Abstract
Undifferentiated or medullary carcinoma (MC) is characterized by its distinct histologic appearance and relatively better prognosis compared to poorly differentiated colonic carcinoma (PDC). These two entities may be difficult to differentiate by light microscopy alone. Only limited immunohistochemical studies investigating MC have been reported. These studies suggest a loss of intestinal differentiation, exemplified by a high percentage of CDX2 negativity. Our aim was to further characterize the immunohistochemical profile of MC, with particular emphasis on intestinal markers. Paraffin blocks from 16 cases of MC and 33 cases of PDC were retrieved and tissue microarrays were constructed and stained with an immunohistochemical panel including, CDX2, CK7, CK20, p53, intestinal trefoil factor 3 (TFF3), chromogranin, synaptophysin, MLH-1, MUC-1, MUC-2 and calretinin. A significantly higher proportion of MC, as opposed to PDC showed loss of staining for MLH-1 and for the intestinal transcription factor CDX2, in accordance with previous studies. MLH-1 staining was present in only 21% of MC cases compared with 60% of the PDC cases (p=0.02), whereas CDX2 was positive in 19% of MCs and 55% of PDCs (p=0.03). Interestingly, calretinin staining was strongly positive in 73% of MCs compared to only 12% of PDCs (p<0.0001). Evidence of intestinal differentiation by MUC-1, MUC-2 and TFF-3 staining was seen in 67, 60 and 53% of the MCs respectively. These three markers were frequently positive in many of the CDX2 negative MC cases. Medullary carcinoma of the colon retains a significant degree of intestinal differentiation as evidenced by its high percentage of staining for MUC-1, MUC-2, and TFF-3. Calretinin, MLH-1 and CDX2 may help to differentiate MC from PDC of the colon.
Keywords: Medullary, Calretinin, Colon, Carcinoma, CDX2
Introduction
Medullary carcinoma of the colon (MC) is a distinct subtype of colonic adenocarcinoma with a unique histologic appearance and a more favorable prognosis in comparison to poorly differentiated colonic adenocarcinomas (PDC). Previous studies have shown that MC as opposed to PDC of the colon are more often right sided, seen in older female patients, and have a lower incidence of lymph node metastases (1–6). Molecular studies have revealed that the majority of MCs exhibit microsatellite instability and show an absence of hMLH1 protein by immunohistochemistry (7). Morphologic features that characterize MC include a syncytial growth pattern, large vesicular nuclei with conspicuous nucleoli and a prominent intertumoral and peritumoral lymphocytic infiltrate (1–3). The tumor cells of PDC are typically more pleomorphic and more commonly exhibit some evidence of glandular differentiation. As there can be significant morphological overlap between MC and PDC, these two entities may be difficult to differentiate by hematoxylin and eosin light microscopy alone.
While the histological appearance of MC has been well described, there have been only limited reports describing its immunohistochemical profile. Typically, MC exhibits loss of MLH-1, variable expression of p53 and neuroendocrine markers and decreased expression of CDX2 (2, 4–7). Only a few studies have directly compared the immunohistochemical phenotype of MC to PDCs. Wick et al found no significant difference between the two entities in expression of p53 or neuroendocrine markers (2). Lanza et al described reduced p53 expression in MC and Sugao et al found that MCs were more likely to be NSE and bcl-2 positive than PDC (4,5).
The aim of this study was to further characterize the immunohistochemical phenotype of MC and to determine whether immunohistochemistry may aid in differentiating MC from PDC. In addition to the markers previously examined in MC we also examined the differential expression of cytokeratin 7 and 20 as well as expression of calretinin which has been shown to be expressed more commonly in PDC as compared to well differentiated cancers (8); however, has not been assessed in MC. Finally, seeing that undifferentiated MC as opposed to PDC have no morphological features of intestinal differentiation we tested whether MC exhibit expression of proteins commonly associated with intestinal differentiation such as trefoil factor 3 (TFF3), MUC1 and MUC2 (9–11).
Material and Methods
Patients and samples
Formalin-fixed paraffin embedded tissues from 16 patients with MC and 33 patients with PDC were collected from the archives of the Department of Pathology at the Rhode Island and Miriam Hospitals in accordance with Institutional Review Board approvals from both hospitals. Cases were selected by reviewing the written reports of all colon cancer patients diagnosed between the years 1984–2008. Histological sections described from tumors as either PDC, MC or undifferentiated were re-reviewed by MR, BW and JG and only those cases that fulfilled the WHO criteria for MC and PDC were selected for this study (12). Pure neuroendocrine carcinomas as identified by H&E staining and signet ring cell carcinomas were excluded from this study.
Tissue microarray construction
Hematoxylin-eosin-stained slides from each case were examined and areas of pure invasive carcinoma were identified. The corresponding areas on the paraffin embedded source blocks were identified and marked. The source blocks were then cored and a 1-mm core was transferred to the “master block” using the Beecher Tissue Microarrayer (Beecher Instruments, Silver Spring, MD). Approximately three to five tissue cores of tumor were obtained for each case. Additionally, two to three cores of histologically normal adjacent colonic mucosal tissue were obtained and arrayed in the same manner.
Immunohistochemistry
Immunohistochemical staining for each antigen was performed on 5 micron paraffin sections of each of the tissue microarray masterblocks. The immunohistochemical panel included CK7, CK20, p53, trefoil factor 3 (TFF3), chromogranin, synaptophysin, MLH-1, MUC-1 and MUC-2. Slides stained for CDX2 (mouse antibody, clone CDX2-88, 1:50, Biogenex, San Ramon, CA), CK7 (mouse antibody, OV-TL12/30, 1:100, DAKO, Carpenteria, CA), CK20 (mouse antibody, clone KS20.8, 1:40, DAKO), p53 (mouse antibody, clone DO-7, 1:15000, DAKO), chromogranin (mouse antibody, clone LK2H10, 1:40, Covance, Princeton, NJ), synaptophysin (mouse antibody, clone SNP88, 1:800, Biogenex), MLH-1 (mouse antibody, clone G168-15, 1:25, BD Bioscience, San Diego, CA), MUC1 (mouse antibody, Neomarkers, 1:40, Fremont, CA), MUC2 (mouse antibody, 1:100, Neomarkers,), TFF3 (mouse antibody, Gift from Felicity E. May,1:40) (13), and calretinin (mouse antibody, clone Z11-E3, 1:100, Invitrogen, Carlsbad, CA), were all performed using the Dako Autostainer with the Dako Envision Plus kit or manually with the Dako Envision Plus kit. The slides were counter stained with hematoxylin and dehydrated in graded alcohols through to xylene and coverslipped. Normal colonic mucosa served as a positive control for cytokeratin 20, CDX2, TFF3, MUC1 and MUC2. A stage II colonic cancer tumor microarray served as the positive control for p53 (14) and a custom made multi-tumor tissue microarray served as the control for cytokeratin 7 and calretinin.
Immunohistochemical assessment
All of the immunohistochemical stains were scored using a two-tiered scoring system: positive or negative. For p53, greater than 10% of nuclear staining was scored as positive (14). For the remainder of the stains, a positive score required moderate to strong staining of more than 25% of the tumor cell population, whereas a negative score included tumors with focal or weak staining. This cutoff was reached based on personal experience and allowed for minimal interobserver variability in the scoring. BW and JG independently scored each of the sections without knowledge of the histologic diagnosis. There was a high correlation between the two scores and in the few discrepant cases, a consensus was reached after joint review.
Statistical analysis
Associations between tumor types and the immunohistochemical staining scores as well as between the tumor types and the survival rates at the end of the follow-up period were evaluated using the Chi square test or the Fisher’s exact test as appropriate. Two-tailed P values of 0.05 or less were considered to be statistically significant.
Results
Clinicopathologic features
The clinicopathologic characteristics are summarized in Table 1. The mean age at the time of surgery of the MC group was 75yrs and 65yrs for the PDC group (p=0.3). The vast majority (87%) of patients with MC were females whereas 42% of patients with PDC were female (p=0.004). MC occurred on the right side in 69% of patients and PDC was right sided in 45% of cases (p=0.07). Most of the poorly differentiated tumors presented at a higher tumor stage as opposed to the medullary group (p=0.002). The mean and median follow-up times were 38.9 and 20.0 months respectively with a range of 0.2 to 199 months. Prognostically the MC group did better with only 15% patients dying of disease as opposed to 53% of the PDC by the end of follow-up. Based on chart review only, three of the PDC patients and none of the MC patients fulfilled the clinical criteria of the Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer testing (15).
Table 1.
Clinicopathologic Characteristics of 49 Cases of Colonic Medullary and Poorly Differentiated Adenocarcinoma
| Variable | MC (n = 16) | PDC (n = 33) | P-value |
|---|---|---|---|
| Age at surgery (y) | NS | ||
| Mean | 75.3 | 69.3 | |
| Range | 55–89 | 33–89 | |
| Sex | 0.004 | ||
| Male | 2 (13%) | 19 (58%) | |
| Female | 14 (87%) | 14 (42%) | |
| Locationa | NS | ||
| Right colon | 11 (69%) | 15 (45%) | |
| Left colon | 5 (31%) | 18 (55%) | |
| Tumor stageb | 0.002 | ||
| I | 3 (19%) | 3 (9%) | |
| II | 7 (44%) | 4 (12%) | |
| III | 5 (31%) | 18 (55%) | |
| IV | 1 (6%) | 8 (24%) |
Cutoff for right vs left is hepatic flexure
AJCC stage
MC: Medullary carcinoma, PDC: Poorly differentiated carcinoma
NS: Not statistically significant (p<0.05)
MCs were comprised of solid sheets and trabeculae of uniform small to medium sized neoplastic cells with a moderate amount of eosinophilic or amphophilic cytoplasm (Figure 1A). The nuclei were relatively uniform, and exhibited a vesicular chromatin pattern with prominent nucleoli. Typically there was an intense intra- and peritumoral lymphoplasmacytic infiltrate as well as a Crohn-like lymphoid reaction.
Figure 1.
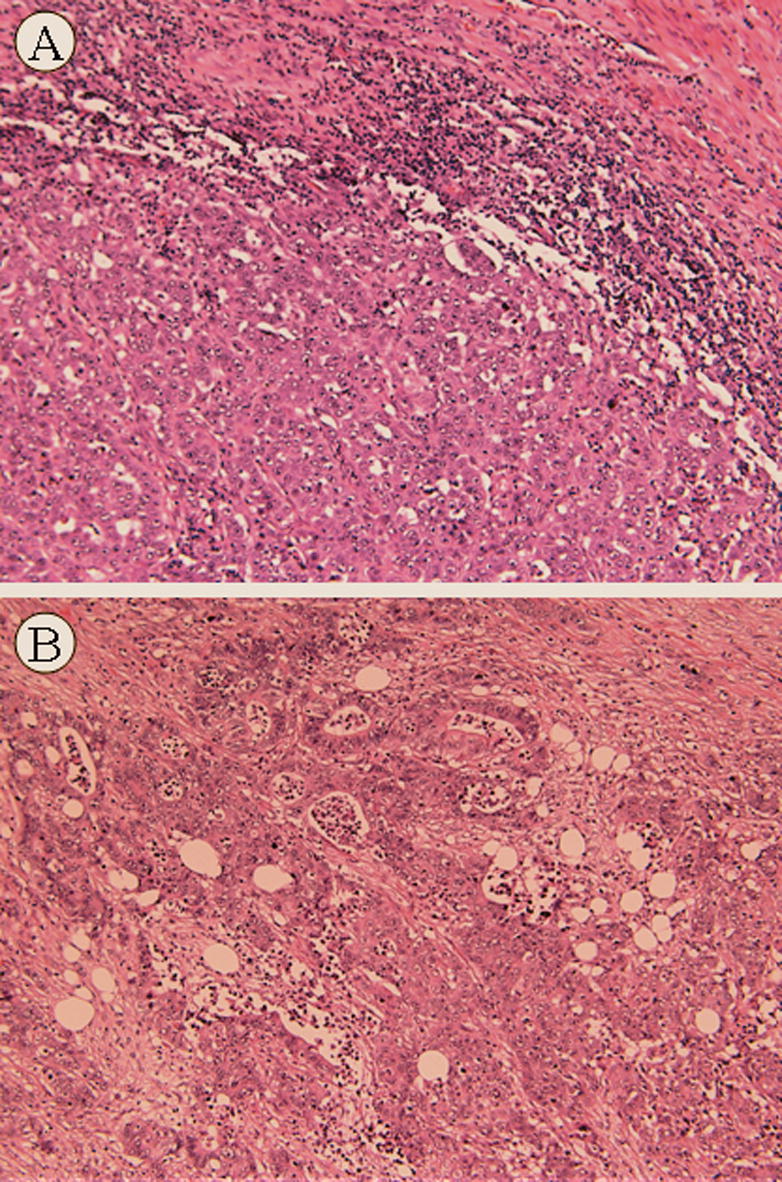
Hematoxylin and eosin stains of Medullary carcinoma (x25) (A). Poorly differentiated adenocarcinoma (x25) (B).
The criterion for the diagnosis of PDC was that over 50% of the tumor cell population grew in solid sheets or single cells. The neoplastic cells were medium to large sized and exhibited more severe nuclear pleomorphism and chromatin variation than the MC group (Figure 1B). The PDC group exhibited at least focal areas of gland formation, while only one of the MC cases exhibited a well-differentiated component comprising less than 5% of the tumor. The majority of both the PDC and MC stained with mucicarmine (73% and 67% respectively).
Immunohistochemical differentiation of MC from PDC
The staining patterns of the neoplastic cells for p53, MLH-1 and CDX2 were nuclear, for chromogranin, synaptophysin, CK7, CK20, TFF3, diffusely cytoplasmic and a combination of nuclear and cytoplasmic for calretinin. The staining pattern for MUC1, MUC2 and TFF3 in MC was diffusely cytoplasmic as opposed to a membranous pattern. The degree of tumor staining (both intensity and extent) was for the most part uniform between the cores, however, some tumor heterogeneity was present especially for the neuroendocrine markers and p53. Eight of the 11 immunohistochemical markers in the study showed no statistically significant differences (p>0.05) in staining between the two groups (MC versus PDC): The markers included cytokeratin 7 and 20, p53, the neuroendocrine markers, synaptophysin and chromogranin and the markers associated with intestinal differentiation MUC1, MUC2 and TFF3 (Table 2). Staining for MUC1, MUC2, and TFF3 were positive in 50% or more of MC cases (Fig. 2A–C).
Table 2.
Immunohistochemical Markers with no Significant Differential Staining
| MC (n=16) | PDC (n=33) | P-value | |
|---|---|---|---|
| CK7 | 2 (13%) | 7 (21%) | 0.96 |
| CK20 | 7 (44%) | 15 (45%) | 0.91 |
| p53 | 6 (38%) | 14 (44%) | 0.67 |
| Chromogranin | 0 (0%) | 5 (15%) | 0.15 |
| Synaptophysin | 0 (0%) | 1 (3%) | 1 |
| TFF3 | 8 (53%) | 20 (63%) | 0.75 |
| MUC1 | 10 (67%) | 21 (72%) | 0.73 |
| MUC2 | 9 (60%) | 19 (59%) | 0.96 |
MC-medullary carcinoma. PDC-poorly differentiated adenocarcinoma.
Figure 2.
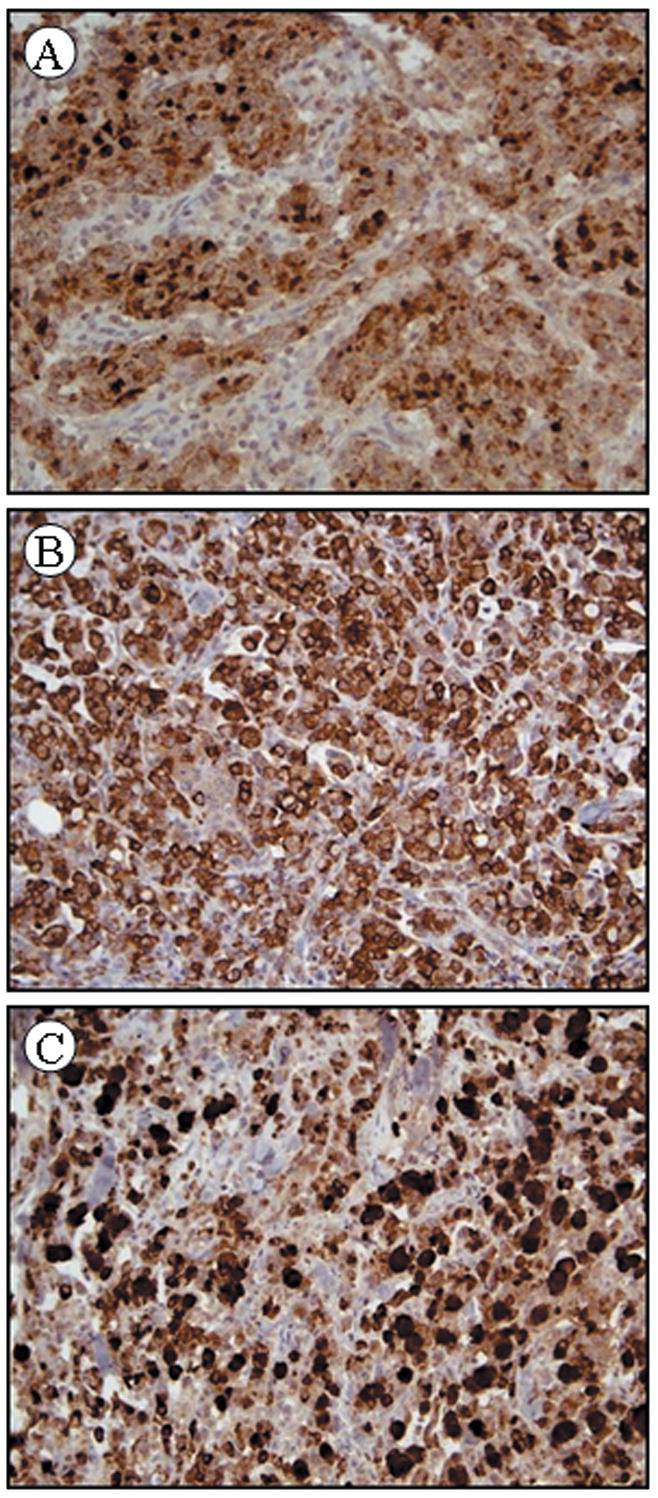
Medullary carcinoma showing diffuse cytoplasmic staining with MUC1 (A), MUC2 (B), and TFF3 (C). (x50 magnification)
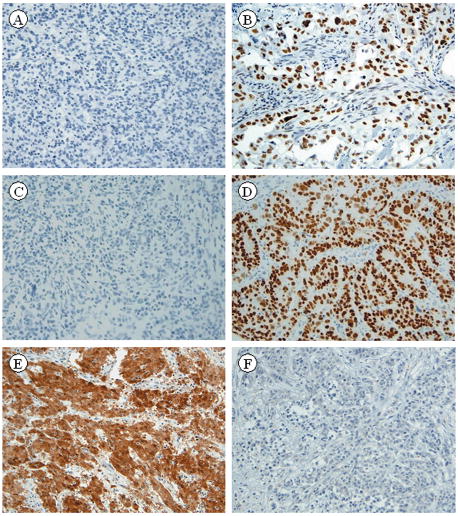
Three immunohistochemical markers, MLH-1, CDX2 and calretinin showed statistically significant differences in staining (p<0.05) between the MC and PDC groups (Table 3). MLH1 and CDX2 were positive in 21% and 19% of the MC as opposed to 60% and 55% of the PDC (p = 0.02 and p = 0.03 respectively). The differential staining pattern for calretinin was the most striking with 73% of the MC as opposed to 12% of the PDC staining positive (p < 0.0001). A CDX2 negative, MLH1 negative, and calretinin positive immunohistochemical phenotype has an 82% PPV for correctly distinguishing MC from PDC. Representative MC and PDC stained with MLH-1, CDX2 and calretinin are shown in figure 3A–F.
Table 3.
Immunohistochemical Markers with Significant Differential Staining
| MC (n=16) | PDC (n=33) | P-value | |
|---|---|---|---|
| MLH1 | 3 (21%) | 18 (60%) | 0.02 |
| CDX2 | 3 (19%) | 18 (55%) | 0.03 |
| Calretinin | 11 (73%) | 4 (12%) | <0.0001 |
MC-medullary carcinoma. PDC-poorly differentiated adenocarcinoma.
Figure 3.
Medullary carcinoma showing negative staining with MLH-1 (A) and poorly differentiated carcinoma showing positive nuclear staining with MLH-1 (B). Medullary carcinoma showing negative staining with CDX2 (C) and poorly differentiated carcinoma showing positive nuclear staining with CDX2 (D). Medullary carcinoma showing positive nuclear and cytoplasmic staining with calretinin (E), and poorly differentiated carcinoma showing negative staining for calretinin (F). (x50 magnification)
Discussion
Medullary carcinoma (MC) of the large intestine is a distinct entity that needs to be differentiated from poorly differentiated colorectal carcinoma (PDC). The neoplastic cells of MC are characteristically composed of sheets of uniform medium to large sized cells with amphiphilic cytoplasm, vesicular nuclei and prominent nucleoli, whereas, the neoplastic cells of PDC are typically more pleomorphic then those of MC, however, there are no strict morphological criteria that serve to differentiate these two entities. This is further compounded by the fact that certain morphological criteria for MC are somewhat vague. For example in the World Health Organization monograph (12) medullary carcinomas are defined as malignant tumors with no glandular structures or features of intestinal differentiation, whereas PDC exhibit at least some gland formation or mucus production (12). However, many previously reported case series of MC accept some evidence of glandular differentiation such as mucicarmine staining or residual areas of glandular differentiation (2–4). The term “large cell carcinoma with minimal differentiation” has also been used to describe what is probably MCs (6). As there is a considerable difference in prognosis between MC and PDC we attempted to determine whether immunohistochemistry might be used as an adjunct to differentiate between these two entities.
Few studies have attempted to compare the immunohistochemical characteristics of MC directly with those of PDC (2,4). Similar to the studies by Wick et al and Sugao et al, we found no difference in the frequency of p53 expression or neuroendocrine differentiation between these two tumors although the latter study found differences in the frequency of NSE expression (2,4). In our study, MLH-1 expression was significantly reduced in the MC as opposed to the PDC groups. This finding further supports the contention proposed by Alexander et al that the medullary phenotype of colon cancer is an effective predictor of microsatellite instability (16).
Calretinin is a calcium binding protein principally expressed in neurons (17). Its been shown to be more commonly expressed by PDC tumors as opposed to well-differentiated cancers; however, in the study by Gotzos et al no distinction was made between MC and PDC (8). Based on our study whereby 73% of MC as opposed to only 12% PDC express calretinin it appears that strong calretinin staining is quite unique to MCs. It is not clear why MC express calretinin, however, in a study using human colonic adenocarcinoma cell lines, Cargnello et al. showed a correlation between calretinin expression and loss of differentiation and proposed a role for calretinin in the maintenance of this undifferentiated state (18).
As MC characteristically have an undifferentiated morphological appearance we thought it would also be of interest to determine whether MC express markers typically associated with intestinal differentiation such as: CDX2, CK20, TFF3, MUC1 and MUC2. CDX2, a gene that encodes an intestinal-specific transcription factor expressed in the nuclei of intestinal epithelial cells (19), is reported to be a sensitive and specific marker of intestinal adenocarcinomas (20). In a recent study CDX2 was shown to stain a greater proportion of well to moderately differentiated than high-grade tumors (21). Hinoi et al reported that CDX2 shows a lower percentage of staining in MC compared to well differentiated colorectal carcinomas (6), suggesting a loss of intestinal differentiation in MC. Our study, the only report which directly compared expression of CDX2 between MC and PDC, found strongly significant decreased expression of CDX2 in MC as opposed to PDC. Although not specific for colorectal neoplasms, CK20 universally stains normal intestinal epithelium and the majority of colonic adenocarcinomas (22). Here we show that approximately one third of MC express CK20, which was not significantly different from the PDC group.
This is the first study to examine TFF3, MUC1 and MUC2 expression in MC. TFF3 otherwise known as the intestinal trefoil factor is a gene normally expressed in goblet cells of the intestinal epithelium (9) and is thought to play a role in epithelial restitution (23). The expression of TFF3 has been shown to be preserved in the human colonic adenocarcinoma sequence (24), and may be associated with aggressive behavior (23). MUC1, a non-secretory glycoprotein expressed along the apical membrane of colonic columnar epithelium, is expressed also by colorectal carcinomas and has been shown to behave as a marker for tumor progression (10,26). MUC2, considered as the principal secretory intestinal mucin, is expressed by goblet cells (11), colonic adenomas and adenocarcinomas (26,27). The staining pattern for MUC1, MUC2 and TFF3 in MC was diffusely cytoplasmic as opposed to a membranous pattern in normal colonic epithelium suggesting that these tumors are not able to glycosylate and transport these proteins into their original apical regions. However despite its undifferentiated morphology a significant proportion of MC express these three intestinal markers as MUC1, MUC2 and TFF3 staining was seen in 67, 60 and 53% of MC.
An immunohistochemical panel including MLH-1, CDX2, and calretinin would likely be useful in cases were the differential diagnosis of a poorly differentiated colorectal neoplasm includes MC and PDC. In our study a MLH-1 negative, CDX2 negative, calretinin positive phenotype had an 82% positive predictive value for correctly identifying MC. It will be of interest to determine whether calretinin is expressed by tumors with similar phenotypes to that of MC originating from other organs such as the stomach, nasopharynx and breast. If these medullary-like tumors do not express calretinin, then calretinin positivity may be suggestive of colonic origin in biopsies of metastatic disease where the primary is unknown and other markers typically positive in colonic adenocarcinoma (CK20 and CDX2) are negative. Finally, while some markers of intestinal differentiation such as CDX2 showed a low frequency of staining, evidence of intestinal differentiation by MUC1, MUC2 and TFF3 staining was seen in the majority of MC confirming that some degree of intestinal differentiation is preserved in MC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ruschoff J, Dietmaier W, Luttges J, Seitz G, Bocker T, Zirngibl H, Schlegel J, Schackert H, Jauch K, Hofstaedter F. Poorly differentiated colonic adenocarcinoma, medullary type: clinical, phenotypic, and molecular characteristics. Am J Pathol. 1997;150:1815–1825. [PMC free article] [PubMed] [Google Scholar]
- 2.Wick MR, Vitsky JL, Ritter JH, Swanson PE, Mills SE. Sporadic medullary carcinoma of the colon. Am J Clin Pathol. 2005;123:56–65. [PubMed] [Google Scholar]
- 3.Jessurun J, Romero-Guadarrama M, Manivel JC. Medullary adenocarcinoma of the colon: clinicopathologic study of 11 cases. Hum Pathol. 1999;30:843–848. doi: 10.1016/s0046-8177(99)90146-6. [DOI] [PubMed] [Google Scholar]
- 4.Sugao Y, Yao T, Kubo C, Tsuneyoshi M. Improved prognosis of solid-type poorly differentiated colorectal adenocarcinoma: a clinicopathological and immunohistochemical study. Histopathology. 1997;31:123–133. doi: 10.1046/j.1365-2559.1997.2320843.x. [DOI] [PubMed] [Google Scholar]
- 5.Lanza G, Gara R, Matteuzzi M, Santini A. Medullary-type poorly differentiated adenocarcinoma of the large bowel: a distinct clinicopathologic entity characterized by microsatellite instability and improved survival. J Clin Oncol. 1999;17:2429–38. doi: 10.1200/JCO.1999.17.8.2429. [DOI] [PubMed] [Google Scholar]
- 6.Hinoi T, Tani M, Lucas PC, Caca K, Dunn RL, Macri E, Loda M, Appelman HD, Cho KR, Fearon ER. Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am J Pathol. 2001;159:2239–2248. doi: 10.1016/S0002-9440(10)63074-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arai T, Esaki Y, Sawabe M, Honma N, Nakamura K, Takubo K. Hypermethylation of the hMLH1 promoter with absent hMLH1 expression in medullary-type poorly differentiated colorectal adenocarcinoma in the elderly. Mod Pathol. 2004;17:172–179. doi: 10.1038/modpathol.3800018. [DOI] [PubMed] [Google Scholar]
- 8.Gotzos V, Wintergerst E, Musy JP, Spichtin HP, Genton CY. Selective distribution of calretinin in adenocarcinomas of the human colon and adjacent tissues. Am J Surg Pathol. 1999;23:701–711. doi: 10.1097/00000478-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Podolsky DK, Lynch-Devaney K, Stow JL, Oates P, Murgue B, DeBeaumont M, Sands BE, Mahida YR. Identification of human intestinal trefoil factor: Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993;268:6694–6702. [PubMed] [Google Scholar]
- 10.Nakamori S, Ota DM, Cleary KR, Shirotani K, Irimura T. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology. 1994;106:353–361. doi: 10.1016/0016-5085(94)90592-4. [DOI] [PubMed] [Google Scholar]
- 11.Tytgat KM, Buller HA, Opdam FJ, Kim YS, Einerhand AW, Dekker J. Biosynthesis of human colonic mucin: MUC2 is the prominent secretory mucin. Gastroenterology. 1994;107:1352–1363. doi: 10.1016/0016-5085(94)90537-1. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton SR, Vogelstein B, Kudo S, Riboli E, Nakamura S, Hainaut P, et al. Carcinoma of the colon and rectum. In: Hamilton SR, Aaltonen L, editors. World Health Organization Classification of Tumors: Pathology and Genetics. Tumors of the Digestive System. IARC Press; Lyon, France: 2000. pp. 103–142. [Google Scholar]
- 13.Lafontaine PO, Arnal M, Buron N, Solary E, Bron AM, Westley BR, May FE, Bara J, Gespach C, Creuzot-Garcher C. Trefoil factor family mRNA and protein expression in pterygium. Int J Oncol. 2005;27:997–1003. [PubMed] [Google Scholar]
- 14.Resnick MB, Routhier J, Konkin T, Sabo E, Pricolo V. Epidermal growth factor receptor, c-MET, beta-catenin and p53 expression in Stage II colon cancer: A tissue microarray study. Clin Cancer Res. 2004;10:3069–75. doi: 10.1158/1078-0432.ccr-03-0462. [DOI] [PubMed] [Google Scholar]
- 15.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158:527–535. doi: 10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers JH. Calretinin: A gene for a novel calcium-binding protein expressed principally in neurons. J Cell Biol. 1987;105:1343–1353. doi: 10.1083/jcb.105.3.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cargnello R, Celio MR, Schwaller B, Gotzos V. Change of calretinin expression in the human colon adenocarcinoma cell line HT29 after differentaition. Biochim Biophys Acta. 1996;1313:201–208. doi: 10.1016/0167-4889(96)00090-0. [DOI] [PubMed] [Google Scholar]
- 19.Drummond F, Putt W, Fox M, et al. Cloning and chromosome assignment of the human CDX2 gene. Ann Hum Genet. 1997;61:393–400. doi: 10.1046/j.1469-1809.1997.6150393.x. [DOI] [PubMed] [Google Scholar]
- 20.Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin. Am J Surg Pathol. 2003;27:303–310. doi: 10.1097/00000478-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 21.De Lott LB, Morrison C, Suster S, Cohn DE, Frankel WL. CDX2 is a useful marker of intestinal type differentiation: A tissue microarray-based study of 629 tumors from various sites. Arch Pathol Lab Med. 2005;129:1100–1105. doi: 10.5858/2005-129-1100-CIAUMO. [DOI] [PubMed] [Google Scholar]
- 22.Miettinen M. Keratin 20: Immunohistochemical marker for gastrointestinal, urothelial, and Merkel cell carcinomas. Mod Pathol. 1995;8:384–388. [PubMed] [Google Scholar]
- 23.Dignass A, Lynch-Devaney K, Kindon H, Thim L, Podolsky Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1995;94:376–83. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taupin D, Ooi K, Yeomans N, Giraud A. Conserved expression of intestinal trefoil factor in the human colonic adenoma-carcinoma sequence. Lab Invest. 1996;75:25–32. [PubMed] [Google Scholar]
- 25.Yio X, Zhang J, Babyatsky M, Chen A, Lin J, Fan Q, Werther JL, Itzkowitz S. Trefoil factor family-3 is associated with aggressive behavior of colon cancer cells. Clin and Exp Metastasis. 2005;22:157–165. doi: 10.1007/s10585-005-6615-z. [DOI] [PubMed] [Google Scholar]
- 26.Ajioka Y, Allison LJ, Jass JR. Significance of MUC1 and MUC2 mucin expression in colorectal cancer. J Clin Pathol. 1996;49:560–564. doi: 10.1136/jcp.49.7.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blank M, Klussmann E, Kruger-Krasagakes S, Schmitt-Graff A, Stolte M, Bornhoeft G, Stein H, Xing PX, McKenzie IF, Verstijnen CP. Expression of MUC2-mucin in colorectal adenomas and carcinomas of different histological types. Int J Cancer. 1994;59:301–306. doi: 10.1002/ijc.2910590302. [DOI] [PubMed] [Google Scholar]