Abstract
Introduction
While epilepsy is a well-characterized disease, the majority of emergency department (ED) visits for “seizure” involve patients without known epilepsy. The epidemiology of seizure presentations and national patterns of management are unclear. The aim of this investigation was to characterize ED visits for seizure in a large representative US sample and investigate any potential impact of race or ethnicity on management.
Methods
Seizure visits from the National Hospital Ambulatory Medical Care Survey (NHAMCS) from 1993 to 2003 were analysed. Demographic factors associated with presentation, neuroimaging and hospital admission in the USA were analysed using controlled multivariate logistic regression.
Results
Seizure accounts for 1 million ED visits annually [95% confidence interval (CI): 926,000–1,040,000], or 1% of all ED visits in the USA. Visits were most common among infants, at 8.0 per 1,000 population (95% CI: 6.0–10.0), and children aged 1–5 years (7.4; 95% CI: 6.4–8.4). Seizure was more likely among those with alcohol-related visits [odds ratio (OR): 3.2; 95% CI: 2.7–3.9], males (OR: 1.4; 95% CI: 1.3–1.5) and Blacks (OR: 1.4; 95% CI: 1.3–1.6). Neuroimaging was used less in Blacks than Whites (OR: 0.6; 95% CI: 0.4–0.8) and less in Hispanics than non-Hispanics (OR: 0.6; 95% CI: 0.4–0.9). Neuroimaging was used less among patients with Medicare (OR: 0.4; 95% CI: 0.2–0.6) or Medicaid (OR: 0.5; 95% CI: 0.4–0.7) vs private insurance and less in proprietary hospitals. Hospital admission was less likely for Blacks vs Whites (OR: 0.6; 95% CI: 0.4–0.8).
Conclusion
Seizures account for 1% of ED visits (1 million annually). Seizure accounts for higher proportions of ED visits among infants and toddlers, males and Blacks. Racial/ethnic disparities in neuroimaging and hospital admission merit further investigation.
Keywords: Seizures, Emergencies, Emergency treatment, Ethnic groups, Vulnerable populations, Racial/ethnic disparities, Neuroimaging
Introduction
Although seizures are very common, with 11% of all people having a seizure at some point in life, epilepsy occurs in only 3% of the population [6, 11]. Thus, most people who have a seizure do not have epilepsy, but rather symptomatic seizures, defined as those caused by well-defined acute insults. These patients are often cared for by non-neurologists, in diverse settings including emergency departments (EDs) and hospital wards.
The epidemiology of epilepsy is well developed, but the epidemiology of acute symptomatic seizures is less so [4]. Most knowledge on the occurrence of seizures in the emergency department (ED) setting is based on small studies, with only two samples of more than 1,000 patients published, these having limited generalizability [12, 20].
Descriptive epidemiology is important for at least three reasons. First, knowledge of health care utilization is essential for planning and management. Second, descriptive studies identify patient characteristics that constitute possible risk factors for disease, which can then be examined in prospective studies. Finally, recent decades have seen the emergence of literature documenting racial/ethnic disparities in care, and descriptive studies are crucial to identify and monitor such disparities [21, 27]. Disparities are known to exist in the treatment of epilepsy, but little is known about racial/ethnic determinants of care for acute symptomatic seizures [28].
This investigation was designed to: (1) define the incidence and epidemiology of seizure presentations to US EDs, in order to provide the basic descriptive information that is necessary for understanding health care utilization and planning future studies; (2) describe demographic features associated with presentation and disposition; and (3) examine whether ED care may be influenced by race, ethnicity or insurance status.
In order to develop a broadly representative sample, we combined 11 years of National Hospital Ambulatory Medical Care Survey (NHAMCS) data, from 1993 to 2003 [18]. Based on information from small regional studies, we hypothesized that there would be increased rates of seizure among children in the febrile seizure age range of 6 months to 6 years, males, and possibly Black patients [4, 13, 22, 24, 25, 29, 30]. In addition, we hypothesized that alcohol use and insurance status would be associated with ED utilization for seizures, and that likelihood of neuroimaging and hospital admission would differ according to race, ethnicity and insurance status [20].
Methods
NHAMCS is a systematic sample of visits to EDs of general and short stay hospitals, excluding federal, military and Veterans Affairs hospitals in the 50 United States and the District of Columbia. Its methods have been described in detail, including the reciprocal inflation procedures used to generate national estimates from the sample [17, 18]. Trained staff collect data using standardized forms during randomly assigned 4-week periods. All collected forms are sent to the Constella Group (Durham, NC, USA) and coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Medications are coded according to the National Drug Code Database and include those administered in the ED or prescribed at discharge [16].
Certain core variables are included in data collection every year (such as demographic characteristics, up to three diagnoses per visit and insurance status). Other variables are collected only during some of the study years, including diagnostic and surgical procedures including lumbar puncture and electroencephalography. The present investigation constitutes a retrospective analysis of this multi-year database, without opportunity to influence what variables were collected. Of note, tracking of chronic conditions such as epilepsy is not within the scope of the NHAMCS as information from the past medical history is not included in the data form. It is not possible to quantify the proportion of ED seizure patients who had pre-existing epilepsy using our data source; nor could we differentiate a first-time seizure from a recurrent seizure.
We assessed the frequency of visits for seizures by identifying visits with ICD-9 codes 345 (epileptic convulsions), 780.3 [convulsions: not otherwise specified (NOS), febrile, or infantile; fit NOS] and 779.0 (convulsions in newborn) in any one of the three diagnosis fields. The NHAMCS database does not differentiate patients whose seizures had resolved upon ED arrival from patients who continued to seize in the ED. US visit rates were computed using mid-year age, sex, race, ethnicity, region and metropolitan status-specific population estimates from the US Census Bureau; all rates were reported per 1,000 individuals in the US resident population per year [1].
We analysed relationships between seizure visit rates, sociodemographic variables, diagnoses other than seizure, performance of neuroimaging, medications prescribed and disposition. We included the following variables in multivariate analyses: urgency at triage, age, sex, race, Hispanic ethnicity, insurance status, metropolitan statistical area (MSA) status of the hospital (i.e. metropolitan vs not metropolitan), hospital ownership category, region (Northeast, Midwest, South and West), presence of a “high-risk” co-diagnosis, whether neuroimaging was performed and ED disposition.
Regarding the coding of urgency at triage, from 1993 to 1996, visits were coded as urgent at triage yes/no. This variable was not collected between 1997 and 2003. To keep analyses between earlier and later years consistent, we coded visits that occurred after a change in coding in 1997 (1997–2003) as “urgent/emergent” if immediacy to be seen was recorded as “less than 15 min” or “15–60 min” and as “non-urgent” if recorded as “> 1–2 h or longer”.
Regarding neuroimaging, our analyses focused on whether neuroimaging was performed at all. Data on computed tomography (CT) scanning were collected alone for years 1995–2000 and magnetic resonance imaging (MRI) alone from 1995 to 2000. Beginning in 2001 CT and MRI were collected as “CT or MRI” and no longer collected separately. We created a new variable for use of either MRI or CT using years 1995–2003 and only analysed 1995–2003 data when evaluating neuroimaging. Thus the fact that these data were included differently in the different years of study had the effect of lowering power for analyses of neuroimaging, but would not be expected to introduce bias.
For the medication analysis, up to five medications were recorded during 1993–1994, up to six between 1995 and 2002 and up to eight for 2003, with medications coded as per published National Center for Health Statistics (NCHS) definitions [16]. Medications recorded included medications administered in the ED or prescribed upon discharge.
To evaluate other diagnoses recorded among patients with seizure (“co-diagnoses”), we focused on those that were seen in ten or more of the visits in our sample. Using ICD-9 codes, we identified 58 such diagnoses. For the purpose of analytic control in our examination of demographic predictors of making a visit for seizure, receiving neuroimaging services and being admitted to the hospital, we created a binary variable to differentiate cases with and without a “high-risk” co-diagnosis. We designated a co-diagnosis “high risk” if, in our judgment, it would tend to change ED management or warrant hospital admission. Thus we categorized the following co-diagnoses as “high risk”: alcohol withdrawal, pneumonia, cerebrovascular disease, hypoglycaemia, septicaemia, hypokalaemia, transient ischaemic attack (TIA), hyponatraemia, brain neoplasm and intracranial injury. We identified alcohol-related visits by ICD-9 codes and by a check box indicating a role for alcohol, present during some of the years of study.
We performed all analyses using STATA 9.0 (StataCorp, College Station, TX, USA). Information from a masked sample design was used to estimate variance and calculate 95% confidence intervals (CI). Associations were examined using Pearson’s chi-square. Linear regression was used for trend analysis and multivariate logistic regression was used to examine factors associated with seizure visit, neuroimaging and hospital admission. Multivariate models were built, and potential confounding variables chosen, based on a priori hypotheses, with one exception: in contemplating our observation of racial and ethnic disparities in neuroimaging and hospital admission, we recognized that not only “high-risk” diagnoses but also “low-risk” diagnoses could have confounded our findings. In particular, if Black and Hispanic children were more likely than other groups to visit the ED after a febrile seizure, the likelihood of neuroimaging and hospital admission would appear lower in these groups. We were not overly concerned about this possibility because our models control for insurance status. Nonetheless, to be safe, in a post hoc analysis, we created a variable to capture “low-risk” diagnoses, chosen from among the diagnoses seen in > 30 cases. A patient was noted to have a “low-risk” diagnosis if s/he had otitis media, acute respiratory infection or urinary tract infection. Addition of this variable to the models resulted in no change, and therefore we did not include it in the final models.
All p values were two-tailed, with p < 0.05 considered statistically significant. If the relative standard error for the NHAMCS data was greater than 30%, or if estimates were based on fewer than 30 cases, confidence intervals were not reported, since the estimates would not be robust. Our human subjects research committee exempted the study from review.
Results
From 1993 through 2003, there were a total of 1.1 billion visits to US EDs, for an annual average of 100.7 million visits per year. Seizure was present at 1.0% (95% CI: 0.9–1.1%) of these visits, an average of 1 million per year (95% CI: 926,000–1,104,000), with no change in seizure frequency over the 11-year study period (p for trend 0.20). These estimates are based on a sample of 341,000 ED visits for all ages, of which 3,215 involved seizure. On a population basis, there were 3.7 (95% CI: 3.4–4.0) ED visits for seizure per 1,000 US residents per year.
For children (age < 18), there were 293 million total visits to US EDs, for an annual average of 27 million per year. Of these, 3.1 million visits involved seizure (95% CI: 2.7–3.5 million) which corresponded to 1.0% (95% CI: 0.9–1.2) of all ED visits for children. On a population basis, there were 4 ED visits (95% CI: 3.5–4.4) for seizure per 1,000 US residents aged < 18 per year.
Table 1 provides the distribution of ED seizure visits by patient and hospital characteristics. Frequency of seizure visits did not differ significantly across any time of day, day of the week, season or year of visit (data not shown).
Table 1.
Distribution of emergency department seizure visits in the USA by patient and hospital characteristics (1993–2003)
| Group | Sample (n) | Cumulative estimate in thousands (95% CI) | Annual average rate per 1,000 US population (95% CI) | Annual average rate per 1,000 ED visits (95% CI) |
|---|---|---|---|---|
| Overall | 3,215 | 11,200 (10,200–12,100) | 3.7 (3.4–4.0) | 10.1 (9.2–10.9) |
| Age (years) | ||||
| < 1 | 106 | 348 (260–437) | 8.0 (6.0–10.1) | 8.5 (6.3–10.6) |
| 1–5 | 450 | 1,613 (1,402–1,824) | 7.4 (6.4–8.4) | 15.1 (13.1–17.0) |
| 6–10 | 121 | 456 (338–575) | 2.1 (1.5–2.6) | 8.1 (6.0–10.2) |
| 11–15 | 101 | 374 (277–472) | 1.7 (1.3–2.2) | 6.5 (4.8–8.2) |
| 16–20 | 197 | 775 (606–944) | 3.6 (2.8–4.4) | 8.9 (7.0–10.8) |
| 21–30 | 436 | 1,497 (1,266–1,727) | 3.6 (3.0–4.1) | 8.1 (6.9–9.4) |
| 31–40 | 552 | 1,880 (1,653–2,107) | 3.9 (3.4–4.4) | 11.1 (9.7–12.4) |
| 41–50 | 564 | 1,848 (1,607–2,090) | 4.2 (3.7–4.8) | 14.2 (12.4–16.1) |
| 51–60 | 249 | 846 (699–994) | 2.8 (2.3–3.3) | 10.1 (8.3–11.8) |
| 61–70 | 177 | 610 (479–741) | 2.8 (2.2–3.4) | 9.1 (7.2–11.1) |
| 71–80 | 155 | 526 (417–634) | 3.2 (2.5–3.8) | 7.6 (6.1–9.2) |
| 81+ | 107 | 393 (266–520) | 0.4 (0.3–0.5) | 7.1 (4.8–9.4) |
| < 18 | 850 | 3,095 (2,726–3,463) | 4.0 (3.5–4.4) | 10.6 (9.3–11.8) |
| ≥ 18 | 2,365 | 8,073 (7,325–8,821) | 3.6 (3.3–3.9) | 9.9 (9.0–10.8) |
| Sex | ||||
| Female | 1,395 | 4,844 (4,358–5,331) | 3.2 (2.8–3.5) | 8.3 (7.4–9.1) |
| Male | 1,820 | 6,323 (5,731–6,915) | 4.3 (3.9–4.7) | 12.1 (11.0–13.2) |
| Race | ||||
| White | 2,186 | 7,827 (7,055–8,600) | 3.2 (2.9–3.5) | 9.3 (8.3–10.2) |
| Black | 910 | 2,995 (2,586–3,403) | 7.9 (6.8–8.9) | 12.9 (11.1–14.6) |
| Othera | 119 | 345 (260–431) | 2.2 (1.6–2.7) | 11.6 (8.7–14.5) |
| Ethnicityb | ||||
| Hispanic | 348 | 1,085 (871–1,299) | 3.1 (2.5–3.7) | 11.4 (9.2–13.7) |
| Non-Hispanic | 2,381 | 8,282 (7,503–9,062) | 3.1 (2.8–3.4) | 11.8 (10.7–12.9) |
| Urban status | ||||
| MSA | 2,844 | 9,231 (8,249–10,200) | 3.8 (3.4–4.2) | 10.6 (9.5–11.8) |
| Non-MSA | 371 | 1,937 (1,296–2,577) | 3.1 (2.1–4.2) | 8.1 (5.4–10.7) |
| US region | ||||
| Northeast | 801 | 2,193 (1,847–2,538) | 3.8 (3.2–4.4) | 10.1 (8.5–11.6) |
| Midwest | 638 | 2,619 (2,132–3,106) | 3.8 (3.1–4.5) | 9.3 (7.6–11.0) |
| South | 1,076 | 4,145 (3,478–4,811) | 3.9 (3.3–4.5) | 10.1 (8.5–11.8) |
| West | 700 | 2,211 (1,818–2,604) | 3.3 (2.7–3.9) | 11.1 (9.1–13.1) |
ED emergency department, CI confidence interval, MSA metropolitan statistical area
a“Other race” includes Asian, Pacific Islander, American Indian or more than one race was reported
b19% of sample missing ethnicity data
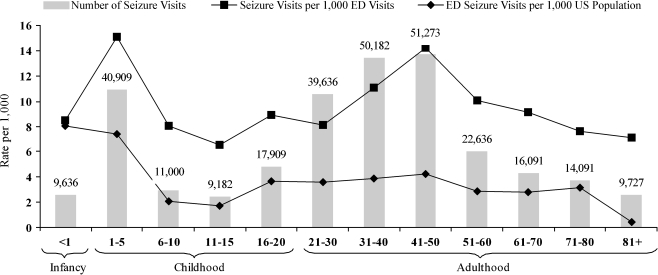
Figure 1 depicts the age dependence of ED utilization for seizure, showing annual estimates based on our 11 years of data. It was necessary to stratify children as shown because the epidemiology of the febrile seizure is an important sub-topic. However, we could not create an age stratum for 6 months to 6 years (corresponding to the defining ages of the febrile seizure), because monthly age data were not usable.
Fig. 1.
Annual population rates for US emergency department visits for seizure (1993–2003)
On a population basis, ED visits for seizure were significantly more common among infants and children aged 1–5 years (approximating the age of the febrile seizure) and the 41–50 year group than among other age groups. Adolescents and the elderly made the fewest visits for seizure on a population basis.
Upon observing the unexpectedly high rate among those aged 41–50, we sought to determine whether alcohol played a role in determining the proportion of ED visits with seizure, by excluding all visits with mention of alcohol (6.4% of all visits). In the 41–50 age group, as a result of this adjustment, the proportion decreased from 1.4 to 1.2% (95% CI: 1.0–1.4%).
Table 1 shows results by gender, race and insurance status. In a multivariate logistic regression (controlling for age, ethnicity, region, metropolitan status and insurance status), Table 3 factors significantly associated with a higher proportion of seizure visits among all ED visits included male gender [odds ratio (OR): 1.4], Black race (OR: 1.4) and alcohol-related visit (OR: 3.2).
Table 3.
Controlled multivariate analysis to predict neuroimaging and hospital admission among emergency department patients with seizure (1993–2003)
| Group | Likelihood of neuroimaging | Likelihood of hospital admission |
|---|---|---|
| Odds ratio (95% CI) | Odds ratio (95% CI) | |
| Age (per decade) | 1.2 (1.2–1.3)a | 1.2 (1.1–1.3)a |
| Sex | ||
| Male (vs female) | 1.0 (0.8–1.4) | 1.0 (0.8–1.3) |
| Race | ||
| Black (vs White) | 0.6 (0.4–0.8)a | 0.6 (0.4–0.8)a |
| Other (vs White) | 0.7 (0.3–1.7) | 1.3 (0.5–3.1) |
| Ethnicity | ||
| Hispanic (vs non-Hispanic) | 0.6 (0.4–0.9)a | 1.4 (0.8–2.3) |
| Urban status | ||
| MSA (vs non-MSA) | 2.0 (1.2–3.1)a | 1.3 (0.8–2.0) |
| US region | ||
| Northeast | 1.0 | 1.0 |
| Midwest | 1.9 (1.2–2.8)a | 1.1 (0.7–1.8) |
| South | 2.0 (1.3–3.0)a | 0.9 (0.6–1.4) |
| West | 1.9 (1.2–2.9)a | 0.5 (0.3–0.8)a |
| Hospital ownership | ||
| Voluntary (non-profit) | 1.0 | 1.0 |
| Government non-federal | 0.8 (0.5–1.2) | 0.9 (0.5–1.4) |
| Proprietary | 0.6 (0.4–0.97)a | 0.8 (0.5–1.3) |
| Insurance status | ||
| Private insurance | 1.0 | 1.0 |
| Medicare | 0.4 (0.2–0.6)a | 1.3 (0.8–2.2) |
| Medicaid | 0.5 (0.4–0.7)a | 1.0 (0.7–1.6) |
| Self-pay | 0.9 (0.6–1.4) | 0.7 (0.4–1.1) |
| Other insurance | 0.5 (0.3–0.9)a | 0.8 (0.4–1.4) |
| Urgency at triage | 1.7 (1.0–2.9)a | 2.3 (1.3–3.9)a |
| Alcohol-related visit | 0.7 (0.4–1.3) | 1.5 (0.8–2.9) |
| High-risk co-diagnosis | 1.8 (1.0–3.1)a | 2.5 (1.4–4.5)a |
| CT or MRI | Outcome variable | 3.2 (2.3–4.4)a |
| Hospital admission | 3.1 (2.2–4.4)a | Outcome variable |
MSA metropolitan statistical area, CT computed tomography, MRI magnetic resonance imaging
aStatistically significant at α = 0.05
At least one co-diagnosis was noted in 66% of cases. There were over 500 different co-diagnoses, and each of these tended to be noted in only a few cases, prohibiting estimation of national totals. Only 9 co-diagnoses were reported in 30 or more cases; see Table 2. Among these, alcohol-related issues were most common. Otitis media was second most common, perhaps as a cause of febrile seizures. “High risk” associated diagnoses (as defined in the “Methods” section) were present in 7.1%; the most prevalent of these was pneumonia, seen in 1.2% of all ED seizure visits. Injuries were less commonly seen in patients with seizures than in non-seizure patients (17.9 vs 34.7%, p < 0.001). Meningitis and tricyclic antidepressant poisoning were seen in too few cases for robust national estimates. Blood glucose checks were documented in 31% of visits, but results from glucose analysis in the prehospital and laboratory settings were not recorded.
Table 2.
Co-diagnoses, diagnostic tests and medications among US emergency department patients with seizure (1993–2003)
| Group | Sample (n) | % (95% confidence interval) |
|---|---|---|
| Co-diagnoses | ||
| Alcohol-related | 224 | 6.4 (5.3–7.6) |
| Otitis media (unspecified) | 103 | 3.4 (2.6–4.3) |
| Hypertension (unspecified) | 59 | 1.4 (0.9–1.8) |
| Acute respiratory infection | 38 | 1.4 (0.9–1.9) |
| Pneumonia | 35 | 1.2 (0.7–1.7) |
| Urinary tract infection | 35 | 1.2 (0.7–1.7) |
| Cerebrovascular disease | 32 | 0.8 (0.4–1.2) |
| All high-risk co-diagnosesa | 217 | 7.1 (5.9–8.4) |
| Diagnostic tests | ||
| Neuroimaging (CT or MRI)b | 626 | 24.2 (22.1–26.3) |
| Lumbar puncturec | 44 | 2.3 (1.5–3.1) |
| EEG | 51 | 4.0 (2.4–5.5) |
| Glucose screenc | 402 | 31.3 (27.4–35.3) |
| Top ten medications | ||
| Phenytoin | 1003 | 29.9 (27.8–32.0) |
| Acetaminophen | 435 | 13.0 (11.5–14.6) |
| Lorazepam | 351 | 10.5 (9.1–11.8) |
| Ibuprofen | 252 | 7.9 (6.6–9.1) |
| Phenobarbital | 247 | 7.4 (6.1–8.7) |
| Carbamazepine | 244 | 7.8 (6.6–9.0) |
| Diazepam | 185 | 6.1 (5.0–7.1) |
| Divalproex sodium | 154 | 4.7 (3.7–5.7) |
| Ceftriaxone | 130 | 4.2 (3.2–5.2) |
| Thiamine | 101 | 2.8 (2.2–3.5) |
aIncludes alcohol withdrawal, pneumonia, cerebrovascular disease, hypoglycaemia, septicaemia, hypokalaemia, TIA, hyponatraemia, brain neoplasm and intracranial injury
bData not available for 1993–1994
cData not available for 2001–2003
At least one medication was noted at 78% of visits; Table 2 shows the ten most common. A benzodiazepine or a barbiturate was prescribed at 54.0% (95% CI: 51.8–56.3) of ED seizure visits. Endotracheal intubation was noted in 1.1% (95% CI: 0.6 - 1.7%) of ED seizure visits.
Neuroimaging (CT or MRI) was noted in 24% of seizure visits (Table 2). Table 3 gives the results of a multivariate logistic regression analysis designed to determine predictors of neuroimaging. The regression model controlled for age, sex, race, ethnicity, hospital ownership category, region, MSA status, payer, urgency at triage, alcohol, “high risk” associated diagnosis and hospital admission. Older age was a significant predictor of higher likelihood of neuroimaging, with each successive age category 1.2 times as likely to receive neuroimaging on average. Despite control for insurance status, Blacks were less likely to receive neuroimaging than Whites (OR: 0.6). Hispanics were less likely to receive neuroimaging than non-Hispanics (OR: 0.6). Relative to patients with private insurance, those with Medicare were less likely to receive neuroimaging (OR: 0.4), and those with Medicaid were also less likely to receive neuroimaging (OR: 0.5). Self-pay patients were no less likely to receive neuroimaging than those with private insurance. Visits by Medicare patients aged 65 or older had a relatively high rate of neuroimaging (34%), and those by Medicare patients aged < 65 had a relatively low rate (19%).
Regarding disposition, none of the patients with seizure in our sample died in the ED. Hospital admission was the endpoint of 23% of seizure visits, versus 13% for non-seizure visits (p < 0.001). Table 3 gives the results of a multivariate logistic regression analysis designed to determine predictors of hospital admission. The regression model controlled for age, sex, race, hospital ownership, ethnicity, hospital status, region, MSA status, payer, urgency at triage, alcohol, “high risk” associated diagnosis and whether neuroimaging was performed. Factors associated with increased likelihood of admission included older age, urgency at triage, “high-risk” diagnosis as defined above and having received neuroimaging. Blacks were less likely to be admitted than Whites (OR: 0.6). There was no relationship between hospital admission and Hispanic ethnicity or insurance status.
Discussion
We estimate that seizures account for 1 million ED visits per year, or 1% of all ED visits, in the US. This is consistent with the findings of three smaller studies that reported the frequency of seizures among general ED visits to be 0.7–1.2% [13, 14, 24]. In our study, among children, 1% of ED visits involved seizure. Only one prior study reported the proportion of children’s ED visits due to seizure (2%), but that study was done at a tertiary referral centre whose population is not representative of that seen in most EDs [26].
On a population basis, the rate of ED visits for seizure was 3.7 per 1,000 population per year. ED visits with seizure were most common among infants, at a rate of 8.0, and children aged 1–5 years, at 7.4. Possible explanations for this high rate of seizure visits include electrolyte abnormalities and febrile seizures [2, 8, 9, 23]. Another possibility is that some of the infants whose diagnosis was coded as seizure may actually have had other conditions, such as myoclonus or other unusual movements that worry parents. No prior study has reported the population rates of ED visits for seizure. One study examined the incidence of acute symptomatic seizures in an entire population, finding 39 seizures per 100,000 person-years of observation [4]. This is not comparable to our findings as it excluded all unprovoked seizures and all febrile seizures. Moreover, that study involved the population of Rochester, MN, one that may not be representative of that served by the nation’s EDs.
Our data revealed a peak in ED utilization for seizure among middle-aged patients. Seizure accounted for a significantly higher proportion of ED visits in this age group than in any other group except for children aged 1–5 years. This was not entirely explained by correlation with alcohol-related diagnoses. In contrast, the population-based study mentioned above found a steady increase in the population incidence of acute symptomatic seizures among adults, with no peak in middle age, from 15 per 100,000 patient-years of observation among those aged 25–34 to 44 among those aged 45–54 and 123 among those aged > 74 years [4]. This is likely because, when compared to the general population of middle-aged adults, those seen in the ED are more at risk for seizure due to influences such as alcohol abuse, trauma and chronic illness, and middle-aged adults may be less likely to use the ED for non-seizure problems than other age groups.
We confirmed our hypothesis that seizures account for a higher proportion of ED visits among males than among females. However, the disparity was not as pronounced as initially expected. Prior studies found that males accounted for nearly twice as many ED visits for seizures [4, 12, 13, 20, 24, 25, 29]. Our study found that males were 1.4 times as likely as females to have a seizure-related ED visit. This could indicate that females with seizure are more likely to seek ED care in general, or that the increased incidence among males is truly not as high as prior studies have suggested.
Visits were proportionally higher among Black patients than among Whites. This is a new finding, except for one prior study that noted slightly increased ED utilization for seizure among Blacks, but relied upon historical controls rather than population data (i.e. the US Census) for a description of the source population, and did not control for insurance status [20, 28]. As predicted, we found that the prominence of seizure among ED visits was higher among those with public insurance, relative to those with private insurance.
We observed racial and ethnic disparities in neuroimaging of ED patients with seizure. Neuroimaging was performed at 24% of all seizure visits, but Blacks and Hispanics were significantly less likely to receive neuroimaging than their White and non-Hispanic counterparts. This finding was robust to analytic control for age, gender, hospital ownership, insurance, region, urgency at triage, alcohol, “high risk” associated diagnosis and hospital admission. This is not likely to be solely a factor of differential rates of known seizure disorder, as only a minority of ED seizure visits are due to a known seizure disorder [12, 20]. For Hispanics, this pattern is the opposite of what has been found for neuroimaging in patients with cerebrovascular disease. One study showed higher utilization among Hispanics compared to Whites, possibly due to language barriers leading to reliance on testing in place of history [7]. The reasons for differential neuroimaging according to race/ethnicity may be different among patients with seizure versus cerebrovascular disease. As the NHAMCS database does not contain details of the presentation or physical exam, it is not possible to determine the “appropriate” rate of neuroimaging according to national guidelines in place at the time. In fact, while some suggest that neuroimaging may be overused [3, 9] the most recent expert guidelines offer few restrictions, finding that acute neuroimaging may be useful in adults or children with first seizure, with acquired immunodeficiency syndrome (AIDS), an abnormal neurologic exam, worrisome history or focal seizure onset [10, 15]. Therefore, our finding of less neuroimaging among minorities resonates with the findings of racial and ethnic disparities in diagnostic procedures in many prior studies [27] and highlights the need for future work to address potential disparities in health care delivery [28].
Interestingly, neuroimaging was substantially less common in proprietary hospitals than in non-profit and non-federal government hospitals. Whether this was due to differing patient characteristics or economic influences could not be ascertained in this study.
We observed an overall admission rate of 23%, identical to the rate seen in a prior multicentre study of 368 unselected seizure patients [13]. Our finding of a lower likelihood of hospital admission among Black ED patients with seizure is difficult to explain.
Racial and ethnic disparities in health care have been documented in a variety of settings, including in the outpatient management of epilepsy [28], but to our knowledge this is the first report of disparities in the emergency care of patients with seizure. The reason for the disparities we observed cannot be ascertained from our source data, but the adjustment for geographic region, hospital characteristics and insurance status rules out several factors often cited as potential explanations for racial/ethnic disparities [5]. Other possibilities include hospital-level variation in key variables such as ED crowding and availability of neuroimaging, socioeconomic or biological determinants of different aetiologies for seizure among different groups or subconscious bias on the part of health care providers [27, 31]. Increased use of the ED for low-acuity problems predisposing to febrile seizure, such as otitis media, is not a plausible explanation for our findings, because: (a) seizure is certainly not a “primary care” type of complaint and (b) inclusion of “any low-risk diagnosis” in our controlled models did not change the findings (see “Methods” section). If alcohol played a role in minorities more frequently than in Whites, both neuroimaging and hospital admission would have been indicated less frequently for minority patients. However, our analysis controlled for alcohol-related visits, and for socioeconomic marginalization as indicated by insurance status. Nonetheless, since this is a secondary data analysis, the robustness of such control cannot be trusted absolutely. Thus it remains possible that socioeconomically disadvantaged groups might have been more likely to use the ED for alcohol-related seizures.
Insurance status was a significant predictor of neuroimaging, but not hospital admission. Medicare and Medicaid patients were significantly less likely to have neuroimaging than patients with private insurance. Medicare patients likely had a low rate of neuroimaging because many patients have Medicare not due to old age, but due to chronic illness. A high rate of unprovoked seizures due to previously diagnosed seizure disorders would lead to many ED visits for seizure without neuroimaging in this group. Indeed, we found that Medicare patients below age 65 had a very low rate of neuroimaging (19%).
Our study has certain limitations. Because NHAMCS data only contain information about the ED visit itself, actions taken before arrival to the ED or after discharge or hospital admission are not available for analysis. The NHAMCS is not designed to collect information about chronic conditions, and thus we were unable to ascertain what proportion of our cases were “breakthrough seizures” occurring in patients who already had a diagnosis of epilepsy, versus acute symptomatic seizures, versus first-time unprovoked seizures. Repeat visits are unlikely to have been a major factor, as individual EDs were sampled only for 4-week periods. Nonetheless, the discrepancies in neuroimaging we observed should be verified in a study of identified cases to remove the unlikely possibility of confounding by repeat visits. Data on Hispanic ethnicity were missing in 19% of our sample. Finally, limitations in the use of ICD-9 coding have been described, and therefore, the accuracy of various co-diagnoses, including electrolyte disturbances and contribution of alcohol, is difficult to ascertain [19]. The NHAMCS database is useful for describing the demographic covariates of a straightforward diagnosis like seizure that is unlikely to go unmentioned in an ED chart, but inferences about causal relationships among seizure and other co-diagnoses would not be appropriate.
Conclusion
In a nationally representative sample of seizure visits to US EDs, we found that 1 million such visits occur each year, with an admission rate of 23%. ED utilization for seizure has a bimodal age distribution, with peaks in the first 5 years of life and in middle age, and is more common in males. Among seizure patients seen in US EDs, neuroimaging services are used more in patients with private insurance, and less among Blacks and Hispanics compared to their White and non-Hispanic counterparts. Hospital admission is also substantially less likely for Blacks than for Whites. These disparities were not explained by alcohol-related seizures in the present secondary data analysis, but future studies should examine this potential confounder. These observations were robust to analytic control for multiple potential confounders including age, sex, insurance status, geography and hospital ownership.
Biography
Dr. Pallin is the Director of Clinical Research of the Department of Emergency Medicine at Brigham and Women’s Hospital. He is also an attending physician in the Division of Emergency Medicine of Children’s Hospital Boston, and an Assistant Professor of Medicine and Pediatrics at Harvard Medical School.
Footnotes
The views expressed in this paper are those of the author(s) and not those of the editors, editorial board or publisher.
References
- 1.National Center for Health Statistics. Bridged-Race Estimates, available at: http://wonder.cdc.gov/population.html. Accessed 5 Nov 2005
- 2.Alam NH, Yunus M, Faruque AS, et al. Symptomatic hyponatremia during treatment of dehydrating diarrheal disease with reduced osmolarity oral rehydration solution. JAMA. 2006;296:567–573. doi: 10.1001/jama.296.5.567. [DOI] [PubMed] [Google Scholar]
- 3.Allen L, Jones CT. Emergency department use of computed tomography in children with epilepsy and breakthrough seizure activity. J Child Neurol. 2007;22:1099–1101. doi: 10.1177/0883073807306249. [DOI] [PubMed] [Google Scholar]
- 4.Annegers JF, Hauser WA, Lee JR, et al. Incidence of acute symptomatic seizures in Rochester, Minnesota, 1935–1984. Epilepsia. 1995;36:327–333. doi: 10.1111/j.1528-1157.1995.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 5.Bach PB. Racial disparities and site of care. Ethn Dis. 2005;15:S31–S33. [PubMed] [Google Scholar]
- 6.Bethune P, Gordon K, Dooley J, et al. Which child will have a febrile seizure? Am J Dis Child. 1993;147:35–39. doi: 10.1001/archpedi.1993.02160250037013. [DOI] [PubMed] [Google Scholar]
- 7.Elixhauser A, Weinick RM, Betancourt JR, et al. Differences between Hispanics and non-Hispanic Whites in use of hospital procedures for cerebrovascular disease. Ethn Dis. 2002;12:29–37. [PubMed] [Google Scholar]
- 8.Farrar HC, Chande VT, Fitzpatrick DF, et al. Hyponatremia as the cause of seizures in infants: a retrospective analysis of incidence, severity, and clinical predictors. Ann Emerg Med. 1995;26:42–48. doi: 10.1016/S0196-0644(95)70236-9. [DOI] [PubMed] [Google Scholar]
- 9.Hampers LC, Thompson DA, Bajaj L, et al. Febrile seizure: measuring adherence to AAP guidelines among community ED physicians. Pediatr Emerg Care. 2006;22:465–469. doi: 10.1097/01.pec.0000226870.49427.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harden CL, Huff JS, Schwartz TH, et al. Reassessment: neuroimaging in the emergency patient presenting with seizure (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2007;69:1772–1780. doi: 10.1212/01.wnl.0000285083.25882.0e. [DOI] [PubMed] [Google Scholar]
- 11.Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: Contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576–586. doi: 10.4065/71.6.576. [DOI] [PubMed] [Google Scholar]
- 12.Henneman PL, DeRoos F, Lewis RJ. Determining the need for admission in patients with new-onset seizures. Ann Emerg Med. 1994;24:1108–1114. doi: 10.1016/S0196-0644(94)70240-3. [DOI] [PubMed] [Google Scholar]
- 13.Huff JS, Morris DL, Kothari RU, et al. Emergency department management of patients with seizures: a multicenter study. Acad Emerg Med. 2001;8:622–628. doi: 10.1111/j.1553-2712.2001.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 14.Krumholz A, Grufferman S, Orr ST, et al. Seizures and seizure care in an emergency department. Epilepsia. 1989;30:175–181. doi: 10.1111/j.1528-1157.1989.tb05451.x. [DOI] [PubMed] [Google Scholar]
- 15.Krumholz A, Wiebe S, Gronseth G, et al. Practice Parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2007;69:1996–2007. doi: 10.1212/01.wnl.0000285084.93652.43. [DOI] [PubMed] [Google Scholar]
- 16.Lewis RJ, Yee L, Inkelis SH, et al. Clinical predictors of post-traumatic seizures in children with head trauma. Ann Emerg Med. 1993;22:1114–1118. doi: 10.1016/S0196-0644(05)80974-6. [DOI] [PubMed] [Google Scholar]
- 17.Marson A, Jacoby A, Johnson A, et al. Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: a randomised controlled trial. Lancet. 2005;365:2007–2013. doi: 10.1016/S0140-6736(05)66694-9. [DOI] [PubMed] [Google Scholar]
- 18.McCaig LF, McLemore T. Plan and operation of the National Hospital Ambulatory Medical Survey. Series 1: programs and collection procedures. Vital Health Stat 1. 1994;34:1–78. [PubMed] [Google Scholar]
- 19.O’Malley KJ, Cook KF, Price MD, et al. Measuring diagnoses: ICD code accuracy. Health Serv Res. 2005;40:1620–1639. doi: 10.1111/j.1475-6773.2005.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong S, Talan DA, Moran GJ, et al. Neurocysticercosis in radiographically imaged seizure patients in U.S. emergency departments. Emerg Infect Dis. 2002;8:608–613. doi: 10.3201/eid0806.010377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards CF, Lowe RA. Researching racial and ethnic disparities in emergency medicine. Acad Emerg Med. 2003;10:1169–1175. doi: 10.1111/j.1553-2712.2003.tb00599.x. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal RH, Heim ML, Waeckerle JF. First time major motor seizures in an emergency department. Ann Emerg Med. 1980;9:242–245. doi: 10.1016/S0196-0644(80)80379-9. [DOI] [PubMed] [Google Scholar]
- 23.Scarfone RJ, Pond K, Thompson K, et al. Utility of laboratory testing for infants with seizures. Pediatr Emerg Care. 2000;16:309–312. doi: 10.1097/00006565-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Schoenenberger RA, Heim SM. Indication for computed tomography of the brain in patients with first uncomplicated generalised seizure. BMJ. 1994;309:986–989. doi: 10.1136/bmj.309.6960.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sempere AP, Villaverde FJ, Martinez-Menendez B, et al. First seizure in adults: a prospective study from the emergency department. Acta Neurol Scand. 1992;86:134–138. doi: 10.1111/j.1600-0404.1992.tb05054.x. [DOI] [PubMed] [Google Scholar]
- 26.Sharma S, Riviello JJ, Harper MB, et al. The role of emergent neuroimaging in children with new-onset afebrile seizures. Pediatrics. 2003;111:1–5. doi: 10.1542/peds.111.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Smedley BD, Stith AY, Nelson AR. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academy Press; 2003. [PubMed] [Google Scholar]
- 28.Szaflarski M, Szaflarski JP, Privitera MD, et al. Racial/ethnic disparities in the treatment of epilepsy: what do we know? What do we need to know? Epilepsy Behav. 2006;9:243–264. doi: 10.1016/j.yebeh.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Tardy B, Lafond P, Convers P, et al. Adult first generalized seizure: etiology, biological tests, EEG, CT scan, in an ED. Am J Emerg Med. 1995;13:1–5. doi: 10.1016/0735-6757(95)90229-5. [DOI] [PubMed] [Google Scholar]
- 30.Turnbull TL, Vanden Hoek TL, Howes DS, et al. Utility of laboratory studies in the emergency department patient with a new-onset seizure. Ann Emerg Med. 1990;19:373–377. doi: 10.1016/S0196-0644(05)82337-6. [DOI] [PubMed] [Google Scholar]
- 31.Ryn M, Fu SS. Paved with good intentions: do public health and human service providers contribute to racial/ethnic disparities in health? Am J Public Health. 2003;93:248–255. doi: 10.2105/ajph.93.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]