mosaic and non-mosaic penA genes with cefixime MIC ≥0.125 mg/L. Modeling showed that the beta-lactam binding pocket was altered both with the mosaic pattern and with the non-mosaic pattern that included the A501V alteration.111 Further, direct assays of PBP2 binding using both wild type and mosaic PBP2 showed that the mosaic PBP2 resisted binding by cefixime and cefdinir, but had no effect on binding of ceftriaxone.150
Whiley et al published reports questioning the importance of the mosaic penA genotype. They sequenced the penA gene in 109 N. gonorrhoeae isolates collected in Australia during 1997–2005 with a range of ceftriaxone MICs. Of the 50 isolates with ceftriaxone MIC ≥0.06 mg/L, only 10 had the mosaic penA and 10 other penA sequences were identified among isolates with ceftriaxone MIC ≥0.06 mg/L. Furthermore, 1 isolate with the mosaic penA had a ceftriaxone MIC of 0.03 mg/L and another isolate with a mosaic variant was completely sensitive to ceftriaxone (0.008 mg/L).142, 151 Those authors report that the PBP2 A501 alteration was present in 22 of the 50 isolates with ceftriaxone MIC ≥0.06 (in 5 of the 10 sequence patterns with ceftriaxone MIC ≥0.06). However, 3 of the 25 isolates with the A501 alteration had MIC of ≤0.008 mg/L raising questions about the specificity of this marker as well.142
Tanaka et al reported an N. gonorrhoeae isolate with ceftriaxone resistance (MIC=0.5 mg/L) that possessed the mosaic PBP2, but also had mutations in ponA (L421P), penB (A120 and A121), and mtrR (See Table 3). They hypothesized that the L421P substitution in the ponA gene coding for PBP1 might also be important in conferring ceftriaxone resistance.109 However, they did not report isolates with cefixime resistance only (ceftriaxone sensitive) and thus could not compare ceftriaxone phenotypes in regard to these non-penA mutations. The possible importance of ponA L421P was further supported by data from Takahata in which strains with the L421P substitution were associated with increased cephalosporin MICs compared with laboratory derived transformants possessing only the mosaic PBP2 (all isolates with the mosaic PBP2 also had the L421P substitution in PBP1)112 However, Nicholas et al found that neither the presence nor absence of ponA affected the cephalosporin MIC.149
These results seem to indicate that the mosaic penA is important but not sufficient to attain a higher level of cefixime resistance and highlights the importance of other chromosomal alterations such as those previously associated with penicillin resistance and perhaps other unknown alterations.
5.3.2 Reduction of intracellular antimicrobial concentration
Another basic mechanism of resistance to antimicrobials includes reducing the intracellular concentration of an antimicrobial either by preventing its entry or by actively pumping antimicrobials out. Like other bacteria, N. gonorrhoeae has a system of efflux pumps. One of these, the MtrC-D-E system, is repressed by the mtrR gene so that mutations in the mtrR gene have been shown to increase efflux and induce resistance to penicillin, tetracycline, macrolides, and possibly fluoroquinolones. Whether this mutation also confers resistance to cephalosporins is not clear. Tanaka et al however reported an isolate with resistance to ceftriaxone (MIC=0.5) that did have an mtrR mutation in addition to others.109 Lindberg et al found that 13 of 18 isolates with ceftriaxone MIC ≥0.06 had the mtrR mutation along with mutations in penA, penB, and ponA.143
Other N. gonorrhoeae mutations can reduce the permeability of the outer membrane. The penB mutation of the porin gene reduces permeability to hydrophilic antimicrobials such as penicillin and tetracycline, but is only apparent when it co-exists with the mtrR mutation. It has not been shown to confer meaningful resistance to cephalosporins.152
Acquisition of beta-lactamases is not thought to play a role in resistance to cephalosporins for N. gonorrhoeae. Nearly all isolates with decreased susceptibility to cephalosporins have not been found to express β-lactamase.106, 108, 109, 143 Cephalosporinases like those seen in other resistant gram negative organisms153 have not been documented in N. gonorrhoeae.
5.4 Is emergence of cephalosporin resistance clonal?
An important question is whether the emerging resistance to cephalosporins is spreading from a common ancestor or whether newly resistant isolates are arising anew as a result of factors such as antimicrobial pressure and transformation from commensal Neisseria spp. Muratani et al found rapid emergence of isolates with resistance to some oral cephalosporins (cefixime MIC ≥0.125), and, on the basis of RFLP analysis, concluded that this was the result of clonal spread.106 Further studies in Japan showed that 55% of 47 isolates with the mosaic PBP2 had identical PFGE patterns and 79% had >90% similarity.154 In addition, the sequence of the mosaic PBP2 found in different areas of Japan differed by only one base pair.154 In Hong Kong, 11 isolates with ceftibuten MIC=8 mg/L had the mosaic penA and identical or nearly identical NG-MAST sequence types.84 In a study of isolates from the United Kingdom, Sweden, and the United States, the isolates with decreased susceptibility to cephalosporins were apparently closely related with only 2 NG-MAST sequence types among 18 isolates.143 Last, in a cluster of isolates from northern Greece with ceftriaxone MIC 0.06–0.125 mg/L (possession of mosaic PBP2 was not determined), the serotypes were unique and PFGE patterns similar.128 However, casting doubt about clonality, other investigations have found the mosaic PBP2 in a diverse set of isolates typed by porin sequence,112 and Whiley et al found no specific correlation between PBP2 pattern and auxotype, serotype, or NG MAST sequence type among a group of isolates with diverse collection years and locations.142 Likely multiple mechanisms of resistance including de novo development of resistance, selection, and clonal spread are involved.
5.5 Methods to detect resistance to cephalosporins
Currently, the only reliable method to detect resistance to cephalosporins is through isolation and susceptibility testing. The gold standard culture method for MIC determination is agar dilution though disk diffusion has also been studied and validated.139 However, with the declining use of culture for routine diagnosis of gonococcal infections, fewer and fewer isolates are available for susceptibility testing outside of established antimicrobial susceptibility surveillance systems. This makes the possibility of using molecular assays to identify markers of resistance in specimens collected for nucleic acid-based diagnostic tests very attractive. Molecular tests have been developed to detect ciprofloxacin resistance in N. gonorrhoeae,155, 156 and azithromycin resistance in Treponema pallidum 157 but are not in widespread clinical use. A major limitation of these tests is that they depend on knowing the importance of particular mutations in conferring resistance and how those mutations correlate with in vitro MIC and with clinical outcomes, information that is not reliably known for cephalosporin resistance. PCR-based assays for identification of the mosaic penA gene have recently been published.158, 159 Such an assay might be useful in identifying organisms with the mosaic penA gene in clinical specimens, however, because the importance of this genotype is not completely understood, the interpretation of the results of the assay is not clear.
6. Treatment options for cephalosporin-resistant infections
The looming question behind this discussion is what treatment options are available when cephalosporins become unreliable? Some possibilities exist and have recently been reviewed,33 but none are likely to be reliable for long. Additionally, in many reports, isolates with increased cephalosporin MICs are resistant to multiple antimicrobials already, further limiting options for treatment.109, 113, 114, 128, 143, 160, 161
Azithromycin is one possible option since 2 grams is generally effective against N. gonorrhoeae. However, isolates with elevated MICs have emerged in multiple locations, including the United States and Europe.83, 162, 163 Additionally 2 grams of azithromycin is poorly tolerated because of gastrointestinal upset though a new timed release formulation might improve that.44 However, azithromycin achieves low serum levels, is frequently prescribed for other conditions such as upper respiratory tract infections, and ongoing antimicrobial pressure from azithromycin use might result in the emergence of azithromycin resistance among N. gonorrhoeae isolates.129 Another option is spectinomycin, an injectable aminocyclitol antimicrobial used for gonococcal infections in a dose of 2 gm IM.164 Spectinomycin is effective for the treatment of anogenital gonococcal infections, but is not effective for treating pharyngeal infections.91, 165 Spectinomycin is one of three first-line antimicrobials for treating gonococcal infections in Japan where oral cephalosporin resistance is common. It has recently been shown to be effective in this setting as well.91 However, N. gonorrhoeae can develop high-level resistance from a single-step mutation. Resistance has quickly developed with widespread use among American soldiers in the past,8, 166 and other reports have documented spectinomycin resistant isolates in areas where it is frequently used.117, 167 Nevertheless, documented resistance to spectinomycin has been rare and sporadic. It has been identified only 5 times in the United States during 1986–2004 where it is very seldom used,33 and has been infrequently and sporadically identified by surveillance systems in the United Kingdom and the WHO Western Pacific Region.115 Spectinomycin can be difficult to obtain; it is not currently available in the United States though it is expected to become available in the future.44
Other antimicrobials might be options but there is currently little clinical evidence of their efficacy. Limited experience exists in treating gonococcal infections with aminoglycosides, though these drugs have been used in Asia and Africa. A number of surveillance studies have not found resistance to kanamycin,168, 169 however, resistance has developed when gentamicin has been used widely in Malawi.44, 170 Rifampin is inexpensive but, like other organisms, N. gonorrhoeae has been shown to develop resistance rapidly when rifampin has been used as a single agent.171 Ertapenem, a parenteral carbapenem, has been studied in vitro against stored specimens from UK surveillance isolates though its activity against cephalosporin non-susceptible isolates has not been studied.172 Similarly, tigecycline, a broad spectrum parenteral glycylcycline tetracycline derivative, has shown activity in vitro against tetracycline resistant N. gonorrhoeae, but has not been tested clinically or against isolates with known increased cephalosporin MICs.173 Although new cephalosporins with broader spectrum of activity against antimicrobial-resistant organisms such as methicillin-resistant Staphylococcus aureus are expected to be approved and become clinically available soon, on the basis of limited in vitro data, these might not have additional activity against antimicrobial-resistant N. gonorrhoeae.174
7. Conclusions
Gonorrhea remains among the most common infectious diseases throughout the world and one that has repeatedly proven its ability to develop resistance to antimicrobial agents. Cephalosporins are now the only first line therapies recommended in many areas worldwide though resistance has begun to emerge and spread in Asia, Australia, and elsewhere. The exact mechanism of this resistance is under study but might be the result of several different chromosomal alterations including in PBP2, other alterations that have been important in conferring penicillin resistance in the past, and other unknown alterations. The most widely studied alteration has been the mosaic penA gene which appears to play a role in resistance to oral third-generation cephalosporins. However, this alteration is likely neither necessary nor sufficient to develop high level cephalosporin resistance and might not play a large role in ceftriaxone resistance.
8. Expert Opinion
If history serves as a pattern for future events, then we can expect wide dissemination of cephalosporin resistance among N. gonorrhoeae isolates in the future. Many questions remain unanswered such as why and how cephalosporin resistance has developed. However, the question at hand now is what can be done to prevent, delay, or at least prepare for this development.
In making plans to prevent the spread of cephalosporin resistance, it is important to know whether resistance is developing anew or is a result of spread of one (or a few) original resistant isolates. Preventing the development of new strains with cephalosporin resistance must necessarily rely on different prevention strategies (limiting antimicrobial use, assuring complete treatment of all gonococcal infections including pharyngeal infections), whereas prevention of the spread of a resistant clone would rely more on early identification and containment of a resistant isolate through interventions focused on travelers and their partners, such as contact tracing, directly observed therapy, and possibly tests of cure. Of course, if new resistant mutants are developing anew, strategies of containment will also be useful. They would likely be less effective if the development of new resistant mutants is widespread and could not necessarily focus on travelers or other likely sources of importation.
8.1 Role of pharyngeal infections
There are several reasons to think that pharyngeal gonorrhea might play a role in the development of cephalosporin resistance. Pharyngeal infections have a lower cure rate than anogenital gonococcal infections.77, 175, 176 Cephalosporins, particularly oral cephalosporins might not consistently achieve adequate tissue levels in the pharyngeal mucosa. This might mean that many pharyngeal infections, which are predominantly asymptomatic,177 are incompletely treated allowing continued growth of the gonococcus in the pharynx in the presence of declining levels of antimicrobials.
One intriguing hypothesis from the reports of mosaic penA genes in Japan highlights this possible role of pharyngeal gonorrhea. Two men with gonococcal urethritis infected with isolates with cefixime MIC of 0.5 mg/L reported exposure only through oral sex. The authors hypothesized that pharyngeal gonorrhea in the source partners allowed N. gonorrhoeae and other commensal Neisseria to coexist and acquire this mosaic,108 possibly aided by low concentrations of cephalosporins in the pharynx.
If that hypothesis is correct, then the prevention of new cephalosporin resistance arising might require focusing more efforts on diagnosing and properly treating pharyngeal gonorrhea. Some researchers have demonstrated that treatment effectiveness for pharyngeal gonorrhea can be increased with the use of more than one type of antimicrobial178 or more than one dose of cephalosporin.179 Prevention and control of cephalosporin resistance might also require modification of current treatment practices making sure that pharyngeal gonorrhea is treated with ceftriaxone or multiple doses of an oral cephalosporin instead of a single dose of oral cephalosporin.
However, controversy exists about the clinical significance of pharyngeal gonococcal infections which are usually asymptomatic and do not result in serious medical sequelae such as infertility or pelvic inflammatory disease. At this point, more research is needed to determine the role of pharyngeal infection in the development of cephalosporin resistance.
9.2 Surveillance programs
Regardless of whether cephalosporin resistance is arising anew or spreading from a few original resistant isolates, surveillance systems are crucial to identify resistant infections for intervention. These systems have already been shown to be critically important in setting treatment guidelines. In the future, these systems should especially focus on cephalosporins and should likely monitor both ceftriaxone and oral third-generation cephalosporin MICs. Unfortunately, most sentinel surveillance systems have important inherent biases such as including only men, usually only those with symptoms who attend STD clinics. Such selection bias might result in the emergence of resistance in other populations being overlooked until resistance has already been established. This has been seen in other sentinel surveillance systems such as for resistant Streptococcus pneumoniae.180 This was also observed in GISP; the local prevalence of fluoroquinolone resistance at nonsentinel sites sometimes differed substantially from sentinel sites.129 As such, these sentinel surveillance systems might need to be augmented with additional testing of non-culture specimens obtained from populations not typically included. The use of molecular assays to monitor molecular markers of resistance likely will be essential in that effort. Because those assays are in development as research tools, their results would necessarily have to be validated and confirmed, but the cost of not developing and using these assays might be that cephalosporin resistance develops and gains a foothold before we know that it is present.
As has been seen in the past, resistant gonorrhea can be spread by international travel.129, 130 As others have pointed out,44, 181 this makes international collaboration among regional and national surveillance systems crucial. This might be particularly true in regard to the surveillance of the Western Pacific Region where resistance to cephalosporins has already been seen, and from where resistance to other antimicrobials has spread worldwide in the past.
Response to newly developed antimicrobial resistance in the past has relied chiefly on the development of new antimicrobials. We are now faced with the fact that we are nearly out of options with no new promising alternative currently on the horizon. Even if there were a new option in development, without other intervention, resistance will no doubt emerge again in the future.
Other pharmaceutical strategies could be considered. The use of more than one agent to treat gonococcal infections in order to prevent emergence and spread of resistance has been suggested on the premise that mutations conferring resistance to both agents would have to develop simultaneously; an unlikely occurrence. There is some data to support the increased efficacy of dual therapy in pharyngeal infections.178 However, dual therapy is already occurring frequently in order to treat simultaneously for gonorrhea and chlamydia and might be playing a role in the spread of azithromycin resistance. Additionally, critics have pointed out that this approach adds costs and adverse events and is not likely to halt the spread of an imported resistant isolate (the most likely scenario for dissemination of resistance to developed countries).181, 182 Alternatively single-dose oral regimens could be eliminated in favor of IM ceftriaxone or multiple doses of an oral agent. However these strategies must be more completely studied and are likely to suffer from increased costs, increased side effects, and would likely adversely affect adherence with partner therapy.
Ultimately, success in preserving cephalosporins as a treatment option for gonorrhea is possible but will likely not be easy and will require a combination of approaches. More powerful than the gonorrhea-focused options discussed here are broader strategies to control and prevent sexually transmitted infections and to limit antimicrobial use worldwide. Sexually transmitted infection control and prevention is hampered by grossly inadequate global funding and political will though there is always hope with new attention focused on STI prevention at the 2006 World Health Assembly.183 A global program focusing on making antimicrobial use more appropriate with the aim of reducing antimicrobial resistance in all pathogenic organisms has been proposed.184 Over the long term, these programs might take selective pressure off N. gonorrhoeae, but significant challenges exist.
Acknowledgments
The authors thank Mark Pandori, PhD and Daniel Deck, PharmD for reviewing the manuscript.
Funding Support: This report was funded in part by US Public Health Service T32 Grant AI007641-06A2 and a California HIV Research Program Grant.
References
- 1.Hook EW, III, Handsfield HH. Gonococcal infections in the adult. In: Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, et al., editors. Sexually Transmitted Diseases. 4. New York: McGraw-Hill Medical; 2008. pp. 627–45. [Google Scholar]
- 2.Brandt AM. No Magic Bullet: A Social History of Venereal Disease in the United States since 1880. 2. New York: Oxford University Press; 1987. [Google Scholar]
- 3.Dees JE, Colston JAC. The Use of Sulfanilamide in Gonococcic Infections. JAMA. 1937 May 29;108:1855–8. [Google Scholar]
- 4.Nelson NA. The Treatment of Syphilis and Gonorrhea As of Today. The American Journal of Nursing 1944. 1944 Aug;44(8):737–41. [Google Scholar]
- 5.Whittington WL, Knapp JS. Trends in resistance of Neisseria gonorrhoeae to antimicrobial agents in the United States. Sex Transm Dis. 1988 Oct-Dec;15(4):202–10. doi: 10.1097/00007435-198810000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Dan M. The use of fluoroquinolones in gonorrhoea: the increasing problem of resistance. Expert Opin Pharmacother. 2004 Apr;5(4):829–54. doi: 10.1517/14656566.5.4.829. *Very thorough review of fluoroquinolone resistance in N. gonorrhoeae.
- 7.Centers for Disease Control and Prevention. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007 Apr 13;56(14):332–6. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006 Aug 4;55(RR11):1–94. **Crucial resource for clinical treatment recommendations especially for the United States.
- 9.Kent CK, Chaw JK, Wong W, Liska S, Gibson S, Hubbard G, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis. 2005 Jul 1;41(1):67–74. doi: 10.1086/430704. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections, Overview and Estimates. Geneva: World Health Organization; 2001. Report No.: WHO/HIV_AIDS/2001.02. [Google Scholar]
- 11.Laga M, Meheus A, Piot P. Epidemiology and control of gonococcal ophthalmia neonatorum. Bull World Health Organ. 1989;67(5):471–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999 Feb;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rottingen JA, Cameron DW, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sex Transm Dis. 2001 Oct;28(10):579–97. doi: 10.1097/00007435-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections - 2002. MMWR. 2002;51(RR15):1–27. [PubMed] [Google Scholar]
- 15.Thayer JD, Martin JE., Jr A Selective Medium for the Cultivation of N. gonorrhoeae and N. meningiditis. Public Health Rep. 1964 Jan;79:49–57. [PMC free article] [PubMed] [Google Scholar]
- 16.Cook RL, Hutchison SL, Ostergaard L, Braithwaite RS, Ness RB. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhoeae. Ann Intern Med. 2005 Jun 7;142(11):914–25. doi: 10.7326/0003-4819-142-11-200506070-00010. [DOI] [PubMed] [Google Scholar]
- 17.Golden MR, Hughes JP, Cles LE, Crouse K, Gudgel K, Hu J, et al. Positive predictive value of Gen-Probe APTIMA Combo 2 testing for Neisseria gonorrhoeae in a population of women with low prevalence of N. gonorrhoeae infection. Clin Infect Dis. 2004 Nov 1;39(9):1387–90. doi: 10.1086/424880. [DOI] [PubMed] [Google Scholar]
- 18.Whiley DM, Garland SM, Harnett G, Lum G, Smith DW, Tabrizi SN, et al. Exploring ‘best practice’ for nucleic acid detection of Neisseria gonorrhoeae. Sex Health. 2008 Mar;5(1):17–23. doi: 10.1071/sh07050. [DOI] [PubMed] [Google Scholar]
- 19.Tapsall J, Whiley D, Sloots T. Applications of molecular testing in clinical laboratories for the diagnosis and control of gonorrhea. Future Microbiol. 2006 Oct;1:317–24. doi: 10.2217/17460913.1.3.317. [DOI] [PubMed] [Google Scholar]
- 20.Dicker LW, Mosure DJ, Steece R, Stone KM. Laboratory tests used in US public health laboratories for sexually transmitted diseases, 2000. Sex Transm Dis. 2004 May;31(5):259–64. doi: 10.1097/01.olq.0000124609.84050.f3. [DOI] [PubMed] [Google Scholar]
- 21.Dicker LW, Mosure DJ, Steece R, Stone KM. Testing for sexually transmitted diseases in U.S. Public health laboratories in 2004. Sex Transm Dis. 2007 Jan;34(1):41–6. doi: 10.1097/01.olq.0000222708.70594.8e. [DOI] [PubMed] [Google Scholar]
- 22.Fredlund H, Falk L, Jurstrand M, Unemo M. Molecular genetic methods for diagnosis and characterisation of Chlamydia trachomatis and Neisseria gonorrhoeae: impact on epidemiological surveillance and interventions. Apmis. 2004 Nov-Dec;112(1112):771–84. doi: 10.1111/j.1600-0463.2004.apm11211-1205.x. [DOI] [PubMed] [Google Scholar]
- 23.Gaydos CA, Quinn TC, Willis D, Weissfeld A, Hook EW, Martin DH, et al. Performance of the APTIMA Combo 2 assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in female urine and endocervical swab specimens. J Clin Microbiol. 2003 Jan;41(1):304–9. doi: 10.1128/JCM.41.1.304-309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adler MW. Sexually transmitted diseases control in developing countries. Genitourin Med. 1996 Apr;72(2):83–8. doi: 10.1136/sti.72.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Department of Reproductive Health and Research WHO. Sexually Transmitted and Other Reproductive Tract Infections: A guide to essential practice. Geneva: World Health Organization; 2005. [Google Scholar]
- 26.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2006. Atlanta, GA: US Department of Health and Human Services; 2007. Available at: http://www.cdc.gov/std/stats/pdf/Surv2006.pdf. [Google Scholar]
- 27.Weinstock H, Berman S, Cates W., Jr Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004 Jan-Feb;36(1):6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 28.GRASP Steering Group. The Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) Year 2007 report. London: Health Protection Agency; 2008. [12/8/2008]. Available at: http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1221117895841. [Google Scholar]
- 29.Stoner BP, Whittington WL, Hughes JP, Aral SO, Holmes KK. Comparative epidemiology of heterosexual gonococcal and chlamydial networks: implications for transmission patterns. Sex Transm Dis. 2000 Apr;27(4):215–23. doi: 10.1097/00007435-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Racial disparities in nationally notifiable diseases--United States, 2002. MMWR Morb Mortal Wkly Rep. 2005 Jan 14;54(1):9–11. [PubMed] [Google Scholar]
- 31.World Health Organization. Guidelines for the management of sexually transmitted infections. Geneva, Switzerland: 2003. Available at: http://www.who.int/hiv/pub/sti/en/STIGuidelines2003.pdf. [Google Scholar]
- 32.Moran JS, Levine WC. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin Infect Dis. 1995 Apr;20(Suppl 1):S47–65. doi: 10.1093/clinids/20.supplement_1.s47. **Classic article stating rationale for selecting antimicrobials for one time treatment regimens.
- 33.Newman LM, Moran JS, Workowski KA. Update on the management of gonorrhea in adults in the United States. Clin Infect Dis. 2007 Apr 1;44(Suppl 3):S84–101. doi: 10.1086/511422. **Thorough review and explanation of the rationale behind US CDC gonorrhea treatment recommendations. Includes review of therapies under investigation.
- 34.Centers for Disease Control and Prevention (CDC) Expedited Partner Therapy in the Management of Sexually Transmitted Diseases. Atlanta, GA: US Department of Health and Human Services; 2006. Available at: http://www.cdc.gov/std/treatment/EPTFinalReport2006.pdf. [Google Scholar]
- 35.Golden MR, Whittington WL, Handsfield HH, Hughes JP, Stamm WE, Hogben M, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005 Feb 17;352(7):676–85. doi: 10.1056/NEJMoa041681. [DOI] [PubMed] [Google Scholar]
- 36.Kissinger P, Mohammed H, Richardson-Alston G, Leichliter JS, Taylor SN, Martin DH, et al. Patient-delivered partner treatment for male urethritis: a randomized, controlled trial. Clin Infect Dis. 2005 Sep 1;41(5):623–9. doi: 10.1086/432476. [DOI] [PubMed] [Google Scholar]
- 37.Fleming A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. 1929. Bull World Health Organ. 2001;79(8):780–90. [PMC free article] [PubMed] [Google Scholar]
- 38.Mahoney JF, Ferguson C, Buchholtz M, Van Slyke CJ. The Use of Penicillin Sodium in the Treatment of Sulfonamide-resistant Gonorrhea in Men American Journal of Syphilis, Gonorrhea, and Venereal Diseases. 1943 Sept;27:525. [Google Scholar]
- 39.Herrell WE, Cook EN, Thompson L. Use of Penicillin in Sulfonamide-resistant Gonorrhea Infections. JAMA. 1943 May 29;132:289. [Google Scholar]
- 40.Van Slyke CJ, Arnold RC, Buchholtz M. Penicillin Therapy in Sulfonamide-resistant Gonorrhea in Men. Am J Pub Health. 1943 Dec;33:1392–4. doi: 10.2105/ajph.33.12.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Catlin BW, Reyn A. Neisseria gonorrhoeae isolated from disseminated and localised infections in pre-penicillin era. Auxotypes and antibacterial drug resistances. Br J Vener Dis. 1982 Jun;58(3):158–65. doi: 10.1136/sti.58.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thayer J, Field F, Magnusos H. The sensitivity of gonococci to penicillin and its relationship to penicillin failures. Antibiot Chemother. 1957;7:306–10. [PubMed] [Google Scholar]
- 43.Jaffe HW, Biddle JW, Thornsberry C, Johnson RE, Kaufman RE, Reynolds GH, et al. National gonorrhea therapy monitoring study: in vitro antibiotic susceptibility and its correlation with treatment results. N Engl J Med. 1976 Jan 1;294(1):5–9. doi: 10.1056/NEJM197601012940102. [DOI] [PubMed] [Google Scholar]
- 44.Workowski KA, Berman SM, Douglas JM., Jr Emerging antimicrobial resistance in Neisseria gonorrhoeae: urgent need to strengthen prevention strategies. Ann Intern Med. 2008 Apr 15;148(8):606–13. doi: 10.7326/0003-4819-148-8-200804150-00005. *Recent review of antimicrobial resistance from a US public health perspective.
- 45.Centers for Disease Control. CDC recommended treatment schedules, 1974. Morbidity and Mortality Weekly Report. 1974;23:341–2. [Google Scholar]
- 46.Ison CA. Antimicrobial agents and gonorrhoea: therapeutic choice, resistance and susceptibility testing. Genitourin Med. 1996 Aug;72(4):253–7. doi: 10.1136/sti.72.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips I. Beta-lactamase-producing, penicillin-resistant gonococcus. Lancet. 1976 Sep 25;2(7987):656–7. doi: 10.1016/s0140-6736(76)92466-1. [DOI] [PubMed] [Google Scholar]
- 48.Ashford WA, Golash RG, Hemming VG. Penicillinase-producing Neisseria gonorrhoeae. Lancet. 1976 Sep 25;2(7987):657–8. doi: 10.1016/s0140-6736(76)92467-3. [DOI] [PubMed] [Google Scholar]
- 49.Lind I. Antimicrobial resistance in Neisseria gonorrhoeae. Clin Infect Dis. 1997 Jan;24(Suppl 1):S93–7. doi: 10.1093/clinids/24.supplement_1.s93. [DOI] [PubMed] [Google Scholar]
- 50.Shigemura K, Shirakawa T, Okada H, Hinata N, Acharya B, Kinoshita S, et al. Mutations in the gyrA and parC genes and in vitro activities of fluoroquinolones in 91 clinical isolates of Neisseria gonorrhoeae in Japan. Sex Transm Dis. 2004 Mar;31(3):180–4. doi: 10.1097/01.olq.0000114654.91972.66. [DOI] [PubMed] [Google Scholar]
- 51.Martin IM, Hoffmann S, Ison CA. European Surveillance of Sexually Transmitted Infections (ESSTI): the first combined antimicrobial susceptibility data for Neisseria gonorrhoeae in Western Europe. J Antimicrob Chemother. 2006 Sep;58(3):587–93. doi: 10.1093/jac/dkl265. [DOI] [PubMed] [Google Scholar]
- 52.Morse SA, Johnson SR, Biddle JW, Roberts MC. High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob Agents Chemother. 1986 Nov;30(5):664–70. doi: 10.1128/aac.30.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott GR, McMillan A, Young H. Ciprofloxacin versus ampicillin and probenecid in the treatment of uncomplicated gonorrhoea in men. J Antimicrob Chemother. 1987 Jul;20(1):117–21. doi: 10.1093/jac/20.1.117. [DOI] [PubMed] [Google Scholar]
- 54.Roddy RE, Handsfield HH, Hook EW., 3rd Comparative trial of single-dose ciprofloxacin and ampicillin plus probenecid for treatment of gonococcal urethritis in men. Antimicrob Agents Chemother. 1986 Aug;30(2):267–9. doi: 10.1128/aac.30.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention. 1989 Sexually Transmitted Diseases Treatment Guidelines. Morbidity and Mortality Weekly Report. 1989 September 01;38(S8):i–xi. 1–43. [Google Scholar]
- 56.Centers for Disease Control and Prevention. 1993 sexually transmitted diseases treatment guidelines. MMWR Recomm Rep. 1993 Sep 24;42(RR14):1–102. [PubMed] [Google Scholar]
- 57.Tanaka M, Kumazawa J, Matsumoto T, Kobayashi I. High prevalence of Neisseria gonorrhoeae strains with reduced susceptibility to fluoroquinolones in Japan. Genitourin Med. 1994 Apr;70(2):90–3. doi: 10.1136/sti.70.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention. Fluoroquinolone resistance in Neisseria gonorrhoeae--Colorado and Washington, 1995. MMWR Morb Mortal Wkly Rep. 1995 Oct 20;44(41):761–4. [PubMed] [Google Scholar]
- 59.Gorwitz RJ, Nakashima AK, Moran JS, Knapp JS. Sentinel surveillance for antimicrobial resistance in Neisseria gonorrhoeae--United States, 1988-1991. The Gonococcal Isolate Surveillance Project Study Group. MMWR CDC Surveill Summ. 1993 Aug 13;42(3):29–39. [PubMed] [Google Scholar]
- 60.Turner A, Gough KR, Jephcott AE, McClean AN. Importation into the UK of a strain of Neisseria gonorrhoeae resistant to penicillin, ciprofloxacin and tetracycline. Genitourin Med. 1995 Oct;71(5):331–2. doi: 10.1136/sti.71.5.331-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tapsall JW, Phillips EA, Shultz TR, Thacker C. Quinolone-resistant Neisseria gonorrhoeae isolated in Sydney, Australia, 1991 to 1995. Sex Transm Dis. 1996 Sep-Oct;23(5):425–8. doi: 10.1097/00007435-199609000-00014. [DOI] [PubMed] [Google Scholar]
- 62.Knapp JS, Ohye R, Neal SW, Parekh MC, Higa H, Rice RJ. Emerging in vitro resistance to quinolones in penicillinase-producing Neisseria gonorrhoeae strains in Hawaii. Antimicrob Agents Chemother. 1994 Sep;38(9):2200–3. doi: 10.1128/aac.38.9.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention. Increases in fluoroquinolone-resistant Neisseria gonorrhoeae among men who have sex with men--United States, 2003, and revised recommendations for gonorrhea treatment, 2004. MMWR Morb Mortal Wkly Rep. 2004 Apr 30;53(16):335–8. [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention. Increases in fluoroquinolone-resistant Neisseria gonorrhoeae--Hawaii and California, 2001. MMWR Morb Mortal Wkly Rep. 2002 Nov 22;51(46):1041–4. [PubMed] [Google Scholar]
- 65.BASHH (British Association for Sexual Health and HIV) National Guideline on the Diagnosis and Treatment of Gonorrhoea in Adults 2005. 2005 [Google Scholar]
- 66.Andes DR, Craig WA. Cephalosporins. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 6. Philadelphia, Pennsylvania: Elsevier Inc; 2005. [Google Scholar]
- 67.Marshall WF, Blair JE. The cephalosporins. Mayo Clin Proc. 1999 Feb;74(2):187–95. doi: 10.4065/74.2.187. [DOI] [PubMed] [Google Scholar]
- 68.Craig WA. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis. 1995 May-Jun;22(12):89–96. doi: 10.1016/0732-8893(95)00053-d. [DOI] [PubMed] [Google Scholar]
- 69.Thorpe EM, Schwebke JR, Hook EW, 3rd, Rompalo A, McCormack WM, Mussari KL, et al. Comparison of single-dose cefuroxime axetil with ciprofloxacin in treatment of uncomplicated gonorrhea caused by penicillinase-producing and non-penicillinase-producing Neisseria gonorrhoeae strains. Antimicrob Agents Chemother. 1996 Dec;40(12):2775–80. doi: 10.1128/aac.40.12.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ison CA, Mouton JW, Jones K, Fenton KA, Livermore DM. Which cephalosporin for gonorrhoea? Sex Transm Infect. 2004 Oct;80(5):386–8. doi: 10.1136/sti.2004.012757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crabbe F, Grobbelaar TM, van Dyck E, Dangor Y, Laga M, Ballard RC. Cefaclor, an alternative to third generation cephalosporins for the treatment of gonococcal urethritis in the developing world? Genitourin Med. 1997 Dec;73(6):506–9. doi: 10.1136/sti.73.6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Handsfield HH, McCormack WM, Hook EW, 3rd, Douglas JM, Jr, Covino JM, Verdon MS, et al. A comparison of single-dose cefixime with ceftriaxone as treatment for uncomplicated gonorrhea. The Gonorrhea Treatment Study Group. N Engl J Med. 1991 Nov 7;325(19):1337–41. doi: 10.1056/NEJM199111073251903. [DOI] [PubMed] [Google Scholar]
- 73.Verdon MS, Douglas JM, Jr, Wiggins SD, Handsfield HH. Treatment of uncomplicated gonorrhea with single doses of 200 mg cefixime. Sex Transm Dis. 1993 Sep-Oct;20(5):290–3. doi: 10.1097/00007435-199309000-00010. [DOI] [PubMed] [Google Scholar]
- 74.Plourde PJ, Tyndall M, Agoki E, Ombette J, Slaney LA, D’Costa LJ, et al. Single-dose cefixime versus single-dose ceftriaxone in the treatment of antimicrobial-resistant Neisseria gonorrhoeae infection. J Infect Dis. 1992 Oct;166(4):919–22. doi: 10.1093/infdis/166.4.919. [DOI] [PubMed] [Google Scholar]
- 75.Portilla I, Lutz B, Montalvo M, Mogabgab WJ. Oral cefixime versus intramuscular ceftriaxone in patients with uncomplicated gonococcal infections. Sex Transm Dis. 1992 Mar-Apr;19(2):94–8. [PubMed] [Google Scholar]
- 76.Novak E, Paxton LM, Tubbs HJ, Turner LF, Keck CW, Yatsu J. Orally administered cefpodoxime proxetil for treatment of uncomplicated gonococcal urethritis in males: a dose-response study. Antimicrob Agents Chemother. 1992 Aug;36(8):1764–5. doi: 10.1128/aac.36.8.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hall C, McElroy M, Samuel M, Newman L, Bauer H, Chambers H, et al. Single-Dose, Oral Cefpodoxime Proxetil is Effective for Treatment of Uncomplicated Urogenital and Rectal Gonorrhea. 17th Biennial Meeting of the International Society for Sexually Transmitted Disease Research; 2007; Seattle. 2007. [Google Scholar]
- 78.Chong LY, Cheung WM, Leung CS, Yu CW, Chan LY. Clinical evaluation of ceftibuten in gonorrhea. A pilot study in Hong Kong. Sex Transm Dis. 1998 Oct;25(9):464–7. doi: 10.1097/00007435-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 79.Cohen MA, Joannides ET, Roland GE, Meservey MA, Huband MD, Shapiro MA, et al. In vitro evaluation of cefdinir (FK482), a new oral cephalosporin with enhanced antistaphylococcal activity and beta-lactamase stability. Diagn Microbiol Infect Dis. 1994 Jan;18(1):31–9. doi: 10.1016/0732-8893(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 80.Hook EW, 3rd, Judson FN, Verdon MS, Ehret JM, Handsfield HH. Comparative study of cefoperazone and spectinomycin for treatment of uncomplicated gonorrhea in men. Antimicrob Agents Chemother. 1986 Oct;30(4):619–21. doi: 10.1128/aac.30.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim JH, Ro YS, Kim YT. Cefoperazone (Cefobid) for treating men with gonorrhoea caused by penicillinase producing Neisseria gonorrhoeae. Br J Vener Dis. 1984 Aug;60(4):238–40. doi: 10.1136/sti.60.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Centers for Disease Control and Prevention. Availability of cefixime 400 mg tablets--United States, April 2008. MMWR Morb Mortal Wkly Rep. 2008 Apr 25;57(16):435. [PubMed] [Google Scholar]
- 83.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2006 Supplement: Gonococcal Isolate Surveillance Project (GISP) Annual Report -- 2006. Atlanta, GA: US Department of Health and Human Services; 2008. Available at: http://www.cdc.gov/STD/gisp2006/GISPSurvSupp2006Complete.pdf. [Google Scholar]
- 84.Lo JY, Ho KM, Leung AO, Tiu FS, Tsang GK, Lo AC, et al. Ceftibuten resistance and treatment failure of Neisseria gonorrhoeae infection. Antimicrob Agents Chemother. 2008 Oct;52(10):3564–7. doi: 10.1128/AAC.00198-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Handsfield HH, Murphy VL. Comparative study of ceftriaxone and spectinomycin for treatment of uncomplicated gonorrhoea in men. Lancet. 1983 Jul 9;2(8341):67–70. doi: 10.1016/s0140-6736(83)90058-2. [DOI] [PubMed] [Google Scholar]
- 86.Collier AC, Judson FN, Murphy VL, Leach LA, Root CJ, Handsfield HH. Comparative study of ceftriaxone and spectinomycin in the treatment of uncomplicated gonorrhea in women. Am J Med. 1984 Oct 19;77(4C):68–72. [PubMed] [Google Scholar]
- 87.Judson FN, Ehret JM, Root CJ. Comparative study of ceftriaxone and aqueous procaine penicillin G in the treatment of uncomplicated gonorrhea in women. Antimicrob Agents Chemother. 1983 Feb;23(2):218–20. doi: 10.1128/aac.23.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rajan VS, Sng EH, Thirumoorthy T, Goh CL. Ceftriaxone in the treatment of ordinary and penicillinase-producing strains of Neisseria gonorrhoeae. Br J Vener Dis. 1982 Oct;58(5):314–6. doi: 10.1136/sti.58.5.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Handsfield HH, Murphy VL, Holmes KK. Dose-ranging study of ceftriaxone for uncomplicated gonorrhea in men. Antimicrob Agents Chemother. 1981 Dec;20(6):839–40. doi: 10.1128/aac.20.6.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eichmann A, Weidmann G, Havas L. One-dose treatment of acute uncomplicated gonorrhoea of male patients with ceftriaxone Ro 13-9904, a new parenteral cephalosporin. A dose-range finding pilot study using doses of 500, 250, 125 and 50 mg respectively, in descending order. Chemotherapy. 1981;27(Suppl 1):62–9. doi: 10.1159/000238031. [DOI] [PubMed] [Google Scholar]
- 91.Kojima M, Masuda K, Yada Y, Hayase Y, Muratani T, Matsumoto T. Single-dose treatment of male patients with gonococcal urethritis using 2g spectinomycin: microbiological and clinical evaluations. Int J Antimicrob Agents. 2008 Jul;32(1):50–4. doi: 10.1016/j.ijantimicag.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 92.Goldstein AM, Clark JH, Wickler MA. Comparison of single-dose ceftizoxime or ceftriaxone in the treatment of uncomplicated urethral gonorrhea. Sex Transm Dis. 1991 Jul-Sep;18(3):180–2. doi: 10.1097/00007435-199107000-00011. [DOI] [PubMed] [Google Scholar]
- 93.Goldstein AM, Clark JH. Treatment of uncomplicated gonococcal urethritis with single-dose ceftizoxime. Sex Transm Dis. 1990 Oct-Dec;17(4):181–3. doi: 10.1097/00007435-199010000-00006. [DOI] [PubMed] [Google Scholar]
- 94.Veeravahu M, Clay JC, Mohanty KC, Tovey SJ, Wanas TM, Roberts KN. Efficacy of ceftizoxime in the treatment of uncomplicated gonorrhoea: comparison with amoxycillin. Br J Clin Pract. 1990 Jun;44(6):216–8. [PubMed] [Google Scholar]
- 95.Berg SW, Kilpatrick ME, Harrison WO, McCutchan JA. Cefoxitin as a single-dose treatment for urethritis caused by penicillinase-producing Neisseria gonorrhoeae. N Engl J Med. 1979 Sep 6;301(10):509–11. doi: 10.1056/NEJM197909063011001. [DOI] [PubMed] [Google Scholar]
- 96.Greaves WL, Kraus SJ, McCormack WM, Biddle JW, Zaidi A, Fiumara NJ, et al. Cefoxitin vs. penicillin in the treatment of uncomplicated gonorrhea. Sex Transm Dis. 1983 Apr-Jun;10(2):53–5. doi: 10.1097/00007435-198304000-00001. [DOI] [PubMed] [Google Scholar]
- 97.Zajdowicz TR, Sanches PL, Berg SW, Kerbs SB, Newquist RL, Harrison WO. Comparison of ceftriaxone with cefoxitin in the treatment of penicillin-resistant gonococcal urethritis. Br J Vener Dis. 1983 Jun;59(3):176–8. doi: 10.1136/sti.59.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Korting HC, Abeck D. One-shot treatment of uncomplicated gonorrhoea with third-generation cephalosporins with differing serum half-life. Results of a controlled trial with ceftriaxone and cefotaxime. Chemotherapy. 1989;35(6):441–8. doi: 10.1159/000238708. [DOI] [PubMed] [Google Scholar]
- 99.Mogabgab WJ, Lutz FB. Randomized study of cefotaxime versus ceftriaxone for uncomplicated gonorrhea. South Med J. 1994 Apr;87(4):461–4. doi: 10.1097/00007611-199404000-00007. [DOI] [PubMed] [Google Scholar]
- 100.McCormack WM, Mogabgab WJ, Jones RB, Hook EW, 3rd, Wendel GD, Jr, Handsfield HH. Multicenter, comparative study of cefotaxime and ceftriaxone for treatment of uncomplicated gonorrhea. Sex Transm Dis. 1993 Sep-Oct;20(5):269–73. doi: 10.1097/00007435-199309000-00006. [DOI] [PubMed] [Google Scholar]
- 101.van der Willigen AH, Wagenvoort JH, Schalla WO, Knapp JS, Boot JM, Heeres-Weststrate PL, et al. Randomized comparative study of 0.5 and 1 g of cefodizime (HR 221) versus 1 g of cefotaxime for acute uncomplicated urogenital gonorrhea. Antimicrob Agents Chemother. 1988 Apr;32(4):426–9. doi: 10.1128/aac.32.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matsumoto T, Muratani T, Takahashi K, Ando Y, Sato Y, Kurashima M, et al. Single dose of cefodizime completely eradicated multidrug-resistant strain of Neisseria gonorrhoeae in urethritis and uterine cervicitis. J Infect Chemother. 2006 Apr;12(2):97–9. doi: 10.1007/s10156-006-0431-5. [DOI] [PubMed] [Google Scholar]
- 103.Tanaka M, Nakayama H, Tunoe H, Egashira T, Kanayama A, Saika T, et al. A remarkable reduction in the susceptibility of Neisseria gonorrhoeae isolates to cephems and the selection of antibiotic regimens for the single-dose treatment of gonococcal infection in Japan. J Infect Chemother. 2002 Mar;8(1):81–6. doi: 10.1007/s101560200011. [DOI] [PubMed] [Google Scholar]
- 104.Akasaka S, Muratani T, Yamada Y, Inatomi H, Takahashi K, Matsumoto T. Emergence of cephem- and aztreonam-high-resistant Neisseria gonorrhoeae that does not produce beta-lactamase. J Infect Chemother. 2001 Mar;7(1):49–50. doi: 10.1007/s101560170034. *Early report of cephalosporin treatment failures in Japan.
- 105.Yamaguchi K, Domon H, Miyazaki S, Tateda K, Ohno A, Ishii K, et al. In vitro and in vivo antibacterial activities of CS-834, a new oral carbapenem. Antimicrob Agents Chemother. 1998 Mar;42(3):555–63. doi: 10.1128/aac.42.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muratani T, Akasaka S, Kobayashi T, Yamada Y, Inatomi H, Takahashi K, et al. Outbreak of cefozopran (penicillin, oral cephems, and aztreonam)-resistant Neisseria gonorrhoeae in Japan. Antimicrob Agents Chemother. 2001 Dec;45(12):3603–6. doi: 10.1128/AAC.45.12.3603-3606.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ito M, Yasuda M, Yokoi S, Ito S, Takahashi Y, Ishihara S, et al. Remarkable increase in central Japan in 2001-2002 of Neisseria gonorrhoeae isolates with decreased susceptibility to penicillin, tetracycline, oral cephalosporins, and fluoroquinolones. Antimicrob Agents Chemother. 2004 Aug;48(8):3185–7. doi: 10.1128/AAC.48.8.3185-3187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ameyama S, Onodera S, Takahata M, Minami S, Maki N, Endo K, et al. Mosaic-like structure of penicillin-binding protein 2 Gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob Agents Chemother. 2002 Dec;46(12):3744–9. doi: 10.1128/AAC.46.12.3744-3749.2002. *First discussion of mosaic PBP2.
- 109.Tanaka M, Nakayama H, Huruya K, Konomi I, Irie S, Kanayama A, et al. Analysis of mutations within multiple genes associated with resistance in a clinical isolate of Neisseria gonorrhoeae with reduced ceftriaxone susceptibility that shows a multidrug-resistant phenotype. Int J Antimicrob Agents. 2006 Jan;27(1):20–6. doi: 10.1016/j.ijantimicag.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 110.Yokoi S, Deguchi T, Ozawa T, Yasuda M, Ito S, Kubota Y, et al. Threat to cefixime treatment for gonorrhea. Emerg Infect Dis. 2007 Aug;13(8):1275–7. doi: 10.3201/eid1308.060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Osaka K, Takakura T, Narukawa K, Takahata M, Endo K, Kiyota H, et al. Analysis of amino acid sequences of penicillin-binding protein 2 in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime and ceftriaxone. J Infect Chemother. 2008 Jun;14(3):195–203. doi: 10.1007/s10156-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 112.Takahata S, Senju N, Osaki Y, Yoshida T, Ida T. Amino acid substitutions in mosaic penicillin-binding protein 2 associated with reduced susceptibility to cefixime in clinical isolates of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2006 Nov;50(11):3638–45. doi: 10.1128/AAC.00626-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tapsall JW, Limnios EA, Murphy D. Analysis of trends in antimicrobial resistance in Neisseria gonorrhoeae isolated in Australia, 1997 2006. J Antimicrob Chemother. 2008 Jan;61(1):150–5. doi: 10.1093/jac/dkm434. [DOI] [PubMed] [Google Scholar]
- 114.Annual report of the Australian Gonococcal Surveillance Programme, 2007. Commun Dis Intell. 2008;32(2):227–31. doi: 10.33321/cdi.2008.32.20. [DOI] [PubMed] [Google Scholar]
- 115.WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific Region, 2006. Commun Dis Intell. 2008 Mar;32(1):48–51. doi: 10.33321/cdi.2008.32.5. [DOI] [PubMed] [Google Scholar]
- 116.Ye S, Su X, Wang Q, Yin Y, Dai X, Sun H. Surveillance of antibiotic resistance of Neisseria gonorrhoeae isolates in China, 1993-1998. Sex Transm Dis. 2002 Apr;29(4):242–5. doi: 10.1097/00007435-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 117.Guoming L, Qun C, Shengchun W. Resistance of Neisseria gonorrhoeae epidemic strains to antibiotics: report of resistant isolates and surveillance in Zhanjiang, China: 1998 to 1999. Sex Transm Dis. 2000 Feb;27(2):115–8. doi: 10.1097/00007435-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 118.Wong WW, Huang CT, Li LH, Chiang CC, Chen BD, Li SY. Molecular Epidemiology of Gonorrhea Identified Clonal Clusters with Distinct Susceptibilities Associated with Specific High-risk Groups. J Clin Microbiol. 2008 Oct 8; doi: 10.1128/JCM.00577-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cao V, Ratsima E, Tri DV, Bercion R, Fonkoua MC, Richard V, et al. Antimicrobial Susceptibility of Neisseria gonorrhoeae Strains Isolated in 2004-2005 in Bangui, Central African Republic; Yaounde, Cameroon; Antananarivo, Madagascar; and Ho Chi Minh Ville and Nha Trang, Vietnam. Sex Transm Dis. 2008 Aug 21; doi: 10.1097/OLQ.0b013e31818318d8. [DOI] [PubMed] [Google Scholar]
- 120.Clendennen TE, 3rd, Hames CS, Kees ES, Price FC, Rueppel WJ, Andrada AB, et al. In vitro antibiotic susceptibilities of Neisseria gonorrhoeae isolates in the Philippines. Antimicrob Agents Chemother. 1992 Feb;36(2):277–82. doi: 10.1128/aac.36.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clendennen TE, Echeverria P, Saengeur S, Kees ES, Boslego JW, Wignall FS. Antibiotic susceptibility survey of Neisseria gonorrhoeae in Thailand. Antimicrob Agents Chemother. 1992 Aug;36(8):1682–7. doi: 10.1128/aac.36.8.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.World Health Organization Regional Office for the Western Pacific. Report: Meeting on Controlling Sexually Transmitted Infections --- Enhancing HIV Prevention in the Western Pacific Region; 2007 29 October – 1 November 2007; Penang, Malaysia: World Health Organization Regional Office for the Western Pacific; [08/11/2008]. Report No.: WPHSI/ICP/HIV/1.4/001 RS/2007/GE/40MAA. Available at: http://www.wpro.who.int/NR/rdonlyres/0C9AD220-9EE9-4F2E-9C44-5405BE5B10AE/0/MeetingReport_STIMtg_Penang.pdf. [Google Scholar]
- 123.Ray K, Bala M, Kumari S, Narain JP. Antimicrobial resistance of Neisseria gonorrhoeae in selected World Health Organization Southeast Asia Region countries: an overview. Sex Transm Dis. 2005 Mar;32(3):178–84. doi: 10.1097/01.olq.0000154490.40381.15. [DOI] [PubMed] [Google Scholar]
- 124.Bala M, Ray K, Gupta SM, Muralidhar S, Jain RK. Changing trends of antimicrobial susceptibility patterns of Neisseria gonorrhoeae in India and the emergence of ceftriaxone less susceptible N. gonorrhoeae strains. J Antimicrob Chemother. 2007 Sep;60(3):582–6. doi: 10.1093/jac/dkm238. [DOI] [PubMed] [Google Scholar]
- 125.Olsen B, Hadad R, Fredlund H, Unemo M. The Neisseria gonorrhoeae population in Sweden during 2005-phenotypes, genotypes and antibiotic resistance. Apmis. 2008 Mar;116(3):181–9. doi: 10.1111/j.1600-0463.2008.00895.x. [DOI] [PubMed] [Google Scholar]
- 126.Hoffmann S, Lambertsen L, Berthelsen L, Cowan S. Neisseria gonorrhoeae with increasing ceftriaxone MIC in Denmark in 2004: Serotyping, bi-locus sequence typing, and sexual preference, Abstract WP-035. 16th Meeting of the International Society for Sexually Transmitted Diseases Research; 2005; Amsterdam, The Netherlands. 2005. [12/4/2008]. Available at: http://www.parthen-impact.com/pco/6_05STD/public/ [Google Scholar]
- 127.Vazquez JA, Martin E, Galarza P, Gimenez MJ, Aguilar L, Coronel P. In vitro susceptibility of Spanish isolates of Neisseria gonorrhoeae to cefditoren and five other antimicrobial agents. Int J Antimicrob Agents. 2007 Apr;29(4):473–4. doi: 10.1016/j.ijantimicag.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 128.Tzelepi E, Daniilidou M, Miriagou V, Siatravani E, Pavlidou E, Flemetakis A. Cluster of multidrug-resistant Neisseria gonorrhoeae with reduced susceptibility to the newer cephalosporins in Northern Greece. J Antimicrob Chemother. 2008 Sep;62(3):637–9. doi: 10.1093/jac/dkn236. [DOI] [PubMed] [Google Scholar]
- 129.Wang SA, Harvey AB, Conner SM, Zaidi AA, Knapp JS, Whittington WL, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: the spread of fluoroquinolone resistance. Ann Intern Med. 2007 Jul 17;147(2):81–8. doi: 10.7326/0003-4819-147-2-200707170-00006. [DOI] [PubMed] [Google Scholar]
- 130.Wang SA, Lee MV, O’Connor N, Iverson CJ, Ohye RG, Whiticar PM, et al. Multidrug-resistant Neisseria gonorrhoeae with decreased susceptibility to cefixime-Hawaii, 2001. Clin Infect Dis. 2003 Sep 15;37(6):849–52. doi: 10.1086/377500. [DOI] [PubMed] [Google Scholar]
- 131.Lewis DA, Scott L, Slabbert M, Mhlongo S, van Zijl A, Sello M, et al. Escalation in the relative prevalence of ciprofloxacin-resistant gonorrhoea among men with urethral discharge in two South African cities: association with HIV seropositivity. Sex Transm Infect. 2008 Oct;84(5):352–5. doi: 10.1136/sti.2007.029611. [DOI] [PubMed] [Google Scholar]
- 132.De Jongh M, Dangor Y, Adam A, Hoosen AA. Gonococcal resistance: evolving from penicillin, tetracycline to the quinolones in South Africa -- implications for treatment guidelines. Int J STD AIDS. 2007 Oct;18(10):697–9. doi: 10.1258/095646207782193768. [DOI] [PubMed] [Google Scholar]
- 133.Moodley P, Martin IM, Pillay K, Ison CA, Sturm AW. Molecular epidemiology of recently emergent ciprofloxacin-resistant Neisseria gonorrhoeae in South Africa. Sex Transm Dis. 2006 Jun;33(6):357–60. doi: 10.1097/01.olq.0000194581.02022.f0. [DOI] [PubMed] [Google Scholar]
- 134.Dillon JA, Ruben M, Li H, Borthagaray G, Marquez C, Fiorito S, et al. Challenges in the control of gonorrhea in South America and the Caribbean: monitoring the development of resistance to antibiotics. Sex Transm Dis. 2006 Feb;33(2):87–95. doi: 10.1097/01.olq.0000187231.28812.29. [DOI] [PubMed] [Google Scholar]
- 135.Sparling PF. Biology of Neisseria gonorrhoeae. In: Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, et al., editors. Sexually Transmitted Diseases. 4. McGraw-Hill Medical: 2008. pp. 607–26. [Google Scholar]
- 136.Chung GT, Yoo JS, Oh HB, Lee YS, Cha SH, Kim SJ, et al. The Complete Genome Sequence of Neisseria gonorrhoeae NCCP11945. J Bacteriol. 2008 Jun 27; doi: 10.1128/JB.00566-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Davidsen T, Rodland EA, Lagesen K, Seeberg E, Rognes T, Tonjum T. Biased distribution of DNA uptake sequences towards genome maintenance genes. Nucleic Acids Res. 2004;32(3):1050–8. doi: 10.1093/nar/gkh255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hamilton HL, Dillard JP. Natural transformation of Neisseria gonorrhoeae: from DNA donation to homologous recombination. Mol Microbiol. 2006 Jan;59(2):376–85. doi: 10.1111/j.1365-2958.2005.04964.x. *Excellent reveiw of this interesting aspect of neisseria genetics and biology.
- 139.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Eighteenth Informational Supplement. CLSI document M100-S18. 2008 January; [Google Scholar]
- 140.Tapsall J. Antimicrobial resistance in Neisseria gonorrhoeae. Sydney, Australia: WHO Collaborating Centre for STD and HIV; 2001. Report No.: WHO/CDS/CSR/DRS/2001.3. **Complete review and guidance for public health programs and laboratories worldwide.
- 141.Ison CA, Martin IM, Lowndes CM, Fenton KA. Comparability of laboratory diagnosis and antimicrobial susceptibility testing of Neisseria gonorrhoeae from reference laboratories in Western Europe. J Antimicrob Chemother. 2006 Sep;58(3):580–6. doi: 10.1093/jac/dkl264. [DOI] [PubMed] [Google Scholar]
- 142.Whiley DM, Limnios EA, Ray S, Sloots TP, Tapsall JW. Diversity of penA alterations and subtypes in Neisseria gonorrhoeae strains from Sydney, Australia, that are less susceptible to ceftriaxone. Antimicrob Agents Chemother. 2007 Sep;51(9):3111–6. doi: 10.1128/AAC.00306-07. *Very well carried-out report questioning significance of the mosaic PBP2 in conferring cephalosporin resistance.
- 143.Lindberg R, Fredlund H, Nicholas R, Unemo M. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrob Agents Chemother. 2007 Jun;51(6):2117–22. doi: 10.1128/AAC.01604-06. *Complete analysis of multiple mutations among recent cephem resistant isolates.
- 144.Dougherty TJ, Koller AE, Tomasz A. Penicillin-binding proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980 Nov;18(5):730–7. doi: 10.1128/aac.18.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brannigan JA, Tirodimos IA, Zhang QY, Dowson CG, Spratt BG. Insertion of an extra amino acid is the main cause of the low affinity of penicillin-binding protein 2 in penicillin-resistant strains of Neisseria gonorrhoeae. Mol Microbiol. 1990 Jun;4(6):913–9. doi: 10.1111/j.1365-2958.1990.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 146.Dowson CG, Jephcott AE, Gough KR, Spratt BG. Penicillin-binding protein 2 genes of non-beta-lactamase-producing, penicillin-resistant strains of Neisseria gonorrhoeae. Mol Microbiol. 1989 Jan;3(1):35–41. doi: 10.1111/j.1365-2958.1989.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 147.Spratt BG. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature. 1988 Mar 10;332(6160):173–6. doi: 10.1038/332173a0. [DOI] [PubMed] [Google Scholar]
- 148.Spratt BG, Zhang QY, Jones DM, Hutchison A, Brannigan JA, Dowson CG. Recruitment of a penicillin-binding protein gene from Neisseria flavescens during the emergence of penicillin resistance in Neisseria meningitidis. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8988–92. doi: 10.1073/pnas.86.22.8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nicholas RA, Zhao S, Tomberg J, Unemo M, Davies C. Genetics of intermediate resistance to expanded-spectrum cephalosporins in Neisseria gonorrhoeae, Abstract P054. 16th International Pathogenic Neisseria Conference; 2008 September; Rotterdam, The Netherlands. 2008. p. 133. Available at: http://www.ipnc2008.org/ [Google Scholar]
- 150.Ochiai S, Sekiguchi S, Hayashi A, Shimadzu M, Ishiko H, Matsushima-Nishiwaki R, et al. Decreased affinity of mosaic-structure recombinant penicillin-binding protein 2 for oral cephalosporins in Neisseria gonorrhoeae. J Antimicrob Chemother. 2007 Jul;60(1):54–60. doi: 10.1093/jac/dkm166. [DOI] [PubMed] [Google Scholar]
- 151.Whiley DM, Limnios EA, Ray S, Sloots TP, Tapsall JW. Further questions regarding the role of mosaic penA sequences in conferring reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2007 Feb;51(2):802–3. doi: 10.1128/AAC.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gill MJ, Simjee S, Al-Hattawi K, Robertson BD, Easmon CS, Ison CA. Gonococcal resistance to beta-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob Agents Chemother. 1998 Nov;42(11):2799–803. doi: 10.1128/aac.42.11.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nordmann P, Mammeri H. Extended-spectrum cephalosporinases: structure, detection and epidemiology. Future Microbiol. 2007 Jun;2:297–307. doi: 10.2217/17460913.2.3.297. [DOI] [PubMed] [Google Scholar]
- 154.Ito M, Deguchi T, Mizutani KS, Yasuda M, Yokoi S, Ito S, et al. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in Central Japan. Antimicrob Agents Chemother. 2005 Jan;49(1):137–43. doi: 10.1128/AAC.49.1.137-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Siedner MJ, Pandori M, Castro L, Barry P, Whittington WL, Liska S, et al. Real-time PCR assay for detection of quinolone-resistant Neisseria gonorrhoeae in urine samples. J Clin Microbiol. 2007 Apr;45(4):1250–4. doi: 10.1128/JCM.01909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li Z, Yokoi S, Kawamura Y, Maeda S, Ezaki T, Deguchi T. Rapid detection of quinolone resistance-associated gyrA mutations in Neisseria gonorrhoeae with a LightCycler. J Infect Chemother. 2002 Jun;8(2):145–50. doi: 10.1007/s101560200025. [DOI] [PubMed] [Google Scholar]
- 157.Lukehart SA, Godornes C, Molini BJ, Sonnett P, Hopkins S, Mulcahy F, et al. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med. 2004 Jul 8;351(2):154–8. doi: 10.1056/NEJMoa040216. [DOI] [PubMed] [Google Scholar]
- 158.Ochiai S, Ishiko H, Yasuda M, Deguchi T. Rapid detection of the mosaic structure of the Neisseria gonorrhoeae penA Gene, which is associated with decreased susceptibilities to oral cephalosporins. J Clin Microbiol. 2008 May;46(5):1804–10. doi: 10.1128/JCM.01800-07. *Real time molecular assay for mosaic penA gene.
- 159.Whiley D, Bates J, Limnios A, Nissen MD, Tapsall J, Sloots TP. Use of a novel screening PCR indicates presence of Neisseria gonorrhoeae isolates with a mosaic penA gene sequence in Australia. Pathology. 2007 Aug;39(4):445–6. doi: 10.1080/00313020701444515. *Real time molecular assay for mosaic penA gene.
- 160.Deguchi T, Yasuda M, Nakano M, Ozeki S, Ezaki T, Saito I, et al. Quinolone-resistant Neisseria gonorrhoeae: correlation of alterations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV with antimicrobial susceptibility profiles. Antimicrob Agents Chemother. 1996 Apr;40(4):1020–3. doi: 10.1128/aac.40.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Roberts MC, DeMaster L, Soge OO, Whittington WLH. Characterization of High-level Multidrug Resistant Neisseria gonorrhoeae Associated with Therapy Failure. 17th Biennial Meeting of the International Society for Sexually Transmitted Disease Research; 2007; Seattle. 2007. [Google Scholar]
- 162.Palmer HM, Young H, Winter A, Dave J. Emergence and spread of azithromycin-resistant Neisseria gonorrhoeae in Scotland. J Antimicrob Chemother. 2008 Sep;62(3):490–4. doi: 10.1093/jac/dkn235. [DOI] [PubMed] [Google Scholar]
- 163.Alcala B, Arreaza L, Salcedo C, Antolin I, Borrell N, Cacho J, et al. Molecular characterization of ciprofloxacin resistance of gonococcal strains in Spain. Sex Transm Dis. 2003 May;30(5):395–8. doi: 10.1097/00007435-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 164.Holloway WJ. Spectinomycin. Med Clin North Am. 1982 Jan;66(1):169–73. [PubMed] [Google Scholar]
- 165.Judson FN, Ehret JM, Handsfield HH. Comparative study of ceftriaxone and spectinomycin for treatment of pharyngeal and anorectal gonorrhea. Jama. 1985 Mar 8;253(10):1417–9. [PubMed] [Google Scholar]
- 166.Boslego JW, Tramont EC, Takafuji ET, Diniega BM, Mitchell BS, Small JW, et al. Effect of spectinomycin use on the prevalence of spectinomycin-resistant and of penicillinase-producing Neisseria gonorrhoeae. N Engl J Med. 1987 Jul 30;317(5):272–8. doi: 10.1056/NEJM198707303170504. [DOI] [PubMed] [Google Scholar]
- 167.Govender S, Lebani T, Nell R. Antibiotic susceptibility patterns of Neisseria gonorrhoeae isolates in Port Elizabeth. S Afr Med J. 2006 Mar;96(3):225–6. [PubMed] [Google Scholar]
- 168.Su X, Hutapea N, Tapsall JW, Lind I. Plasmid-mediated resistance of Neisseria gonorrhoeae strains isolated from female sex workers in North Sumatra, Indonesia, 1996. Sex Transm Dis. 2003 Feb;30(2):178–82. doi: 10.1097/00007435-200302000-00016. [DOI] [PubMed] [Google Scholar]
- 169.Van Dyck E, Karita E, Abdellati S, Dirk VH, Ngabonziza M, Lafort Y, et al. Antimicrobial susceptibilities of Neisseria gonorrhoeae in Kigali, Rwanda, and trends of resistance between 1986 and 2000. Sex Transm Dis. 2001 Sep;28(9):539–45. doi: 10.1097/00007435-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 170.Daly CC, Hoffman I, Hobbs M, Maida M, Zimba D, Davis R, et al. Development of an antimicrobial susceptibility surveillance system for Neisseria gonorrhoeae in Malawi: comparison of methods. J Clin Microbiol. 1997 Nov;35(11):2985–8. doi: 10.1128/jcm.35.11.2985-2988.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sutrisna A, Soebjakto O, Wignall FS, Kaul S, Limnios EA, Ray S, et al. Increasing resistance to ciprofloxacin and other antibiotics in Neisseria gonorrhoeae from East Java and Papua, Indonesia, in 2004 - implications for treatment. Int J STD AIDS. 2006 Dec;17(12):810–2. doi: 10.1258/095646206779307595. [DOI] [PubMed] [Google Scholar]
- 172.Livermore DM, Alexander S, Marsden B, James D, Warner M, Rudd E, et al. Activity of ertapenem against Neisseria gonorrhoeae. J Antimicrob Chemother. 2004 Jul;54(1):280–1. doi: 10.1093/jac/dkh272. [DOI] [PubMed] [Google Scholar]
- 173.Deshpande LM, Gales AC, Jones RN. GAR-936 (9-t-butylglycylamido-minocycline) susceptibility test development for streptococci, Haemophilus influenzae and Neisseria gonorrhoeae: preliminary guidelines and interpretive criteria. Int J Antimicrob Agents. 2001 Jul;18(1):29–35. doi: 10.1016/s0924-8579(01)00355-7. [DOI] [PubMed] [Google Scholar]
- 174.Barry PM, Lenderman C, Melendez J, Whittington W, Hook E, Zenilman J, et al. In vitro Activity of Ceftaroline Against Recent US Isolates of Neisseria gonorrhoeae, Poster C1-163. 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) and the Infectious Diseases Society of America (IDSA) 46th Annual Meeting; Washington, DC. 2008. [Google Scholar]
- 175.Moran JS. Treating uncomplicated Neisseria gonorrhoeae infections: is the anatomic site of infection important? Sex Transm Dis. 1995 Jan-Feb;22(1):39–47. doi: 10.1097/00007435-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 176.Manavi K, Young H, McMillan A. The outcome of oropharyngeal gonorrhoea treatment with different regimens. Int J STD AIDS. 2005 Jan;16(1):68–70. doi: 10.1258/0956462052932566. [DOI] [PubMed] [Google Scholar]
- 177.Morris SR, Klausner JD, Buchbinder SP, Wheeler SL, Koblin B, Coates T, et al. Prevalence and incidence of pharyngeal gonorrhea in a longitudinal sample of men who have sex with men: the EXPLORE study. Clin Infect Dis. 2006 Nov 15;43(10):1284–9. doi: 10.1086/508460. [DOI] [PubMed] [Google Scholar]
- 178.Sathia L, Ellis B, Phillip S, Winston A, Smith A. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007 Sep;18(9):647–8. doi: 10.1258/095646207781568556. [DOI] [PubMed] [Google Scholar]
- 179.Matsumoto T, Muratani T, Takahashi K, Ikuyama T, Yokoo D, Ando Y, et al. Multiple doses of cefodizime are necessary for the treatment of Neisseria gonorrhoeae pharyngeal infection. J Infect Chemother. 2006 Jun;12(3):145–7. doi: 10.1007/s10156-006-0444-0. [DOI] [PubMed] [Google Scholar]
- 180.Schrag SJ, Zell ER, Schuchat A, Whitney CG. Sentinel surveillance: a reliable way to track antibiotic resistance in communities? Emerg Infect Dis. 2002 May;8(5):496–502. doi: 10.3201/eid0805.010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tapsall J. Antibiotic resistance in Neisseria gonorrhoeae is diminishing available treatment options for gonorrhea: some possible remedies. Expert Rev Anti Infect Ther. 2006 Aug;4(4):619–28. doi: 10.1586/14787210.4.4.619. [DOI] [PubMed] [Google Scholar]
- 182.Tapsall JW. What management is there for gonorrhea in the postquinolone era? Sex Transm Dis. 2006 Jan;33(1):8–10. doi: 10.1097/01.olq.0000194599.97426.a3. [DOI] [PubMed] [Google Scholar]
- 183.World Health Organization. Fifty-Ninth World Health Assembly Provisional agenda item 11.6: Prevention and control of sexually transmitted infections: draft global strategy. 2006 May 18; Available at: http://www.who.int/gb/ebwha/pdf_files/WHA59/A59_11-en.pdf.
- 184.Simonsen GS, Tapsall JW, Allegranzi B, Talbot EA, Lazzari S. The antimicrobial resistance containment and surveillance approach--a public health tool. Bull World Health Organ. 2004 Dec;82(12):928–34. [PMC free article] [PubMed] [Google Scholar]
- 185.Ropp PA, Hu M, Olesky M, Nicholas RA. Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2002 Mar;46(3):769–77. doi: 10.1128/AAC.46.3.769-777.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology. 1995 Mar;141(Pt 3):611–22. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- 187.Warner DM, Folster JP, Shafer WM, Jerse AE. Regulation of the MtrC-MtrD-MtrE efflux-pump system modulates the in vivo fitness of Neisseria gonorrhoeae. J Infect Dis. 2007 Dec 15;196(12):1804–12. doi: 10.1086/522964. [DOI] [PubMed] [Google Scholar]


